
应用化学 ›› 2022, Vol. 39 ›› Issue (5): 697-706.DOI: 10.19894/j.issn.1000-0518.210129
• 综合评述 • 下一篇
锂离子电池电解液除酸除水添加剂的研究进展
宋林虎1,2, 李世友1,2,3( ), 王洁1,2, 张晶晶1,2, 张宁霜1,2,3, 赵冬妮1,2,3, 徐菲1,2
), 王洁1,2, 张晶晶1,2, 张宁霜1,2,3, 赵冬妮1,2,3, 徐菲1,2
- 1.兰州理工大学石油化工学院,兰州 730050
2.甘肃省低碳能源化工重点实验室,兰州 730050
3.甘肃省锂离子电池电解液材料工程实验室,兰州 730050
-
收稿日期:2021-03-19接受日期:2021-08-24出版日期:2022-05-01发布日期:2022-05-24 -
通讯作者:李世友 -
基金资助:国家自然基金(21766017)
Research Progress of Additives for Acid and Water Removal in Electrolyte of Lithium Ion Battery
Lin-Hu SONG1,2, Shi-You LI1,2,3( ), Jie WANG1,2, Jing-Jing ZHANG1,2, Ning-Shuang ZHANG1,2,3, Dong-Ni ZHAO1,2,3, Fei XU1,2
), Jie WANG1,2, Jing-Jing ZHANG1,2, Ning-Shuang ZHANG1,2,3, Dong-Ni ZHAO1,2,3, Fei XU1,2
- 1.School of Petrochemical Technology,Lanzhou University of Technology,Lanzhou 730050,China
2.Gansu Key Laboratory of Low-Carbon Energy and Chemical Engineering,Lanzhou 730050,China
3.Gansu Engineering Laboratory of Electrolyte Material for Lithium-ion Battery,Lanzhou 730050,China
-
Received:2021-03-19Accepted:2021-08-24Published:2022-05-01Online:2022-05-24 -
Contact:Shi-You LI -
About author:lishiyoulw@163.com
-
Supported by:the National Natural Science Foundation of China(21766017)
摘要:
商用锂离子电池电解液在应用过程中存在电解质锂盐六氟磷酸锂(LiPF6)易在痕量水环境中发生水解反应,进而导致锂离子电池体系的综合电化学性能受损。因此,亟需控制电解液本体中痕量水的引入以及减小锂盐与痕量水反应产物对电池体系影响的措施。本文主要综述了含有不同官能团的添加剂在除去电解液中痕量水和酸时所具有的特性,并重点分析介绍了其除酸除水的作用机理。 最后,对除酸、除水型添加剂未来的研究方向和应用前景进行了展望。
中图分类号:
引用本文
宋林虎, 李世友, 王洁, 张晶晶, 张宁霜, 赵冬妮, 徐菲. 锂离子电池电解液除酸除水添加剂的研究进展[J]. 应用化学, 2022, 39(5): 697-706.
Lin-Hu SONG, Shi-You LI, Jie WANG, Jing-Jing ZHANG, Ning-Shuang ZHANG, Dong-Ni ZHAO, Fei XU. Research Progress of Additives for Acid and Water Removal in Electrolyte of Lithium Ion Battery[J]. Chinese Journal of Applied Chemistry, 2022, 39(5): 697-706.
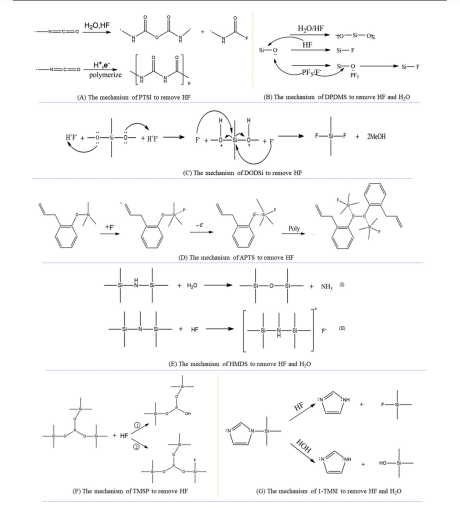
图2 各类添加剂除HF、H2O的作用机理:(A)PTSI除HF的作用机理[8],(B)DPDMS除HF、H2O的作用机理[9], (C)DODSi除HF的作用机理[11],(D) APTS除HF的作用机理[12],(E) HMDS除HF、H2O的作用机理[25,28],(F) TMSP除HF的作用机理[33],(G) 1-TMSI除HF、H2O的作用机理[34]
Fig.2 The action mechanisms of various additives to remove HF and H2O: (A) Mechanism of PTSI to remove HF[8], (B) Mechanism of DPDMS to remove HF and H2O[9], (C) Mechanism of DODSi to remove HF[11], (D) Mechanism of APTS to remove HF[12], (E) Mechanism of HMDS to remove HF and H2O[25,28], (F) Mechanism of TMSP to remove HF[33], and (G) Mechanism of 1-TMSI to remove HF and H2O[34]
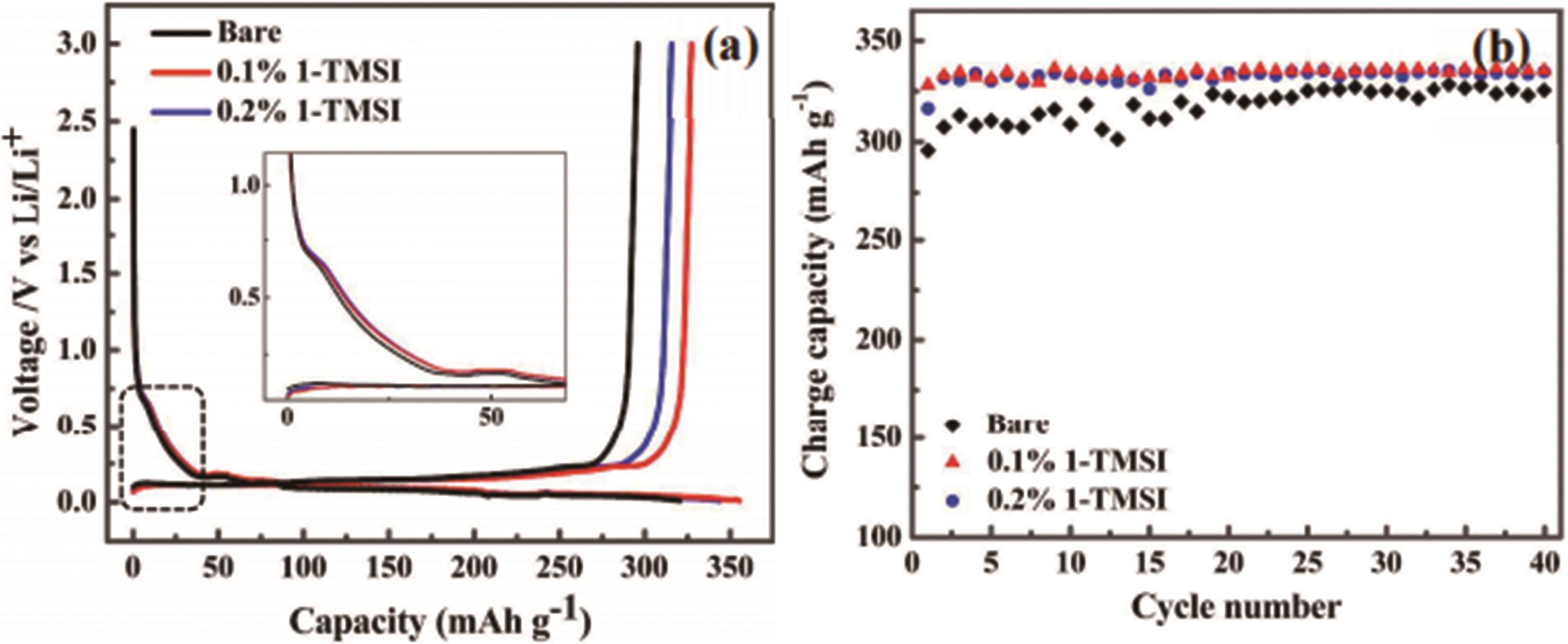
图3 (a)初始充电/放电容量,(b)Li/MCMB 电池的充电容量保持率,测试分别在有和无1-TMSI 添加剂的电解液下进行[34]
Fig.3 (a) Initial charge/discharge capacities, and (b) Charge capacity retention of Li/MCMB cells that are tested in the electrolytes without and with 1-TMSI additive[34]
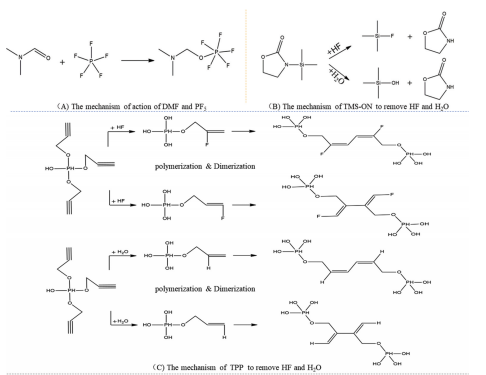
图4 新型化合物添加剂除HF、H2O的作用机理:(A)DMF与PF5的作用机理[13],(B)TMS-ON除HF、H2O的作用机理[15],(C)TPP除HF、H2O的作用机理[16]
Fig.4 Mechanism of new additives to remove HF and H2O: (A) Mechanism of action of DMF and PF5[13],(B) Mechanism of TMS?ON to remove HF and H2O[15], (C) Mechanism of TPP to remove HF and H2O[16]
添加剂 Additive | 特点 Characteristics | 不足 Insufficiencies |
|---|---|---|
异氰酸酯(—N Isocyanate (—N | —N The N atoms in —N | 该化合物作为添加剂在电解液中除杂的研究较少,机理仍需进一步探究 There's not a lot of research on using these compounds as additives to remove impurities in the electrolyte, and the mechanism still needs to be further explored |
硅烷类(Si—O)化合物 Silane (Si—O) compounds | Si—O烷基属于碱类化合物,可与H+结合,除去电解液中酸性杂质,并且Si对F较高的亲和力可清除分离出的F-,同时参与SEI膜的构建 Si—O alkyl can be combined with H+ to remove acidic impurities in electrolyte, due to its basic functional groups, and the separated F can be removed by Si with higher affinity to F-,participating in the construction of SEI film | Si—O与杂质反应生产络合物参与SEI膜的构建机理目前还不明确,仍需进一步研究 The mechanism of Si—O reacting with impurities to produce complexes and participating in the construction of SEI film is still unclear, and further research is needed |
新型化合物添加剂 Other new compound additives | 在极端环境下(如高温高压),亲F-或亲H+型添加剂自身可清除电解液中的HF杂质 The additives can remove HF impurities in the electrolyte by their affinity with F- or H+ under extreme environments (such as high temperature and high potential) | 高温高压条件易导致电解液分解产生其它杂质,故在该环境下研究除酸、除水型添加剂较为困难 Electrolyte is easy to decompose to produce other impurities at high temperature and potential, so it is difficult to study acid and water removing additives under this condition |
表1 各类除酸除水添加剂的特点
Table 1 Characteristics of various acid?removing and water?removing additives
添加剂 Additive | 特点 Characteristics | 不足 Insufficiencies |
|---|---|---|
异氰酸酯(—N Isocyanate (—N | —N The N atoms in —N | 该化合物作为添加剂在电解液中除杂的研究较少,机理仍需进一步探究 There's not a lot of research on using these compounds as additives to remove impurities in the electrolyte, and the mechanism still needs to be further explored |
硅烷类(Si—O)化合物 Silane (Si—O) compounds | Si—O烷基属于碱类化合物,可与H+结合,除去电解液中酸性杂质,并且Si对F较高的亲和力可清除分离出的F-,同时参与SEI膜的构建 Si—O alkyl can be combined with H+ to remove acidic impurities in electrolyte, due to its basic functional groups, and the separated F can be removed by Si with higher affinity to F-,participating in the construction of SEI film | Si—O与杂质反应生产络合物参与SEI膜的构建机理目前还不明确,仍需进一步研究 The mechanism of Si—O reacting with impurities to produce complexes and participating in the construction of SEI film is still unclear, and further research is needed |
新型化合物添加剂 Other new compound additives | 在极端环境下(如高温高压),亲F-或亲H+型添加剂自身可清除电解液中的HF杂质 The additives can remove HF impurities in the electrolyte by their affinity with F- or H+ under extreme environments (such as high temperature and high potential) | 高温高压条件易导致电解液分解产生其它杂质,故在该环境下研究除酸、除水型添加剂较为困难 Electrolyte is easy to decompose to produce other impurities at high temperature and potential, so it is difficult to study acid and water removing additives under this condition |
| 1 | AURBACH D, MARKOVSKY B, SALITRA G, et al. Review on electrode-electrolyte solution interactions, related to cathode materials for Li-ion batteries[J]. J Power Sources, 2007, 165(2): 491-499. |
| 2 | BING J, HWANG, CHIEN Y, et al. Structure, morphology, and electrochemical investigation of LiMn2O4 thin film cathodes deposited by radio frequency sputtering for lithium microbatteries[J]. J Phys Chem C, 2009, 113(26): 11373-11380. |
| 3 | LI W T, CAMPION C, LUCHT B L, et al. Additives for stabilizing LiPF6-based electrolytes against thermal decomposition[J]. J Electrochem Soc, 2005, 152(7): A1361-A1365. |
| 4 | XU G J, LIU Z H, ZHANG C J, et al. Strategies for improving the cyclability and thermo-stability of LiMn2O4-based batteries at elevated temperatures[J]. J Mater Chem A, 2015, 3(8): 4092-4123. |
| 5 | 王洁, 崔孝玲, 赵冬妮, 等. 适配于富锂锰基正极材料电解液体系的研究[J]. 现代化工, 2020, 40(1): 19-24. |
| WANG J, CUI X L, ZHAO D N, et al. Study on electrolyte adapted to lithium-rich layered oxide cathode materials[J]. Mod Chem Ind, 2020, 40(1): 19-24. | |
| 6 | 徐菲, 李世友, 赵冬妮, 等. 硅烷添加剂在锂离子电池电解液中的应用[J]. 硅酸盐学报, 2021, 49(1): 161-166. |
| XU F, LI S Y, ZHAO D N, et al. Application of silane additive in electrolyte of lithium ion battery[J]. J Chinese Ceram Soc, 2021, 49(1): 161-166. | |
| 7 | ZHANG S S. Aromatic isocyanate as a new type of electrolyte additive for the improved performance of Li-ion batteries[J]. J Power Sources, 2006, 163(1): 567-572. |
| 8 | DONG P, WANG D, YAO Y, et al. Stabilizing interface layer of LiNi0.5Co0.2Mn0.3O2 cathode materials under high voltage using p-toluenesulfonyl isocyanate as film forming additive[J]. J Power Sources, 2017, 344: 111-118. |
| 9 | DENG B W, WANG H, GE W J, et al. Investigating the influence of high temperatures on the cycling stability of a LiNi0.6Co0.2Mn0.2O2 cathode using an innovative electrolyte additive[J]. Electrochim Acta, 2017, 236: 61-71. |
| 10 | WANG S L, CHEN S M, GAO W Q, et al. A new additive 3-isocyanatopropyltriethoxysilane to improve electrochemical performance of Li/NCM622 half-cell at high voltage[J]. J Power Sources, 2019, 423: 90-97. |
| 11 | JANG S H, YIM T. Effect of silyl ether-functinoalized dimethoxydimethylsilane on electrochemical performance of a Ni-rich NCM cathode[J]. ChemPhysChem, 2017, 18(23): 3402-3406. |
| 12 | YE C C, TU W Q, YIN L M, et al. Converting detrimental HF in electrolyte into a highly-fluorinated interphase on cathode[J]. J Mater Chem A, 2018, 6(36): 17642-17652. |
| 13 | YOU L, DUAN K, ZHANG G, et al. N,N-Dimethylformamide electrolyte additive via a blocking strategy enables high performance lithium ion battery under high temperature[J]. J Phys Chem C, 2019, 123: 5942-5950. |
| 14 | 陈家辉. 锂离子电池高电压电解液制备及其作用机理研究[D]. 深圳: 深圳大学, 2016. |
| CHEN J H. Preparation and investigation of high voltage electrolyte for lithium-ion battery[D]. Shenzhen: Shenzhen University, 2016. | |
| 15 | KIM K, HWANG D, KIM S, et al. Cyclic aminosilane-based additive ensuring stable electrode-electrolyte interfaces in Li-ion batteries[J]. Adv Energy Mater, 2020, 10(15): 2000012. |
| 16 | ZHAO W M, ZHENG B Z, LI H D, et al. Toward a durable solid electrolyte film on the electrodes for Li-ion batteries with high performance[J]. Nano Energy, 2019, 63: 103815. |
| 17 | JOW R T, ZHANG S, XU K, et al. Non-aqueous electrolyte solutions comprising additives and non-aqueous electrolyte cells comprising the same: US, US6905762 B1[P]. 2005. |
| 18 | HAN J G, JEONG M Y, KIM K, et al. An electrolyte additive capable of scavenging HF and PF5 enables fast charging of lithium-ion batteries in LiPF6-based electrolytes[J]. J Power Sources, 2020, 446: 227366. |
| 19 | AMINE K, WANG Q, VISSERS, et al. Novel silane compounds as electrolyte solvents for Li-ion batteries[J]. Electrochem Commun, 2006, 8(3): 429-433. |
| 20 | WANG J, ZHAO X, LUO H, et al. A novel aminoalkyldisiloxane compound as a film-forming electrolyte additive for graphite anode[J]. Electrochemistry, 2015, 83(7): 537-540. |
| 21 | WANG J L, HAO L, MAI Y J, et al. Synthesis of aminoalkylsilanes with oligo(ethylene oxide) unit as multifunctional electrolyte additives for lithium-ion batteries[J]. Sci China Chem, 2013, 56: 739-745. |
| 22 | WANG S Q, WANG J L, HAO L, et al. A novel aminoalkylsilane compound with oligo(ethylene oxide) units as effective additive for improving cyclability of lithium-ion batteries[J]. J Mater Sci Technol, 2013, 29: 53-57. |
| 23 | ZHANG L, LYONS L, NEWHOUSE J, et al. Synthesis and characterization of alkylsilane ethers with oligo(ethylene oxide) substituents for safe electrolytes in lithium-ion batteries[J]. J Mater Chem, 2010, 20(38): 8224-8226. |
| 24 | YAN X D, CHEN C, ZHU X Q, et al. Aminoalkyldisiloxane as effective electrolyte additive for improving high temperature cycle life of nickel-rich LiNi0.6Co0.2Mn0.2O2/graphite batteries[J]. J Power Sources, 2020, 461: 228099. |
| 25 | YAMANE H, INOUE T, FUJITA M, et al. A causal study of the capacity fading of Li1.01Mn1.99O4 cathode at 80 ℃, and the suppressing substances of its fading[J]. J Power Sources, 2001, 99(1): 60-65. |
| 26 | LI Y K, ZHANG R X, LIU J S, et al. Effect of heptamethyldisilazane as an additive on the stability performance of LiMn2O4 cathode for lithium-ion battery[J]. J Power Sources, 2009, 189(1): 685-688. |
| 27 | WU X W, GUO H J, LI X H, et al. Effect of heptamethyldisilazane on the electrochemical performance of LiMn2O4/Li[J]. Ionics, 2013, 19(3): 429-435. |
| 28 | WU X W, LI X H, WANG Z X, et al. Improvement on the storage performance of LiMn2O4 with the mixed additives of ethanolamine and heptamethyldisilazane[J]. Appl Surf Sci, 2013, 268: 349-354. |
| 29 | LI Y K, ZHANG R X, LIU J S, et al. Effect of heptamethyldisilazane as an additive on the stability performance of LiMn2O4 cathode for lithium-ion battery[J]. J Power Sources, 2009, 189(1): 685-688. |
| 30 | SAIDI Y M, GAO F, BARKER J, et al. Additive to stabilize electrochemical cell: US, US5846673 A[P]. 1998. |
| 31 | TAKECHI K, KOIWAI A, SHIGA T. Nonaqueous electrolytic solution for battery and nonaqueous electrolytic solution battery: US, US6077628 A[P]. 2000. |
| 32 | ZHANG S S. A review on electrolyte additives for lithium-ion batteries[J]. J Power Sources, 2006, 162(2): 1379-1394. |
| 33 | JUNG G H, SUNG J L, JAEGI L, et al. Tunable and robust phosphite-derived surface film to protect lithium-rich cathodes in lithium-ion batteries[J]. ACS Appl Mater Interfaces, 2015, 7(15): 8319-8329. |
| 34 | WOTANGO A S, SU W N, LEGGESSE E G, et al. Improved interfacial properties of MCMB electrode by 1-(trimethylsilyl)imidazole as new electrolyte additive to suppress LiPF6 decomposition[J]. ACS Appl Mater Interfaces, 2017, 9(3): 2410-2420. |
| [1] | 陈兵帅, 卓海涛, 黄书, 陈少军. 高性能硅基负极聚合物粘结剂的研究进展[J]. 应用化学, 2023, 40(5): 625-639. |
| [2] | 胡方正, 高兴, 刘雷, 袁天恒, 曹宁, 李凯, 王亚涛, 李建华, 连慧琴, 汪晓东, 崔秀国. 锂离子电池黑磷负极的储能优势及其优化的研究进展[J]. 应用化学, 2023, 40(4): 571-582. |
| [3] | 王路飞, 甄蒙蒙, 沈伯雄. 贫电解液下电催化剂对调控锂硫电池性能的研究进展[J]. 应用化学, 2023, 40(2): 188-209. |
| [4] | 石雪建, 刘万强, 王春丽, 程勇, 王立民. 钾离子电池用Sb基负极材料研究进展[J]. 应用化学, 2023, 40(2): 210-228. |
| [5] | 王恩通, 杨林芳. 高比容量锂离子电池正极材料LiNi0.6Co0.2Mn0.2O2的制备及性能[J]. 应用化学, 2022, 39(8): 1209-1215. |
| [6] | 张琦, 张乾, 师晓梦, 孔娅淇, 高可心, 杜亚平. 稀土溴化物固态电解质材料在全固态电池中的应用研究进展[J]. 应用化学, 2022, 39(4): 585-598. |
| [7] | 王玉乐, 杨柯利, 高艳芳. 碳化钼改性二氧化硅的制备及其电化学性能[J]. 应用化学, 2022, 39(11): 1716-1725. |
| [8] | 赵莹, 邵奕嘉, 李罗钱, 任建伟, 廖世军. 富锂正极材料的衰减机理及循环稳定性提升的研究进展[J]. 应用化学, 2022, 39(02): 205-222. |
| [9] | 王旭尧, 方应军, 张灵志. 氰基功能化聚乙烯亚胺交联固态聚合物电解质的制备及理化性能[J]. 应用化学, 2021, 38(8): 946-953. |
| [10] | 陶承业, 杨占旭, 李玥. 红磷/石墨复合材料的制备及电化学性能[J]. 应用化学, 2020, 37(9): 1056-1061. |
| [11] | 刘学, 马华, 徐恒, 计海聪, 王栋. 无纺布基聚吡咯柔性电极的储锂性能[J]. 应用化学, 2020, 37(5): 555-561. |
| [12] | 王金莹, 曲江英, 李杰兰, 汤占磊, 臧云浩, 王涛, 顾建峰, 周钢, 高峰. 二次包覆法制备煤沥青基硅/碳复合物及其锂离子电池性能[J]. 应用化学, 2020, 37(5): 562-569. |
| [13] | 胡晨,金翼,朱少青,徐晔,水江澜. 磷酸铁锂电池低温性能的改性方法简述[J]. 应用化学, 2020, 37(4): 380-386. |
| [14] | 崔华敏, 掌学谦, 胡攀登, 闫冰, 黄苇苇, 郭文锋. 杯[4]醌/N掺杂的无定形碳纳米纤维复合材料储锂性能[J]. 应用化学, 2020, 37(2): 198-204. |
| [15] | 惠康龙, 傅继澎, 高湉, 唐明学. 金属硫化物在可充电电池中的研究进展[J]. 应用化学, 2020, 37(12): 1384-1402. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 C
C O)基化合物
O)基化合物 C
C O)-based compounds
O)-based compounds C
C O中的N原子与H原子结合形成络合物,不仅清除了副产物HF和PF5,还参与生成优良的界面膜
O中的N原子与H原子结合形成络合物,不仅清除了副产物HF和PF5,还参与生成优良的界面膜 C
C O combine with H atoms to form complexes, which not only remove the by-products HF and PF5, but also participate in the formation of the excellent interfacial film
O combine with H atoms to form complexes, which not only remove the by-products HF and PF5, but also participate in the formation of the excellent interfacial film