应用化学 ›› 2022, Vol. 39 ›› Issue (5): 797-808.DOI: 10.19894/j.issn.1000-0518.210148
Pr0.8Sr0.2Fe0.7Ni0.3O3-δ -Pr1.2Sr0.8Ni0.6Fe0.4O4+δ 复合阴极的制备及其电化学性能
- 长春工业大学化学与生命科学学院,长春 130012
-
收稿日期:2021-03-26接受日期:2021-07-19出版日期:2022-05-01发布日期:2022-05-24 -
通讯作者:朱晓飞,周德凤 -
基金资助:国家自然科学基金(21471022);吉林省科学基金(20190201230JC);吉林省教育厅“十三五科技研究计划”(JJKH20200647KJ)
Preparation and Electrochemical Performance of Pr0.8Sr0.2Fe0.7Ni0.3O3-δ ⁃Pr1.2Sr0.8Ni0.6Fe0.4O4+δ Composite Cathode
Yu MENG, Qing ZHANG, Wen-Hao PENG, Xiao-Fei ZHU( ), De-Feng ZHOU(
), De-Feng ZHOU( )
)
- School of Chemistry and Life Science,Changchun University of Technology,Changchun 130012,China
-
Received:2021-03-26Accepted:2021-07-19Published:2022-05-01Online:2022-05-24 -
Contact:Xiao-Fei ZHU,De-Feng ZHOU -
About author:defengzhou65@126.com
zhuxiaofei@ccut.edu.cn
-
Supported by:the National Natural Science Foundation of China(21471022);Jilin Provincial Science Research Foundation of China(20190201230JC);the 13th Five?Year Plan for Science & Technology Research is sponsored by Department of Education of Jilin Province(JJKH20200647KJ)
摘要:
采用溶胶凝胶法制备单相Pr0.8Sr0.2Fe0.7Ni0.3O3-δ (PSFN113)和Pr1.2Sr0.8Ni0.6Fe0.4O4+δ (PSNF214),并将二者以3∶7的质量比固态混合制备PSFN113-PSNF214异质复合阴极,系统地研究异质界面的存在对样品的结构和性能的影响,并评估PSFN113-PSNF214复合材料作为中温固体氧化物燃料电池(IT-SOFC)阴极的潜力。结果表明,异质复合材料的两相间具有良好的化学稳定性和热稳定性,与Ce0.8Gd0.2O1.9(GDC)电解质具有良好的兼容性和界面粘附性,具有长期运行稳定性。PSNF113加入到PSFN214中形成异质结构,可提高氧空位含量,改善PSFN113的氧离子传输。两相颗粒紧密缠绕以最大化形成异质界面,提高比表面积。800 ℃时,PSFN113-PSNF214的极化电阻值为0.053 Ω·cm2,仅为PSFN113的41%和PSNF214的61%;相应单电池的最大功率密度为496.80 mW/cm2,高达PSFN113的2.5倍和PSNF214的1.6倍。60 h长期稳定性测试的电压衰减率仅为0.07%/h。因此,PSFN113-PSNF214异质复合阴极优异的性能和杰出的稳定性,使其可作为IT-SOFC阴极的候选材料。
中图分类号:
引用本文
孟玉, 张晴, 彭文浩, 朱晓飞, 周德凤. Pr0.8Sr0.2Fe0.7Ni0.3O3-δ -Pr1.2Sr0.8Ni0.6Fe0.4O4+δ 复合阴极的制备及其电化学性能[J]. 应用化学, 2022, 39(5): 797-808.
Yu MENG, Qing ZHANG, Wen-Hao PENG, Xiao-Fei ZHU, De-Feng ZHOU. Preparation and Electrochemical Performance of Pr0.8Sr0.2Fe0.7Ni0.3O3-δ ⁃Pr1.2Sr0.8Ni0.6Fe0.4O4+δ Composite Cathode[J]. Chinese Journal of Applied Chemistry, 2022, 39(5): 797-808.
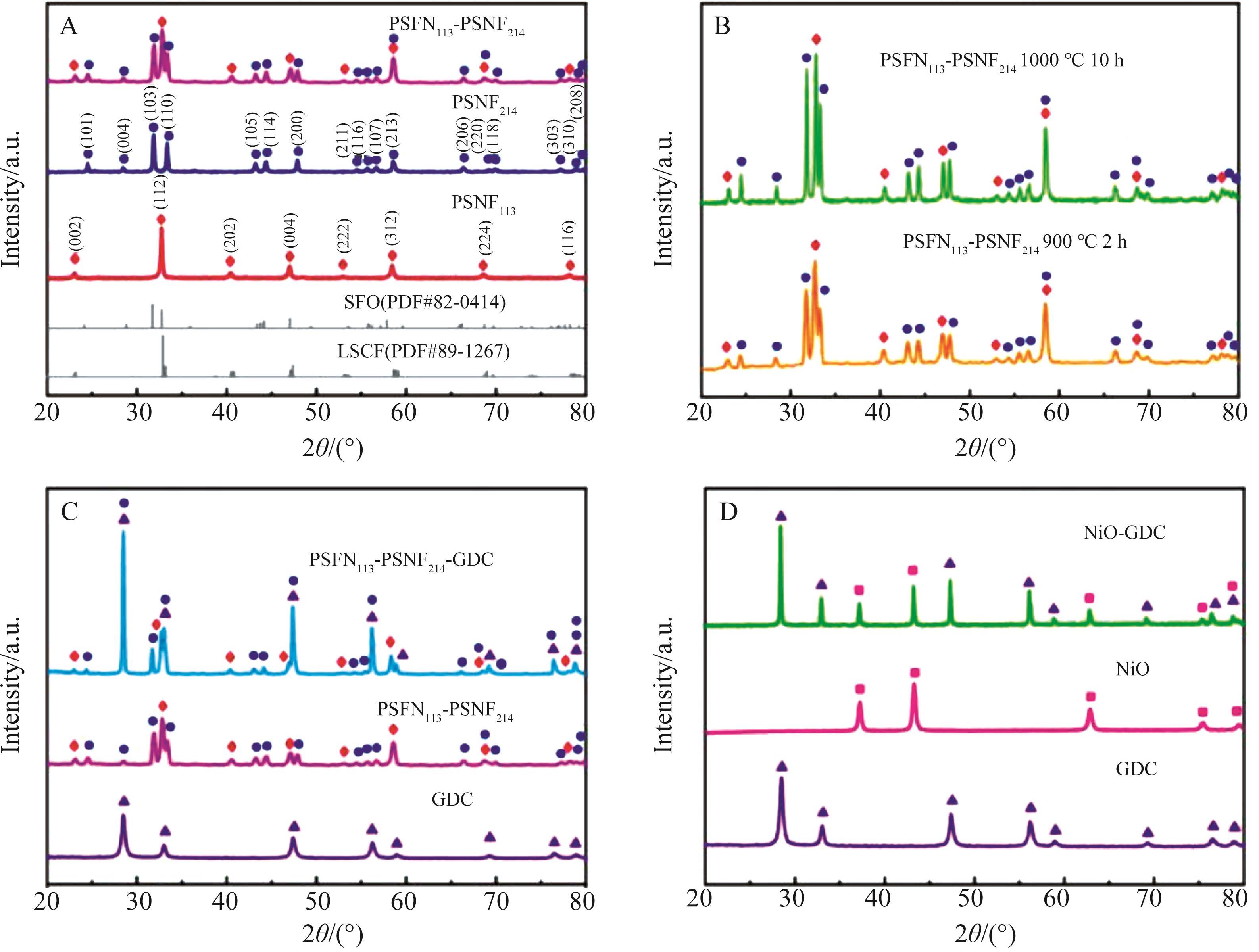
图1 (A)所有样品的XRD图; (B)不同煅烧温度下,PSFN113-PSNF214的XRD图; (C)PSFN113-PSNF214与GDC的XRD图; (D)NiO与GDC的XRD图
Fig.1 (A) XRD patterns of all samples; (B) XRD patterns of PSFN113-PSNF214 at different calcination temperatures; (C) XRD patterns of PSFN113-PSNF214 and GDC; (D) XRD patterns of NiO and GDC
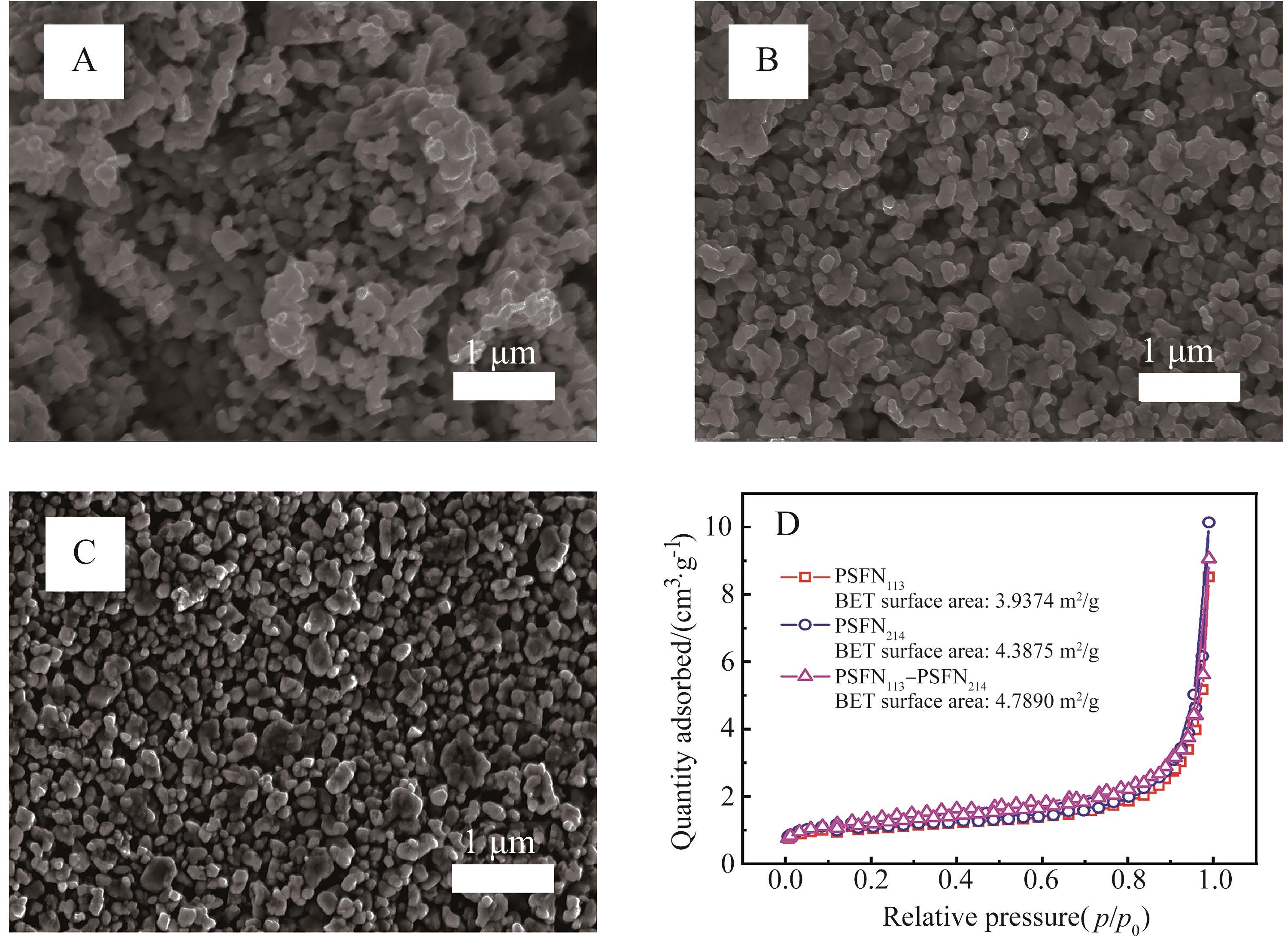
图3 (A)PSFN113、(B)PSNF214和(C)PSFN113-PSNF214粉末的FE-SEM图及(D)样品的N2吸附解吸曲线
Fig.3 FE-SEM images of (A) PSFN113, (B) PSNF214, (C) PSFN113-PSNF214 powders and (D) N2 adsorption desorption curves of all samples
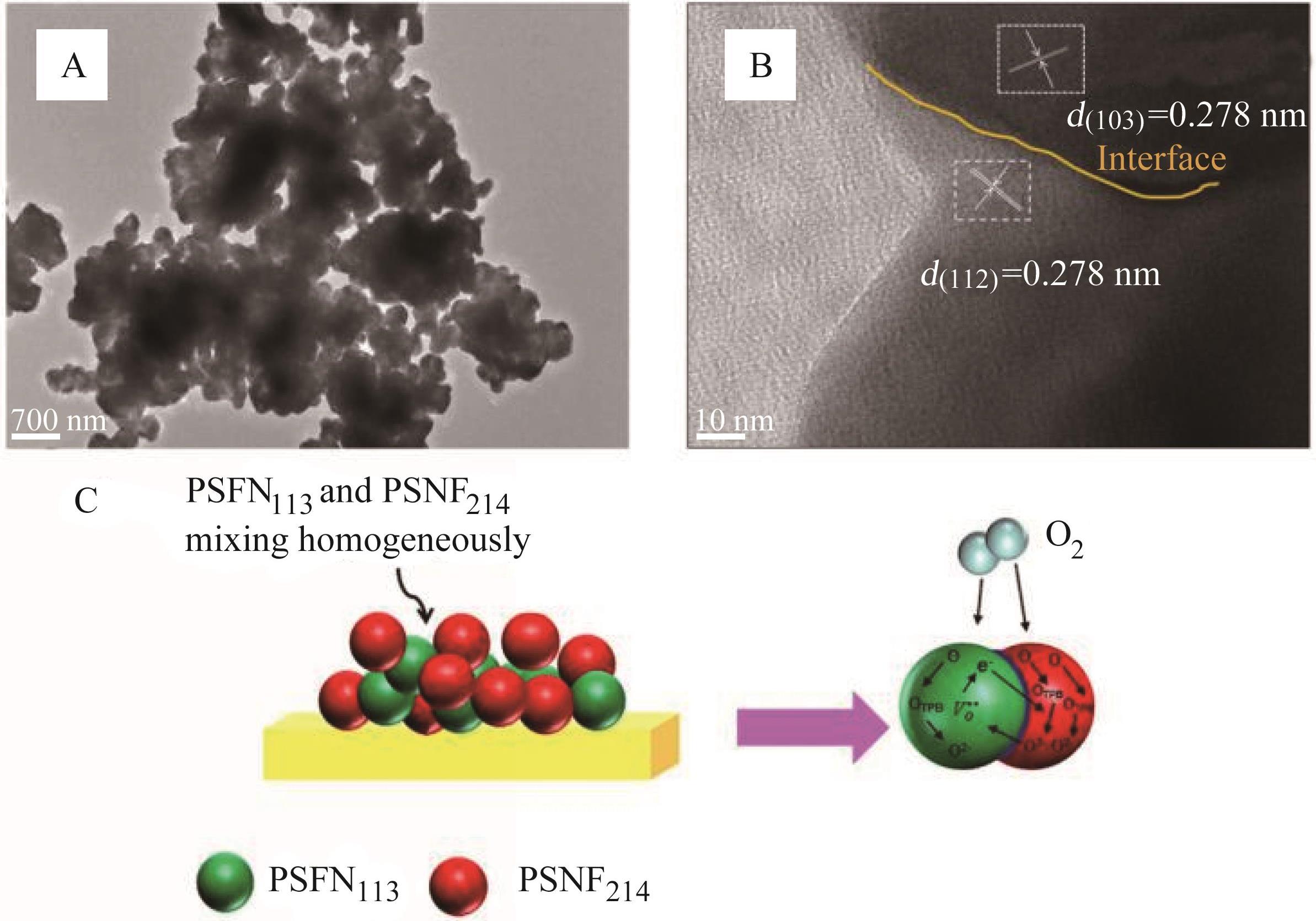
图4 PSFN113-PSNF214异质复合阴极的(A)TEM图像、(B)HR-TEM图像(黄色实线表示PSFN113和PSNF214之间的界面)和(C)性能提升机理图
Fig.4 (A) Bright field TEM image, (B) HR-TEM image and (C) Performance improvement mechanism diagram of PSFN113-PSNF214 heterogeneous composite cathode (the yellow solid line represents the interface between PSFN113 and PSNF214)
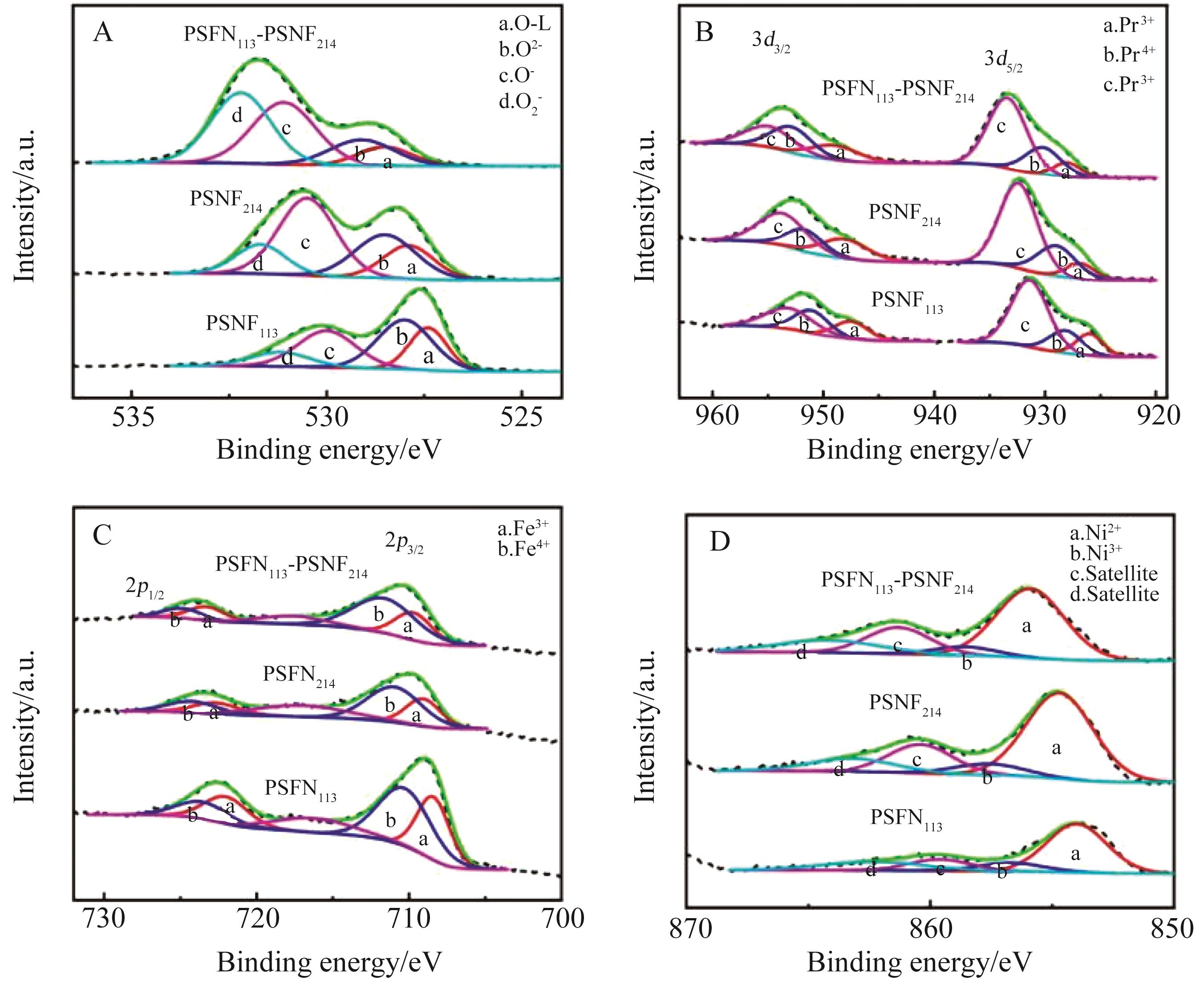
阴极 Cathode | PSFN113 | PSNF214 | PSFN113?PSNF214 |
|---|---|---|---|
| Olattice | 22.1% | 16.6% | 9.3% |
| Osurface | 77.9% | 83.4% | 90.7% |
| Pr4+ | 75.2% | 75.5% | 70.3% |
| Pr3+ | 24.8% | 24.5% | 29.7% |
| Fe4+ | 54.7% | 63.0% | 62.8% |
| Fe3+ | 45.3% | 37.0% | 37.2% |
| Ni3+ | 15.8% | 11.8% | 10.3% |
| Ni2+ | 84.2% | 88.2% | 89.7% |
表1 从XPS光谱分析中获得的相关元素含量
Table 1 Contents of relevant elements obtained from XPS spectrum analysis
阴极 Cathode | PSFN113 | PSNF214 | PSFN113?PSNF214 |
|---|---|---|---|
| Olattice | 22.1% | 16.6% | 9.3% |
| Osurface | 77.9% | 83.4% | 90.7% |
| Pr4+ | 75.2% | 75.5% | 70.3% |
| Pr3+ | 24.8% | 24.5% | 29.7% |
| Fe4+ | 54.7% | 63.0% | 62.8% |
| Fe3+ | 45.3% | 37.0% | 37.2% |
| Ni3+ | 15.8% | 11.8% | 10.3% |
| Ni2+ | 84.2% | 88.2% | 89.7% |
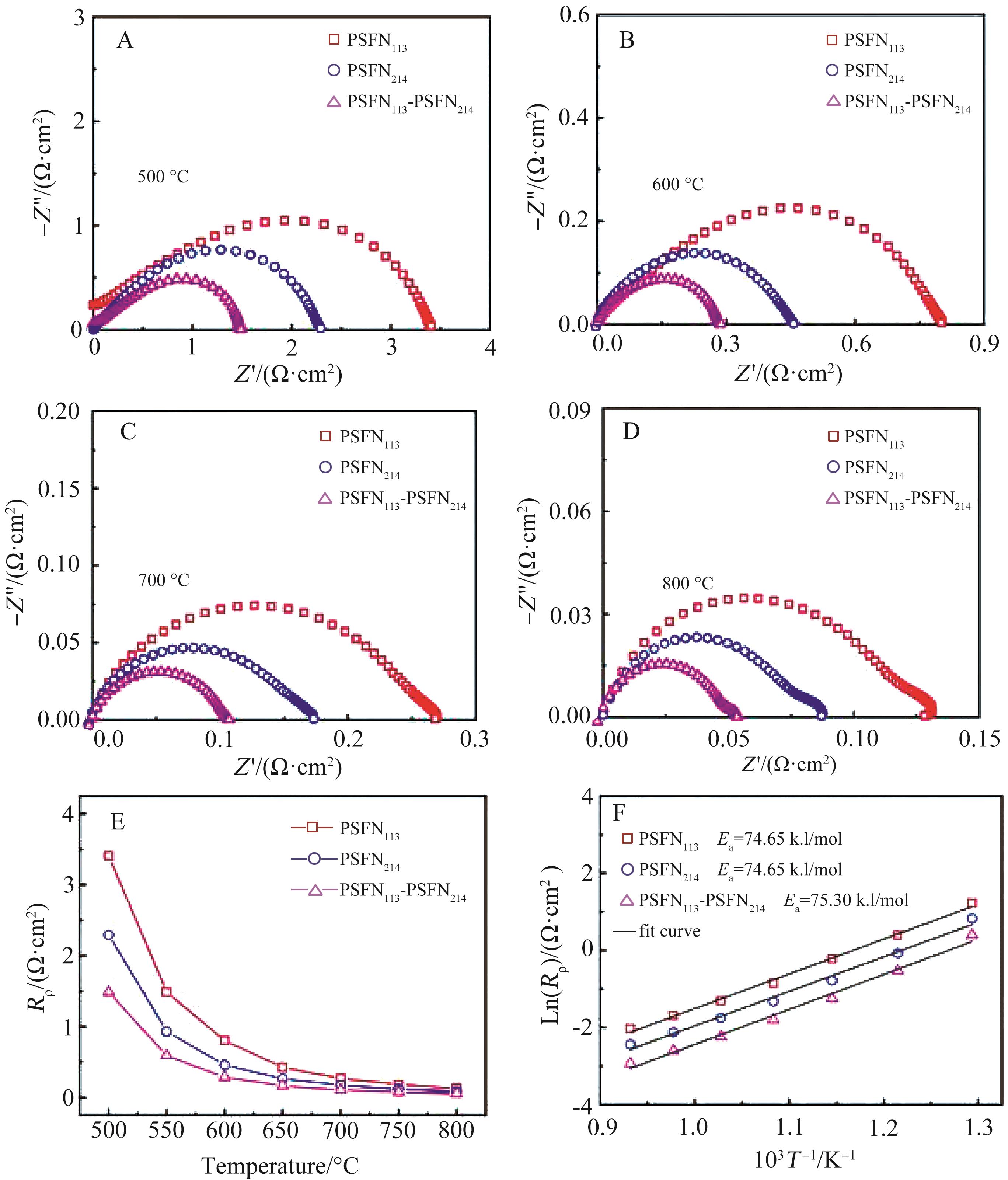
图6 对称电池的电化学阻抗谱:(A)500 ℃, (B)600 ℃, (C)700 ℃, (D)800 ℃; (E)极化电阻随温度的变化曲线及(F)相应的Arrhenius曲线
Fig.6 Electrochemical impedance spectra of symmetrical cells: (A) 500 ℃, (B) 600 ℃, (C) 700 ℃, (D) 800 ℃; (E) The change curves of polarization resistance with temperature and (F) the corresponding Arrhenius curves
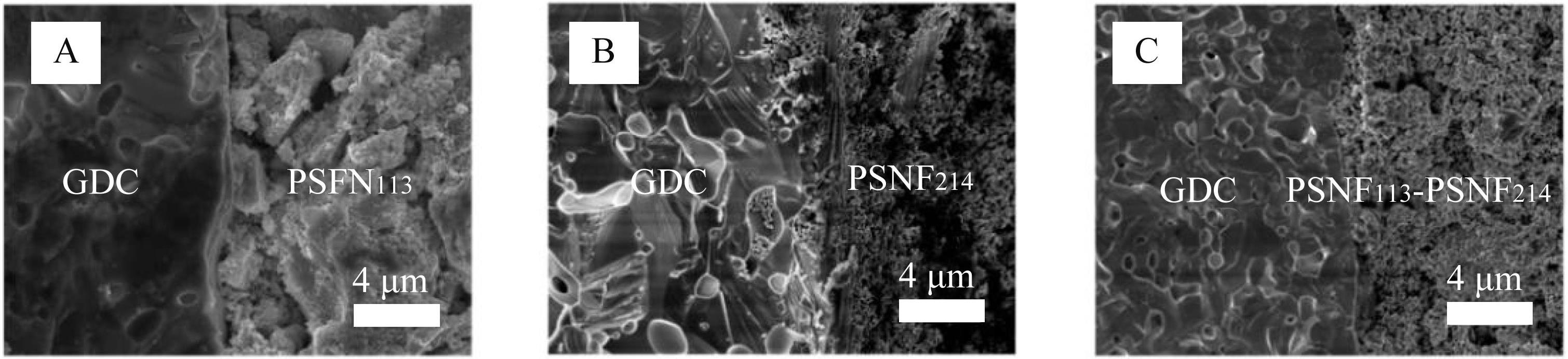
图7 EIS测试后,以(A)PSFN113、 (B)PSNF214和(C)PSFN113-PSNF214作为阴极的对称电池的横截面SEM图
Fig.7 Cross-sectional SEM images of symmetrical cells with (A) PSFN113, (B) PSNF214 and (C) PSFN113-PSNF214 cathode after EIS test
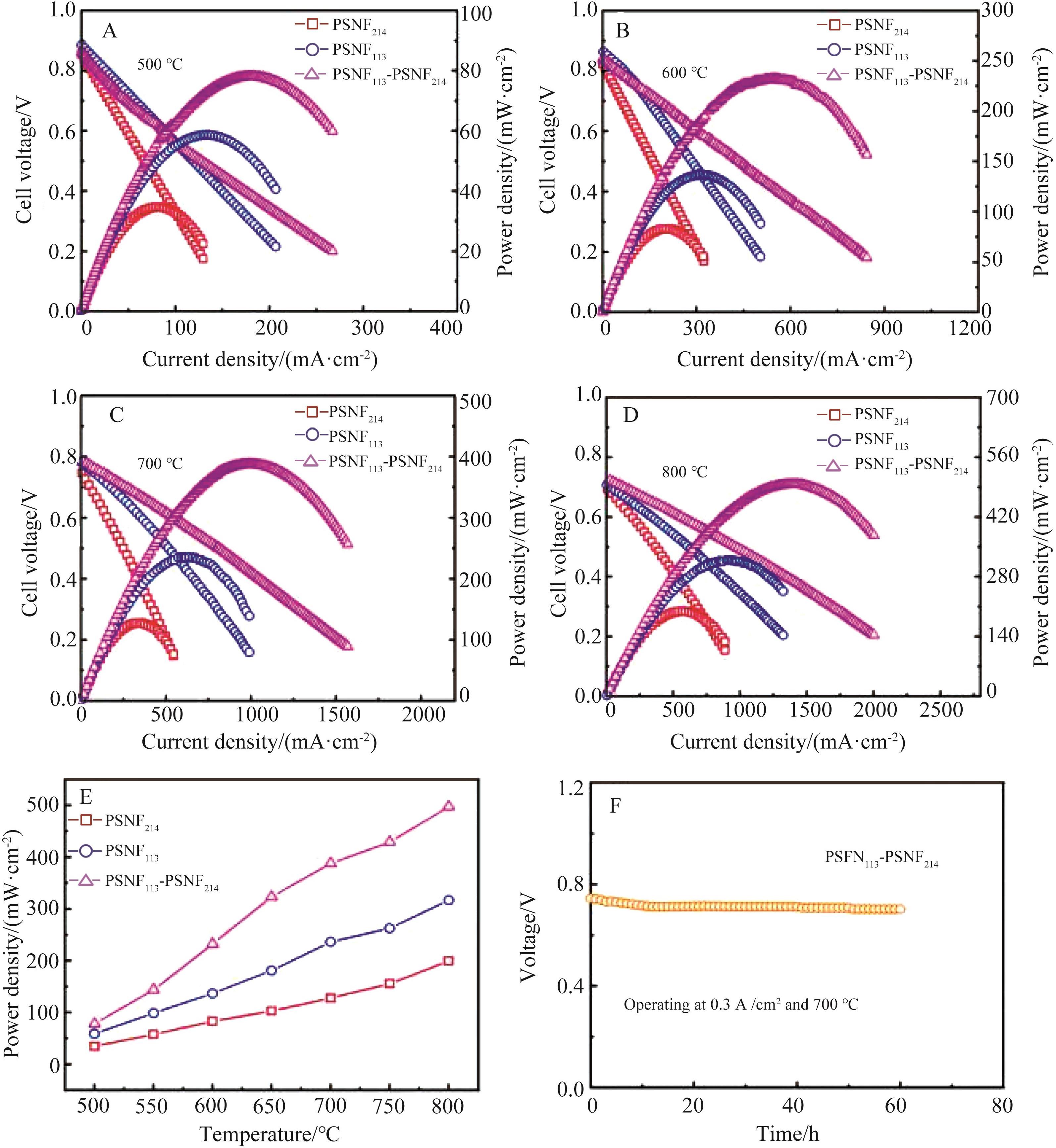
图8 单电池的I?V?P曲线:(A)500 ℃、(B)600 ℃、(C)700 ℃和(D)800 ℃下I-V曲线;(E)功率密度随温度的变化曲线;(F)以PSFN113-PSNF214为阴极构建的单电池的长期稳定性测试图
Fig.8 I?V?P curves of single cells: I-V curves at (A) 500 ℃, (B) 600 ℃, (C) 700 ℃, (D) 800 ℃; (E) Power density change curves with temperature; (F) Long-term stability test curve of a single cell constructed with PSFN113-PSNF214 cathode
阴极 Cathode | 电池构造 Cell configuration | 最大功率密度 PPD/(W·cm-2) | 温度 Temperature/℃ | 参考文献 Reference |
|---|---|---|---|---|
| *Nd0.5Ba0.5Fe0.9Co0.1O3-δ | Ni?BCZD/BCZD/NBFCo | 390 | 700 | |
| La0.3Sr0.7Ti0.3Fe0.70O3-δ | LSTF/SDC/YSZ/SDC/LSTF | 374 | 900 | |
| *Nd1.7Ca0.3NiO4+δ | NiO?GDC/GDC/GDC?Nd1.7Ca0.3NiO4 | 188 | 800 | |
| *La1.5Pr0.5Ni0.8Co0.2O4+δ | LPNCO/CGD/YSZ/Ni?YSZ | 385 | 700 | |
| *Pr2Ni0.5Mn0.5O4-δ | NiO?YSZ/YSZ/GDC/PNM | 485 | 800 | |
| *Bi0.5Ba0.5FeO3-δ ?Ba(Zr0.1Ce0.7Y0.2)O3 | NiO?BZCY/BZCY/BBFO?BZCY | 120 | 700 | |
| Ba0.5Sr0.5Co0.8Fe0.2O3-δ ?Ce0.8Sm0.2O2-δ | LSCF?SDC/SDC/YSZ/SDC/BSCF?SDC | 290 | 850 | |
| Pr2NiO4?40%Sm0.2Ce0.8O1.9 | PNO?40SDC/SDC/PNO?40SDC | 375 | 800 | |
| La0.6Sr0.4Co0.2Fe0.8O3-δ ?Gd0.1Ce0.9O2-δ | SNNV10?GDC/GDC/LSCF?GDC | 280 | 650 | |
| La0.4Sr0.6Co0.2Fe0.7Nb0.1O3-δ ?Gd0.1Ce0.9O2-δ | LSCFN?GDC/GDC/YSZ/GDC/LSCFN?GDC | 348 | 850 | |
| PSFN113-214(5∶5) | NiO?GDC/GD C/PSFN113-214(5∶5) | 496.80 | 800 | This work |
表2 文献中报道的不同阴极材料单电池的最大功率密度比较总结
Table 2 Comparison and summary of the maximum power density of single cells with different cathode materials reported in the literature
阴极 Cathode | 电池构造 Cell configuration | 最大功率密度 PPD/(W·cm-2) | 温度 Temperature/℃ | 参考文献 Reference |
|---|---|---|---|---|
| *Nd0.5Ba0.5Fe0.9Co0.1O3-δ | Ni?BCZD/BCZD/NBFCo | 390 | 700 | |
| La0.3Sr0.7Ti0.3Fe0.70O3-δ | LSTF/SDC/YSZ/SDC/LSTF | 374 | 900 | |
| *Nd1.7Ca0.3NiO4+δ | NiO?GDC/GDC/GDC?Nd1.7Ca0.3NiO4 | 188 | 800 | |
| *La1.5Pr0.5Ni0.8Co0.2O4+δ | LPNCO/CGD/YSZ/Ni?YSZ | 385 | 700 | |
| *Pr2Ni0.5Mn0.5O4-δ | NiO?YSZ/YSZ/GDC/PNM | 485 | 800 | |
| *Bi0.5Ba0.5FeO3-δ ?Ba(Zr0.1Ce0.7Y0.2)O3 | NiO?BZCY/BZCY/BBFO?BZCY | 120 | 700 | |
| Ba0.5Sr0.5Co0.8Fe0.2O3-δ ?Ce0.8Sm0.2O2-δ | LSCF?SDC/SDC/YSZ/SDC/BSCF?SDC | 290 | 850 | |
| Pr2NiO4?40%Sm0.2Ce0.8O1.9 | PNO?40SDC/SDC/PNO?40SDC | 375 | 800 | |
| La0.6Sr0.4Co0.2Fe0.8O3-δ ?Gd0.1Ce0.9O2-δ | SNNV10?GDC/GDC/LSCF?GDC | 280 | 650 | |
| La0.4Sr0.6Co0.2Fe0.7Nb0.1O3-δ ?Gd0.1Ce0.9O2-δ | LSCFN?GDC/GDC/YSZ/GDC/LSCFN?GDC | 348 | 850 | |
| PSFN113-214(5∶5) | NiO?GDC/GD C/PSFN113-214(5∶5) | 496.80 | 800 | This work |
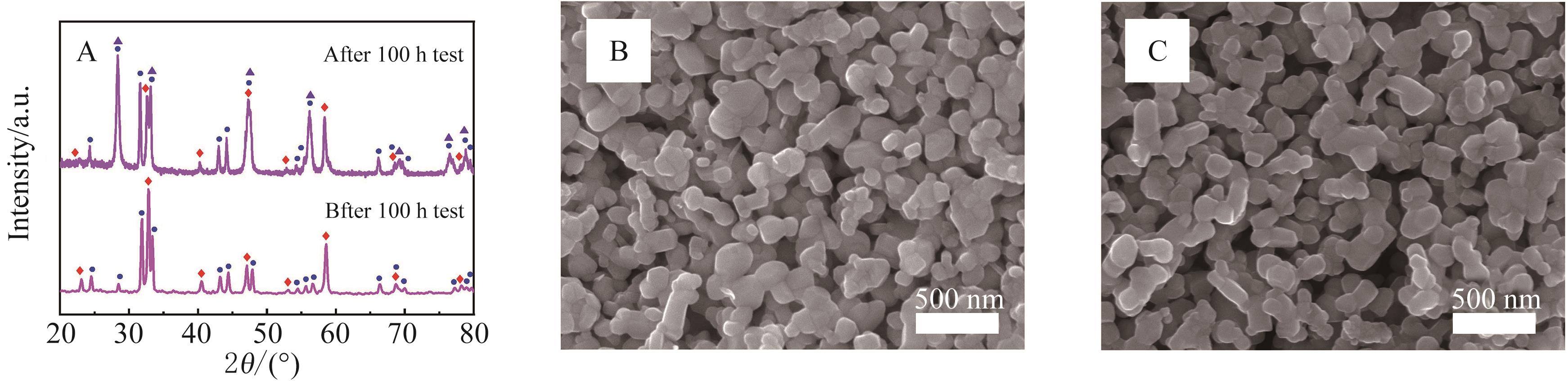
图9 (A)长期稳定性测试前后阴极XRD对比图; (B)测试前复合阴极粉末SEM图; (C)测试前复合阴极粉末SEM图
Fig.9 (A) Comparison of cathode XRD images before and after long-term stability test; (B) SEM image of heterogeneous composite cathode powder before test; (C) SEM image of heterogeneous composite cathode powder after test
| 1 | QIU P, YANG X, ZHU T Y, et al. Review on core-shell structured cathode for intermediate temperature solid oxide fuel cells[J]. Int J Hydrogen Energy, 2020, 45(43): 23160-23173. |
| 2 | ZHAO C H, LI Y F, ZHANG W Q, et al. Heterointerface engineering for enhancing the electrochemical performance of solid oxide cells[J]. Energy Environ Sci, 2020, 13(1): 53-85. |
| 3 | AJAA A, NAB A, MRS A, et al. Review of composite cathodes for intermediate-temperature solid oxide fuel cell applications[J]. Ceram Int, 2020, 46(15): 23314-23325. |
| 4 | KIM D, PARK J W, CHAE M S, et al. An efficient and robust lanthanum strontium cobalt ferrite catalyst as a bifunctional oxygen electrode for reversible solid oxide cells[J]. J Mater Chem A, 2021, 9: 5507-5521. |
| 5 | SHAO Z P, HAILE S M. A high-performance cathode for the next generation of solid-oxide fuel cells[J]. Nature, 2004, 431: 170-173. |
| 6 | HASHIMOTO S I, KAMMER K, LARSEN P H, et al. A study of Pr0.7Sr0.3Fe1- xNixO3- δ as a cathode material for SOFCs with intermediate operating temperature[J]. Solid State Ionics, 2005, 176(11/12): 1013-1020. |
| 7 | GIULIANO A, NICOLLET C, FOURCADE S, et al. Influence of the electrode/electrolyte interface structure on the performance of Pr0.8Sr0.2Fe0.7Ni0.3O3- δ as solid oxide fuel cell cathode[J]. Electrochim Acta, 2017, 236: 328-336. |
| 8 | NIRALA G, YADAV D, UPADHYAY S. Review ruddlesden-popper phase A2BO4 oxides:recent studies on structure, electrical, dielectric, and optical properties[J]. J Adv Ceram, 2020, 9(2): 129-148. |
| 9 | YASHIMA M, YAMADA H, NUANSAENG S, et al. Role of Ga3+ and Cu2+ in the high interstitial oxide-ion diffusivity of Pr2NiO4-based oxides: design concept of interstitial ion conductors through the higher-valence d10 dopant and Jahn-Teller effect[J]. Chem Mater, 2012, 24(21): 4100-4113. |
| 10 | CHEN X, WANG J Q, LIANG Q W, et al. Pr2NiO4-Pr0.2Ce0.8O1.9 composite cathode as a potential cathode material for intermediate temperature solid oxide fuel cells[J]. Solid State Sci, 2020, 100: 106108. |
| 11 | BHOGA S S, KHANDALE A P, PAHUNE B S. Investigation on Pr2- xSrxNiO4+ δ (x=0.3-1.0) cathode materials for intermediate temperature solid oxide fuel cell[J]. Solid State Ionics, 2014, 262: 340-344 |
| 12 | KIM D, LEE K T. Effect of lanthanide (Ln=La, Nd, and Pr) doping on electrochemical performance of Ln2NiO4+ δ-YSZ composite cathodes for solid oxide fuel cells[J]. Ceram Int, 2021, 47(2): 2493-2498. |
| 13 | YANG J F, CHENG J G, JIANG Q M, et al. Preparation and electrochemical properties of strontium doped Pr2NiO4 cathode materials for intermediate-temperature solid oxide fuel cells[J]. Int J Hydrogen Energy, 2012, 37(2): 1746-1751 . |
| 14 | GILEV A R, KISELEV E A, CHEREPANOV V A. Oxygen transport phenomena in (La,Sr)2(Ni,Fe)O4 materials[J]. J Mater Chem A, 2018, 6(13): 5304-5312. |
| 15 | MIAO L N, HOU J, GONG Z, et al. A high-performance cobalt-free ruddlesden-popper phase cathode La1.2Sr0.8Ni0.6Fe0.4O4+ δ for low temperature proton-conducting solid oxide fuel cells[J]. Int J Hydrogen Energy, 2019, 44(14): 7531-7537. |
| 16 | WANG L, WANG P P, GENG C L, et al. A novel core-shell LSCF perovskite structured electrocatalyst with local hetero-interface for solid oxide fuel cells[J]. Int J Hydrogen Energy, 2020, 45(20): 11824-11833. |
| 17 | YU X D, SUI C, REN R Z, et al. Construction of heterointerfaces with enhanced oxygen reduction kinetics for intermediate-temperature solid oxide fuel cells[J]. ACS Appl Energy Mater, 2019, 3(1): 447-455. |
| 18 | ZHENG Y, ZHAO C H, WU T, et al. Enhanced oxygen reduction kinetics by a porous heterostructured cathode for intermediate temperature solid oxide fuel cells[J]. Energy AI, 2020, 2: 100027. |
| 19 | ZHANG W W, ZHANG L F, GUAN K, et al. Effective promotion of oxygen reduction activity by rare earth doping in simple perovskite cathodes for intermediate-temperature solid oxide fuel cells[J]. J Power Sources, 2020, 446: 227360. |
| 20 | HUSSAIN M, MUNEER M, ABBAS G, et al. Cobalt free LaxSr1- xFe1- yCuyO3- δ(x=0.54,0.8,y=0.2,0.4) perovskite structured cathode for SOFC[J]. Ceram Int, 2020, 46(11): 18208-18215. |
| 21 | KHOSHKALAM M, TRIPKOVI O, TONG X, et al. Improving oxygen incorporation rate on (La0.6Sr0.4)0.98FeO3- δ via Pr2Ni1- xCuxO4+ δ surface decoration[J]. J Power Sources, 2020, 457: 228035. |
| 22 | BAI J H, HAN Z Y, LV B, et al. Preparation of 3D structure high performance Ba0.5Sr0.5Fe0.8Cu0.2O3- δ nanofiber SOFC cathode material by low-temperature calcination method[J]. Int J Hydrogen Energy, 2020, 46(11): 8132-8142. |
| 23 | ZHANG W W, ZHANG L F, GUAN K, et al. Effective promotion of oxygen reduction activity by rare earth doping in simple perovskite cathodes for intermediate-temperature solid oxide fuel cells[J]. J Power Sources, 2020, 446: 227360. |
| 24 | WANG H C, ZHANG W W, GUAN K, et al. Enhancing activity and durability of a-site-deficient (La0.6Sr0.4)0.95Co0.2Fe0.8O3- δ cathode by surface modification with PrO2- δ nanoparticles[J]. ACS Sustain Chem Eng, 2020, 8: 3367-3380. |
| 25 | LI F S, JIANG L, ZENG R, et al. Hetero-structured La0.5Sr0.5CoO3- δ/LaSrCoO4± δ cathode with high electro-catalytic activity for solid-oxide fuel cells[J]. Int J Hydrogen Energy, 2017, 42(49): 29463-29471. |
| 26 | 魏振业, 孟君玲, 王浩聪, 等. 同质异形表面修饰提高La2NiO4+ δ阴极的电催化活性[J]. 应用化学, 2020, 8(37): 940-951. |
| WEI Z Y, MENG J L, WANG H C, et al. Improving tne electrocatalytic activity of La2NiO4+ δ cathode by surface modification with conformal heterojunction[J]. Chinese J Appl Chem, 2020, 8(37): 940-951. | |
| 27 | KUZMIN A V, LESNICHYOVA A S, TROPIN E S, et al. LaScO3-based electrolyte for protonic ceramic fuel cells:Influence of sintering additives on the transport properties and electrochemical performance[J]. J Power Sources, 2020, 466: 228255. |
| 28 | LI F S, XU Y X, CHENG F X, et al. Composite fibers of La0.5Sr0.5CoO3- δ-LaSrCoO4± δ with high catalytic activity toward oxygen reduction reaction [J]. Ceram Int, 2020, 46(5): 6191-6198. |
| 29 | YANG Y, LI R Y, WU Y J, et al. Highly active self-assembled hybrid catalyst with multiphase heterointerfaces to accelerate cathodic oxygen reduction of intermediate-temperature solid oxide fuel cells[J]. Ceram Int, 2020, 46(7): 9661-9668. |
| 30 | LYAGAEVA J, DANILOV N, TARUTIN A, et al. Designing a protonic ceramic fuel cell with novel electrochemically active oxygen electrodes based on doped Nd0.5Ba0.5FeO3- δ[J]. Dalton T, 2018, 47: 8149-8157. |
| 31 | CAO Z Q, ZHANG Y H, MIAO J P, et al. Titanium-substituted lanthanum strontium ferrite as a novel electrode material for symmetrical solid oxide fuel cell[J]. Int J Hydrogen Energy, 2015, 40(46): 16572-16577. |
| 32 | LENKA R K, PATRO P K, PATEL V, et al. Comparative investigation on the functional properties of alkaline earth metal (Ca, Ba, Sr) doped Nd2NiO4+ δ oxygen electrode material for SOFC applications[J]. J Alloy Compd, 2020, 860: 158490. |
| 33 | NAVARRETE L, FABUEL M, YOO C Y, et al. Tailoring La2- XAXNi1- YBYO4+ δ cathode performance by simultaneous A and B doping for IT-SOFC[J]. Int J Hydrogen Energy, 2020, 45( 31): 15589-15599. |
| 34 | CUI J J, WANG J K, ZHANG X W, et al. Low thermal expansion material Bi0.5Ba0.5FeO3- δ in application for proton-conducting ceramic fuel cells cathode[J]. Int J Hydrogen Energy, 2019, 44(38): 21127-21135. |
| 35 | CHANG H, YAN J, CHEN H L, et al. Preparation of thin electrolyte film via dry pressing/heating/quenching/calcining for electrolyte-supported SOFCs[J]. Ceram Int, 2019, 45(8): 9866-9870. |
| 36 | GAO Z P, DING X F, DING D, et al. Infiltrated Pr2NiO4 as promising bi-electrode for symmetrical solid oxide fuel cells[J]. Int J Hydrogen Energy, 2018, 43(18): 8953-8961. |
| 37 | PAN K J, HUSSAIN A M, WACHSMAN E D. Investigation on Sr0.2Na0.8Nb1-xVxO3 (x=0.1, 0.2, 0.3) as new ceramic anode materials for low-temperature solid oxide fuel cells[J]. J Power Sources, 2017, 347: 277-282. |
| 38 | XU N, ZHU T, YANG Z, et al. Co-synthesis of LSCFN-GDC electrode for symmetric solid oxide fuel cell running on propane[J]. Electrochim Acta, 2017, 265: 259-264. |
| [1] | 石雪建, 刘万强, 王春丽, 程勇, 王立民. 钾离子电池用Sb基负极材料研究进展[J]. 应用化学, 2023, 40(2): 210-228. |
| [2] | 纪全增, 孟君玲, 王浩聪, 安涛, 刘孝娟, 孟健. La2CuO4阴极薄膜的相转变及其对电化学性能的影响[J]. 应用化学, 2021, 38(8): 976-985. |
| [3] | 纪全增, 孟君玲, 王浩聪, 安涛, 刘孝娟, 孟健. La2CuO4阴极薄膜的相转变及其对电化学性能的影响[J]. 应用化学, 2021, 38(8): 0-0. |
| [4] | 王春丽,孙连山,钟鸣,王立民,程勇. 过渡金属及其化合物应用于锂硫电池的研究进展[J]. 应用化学, 2020, 37(4): 387-404. |
| [5] | 惠康龙, 傅继澎, 高湉, 唐明学. 金属硫化物在可充电电池中的研究进展[J]. 应用化学, 2020, 37(12): 1384-1402. |
| [6] | 蔡敏, 吴春燕, 谢贻顺, 班贵, 赖飞燕, 黄小英, 张晓辉. 正硅酸乙酯对高电压镍锰酸锂基全电池电化学性能的影响[J]. 应用化学, 2020, 37(11): 1301-1308. |
| [7] | 王照民, 易政, 钟鸣, 程勇, 王立民. 锂离子电池Sb基负极材料研究进展[J]. 应用化学, 2018, 35(7): 745-755. |
| [8] | 陈丽辉,吴秋晗,潘佩,宋子轩,王锋,丁瑜. 尖晶石型八面体结构锰酸锂的制备及其电化学性能[J]. 应用化学, 2018, 35(11): 1384-1390. |
| [9] | 万露, 付争兵. 锂离子电池负极材料碳氮包覆钛酸锂的制备及表征[J]. 应用化学, 2018, 35(1): 116-122. |
| [10] | 焦连升, 孟玲菊, 吴同舜, 李风华, 牛利. 磷酸钒锂/石墨烯复合正极材料的制备及表征[J]. 应用化学, 2017, 34(6): 712-722. |
| [11] | 冯雪娇, 崔红敏, 肖正强, 晏南富, 李敏. 微米级SiO合成多孔氧化硅/硅/碳储锂复合材料[J]. 应用化学, 2017, 34(1): 76-82. |
| [12] | 朱小明, 冯泳兰, 张复兴, 庾江喜, 蒋伍玖, 欧亚平, 邝代治. 双核铜(Ⅱ)配合物{[Cu(Phen)(Nap)2]2·(EtOH)2·(H2O)2}(Phen=1,10-菲咯啉,Nap=1-萘甲酸,EtOH=乙醇)的合成、晶体结构及性质[J]. 应用化学, 2016, 33(8): 932-938. |
| [13] | 王新颖, 黄红霞, 谢文强, 孔翔飞, 于文婉. 席夫碱对La-Mg-Ni基储氢合金电化学性能的影响[J]. 应用化学, 2016, 33(7): 813-819. |
| [14] | 徐圣楠, 那兆霖, 尹东明, 吴耀明, 王立民. 铜-铈氧化还原液流电池性能[J]. 应用化学, 2016, 33(12): 1462-1464. |
| [15] | 杨健, 陈步明, 王帅, 郭忠诚. 氯离子浓度对锌电积用铝基铅合金复合β-PbO2颗粒阳极行为的影响[J]. 应用化学, 2015, 32(8): 955-962. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||