应用化学 ›› 2023, Vol. 40 ›› Issue (8): 1126-1139.DOI: 10.19894/j.issn.1000-0518.230129
酸性环境下析氧反应Ir、Ru贵金属电催化剂的研究进展
- 深圳大学化学与环境工程学院,深圳 518000
-
收稿日期:2023-05-04接受日期:2023-07-08出版日期:2023-08-01发布日期:2023-08-24 -
通讯作者:罗兆艳 -
基金资助:国家自然科学基金(22109100)
Research Progress of Noble Metal Electrocatalysts for Oxygen Evolution Reaction in Acidic Environment
Yin-Nan QIAN, Chuan SHI, Wei ZHANG, Zhao-Yan LUO( )
)
- School of Chemical and Environmental Engineering,Shenzhen University,Shenzhen 518000,China
-
Received:2023-05-04Accepted:2023-07-08Published:2023-08-01Online:2023-08-24 -
Contact:Zhao-Yan LUO -
About author:luozhaoyan@szu.edu.cn
-
Supported by:the National Natural Science Foundation of China(22109100)
摘要:
水电解法是利用可再生能源生产氢气的最有效、最环保的方法之一。质子交换膜(PEM)水电解槽对可再生能源的储存和转化具有重要意义,与碱性电解水相比,具有设计紧凑、电压效率高和气体纯度高等优点。然而,阳极电催化剂的低效率、不稳定性和高成本阻碍了PEM水电解。许多析氧反应(OER)电催化剂在恶劣的酸性环境下,在OER电位作用下容易发生溶解或表面结构转变,最终导致催化性能急剧下降,因此酸性OER是阻碍PEM水电解槽实际应用的主要因素之一。高效、经济和耐用的电催化剂可降低OER的高动力学势垒,加速其反应动力学。迄今为止,Ir和Ru基纳米材料仍然代表着最先进的催化剂,已经设计和合成了多种先进的贵金属电催化剂,以增强酸性OER性能。本文综述了近5年性能优异的酸性OER新型电催化剂的研究进展。首先,讨论了对酸性OER的基本认识,包括其反应机理。在此基础上,对贵金属Ir、Ru单原子、合金和氧化物等方面综述了贵金属酸性OER电催化剂的设计和合成进展。最后,从反应机理研究和更高效的电催化剂设计等方面对酸性OER的未来发展提出了展望。
中图分类号:
引用本文
钱音男, 石钏, 张卫, 罗兆艳. 酸性环境下析氧反应Ir、Ru贵金属电催化剂的研究进展[J]. 应用化学, 2023, 40(8): 1126-1139.
Yin-Nan QIAN, Chuan SHI, Wei ZHANG, Zhao-Yan LUO. Research Progress of Noble Metal Electrocatalysts for Oxygen Evolution Reaction in Acidic Environment[J]. Chinese Journal of Applied Chemistry, 2023, 40(8): 1126-1139.
| (Ⅰ) Krasil'shchikov (1963) | (Ⅱ) O'Grady et al. (1974) |
|---|---|
1) 2) 3) 4) | 1) 2) 3) |
| (Ⅲ) Bockris Oxide Path (1983) | (Ⅳ) Bockris Electro Oxide Path (1983) |
1) 2) 3) | 1) 2) 3) |
| (Ⅴ) The Metal Peroxide Path | (Ⅵ) DFT-predicted Path |
1) 2) 3) 4) | 1) 2) 3) 4) |
图1 典型的6种OER路径[23-25]
Fig.1 Six classical OER mechanistic pathways[23-25]
| (Ⅰ) Krasil'shchikov (1963) | (Ⅱ) O'Grady et al. (1974) |
|---|---|
1) 2) 3) 4) | 1) 2) 3) |
| (Ⅲ) Bockris Oxide Path (1983) | (Ⅳ) Bockris Electro Oxide Path (1983) |
1) 2) 3) | 1) 2) 3) |
| (Ⅴ) The Metal Peroxide Path | (Ⅵ) DFT-predicted Path |
1) 2) 3) 4) | 1) 2) 3) 4) |
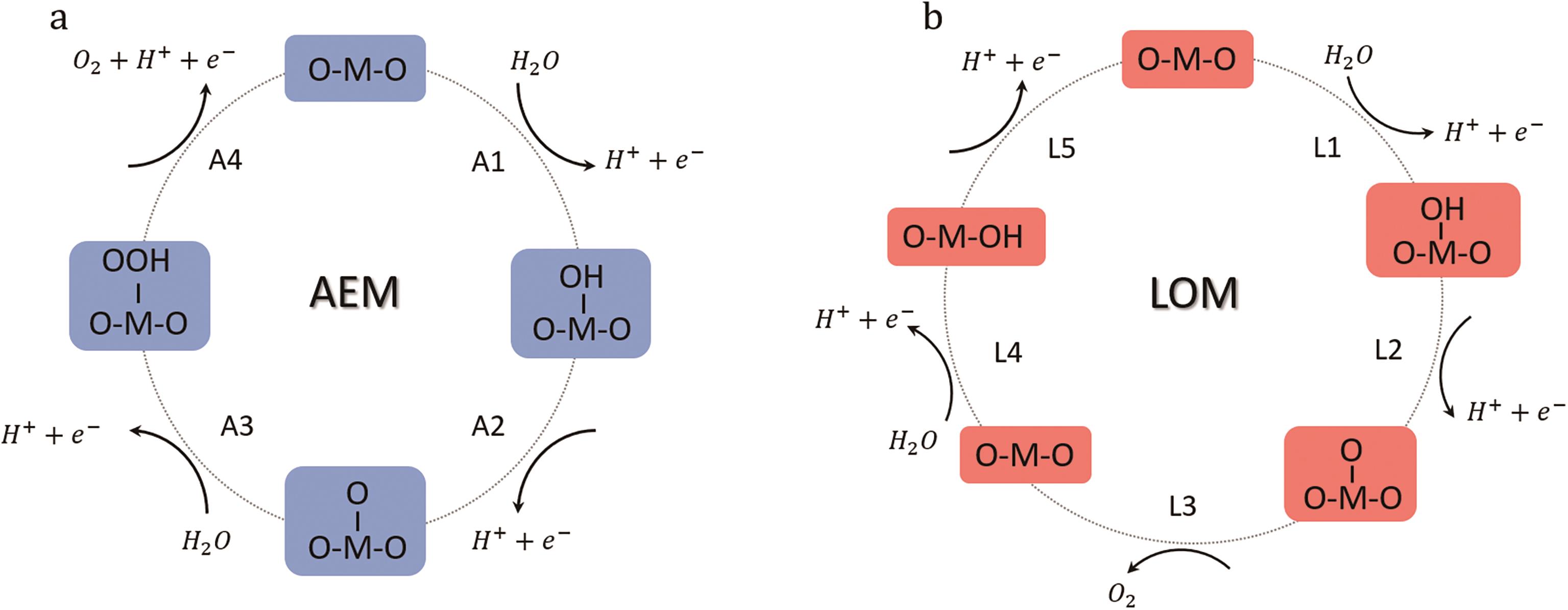
图2 在酸性环境下OER的AEM(a)和LOM(b)[29]
Fig.2 Adsorbates evolution mechanism (AEM) (a) and lattice-oxygen participation mechanism (LOM) (b) for OER in the acidic environment[29]
| Catalyst | Electrolyte | Mechanism | Overpotential/mV (at 10 mA/cm2) | Stability | Tafel slope |
|---|---|---|---|---|---|
| Ir NPs[ | 0.5 mol/L HClO4 | NA | 278 | NA | 56 |
| Ir (PEO-b-PS)[ | 0.5 mol/L H2SO4 | NA | 240 | NA | 49 |
| 3D Ir superstructures[ | 0.5 mol/L H2SO4 | NA | 270 | NA | 50 |
| Ir/GF[ | 0.5 mol/L H2SO4 | NA | 290 | NA | NA |
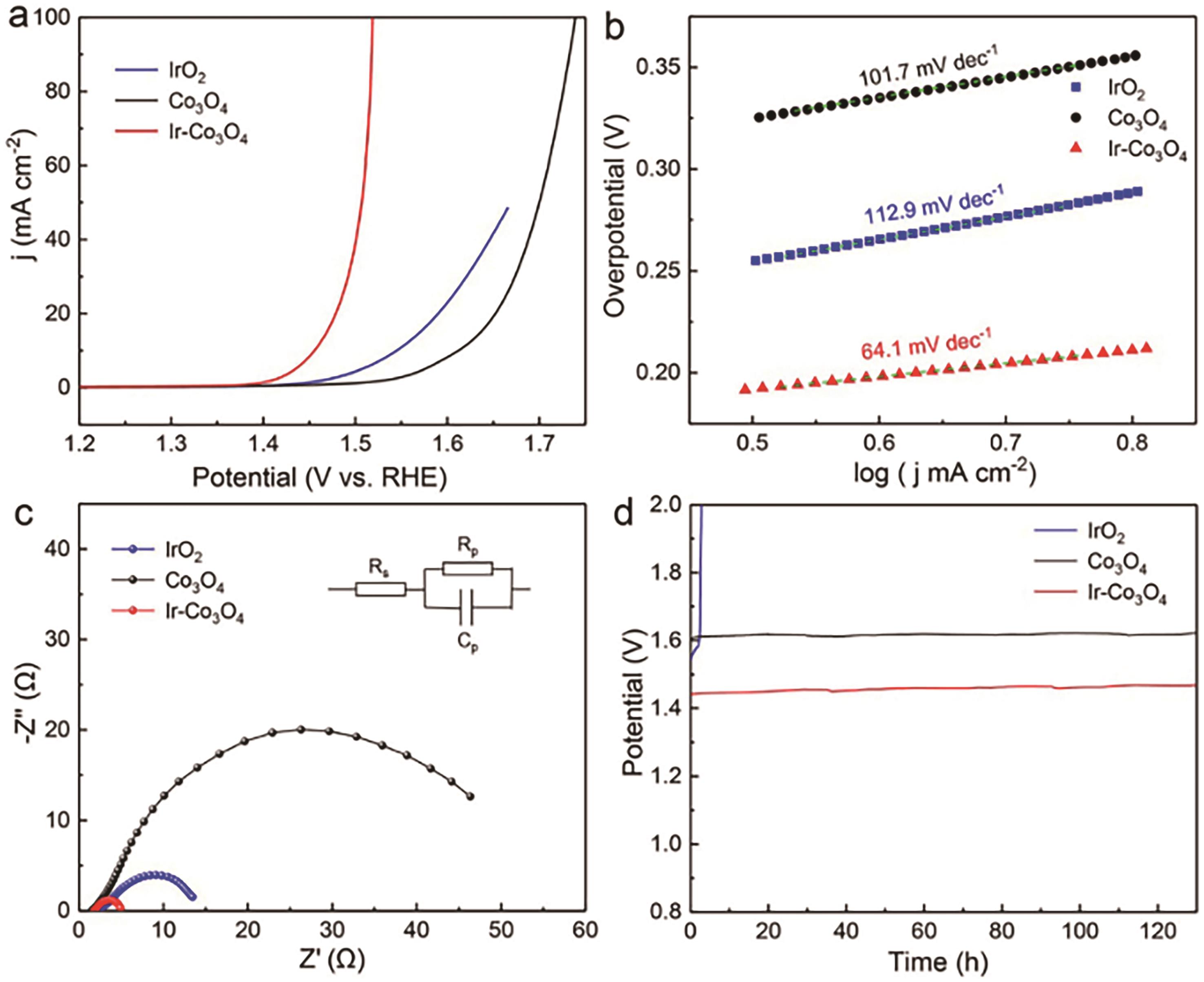
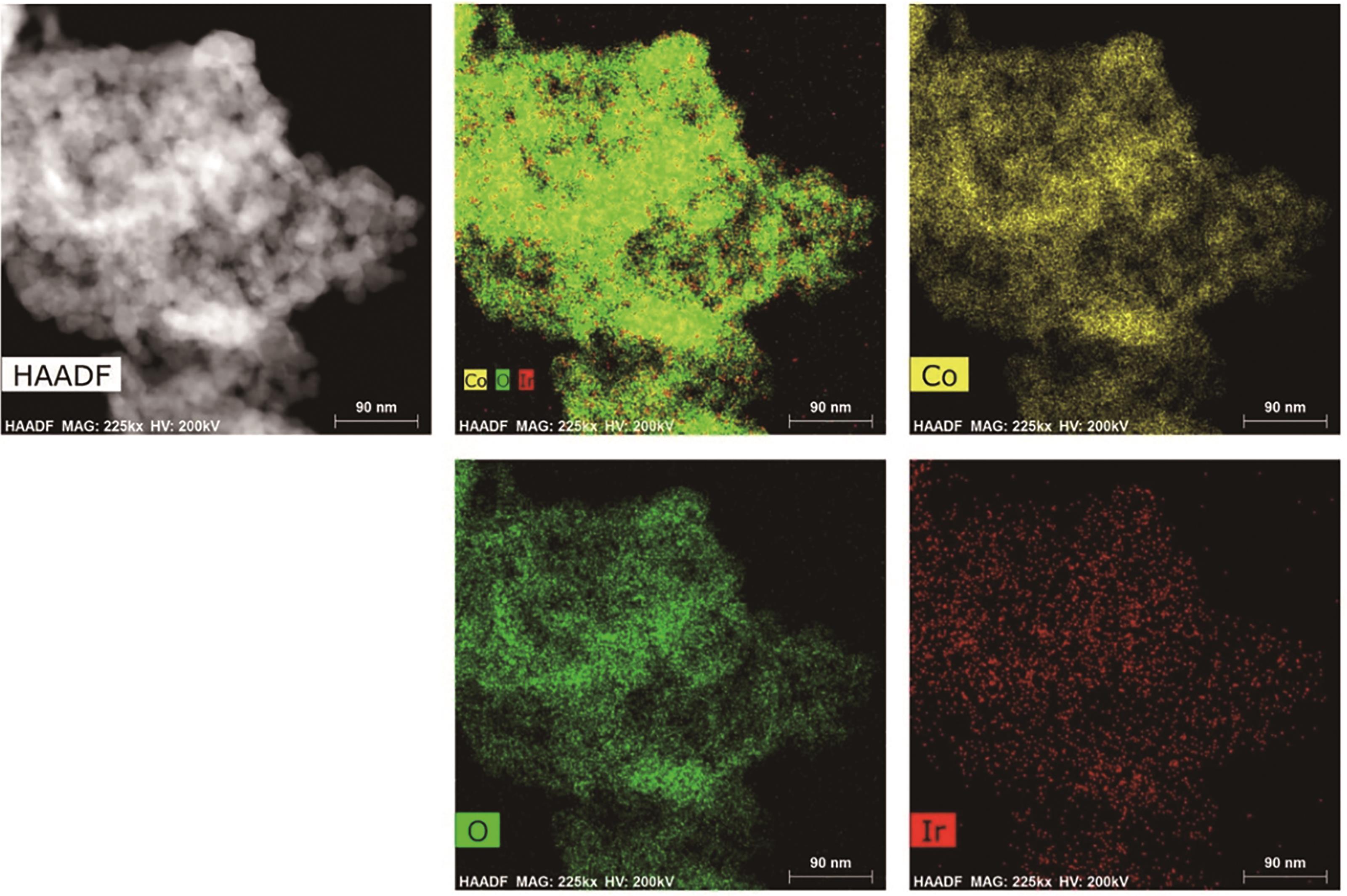
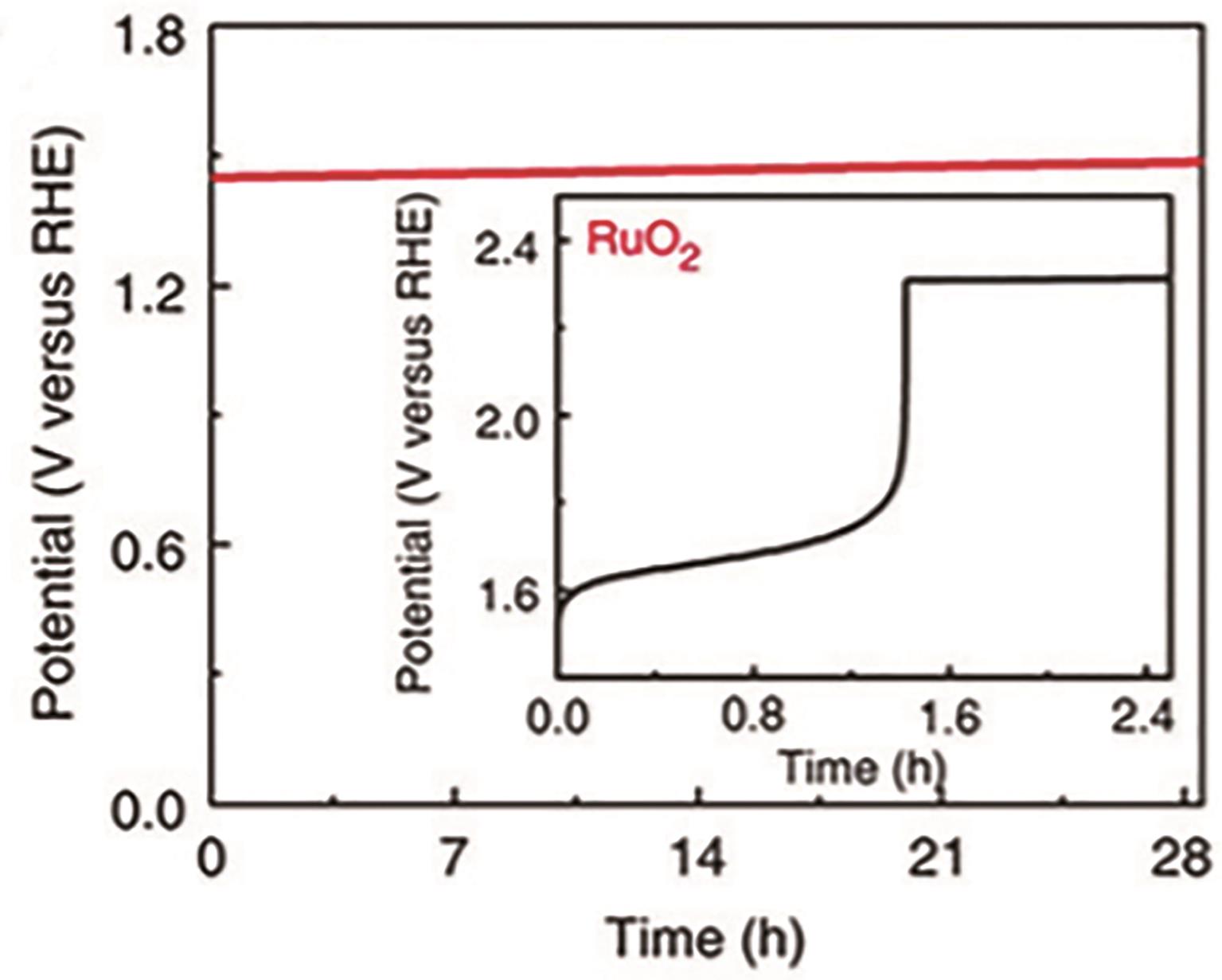
| Ir Co3O4[ | 0.5 mol/L H2SO4 | NA | 225 | Continuous operation 130 h | 64.1 |
| 2D Ru nanosheet[ | 0.5 mol/L H2SO4 | NA | 260 | Reasonable stability | NA |
| IrO2[ | 0.1 mol/L HClO4 | LOM | 382 | Deactivation after 0.5 h | 43.9 |
| RuO2[ | 0.1 mol/L HClO4 | LOM | 310 | 100% dissolution after 2 h | 38 |
| IrO2@RuO2[ | 0.5 mol/L H2SO4 | LOM | 270 | 1 000 cycles at 0.3~1.2 V | 57.8 |
| Ru1-Pt3Cu[ | 0.1 mol/L HClO4 | NA | 220 | NA | NA |
| Ir-Pd nanowires[ | 0.5 mol/L H2SO4 | NA | 300 | 5.56 h at10 mA/cm2 | 60 |
| Ir-Ni lumps connected[ | 0.5 mol/L H2SO4 | NA | NA | 8 h at 1 mA/cm2 | NA |
| Ir-Ni0.57Fe0.82[ | 0.5 mol/L HClO4 | NA | 284 | 5.6 h at 10 mA/cm2 | 48.6 |
| Co-doped IrCu[ | 0.1 mol/L HClO4 | NA | 293 | 2 000 cyclesat 1.2 V | 50 |
| Ir0.5W0.5 nanobranches[ | 0.5 mol/L HClO4 | NA | 250 | NA | 56.6 |
| IrCoNi PHNCs[ | 0.1 mol/L HClO4 | NA | 303 | 0.56 h at 15 mA/cm2 | 53.8 |
| IrNiCu DNF[ | 0.1 mol/L HClO4 | NA | NA | 2 500 cycles at 1.0~1.7 V | 48 |
| AlNiCoIrMo[ | 0.5 mol/L H2SO4 | NA | 233 | 7 000 cycles at 1.20~1.48 V | 55.2 |
| IrNiCu alloy[ | 0.5 mol/L H2SO4 | NA | 262 | NA | 71.4 |
| RuCu nanosheets[ | 0.5 mol/L H2SO4 | NA | 236 | 12 h at 10 mA/cm2 | 40.4 |
| RuCu nanoalloy[ | 0.5 mol/L H2SO4 | NA | 270 | NA | 75.8 |
| Ir0.7Ru0.3O2[ | 0.5 mol/L H2SO4 | NA | 250 | NA | 60.4 |
| Co-doped RuO2[ | 0.5 mol/L H2SO4 | LOM | 169 | Stable at 10 mA/cm2 for 50 h | 66.9 |
| IrO x /SrIrO3[ | 0.5 mol/L H2SO4 | AEM | 270~290 | 30 h at 10 mA/cm2 | NA |
| Y2Ir2O7[ | 0.1 mol/L HClO4 | NA | NA | NA | 51.8 |
表1 具有代表性的贵金属OER电催化剂及其催化性能综述
Table 1 Summary of representative noble metal based OER electrocatalysts and their catalytic performances
| Catalyst | Electrolyte | Mechanism | Overpotential/mV (at 10 mA/cm2) | Stability | Tafel slope |
|---|---|---|---|---|---|
| Ir NPs[ | 0.5 mol/L HClO4 | NA | 278 | NA | 56 |
| Ir (PEO-b-PS)[ | 0.5 mol/L H2SO4 | NA | 240 | NA | 49 |
| 3D Ir superstructures[ | 0.5 mol/L H2SO4 | NA | 270 | NA | 50 |
| Ir/GF[ | 0.5 mol/L H2SO4 | NA | 290 | NA | NA |
| Ir Co3O4[ | 0.5 mol/L H2SO4 | NA | 225 | Continuous operation 130 h | 64.1 |
| 2D Ru nanosheet[ | 0.5 mol/L H2SO4 | NA | 260 | Reasonable stability | NA |
| IrO2[ | 0.1 mol/L HClO4 | LOM | 382 | Deactivation after 0.5 h | 43.9 |
| RuO2[ | 0.1 mol/L HClO4 | LOM | 310 | 100% dissolution after 2 h | 38 |
| IrO2@RuO2[ | 0.5 mol/L H2SO4 | LOM | 270 | 1 000 cycles at 0.3~1.2 V | 57.8 |
| Ru1-Pt3Cu[ | 0.1 mol/L HClO4 | NA | 220 | NA | NA |
| Ir-Pd nanowires[ | 0.5 mol/L H2SO4 | NA | 300 | 5.56 h at10 mA/cm2 | 60 |
| Ir-Ni lumps connected[ | 0.5 mol/L H2SO4 | NA | NA | 8 h at 1 mA/cm2 | NA |
| Ir-Ni0.57Fe0.82[ | 0.5 mol/L HClO4 | NA | 284 | 5.6 h at 10 mA/cm2 | 48.6 |
| Co-doped IrCu[ | 0.1 mol/L HClO4 | NA | 293 | 2 000 cyclesat 1.2 V | 50 |
| Ir0.5W0.5 nanobranches[ | 0.5 mol/L HClO4 | NA | 250 | NA | 56.6 |
| IrCoNi PHNCs[ | 0.1 mol/L HClO4 | NA | 303 | 0.56 h at 15 mA/cm2 | 53.8 |
| IrNiCu DNF[ | 0.1 mol/L HClO4 | NA | NA | 2 500 cycles at 1.0~1.7 V | 48 |
| AlNiCoIrMo[ | 0.5 mol/L H2SO4 | NA | 233 | 7 000 cycles at 1.20~1.48 V | 55.2 |
| IrNiCu alloy[ | 0.5 mol/L H2SO4 | NA | 262 | NA | 71.4 |
| RuCu nanosheets[ | 0.5 mol/L H2SO4 | NA | 236 | 12 h at 10 mA/cm2 | 40.4 |
| RuCu nanoalloy[ | 0.5 mol/L H2SO4 | NA | 270 | NA | 75.8 |
| Ir0.7Ru0.3O2[ | 0.5 mol/L H2SO4 | NA | 250 | NA | 60.4 |
| Co-doped RuO2[ | 0.5 mol/L H2SO4 | LOM | 169 | Stable at 10 mA/cm2 for 50 h | 66.9 |
| IrO x /SrIrO3[ | 0.5 mol/L H2SO4 | AEM | 270~290 | 30 h at 10 mA/cm2 | NA |
| Y2Ir2O7[ | 0.1 mol/L HClO4 | NA | NA | NA | 51.8 |
| 1 | ALIA S M, SHULDA S, NGO C, et al. Iridium-based nanowires as highly active, oxygen evolution reaction electrocatalysts[J]. ACS Catal, 2018, 8(3): 2111-2120. |
| 2 | ANTOLINI E. Iridium as catalyst and cocatalyst for oxygen evolution/reduction in acidic polymer electrolyte membrane electrolyzers and fuel cells[J]. ACS Catal, 2014, 4(5): 1426-1440. |
| 3 | CHANG X, WANG T, YANG P, et al. The development of cocatalysts for photoelectrochemical CO2 reduction[J]. Adv Mater, 2019, 31(31): 1804710. |
| 4 | JIAO Y, ZHENG Y, JARONIEC M, et al. Design of electrocatalysts for oxygen-and hydrogen-involving energy conversion reactions[J]. Chem Soc Rev, 2015, 44(8): 2060-2086. |
| 5 | JIN H, JOO J, CHAUDHARI N K, et al. Recent progress in bifunctional electrocatalysts for overall water splitting under acidic conditions[J]. ChemElectroChem, 2019, 6(13): 3244-3253. |
| 6 | PARK S, SHAO Y, LIU J, et al. Oxygen electrocatalysts for water electrolyzers and reversible fuel cells: status and perspective[J]. Energy Environ Sci, 2012, 5(11): 9331-9344. |
| 7 | SHAN J, LING T, DAVEY K, et al. Transition-metal-doped RuIr bifunctional nanocrystals for overall water splitting in acidic environments[J]. Adv Mater, 2019, 31(17): 1900510. |
| 8 | SARDAR K, PETRUCCO E, HILEY C I, et al. Water-splitting electrocatalysis in acid conditions using ruthenate-iridate pyrochlores[J]. Angew Chem Int Ed, 2014, 53(41): 10960-10964. |
| 9 | FIRMIANO E G S, CORDEIRO M A L, RABELO A C, et al. Graphene oxide as a highly selective substrate to synthesize a layered MoS2 hybrid electrocatalyst[J]. Chem Commun, 2012, 48(62): 7687-7689. |
| 10 | GHADGE S D, VELIKOKHATNYI O I, DATTA M K, et al. Experimental and theoretical validation of high efficiency and robust electrocatalytic response of one-dimensional (1D) (Mn,Ir)O2:10F nanorods for the oxygen evolution reaction in PEM-based water electrolysis[J]. ACS Catal, 2019, 9(3): 2134-2157. |
| 11 | ANDRONESCU C, BARWE S, VENTOSA E, et al. Powder catalyst fixation for post-electrolysis structural characterization of NiFe layered double hydroxide based oxygen evolution reaction electrocatalysts[J]. Angew Chem Int Ed, 2017, 56(37): 11258-11262. |
| 12 | SHIVA KUMAR S, RAMAKRISHNA S U B, BHAGAWAN D, et al. Preparation of RuxPd1- xO2 electrocatalysts for the oxygen evolution reaction (OER) in PEM water electrolysis[J]. Ionics, 2018, 24(8): 2411-2419. |
| 13 | ZHANG C, WANG B, SHEN X, et al. A nitrogen-doped ordered mesoporous carbon/graphene framework as bifunctional electrocatalyst for oxygen reduction and evolution reactions[J]. Nano Energy, 2016, 30: 503-510. |
| 14 | TAHIR M, PAN L, IDREES F, et al. Electrocatalytic oxygen evolution reaction for energy conversion and storage: a comprehensive review[J]. Nano Energy, 2017, 37: 136-157. |
| 15 | BUSCH M, HALCK N B, KRAMM U I, et al. Beyond the top of the volcano?–a unified approach to electrocatalytic oxygen reduction and oxygen evolution[J]. Nano Energy, 2016, 29: 126-135. |
| 16 | LIU Y, WANG Q, ZHANG J, et al. Recent advances in carbon-supported noble-metal electrocatalysts for hydrogen evolution reaction: syntheses, structures, and properties[J]. Adv Energy Mater, 2022, 12(28): 2200928 |
| 17 | CHEREVKO S, ZERADJANIN A R, TOPALOV A A, et al. Dissolution of noble metals during oxygen evolution in acidic media[J]. ChemCatChem, 2014, 6(8): 2219-2223. |
| 18 | ZHU J Y, XUE Q, XUE Y Y, et al. Iridium nanotubes as bifunctional electrocatalysts for oxygen evolution and nitrate reduction reactions[J]. ACS Appl Mater Interfaces, 2020, 12(12): 14064-14070. |
| 19 | CARMO M, FRITZ D L, MERGEL J, et al. A comprehensive review on PEM water electrolysis[J]. Int J Hydrogen Energy, 2013, 38(12): 4901-4934. |
| 20 | DA SILVA G C, FERNANDES M R, TICIANELLI E A. Activity and stability of Pt/IrO2 bifunctional materials as catalysts for the oxygen evolution/reduction reactions[J]. ACS Catal, 2018, 8(3): 2081-2092. |
| 21 | CHEN Y X, LAVACCHI A, MILLER H A, et al. Nanotechnology makes biomass electrolysis more energy efficient than water electrolysis[J]. Nat Commun, 2014, 5(1): 4036. |
| 22 | CHEN Z, DUAN X, WEI W, et al. Electrocatalysts for acidic oxygen evolution reaction: achievements and perspectives[J]. Nano Energy, 2020, 78: 105392. |
| 23 | GIMÉNEZ S, BISQUERT J. Photoelectrochemical solar fuel production[M]Cham, Switzerland: Springer, 2016. |
| 24 | BOCKRIS J O M. Kinetics of activation controlled consecutive electrochemical reactions: anodic evolution of oxygen[J]. J Chem Phys, 1956, 24(4): 817-827. |
| 25 | ROSSMEISL J, QU Z W, ZHU H, et al. Electrolysis of water on oxide surfaces[J]. J Electroanal Chem, 2007, 607(1): 83-89. |
| 26 | REIER T, NONG H N, TESCHNER D, et al. Electrocatalytic oxygen evolution reaction in acidic environments-reaction mechanisms and catalysts[J]. Adv Energy Mater, 2017, 7(1): 1601275. |
| 27 | SPöRI C, KWAN J T H, BONAKDARPOUR A, et al. The stability challenges of oxygen evolving catalysts: towards a common fundamental understanding and mitigation of catalyst degradation[J]. Angew Chem Int Ed, 2017, 56(22): 5994-6021. |
| 28 | ZHANG J, YANG H B, ZHOU D, et al. Adsorption energy in oxygen electrocatalysis[J]. Chem Rev, 2022, 122(23): 17028-17072. |
| 29 | SHAN J, ZHENG Y, SHI B, et al. Regulating electrocatalysts via surface and interface engineering for acidic water electrooxidation[J]. ACS Energy Lett, 2019, 4(11): 2719. |
| 30 | LIN Y, DONG Y, WANG X, et al. Electrocatalysts for the oxygen evolution reaction in acidic media[J]. Adv Mater,2023, 35(22): 2210565. |
| 31 | SUEN N T, HUNG S F, QUAN Q, et al. Electrocatalysis for the oxygen evolution reaction: recent development and future perspectives[J]. Chem Soc Rev, 2017, 46(2): 337-365. |
| 32 | MAN I C, SU H Y, CALLE-VALLEJO F, et al. Universality in oxygen evolution electrocatalysis on oxide surfaces[J]. ChemCatChem, 2011, 3(7): 1159-1165. |
| 33 | ROSSMEISL J, LOGADOTTIR A, NØRSKOV J K. Electrolysis of water on (oxidized) metal surfaces[J]. Chem Phys, 2005, 319(1): 178-184. |
| 34 | LÓPEZ I, ERTEM M Z, MAJI S, et al. A self-improved water-oxidation catalyst: is one site really enough?[J]. Angew Chem Int Ed, 2014, 53(1): 205-209. |
| 35 | CRABTREE R H. Resolving heterogeneity problems and impurity artifacts in operationally homogeneous transition metal catalysts[J]. Chem Rev, 2012, 112(3): 1536-1554. |
| 36 | LEWIS L N. Chemical catalysis by colloids and clusters[J]. Chem Rev, 1993, 93(8): 2693-2730. |
| 37 | KOPER M T M. Analysis of electrocatalytic reaction schemes: distinction between rate-determining and potential-determining steps[J]. J Solid State Electrochem, 2013, 17(2): 339-344. |
| 38 | EXNER K S, OVER H. Kinetics of electrocatalytic reactions from first-principles: a critical comparison with the ab initio thermodynamics approach[J]. Acc Chem Res, 2017, 50(5): 1240-1247. |
| 39 | KELLY S R, KIRK C, CHAN K, et al. Electric field effects in oxygen reduction kinetics: rationalizing pH dependence at the Pt(111), Au(111), and Au(100) electrodes[J]. J Phys Chem C, 2020, 124(27): 14581-14591. |
| 40 | RONG X, PAROLIN J, KOLPAK A M. A fundamental relationship between reaction mechanism and stability in metal oxide catalysts for oxygen evolution[J]. ACS Catal, 2016, 6(2): 1153-1158. |
| 41 | YOO J S, RONG X, LIU Y, et al. Role of lattice oxygen participation in understanding trends in the oxygen evolution reaction on perovskites[J]. ACS Catal, 2018, 8(5): 4628-4636. |
| 42 | HIBBERT D B, CHURCHILL C R. Kinetics of the electrochemical evolution of isotopically enriched gases. Part 2.—18O16O evolution on NiCo2O4 and LiCo3- xO4 in alkaline solution[J]. J Chem Soc, Faraday Transact 1: Phys Chem Condensed Phases, 1984, 80(7): 1965-1975. |
| 43 | WOHLFAHRT-MEHRENS M, HEITBAUM J. Oxygen evolution on Ru and RuO2 electrodes studied using isotope labelling and on-line mass spectrometry[J]. J Electroanal Chem Interfacial Electrochem, 1987, 237(2): 251-260. |
| 44 | AN L, WEI C, LU M, et al. Recent development of oxygen evolution electrocatalysts in acidic environment [J]. Adv Mater, 2021, 33(20): 2006328. |
| 45 | YAO Y, HU S, CHEN W, et al. Engineering the electronic structure of single atom Ru sites via compressive strain boosts acidic water oxidation electrocatalysis[J]. Nat Catal, 2019, 2(4): 304-313. |
| 46 | GIORDANO L, HAN B, RISCH M, et al. pH dependence of OER activity of oxides: current and future perspectives[J]. Catal Today, 2016, 262: 2-10. |
| 47 | HODNIK N, JOVANOVIČ P, PAVLIŠIČ A, et al. New insights into corrosion of ruthenium and ruthenium oxide nanoparticles in acidic media[J]. J Phys Chem C, 2015, 119(18): 10140-10147. |
| 48 | REIER T, OEZASLAN M, STRASSER P. Electrocatalytic oxygen evolution reaction (OER) on Ru, Ir, and Pt catalysts: a comparative study of nanoparticles and bulk materials[J]. ACS Catal, 2012, 2(8): 1765-1772. |
| 49 | DAMJANOVIC A, DEY A, BOCKRIS J O M. Electrode kinetics of oxygen evolution and dissolution on Rh, Ir, and Pt-Rh alloy electrodes[J]. J Electrochem Soc, 1966, 113(7): 739. |
| 50 | MILES M H, KLAUS E A, GUNN B P, et al. The oxygen evolution reaction on platinum, iridium, ruthenium and their alloys at 80 ℃ in acid solutions[J]. Electrochim Acta, 1978, 23(6): 521-526. |
| 51 | PING Y, NIELSEN R J, GODDARD W A. The reaction mechanism with free energy barriers at constant potentials for the oxygen evolution reaction at the IrO2 (110) surface[J]. J Am Chem Soc, 2017, 139(1): 149-155. |
| 52 | KÖTZ R, STUCKI S, SCHERSON D, et al. In-situ identification of RuO4 as the corrosion product during oxygen evolution on ruthenium in acid media[J]. J Electroanal Chem Interfacial Electrochem, 1984, 172(1): 211-219. |
| 53 | FERRER J E, VICTORI L. Study of the oxygen evolution reaction on the iridium electrode in acid medium by EIS[J]. Electrochim Acta, 1994, 39(5): 667-672. |
| 54 | LEDENDECKER M, GEIGER S, HENGGE K, et al. Towards maximized utilization of iridium for the acidic oxygen evolution reaction[J]. Nano Res, 2019, 12(9): 2275-2280. |
| 55 | ZHU J Y, XUE Q, XUE Y Y, et al. Iridium nanotubes as bifunctional electrocatalysts for oxygen evolution and nitrate reduction reactions[J]. ACS Appl Mater Interfaces, 2020, 12(12): 14064-14070. |
| 56 | FU L, CAI P, CHENG G, et al. Colloidal synthesis of iridium-iron nanoparticles for electrocatalytic oxygen evolution[J]. Sustainable Energy Fuels, 2017, 1(5): 1199-1203. |
| 57 | JIANG B, GUO Y, KIM J, et al. Mesoporous metallic iridium nanosheets[J]. J Am Chem Soc, 2018, 140(39): 12434-12441. |
| 58 | WU X, FENG B, LI W, et al. Metal-support interaction boosted electrocatalysis of ultrasmall iridium nanoparticles supported on nitrogen doped graphene for highly efficient water electrolysis in acidic and alkaline media[J]. Nano Energy, 2019, 62: 117-126. |
| 59 | JIANG B, WANG T, CHENG Y, et al. Ir/g-C3N4/nitrogen-doped graphene nanocomposites as bifunctional electrocatalysts for overall water splitting in acidic electrolytes[J]. ACS Appl Mater Interfaces, 2018, 10(45): 39161-39167. |
| 60 | ROY S B, AKBAR K, JEON J H, et al. Iridium on vertical graphene as an all-round catalyst for robust water splitting reactions[J]. J Mater Chem A, 2019, 7(36): 20590-20596. |
| 61 | XUE Q, GAO W, ZHU J, et al. Carbon nanobowls supported ultrafine iridium nanocrystals: an active and stable electrocatalyst for the oxygen evolution reaction in acidic media[J]. J Colloid Interface Sci, 2018, 529: 325-31 |
| 62 | HARTIG-WEISS A, MILLER M, BEYER H, et al. Iridium oxide catalyst supported on antimony-doped tin oxide for high oxygen evolution reaction activity in acidic media[J]. ACS Appl Nano Mater, 2020, 3(3): 2185-2196. |
| 63 | BOSHNAKOVA I, LEFTEROVA E, SLAVCHEVA E. Investigation of montmorillonite as carrier for OER[J]. Int J Hydrogen Energy, 2018, 43(35): 16897-16904. |
| 64 | ZHANG J, WANG G, LIAO Z, et al. Iridium nanoparticles anchored on 3D graphite foam as a bifunctional electrocatalyst for excellent overall water splitting in acidic solution[J]. Nano Energy, 2017, 40: 27-33. |
| 65 | XIE Y, SU Y, QIN H, et al. Ir-doped Co3O4 as efficient electrocatalyst for acidic oxygen evolution reaction[J]. Int J Hydrogen Energy, 2023, 48(39): 14642-14649. |
| 66 | CAO L, LUO Q, CHEN J, et al. Dynamic oxygen adsorption on single-atomic ruthenium catalyst with high performance for acidic oxygen evolution reaction[J]. Nat Commun, 2019, 10(1): 4849. |
| 67 | KÖTZ R, LEWERENZ H J, STUCKI S. XPS studies of oxygen evolution on Ru and RuO2 anodes[J]. J Electrochem Soc, 1983, 130(4): 825. |
| 68 | KONG X, XU K, ZHANG C, et al. Free-standing two-dimensional Ru nanosheets with high activity toward water splitting[J]. ACS Catal, 2016, 6(3): 1487-1492. |
| 69 | SUN Y, HUANG B, LI Y, et al. Trifunctional fishbone-like PtCo/Ir enables high-performance zinc-air batteries to drive the water-splitting catalysis[J]. Chem Mater, 2019, 31(19): 8136-8144. |
| 70 | KÖTZ R, STUCKI S. Oxygen evolution and corrosion on ruthenium-iridium alloys[J]. J Electrochem Soc, 1985, 132(1): 103. |
| 71 | ZHANG T, LIAO S A, DAI L X, et al. Ir-Pd nanoalloys with enhanced surface-microstructure-sensitive catalytic activity for oxygen evolution reaction in acidic and alkaline media[J]. Sci China Mater, 2018, 61(7): 926-938. |
| 72 | PI Y, SHAO Q, WANG P, et al. General formation of monodisperse IrM (M=Ni, Co, Fe) bimetallic nanoclusters as bifunctional electrocatalysts for acidic overall water splitting[J]. Adv Funct Mater, 2017, 27(27): 1700886. |
| 73 | LIM J, YANG S, KIM C, et al. Shaped Ir-Ni bimetallic nanoparticles for minimizing Ir utilization in oxygen evolution reaction[J]. Chem Commun, 2016, 52(32): 5641-5644. |
| 74 | CHEN D, ZHANG P, FANG Q, et al. Coordination-supported organic polymers: mesoporous inorganic-organic materials with preferred stability[J]. Inorg Chem Front, 2018, 5(8): 2018-2022. |
| 75 | FU L, CHENG G, LUO W. Colloidal synthesis of monodisperse trimetallic IrNiFe nanoparticles as highly active bifunctional electrocatalysts for acidic overall water splitting[J]. J Mater Chem A, 2017, 5(47): 24836-24841. |
| 76 | KWON T, HWANG H, SA Y J, et al. Cobalt assisted synthesis of IrCu hollow octahedral nanocages as highly active electrocatalysts toward oxygen evolution reaction[J]. Adv Funct Mater, 2017, 27(7): 1604688. |
| 77 | LV F, FENG J, WANG K, et al. Iridium-tungsten alloy nanodendrites as pH-universal water-splitting electrocatalysts[J]. ACS Cent Sci, 2018, 4(9): 1244-1252 |
| 78 | FENG J, LV F, ZHANG W, et al. Iridium-based multimetallic porous hollow nanocrystals for efficient overall-water-splitting catalysis[J]. Adv Mater, 2017, 29(47): 1703798. |
| 79 | PARK J, SA Y J, BAIK H, et al. Iridium-based multimetallic nanoframe@nanoframe structure: an efficient and robust electrocatalyst toward oxygen evolution reaction[J]. ACS Nano, 2017, 11(6): 5500-5509. |
| 80 | JIN Z, LV J, JIA H, et al. Nanoporous Al-Ni-Co-Ir-Mo high-entropy alloy for record-high water splitting activity in acidic environments[J]. Small, 2019, 15(47): 1904180. |
| 81 | ZHANG J, CHEN Z, LIU C, et al. Hierarchical iridium-based multimetallic alloy with double-core-shell architecture for efficient overall water splitting[J]. Sci China Mater, 2020, 63(2): 249-257. |
| 82 | LI L, WANG P, SHAO Q, et al. Recent progress in advanced electrocatalyst design for acidic oxygen evolution reaction[J]. Adv Mater, 2021, 33(50): 2004243. |
| 83 | FORGIE R, BUGOSH G, NEYERLIN K C, et al. Bimetallic Ru electrocatalysts for the OER and electrolytic water splitting in acidic media[J]. Electrochem Solid-State Lett, 2010, 13(4): B36. |
| 84 | YAO Q, HUANG B, ZHANG N, et al. Channel-rich RuCu nanosheets for pH-universal overall water splitting electrocatalysis[J]. Angew Chem Int Ed, 2019, 58(39): 13983-13988. |
| 85 | YANG J, JI Y, SHAO Q, et al. A universal strategy to metal wavy nanowires for efficient electrochemical water splitting at pH-universal conditions[J]. Adv Funct Mater, 2018, 28(41): 1803722. |
| 86 | LI Y, ZHOU W, ZHAO X, et al. Donutlike RuCu nanoalloy with ultrahigh mass activity for efficient and robust oxygen evolution in acid solution[J]. ACS Appl Energy Mater, 2019, 2(10): 7483-7489. |
| 87 | WANG L, SAVELEVA V A, ZAFEIRATOS S, et al. Highly active anode electrocatalysts derived from electrochemical leaching of Ru from metallic Ir0.7Ru0.3 for proton exchange membrane electrolyzers[J]. Nano Energy, 2017, 34: 385-391. |
| 88 | WANG X, ZHONG H, XI S, et al. Understanding of oxygen redox in the oxygen evolution reaction[J]. Adv Mater, 2022, 34(50): 2107956. |
| 89 | SHI Q, ZHU C, DU D, et al. Robust noble metal-based electrocatalysts for oxygen evolution reaction[J]. Chem Soc Rev, 2019, 48(12): 3181-3192. |
| 90 | SEITZ L C, DICKENS C F, NISHIO K, et al. A highly active and stable IrOx/SrIrO3 catalyst for the oxygen evolution reaction[J]. Science, 2016, 353(6303): 1011-1014. |
| 91 | SHANG C, CAO C, YU D, et al. Electron correlations engineer catalytic activity of pyrochlore iridates for acidic water oxidation[J]. Adv Mater, 2019, 31(6): 1805104. |
| 92 | SHIH P C, KIM J, SUN C J, et al. Single-phase pyrochlore Y2Ir2O7 electrocatalyst on the activity of oxygen evolution reaction[J]. ACS Appl Energy Mater, 2018, 1(8): 3992-3998. |
| 93 | SHI Z, WANG X, GE J, et al. Fundamental understanding of the acidic oxygen evolution reaction: mechanism study and state-of-the-art catalysts[J]. Nanoscale, 2020, 12(25): 13249-13275. |
| [1] | 徐三魁, 王晓栋, 李利民, 许群, 李蕊. 超临界甲醇处理对活性炭表面性质及钌炭催化性能的影响[J]. 应用化学, 2011, 28(08): 936-941. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||