应用化学 ›› 2022, Vol. 39 ›› Issue (5): 809-818.DOI: 10.19894/j.issn.1000-0518.210150
MgO和Fe2O3的添加对GDCSi体系微观结构及电化学性能的影响
- 长春工业大学化学与生命科学学院,长春 130012
-
收稿日期:2021-03-29接受日期:2021-07-29出版日期:2022-05-01发布日期:2022-05-24 -
通讯作者:朱晓飞,周德凤 -
基金资助:国家自然科学基金(21471022);吉林省科学研究基金(20190201230JC);吉林省教育厅“十三五”科技攻关计划(JJKKH20200647KJ)
Effect of MgO and Fe2O3 Incorporation on the Microstructure and Electrochemical Performance of GDCSi System
Jing ZHOU, Yu-Xuan CHEN, Jun-Ming MA, Xiao-Fei ZHU( ), De-Feng ZHOU(
), De-Feng ZHOU( )
)
- School of Chemistry and Life Science,Changchun University of Technology,Changchun 130012,China
-
Received:2021-03-29Accepted:2021-07-29Published:2022-05-01Online:2022-05-24 -
Contact:Xiao-Fei ZHU,De-Feng ZHOU -
About author:defengzhou65@126.com
zhuxiaofei@ccut.edu.cn
-
Supported by:the National Natural Science Foundation of China(21471022);the Jilin Provincial Science Research Foundation of China(20190201230JC);the 13th Five?Year Plan for Science & Technology Research Sponsored by Department of Education of Jilin Province(JJKH20200647KJ)
摘要:
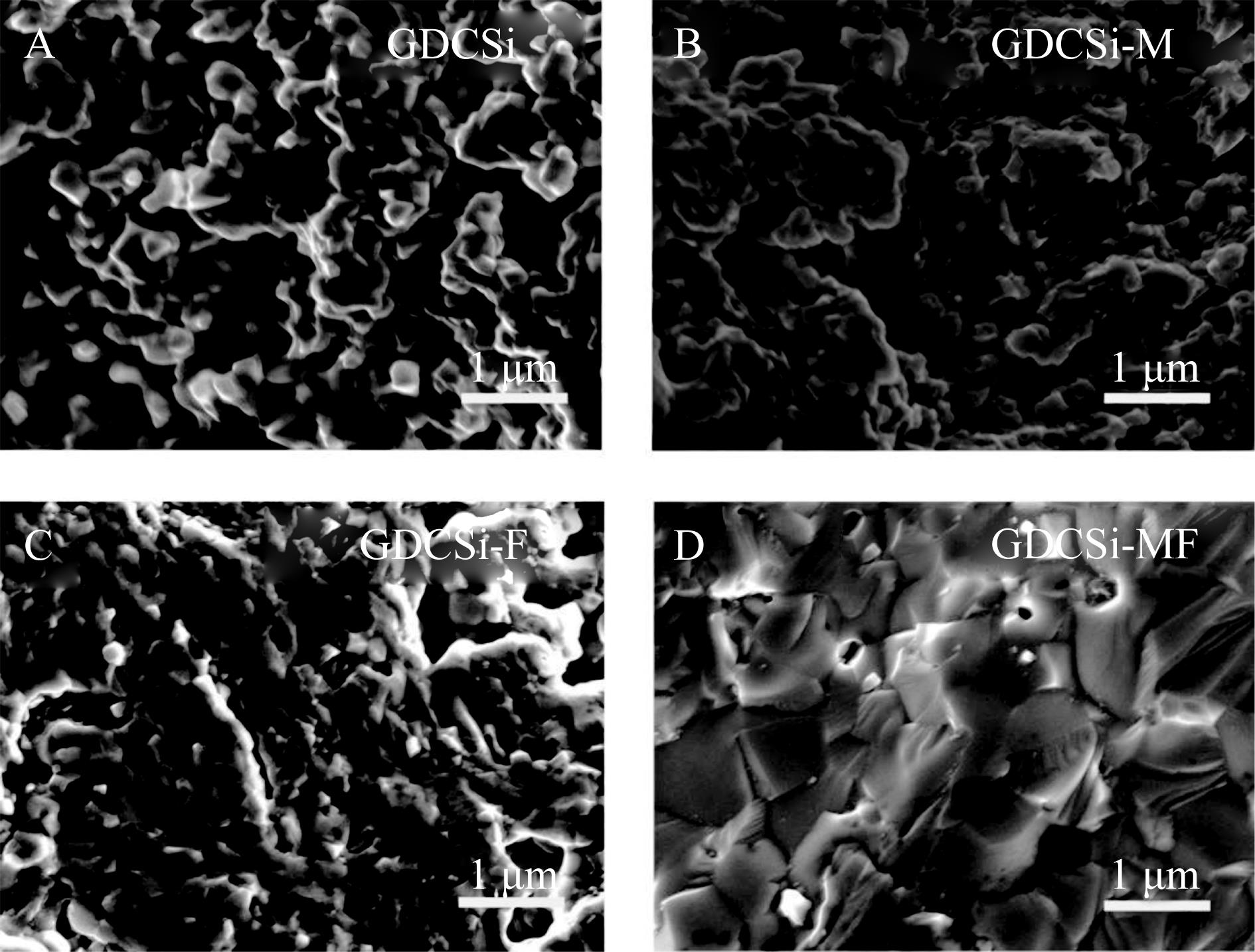
采用溶胶凝胶法制备Gd0.2Ce0.8O3-δ +0.05%(质量分数)SiO2(GDCSi)电解质。在GDCSi体系中加入Fe2O3及MgO可达到降低烧结温度的同时提高晶界电导率,并减小杂质SiO2对氧离子在晶界处传输的阻碍的目的。将MgO和Fe2O3单掺杂或双掺杂在GDCSi体系中并对GDCSi基电解质的微观形貌及电性能进行研究。结果表明,所有样品主要由立方萤石结构相组成;物质的量分数4%MgO单掺杂的GDCSi-M、物质的量分数4%Fe2O3单掺杂的GDCSi-F以及物质的量分数2%MgO-物质的量分数2%Fe2O3共掺杂的GDCSi-MF均可促进GDCSi体系晶粒增长,降低晶粒间孔隙率,提高电解质的相对密度,降低晶粒电阻Rgi、晶界电阻Rgb及总电阻Rt;GDCSi-MF具有最高晶界电导率和总电导率,在400 ℃时GDCSi-MF的晶界电导率σgb和总电导率σt分别是GDCSi的10.41和1.82倍。
中图分类号:
引用本文
周晶, 陈俞萱, 马焌铭, 朱晓飞, 周德凤. MgO和Fe2O3的添加对GDCSi体系微观结构及电化学性能的影响[J]. 应用化学, 2022, 39(5): 809-818.
Jing ZHOU, Yu-Xuan CHEN, Jun-Ming MA, Xiao-Fei ZHU, De-Feng ZHOU. Effect of MgO and Fe2O3 Incorporation on the Microstructure and Electrochemical Performance of GDCSi System[J]. Chinese Journal of Applied Chemistry, 2022, 39(5): 809-818.
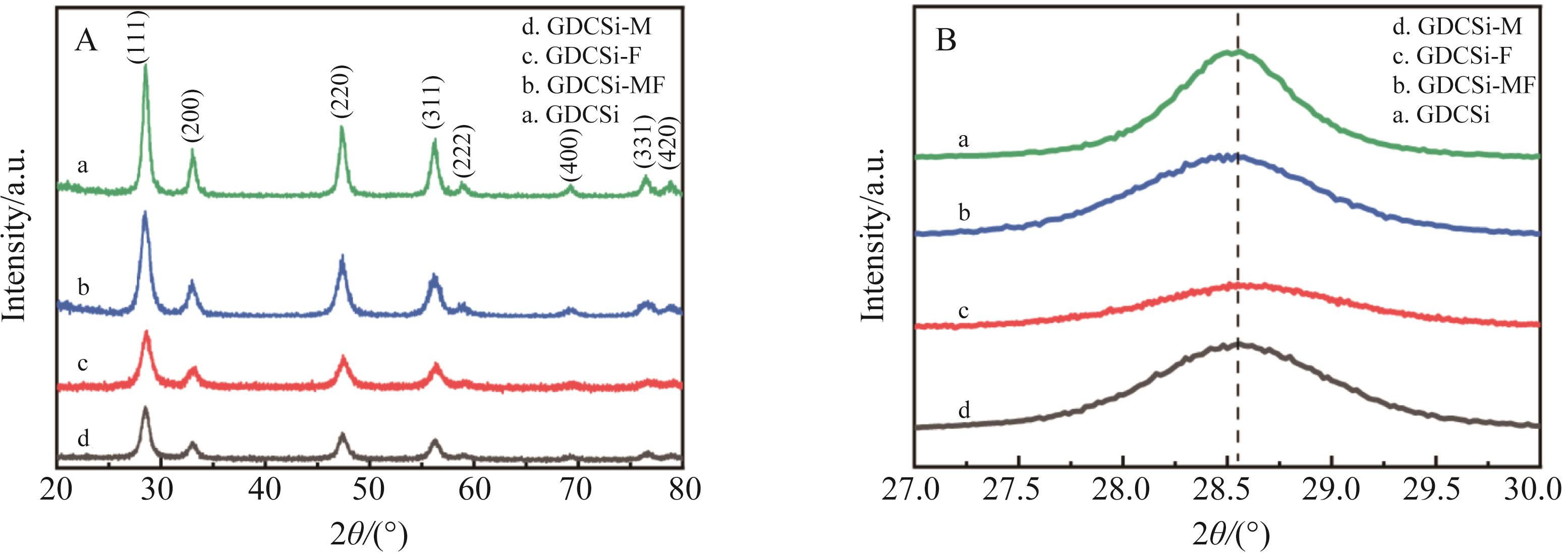
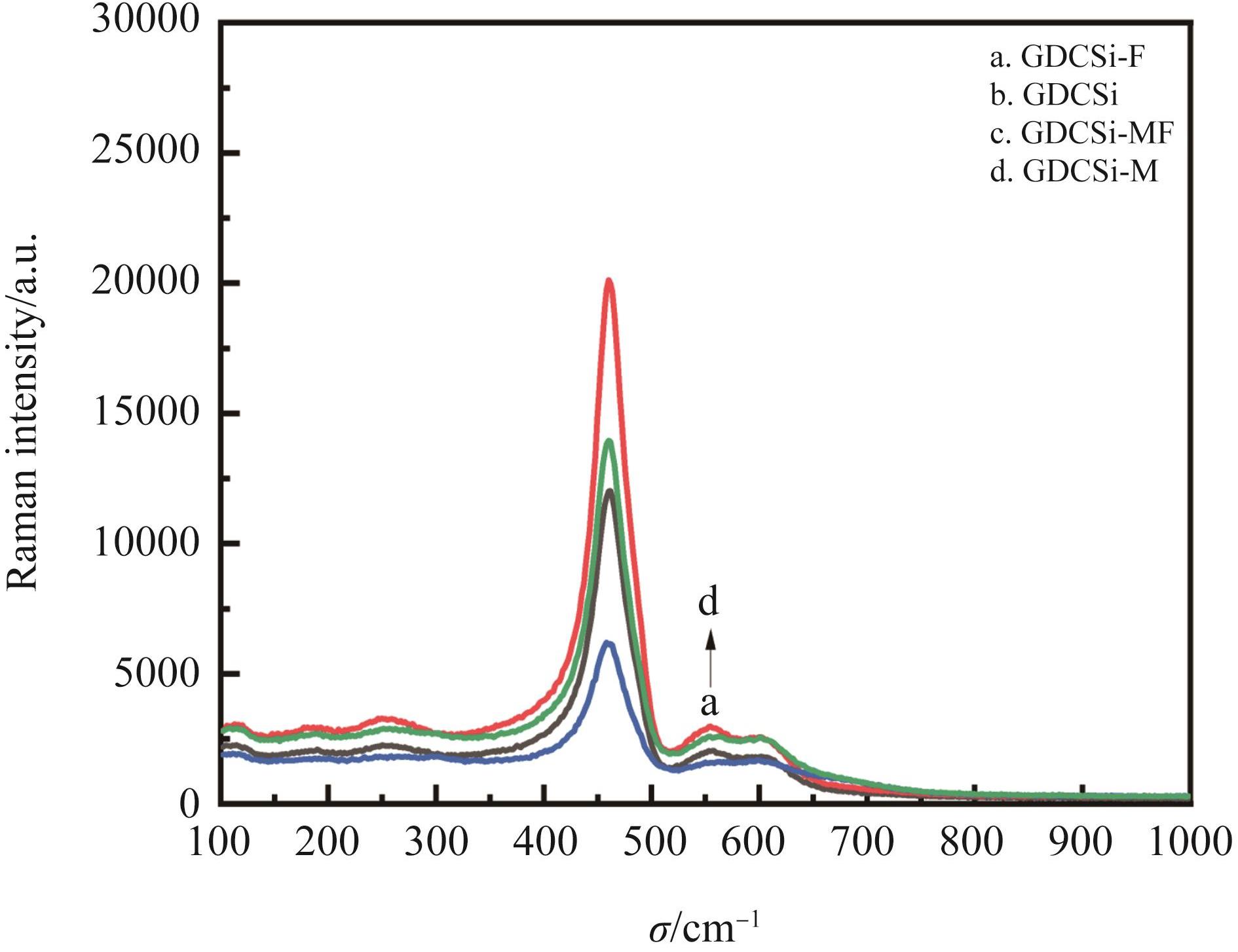
图1 (A) GDCSi基电解质X射线衍射(XRD)图;(B) 2θ范围为27.0~30.0(°)的XRD图
Fig.1 (A) XRD spectra of synthesized GDCSi-based samples; (B) XRD spectra in the 2θ range from 27.0(°) to 30.0(°)
样品 Samples | 晶胞参数 a/nm | 晶胞体积 V/nm3 | 理论密度 ρT/ (g·cm-3) | 实际密度 ρa/(g·cm-3) | 相对密度 ρRel/% | 微晶尺寸 d/nm |
|---|---|---|---|---|---|---|
| GDCSi | 0.541 30 | 15.860 | 7.247 | 6.203 | 85.6 | 14.85 |
| GDCSi?M | 0.541 31 | 15.861 | 7.238 | 6.608 | 91.3 | 15.40 |
| GDCSi?F | 0.541 04 | 15.837 | 7.249 | 6.879 | 94.9 | 18.51 |
| GDCSi?MF | 0.541 29 | 15.862 | 7.251 | 6.939 | 95.7 | 25.20 |
表1 1200 ℃烧结10 h的GDCSi基电解质的相关参数
Table 1 Related parameters of GDCSi?based electrolyte sintered at 1200 ℃ for 10 h
样品 Samples | 晶胞参数 a/nm | 晶胞体积 V/nm3 | 理论密度 ρT/ (g·cm-3) | 实际密度 ρa/(g·cm-3) | 相对密度 ρRel/% | 微晶尺寸 d/nm |
|---|---|---|---|---|---|---|
| GDCSi | 0.541 30 | 15.860 | 7.247 | 6.203 | 85.6 | 14.85 |
| GDCSi?M | 0.541 31 | 15.861 | 7.238 | 6.608 | 91.3 | 15.40 |
| GDCSi?F | 0.541 04 | 15.837 | 7.249 | 6.879 | 94.9 | 18.51 |
| GDCSi?MF | 0.541 29 | 15.862 | 7.251 | 6.939 | 95.7 | 25.20 |
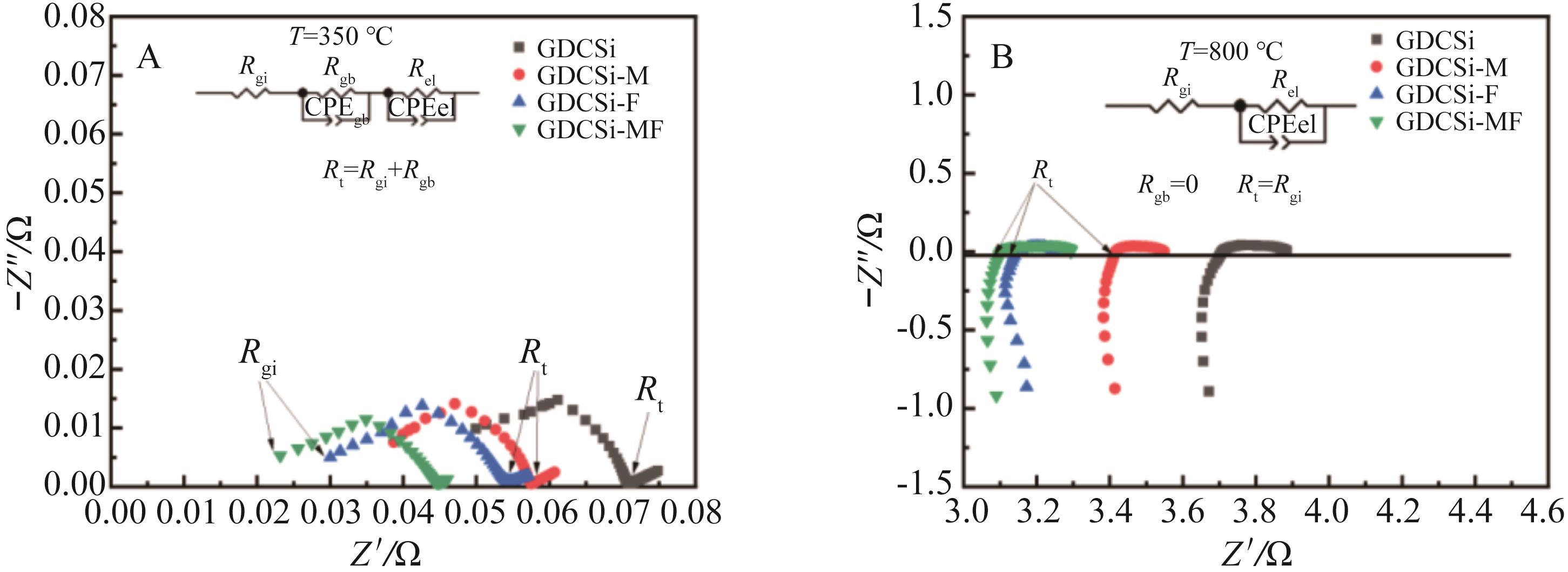
图4 在(A) 350 ℃和(B) 800 ℃空气中测量的GDCSi基电解质的电化学阻抗,插图显示了EIS分析的等效电路图
Fig.4 Electrochemical impedances of GDCSi-based electrolyte measured at (A) 350 ℃, (B) 800 ℃ in air. The illustration shows equivalent circuit diagrams for EIS analysis
样品 Samples | 350 ℃ | 800 ℃ | |||
|---|---|---|---|---|---|
晶粒电阻 Rgi/Ω | 晶界电阻 Rgb/Ω | 总电阻 Rt/Ω | 晶界电阻/总电阻 Rgb/Rt | 总电阻=晶粒电阻 Rt =Rgi/Ω | |
| GDCSi | 1218.9 | 1269.6 | 2488.5 | 0.51 | 3.71 |
| GDCSi?M | 470.61 | 138.05 | 575.22 | 0.24 | 3.42 |
| GDCSi?F | 425.78 | 119.48 | 545.26 | 0.22 | 3.14 |
| GDCSi?MF | 348.89 | 98.35 | 447.24 | 0.21 | 3.10 |
表2 GDCSi基电解质在350和800 ℃下的电阻
Table 2 The resistance values of GDCSi?based electrolytes measured at 350 and 800 ℃
样品 Samples | 350 ℃ | 800 ℃ | |||
|---|---|---|---|---|---|
晶粒电阻 Rgi/Ω | 晶界电阻 Rgb/Ω | 总电阻 Rt/Ω | 晶界电阻/总电阻 Rgb/Rt | 总电阻=晶粒电阻 Rt =Rgi/Ω | |
| GDCSi | 1218.9 | 1269.6 | 2488.5 | 0.51 | 3.71 |
| GDCSi?M | 470.61 | 138.05 | 575.22 | 0.24 | 3.42 |
| GDCSi?F | 425.78 | 119.48 | 545.26 | 0.22 | 3.14 |
| GDCSi?MF | 348.89 | 98.35 | 447.24 | 0.21 | 3.10 |
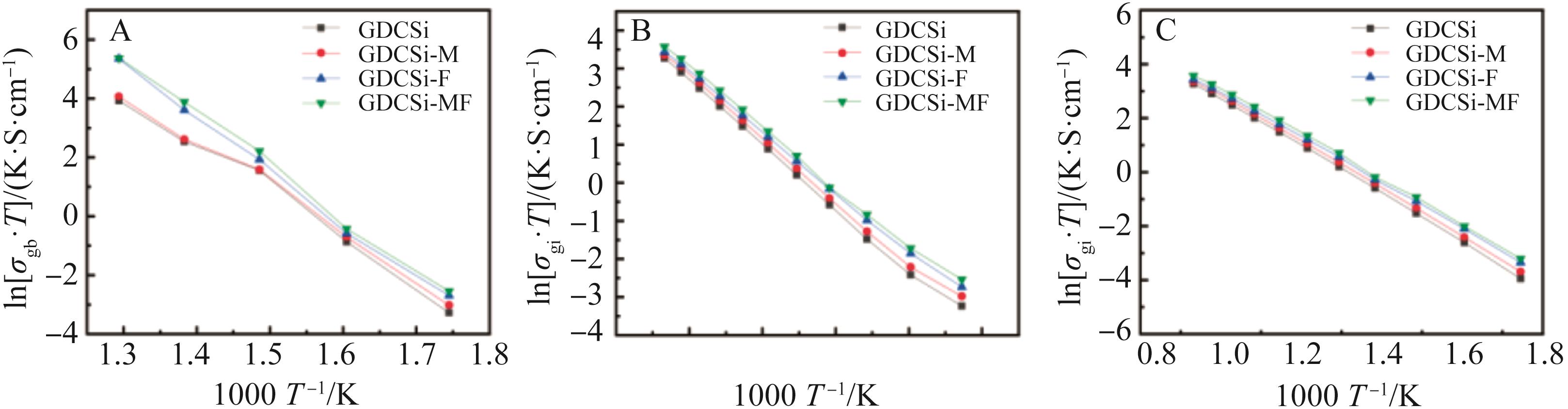
图5 GDCSi基复合电解质的Arrhenius图: (A) 晶界电导率、(B) 晶粒电导率和 (C) 总电导率
Fig.5 Arrhenius curves of (A) the grain boundary conductivity, (B) grain conductivity and (C) total conductivity of GDCSi-based electrolytes
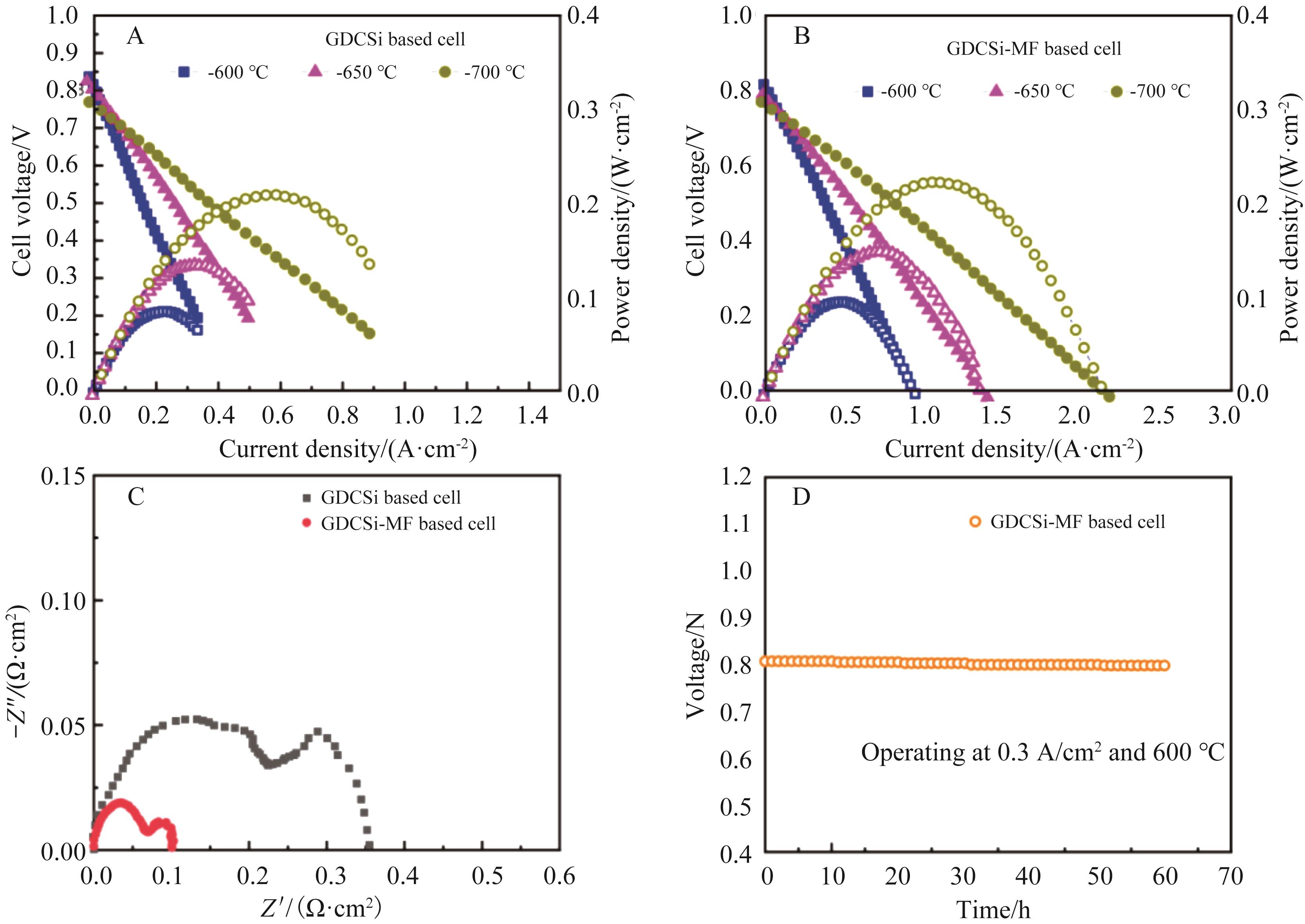
图6 (A)GDCSi为电解质的单电池(Cell-Ⅰ)和(B)GDCSi-MF为电解质的单电池(Cell-Ⅱ)在600~700 ℃的峰值功率密度(C)在开路电压(OCV)下于700 ℃下测得的单电池(Cell-Ⅰ,Cell-Ⅱ)的阻抗谱。(D) Cell-Ⅱ在700 ℃,恒流0.3 A/cm2环境下进行60 h的长期稳定性测试
Fig.6 Peak power density (PPD) of (A) NiO-GDCSi/ GDCSi/LSCF (Cell-Ⅰ) and (B) NiO-GDCSi-MF/GDCSi-MF/LSCF (Cell-Ⅱ) at 600~700 °C. (C) Impedance spectra of single cells (Cell-Ⅰ, Cell-Ⅱ) measured at 700 °C under open circuit voltage (OCV). (D) Long-term stability test of Cell-Ⅱ is conducted for 60 h at 600 °C and at a constant current of 0.3 A/cm2
| 1 | 刘世伟, 梁亮, 李晨阳, 等. 高温质子交换膜燃料电池的复合催化层电极[J]. 应用化学, 2019, 36(9): 1085-1090. |
| LIU S W, LIANG L, LI C Y, et al. Composite catalytic layer electrode for high temperature proton exchange membrane fuel cell[J]. Chinese J Appl Chem, 2019, 36(9): 1085-1090. | |
| 2 | AUCKETT J E, ODRIOZOLA L L, CLARK S, et al. Exploring the nature of the fergusonite-scheelite phase transition and ionic conductivity enhancement by Mo6+ doping in LaNbO4[J]. J Mater Chem A, 2021, 9: 4091-4102. |
| 3 | HOSSEINPOUR J, CHITSAZ A, EISAVI B, et al. Investigation on performance of an integrated SOFC-Goswami system using wood gasification[J]. Energy, 2018, 148: 614-628. |
| 4 | CHEN X, SUN X, ZHOU J, et al. Effects of CoO and Bi2O3 single/dual sintering aids doping on structure and properties of Ce0.8Nd0.2O1.9[J]. Ceram Int, 2020, 46(14): 22727-22732. |
| 5 | PUENTE-MARTÍNEZ D E, DÍAZ-GUILLÉN J A, MONTEMAYOR S M, et al. High ionic conductivity in CeO2 SOFC solid electrolytes; effect of Dy doping on their electrical properties[J]. Int J Hydrogen Energy, 2019, 45(27): 14062-14070. |
| 6 | RAFIQUE A, AHMAD M A, SHAKIR I, et al. Multioxide phase-based nanocomposite electrolyte (M@SDC where M= Zn2+/Ba2+/La3+/Zr2+/Al3+) materials[J]. Ceram Int, 2019, 46(5): 6882-6888. |
| 7 | TOOR S Y, CROISET E. Reducing sintering temperature while maintaining high conductivity for SOFC electrolyte: copper as sintering aid for Samarium Doped Ceria[J]. Ceram Int, 2019, 46(1): 1148-1157. |
| 8 | 刘建伟, 周德凤, 杨梅, 等. MgO或Fe2O3掺杂Ce0.8Nd0.2O1.9固体电解质的结构和电性能[J]. 物理化学学报, 2012, 28(6): 1380-1386. |
| LIU J W, ZHOU D F, YANG M, et al. Structure and electrical properties of Ce0.8Nd0.2O1.9 solid electrolyte doped with MgO or Fe2O3[J]. Chinese J Phy Chem, 2012, 28(6): 1380-1386. | |
| 9 | STEELE B C H. Material science and engineering: the enabling technology for the commercialisation of fuel cell systems[J]. J Mater Sci, 2001, 5: 1053-1068. |
| 10 | XIN G, SIGLE W, MAIER J. Blocking grain boundaries in yttria-doped and undoped ceria ceramics of high purity[J]. J Am Ceram Soc, 2010, 86(1): 77-87. |
| 11 | TIAN N, QU Y, MEN H, et al. Properties of Ce0.85Sm0.15O2- δ-CuO electrolytes for intermediate-temperature solid oxide fuel cells[J]. Solid State Ionics, 2020, 351: 115331. |
| 12 | TIAN N, YU J, DENG Y F, et al. Electrical properties of Ce0.85Sm0.15O1.925-Fe2O3 electrolytes for IT-SOFCs[J]. J Alloy Compd, 2016, 655: 215-219. |
| 13 | XU D, LIU X M, XU S F, et al. Fabrication and performance of Ce0.85Sm0.15O1.925-Fe2O3 electrolytes in IT-SOFCs[J]. Solid State Ionics, 2011, 192(1): 510-514. |
| 14 | BI H L, LIU X M, ZHU L L, et al. Effect of MgO addition and grain size on the electrical properties of Ce0.9Gd0.1O1.95 electrolyte for IT-SOFCs[J]. Int J Hydrogen Energy, 2017, 42(16): 11735-11744. |
| 15 | CHO Y H, CHO P S, AUCHTERLONIE G, et al. Enhancement of grain-boundary conduction in gadolinia-doped ceria by the scavenging of highly resistive siliceous phase[J]. Acta Mater, 2007, 55: 4807-4815. |
| 16 | CHO P S, LEE S B, CHO Y H, et al. Effect of CaO concentration on enhancement of grain-boundary conduction in gadolinia-doped ceria[J]. J Power Sources, 2008, 183(2): 518-523. |
| 17 | GE L, LI R F, HE S C, et al. Enhanced grain-boundary conduction in polycrystalline Ce0.8Gd0.2O1.9 by zinc oxide doping: scavenging of resistive impurities[J]. J Power Sources, 2012: 230: 161-168. |
| 18 | XU S F, LIU J, LI K H, et al. The role of TiO2 on the microstructure and the electrochemical behavior of Ce0.8Gd0.2O2- δ for solid oxide fuel cell electrolyte[J]. J Alloy Compd, 2019, 780: 711-717. |
| 19 | ZHAO G C, ZHOU D F, ZHU J X, et al. Effect of MoO3 concentration on sintering and electrical properties of SiO2-containing neodymium-doped ceria electrolytes[J]. Solid State Sci, 2011, 13(5): 1072-1075. |
| 20 | CHO P S, CHO Y H, PARK S Y, et al. Grain-boundary conduction in gadolinia-doped ceria: the effect of SrO addition[J]. J Electrochem Soc, 2009, 156(3): B339-B344. |
| 21 | ZHANG C, SUNARSO J, ZHU Z H, et al. Enhanced oxygen permeability and electronic conductivity of Ce0.8Gd0.2O2- δ membrane via the addition of sintering aids[J]. Solid State Ionics, 2017, 310: 121-128. |
| 22 | HAN J X, ZHANG J D, LI F, et al. Low-temperature sintering and microstructure evolution of Bi2O3-doped YSZ[J]. Ceram Int, 2018, 44(1): 1026-1033. |
| 23 | MCBRIDE J R, HASS K C, POINDEXTER B D, et al. Raman and X-ray studies of Ce1- xRExO2- y, where RE=La, Pr, Nd, Eu, Gd, and Tb[J]. J Appl Phy, 1994, 76(4):2435-2441. |
| 24 | PIUMETTI M, BENSAID S, ANDANA T, et al. Nanostructured ceria-based materials: effect of the hydrothermal synthesis conditions on the structural properties and catalytic activity[J]. Catalysts, 2017, 7(6): 174. |
| 25 | SPANIER J E, ROBINSON R D, ZHANG F, et al. Size-dependent properties of CeO2- y nanoparticles as studied by Raman scattering[J]. Phys Rev B, 2001, 64(24): 245407. |
| 26 | ZHANG T S, MA J, LENG Y J, et al. Effect of transition metal oxides on densification and electrical properties of Si-containing Ce0.8Gd0.2O2- δ ceramics[J]. Solid State Ionics, 2004, 168: 187-195. |
| 27 | ZHENG Y, ZHOU M, GE L, et al. Effect of Fe2O3 on Sm-doped ceria system solid electrolyte for IT-SOFCs[J]. J Alloy Compd, 2011, 509: 546-550. |
| 28 | ZHANG T S, MA J, CHAN S H, et al. Improvements in sintering behavior and grain-boundary conductivity of ceria-based electrolytes by a small addition of Fe2O3[J]. J Electrochem Soc, 2004, 151(10): J84-J90. |
| 29 | STEELE B C H. Appraisal of Ce1- yGdyO2- y/2 electrolytes for IT-SOFC operation at 500 ℃[J]. Solid State Ionics, 2000, 129(1-4): 95-110. |
| 30 | LEAH R T, BRANDON N P, AGUIAR P. Modelling of cells, stacks and systems based around metal-supported planar IT-SOFC cells with CGO electrolytes operating at 500~600 ℃[J]. J Power Sources, 2005, 145(2): 336-352. |
| 31 | WANG J Q, CHEN X, XIE S K, et al. Bismuth tungstate/neodymium-doped ceria composite electrolyte for intermediate-temperature solid oxide fuel cell: sintering aid and composite effect[J]. J Power Sources, 2019, 428: 105-114. |
| 32 | ZHANG Y, KNIBBE R, SUNARSO J, et al. Recent progress on advanced materials for solid-oxide fuel cells operating below 500 °C[J]. Adv Mater, 2017, 29(48): 1700132. |
| 33 | LIN D, WANG Q, PENG K, et al. Phase formation and properties of composite electrolyte BaCe0.8Y0.2O3- δ-Ce0.8Gd0.2O1.9 for intermediate temperature solid oxide fuel cells[J]. J Power Sources, 2012, 205: 100-107. |
| 34 | ZHAO L, DRENNAN J, KONG C, et al. Insight into surface segregation and chromium deposition on La0.6Sr0.4Co0.2Fe0.8O3- δ cathodes of solid oxide fuel cells[J]. J Mater Chem A, 2014, 2(29): 11114-11123. |
| [1] | 卜芃, 李宏亮. 稀土Yb3+/Tm3+掺杂NaGd(MO4)2荧光粉的制备及其光致发光[J]. 应用化学, 2023, 40(3): 374-379. |
| [2] | 熊波, 黎泰华, 周武平, 刘长宇, 徐晓龙. 一步热聚合法制备Cu2O/CuO-g-C3N4吸附剂及其对甲基橙吸附的性能[J]. 应用化学, 2023, 40(3): 420-429. |
| [3] | 谷欣, 王文庆, 侯钧贺, 高露, 黄明华, 苏革. 无机-无机复合电致变色材料的研究进展[J]. 应用化学, 2022, 39(9): 1345-1359. |
| [4] | 王恩通, 杨林芳. 高比容量锂离子电池正极材料LiNi0.6Co0.2Mn0.2O2的制备及性能[J]. 应用化学, 2022, 39(8): 1209-1215. |
| [5] | 郭峤志, 杨振华, 张月霞, 孟雅婷, 曹宇娟, 孙宣森, 张琪琦, 双少敏, 董川. 基于柠檬酸的石墨烯量子点的制备及其应用[J]. 应用化学, 2022, 39(6): 888-899. |
| [6] | 周友三, 张毅城, 吴思宇, 查飞, 陈德军, 常玥. 铜掺杂钙铝水滑石催化氧化异丙苯制备异丙苯过氧化氢[J]. 应用化学, 2022, 39(6): 941-948. |
| [7] | 齐海燕, 张琛琪, 李金龙, 李军. 氮硫掺杂碳点的制备及检测铜离子[J]. 应用化学, 2022, 39(6): 980-989. |
| [8] | 薛松松, 解正峰, 何佳伟, 张天怡, 夏保平, 李雨芹. 高选择性快速识别汞(Ⅱ)离子的磺酰腙型探针的合成及在吸附中的应用[J]. 应用化学, 2022, 39(5): 760-768. |
| [9] | 张琦, 张乾, 师晓梦, 孔娅淇, 高可心, 杜亚平. 稀土溴化物固态电解质材料在全固态电池中的应用研究进展[J]. 应用化学, 2022, 39(4): 585-598. |
| [10] | 杜慧, 姚晨阳, 彭皓, 姜波, 李顺祥, 姚俊烈, 郑方, 杨方, 吴爱国. 过渡金属掺杂磁性纳米粒子在生物医学领域中的研究进展[J]. 应用化学, 2022, 39(3): 391-406. |
| [11] | 曹桐, 彭军, 冯炎, 刘孝波, 黄宇敏. 侧链磺化聚芳醚质子交换膜的研究进展[J]. 应用化学, 2022, 39(12): 1783-1802. |
| [12] | 孟丹, 郑开元, 陈珊珊, 卓钊龙, 王丽丽. 硅、氮共掺杂碳点荧光粉的制备及发光性能[J]. 应用化学, 2022, 39(11): 1766-1773. |
| [13] | 唐雅薇, 徐兰兰, 刘孝娟. Co、Ni、Fe掺杂有效提升PrBaMn2O5+δ 阳极材料的催化活性[J]. 应用化学, 2022, 39(10): 1543-1553. |
| [14] | 赵莹, 邵奕嘉, 李罗钱, 任建伟, 廖世军. 富锂正极材料的衰减机理及循环稳定性提升的研究进展[J]. 应用化学, 2022, 39(02): 205-222. |
| [15] | 王旭尧, 方应军, 张灵志. 氰基功能化聚乙烯亚胺交联固态聚合物电解质的制备及理化性能[J]. 应用化学, 2021, 38(8): 946-953. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||