应用化学 ›› 2023, Vol. 40 ›› Issue (8): 1109-1125.DOI: 10.19894/j.issn.1000-0518.230126
超亲水/超疏气电解水催化剂的研究进展
谭翠盈1,2, 丁威超1, 马婷婷1, 肖瑶1( ), 刘健2(
), 刘健2( )
)
- 1.青岛科技大学材料科学与工程学院,青岛 266042
2.中国科学院青岛生物能源与过程研究所,青岛 266101
-
收稿日期:2023-04-29接受日期:2023-07-06出版日期:2023-08-01发布日期:2023-08-24 -
通讯作者:肖瑶,刘健 -
基金资助:国家自然科学基金(22109081);青岛科技大学2022年大学生创新训练计划项目(202210426071)
Research Progress on Superhydrophilic/Superaerophobic Electrocatalysts for Water Splitting
Cui-Ying TAN1,2, Wei-Chao DING1, Ting-Ting MA1, Yao XIAO1( ), Jian LIU2(
), Jian LIU2( )
)
- 1.College of Materials Science and Engineering,Qingdao University of Science and Technology,Qingdao 266042,China
2.Qingdao Institute of Bioenergy and Bioprocess Technology,Chinese Academy of Science,Qingdao 266101,China
-
Received:2023-04-29Accepted:2023-07-06Published:2023-08-01Online:2023-08-24 -
Contact:Yao XIAO,Jian LIU -
About author:liujian@qibebt.ac.cn
xiaoyao@qust.edu.cn
-
Supported by:the National Natural Science Foundation of China(22109081);the National Undergraduate Innovation and Entrepreneurship Training Program(202210426071)
摘要:
电解水制氢是一种环保、简便且易于操控的制氢技术。工业化电解水制氢通常在高电流密度下进行,在制氢过程中会产生大量气泡,而气泡在电极表面聚集粘附会覆盖大量活性位点,导致电解水效率降低。因此,调控气体扩散行为对于工业电解水应用来说至关重要。近年来,超浸润材料因为其独特的润湿性能而备受关注。通过控制催化剂表面的化学组成和多尺度微纳米结构可以构建出超浸润界面材料。此类材料具有超亲水/超疏气的界面结构,有助于水相电解液的有效浸润和原位生成气泡的快速释放,从而提升催化剂的水电解性能。系统介绍了2014年至2023年期间报道的部分具有超亲水/超疏气界面结构的电解水催化剂的现状,概述其材料的合成设计策略和水电解催化性能,并对超浸润水电解催化剂的研究现状、面临的挑战和应用前景进行了总结和展望。
中图分类号:
引用本文
谭翠盈, 丁威超, 马婷婷, 肖瑶, 刘健. 超亲水/超疏气电解水催化剂的研究进展[J]. 应用化学, 2023, 40(8): 1109-1125.
Cui-Ying TAN, Wei-Chao DING, Ting-Ting MA, Yao XIAO, Jian LIU. Research Progress on Superhydrophilic/Superaerophobic Electrocatalysts for Water Splitting[J]. Chinese Journal of Applied Chemistry, 2023, 40(8): 1109-1125.
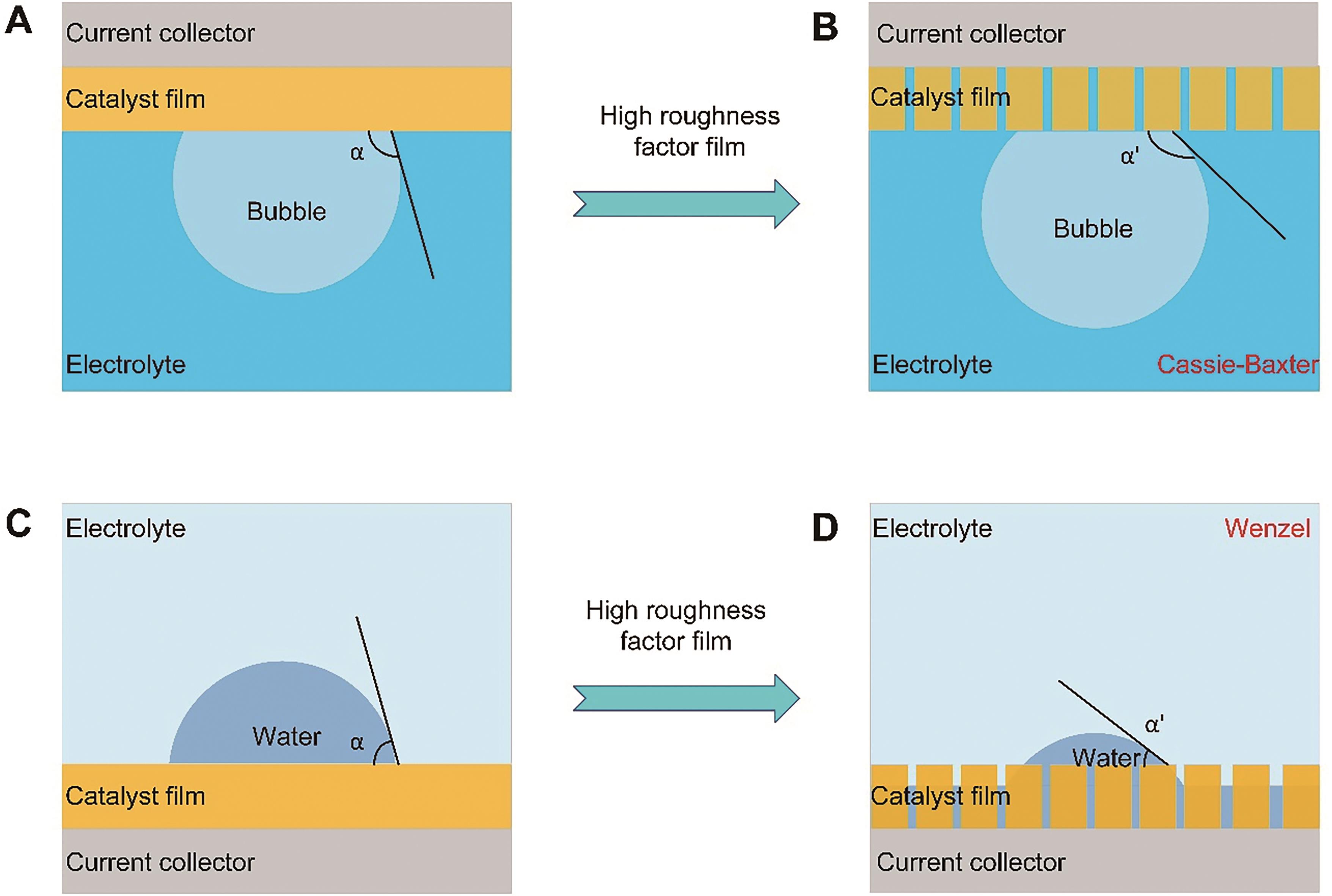
图1 表面粗糙度如何影响气泡、液滴接触角的示意图: (A)平坦的疏气性表面,(B)粗糙的超疏气性表面,(C)平坦的亲水性表面,(D)粗糙的超亲水性表面
Fig.1 Schematic illustration of how the surface roughness affecting the bubble contact angle: (A) flat aerophobic surface, (B) rough superaerophobic surface, (C) flat hydrophilic surface, (D) rough superhydrophilic surface
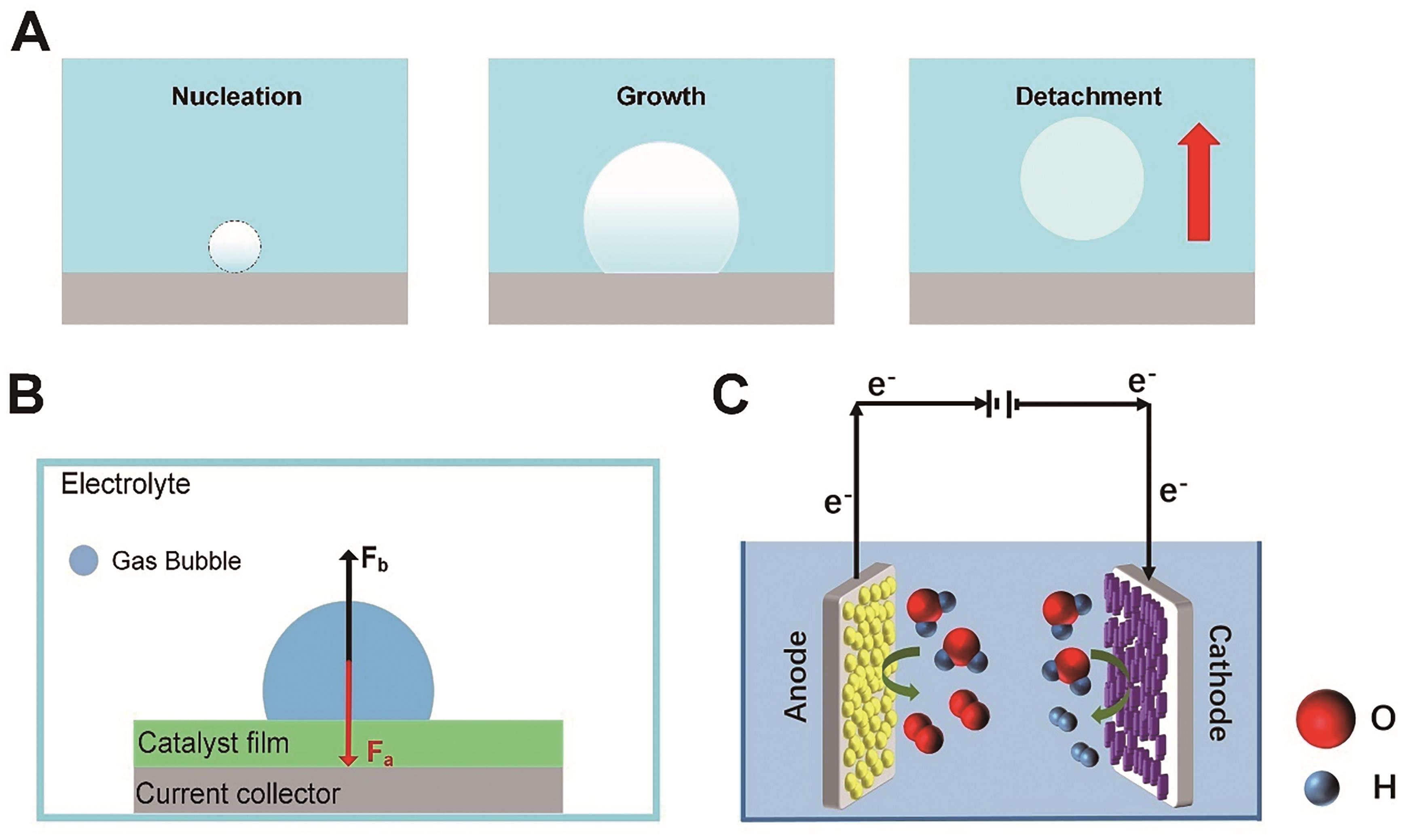
图2 (A)气泡演化过程; (B)电极表面单个气泡的应力分析; (C)电解水示意图
Fig.2 (A) Bubble evolution process; (B) Stress analysis of one single bubble on the electrode surface; (C) Illustration of electrodes for water splitting
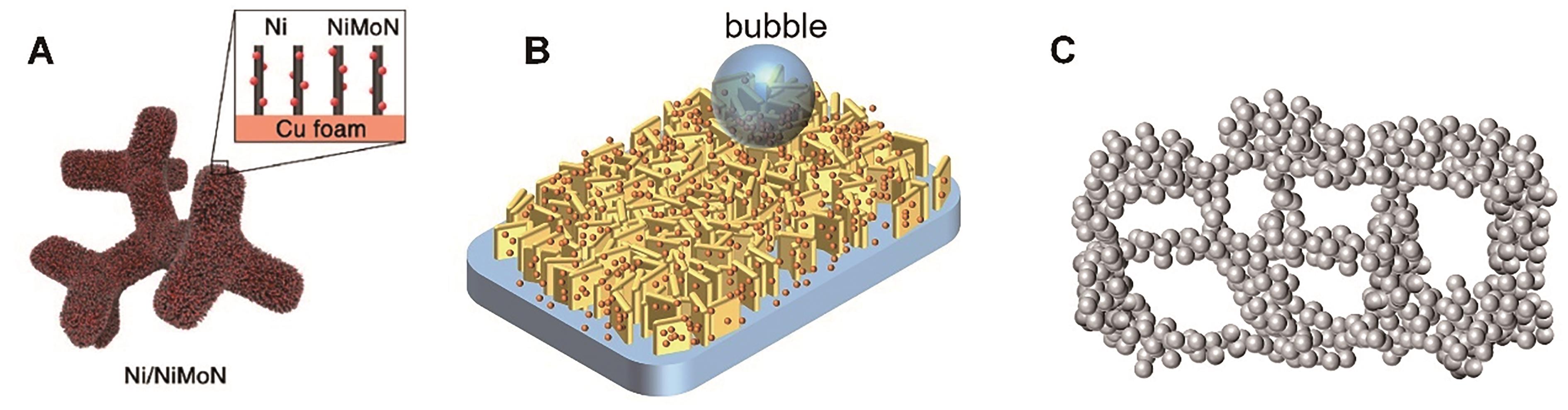
图3 具有纳米复合结构的催化剂: (A) Ni/NiMoN 纳米阵列[99]; (B)纳米片上均匀分布纳米颗粒; (C)相互连接的纳米颗粒结构
Fig.3 Nanocomposite catalyst: (A) Ni/NiMoN nanowire array[99]; (B) Nanoparticles distributed on the nanoparticle; (C) Interconnected nanoparticle structures
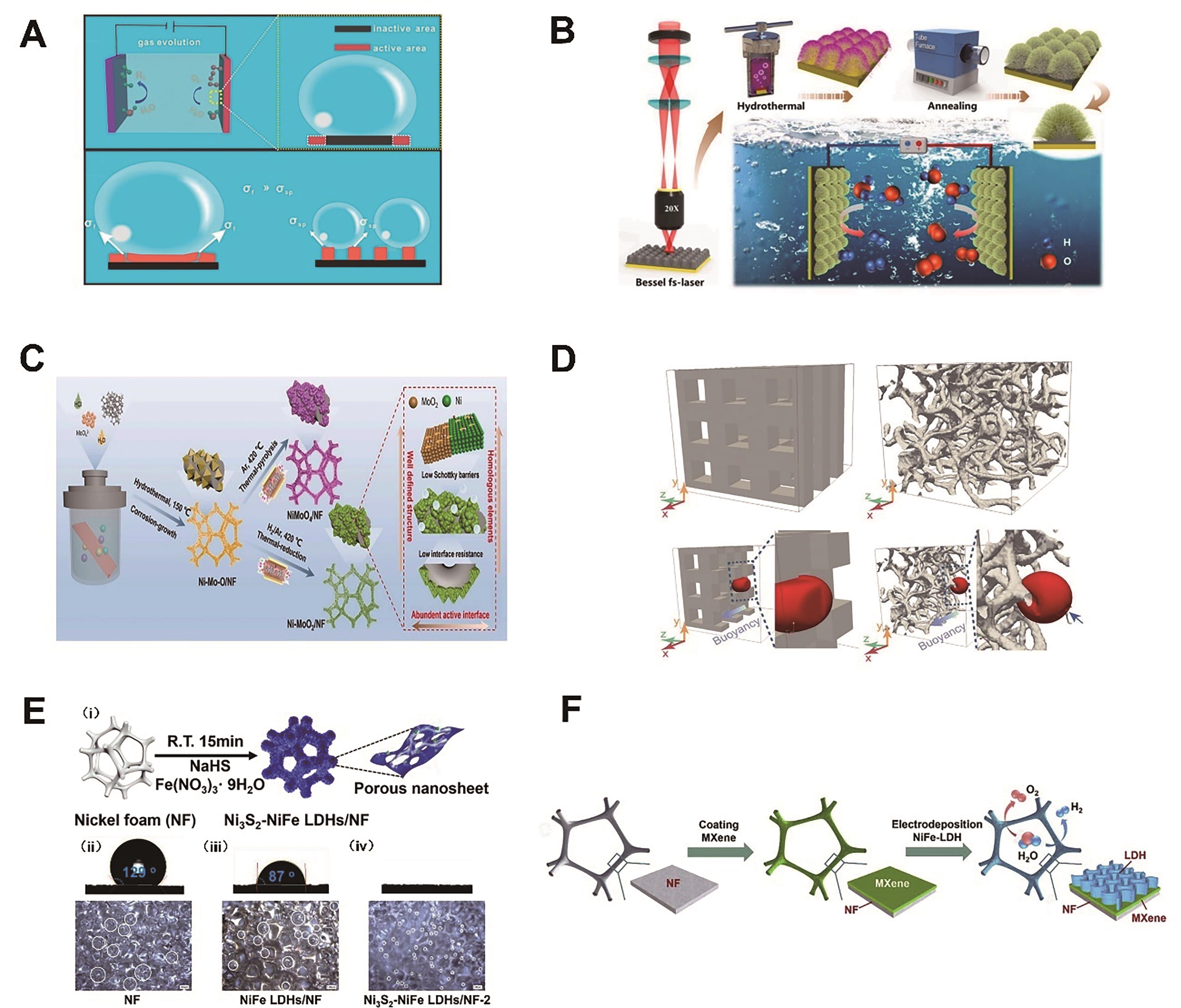
图4 (A)平面薄膜电极和条带电极上气泡的生长示意图[66]; (B)具有分级毛刺状纳米结构的自支撑氧化钴电催化剂的制备工艺及全解水原理图[69]; (C) Ni-MoO2/NF和NiMoO4/NF的制备示意图[70]; (D) 3DPNi和NF中传输过程中气泡形状模拟图[71]; (E) Ni3S2-NiFe LDHs/NF的合成示意图(i),NF(ii)、NiFe LDHs/NF(iii)和Ni3S2-NiFe LDHs/NF-2(iv)的表面浸润性和气泡脱附行为[77]; (F)在大孔MXene/NF框架上生长NiFe-LDH纳米片介孔网络制备多层结构的三维电催化电极示意图[79]
Fig.4 (A) Schematic illustration of the growth of gas bubbles on a flat film electrode and SP assemblies[66]; (B) The preparation process of the self-supported electrocatalysts with hierarchical chestnut burr-like structures and schematic diagram for overall water splitting application[69]; (C) Schematic illustration of the fabrication of Ni-MoO2/NF and NiMoO4/NF[70]; (D) Simulation frames showing bubble shape during transport in 3DPNi and NF[71]; (E) (i) Schemic of the synthesis of Ni3S2-NiFe LDHs/NF and surface wettability and bubble releasing behavior of the (ii) NF, (iii) NiFe LDHs/NF and (iv) Ni3S2-NiFe LDHs/NF-2[77]; (F) Schematic illustration of the fabrication of hierarchically structured 3D electrocatalytic electrode by growing me-soporous network of NiFe-LDH nanosheets onto macroporous MXene/NF frame[79]
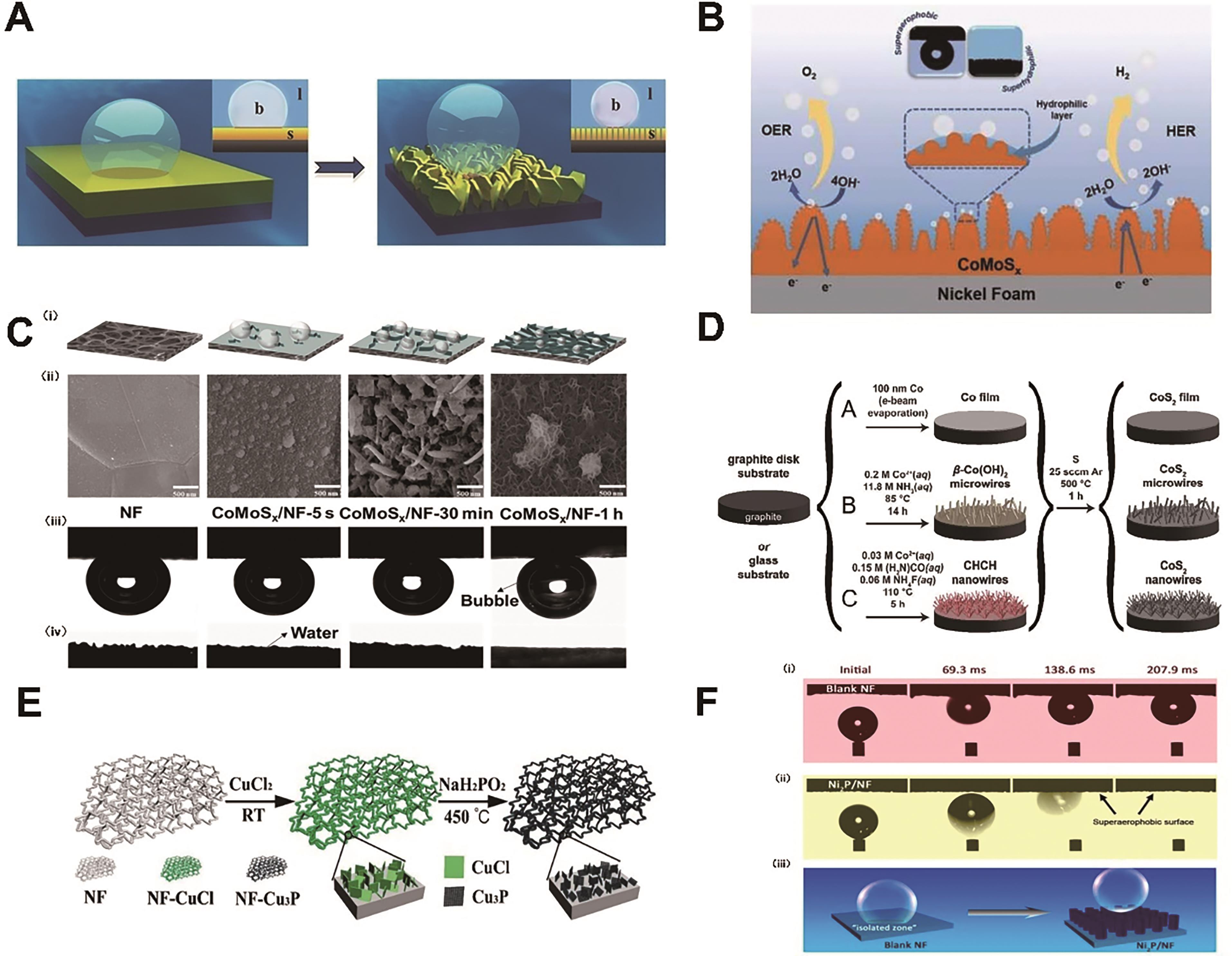
图5 (A)气泡在平面薄膜(左)和纳米结构薄膜(右)上的粘附行为示意图[9]; (B)超亲水/超疏气CoMoS x /NF全解水电催化剂的示意图[84]; (C)具有不同表面结构的CoMoS x /NF示意图(i),SEM图像(ii),水下气泡接触角(iii)和静态水滴接触角(iv)的光学图片[83]; (D)在石墨盘(或玻璃)衬底上制备CoS2薄膜、微米线阵列和纳米线阵列的示意图[15]; (E) Cu3P微米片的制备示意图[92]; (F)Ni2P纳米阵列的合成过程示意图[93]
Fig.5 (A) Schematic illustration of adhesion behaviors of gas bubbles on flat film (left) and nanostructured film (right)?[9]; (B) Design of the superhydrophilic/superaerophobic CoMoS x /NF electrocatalysts for overall water splitting[84]; (C) (i)Schematic illustration of CoMoS x supported on the NF with three distinct geometries, (ii) SEM images of CoMoS x /NF at different reaction times, (iii) air-bubble contact angles under water and (iv) static water-droplet contact angles[83]; (D) Schematic depictions of the preparation of a cobalt pyrite (CoS2) film, microwire array, or nanowire array on a graphite disk (or glass) substrate[15]; (E) Schematic illustration of the preparation of Cu3P microsheets[92]; (F) Schematic illustration of the synthetic process for Ni2P nanoarrays[93]
| Catalyst | ηa (HER/OER)/mV | Bubble contact angle/(°) | Stability/h | Ref. |
|---|---|---|---|---|
| Pt nanoarray | -/- | 161.3±3.4 | 36(-0.5 V) | [ |
| Pt SP5 | η14.3=15 mV/- | — | 11(30 mA/cm2) | [ |
| Ni/NiO@MoO3-x | η10=7 mV/- | 149.4 | 40(100 mA/cm2) | [ |
| Ni-MoO2/NF | η20=1.53 V/- | — | 120(1.53 V) | [ |
| CoO/Co3O4 | η10=105 mV/η10=235 mV | — | 72 | [ |
| C-Ni1-x O/3DPNi | η1000=245 mV/- | — | 16(2.2 V) | [ |
| Ru/Co(OH)2 | η10=35 mV/- | 141 | 14(500 mA/cm2) | [ |
| P-Ni(OH)2/NiMoO4 | η10=60 mV/- | 153.8 | 30 | [ |
| NiFe-LDHs/NF | -/η1000=303 mV | — | 240 | [ |
| Pt@S-NiFe LDHs | η10=60 mV/- | 163.6 | 200 (100 mA/cm2) | [ |
| 3D NiFe/MXene | η500=205 mV/η500=30 200 mV | — | 280(10 mA/cm)2 | [ |
| CoS2 | η10=145 mV/- | — | 40 (10 mA) | [ |
| MoS2 | — | 153.6±2.4 | — | [ |
| CoMoS x /NF | η10=89 mV/- | — | 100 (500 mA/cm2) | [ |
| MoS2/Mo2C | η1000=220 mV/- | — | 24 | [ |
| FeCoNi-HNTAs | η10=58 mV/η10=184 mV | 171.0 | 100(50 mA/cm2) | [ |
| FeS/IF | η1000=336 mV/- | 151.7 | 30 | [ |
| Fe-Ni-P-S | — | 142.3 | 300(2 500 mA/cm2) | [ |
| CuMo6S8/Cu | η2500=321 mV/- | — | 100(2 500 mA/cm2) | [ |
| Cu3P | η10=130 mV/η10=290 mV | 155.7 | 24 | [ |
| Ni2P/NF | η1000=306 mV/- | — | 10(25000 mA/cm2) | [ |
| Ni2P NV/CF | η10=1.48 V/- | — | 50 | [ |
| NF@Co x P | η10=185 mV/- | — | 12 (800 mA/cm2) | [ |
表 1 2014-2023年文献报道的部分超亲水/超疏气电解水催化剂的性能总结
Table 1 The performance of electrolytic water catalysts with superhydrophilic/superaerophobic interface structure in 2014-2023
| Catalyst | ηa (HER/OER)/mV | Bubble contact angle/(°) | Stability/h | Ref. |
|---|---|---|---|---|
| Pt nanoarray | -/- | 161.3±3.4 | 36(-0.5 V) | [ |
| Pt SP5 | η14.3=15 mV/- | — | 11(30 mA/cm2) | [ |
| Ni/NiO@MoO3-x | η10=7 mV/- | 149.4 | 40(100 mA/cm2) | [ |
| Ni-MoO2/NF | η20=1.53 V/- | — | 120(1.53 V) | [ |
| CoO/Co3O4 | η10=105 mV/η10=235 mV | — | 72 | [ |
| C-Ni1-x O/3DPNi | η1000=245 mV/- | — | 16(2.2 V) | [ |
| Ru/Co(OH)2 | η10=35 mV/- | 141 | 14(500 mA/cm2) | [ |
| P-Ni(OH)2/NiMoO4 | η10=60 mV/- | 153.8 | 30 | [ |
| NiFe-LDHs/NF | -/η1000=303 mV | — | 240 | [ |
| Pt@S-NiFe LDHs | η10=60 mV/- | 163.6 | 200 (100 mA/cm2) | [ |
| 3D NiFe/MXene | η500=205 mV/η500=30 200 mV | — | 280(10 mA/cm)2 | [ |
| CoS2 | η10=145 mV/- | — | 40 (10 mA) | [ |
| MoS2 | — | 153.6±2.4 | — | [ |
| CoMoS x /NF | η10=89 mV/- | — | 100 (500 mA/cm2) | [ |
| MoS2/Mo2C | η1000=220 mV/- | — | 24 | [ |
| FeCoNi-HNTAs | η10=58 mV/η10=184 mV | 171.0 | 100(50 mA/cm2) | [ |
| FeS/IF | η1000=336 mV/- | 151.7 | 30 | [ |
| Fe-Ni-P-S | — | 142.3 | 300(2 500 mA/cm2) | [ |
| CuMo6S8/Cu | η2500=321 mV/- | — | 100(2 500 mA/cm2) | [ |
| Cu3P | η10=130 mV/η10=290 mV | 155.7 | 24 | [ |
| Ni2P/NF | η1000=306 mV/- | — | 10(25000 mA/cm2) | [ |
| Ni2P NV/CF | η10=1.48 V/- | — | 50 | [ |
| NF@Co x P | η10=185 mV/- | — | 12 (800 mA/cm2) | [ |
| 1 | YAN D, LI Y, HUO J, et al. Defect chemistry of nonprecious-metal electrocatalysts for oxygen reactions[J]. Adv Mater, 2017, 29(48): 1606459. |
| 2 | SCHLAPBACH L. Hydrogen-fuelled vehicles[J]. Nature, 2009, 460(7257): 809-811. |
| 3 | MORALES-GUIO C G, STERN L A, HU X. Nanostructured hydrotreating catalysts for electrochemical hydrogen evolution[J]. Chem Soc Rev, 2014, 43(18): 6555-6569. |
| 4 | GAO N, CHENG M, QUAN C, et al. Syngas production via combined dry and steam reforming of methane over Ni-Ce/ZSM-5 catalyst[J]. Fuel, 2020, 273: 117702. |
| 5 | CHI J, YU H. Water electrolysis based on renewable energy for hydrogen production[J]. Chin J Catal, 2018, 39(3): 390-394. |
| 6 | WANG J, YUE X, YANG Y, et al. Earth-abundant transition-metal-based bifunctional catalysts for overall electrochemical water splitting: a review[J]. J Alloys Compd, 2020, 819: 153346. |
| 7 | HU S, GE S, LIU H, et al. Low-dimensional electrocatalysts for acidic oxygen evolution: intrinsic activity, high current density operation, and long‐term stability[J]. Adv Funct Mater, 2022, 32(23): 2201726. |
| 8 | WU Z, LU X, ZANG S, et al. Non‐noble‐metal‐based electrocatalysts toward the oxygen evolution reaction[J]. Adv Funct Mater, 2020, 30(15): 1910274. |
| 9 | LU Z, ZHU W, YU X, et al. Ultrahigh hydrogen evolution performance of under-water “superaerophobic” MoS2 nanostructured electrodes[J]. Adv Mater, 2014, 26(17): 2683-2687, 2615. |
| 10 | CHU S, MAJUMDAR A. Opportunities and challenges for a sustainable energy future[J]. Nature, 2012, 488(7411): 294-303. |
| 11 | JUNG W B, YUN G T, KIM Y, et al. Relationship between hydrogen evolution and wettability for multiscale hierarchical wrinkles[J]. ACS Appl Mater Interfaces, 2019, 11(7): 7546-7552. |
| 12 | ZHANG C, XU Z, HAN N, et al. Superaerophilic/superaerophobic cooperative electrode for efficient hydrogen evolution reaction via enhanced mass transfer[J]. Sci Adv, 2023, 9(3): eadd6978. |
| 13 | MILLER H A, BOUZEK K, HNAT J, et al. Green hydrogen from anion exchange membrane water electrolysis: a review of recent developments in critical materials and operating conditions[J]. Sustainable Energy Fuels, 2020, 4(5): 2114-2133. |
| 14 | LUO Y, ZHANG Z, CHHOWALLA M, et al. Recent advances in design of electrocatalysts for high-current-density water splitting[J]. Adv Mater, 2022, 34(16): e2108133. |
| 15 | FABER M S, DZIEDZIC R, LUKOWSKI M A, et al. High-performance electrocatalysis using metallic cobalt pyrite (CoS2) micro- and nanostructures[J]. J Am Chem Soc, 2014, 136(28): 10053-10061. |
| 16 | QU M, MA L, WANG J, et al. Multifunctional superwettable material with smart pH responsiveness for efficient and controllable oil/water separation and emulsified wastewater purification[J]. ACS Appl Mater Interfaces, 2019, 11(27): 24668-24682. |
| 17 | YU S Q, LING Y H, WANG R G, et al. Constructing superhydrophobic WO3@TiO2 nanoflake surface beyond amorphous alloy against electrochemical corrosion on iron steel[J]. Appl Surf Sci, 2018, 436: 527-535. |
| 18 | PAN R, ZHANG H, ZHONG M. Triple-scale superhydrophobic surface with excellent anti-icing and icephobic performance via ultrafast laser hybrid fabrication[J]. ACS Appl Mater Interfaces, 2021, 13(1): 1743-1753. |
| 19 | ZHAO Q, AN J, WANG S, et al. Superhydrophobic air-breathing cathode for efficient hydrogen peroxide generation through two-electron pathway oxygen reduction reaction[J]. ACS Appl Mater Interfaces, 2019, 11(38): 35410-35419. |
| 20 | SU B, TIAN Y, JIANG L. Bioinspired Interfaces with superwettability: from materials to chemistry[J]. J Am Chem Soc, 2016, 138(6): 1727-1748. |
| 21 | LI Y, ZHANG H, XU T, et al. Under-water superaerophobic pine-shaped Pt nanoarray electrode for ultrahigh-performance hydrogen evolution[J]. Adv Funct Mater, 2015, 25(11): 1737-1744. |
| 22 | AKBAR K, HUSSAIN S, TRUONG L, et al. Induced superaerophobicity onto a non-superaerophobic catalytic surface for enhanced hydrogen evolution reaction[J]. ACS Appl Mater Interfaces, 2017, 9(50): 43674-43680. |
| 23 | ZHANG J, ZHANG Q, FENG X. Support and interface effects in water-splitting electrocatalysts[J]. Adv Mater, 2019, 31(31): e1808167. |
| 24 | THOMAS Y. An essay on the cohesion of fluids[J]. Proc R Soc, 1805, 95: 65-87. |
| 25 | LIU M, WANG S, JIANG L. Nature-inspired superwettability systems[J]. Nat Rev Mater, 2017, 2(7): 17036. |
| 26 | WENZEL P, ROBERT N. Surface roughness and contact angle[J]. J Phys Chem C, 1949, 53(9): 1466-1467. |
| 27 | CASSIE A B D, BAXTER S. Wettability of porous surfaces[J]. Trans Faraday Soc, 1944, 40: 546-551. |
| 28 | WENZEL P, ROBERT N. Resistance of solid surfaces to wetting by water[J]. Trans Faraday Soc, 1936, 28(8): 988-994. |
| 29 | WANG S, JIANG L. Definition of superhydrophobic states [J]. Adv Mater, 2007, 19(21): 3423-3424. |
| 30 | FENG L, ZHANG Z, MAI Z, et al. A super-hydrophobic and super-oleophilic coating mesh film for the separation of oil and water[J]. Angew Chem Int Ed Engl, 2004, 43(15): 2012-2014. |
| 31 | WANG J, ZHENG Y, NIE F Q, et al. Air bubble bursting effect of lotus leaf[J]. Langmuir, 2009, 25(24): 14129-14134. |
| 32 | DE MALEPRADE H, CLANET C, QUERE D. Spreading of bubbles after contacting the lower side of an aerophilic slide immersed in water[J]. Phys Rev Lett, 2016, 117(9): 094501. |
| 33 | LI Z, HU R, SONG J, et al. Gas-liquid-solid triphase interfacial chemical reactions associated with gas wettability[J]. Adv Mater Interfaces, 2021, 8(6): 2001636. |
| 34 | ZOU X, ZHANG Y. Noble metal-free hydrogen evolution catalysts for water splitting[J]. Chem Soc Rev, 2015, 44(15): 5148-5180. |
| 35 | GUO Y, PARK T, YI J W, et al. Nanoarchitectonics for transition-metal-sulfide-based electrocatalysts for water splitting[J]. Adv Mater, 2019, 31(17): e1807134. |
| 36 | MAN I C, SU H Y, CALLE‐VALLEJO F, et al. Universality in oxygen evolution electrocatalysis on oxide surfaces[J]. ChemCatChem, 2011, 3(7): 1159-1165. |
| 37 | FABBRI E, SCHMIDT T J. Oxygen evolution reaction-the enigma in water electrolysis[J]. ACS Catal, 2018, 8(10): 9765-9774. |
| 38 | HE Y, CUI Y, SHANG W, et al. Insight into the bubble-induced overpotential towards high-rate charging of Zn-air batteries[J]. Chem Eng J, 2022, 448: 137782. |
| 39 | YANG L, LI H, YU Y, et al. Assembled 3D MOF on 2D nanosheets for self-boosting catalytic synthesis of N-doped carbon nanotube encapsulated metallic Co electrocatalysts for overall water splitting[J]. Appl Catal B: Environ, 2020, 271: 118939. |
| 40 | EIGELDINGER J, VOGT H. The bubble coverage of gas-evolving electrodes in a flowing electrolyte[J]. Electrochim Acta, 2000, 45(27): 4449-4456. |
| 41 | ZHANG L, XIONG K, CHEN S, et al. In situ growth of ruthenium oxide-nickel oxide nanorod arrays on nickel foam as a binder-free integrated cathode for hydrogen evolution[J]. J Power Sources, 2015, 274: 114-120. |
| 42 | MANI-LATA C, HUSSAKAN C, PANOMSUWAN G. Fast and facile synthesis of Pt nanoparticles supported on ketjen black by solution plasma sputtering as bifunctional HER/ORR catalysts[J]. J Compos Sci, 2020, 4(3): 121. |
| 43 | DERJAGUIN B V, MULLER V M, TOPOROV Y P. Effect of contact deformations on the adhesion of particles[J]. J Colloid Interface Sci, 1975, 53(2): 314-326. |
| 44 | WANG M, WANG Z, GUO Z. Water electrolysis enhanced by super gravity field for hydrogen production[J]. Int J Hydrogen Energy, 2010, 35(8): 3198-3205. |
| 45 | LI S, WANG C, CHEN C. Water electrolysis in the presence of an ultrasonic field[J]. Electrochim Acta, 2009, 54(15): 3877-3883. |
| 46 | WANG K, LIU X, PEI P, et al. Guiding bubble motion of rechargeable zinc-air battery with electromagnetic force[J]. Chem Eng J, 2018, 352: 182-187. |
| 47 | DARBAND G B, ALIOFKHAZRAEI M, SHANMUGAM S. Recent advances in methods and technologies for enhancing bubble detachment during electrochemical water splitting[J]. Renew Sustainable Energy Rev, 2019, 114: 109300. |
| 48 | SWIEGERS G F, TERRETT R N L, TSEKOURAS G, et al. The prospects of developing a highly energy-efficient water electrolyser by eliminating or mitigating bubble effects[J]. Sustainable Energy Fuels, 2021, 5(5): 1280-1310. |
| 49 | KANG E, LEE D H, KIM C B, et al. A hemispherical microfluidic channel for the trapping and passive dissipation of microbubbles[J]. J Micromech Microeng, 2010, 20(4): 045009. |
| 50 | BAE M, KANG Y, LEE D W, et al. Superaerophobic polyethyleneimine hydrogels for improving electrochemical hydrogen production by promoting bubble detachment[J]. Adv Energy Mater, 2022, 12(29): 2201452. |
| 51 | ZHANG J, DONG F, WANG C, et al. Integrated bundle electrode with wettability-gradient copper cones inducing continuous generation, directional transport, and efficient collection of H2 bubbles[J]. ACS Appl Mater Interfaces, 2021, 13(27): 32435-32441. |
| 52 | KIM M, ANJUM M A R, CHOI M, et al. Covalent 0D-2D heterostructuring of Co9S8-MoS2 for enhanced hydrogen evolution in all pH electrolytes[J]. Adv Funct Mater, 2020, 30(40): 2002536. |
| 53 | ANGULO A, VAN DER LINDE P, GARDENIERS H, et al. Influence of bubbles on the energy conversion efficiency of electrochemical reactors[J]. Joule, 2020, 4(3): 555-579. |
| 54 | ANDAVEH R, BARATI DARBAND G, MALEKI M, et al. Superaerophobic/superhydrophilic surfaces as advanced electrocatalysts for the hydrogen evolution reaction: a comprehensive review[J]. J Mater Chem A, 2022, 10(10): 5147-5173. |
| 55 | LIU W, HU E, JIANG H, et al. A highly active and stable hydrogen evolution catalyst based on pyrite-structured cobalt phosphosulfide[J]. Nat Commun, 2016, 7: 10771. |
| 56 | LIU R, GU S, DU H, et al. Controlled synthesis of FeP nanorod arrays as highly efficient hydrogen evolution cathode[J]. J Mater Chem A, 2014, 2(41): 17263-17267. |
| 57 | XIE J, QU H, XIN J, et al. Defect-rich MoS2 nanowall catalyst for efficient hydrogen evolution reaction[J]. Nano Res, 2017, 10(4): 1178-1188. |
| 58 | LIU Y, ZHOU X, DING T, et al. 3D architecture constructed via the confined growth of MoS2 nanosheets in nanoporous carbon derived from metal-organic frameworks for efficient hydrogen production[J]. Nanoscale, 2015, 7(43): 18004-18009. |
| 59 | XU W, LU Z, SUN X, et al. Superwetting electrodes for gas-involving electrocatalysis[J]. Acc Chem Res, 2018, 51(7): 1590-1598. |
| 60 | JIA Y, ZHANG L, GAO G, et al. A heterostructure coupling of exfoliated Ni-Fe hydroxide nanosheet and defective graphene as a bifunctional electrocatalyst for overall water splitting[J]. Adv Mater, 2017, 29(17): 201700017. |
| 61 | TANG T, JIANG W J, NIU S, et al. Electronic and morphological dual modulation of cobalt carbonate hydroxides by Mn doping toward highly efficient and stable bifunctional electrocatalysts for overall water splitting[J]. J Am Chem Soc, 2017, 139(24): 8320-8328. |
| 62 | LI Y, YIN K, WANG L, et al. Engineering MoS2 nanomesh with holes and lattice defects for highly active hydrogen evolution reaction[J]. Appl Catal B: Environ, 2018, 239: 537-544. |
| 63 | LING W, LU G, NG T. Increased stability and size of a bubble on a superhydrophobic surface[J]. Langmuir, 2011, 27(7): 3233-3237. |
| 64 | ZHANG P, WANG S, WANG S, et al. Superwetting surfaces under different media: effects of surface topography on wettability[J]. Small, 2015, 11(16): 1939-1946. |
| 65 | TAN Y, XIE R, ZHAO S, et al. Facile fabrication of robust hydrogen evolution electrodes under high current densities via Pt@Cu interactions[J]. Adv Funct Mater, 2021, 31(45): 2105579. |
| 66 | SONG Q, XUE Z, LIU C, et al. General strategy to optimize gas evolution reaction via assembled striped-pattern superlattices[J]. J Am Chem Soc, 2020, 142(4): 1857-1863. |
| 67 | ZHANG J, LIANG J, MEI B, et al. Synthesis of Ni/NiO@MoO3- x composite nanoarrays for high current density hydrogen evolution reaction[J]. Adv Energy Mater, 2022, 12(22): 2200001. |
| 68 | MA T Y, DAI S, JARONIEC M, et al. Metal-organic framework derived hybrid Co3O4-carbon porous nanowire arrays as reversible oxygen evolution electrodes[J]. J Am Chem Soc, 2014, 136(39): 13925-13931. |
| 69 | LIU H, LI Z, HU J, et al. Self-supported cobalt oxide electrocatalysts with hierarchical chestnut burr-like nanostructure for efficient overall water splitting[J]. Chem Eng J, 2022, 435: 134995. |
| 70 | REN J, WU X, LIU T, et al. Interfacing nickel with molybdenum oxides as monolithic catalyst to accelerate alkaline hydrogen electrocatalysis with robust stability[J]. Appl Catal B: Environ, 2022, 317: 121786. |
| 71 | KOU T, WANG S, SHI R, et al. Periodic porous 3D electrodes mitigate gas bubble traffic during alkaline water electrolysis at high current densities[J]. Adv Energy Mater, 2020, 10(46): 2002955. |
| 72 | GAO X, ZHANG H, LI Q, et al. Hierarchical NiCo2O4 hollow microcuboids as bifunctional electrocatalysts for overall water-splitting[J]. Angew Chem Int Ed Engl, 2016, 55(21): 6290-6294. |
| 73 | GUAN C, LIU X, REN W, et al. Rational design of metal-organic framework derived hollow NiCo2O4 arrays for flexible supercapacitor and electrocatalysis[J]. Adv Energy Mater, 2017, 7(12): 1602391. |
| 74 | XIAO C, LI Y, LU X, et al. Bifunctional porous NiFe/NiCo2O4/Ni foam electrodes with triple hierarchy and double synergies for efficient whole cell water splitting[J]. Adv Funct Mater, 2016, 26(20): 3515-3523. |
| 75 | SHEN J, LI B, ZHENG Y, et al. Engineering the composition and structure of superaerophobic nanosheet array for efficient hydrogen evolution[J]. Chem Eng J, 2022, 433: 133517. |
| 76 | XI W, YAN G, TAN H, et al. Superaerophobic P-doped Ni(OH)2/NiMoO4 hierarchical nanosheet arrays grown on Ni foam for electrocatalytic overall water splitting[J]. Dalton Trans, 2018, 47(26): 8787-8793. |
| 77 | WU S, LIU S, TAN X, et al. Ni3S2-embedded NiFe LDH porous nanosheets with abundant heterointerfaces for high-current water electrolysis[J]. Chem Eng J, 2022, 442: 136105. |
| 78 | LEI H, WAN Q, TAN S, et al. Pt quantum dots modified S-NiFe layered double hydroxide for high-current-density alkaline water splitting at industrial temperature[J]. Adv Mater, 2023: e2208209. |
| 79 | YU M, WANG Z, LIU J, et al. A hierarchically porous and hydrophilic 3D nickel-iron/MXene electrode for accelerating oxygen and hydrogen evolution at high current densities[J]. Nano Energy, 2019, 63: 103880. |
| 80 | YE Y, ZHANG N, LIU X. Amorphous NiFe(oxy)hydroxide nanosheet integrated partially exfoliated graphite foil for high efficiency oxygen evolution reaction[J]. J Mater Chem A, 2017, 5(46): 24208-24216. |
| 81 | LUO Y, TANG L, KHAN U, et al. Morphology and surface chemistry engineering toward pH-universal catalysts for hydrogen evolution at high current density[J]. Nat Commun, 2019, 10(1): 269. |
| 82 | LIU H, XIE R, LUO Y, et al. Dual interfacial engineering of a chevrel phase electrode material for stable hydrogen evolution at 2500 mA·cm-2[J]. Nat Commun, 2022, 13(1): 6382. |
| 83 | MU H, LIN G, ZHANG Y, et al. Rational engineering of superaerophobic CoMoSx electrocatalysts for overall water splitting[J]. Colloids Surf A Physicochem Eng Asp, 2021, 623: 126734. |
| 84 | SHAN X, LIU J, MU H, et al. An engineered superhydrophilic/superaerophobic electrocatalyst composed of the supported CoMoSx chalcogel for overall water splitting[J]. Angew Chem Int Ed Engl, 2020, 59(4): 1659-1665. |
| 85 | WAN L, XU Z, WANG P, et al. Dual regulation both intrinsic activity and mass transport for self-supported electrodes using in anion exchange membrane water electrolysis[J]. Chem Eng J, 2022, 431: 133942. |
| 86 | LI H, CHEN S, ZHANG Y, et al. Systematic design of superaerophobic nanotube-array electrode comprised of transition-metal sulfides for overall water splitting[J]. Nat Commun, 2018, 9(1): 2452. |
| 87 | LONG X, LI G, WANG Z, et al. Metallic iron-nickel sulfide ultrathin nanosheets as a highly active electrocatalyst for hydrogen evolution reaction in acidic media[J]. J Am Chem Soc, 2015, 137(37): 11900-11903. |
| 88 | YANG Y, YAO H, YU Z, et al. Hierarchical nanoassembly of MoS2/Co9S8/Ni3S2/Ni as a highly efficient electrocatalyst for overall water splitting in a wide pH range[J]. J Am Chem Soc, 2019, 141(26): 10417-10430. |
| 89 | YU Q, ZHANG Z, QIU S, et al. A Ta-TaS2 monolith catalyst with robust and metallic interface for superior hydrogen evolution[J]. Nat Commun, 2021, 12(1): 6051. |
| 90 | ZHANG C, LUO Y, TAN J, et al. High-throughput production of cheap mineral-based two-dimensional electrocatalysts for high-current-density hydrogen evolution[J]. Nat Commun, 2020, 11(1): 3724. |
| 91 | ZOU X, WU Y, LIU Y, et al. In situ generation of bifunctional, efficient Fe-based catalysts from mackinawite iron sulfide for water splitting[J]. Chem, 2018, 4(5): 1139-1152. |
| 92 | HAO J, YANG W, HUANG Z, et al. Superhydrophilic and superaerophobic copper phosphide microsheets for efficient electrocatalytic hydrogen and oxygen evolution[J]. Adv Mater Interfaces, 2016, 3(16): 1600236. |
| 93 | YU X, YU Z, ZHANG X, et al. “Superaerophobic” nickel phosphide nanoarray catalyst for efficient hydrogen evolution at ultrahigh current densities[J]. J Am Chem Soc, 2019, 141(18): 7537-7543. |
| 94 | YIN Y, TAN Y, WEI Q, et al. Nanovilli electrode boosts hydrogen evolution: a surface with superaerophobicity and superhydrophilicity[J]. Nano Res, 2020, 14(4): 961-968. |
| 95 | CHEN X, SHENG L, LI S, et al. Facile syntheses and in-situ study on electrocatalytic properties of superaerophobic CoxP-nanoarray in hydrogen evolution reaction[J]. Chem Eng J, 2021, 426: 131029. |
| 96 | LI Y, ZHANG H, JIANG M, et al. 3D self-supported Fe-doped Ni2P nanosheet arrays as bifunctional catalysts for overall water splitting [J]. Adv Funct Mater, 2017, 27(37): 1702513. |
| 97 | YU X, WANG M, GONG X, et al. Self-supporting porous CoP-based films with phase-separation structure for ultrastable overall water electrolysis at large current density[J]. Adv Energy Mater, 2018, 8(34): 1802445. |
| 98 | ZHANG X, LI J, YANG Y, et al. Co3O4/Fe0.33Co0.66P interface nanowire for enhancing water oxidation catalysis at high current density[J]. Adv Mater, 2018, 30(45): e1803551. |
| 99 | SHANG L, ZHAO Y, KONG X Y, et al. Underwater superaerophobic Ni nanoparticle-decorated nickel-molybdenum nitride nanowire arrays for hydrogen evolution in neutral media[J]. Nano Energy, 2020, 78: 105375. |
| 100 | SHI Y, ZHANG B. Recent advances in transition metal phosphide nanomaterials: synthesis and applications in hydrogen evolution reaction[J]. Chem Soc Rev, 2016, 45(6): 1529-1541. |
| 101 | NøRSKOV J K, BLIGAARD T, LOGADOTTIR A, et al. Trends in the exchange current for hydrogen evolution[J]. J Electrochem Soc, 2005, 152(3): J23-J26. |
| 102 | MAHMOOD J, LI F, JUNG S M, et al. An efficient and pH-universal ruthenium-based catalyst for the hydrogen evolution reaction[J]. Nat Nanotechnol, 2017, 12(5): 441-446. |
| 103 | YAO H, WANG X, LI K, et al. Strong electronic coupling between ruthenium single atoms and ultrafine nanoclusters enables economical and effective hydrogen production[J]. Appl Catal B: Environ, 2022, 312: 121378. |
| 104 | ZHAO Q, YAN Z, CHEN C, et al. Spinels: controlled preparation, oxygen reduction/evolution reaction application, and beyond[J]. Chem Rev, 2017, 117(15): 10121-10211. |
| 105 | BAO J, ZHANG X, FAN B, et al. Ultrathin spinel-structured nanosheets rich in oxygen deficiencies for enhanced electrocatalytic water oxidation[J]. Angew Chem Int Ed Engl, 2015, 54(25): 7399-7404. |
| 106 | JIN Y, WANG H, LI J, et al. Porous MoO2 nanosheets as non-noble bifunctional electrocatalysts for overall water splitting[J]. Adv Mater, 2016, 28(19): 3785-3790. |
| 107 | LI C, HAN X, CHENG F, et al. Phase and composition controllable synthesis of cobalt manganese spinel nanoparticles towards efficient oxygen electrocatalysis[J]. Nat Commun, 2015, 6: 7345. |
| 108 | PAN Y, REN H, DU H, et al. Active site engineering by surface sulfurization for a highly efficient oxygen evolution reaction: a case study of Co3O4 electrocatalysts[J]. J Mater Chem A, 2018, 6(45): 22497-22502. |
| 109 | HE Y, CUI Y, ZHAO Z, et al. Strategies for bubble removal in electrochemical systems[J]. Energy Rev, 2023, 2(1): 100015. |
| 110 | INAMDAR A I, CHAVAN H S, SEOK J H, et al. Optimal rule-of-thumb design of NiFeMo layered double hydroxide nanoflakes for highly efficient and durable overall water-splitting at large currents[J]. J Mater Chem A, 2022, 10(38): 20497-20508. |
| 111 | NIU S, JIANG W, WEI Z, et al. Se-doping activates FeOOH for cost-effective and efficient electrochemical water oxidation[J]. J Am Chem Soc, 2019, 141(17): 7005-7013. |
| 112 | ZHOU J, YU L, ZHU Q, et al. Defective and ultrathin NiFe LDH nanosheets decorated on V-doped Ni3S2 nanorod arrays: a 3D core-shell electrocatalyst for efficient water oxidation[J]. J Mater Chem A, 2019, 7(30): 18118-18125. |
| 113 | ZHANG L, KAN X, HUANG T, et al. Electric field modulated water permeation through laminar Ti3C2Tx MXene membrane[J]. Water Res, 2022, 219: 118598. |
| 114 | CAI P, HUANG J, CHEN J, et al. Oxygen-containing amorphous cobalt sulfide porous nanocubes as high-activity electrocatalysts for the oxygen evolution reaction in an alkaline/neutral medium[J]. Angew Chem Int Ed Engl, 2017, 56(17): 4858-4861. |
| 115 | CHENG Y, GUO H, ZHANG L, et al. Mo‐mediated transition of the lattice to long‐range disorder enables ultra-high current density hydrogen production at low potentials[J]. Adv Funct Mater, 2023, 33(12): 2208718. |
| 116 | FENG L L, YU G, WU Y, et al. High-index faceted Ni3S2 nanosheet arrays as highly active and ultrastable electrocatalysts for water splitting[J]. J Am Chem Soc, 2015, 137(44): 14023-14026. |
| 117 | LI H, CHEN S, JIA X, et al. Amorphous nickel-cobalt complexes hybridized with 1T-phase molybdenum disulfide via hydrazine-induced phase transformation for water splitting[J]. Nat Commun, 2017, 8: 15377. |
| 118 | LYU F, ZENG S, JIA Z, et al. Two-dimensional mineral hydrogel-derived single atoms-anchored heterostructures for ultrastable hydrogen evolution[J]. Nat Commun, 2022, 13(1): 6249. |
| 119 | SINGH S, NGUYEN D C, KIM N H, et al. Interface engineering induced electrocatalytic behavior in core-shelled CNTs@NiP2/NbP heterostructure for highly efficient overall water splitting[J]. Chem Eng J, 2022, 442: 136120. |
| 120 | CHENG X, PAN Z, LEI C, et al. A strongly coupled 3D ternary Fe2O3@Ni2P/Ni(PO3)2 hybrid for enhanced electrocatalytic oxygen evolution at ultra-high current densities[J]. J Mater Chem A, 2019, 7(3): 965-971. |
| 121 | KUANG Y, FENG G, LI P, et al. Single-crystalline ultrathin nickel nanosheets array from in situ topotactic reduction for active and stable electrocatalysis[J]. Angew Chem Int Ed Engl, 2016, 55(2): 693-697. |
| 122 | JIANG M, WANG H, LI Y, et al. Superaerophobic RuO2-based nanostructured electrode for high-performance chlorine evolution reaction[J]. Small, 2017, 13(4): 1602240. |
| 123 | LU Z, XU W, MA J, et al. Superaerophilic carbon-nanotube-array electrode for high-performance oxygen reduction reaction[J]. Adv Mater, 2016, 28(33): 7155-7161. |
| 124 | LONG C, WANG K, SHI Y, et al. Tuning the electronic structure of PtRu bimetallic nanoparticles for promoting the hydrogen oxidation reaction in alkaline media[J]. Inorg Chem Front, 2019, 6(10): 2900-2905. |
| 125 | LIN G, ZHANG Y, HUA Y, et al. Bioinspired metalation of the metal-organic framework MIL-125-NH2 for photocatalytic NADH regeneration and gas-liquid-solid three-phase enzymatic CO2 reduction[J]. Angew Chem Int Ed Engl, 2022, 61(31): e202206283. |
| [1] | 李慧慧, 姚开胜, 赵亚南, 范李娜, 田钰琳, 卢伟伟. 离子液体调控合成Pt-Pd双金属纳米材料及其催化氨硼烷水解释氢[J]. 应用化学, 2023, 40(4): 597-609. |
| [2] | 林楠煜, 高峰, 曲江英, 涂晶晶, 钟伟军, 臧云浩. 超亲水/水下疏油高硅布的制备及其油水分离性能[J]. 应用化学, 2023, 40(3): 449-459. |
| [3] | 柳小虎, 赖小娟, 曹红燕, 王婷婷, 党志强. 起泡剂/稳泡剂/SiO2复合泡沫缓速酸液体系协同增效性能[J]. 应用化学, 2023, 40(1): 91-99. |
| [4] | 赵跃华, 王大鹏. 氨基化氧化石墨烯和脂肪酸在水-油界面的共吸附动力学[J]. 应用化学, 2022, 39(8): 1274-1284. |
| [5] | 孟玉, 张晴, 彭文浩, 朱晓飞, 周德凤. Pr0.8Sr0.2Fe0.7Ni0.3O3-δ -Pr1.2Sr0.8Ni0.6Fe0.4O4+δ 复合阴极的制备及其电化学性能[J]. 应用化学, 2022, 39(5): 797-808. |
| [6] | 黎振华, 诸颖, 陈静, 宋世平. 抗污界面构建及其电化学生物传感应用进展[J]. 应用化学, 2022, 39(5): 736-748. |
| [7] | 陶荟冰, 田震, 谢勇, 孙瑜, 汪莉, 康卓, 张跃. 电解水催化材料动态构效关系的原位拉曼研究进展[J]. 应用化学, 2022, 39(4): 528-539. |
| [8] | 杜慧, 姚晨阳, 彭皓, 姜波, 李顺祥, 姚俊烈, 郑方, 杨方, 吴爱国. 过渡金属掺杂磁性纳米粒子在生物医学领域中的研究进展[J]. 应用化学, 2022, 39(3): 391-406. |
| [9] | 范岳, 田雪林. 类液体动态界面材料:近期应用进展[J]. 应用化学, 2022, 39(1): 131-141. |
| [10] | 刘旭, 李杨可欣, 杜黎, 于健, 王佳程, 耿阳, 韩广, 孙宽, 李猛. 水凝胶的制备及仿生设计在能源领域应用的研究进展[J]. 应用化学, 2022, 39(1): 35-54. |
| [11] | 张云雷, 赵蔚祎, 麻拴红, 周峰. 仿生干湿摩擦黏附器件的研究进展[J]. 应用化学, 2022, 39(1): 86-98. |
| [12] | 赵春梅, 周秀苗, 金茜茜, 王雨杭, 党祎静. 基于马鞍形环八四噻吩的复合纳米材料的制备及发光性能[J]. 应用化学, 2022, 39(02): 283-288. |
| [13] | 黄晓桐, 陈颖欣, 朱泽滨, 周丽华. 基于纳米材料光谱分析法检测抗坏血酸的研究进展[J]. 应用化学, 2021, 38(6): 637-650. |
| [14] | 刘娇, 邹鹏飞, 李平, 张潇, 王欣欣, 高媛媛, 李莉莉. 多肽类自组装纳米材料对抗细菌耐药的研究进展[J]. 应用化学, 2021, 38(5): 546-558. |
| [15] | 刘林昌, 郭亚君, 朱红林, 马静静, 李忠义, 水淼, 郑岳青. 负载型超细纳米材料催化氨硼烷水解制氢的研究进展[J]. 应用化学, 2021, 38(11): 1405-1422. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||