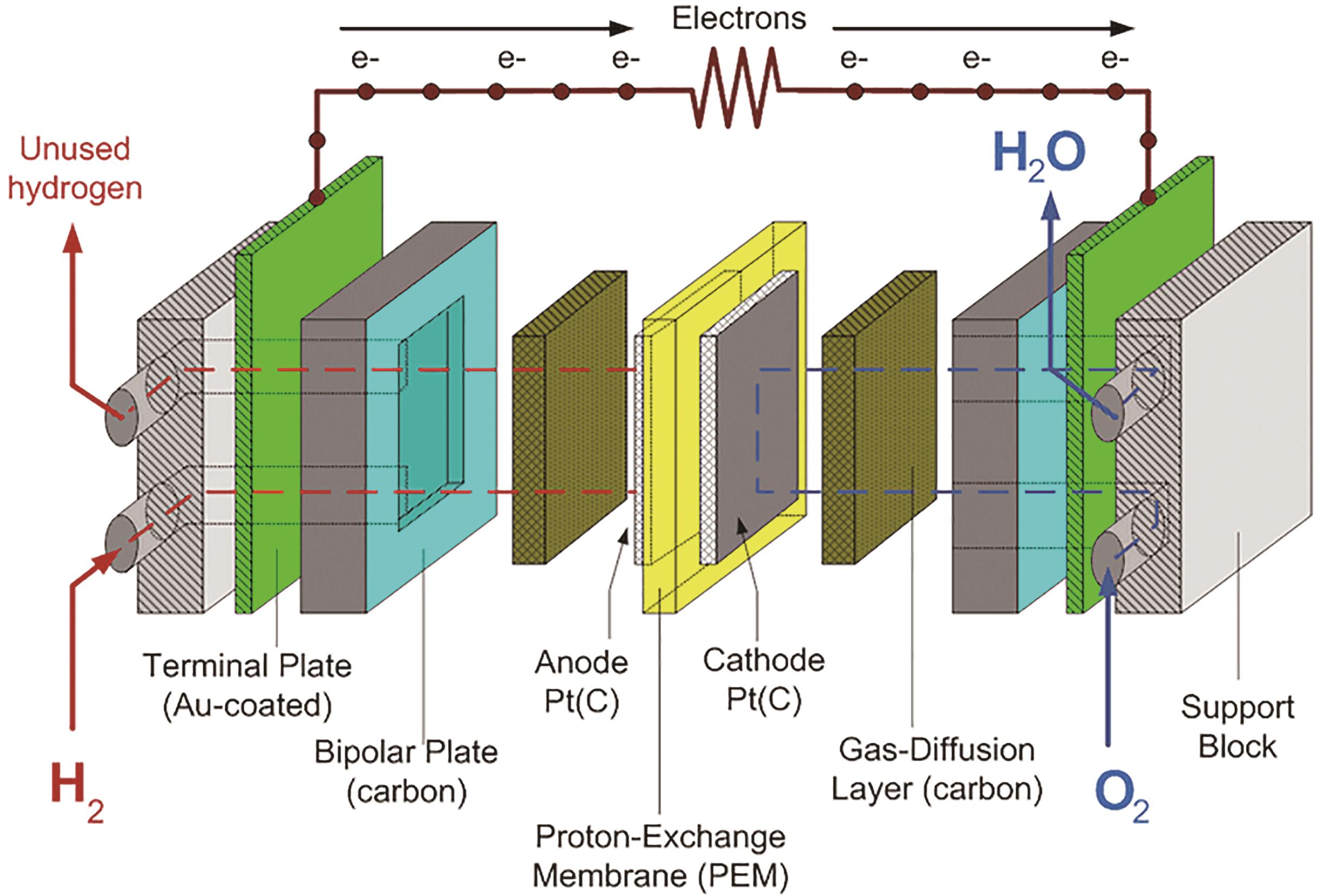
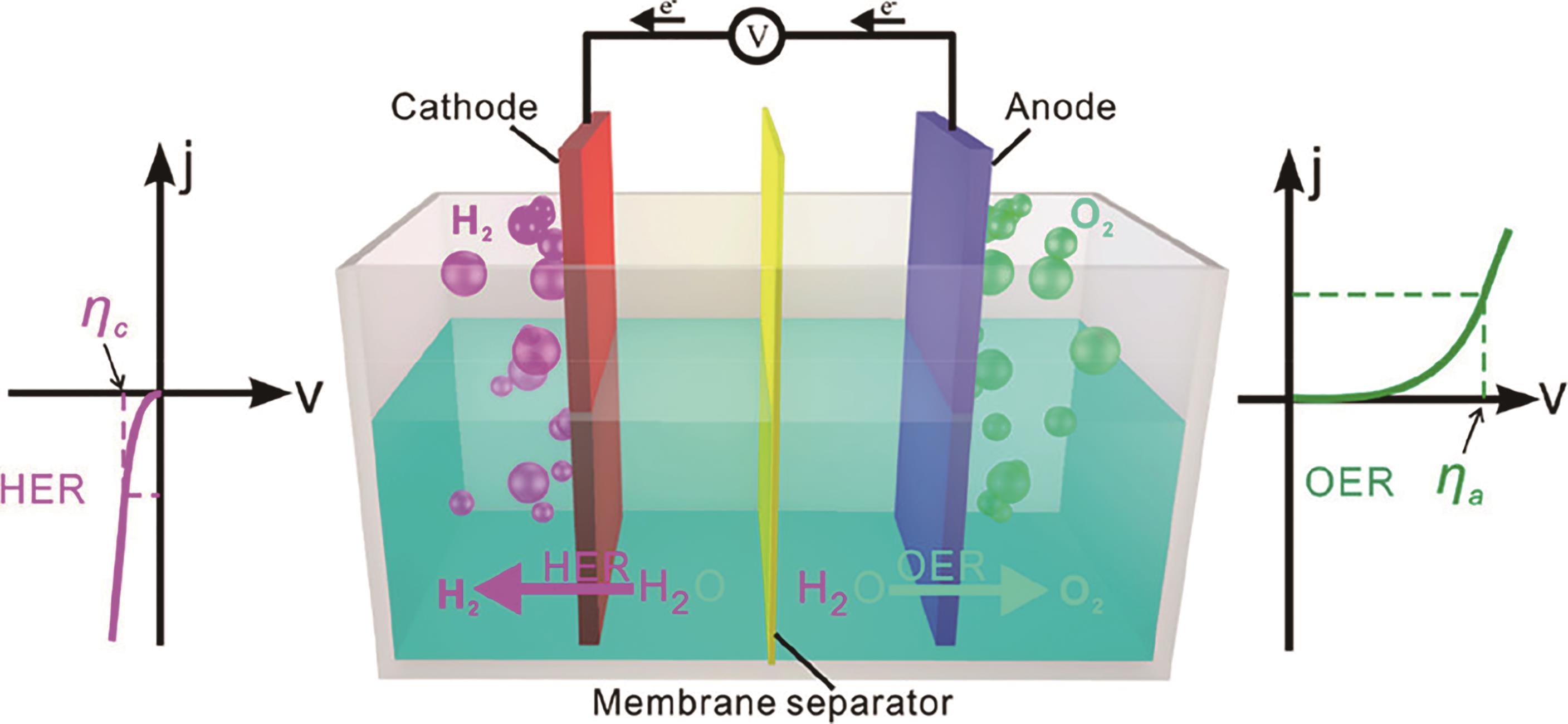
应用化学 ›› 2023, Vol. 40 ›› Issue (8): 1140-1157.DOI: 10.19894/j.issn.1000-0518.230130
金属间化合物电催化剂在氢电转换器件应用中的研究进展
- 华南理工大学化学与化工学院,广州 510641
-
收稿日期:2023-05-04接受日期:2023-07-06出版日期:2023-08-01发布日期:2023-08-24 -
通讯作者:崔志明 -
基金资助:国家自然科学基金(22072048);广东省自然科学基金(2021A1515010128);电分析化学国家重点实验室科学基金(SKLEAC202208)
Research Progress on Intermetallic Compound Electrocatalysts Applied in the Interconversion Between Hydrogen and Electric Power
Jin-Hui LIANG, Le-Cheng LIANG, Zhi-Ming CUI( )
)
- School of Chemistry and Chemical Engineering,South China University of Technology,Guangzhou 510641,China
-
Received:2023-05-04Accepted:2023-07-06Published:2023-08-01Online:2023-08-24 -
Contact:Zhi-Ming CUI -
About author:zmcui@scut.edu.cn
-
Supported by:the National Natural Science Foundation of China (No.?22072048)?, Guangdong Provincial Department of Science and Technology (Nos.?2021A1515010128, 2022A0505050013)and the Open Funds of the State Key Laboratory of Electroanalytical Chemistry(SKLEAC202208)
摘要:
人类对可持续能源的高度重视,使得绿色环保的氢能成为研究热点。能量转换和存储技术(如燃料电池和电解水)可实现氢能源和电力的相互转换,但实现这些技术的前提是需要开发高效稳定的催化剂。结构有序的金属间化合物具有确定的组成和可调控的几何效应和电子效应的优势,已被探索作为能量转换和存储技术的有效电催化剂,金属间化合物表面均匀的活性位点分布也有利于研究催化剂活性和结构之间联系(即构效关系)的理想模型。本综述首先介绍了氢电转换装置催化剂所面临的挑战以及金属间化合物在电催化中的优势,其次从活性和稳定性的角度重点论述金属间化合物在氢电转换装置中的研究进展,最后总结并展望了金属间化合物电催化剂未来的发展前景。
中图分类号:
引用本文
梁锦辉, 梁乐程, 崔志明. 金属间化合物电催化剂在氢电转换器件应用中的研究进展[J]. 应用化学, 2023, 40(8): 1140-1157.
Jin-Hui LIANG, Le-Cheng LIANG, Zhi-Ming CUI. Research Progress on Intermetallic Compound Electrocatalysts Applied in the Interconversion Between Hydrogen and Electric Power[J]. Chinese Journal of Applied Chemistry, 2023, 40(8): 1140-1157.
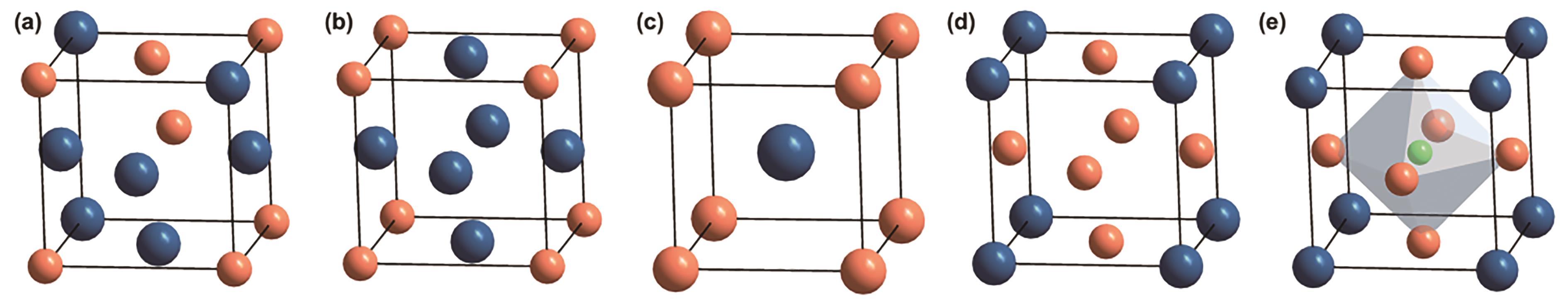
图3 晶胞示意图(a)无序PtM合金(面心立方), (b) Pt3M金属间化合物(面心立方), (c) PtM金属间化合物(面心四方), (d) PtM3金属间化合物(面心立方), (e) PtM3N金属间化合物
Fig.3 Cell diagram of (a) Disordered PtM alloy (face-centered cubic), (b)Pt3M intermetallic compound (face-centered cubic), (c) PtM intermetallic compound (body-centered cubic), (d) PtM3 intermetallic compound (face-centered cubic), (e) PtM3Nintermetallic compound
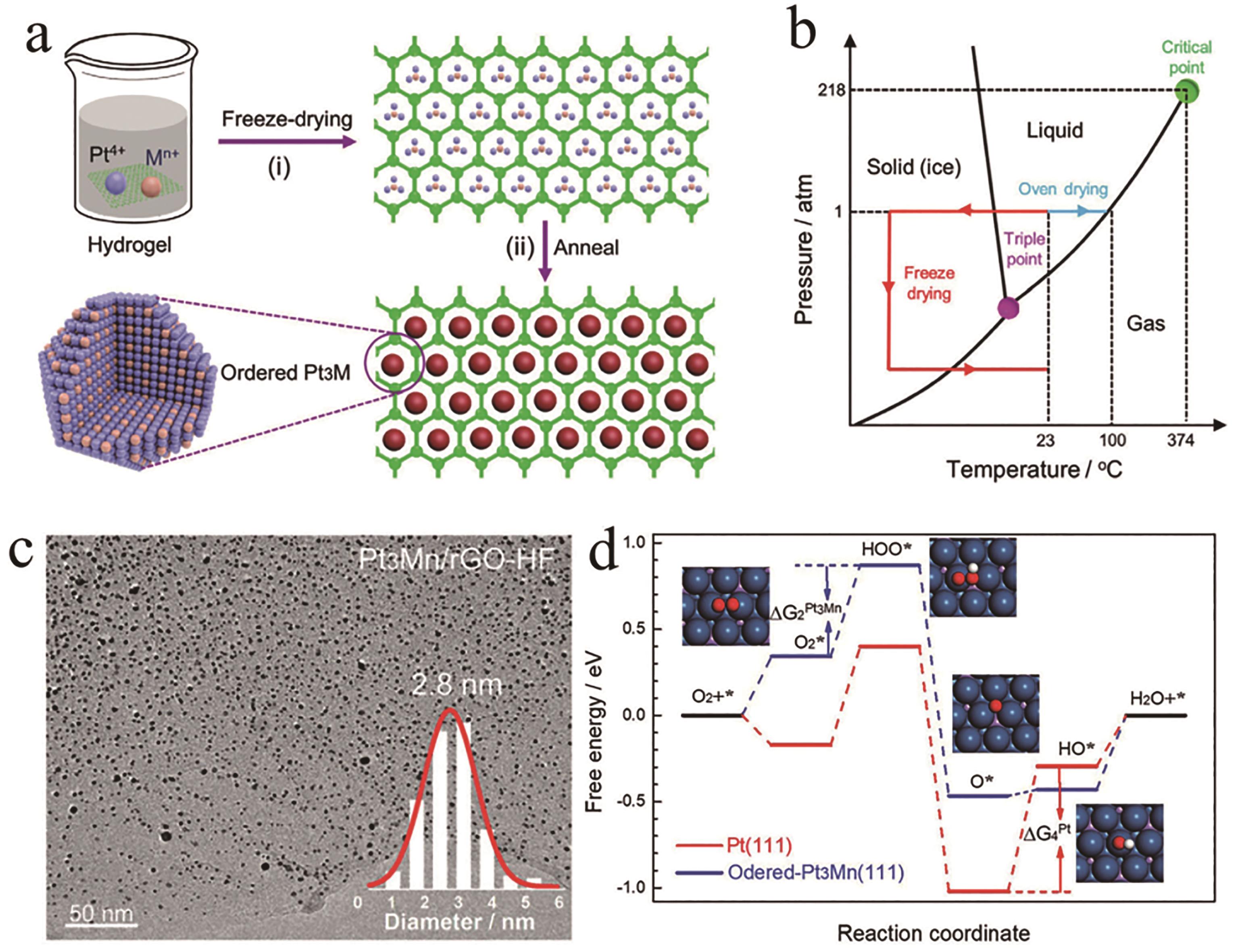
图4 (a)有序的Pt3Mn/rGO-HF制备流程, (b)水的相图, (c) Pt3Mn/rGO-HF透射电镜图展现出有序金属间化合物Pt3Mn的超小尺寸, (d)Pt(111) 和Pt3Mn(111)晶面对氧还原反应不同中间体吸附台阶图[38]
Fig.4 (a) Preparation scheme of ordered Pt3Mn/rGO-HF, (b) Phase diagram of water, (c) TEM image of Pt3Mn/rGO-HF showing the ultra-small particle size of the synthesized ordered Pt3Mn, (d) The free energies of the intermediates along with the reaction on Pt(111) and ordered Pt3Mn(111) toward ORR[38]
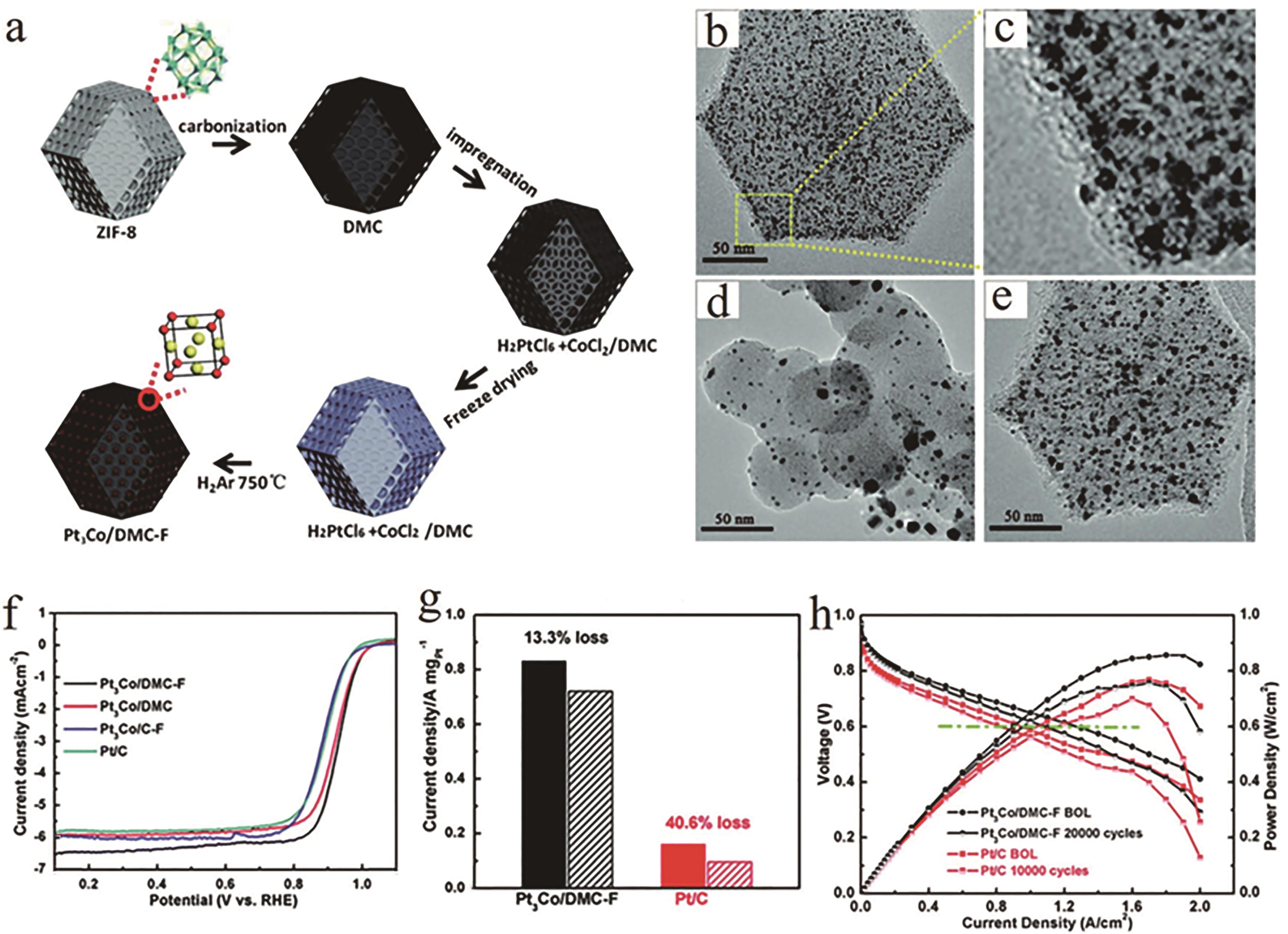
图5 (a)通过冷冻干燥浸渍和热处理方法制备限域于MOF衍生介孔碳Pt3Co纳米颗粒的流程图, (b)和(c) Pt3Co/DMC-F透射电子显微镜图, (d) Pt3Co/C-F透射电子显微镜图, (e) Pt3Co/DMC透射电子显微镜图, (f)不同氧还原极化曲线的性能曲线对比, (g) Pt3Co/DMC-F和商业 Pt/C的稳定性对比, (h) Pt3Co/DMC-F和商业Pt/C的氢-空气膜电极组装单电池极化曲线[39]
Fig.5 (a) Schematic of the preparation for Pt3Co nanoparticles confined in MOF-derived mesoporous carbon via the freeze dry impregnation and high temperature treatment method. TEM images of different Pt3Co electrocatalysts: (b and c) Pt3Co/DMC-F, (d) Pt3Co/C-F, and (e) Pt3Co/DMC. (f) The comparison of the ORR LSV curves between different electrocatalysts, (g) The comparison of the stability between Pt3Co/DMC-F and commercial Pt/C, (h) H2-air single cell polarization plots of the Pt3Co/DMC-F and the commercial Pt/C enabled MEAs, respectively[39]
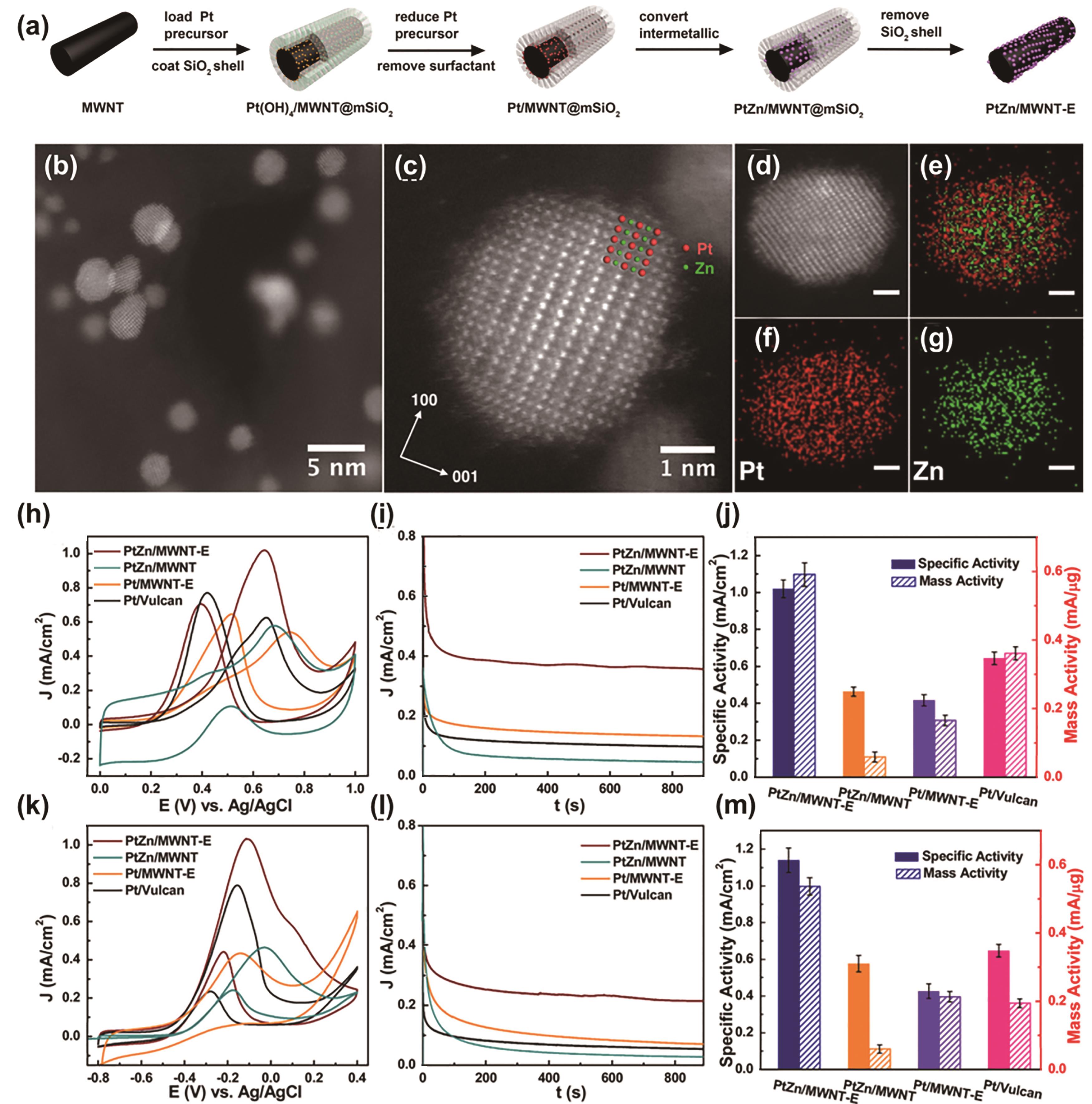
图6 (a) PtZn/MWNT@mSiO2合成路线示意图, (b, c) PtZn纳米颗粒的高分辨率高角环形暗场-透射电子显微镜图。图b中的红色和绿色球分别代表Pt和Zn原子,(c, d)单个PtZn纳米颗粒的高分辨率高角环形暗场-透射电子显微镜图, (e-g) Pt与Zn(e)、 Pt (f)和Zn(g)的元素映射图(标尺为1 nm), (h)循环伏安法, (i) PtZn/MWNT-E、Pt/MWNT-E和PtZn/MWNT(在没有mSiO2的情况下合成)的恒电位计时电流法,以及在室温下氩气饱和的0.5 mol/L H2SO4和1 mol/L甲醇中测量的商业Pt/Vulcan催化剂, (j) PtZn/MWNT-E、Pt/MWNT-E、PtZn/MWNT(在不含mSiO2的情况下合成)和商用Pt/Vulcan催化剂在0.65 V(vs.Ag/AgCl)参比电极下的本征活性和质量活性, (k)循环伏安法和(l)计时电流法,在氩气饱和的0.1 mol/L KOH和0.5 mol/L甲醇中测量。 (m)在-0.1 V(vs.Ag/AgCl)参比电极的本征活性和质量活性。测试重复3次,电流归一化到电化学活性面积(ECSA)[48]
Fig.6 (a) Schematic representation of the synthesis route to PtZn/MWNT@mSiO2. (b, c) High-resolution HAADF- STEM images of PtZn nanoparticles. The red and green spheres in panel b represent Pt and Zn atoms. (c, d) HAADF-STEM image of a single PtZn nanoparticles. (e-g) Elemental mappings of Pt versus Zn (e), Pt (f), and Zn (g). Scale bar, 1 nm. (h) Cyclic voltammetry and (i) Chronoamperometry of PtZn/MWNT-E, Pt/MWNT-E, PtZn/MWNT (synthesized in the absence of mSiO2), and commercial Pt/Vulcan catalysts measured at room temperature in argon-purged 0.5 mol/L H2SO4 and 1 mol/L methano. (j) Specific activity and mass activity at 0.65 V(vs.Ag/AgCl) reference electrode, (k) Cyclic voltammetry and (l) Chronoamperometry of PtZn/MWNT-E, Pt/MWNT-E, PtZn/MWNT (synthesized in absence of mSiO2) and commercial Pt/Vulcan catalysts measured at room temperature in argon-purged 0.1 mol/L KOH and 0.5 mol/L methanol. (m) Specific activity and mass activity at -0.1 V(vs.Ag/AgCl) electrode. All the measurements were repeated 3 times, and currents were normalized to the electrochemical surface area (ECSA)[48]
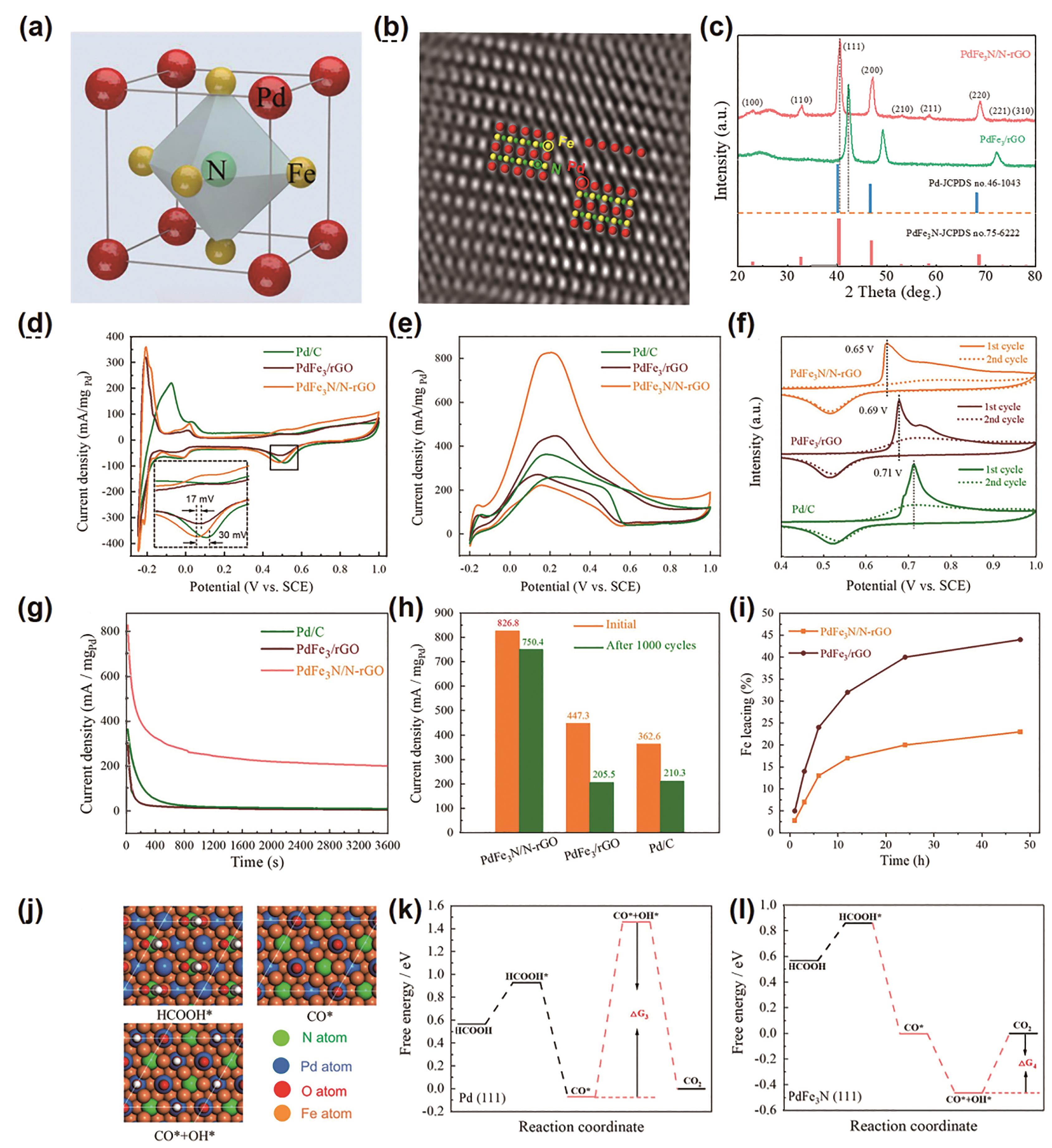
图7 (a) PdFe3N晶胞图, (b) PdFe3N/N-rGO纳米颗粒的高分辨率高角环形暗场-透射电镜图。红色、黄色和绿色小球分别代表Pd、Fe和N原子, (c) PdFe3N/N-rGO 和PdFe3/rGO的XRD图, (d)循环伏安(CV)曲线(0.5 mol/L H2SO4溶液,扫描速率为50 mV/s), (e)甲酸氧化曲线 (0.5 mol/L H2SO4+0.5 mol/L HCOOH溶液,扫描速率为20 mV/s), (f) CO的脱附测试, (g)恒电压的计时电流曲线(0.5 mol/L H2SO4+0.5 mol/L HCOOH溶液,0.2 V), (h)3种催化在测试前和测试后的质量活性对比柱状图, (i) ICP测试Fe在0.5 mol/L H2SO4溶液中析出量, (j) HCOOH*、CO*和CO*+OH*吸附在已优化的PdFe3N(111)晶面示意图, (k) Pd(111)和(l) PdFe3N(111)间接反应途径上甲酸氧化的吉布斯自由能图[54]
Fig.7 (a) The unit cell of PdFe3N. (b) High-resolution HAADF STEM images of PdFe3N/N-rGO nanoparticles. The red, yellow and green spheres in panel represent Pd, Fe and N atoms, respectively. (c) X-ray diffraction (XRD) patterns of PdFe3N/N-rGO and PdFe3/rGO. (d) Cyclic voltammogram (CV) curves recorded in 0.5 mol/L H2SO4 solution with a scan rate of 50 mV/s. (e) Formic acid oxidation reaction (FAOR) curves for catalysts recorded in 0.5 mol/L H2SO4+0.5 mol/L HCOOH solution with a scan rate of 20 mV/s. (f) CO-stripping test. (g) The chronoamperometry test for three catalysts in 0.5 mol/L H2SO4+0.5 mol/L HCOOH solution at 0.2 V. (h) Comparison of the mass activity of three catalysts before and after CV cyclic stability test. (i) The leaching of Fe in the samples in 0.5 mol/L H2SO4 solution. (j) Optimized surface slabs with HCOOH*, CO*, and CO*+OH* on PdFe3N (111) (H in white). Gibbs free energy diagrams of formic acid oxidation for indirect reaction pathway on (k) Pd (111) and (l) PdFe3N (111) at U=0 V, respectively[54]
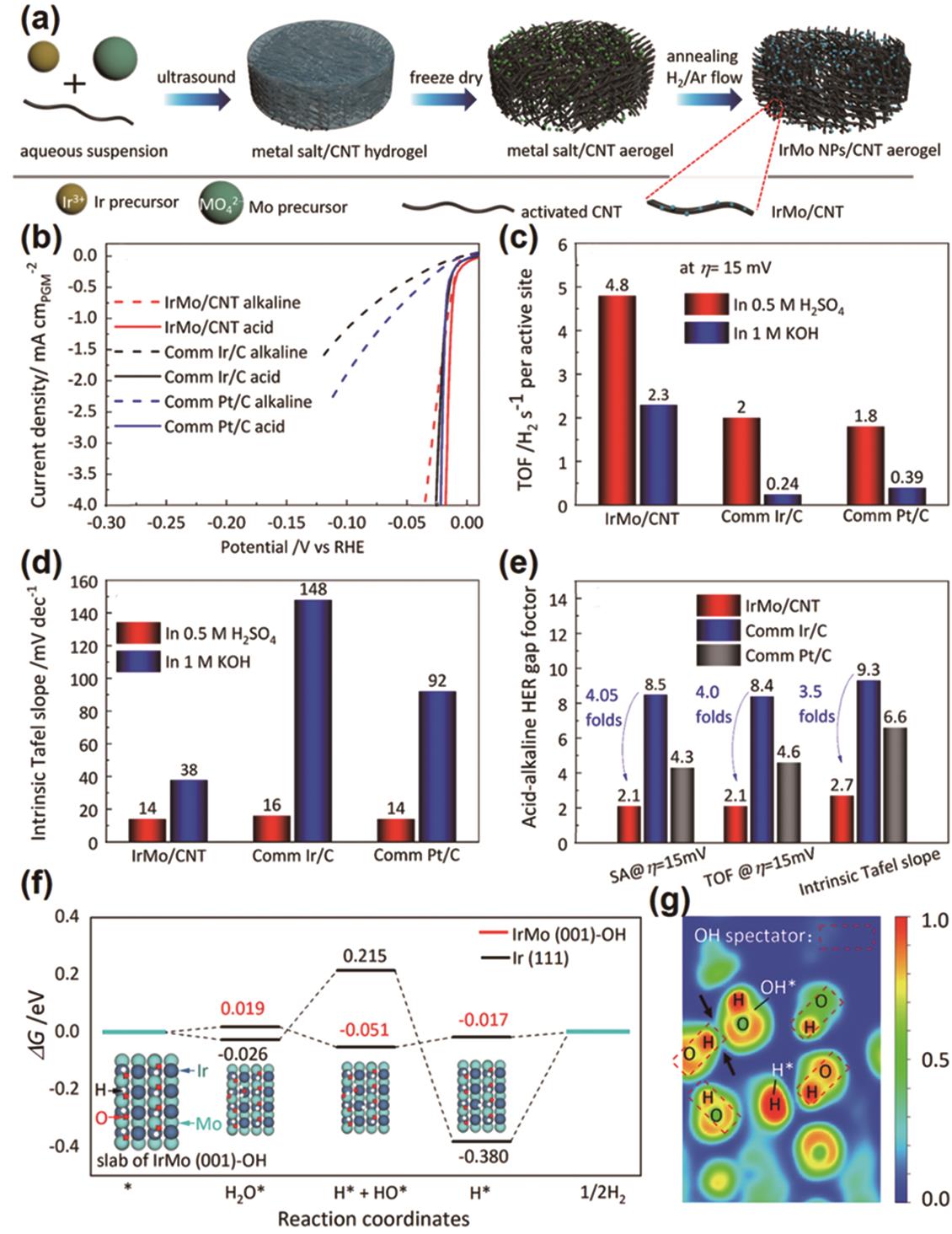
图8 (a)采用冷冻干燥浸渍和退火还原法制备碳纳米管负载的金属间化合物IrMo纳米颗粒 IrMo/CNT、Ir/C和Pt/C的酸性和碱性HER动力学和本征活性比较。 (b)经ECSA归一化电流密度后HER极化曲线, (c) η= 15 mV时转换频率的比较, (d)在酸性和碱性电解质中Tafel斜率与ECSA归一化的电流密度比较, (e)酸碱HER活性差异对比, (f)基于Volmer-Heyrovsky机理的HER反应吉布斯自由能图, (g)吸附H * 和OH * 的IrMo (001)-OH表面的电子局域函数图[57]
Fig.8 (a) Schematic of the preparation for the CNT-supported Intermetallic IrMo nanoparticle by freeze-drying impregnation and annealing reduction. (b) Alkaline and acidic HER polarization curves with the current density normalized by ECSA for comparison of the kinetics and intrinsic activity between acidic and alkaline HER for IrMo/CNT, Ir/C, and Pt/C. (c) Comparison of the TOF at η=15 mV. (d) Comparison of the intrinsic Tafel slopes extracted from the Tafel plots with the current density normalized by the ECSA of the electrocatalysts in acidic and alkaline electrolytes. (e) Comparison of the acid-alkaline HER gap factor on specific activity (SA), TOF, and Tafel slope. The acid-alkaline HER gap factor can be determined by SAacid/SAalkaline, TOFacid/TOFalkaline, or intrinsic Tafel slopealkaline/intrinsic Tafel slopeacid. (f) Gibbs free energy diagram for HER reaction based on Volmer-Heyrovsky process, (g) Electron localization function (ELF) plot of the IrMo (001)-OH slab adsorbed with a H* and OH*[57]
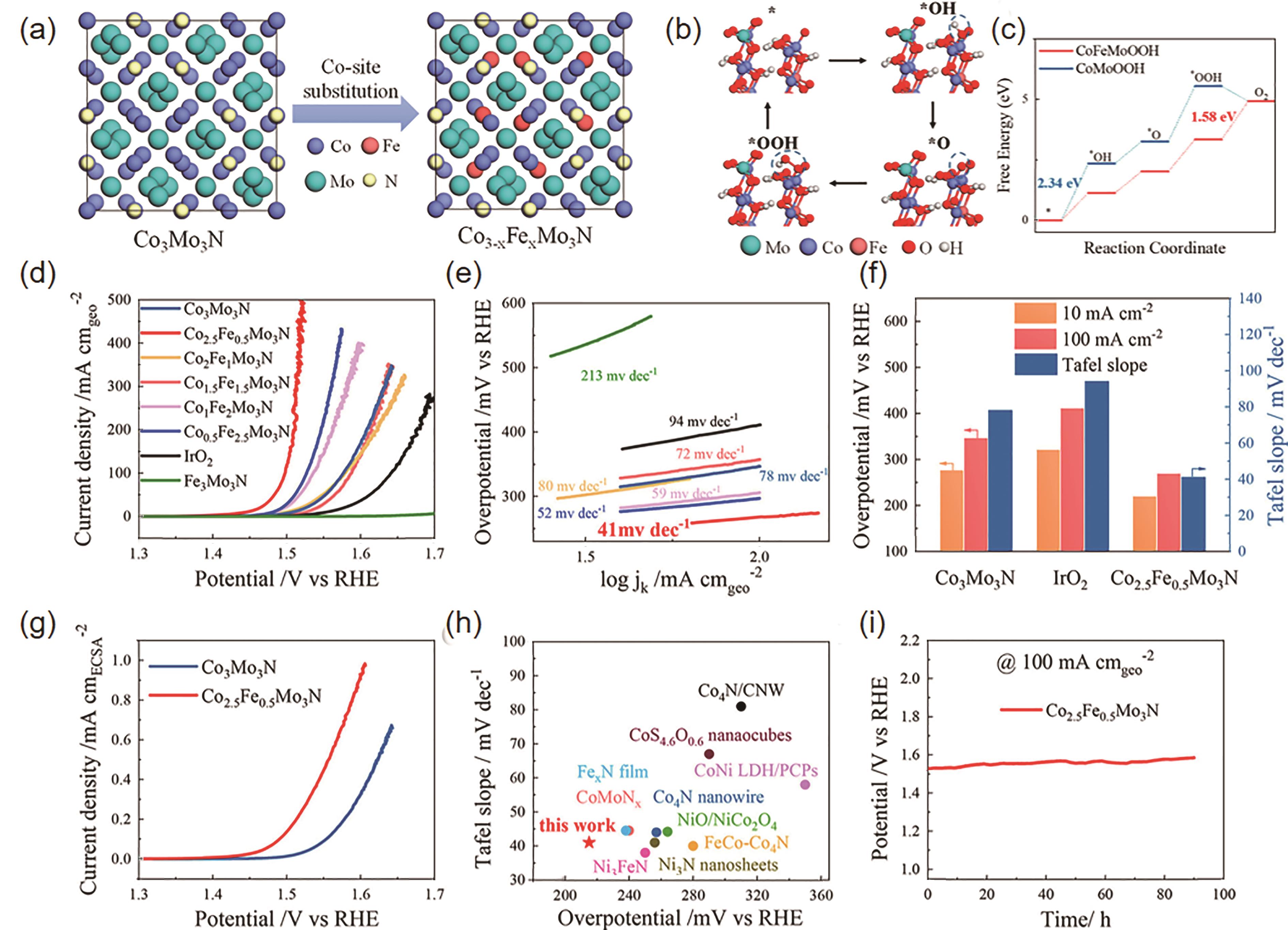
图9 (a)Fe取代Co3-x Fe x Mo3N中Co位点的机理图, (b) CoFeMoOOH表面OER机理图, (c) CoMoOOH和CoMoOOH表面Co位点OER的吉布斯自由能图, (d) Co3-x Fe x Mo3N和商业IrO2的线性扫描伏安曲线(O2饱和的1 mol/L KOH,扫速为1 mV/s), (e)塔菲尔曲线, (f) Co2.5Fe0.5Mo3N、Co3Mo3N 和IrO2在10和100 mA/cm2对应的过电位以及塔菲尔斜率对比, (g) Co3Mo3N和Co2.5Fe0.5Mo3N经电化学活性面积归一化后的线性扫描伏安曲线, (h)目前报道的OER催化剂性能对比, (i) Co2.5Fe0.5Mo3N在电流密度为100 mA/cm2测试恒电流的计时电压曲线[64]
Fig.9 (a) Scheme of partial substitution of the Co site of Co3-x Fe x Mo3N (0≤x≤3), (b) Scheme of the OER mechanism on the CoFeMoOOH surface. (c) Gibbs free energy diagram of the OER at the Co sites on the CoFeMoOOH and CoMoOOH surfaces. (d) Linear sweeping voltammetry (LSV) curves of Co3-x Fe x Mo3N and commercial IrO2 measured in O2 saturated 1.0 mol/L KOH solutions at the scan rate of 1 mV/s and (e) its derived Tafel plots. (f) Comparison of the overpotential at 10 mA/m2, the overpotential at 100 mA/cm2, and the Tafel slopes of Co2.5Fe0.5Mo3N, Co3Mo3N and IrO2, (g) LSV curves of Co3Mo3N and Co2.5Fe0.5Mo3N normalized by their ECSAs. (h) Comparison of the selected OER electrocatalysts, (i) Chronopotentiometric curves of Co2.5Fe0.5Mo3N at the current density of 100 mA/cm2[64]
| 1 | KULKARNI A, SIAHROSTAMI S, PATEL A, et al. Understanding catalytic activity trends in the oxygen reduction reaction[J]. Chem Rev, 2018, 118(5): 2302-2312. |
| 2 | CANO Z P, BANHAM D, YE S Y, et al. Batteries and fuel cells for emerging electric vehicle markets[J]. Nat Energy, 2018, 3(4): 279-289. |
| 3 | YAN Y C, DU J S, GILROY K D, et al. Intermetallic nanocrystals: syntheses and catalytic applications[J]. Adv Mater, 2017, 29(14): 1605997. |
| 4 | ZHANG J W, YUAN Y L, GAO L, et al. Stabilizing Pt-based electrocatalysts for oxygen reduction reaction: fundamental understanding and design strategies[J]. Adv Mater, 2021, 33(20): e2006494. |
| 5 | ZHOU M, LI C, FANG J Y. Noble-metal based random alloy and intermetallic nanocrystals: syntheses and applications[J]. Chem Rev, 2021, 121(2): 736-795. |
| 6 | DEBE M K. Electrocatalyst approaches and challenges for automotive fuel cells[J]. Nature, 2012, 486(7401): 43-51. |
| 7 | SEH Z W, KIBSGAARD J, DICKENS C F, et al. Combining theory and experiment in electrocatalysis: insights into materials design[J]. Science, 2017, 355(6321): eaad4998. |
| 8 | LI Y, WANG H H, PRIEST C, et al. Advanced electrocatalysis for energy and environmental sustainability via water and nitrogen reactions[J]. Adv Mater, 2021, 33(6): e2000381. |
| 9 | WANG X X, SWIHART M T, WU G. Achievements, challenges and perspectives on cathode catalysts in proton exchange membrane fuel cells for transportation[J]. Nat Catal, 2019, 2(7): 578-589. |
| 10 | MA Z, CANO Z P, YU A P, et al. Enhancing oxygen reduction activity of Pt-based electrocatalysts: from theoretical mechanisms to practical methods[J]. Angew Chem Int Ed, 2020, 59(42): 18334-18348. |
| 11 | TIAN X L, LU X F, XIA B Y, et al. Advanced electrocatalysts for the oxygen reduction reaction in energy conversion technologies[J]. Joule, 2020, 4(1): 45-68. |
| 12 | FEI H L, DONG J C, FENG Y X, et al. General synthesis and definitive structural identification of MN4C4 single-atom catalysts with tunable electrocatalytic activities[J]. Nat Catal, 2018, 1(1): 63-72. |
| 13 | FU X Y, WAN C Z, HUANG Y, et al. Noble metal based electrocatalysts for alcohol oxidation reactions in alkaline media[J]. Adv Funct Mater, 2022, 32(11): 2106401. |
| 14 | ANTOLINI E. Effect of atomic ordering on the activity for methanol and formic acid oxidation of Pt-based electrocatalysts[J]. Energy Technol, 2019, 7(5): 1800553. |
| 15 | LYU F, CAO M H, MAHSUD A, et al. Interfacial engineering of noble metals for electrocatalytic methanol and ethanol oxidation[J]. J Mater Chem A, 2020, 8(31): 15445-15457. |
| 16 | OSMIERI L, ESCUDERO-CID R, MONTEVERDE VIDELA A H A, et al. Performance of a Fe-N-C catalyst for the oxygen reduction reaction in direct methanol fuel cell: cathode formulation optimization and short-term durability[J]. Appl Catal B: Environ, 2017, 201: 253-265. |
| 17 | BAI G L, LIU C, GAO Z, et al. Atomic carbon layers supported pt nanoparticles for minimized CO poisoning and maximized methanol oxidation[J]. Small, 2019, 15(38): e1902951. |
| 18 | XIA Z X, ZHANG X M, SUN H, et al. Recent advances in multi-scale design and construction of materials for direct methanol fuel cells[J]. Nano Energy, 2019, 65: 104048. |
| 19 | LIU Z Y, FU G T, LI J H, et al. Facile synthesis based on novel carbon-supported cyanogel of structurally ordered Pd3Fe/C as electrocatalyst for formic acid oxidation[J]. Nano Res, 2018, 11(9): 4686-4696. |
| 20 | RÖßNER L, ARMBRÜSTER M. Electrochemical energy conversion on intermetallic compounds: a review[J]. ACS Catal, 2019, 9(3): 2018-2062. |
| 21 | YE L, MAHADI A H, SAENGRUENGRIT C, et al. Ceria nanocrystals supporting Pd for formic acid electrocatalytic oxidation: prominent polar surface metal support interactions[J]. ACS Catal, 2019, 9(6): 5171-5177. |
| 22 | XIONG Y, DONG J C, HUANG Z Q, et al. Single-atom Rh/N-doped carbon electrocatalyst for formic acid oxidation[J]. Nat Nanotechnol, 2020, 15(5): 390-397. |
| 23 | CHEN X T, GRANDA-MARULANDA L P, MCCRUM I T, et al. How palladium inhibits CO poisoning during electrocatalytic formic acid oxidation and carbon dioxide reduction[J]. Nat Commun, 2022, 13(1): 38-48. |
| 24 | STAMENKOVIC V R, STRMCNIK D, LOPES P P, et al. Energy and fuels from electrochemical interfaces[J]. Nat Mater, 2016, 16(1): 57-69. |
| 25 | ZHANG R H, DUBOUIS N, BEN OSMAN M, et al. A dissolution/precipitation equilibrium on the surface of iridium-based perovskites controls their activity as oxygen evolution reaction catalysts in acidic media[J]. Angew Chem Int Ed, 2019, 58(14): 4571-4575. |
| 26 | JOVANOVIC P, HODNIK N, RUIZ-ZEPEDA F, et al. Electrochemical dissolution of iridium and iridium oxide particles in acidic media: transmission electron microscopy, electrochemical flow cell coupled to inductively coupled plasma mass spectrometry, and X-ray absorption spectroscopy study[J]. J Am Chem Soc, 2017, 139(36): 12837-12846. |
| 27 | ZHU J, HU L S, ZHAO P X, et al. Recent advances in electrocatalytic hydrogen evolution using nanoparticles[J]. Chem Rev, 2020, 120(2): 851-918. |
| 28 | WANG Q, ZHANG Z, CAI C, et al. Single iridium atom doped Ni2P catalyst for optimal oxygen evolution[J]. J Am Chem Soc, 2021, 143(34): 13605-13615. |
| 29 | ZHOU W, WU J B, YANG H. Highly uniform platinum icosahedra made by hot injection-assisted grails method[J]. Nano Lett, 2013, 13(6): 2870-2874. |
| 30 | GILROY K D, RUDITSKIY A, PENG H C, et al. Bimetallic nanocrystals: syntheses, properties, and applications[J]. Chem Rev, 2016, 116(18): 10414-10472. |
| 31 | ZHANG J T, QU L T, SHI G Q, et al. N,P-codoped carbon networks as efficient metal-free bifunctional catalysts for oxygen reduction and hydrogen evolution reactions[J]. Angew Chem Int Ed, 2016, 55(6): 2230-2234. |
| 32 | LUO M C, SUN Y J, WANG L, et al. Tuning multimetallic ordered intermetallic nanocrystals for efficient energy electrocatalysis[J]. Adv Energy Mater, 2017, 7(11): 1602073. |
| 33 | GAMLER J T L, ASHBERRY H M, SKRABALAK S E, et al. Random alloyed versus intermetallic nanoparticles: a comparison of electrocatalytic performance[J]. Adv Mater, 2018, 30(40): e1801563. |
| 34 | XIAO W P, LEI W, GONG M X, et al. Recent advances of structurally ordered intermetallic nanoparticles for electrocatalysis[J]. ACS Catal, 2018, 8(4): 3237-3256. |
| 35 | LIANG J S, MA F, HWANG S, et al. Atomic arrangement engineering of metallic nanocrystals for energy-conversion electrocatalysis[J]. Joule, 2019, 3(4): 956-991. |
| 36 | ZHANG J X, ZHANG L H, CUI Z M. Strategies to enhance the electrochemical performances of Pt-based intermetallic catalysts[J]. Chem Commun, 2021, 57(1): 11-26. |
| 37 | LI J R, SUN S H. Intermetallic nanoparticles: synthetic control and their enhanced electrocatalysis[J]. Acc Chem Res, 2019, 52(7): 2015-2025. |
| 38 | ZHANG B T, FU G T, LI Y T, et al. General strategy for synthesis of ordered Pt3M intermetallics with ultrasmall particle size[J]. Angew Chem Int Ed, 2020, 59(20): 7857-7863. |
| 39 | ZHAO W Y, YE Y K, JIANG W J, et al. Mesoporous carbon confined intermetallic nanoparticles as highly durable electrocatalysts for the oxygen reduction reaction[J]. J Mater Chem A, 2020, 8(31): 15822-15828. |
| 40 | HODNIK N, JEYABHARATHI C, MEIER J C, et al. Effect of ordering of PtCu3 nanoparticle structure on the activity and stability for the oxygen reduction reaction[J]. Phys Chem Chem Phys, 2014, 16(27): 13610-13615. |
| 41 | KIM D, XIE C, BECKNELL N, et al. Electrochemical activation of CO2 through atomic ordering transformations of AuCu nanoparticles[J]. J Am Chem Soc, 2017, 139(24): 8329-8336. |
| 42 | XIA B Y, WU H B, WANG X, et al. One-pot synthesis of cubic PtCu3 nanocages with enhanced electrocatalytic activity for the methanol oxidation reaction[J]. J Am Chem Soc, 2012, 134(34): 13934-13937. |
| 43 | CUI Z M, CHEN H, ZHAO M T, et al. Synthesis of structurally ordered Pt3Ti and Pt3V nanoparticles as methanol oxidation catalysts[J]. J Am Chem Soc, 2014, 136(29): 10206-10209. |
| 44 | CUI Z M, CHEN H, ZHOU W D, et al. Structurally ordered Pt3Cr as oxygen reduction electrocatalyst: ordering control and origin of enhanced stability[J]. Chem Mater, 2015, 27(21): 7538-7545. |
| 45 | PAN X K, YU S Y, ZHANG J X, et al. Universal solid-phase seed-mediated synthesis of monodisperse and ultrasmall L10-PtM intermetallic nanocrystals[J]. Chem Mater, 2023, 35(6): 2559-2568. |
| 46 | ZHAO W Y, CHI B, LIANG L C, et al. Optimizing the electronic structure of ordered Pt-Co-Ti ternary intermetallic catalyst to boost acidic oxygen reduction[J]. ACS Catal, 2022, 12(13): 7571-7578. |
| 47 | FENG Q C, ZHAO S, HE D S, et al. Strain engineering to enhance the electrooxidation performance of atomic-layer Pt on intermetallic Pt3Ga[J]. J Am Chem Soc, 2018, 140(8): 2773-2776. |
| 48 | QI Z Y, XIAO C X, LIU C, et al. Sub-4 nm PtZn intermetallic nanoparticles for enhanced mass and specific activities in catalytic electrooxidation reaction[J]. J Am Chem Soc, 2017, 139(13): 4762-4768. |
| 49 | KWON T, LIM S, JUN M, et al. Pt2+-exchanged ZIF-8 nanocube as a solid-state precursor for L10-PtZn intermetallic nanoparticles embedded in a hollow carbon nanocage[J]. Nanoscale, 2020, 12(2): 1118-1127. |
| 50 | CHEN D J, TONG Y J. Irrelevance of carbon monoxide poisoning in the methanol oxidation reaction on a PtRu electrocatalyst[J]. Angew Chem Int Ed, 2015, 54(32): 9394-9398. |
| 51 | WEI D Y, YUE M F, QIN S N, et al. In situ Raman observation of oxygen activation and reaction at platinum-ceria interfaces during CO oxidation[J]. J Am Chem Soc, 2021, 143(38): 15635-15643. |
| 52 | LEONARD B M, ZHOU Q, WU D, et al. Facile synthesis of PtNi intermetallic nanoparticles: influence of reducing agent and precursors on electrocatalytic activity[J]. Chem Mater, 2011, 23(5): 1136-1146. |
| 53 | ZHU J, ZHENG X, WANG J, et al. Structurally ordered PtZn/C series nanoparticles as efficient anode catalysts for formic acid electrooxidation[J]. J Mater Chem A, 2015, 3(44): 22129-22135. |
| 54 | LIANG L C, LI M, ZHANG B T, et al. Ordered and isolated Pd sites endow antiperovskite-type PdFe3N with high CO-tolerance for formic acid electrooxidation[J]. Adv Energy Mater, 2023, 13(10): 2203803. |
| 55 | YAO Y C, GU X K, HE D S, et al. Engineering the electronic structure of submonolayer Pt on intermetallic Pd3Pb via charge transfer boosts the hydrogen evolution reaction[J]. J Am Chem Soc, 2019, 141(51): 19964-19968. |
| 56 | CHEN L W, GUO X, SHAO R Y, et al. Structurally ordered intermetallic Ir3V electrocatalysts for alkaline hydrogen evolution reaction[J]. Nano Energy, 2021, 81: 105636. |
| 57 | ZHANG J X, ZHANG L H, LIU J M, et al. OH spectator at IrMo intermetallic narrowing activity gap between alkaline and acidic hydrogen evolution reaction[J]. Nat Commun, 2022, 13(1): 5497-5505. |
| 58 | ZHANG J X, ZHANG L H, DU L, et al. Composition-tunable antiperovskite CuxIn1- xNNi3 as superior electrocatalysts for the hydrogen evolution reaction[J]. Angew Chem Int Ed, 2020, 59(40): 17488-17493. |
| 59 | GRIMAUD A, DIAZ-MORALES O, HAN B, et al. Activating lattice oxygen redox reactions in metal oxides to catalyse oxygen evolution[J]. Nat Chem, 2017, 9(5): 457-465. |
| 60 | KUZNETSOV D A, NAEEM M A, KUMAR P V, et al. Tailoring lattice oxygen binding in ruthenium pyrochlores to enhance oxygen evolution activity[J]. J Am Chem Soc, 2020, 142(17): 7883-7888. |
| 61 | LIANG X, SHI L, LIU Y P, et al. Activating inert, nonprecious perovskites with iridium dopants for efficient oxygen evolution reaction under acidic conditions[J]. Angew Chem Int Ed, 2019, 58(23): 7631-7635. |
| 62 | ZHANG J X, ZHAO X, DU L, et al. Antiperovskite nitrides CuNCo3- xVx: highly efficient and durable electrocatalysts for the oxygen-evolution reaction[J]. Nano Lett, 2019, 19(10): 7457-7463. |
| 63 | ZHANG J X, LIU J M, ZHANG L H, et al. Fe3+-preactivated Ni/Co-based antiperovskite nitrides for boosting oxygen evolution: surface tuning and catalytic mechanism[J]. ACS Catal, 2023, 13(7): 5043-5052. |
| 64 | ZHONG C Z, ZHANG J X, ZHANG L H, et al. Composition-tunable Co3- xFexMo3N electrocatalysts for the oxygen evolution reaction[J]. ACS Energy Lett, 2023, 8(3): 1455-1462. |
| [1] | 王伟, 李家源. 电解水析氢反应磷化钴异质结催化剂的研究进展[J]. 应用化学, 2023, 40(8): 1175-1186. |
| [2] | 刘嘉欣, 范家禾, 李曙辉, 马亮. Rh@Pt内凹立方体核壳催化剂的制备及其乙醇电氧化性能[J]. 应用化学, 2023, 40(8): 1195-1204. |
| [3] | 惠连成, 庄鉴行, 肖顺, 李美平, 靳梦圆, 吕青. 镍氮掺杂石墨炔用作高效氧还原电催化剂[J]. 应用化学, 2023, 40(8): 1205-1213. |
| [4] | 王路飞, 甄蒙蒙, 沈伯雄. 贫电解液下电催化剂对调控锂硫电池性能的研究进展[J]. 应用化学, 2023, 40(2): 188-209. |
| [5] | 杜卫民, 刘欣, 朱琳, 付佳敏, 郭文山, 杨晓晴, 双培硕. 三元镍基硫属化物纳米棒阵列的简单合成及其高效的电催化析氧性能[J]. 应用化学, 2022, 39(8): 1252-1261. |
| [6] | 张超. 单原子催化剂电催化还原二氧化碳研究进展[J]. 应用化学, 2022, 39(6): 871-887. |
| [7] | 王岩, 张树聪, 汪兴坤, 刘志承, 王焕磊, 黄明华. 电解海水析氢反应过渡金属基催化剂的研究进展[J]. 应用化学, 2022, 39(6): 927-940. |
| [8] | 曹维锦, 白璐, 武兰兰, 李敬德, 宋术岩. 多壳层中空镍钴双金属磷化物纳米球用于高效电催化析氧[J]. 应用化学, 2022, 39(4): 666-672. |
| [9] | 商林杰, 刘江, 兰亚乾. 共价有机框架材料用于光/电催化CO2还原的研究进展[J]. 应用化学, 2022, 39(4): 559-584. |
| [10] | 张艺潆, 李翠艳, 赵杰, 余笑明, 方千荣. 卟啉-硫醚基共价有机框架材料用于氧还原反应电催化剂[J]. 应用化学, 2022, 39(4): 647-656. |
| [11] | 孙立智, 吕浩, 闵晓文, 刘犇. 介孔钯-硼合金纳米颗粒的制备和甲醇氧化电催化性能[J]. 应用化学, 2022, 39(4): 673-684. |
| [12] | 吴小峰, 陈德顺, 马伟, 黄科科. 电喷雾沉积WO3/Fe2TiO5复合光阳极及其光电催化水裂解性能[J]. 应用化学, 2022, 39(4): 694-696. |
| [13] | 毕一飘, 宫雪, 杨发, 阮明波, 宋平, 徐维林. 多价MnOx/C电催化剂用于高效氮还原反应[J]. 应用化学, 2020, 37(9): 1048-1055. |
| [14] | 李琳, 任慧敏, 卫博慧, 李军, 王杰, 李晖, 姚陈忠. 钒-氮共掺杂碳基介孔纳米材料的制备及氮还原电催化性能[J]. 应用化学, 2020, 37(8): 930-938. |
| [15] | 蒙阳, 杨婵, 彭娟. 基于铁、钴、镍金属磷化物纳米催化剂的碱性条件下电解水制氢的研究进展[J]. 应用化学, 2020, 37(7): 733-745. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||