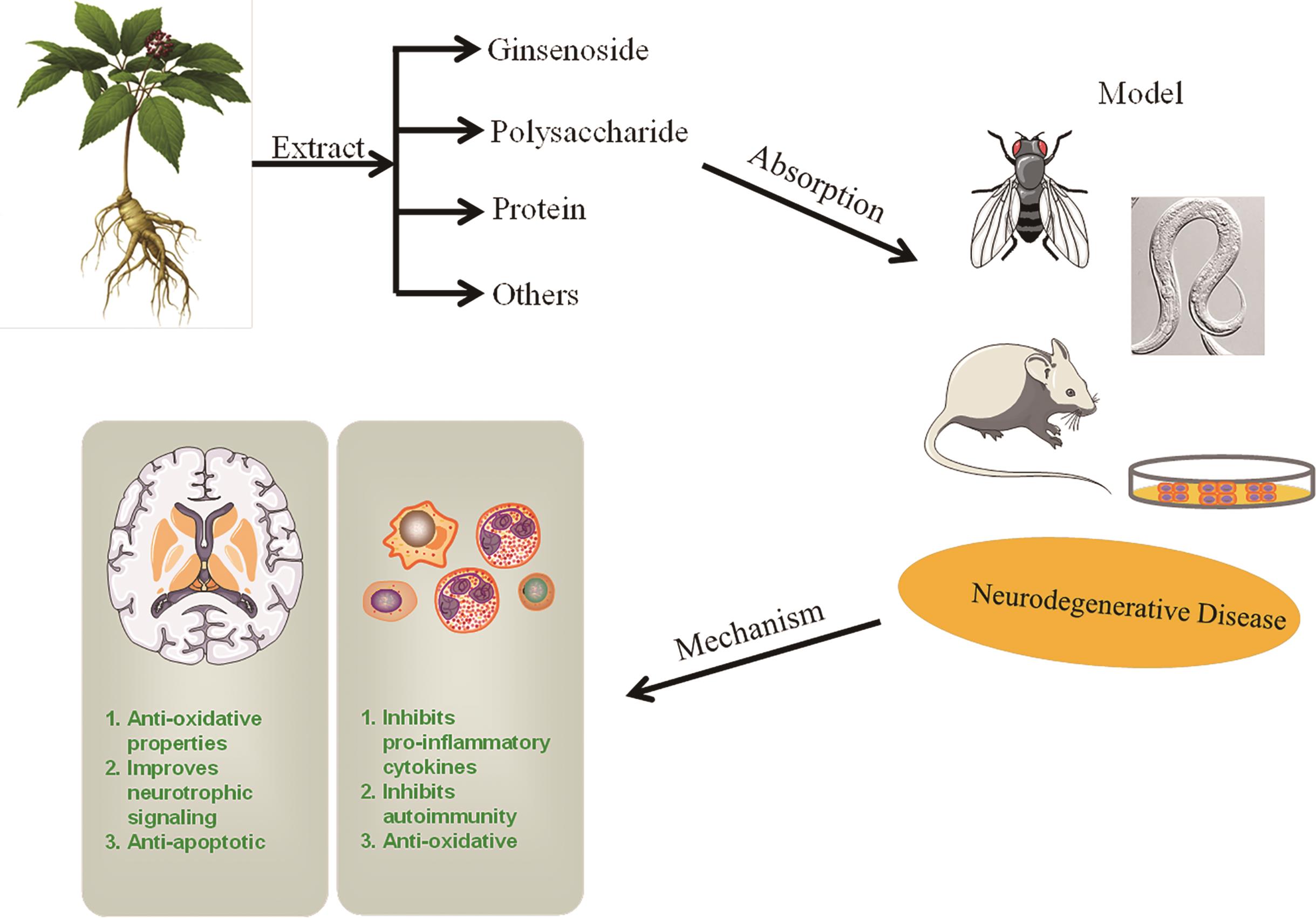
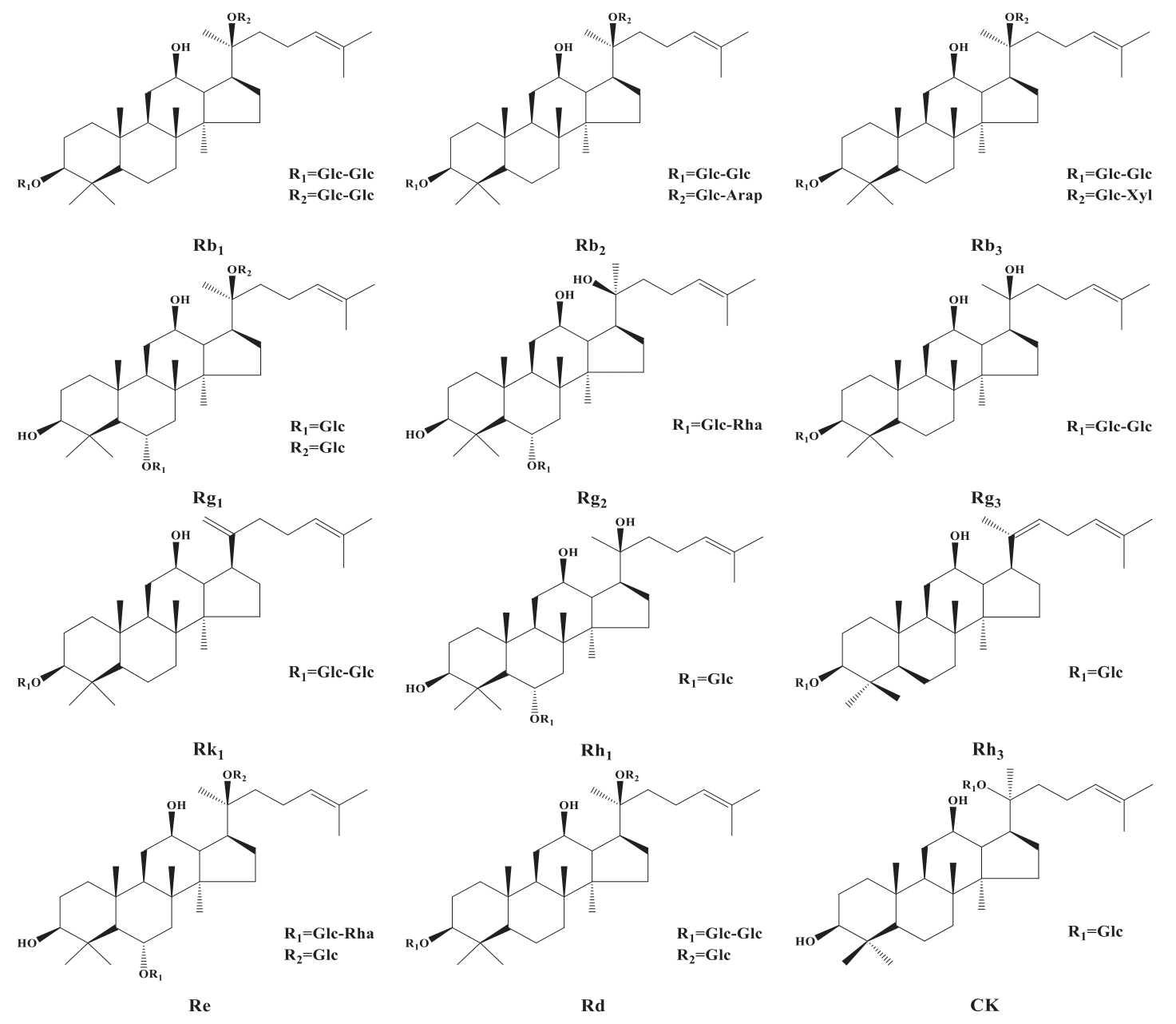
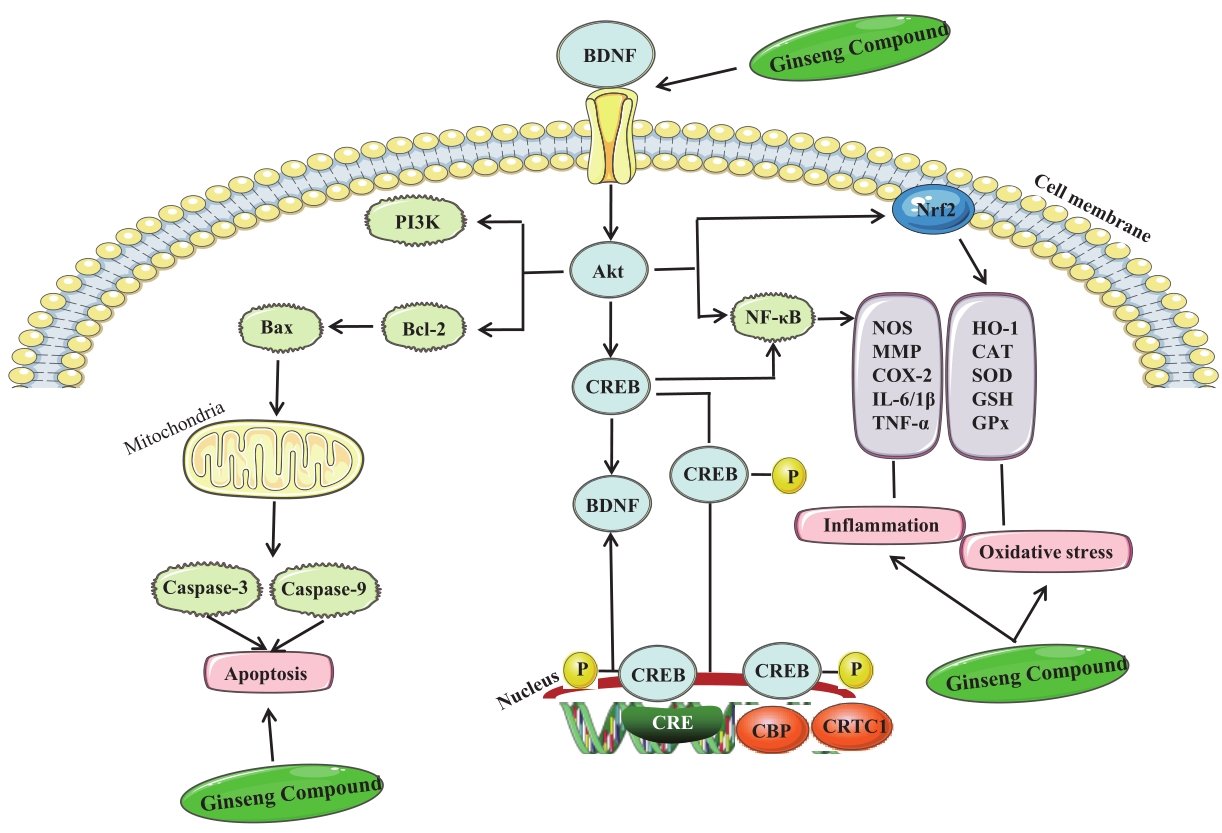
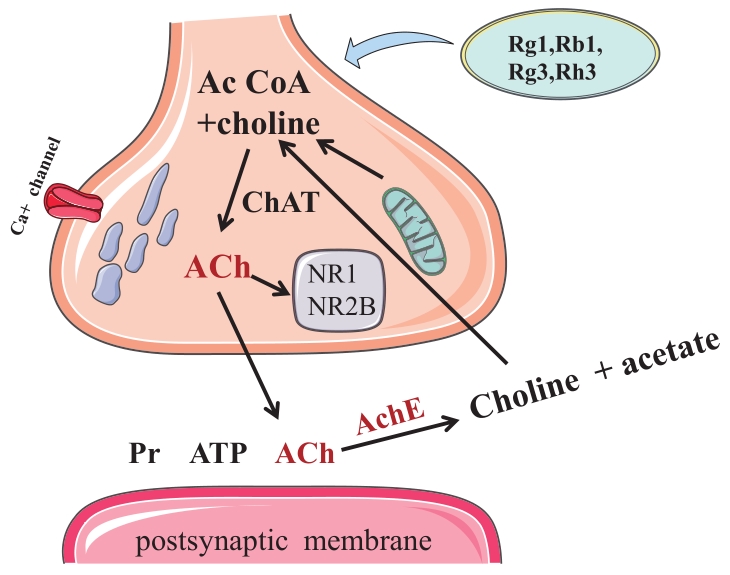
Chinese Journal of Applied Chemistry ›› 2023, Vol. 40 ›› Issue (4): 486-499.DOI: 10.19894/j.issn.1000-0518.220264
• Review • Previous Articles Next Articles
Research Progress of the Role of Chemical Active Components of Ginseng in Prevention and Treatment of Neurodegenerative Diseases
Wei-Yin XU1, Tian-Yang XU3, Si-Meng SHAO2, Zhao-Yang XIE2, Hong-Mei YANG2( ), Peng YU1(
), Peng YU1( )
)
- 1.School of Pharmacy,Changchun University of Traditional Chinese Medicine,Changchun 130117,China
2.Northeast Asia Institute of Chinese Medicine,Changchun University of Traditional Chinese Medicine,Changchun 130117,China
3.Centre for Innovation and Practice,Changchun University of Chinese Medicine,Changchun 130117,China
-
Received:2022-08-02Accepted:2023-01-28Published:2023-04-01Online:2023-04-17 -
Contact:Hong-Mei YANG,Peng YU -
Supported by:Jilin Province Science and Technology Development Plan Project(20220508086RC);the Health Technology Innovation Project of Jilin Province(2021JC075)
CLC Number:
Cite this article
Wei-Yin XU, Tian-Yang XU, Si-Meng SHAO, Zhao-Yang XIE, Hong-Mei YANG, Peng YU. Research Progress of the Role of Chemical Active Components of Ginseng in Prevention and Treatment of Neurodegenerative Diseases[J]. Chinese Journal of Applied Chemistry, 2023, 40(4): 486-499.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: http://yyhx.ciac.jl.cn/EN/10.19894/j.issn.1000-0518.220264
| Active ingredient | Activities | Neuropharmacological mechanisms | Ref. |
|---|---|---|---|
| Rb1, Rg5, Rh3 | Oxidative stress | Nrf2/HO-1, HO-1 | [ |
| Rg1, Rb1, Rg1/Rb1, Rh2 | SOD, GSH, CAT, MDA, BDNF | [ | |
| Rg1, Rg5 | Neuroinflammation | iNOS, COX-2, TNF-α, IL-1β, NF-κB, CREB, IL-6, PI3K/Akt | [ |
| Rg3 or Rh2 | IL-1α/β, IL-6, TNF-α, MCP-1, PI3K/Akt | [ | |
| Rg1 | TNF-α, IL-1β | [ | |
| Rg5 | TNF-α, IL-6, PI3K/Akt | [ | |
| RGO | IL-1β, IL-6, TNF-α, iNOS, COX-2 | [ | |
| Rg1 | Apoptosis | Akt, caspase-3, Bcl-xl | [ |
| Rg1, Rb1, Rg1/Rb1, Rg3, Rg2 | PI3K/Akt, Bcl-2/Bax, caspase-3, caspase-9 | [ | |
| Rd1, Rg2 | CREB, BDNF, caspase-3 | [ | |
| Rg1 | Akt, BDNF/TrkB | [ | |
| RGO, GP | PI3K/Akt, Bcl-2/Bax | [ | |
| Rg1, Rg3, Rb1, Rh3 | Cholinergic synapses | AchE, ACh, BDNF, p-TrkB,NR1, NR2B | [ |
Table 1 Relevant pathways of active components of ginseng in neurodegenerative diseases
| Active ingredient | Activities | Neuropharmacological mechanisms | Ref. |
|---|---|---|---|
| Rb1, Rg5, Rh3 | Oxidative stress | Nrf2/HO-1, HO-1 | [ |
| Rg1, Rb1, Rg1/Rb1, Rh2 | SOD, GSH, CAT, MDA, BDNF | [ | |
| Rg1, Rg5 | Neuroinflammation | iNOS, COX-2, TNF-α, IL-1β, NF-κB, CREB, IL-6, PI3K/Akt | [ |
| Rg3 or Rh2 | IL-1α/β, IL-6, TNF-α, MCP-1, PI3K/Akt | [ | |
| Rg1 | TNF-α, IL-1β | [ | |
| Rg5 | TNF-α, IL-6, PI3K/Akt | [ | |
| RGO | IL-1β, IL-6, TNF-α, iNOS, COX-2 | [ | |
| Rg1 | Apoptosis | Akt, caspase-3, Bcl-xl | [ |
| Rg1, Rb1, Rg1/Rb1, Rg3, Rg2 | PI3K/Akt, Bcl-2/Bax, caspase-3, caspase-9 | [ | |
| Rd1, Rg2 | CREB, BDNF, caspase-3 | [ | |
| Rg1 | Akt, BDNF/TrkB | [ | |
| RGO, GP | PI3K/Akt, Bcl-2/Bax | [ | |
| Rg1, Rg3, Rb1, Rh3 | Cholinergic synapses | AchE, ACh, BDNF, p-TrkB,NR1, NR2B | [ |
| 1 | XIANG Y Z, SHANG H C, GAO X M, et al. A comparison of the ancient use of ginseng in traditional Chinese medicine with modern pharmacological experiments and clinical trials[J]. Phytother Res, 2010, 22(7): 851-858. |
| 2 | 陈林, 郑红英, 郭建鹏. 基于响应面法的人参新炮制品“黑参”提取工艺研究[J]. 中华中医药杂志, 2021, 36(3): 1607-1611. |
| CHEN L, ZHENG H Y, GUO J P. Study on extraction process of new ginseng products ‘black Ginseng’ based on response surface method[J]. China J Tradit Chin Med Pharm, 2021, 36(3): 1607-1611. | |
| 3 | 曾琪, 刘杨波, 谌浩东, 等. 高效液相色谱指纹图谱及一测多评法评价黑参的质量[J]. 中药新药与临床药理, 2021, 32(11): 1710-1715. |
| ZENG Q, LIU Y B, CHEN H D, et al. Quality evaluation of black ginseng based on HPLC fingerprint and QAMS[J]. Tradit Chin Drug Res Clin Pharmacol, 2021, 32(11): 1710-1715. | |
| 4 | 卢蕾, 敖曼, 陈舒雅, 等. 一测多评法同时测定黑参中12种单体皂苷含量[J]. 中药材, 2020, 43(2): 394-397. |
| LU L, AO M, CHEN S Y, et al. Simultaneous determination of 12 monomeric saponins in black ginseng by one test and multiple evaluations[J]. J Chin Med Mater 2020, 43(2): 394-397. | |
| 5 | METWALY A M, ZHU L, HUANG L, et al. Black ginseng and its saponins: preparation, phytochemistry and pharmacological effects[J]. Molecules, 2019, 24(10): 1856. |
| 6 | LEE D K, PARK S, LONG N P, et al. Research quality-based multivariate modeling for comparison of the pharmacological effects of black and red ginseng[J]. Nutrients, 2020, 12(9): 2590. |
| 7 | KWON H S, KOH S H. Neuroinflammation in neurodegenerative disorders: the roles of microglia and astrocytes[J]. Transl Neurodegener, 2020, 9(4): 12. |
| 8 | JEFFREY L C, GARY T, CLIVE B. Treatment combinations for Alzheimer′s disease: current and future pharmacotherapy options[J]. J Alzheimers Dis, 2019, 67(3): 779-794. |
| 9 | AMMAR A C. Preventing neurodegenerative disease[J]. Brain, 2021, 144(5): 1279-1280. |
| 10 | PENG C, TROJANOWSKI J Q, LEE M Y. Protein transmission in neurodegenerative disease[J]. Nat Rev Neurol, 2020, 16(4): 199-212. |
| 11 | GHIDONI R, PATERLINI A, BENUSSI L. Translational proteomics in Alzheimer′s disease and related disorders[J]. Clin Biochem, 2013, 46(6): 480-486. |
| 12 | BUTTERFIELD D A, SULATANA R. Proteomics analysis in Alzheimer′s disease: new insights into mechanisms of neurodegeneration[J]. Int Rev Neurobiol, 2004, 61(12): 159-188. |
| 13 | BLOEM B R, OKUN M S, KLEIN C. Parkinson′s disease[J]. Lancet, 2021, 397(10291): 2284-2303. |
| 14 | OH J, KIM J S. Compound K derived from ginseng: neuroprotection and cognitive improvement[J]. Food Funct, 2016, 7(11): 4506-4515. |
| 15 | SEO J Y, JU S H, OH J, et al. Neuroprotective and cognition-enhancing effects of compound K isolated from Red ginseng[J]. Food Chem, 2016, 64(14): 2855-2864. |
| 16 | JAKARIA M, HAQUE M E, KIM J, et al. Active ginseng components in cognitive impairment: therapeutic potential and prospects for delivery and clinical study[J]. Oncotarget, 2018, 9(71): 33601-33620. |
| 17 | HENEKA M T, KUMMER M P, LATZ E. Innate immune activation in neurodegenerative disease[J]. Nat Rev Immunol, 2014, 14(7): 463-477. |
| 18 | HICKMAN S, IZZY S, SEN P, et al. Microglia in neurodegeneration[J]. Nat Neurosci, 2018, 21: 1359-1369. |
| 19 | UDDIN M S, KABIR M T, JALOULI M, et al. Neuroinflammatory signaling in the pathogenesis of Alzheimer′s disease[J]. Curr Neuropharmacol, 2022, 20(1): 126-146. |
| 20 | BERG J V, PROKOP S, MILLER K R, et al. Inhibition of IL-12/IL-23 signaling reduces Alzheimer′s disease-like pathology and cognitive decline[J]. Nat Med, 2012, 18(12): 1812-1819. |
| 21 | KANG A, XIE T, ZHU D, et al. Suppressive effect of Ginsenoside Rg3 against lipopolysaccharide-induced depression-like behavior and neuroinflammation in mice[J]. Agric Food Chem, 2017, 65(32): 6861-6869. |
| 22 | KUMAR A, RINWA P, DHAR H. Microglial inhibitory effect of ginseng ameliorates cognitive deficits and neuroinflammation following traumatic head injury in rats[J]. Inflammopharmacology, 2014, 22(3): 155-168. |
| 23 | WAN J, DENG L, ZHANG C, et al. Chikusetsu saponin V attenuates H2O2-induced oxidative stress in human neuroblastoma SH-SY5Y cells through Sirt1/PGC-1α/Mn-SOD signaling pathways[J]. Can J Physiol Pharmacol, 2016, 94(9): 919-928. |
| 24 | SELIM G N, IBRAHIM Y, BARAN K, et al. A comparison of the effects of neuronal nitric oxide synthase and inducible nitric oxide synthase inhibition on cartilage damage[J]. Bio Med Res Int, 2016, 2016: 7857345. |
| 25 | CHEN C, CAO J, MA X, et al. Neuroprotection by polynitrogen manganese complexes: regulation of reactive oxygen species-related pathways[J]. Sci Rep, 2016, 6: 20853. |
| 26 | ILARIA L, GENNARO R, FRANCESCO C, et al. Oxidative stress, aging, and diseases[J]. Clin Interventions Aging, 2018, 13: 757-772. |
| 27 | NABAVI S F, SUREDA A, HABTEMARIAM S, et al. Ginsenoside Rd and ischemic stroke; a short review of literatures[J]. J Ginseng Res, 2015, 39(4): 299-303. |
| 28 | ZHOU T T, ZU G, WANG X, et al. Immunomodulatory and neuroprotective effects of ginsenoside Rg1 in the MPTP(1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) -induced mouse model of Parkinson′s disease[J]. Int Immunopharmacol, 2015, 29(2): 334-343. |
| 29 | PARK Y J, CHO M, CHOI G, et al. A critical regulation of Th17 cell responses and autoimmune neuro-inflammation by ginsenoside Rg3[J]. Biomolecules, 2020, 10(1): 122-133. |
| 30 | LONG J M, HOLTZMAN D M. Alzheimer disease: an update on pathobiology and treatment strategies[J]. Cell, 2019, 179(2): 312-339. |
| 31 | BUDZYNSKA B, BOGUSZEWSKA C A, KRUK S M, et al. Effects of imperatorin on scopolamine-induced cognitive impairment and oxidative stress in mice[J]. Psychopharmacology, 2015, 232(5): 931-942. |
| 32 | WANG Q, SUN L H, JIA W, et al. Comparison of ginsenosides Rg1 and Rb1 for their effects on improving scopolamine‐induced learning and memory impairment in mice[J]. Phytother Res, 2010, 24(12): 1748-1754. |
| 33 | TODOROVA V, BLOKLAND A. Mitochondria and synaptic plasticity in the mature and aging nervous system[J]. Curr Neuropharmacol, 2017, 15(1): 166-173. |
| 34 | SONG Z, SHEN F, ZHANG Z, et al. Calpain inhibition ameliorates depression-like behaviors by reducing inflammation and promoting synaptic protein expression in the hippocampus[J]. Neuropharmacology, 2020, 174: 108175. |
| 35 | 王娟, 申丰铭, 张峥嵘, 等. 人参皂苷Rg1对慢性应激小鼠抑郁样行为、海马突触蛋白及胶质细胞的作用[J]. 生物学杂志, 2021, 38(3): 26-30. |
| WANG J, SHEN F M, ZHANG Z R, et al. Effects of ginsenoside Rg1 on depression-like behaviors,expression of hippocampal synaptic proteins and activation of glial cells in stressed mice[J]. J Biol, 2021, 38(3): 26-30. | |
| 36 | BIKASH C, ECKHARD M, EVA-MARIA M, et al. Glutamatergic nervous system degeneration in a C. elegans. Tau A152T tauopathy model involves pathways of excitotoxicity and Ca2+ dysregulation[J]. Neurobiol Dis, 2018, 117: 189-202. |
| 37 | BIKASH C, ECKHARD M, EVA-MARIA M, et al. Glutamatergic nervous system degeneration in a C.elegans TauA152T tauopathy model involves pathways of excitotoxicity and Ca2+ dysregulation[J]. Neurobiol Dis, 2018, 117: 189-202. |
| 38 | ZHANG Y, YANG X M, WANG S, et al. Ginsenoside Re3 prevents cognitive impairment by improoing mitochondrial dysfunction in the rat model of Alzheimer's disease[J]. Agric Food Chem, 2019, 67(36): 10048-10058. |
| 39 | KWAN K, YUN H, DONG T, et al. Ginsenosides attenuate bioenergetics and morphology of mitochondria in cultured PC12 cells under the insult of amyloid beta-peptide[J]. J Ginseng Res, 2021, 45(4): 473-481. |
| 40 | SHIN S J, NAM Y, PARK Y H, et al. Therapeutic effects of non-saponin fraction with rich polysaccharide from Korean red ginseng on aging and Alzheimer′s disease[J]. Free Radical Biol Med, 2021, 164: 233-248. |
| 41 | NOUR S E. Apoptosis and its therapeutic implications in neurodegenerative diseases[J]. Clin Anat, 2022, 35(1): 65-78. |
| 42 | LIN M T, BEAL M F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases[J]. Nature, 2006, 443: 787-795. |
| 43 | SANG E P, PARK C, SUN H K, et al. Korean red ginseng extract induces apoptosis and decreases telomerase activity in human leukemia cells[J]. J Ethnopharmacol, 2009, 121(2): 304-312. |
| 44 | HWANG J Y, SHIM J S, SONG M Y, et al. Proteomic analysis reveals that the protective effects of ginsenoside Rb1 are associated with the actin cytoskeleton in beta-amyloid-treated neuronal cells[J]. J Ginseng Res, 2016, 40(3): 278-284. |
| 45 | WANG Y, LI Y, YANG W, et al. Ginsenoside Rb1 inhibit apoptosis in rat model of Alzheimer′s disease induced by Aβ1-40[J]. Am J Transl Res, 2018, 10(3): 796-805. |
| 46 | LIU Y, ZONG X, HUANG J, et al. Ginsenoside Rb1 regulates prefrontal cortical GABAergic transmission in MPTP-treated mice[J]. Aging, 2019, 11(14): 5008-5034. |
| 47 | CUI J, SHAN R, CAO Y, et al. Protective effects of ginsenoside Rg2 against memory impairment and neuronal death induced by Abeta25-35 in rats[J]. J Ethnopharmacol, 2021, 266: 113466. |
| 48 | CHRISRTENSEN L P. Ginsenosides: chemistry, biosynthesis, analysis, and potential health effects[J]. Food Nutr Res, 2008, 55: 1-99. |
| 49 | KENNEDY D O, SCHOLEY A B. Ginseng: potential for the enhancement of cognitive performance and mood[J]. Pharmacol Biochem Behav, 2003, 75(3): 687-700. |
| 50 | PARK C S, YOO M H, NOH K H, et al. Biotransformation of ginsenosides by hydrolyzing the sugar moieties of ginsenosides using microbial glycosidases[J]. Appl Microbiol Biotechnol, 2010, 87(1): 9-19. |
| 51 | KIM E J, JUNG I H, VAN LE T K, et al. Ginsenosides Rg5 and Rh3 protect scopolamine-induced memory deficits in mice[J]. J Ethnopharmacol, 2013, 146(1): 294-299. |
| 52 | WANG Y Z, CHEN J, CHU S F, et al. Improvement of memory in mice and increase of hippocampal excitability in rats by ginsenoside Rg1′s metabolites ginsenoside Rh1 and protopanaxatriol[J]. J Pharmacol Sci, 2009, 109(4): 504-510. |
| 53 | XU K, ZHANG Y, WANG Y, et al. Ginseng Rb fraction protects glia, neurons and cognitive function in a rat model of neurodegeneration[J]. PLoS One, 2014, 9(6): e101077. |
| 54 | SONG X Y, HU J F, CHU S F, et al. Ginsenoside Rg1 attenuates okadaic acid induced spatial memory impairment by the GSK3beta/tau signaling pathway and the Abeta formation prevention in rats[J]. Eur J Pharmacol, 2013, 710(1/2/3): 29-38. |
| 55 | ZHOU T T, ZU G, WANG X, et al. Immunomodulatory and neuroprotective effects of ginsenoside Rg1 in the MPTP(1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine)-induced mouse model of Parkinson′s disease[J]. Int Immunopharmacol, 2015, 29(2): 334-343. |
| 56 | HENG Y, ZHANG Q S, MU Z, et al. Ginsenoside Rg1 attenuates motor impairment and neuroinflammation in the MPTP-probenecid-induced parkinsonism mouse model by targeting alpha-synuclein abnormalities in the substantia nigra[J]. Toxicol Lett, 2016, 243: 7-21. |
| 57 | JIN Y, PENG J, WANG X, et al. Ameliorative effect of Ginsenoside Rg1 on lipopolysaccharide-induced cognitive impairment: role of cholinergic system[J]. Neurochem Res, 2017, 42(5): 1299-1307. |
| 58 | HUANG L, PENG Z, LU C, et al. Ginsenoside Rg1 alleviates repeated alcohol exposure-induced psychomotor and cognitive deficits[J]. Chin Med, 2020, 15: 44-55. |
| 59 | ZHANG H, SU Y, SUN Z, et al. Ginsenoside Rg1 alleviates Abeta deposition by inhibiting NADPH oxidase 2 activation in APP/PS1 mice[J]. J Ginseng Res, 2021, 45(6): 665-675. |
| 60 | KIM J, SJIM J, LEE S, et al. Rg3-enriched ginseng extract ameliorates scopolamine-induced learning deficits in mice[J]. BMC Complementary Altern Med, 2016, 16: 66-75. |
| 61 | CHALOTAK P, SANGUANPHUN T, LIMBOONEREUNG T, et al. Neurorescue effects of frondoside A and Ginsenoside Rg3 in C. elegans model of Parkinson′s disease[J]. Molecules, 2021, 26(16): 4843-4859. |
| 62 | JINGANG H, SUNCHANG K, CHANGKEUN S, et al. Ginsenoside Rg3 prevents oxidative stress-induced astrocytic senescence and ameliorates senescence paracrine effects on glioblastoma[J]. Molecules, 2017, 22(9): 1516-1532. |
| 63 | CUI J, SHAN R, CAO Y, et al. Protective effects of ginsenoside Rg2 against memory impairment and neuronal death induced by Abeta25-35 in rats[J]. J Ethnopharmacol, 2021, 266: 113466. |
| 64 | HOU J, XUE J, LEE M, et al. Ginsenoside Rd as a potential neuroprotective agent prevents trimethyltin injury[J]. Biomed Rep, 2017, 22: 435-440. |
| 65 | LIU J F, YAN X D, LI L, et al. Ginsenoside Rd improves learning and memory ability in APP transgenic mice[J]. J Mol Neurosci, 2015, 57(4): 522-528. |
| 66 | LEE K W, JUNG S Y, CHOI S M, et al. Effects of ginsenoside Re on LPS-induced inflammatory mediators in BV2 microglial cells[J]. BMC Complementary Altern Med, 2012, 12(1): 196-204. |
| 67 | SHI J, XUE W, ZHAO W J, et al. Pharmacokinetics and dopamine/acetylcholine releasing effects of ginsenoside Re in hippocampus and mPFC of freely moving rats[J]. Acta Pharmacol Sin, 2013, 2: 214-220. |
| 68 | CAI M, YANG E J. Ginsenoside Re attenuates neuroinflammation in a symptomatic ALS animal model[J]. Am J Chin Med, 2016, 44(2): 401-413. |
| 69 | YANG Q, LIN J, ZHANG H, et al. Ginsenoside compound K regulates amyloid beta via the Nrf2/Keap1 signaling pathway in mice with scopolamine hydrobromide-induced memory impairments[J]. J Mol Neurosci, 2019, 67(1): 62-71. |
| 70 | HOU J G, XUE J J, LEE M R, et al. Compound K is able to ameliorate the impaired cognitive function and hippocampal neurogenesis following chemotherapy treatment[J]. Biochem Biophys Res Commun, 2013, 436(1): 104-109. |
| 71 | HUA K F, CHAO A C, LIN T Y, et al. Ginsenoside compound K reduces the progression of Huntington′s disease via the inhibition of oxidative stress and overactivation of the ATM/AMPK pathway[J]. J Ginseng Res, 2021, 46(4):572-584. |
| 72 | LI Z, ZHAO L, CHEN J, et al. Ginsenoside Rk1 alleviates LPS-induced depression-like behavior in mice by promoting BDNF and suppressing the neuroinflammatory response[J]. Biochem Biophys Res Commun, 2020, 530(4): 658-664. |
| 73 | JU S, SEO J Y, LEE S K, et al. Oral administration of hydrolyzed red ginseng extract improves learning and memory capability of scopolamine-treated C57BL/6J mice via upregulation of Nrf2-mediated antioxidant mechanism[J]. Ginseng Res, 2021, 45(1): 108-118. |
| 74 | CHEN J, LI M, QU D, et al. Neuroprotective effects of red ginseng saponins in scopolamine-treated rats and activity screening based on pharmacokinetics[J]. Molecules, 2019, 24(11): 2136. |
| 75 | IQBAL H, KIM S K, CHA K M, et al. Korean red ginseng alleviates neuroinflammation and promotes cell survival in the intermittent heat stress-induced rat brain by suppressing oxidative stress via estrogen receptorbeta and brain-derived neurotrophic factor upregulation[J]. J Ginseng Res, 2020, 44(4): 593-602. |
| 76 | SHIN S J, PARK Y H, JEON S G, et al. Red ginseng inhibits Tau aggregation and promotes Tau dissociation in vitro[J]. Oxid Med Cell Longevity, 2020, 2020: 7829842. |
| 77 | CHOI J H, JANG M, NAH S Y, et al. Multitarget effects of Korean red ginseng in animal model of Parkinson′s disease: antiapoptosis, antioxidant, antiinflammation, and maintenance of blood-brain barrier integrity[J]. J Ginseng Res, 2018, 42(3): 379-388. |
| 78 | KIM J, KIM S H, LEE D S, et al. Effects of fermented ginseng on memory impairment and beta-amyloid reduction in Alzheimer′s disease experimental models[J]. J Ginseng Res, 2013, 37(1): 100-107. |
| 79 | CHPI J G, KIM N, HUH E, et al. White ginseng protects mouse hippocampal cells against amyloid-beta oligomer toxicity[J]. Phytother Res, 2017, 31(3): 497-506. |
| 80 | 赵莉, 郜玉钢, 姬庆. 人参化学成分的免疫作用及其机制的研究进展[J]. 中南药学, 2015, 13(7): 741-745. |
| ZHAO L, GAO Y G, JI Q. Immune effects and mechanism of ginseng[J]. Cent South Pharm, 2015, 13(7): 741-745. | |
| 81 | LOU H, HU J, WANG Y, et al. In vivo and in vitro neuroprotective effects of Panax ginseng glycoproteins[J]. Int J Biol Macromol, 2018, 113: 607-615. |
| 82 | SHIN S J, NAM Y, PARK Y H, et al. Therapeutic effects of non-saponin fraction with rich polysaccharide from Korean red ginseng on aging and Alzheimer′s disease[J]. Free Radical Biol Med, 2021, 164: 233-248. |
| 83 | XU T, SHEN X, YU H, et al. Water-soluble ginseng oligosaccharides protect against scopolamine-induced cognitive impairment by functioning as an antineuroinflammatory agent[J]. J Ginseng Res, 2016, 40(3): 211-219. |
| 84 | LI H, KANG T, QI B, et al. Neuroprotective effects of ginseng protein on PI3K/Akt signaling pathway in the hippocampus of D-galactose/AlCl3 inducing rats model of Alzheimer′s disease[J]. J Ethnopharmacol, 2016, 179: 162-169. |
| 85 | LIU M, YU S, WANG J, et al. Ginseng protein protects against mitochondrial dysfunction and neurodegeneration by inducing mitochondrial unfolded protein response in Drosophila melanogaster PINK1 model of Parkinson′s disease[J]. J Ethnopharmacol, 2020, 247: 112213. |
| 86 | LEE S, YOUN K, JOENG W S, et al. Protective effects of Red ginseng oil against Abeta25-35-induced neuronal apoptosis and inflammation in PC12 cells[J]. Int J Mol Sci, 2017, 18(10): 2218-2238. |
| 87 | LEE S, YOUN K, JUN M. Major compounds of red ginseng oil attenuate Abeta25-35-induced neuronal apoptosis and inflammation by modulating MAPK/NF-kappaB pathway[J]. Food Funct, 2018, 9(8): 4122-4134. |
| 88 | HOU J, XUE J, WANG Z, et al. Ginsenoside Rg3 and Rh2 protect trimethyltin-induced neurotoxicity via prevention on neuronal apoptosis and neuroinflammation[J]. Phytother Res, 2018, 32(12): 2531-2540. |
| 89 | MD J, JOONSOO K, GOVINDARAJAN K, et al. Emerging signals modulating potential of ginseng and its active compounds focusing on neurodegenerative diseases[J]. J Ginseng Res, 2019, 43(2): 163-171. |
| 90 | LI Z, ZHAO L, CHEN J, et al. Ginsenoside Rk1 alleviates LPS-induced depression-like behavior in mice by promoting BDNF and suppressing the neuroinflammatory response[J]. Biochem Biophys Res Commun, 2020, 530(4): 658-664. |
| 91 | XU T, SHEN X, YU H, et al. Water-soluble ginseng oligosaccharides protect against scopolamine-induced cognitive impairment by functioning as an antineuroinflammatory agent[J]. J Ginseng Res, 2016, 40(3): 211-219. |
| 92 | LEE S, YOUN K, JEONG W S, et al. Protective effects of red ginseng oil against Abeta25-35-induced neuronal apoptosis and inflammation in PC12 cells[J]. Int J Mol Sci, 2017, 18(10): 2218-2238. |
| 93 | LEE J S, SONG J H, SOHN N W, et al. Inhibitory effects of ginsenoside Rb1 on neuroinflammation following systemic lipopolysaccharide treatment in mice[J]. Phytother Res, 2013, 27(9): 1270-1276. |
| 94 | GH A, DU A, KM B, et al. Inhibitory effect of panaxytriol on BV-2 microglial cell activation[J]. J Pharmacol Sci, 2021, 145(3): 273-278. |
| 95 | LU D, ZHU L H, SHU X M, et al. Ginsenoside Rg1 relieves tert-butyl hydroperoxide-induced cell impairment in mouse microglial BV2 cells[J]. J Asian Nat Prod Res, 2015, 17(9): 930-945. |
| 96 | YE R, YANG Q, KONG X, et al. Ginsenoside Rd attenuates early oxidative damage and sequential inflammatory response after transient focal ischemia in rats[J]. Neurochem Int, 2011, 58(3): 391-398. |
| 97 | CHOI S, LIM J W, KIM H. Korean red ginseng inhibits amyloid-β-induced apoptosis and nucling expression in human neuronal cells[J]. Pharmacology, 2020, 105(9/10): 586-597. |
| 98 | ZHANG G, LIU A, ZHOU Y, et al. Panax ginseng ginsenoside-Rg2 protects memory impairment via anti-apoptosis in a rat model with vascular dementia[J]. J Ethnopharmacol, 2008, 115(3): 441-448. |
| 99 | WANG J, LAISHER-GRINBERG S, LI S, et al. Antidepressant-like effects of the active acidic polysaccharide portion of ginseng in mice[J]. J Ethnopharmacol, 2010, 132(1): 65-69. |
| 100 | LI H, KANG T, QI B, et al. Neuroprotective effects of ginseng protein on PI3K/Akt signaling pathway in the hippocampus of D-galactose/AlCl3 inducing rats model of Alzheimer′s disease[J]. J Ethnopharmacol, 2016, 179: 162-169. |
| 101 | ZONG Y, AI Q L, ZHONG L M, et al. Ginsenoside Rg1 attenuates lipopolysaccharide-induced inflammatory responses via the phospholipase C-γ1 signaling pathway in murine BV-2 microglial cells[J]. Curr Med Chem, 2012, 19(5): 770-779. |
| 102 | CUI J, SHAN R, CAO Y, et al. Protective effects of ginsenoside Rg2 against memory impairment and neuronal death induced by Aβ25-35 in rats[J]. J Ethnopharmacol, 2021, 266: 113466. |
| 103 | CHOI S, LIM J W, KIM H. Korean red ginseng inhibits amyloid-β-induced apoptosis and nucling expression in human neuronal cells[J]. Pharmacology, 2020, 105: 586-597. |
| 104 | LEE S, YOUN K, JEONG W S, et al. Protective effects of red ginseng oil against Abeta25-35-induced neuronal apoptosis and inflammation in PC12 cells[J]. Food Funct, 2018, 9(8): 4122-4134. |
| 105 | YE J, YAO J P, XU W, et al. Neuroprotective effects of ginsenosides on neural progenitor cells against oxidative injury[J]. Mol Med Rep, 2016, 13(4): 3083-3091. |
| 106 | MA J, LIU J, QI W, et al. The beneficial effect of Ginsenoside Rg1 on schwann cells subjected to hydrogen peroxide induced oxidative injury[J]. Int J Biol Sci, 2013, 9(6): 624-636. |
| 107 | ZENG X S, ZHOU X S, LUO F C, et al. Comparative analysis of the neuroprotective effects of ginsenosides Rg1 and Rb1 extracted from Panax notoginseng against cerebral ischemia[J]. Can J Physiol Pharmacol, 2014, 92(2): 102-108. |
| 108 | LV J, LU C, JIANG N, et al. Protective effect of ginsenoside Rh2 on scopolamine-induced memory deficits through regulation of cholinergic transmission, oxidative stress and the ERK-CREB-BDNF signaling pathway[J]. Phytother Res, 2021, 35(1): 337-345. |
| 109 | LEE Y, PARK J S, JUNG J S, et al. Anti-inflammatory effect of Ginsenoside Rg5 in lipopolysaccharide-stimulated BV2 microglial cells[J]. Int J Mol Sci, 2013, 14(5): 9820-9833. |
| 110 | NING J A, JI A, HW A, et al. Ginsenoside Rg1 ameliorates chronic social defeat stress-induced depressive-like behaviors and hippocampal neuroinflammation[J]. Life Sci, 2020, 252: 117669. |
| 111 | XUE J, WANG Z, LI W, et al. Ginsenoside Rg3 and Rh2 protect trimethyltin-induced neurotoxicity via prevention on neuronal apoptosis and neuroinflammation, Phytother[J]. Res, 2018,32(12): 2531-2540. |
| 112 | HENG Y, ZHANG Q S, MU Z, et al. Ginsenoside Rg1 attenuates motor impairment and neuroinflammation in the MPTP-probenecid-induced parkinsonism mouse model by targeting alpha-synuclein abnormalities in the substantia nigra[J]. Toxicol Lett, 2016, 243: 7-21. |
| 113 | LEE Y Y, PARK J S, LEE E J, et al. Anti-inflammatory mechanism of ginseng saponin metabolite Rh3 in lipopolysaccharide-stimulated microglia: critical role of 5'-adenosine monophosphate-activated protein kinase signaling pathway[J]. ACS Food Sci Technol, 2015, 63(13): 3472-3480. |
| 114 | NAN F, SUN G, XIE W, et al. Ginsenoside Rb1 mitigates oxidative stress and apoptosis induced by methylglyoxal in SH-SY5Y cells via the PI3K/Akt pathway[J]. Mol Cell Probes, 2019, 48: 101469. |
| 115 | HASHIMOTO R, YU J, KOIZUMI H, et al. Ginsenoside Rb1 prevents MPP+-induced apoptosis in PC12 Cells by stimulating estrogen receptors with consequent activation of ERK1/2, Akt and inhibition of SAPK/JNK, p38 MAPK[J]. J Evidence-Based Complementary Altern Med, 2012, 2012: 693717. |
| 116 | LEUMG K W, YUNG K K L, MAK N K, et al. Neuroprotective effects of ginsenoside-Rg1 in primary nigral neurons against rotenone toxicity-ScienceDirect[J]. Neuropharmacology, 2007, 52(3): 827-835. |
| 117 | CUI J, WANG J, ZHENG M, et al. Ginsenoside Rg2 protects PC12 cells against β-amyloid 25-35 -induced apoptosis via the phosphoinositide 3-kinase/Akt pathway[J]. Chem-Biol Interact, 2017, 275: 152-161. |
| 118 | ZHANG H, ZHOU Z, CHEN Z, et al. Ginsenoside Rg3 exerts anti-depressive effect on an NMDA-treated cell model and a chronic mild stress animal model[J]. J Pharmacol Sci, 2017, 134(1): 45-54. |
| 119 | ZHONG S J, WANG L, GU R Z, et al. Ginsenoside Rg1 ameliorates the cognitive deficits in D-galactose and AlCl3-induced aging mice by restoring FGF2-Akt and BDNF-TrkB signaling axis to inhibit apoptosis[J]. Int J Med Sci, 2020, 17(8): 1048-1055. |
| 120 | KIM H J, SHIN E J, LEE B H, et al. Oral administration of gintonin attenuates cholinergic impairments by scopolamine, amyloid-β protein, and mouse model of Alzheimer′s disease[J]. Mol Cells, 2015, 38(9): 796-805. |
| 121 | NIE L, XIA J, LI H, et al. Ginsenoside Rg1 ameliorates behavioral abnormalities and modulates the hippocampal proteomic change in triple transgenic mice of Alzheimer′s disease[J]. Oxid Med Cell Longevity, 2017, 2017: 6473506. |
| 122 | LI F, WU X, LI J, et al. Ginsenoside Rg1 ameliorates hippocampal long-term potentiation and memory in an Alzheimer′s disease model[J]. Mol Med Rep, 2016, 13(6): 4904-4910. |
| [1] | Yong-Yu CAI, Yong-Xi WU, Fang-Tong LI, Dong XIE, Yi-Zhu WANG, Mei-Yu ZHANG, Yu-Lin DAI, Fei ZHENG, Hao YUE. Research Progress on the Relationship Between Gut Microbiota and Its Metabolites and Neurodegenerative Diseases [J]. Chinese Journal of Applied Chemistry, 2023, 40(3): 309-316. |
| [2] | Rui WANG, Xiang-Ru MENG, Qiong LI, En-Peng WANG, Xin HUANG, Chang-Bao CHEN. Research Progress on the Decomposed Allelopathy of Panax Genus [J]. Chinese Journal of Applied Chemistry, 2023, 40(1): 1-8. |
| [3] | Yan-Long SHEN, Li-Ye CHENG, Xiang-Ru MENG, Qiong LI, Lian-Yun DU, En-Peng WANG, Chang-Bao CHEN. Effects of Ginseng Continuous Soil Crop on Growth Development and Antioxidant System of Ginseng at Different Fertility Stages [J]. Chinese Journal of Applied Chemistry, 2023, 40(1): 109-115. |
| [4] | Jun-Jie ZHANG, Yun-Jiao SHEN, Li-Ying MA, Peng-Hui WANG, Lei WANG, Yu-Lin DAI, Lei ZHAO. Study on Extract Composition of American Ginseng Flower in Oxidative-Induced H9c2 Cardromyocytes by LC-MS [J]. Chinese Journal of Applied Chemistry, 2023, 40(1): 126-133. |
| [5] | Jing-Wan LIU, Qiong LI, Tao ZHANG, En-Peng WANG, Huan WANG, Xue CHEN, Chang-Bao CHEN. Research Progress on the Continuous Cropping Obstacles of Ginseng from Soil Improvement [J]. Chinese Journal of Applied Chemistry, 2022, 39(12): 1818-1832. |
| [6] | Jing-Wan LIU, Qiong LI, En-Peng WANG, Tao ZHANG, Huan WANG, Zhe ZHANG, Xue CHEN, Chang-Bao CHEN. Research Progress on Cultivation of Panax Ginseng C.A.Meyer [J]. Chinese Journal of Applied Chemistry, 2022, 39(11): 1641-1651. |
| [7] | ZHANG Na, LI Le-Le, HUANG Xin, LIU Shu-Ying. Determination of Oligosaccharides in Ginseng from Different Growth Environments by Ultra Performance Liquid Chromatography Triple Quadrupole Tandem Mass Spectrometry Combined with Solid Phase Methylation [J]. Chinese Journal of Applied Chemistry, 2021, 38(3): 247-255. |
| [8] | LI Le, TAN Lu-Ying, WANG Cai-Xia, LI Kun, LI Ping-Ya, LIU Jin-Ping, LIU Yun-He. Identification of Chemical Constituents of American Ginseng Fruit Pedicels by Ultra-performance Liquid Chromatography Quadrupole Time-of-Flight Mass Spectrometry [J]. Chinese Journal of Applied Chemistry, 2021, 38(3): 256-270. |
| [9] | WANG En-Peng, DU Lian-Yun, JIANG Tao, LI Guang, WEI Kun, ZHU Shuang, YUE Hao, CHEN Chang-Bao. Whitening Activity, Antioxidant Activity and Ginsenosides Analysis of Ginseng Wash Water [J]. Chinese Journal of Applied Chemistry, 2021, 38(3): 289-297. |
| [10] | ZHANG Hui-E, HOU Jian-Feng, WANG Jing-Yuan, ZHU Shuang, DU Lian-Yun, YE Ping, WEI Kun, CHEN Chang-Bao, LI Guang, WANG En-Peng. A Differential Study on in vitro Antioxidant Activity and Extract Composition of Different Parts of Panax Ginseng [J]. Chinese Journal of Applied Chemistry, 2021, 38(11): 1531-1540. |
| [11] | WANG Wei, ZHENG Fei, GE Yan, QIAO Mengdan, YUE Hao, LIU Shuying. Analysis of Volatile Components in Processed Ginseng by GC-MS/MS [J]. Chinese Journal of Applied Chemistry, 2017, 34(8): 965-970. |
| [12] | WANG Haiwei, XU Kun, TAN Ying, LU Cuige, NA Hui, WANG Pixin, WANG Yang, LIU Xiusheng. Synthesis and Application of Hyperbranched Poly(amido amine) Demulsifier [J]. Chinese Journal of Applied Chemistry, 2017, 34(4): 423-429. |
| [13] | DU Qin-Qin1,2, ZHANG Xu1, SONG Feng-Rui1, LIU Zhi-Qiang1, LIU Shu-Ying1*. The Application of HPLC-ESI-MS or FRAP in Studying the Variation of Ginsenosides and Their Anti-oxidation after the Combination of Ginseng with Zingiber officinale Rosc or Radix Paeoniae Rubra [J]. Chinese Journal of Applied Chemistry, 2010, 27(10): 1209-1214. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||