
应用化学 ›› 2023, Vol. 40 ›› Issue (8): 1094-1108.DOI: 10.19894/j.issn.1000-0518.230108
过渡金属磷酸盐电解水催化剂调控策略研究进展
- 中国地质大学(武汉)材料与化学学院,湖北省氢能创新中心,武汉 430000
-
收稿日期:2023-04-17接受日期:2023-07-07出版日期:2023-08-01发布日期:2023-08-24 -
通讯作者:蔡卫卫 -
基金资助:国家自然科学基金(22179121)
Research Progress in Regulation Strategy of Transition Metal Phosphate Catalyst for Electrochemical Water Splitting
Ying-Hua GUO, Shun-Fa ZHOU, Jing LI, Wei-Wei CAI( )
)
- Hydrogen Energy Technology Innovation Center of Hubei Province,Faculty of Materials Science and Chemistry,China University of Geosciences,Wuhan 430074,China
-
Received:2023-04-17Accepted:2023-07-07Published:2023-08-01Online:2023-08-24 -
Contact:Wei-Wei CAI -
About author:caiww@cug.edu.cn
-
Supported by:the National Natural Science Foundation of China(22179121)
摘要:
长期以来,过渡金属磷酸盐因其安全清洁、低廉高效的优点在电解水催化剂领域备受研究者们的关注。磷酸盐中的磷酸基团具有独特的原子几何结构、较强的协调性以及多种取向性,使其有利于稳定过渡金属的中间价态并加速质子传导速率。然而其电导率差、孔隙率低的缺点则促使研究者们探究设计更加高效的过渡金属磷酸盐电催化剂。虽然科研人员为此投入了大量的时间和精力,在过渡金属磷酸盐电催化剂高效开发利用上仍有许多问题亟待解决。在此,结合过渡金属磷酸盐电催化剂近10年的最新研究进展,重点从形貌调控、缺陷工程和界面工程等几方面介绍了近几年科研工作者对于磷酸盐的开发设计策略。同时,从科学研究及实际应用方面讨论了该类催化剂在未来材料领域需要面对的机遇与挑战。
中图分类号:
引用本文
郭颖华, 周顺发, 李静, 蔡卫卫. 过渡金属磷酸盐电解水催化剂调控策略研究进展[J]. 应用化学, 2023, 40(8): 1094-1108.
Ying-Hua GUO, Shun-Fa ZHOU, Jing LI, Wei-Wei CAI. Research Progress in Regulation Strategy of Transition Metal Phosphate Catalyst for Electrochemical Water Splitting[J]. Chinese Journal of Applied Chemistry, 2023, 40(8): 1094-1108.
| Catalyst | Electrolyte | Overpotential (η10)/V | Tafel slope/(mV·dec-1) | Ref. |
|---|---|---|---|---|
| Fe0.72Co0.42PO4/Ni* | 1.0 mol/L KOH | 77 | 80.7 | [ |
| δ-FeOOH NSs/NF | 1.0 mol/L KOH | 265 | 36 | [ |
| CoPO/NF* | 1.0 mol/L KOH | 116 | 65.6 | [ |
| S-doped Co-Fe-Pi | 1.0 mol/L KOH | 273 | 40 | [ |
| Gly-NCP | 1.0 mol/L KOH 1.0 mol/L KOH+0.5 mol/L NaCl | 265 252 | 57 39 | [ |
| NiFeP/Pi | 0.1 mol/L KOH | 210 | 39 | [ |
| α-ZrP | 0.1 mol/L KOH | 450 | 53.4 | [ |
| HEPi | 1.0 mol/L KOH | 270 | 74 | [ |
Ni5P4@ Ni2+δ O δ (OH)2-δ* | 1.0 mol/L KOH Seawater 0.5 mol/L H2SO4 | 87 144 66 | 69 108 33 | [ |
| Nd2O3∶NdPO4* | 0.5 mol/L H2SO4 | 134 | 55.6 | [ |
| CoFeP/NF | 1.0 mol/L KOH | 253 | 36 | [ |
| CoFePi | 1.0 mol/L KOH | 225 | 34 | [ |
| (Fe x Co y )P2O7@N-C | 1.0 mol/L KOH | 341 | 34.9 | [ |
| FMZP4* | 1.0 mol/L KOH | 53 | 53.2 | [ |
| RuFeP-NCs/CNF* | 0.5 mol/L H2SO4 1.0 mol/L KOH | 65.8 16.0 | 50 90.24 | [ |
| NiCo-2.0 | 1.0 mol/L KOH | 320 | 84 | [ |
| Co7Fe3-P/C | 1.0 mol/L KOH | 260 | 37.8 | [ |
| Ni-Co-TEP-600 | 1.0 mol/L KOH | 310 | 68 | [ |
| CoPiNF-800 | 1.0 mol/L KOH | 222 (η100) | 62 | [ |
| NiCoPO@NC/P-NF-e | 1.0 mol/L KOH | 221 | 87.8 | [ |
| FPO3/NF | 1.0 mol/L KOH | 309 | 96.9 | [ |
| A-Ni2P/Cu3P | 1.0 mol/L KOH | 262 | 78.1 | [ |
| NiCoPi/Ni-P/NiCoPi | 1.0 mol/L KOH | 234 | 87 | [ |
| Ni∶Pi-Fe/NF | 1.0 mol/L KOH | 220 | 37 | [ |
| Co-Zn-P/NiFoam | 1.0 mol/L KOH | 307 | 56.6 | [ |
| NiFeO x H y /NF-0H | 1.0 mol/L KOH | 205 (η50) | 30 | [ |
| Ni3Fe-LDHs@CoP x /NF | 1 mol/L phosphate buffer+0.5 mol/L NaCl | 370 | 76 | [ |
| NiCoFe phosphate NSs/NF | 1.0 mol/L KOH | 240 | 58 | [ |
| Ni(PO3)2-CoP4/CoMoO4/NF* | 1.0 mol/L KOH | 79 (η100) | 27.75 | [ |
| De-LCoFeP/rGO | 0.1 mol/L KOH | 270 | 57.5 | [ |
| NiMo-Fe-P | 1.0 mol/L KOH | 215.2 | 37.52 | [ |
表1 过渡金属磷酸盐电解水催化剂最新研究结果
Table 1 Latest research results of transition metal phosphate electrolytic water catalyst
| Catalyst | Electrolyte | Overpotential (η10)/V | Tafel slope/(mV·dec-1) | Ref. |
|---|---|---|---|---|
| Fe0.72Co0.42PO4/Ni* | 1.0 mol/L KOH | 77 | 80.7 | [ |
| δ-FeOOH NSs/NF | 1.0 mol/L KOH | 265 | 36 | [ |
| CoPO/NF* | 1.0 mol/L KOH | 116 | 65.6 | [ |
| S-doped Co-Fe-Pi | 1.0 mol/L KOH | 273 | 40 | [ |
| Gly-NCP | 1.0 mol/L KOH 1.0 mol/L KOH+0.5 mol/L NaCl | 265 252 | 57 39 | [ |
| NiFeP/Pi | 0.1 mol/L KOH | 210 | 39 | [ |
| α-ZrP | 0.1 mol/L KOH | 450 | 53.4 | [ |
| HEPi | 1.0 mol/L KOH | 270 | 74 | [ |
Ni5P4@ Ni2+δ O δ (OH)2-δ* | 1.0 mol/L KOH Seawater 0.5 mol/L H2SO4 | 87 144 66 | 69 108 33 | [ |
| Nd2O3∶NdPO4* | 0.5 mol/L H2SO4 | 134 | 55.6 | [ |
| CoFeP/NF | 1.0 mol/L KOH | 253 | 36 | [ |
| CoFePi | 1.0 mol/L KOH | 225 | 34 | [ |
| (Fe x Co y )P2O7@N-C | 1.0 mol/L KOH | 341 | 34.9 | [ |
| FMZP4* | 1.0 mol/L KOH | 53 | 53.2 | [ |
| RuFeP-NCs/CNF* | 0.5 mol/L H2SO4 1.0 mol/L KOH | 65.8 16.0 | 50 90.24 | [ |
| NiCo-2.0 | 1.0 mol/L KOH | 320 | 84 | [ |
| Co7Fe3-P/C | 1.0 mol/L KOH | 260 | 37.8 | [ |
| Ni-Co-TEP-600 | 1.0 mol/L KOH | 310 | 68 | [ |
| CoPiNF-800 | 1.0 mol/L KOH | 222 (η100) | 62 | [ |
| NiCoPO@NC/P-NF-e | 1.0 mol/L KOH | 221 | 87.8 | [ |
| FPO3/NF | 1.0 mol/L KOH | 309 | 96.9 | [ |
| A-Ni2P/Cu3P | 1.0 mol/L KOH | 262 | 78.1 | [ |
| NiCoPi/Ni-P/NiCoPi | 1.0 mol/L KOH | 234 | 87 | [ |
| Ni∶Pi-Fe/NF | 1.0 mol/L KOH | 220 | 37 | [ |
| Co-Zn-P/NiFoam | 1.0 mol/L KOH | 307 | 56.6 | [ |
| NiFeO x H y /NF-0H | 1.0 mol/L KOH | 205 (η50) | 30 | [ |
| Ni3Fe-LDHs@CoP x /NF | 1 mol/L phosphate buffer+0.5 mol/L NaCl | 370 | 76 | [ |
| NiCoFe phosphate NSs/NF | 1.0 mol/L KOH | 240 | 58 | [ |
| Ni(PO3)2-CoP4/CoMoO4/NF* | 1.0 mol/L KOH | 79 (η100) | 27.75 | [ |
| De-LCoFeP/rGO | 0.1 mol/L KOH | 270 | 57.5 | [ |
| NiMo-Fe-P | 1.0 mol/L KOH | 215.2 | 37.52 | [ |
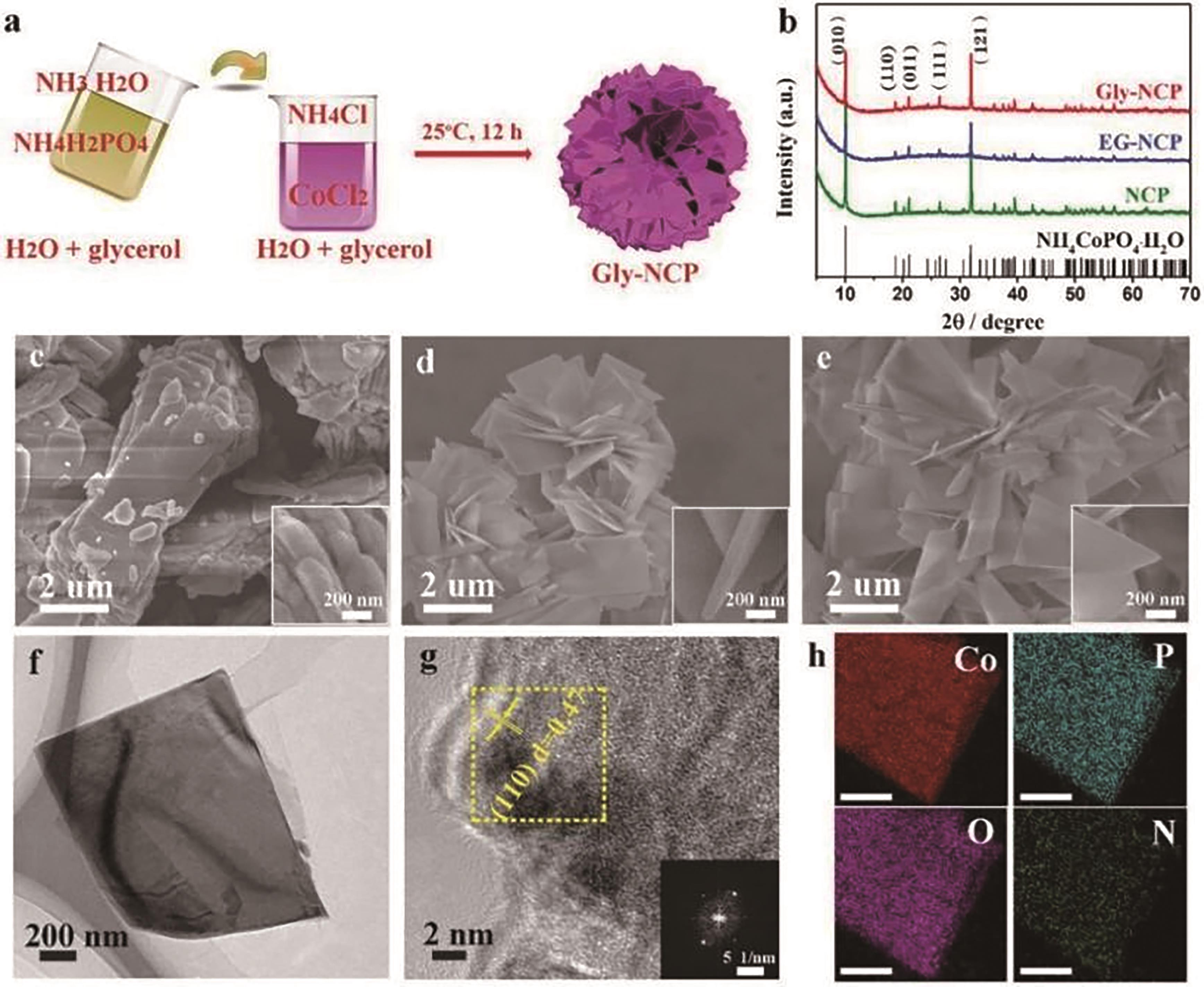
图2 NCP、EG-NCP和Gly-NCP材料的合成和微观表征。?(a) Gly-NCP纳米片的合成工艺示意图。(b) NCP、EG-NCP和Gly-NCP样品的XRD图谱。(c) NCP、(d) EG-NCP和(e) Gly-NCP在的SEM图像。(f)从黄色矩形区域获得的TEM图像, (g) HRTEM图像和SAED图(插图), (h) Gly-NCP纳米片的EDS元素图[57]
Fig.2 Synthesis and microscopic characterization of NCP, EG-NCP and Gly-NCP materials. (a) Schematic diagram of the synthesis process of Gly-NCP nanosheets. (b) XRD pattern of NCP, EG-NCP and Gly-NCP sample. (c) SEM images of NCP, (d) EG-NCP and (e) Gly-NCP. (f) TEM images obtained from yellow rectangular areas, (g) HRTEM images and SAED diagrams (illustrations), and (h) EDS element diagrams of Gly-NCP nanosheets[57]
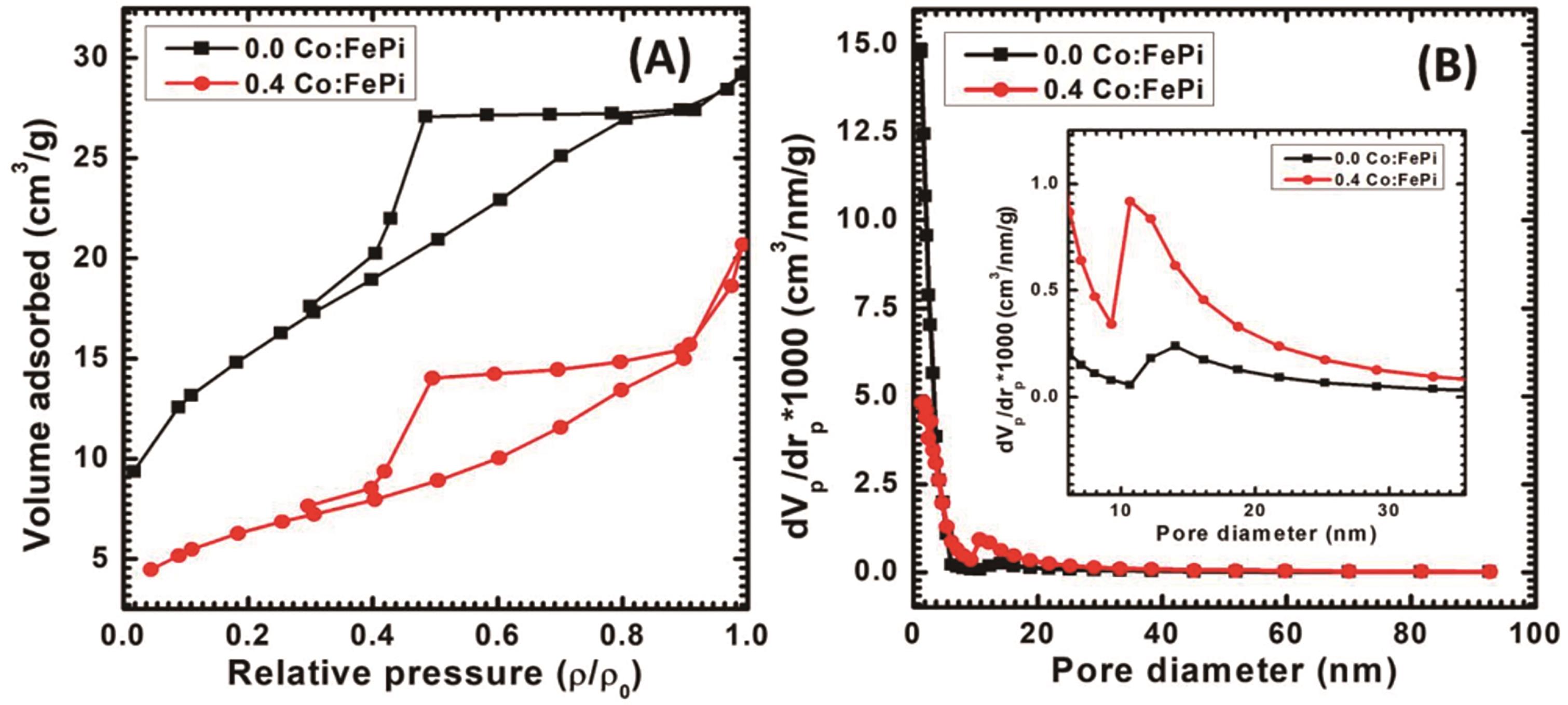
图4 0.0 Co∶FePi和0.4 Co∶FePi的(A)氮吸附-解吸等温线和相应的(B)孔径分布曲线[105]
Fig.4 Nitrogen adsorption-desorption isotherms and corresponding pore-size distribution of 0.0 Co∶FePi and 0.4 Co∶FePi[105]
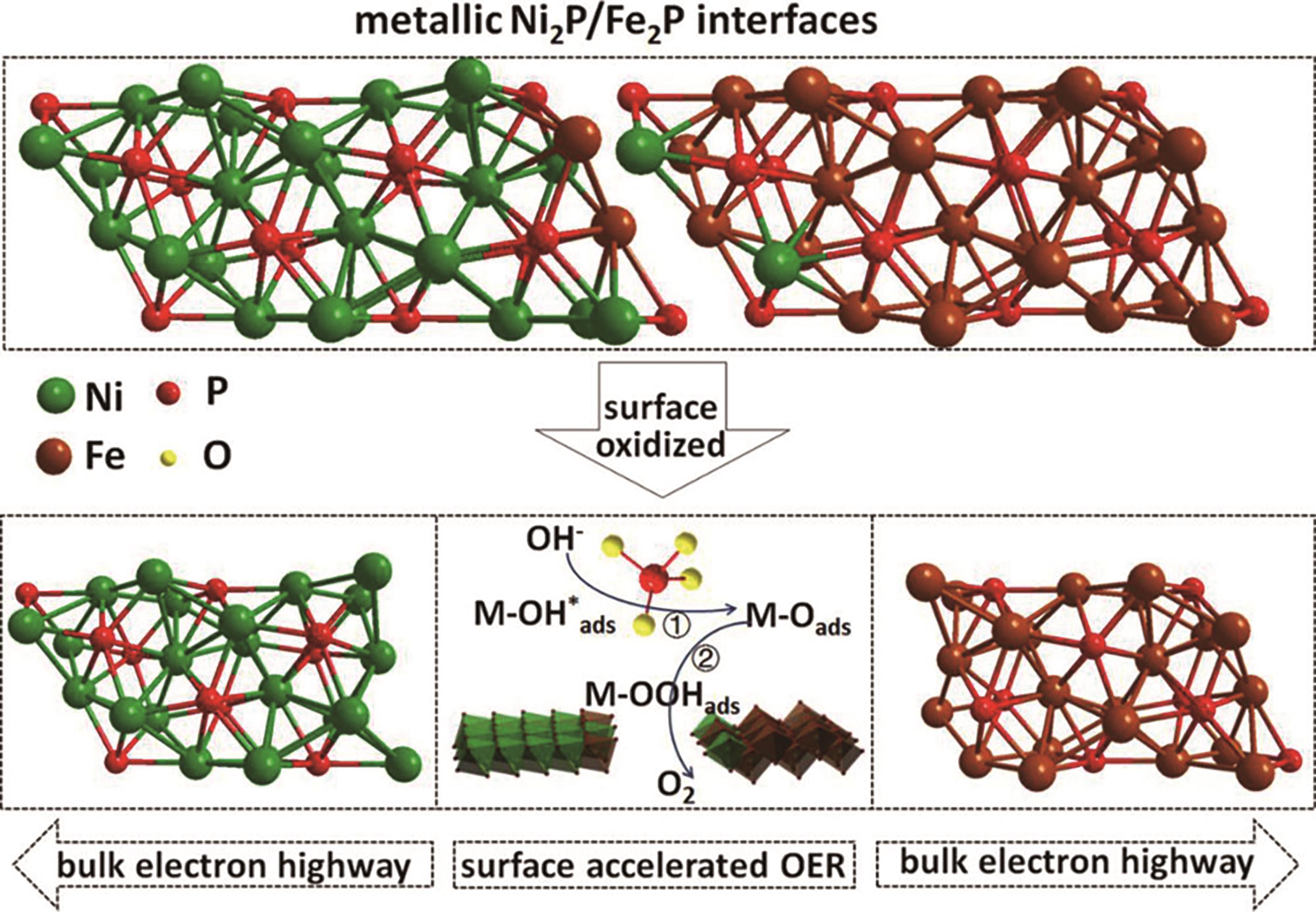
图5 具有活跃的表面氧化重组(掺杂氢氧化物和磷酸盐)和持久的导电磷化体Ni2P(O)/Fe2P(O)界面示意图[115]
Fig.5 Schematic diagram of Ni2P(O)/Fe2P(O) interface with active surface oxidation recombination (doped with hydroxide and phosphate) and durable conductive phosphide[115]
| 1 | SHRESTHA A, MUSTAFA A A, HTIKE M M, et al. Evolution of energy mix in emerging countries: modern renewable energy, traditional renewable energy, and non-renewable energy[J]. Renew Energy, 2022, 199: 419-432. |
| 2 | SCHEFFE J R, HAUSSENER S, PATZKE G R. Solar hydrogen production[J]. Energy Technol, 2022, 10(1): 2101021. |
| 3 | DOS SANTOS K G, ECKERT C T, DE ROSSI E, et al. Hydrogen production in the electrolysis of water in Brazil, a review[J]. Renew Sustainable Energy Rev, 2017, 68: 563-571. |
| 4 | ZHANG Q, JIAO S, WANG B, et al. Accelerate the alkaline hydrogen evolution reaction of the heterostructural Ni2P@Ni(OH)2/NF by dispersing a trifle of Ru on the surface[J]. Int J Hydrogen Energy, 2021, 46(52): 26329-26339. |
| 5 | SORSA O, BACKHOUSE R, SAXELIN S, et al. Optimization and aging of Pt nanowires supported on single-walled carbon nanotubes as a cathode catalyst in polymer electrolyte membrane water electrolyser[J]. Int J Hydrogen Energy, 2020, 45(38): 19121-19132. |
| 6 | HUANG J, JIANG Y. Tailoring resource-efficient catalysts for sustainable energy and chemical processes[J]. ACS Sustainable Chem Eng, 2019, 7(7): 6423. |
| 7 | HERAVI M M, MOHAMMADI P. Layered double hydroxides as heterogeneous catalyst systems in the cross-coupling reactions: an overview[J]. Mol Diversity, 2022, 26(1): 569-587. |
| 8 | CHEN J, LI H, CHEN S, et al. Co-Fe-Cr(oxy) hydroxides as efficient oxygen evolution reaction catalysts[J]. Adv Energy Mater, 2021, 11(11): 2003412. |
| 9 | JOO J, KIM T, LEE J, et al. Morphology-controlled metal sulfides and phosphides for electrochemical water splitting[J]. Adv Mater, 2019, 31(14): e1806682. |
| 10 | DING X, YANG T, WEI W, et al. An in situ grown lanthanum sulfide/molybdenum sulfide hybrid catalyst for electrochemical hydrogen evolution[J]. Catal Sci Technol, 2020, 10(10): 3247-3254. |
| 11 | MOYER M M, KARAKAYA C, KEE R J, et al. In situ formation of metal carbide catalysts[J]. ChemCatChem, 2017, 9(16): 3090-3101. |
| 12 | MA Y, GUAN G, HAO X, et al. Molybdenum carbide as alternative catalyst for hydrogen production-a review[J]. Renew Sustainable Energy Rev, 2017, 75: 1101-1129. |
| 13 | WU Z, GAN Q, LI X, et al. Elucidating surface restructuring-induced catalytic reactivity of cobalt phosphide nanoparticles under electrochemical conditions[J]. J Phys Chem C, 2018, 122(5): 2848-2853. |
| 14 | ANANTHARAJ S, REDDY P N, KUNDU S. Core-oxidized amorphous cobalt phosphide nanostructures: an advanced and highly efficient oxygen evolution catalyst[J]. Inorg Chem, 2017, 56(3): 1742-1756. |
| 15 | LI X, XIAO X, LI Q, et al. Metal (M=Co, Ni) phosphate based materials for high-performance supercapacitors[J]. Inorg Chem Front, 2018, 5(1): 11-28. |
| 16 | LI C, MEI X, LAM F L Y, et al. Amorphous iron and cobalt based phosphate nanosheets supported on nickel foam as superior catalysts for hydrogen evolution reaction[J]. ACS Appl Energy Mater, 2018, 1(12): 6764-6768. |
| 17 | GUI L, MIAO X, LEI C, et al. Co3+-rich Na1.95CoP2O7 phosphates as efficient bifunctional catalysts for oxygen evolution and reduction reactions in alkaline solution[J]. Chemistry, 2019, 25(47): 11007-11014. |
| 18 | ESSWEIN A J, SURENDRANATH Y, REECE S Y, et al. Highly active cobalt phosphate and borate based oxygen evolving catalysts operating in neutral and natural waters[J]. Energy Environ Sci, 2011, 4(2): 499-504. |
| 19 | KING H J, BONKE S A, CHANG S L Y, et al. Engineering disorder into heterogenite-like cobalt oxides by phosphate doping: implications for the design of water-oxidation catalysts[J]. ChemCatChem, 2017, 9(3): 511-521. |
| 20 | GUO R, LAI X, HUANG J, et al. Phosphate-based electrocatalysts for water splitting: recent progress[J]. ChemElectroChem, 2018, 5(24): 3822-3834. |
| 21 | ZHAO H, YUAN Z Y. Design strategies of transition-metal phosphate and phosphonate electrocatalysts for energy-related reactions[J]. ChemSusChem, 2021, 14(1): 130-149. |
| 22 | ZHANG L, YE F, WU Z, et al. Carbonate-hydroxide induced metal-organic framework transformation strategy for honeycomb-like NiCoP nanoplates to drive enhanced pH-universal hydrogen evolution[J]. Small Methods, 2022, 6(8): e2200515. |
| 23 | DUAN R, LI Y, WANG S, et al. Fast and deep reconstruction of coprecipitated Fe phosphates on nickel foams for an alkaline oxygen evolution reaction[J]. J Phys Chem Lett, 2022, 13(6): 1446-1452. |
| 24 | LIU S, ZAHARIEVA I, D'AMARIO L, et al. Electrocatalytic water oxidation at neutral pH-deciphering the rate constraints for an amorphous cobalt-phosphate catalyst system[J]. Adv Energy Mater, 2022, 12(46): 2202914. |
| 25 | NAITO T, SHINAGAWA T, NISHIMOTO T, et al. Water electrolysis in saturated phosphate buffer at neutral pH[J]. ChemSusChem, 2020, 13(22): 5921-5933. |
| 26 | LIU H, CAO S, ZHANG J, et al. Facile control of surface reconstruction with Co2+ or Co3+-rich (oxy)hydroxide surface on ZnCo phosphate for large-current-density hydrogen evolution in alkali[J]. Mater Today Phys, 2021, 20: 100448. |
| 27 | CHEN J, JAYABAL S, GENG D, et al. Stable water oxidation catalysts based on in-situ electrochemical transition of nickel phosphate[J]. Catal Lett, 2021, 152(8): 2333-2341. |
| 28 | ZHAO H, YUAN Z Y. Insights into transition metal phosphate materials for efficient electrocatalysis[J]. ChemCatChem, 2020, 12(15): 3797-810. |
| 29 | ZHOU Q, LIAO L, ZHOU H, et al. Innovative strategies in design of transition metal-based catalysts for large-current-density alkaline water/seawater electrolysis[J]. Mater Today Phys, 2022, 26: 100727. |
| 30 | ZHOU B, GAO R, ZOU J J, et al. Surface design strategy of catalysts for water electrolysis[J]. Small, 2022, 18(27): e2202336. |
| 31 | CHEN L, REN J T, YUAN Z Y. Design strategies of phosphorus-containing catalysts for photocatalytic, photoelectrochemical and electrocatalytic water splitting[J]. Green Chem, 2022, 24(2): 713-747. |
| 32 | CHOI S, PARK Y, YANG H, et al. Vacancy-engineered catalysts for water electrolysis[J]. CrystEngComm, 2020, 22(9): 1500-1513. |
| 33 | ZHANG R, PEARCE P E, DUAN Y, et al. Importance of water structure and catalyst-electrolyte interface on the design of water splitting catalysts[J]. Chem Mater, 2019, 31(20): 8248-8259. |
| 34 | CHINNADURAI D, NALLAL M, KIM H J, et al. Mn3+ active surface site enriched manganese phosphate nano-polyhedrons for enhanced bifunctional oxygen electrocatalyst[J]. ChemCatChem, 2020, 12(8): 2348-2355. |
| 35 | GOND R, SADA K, SENTHILKUMAR B, et al. Bifunctional electrocatalytic behavior of sodium cobalt phosphates in alkaline solution[J]. ChemElectroChem, 2018, 5(1): 153-158. |
| 36 | ZHANG R, VAN STRAATEN G, DI PALMA V, et al. Electrochemical activation of atomic layer-deposited cobalt phosphate electrocatalysts for water oxidation[J]. ACS Catal, 2021, 11(5): 2774-2785. |
| 37 | FANG Z, PENG L, QIAN Y, et al. Dual tuning of Ni-Co-A (A=P, Se, O) nanosheets by anion substitution and holey engineering for efficient hydrogen evolution[J]. J Am Chem Soc, 2018, 140(15): 5241-5247. |
| 38 | WU R, XIAO B, GAO Q, et al. Inside back cover: a janus nickel cobalt phosphide catalyst for high-efficiency neutral-pH water splitting[J]. Angew Chem Int Ed, 2018, 57(47): 15607. |
| 39 | LUO F, ZHANG Q, YU X, et al. Palladium phosphide as a stable and efficient electrocatalyst for overall water splitting[J]. Angew Chem, 2018, 57(45): 14862-14867. |
| 40 | LI L, LU X, CHEN W, et al. A new strategy to hydrothermally synthesize olivine phosphates[J]. Chem Commun, 2019, 55(80): 12092-12095. |
| 41 | VECSTAUDZA J, LOCS J. Novel preparation route of stable amorphous calcium phosphate nanoparticles with high specific surface area[J]. J Alloys Compd, 2017, 700: 215-222. |
| 42 | CAO Y, CHEN Z, YE F, et al. One-step synthesis of amorphous NiCoP nanoparticles by electrodeposition as highly efficient electrocatalyst for hydrogen evolution reaction in alkaline solution[J]. J Alloys Compd, 2022, 896: 163103. |
| 43 | CHINNADURAI D, RAJENDIRAN R, LI O L, et al. Mn-Co bimetallic phosphate on electrodeposited PANI nanowires with composition modulated structural morphology for efficient electrocatalytic water splitting[J]. Appl Catal B, 2021, 292: 120202. |
| 44 | KIM J, KIM H, MYUNG S T, et al. Exceptional effect of glassy lithium fluorophosphate on Mn-rich olivine cathode material for high-performance Li ion batteries[J]. J Power Sources, 2018, 374: 55-60. |
| 45 | YIN D, JIN Z, LIU M, et al. Microwave-assisted synthesis of the cobalt-iron phosphates nanosheets as an efficient electrocatalyst for water oxidation[J]. Electrochim Acta, 2018, 260: 420-429. |
| 46 | LIU Y, YANG D, LIU Z, et al. Nickel foam supported cobalt phosphate electrocatalyst for alkaline oxygen evolution reaction[J]. J Power Sources, 2020, 461: 228165. |
| 47 | WANG Z, ZHANG X, WU X, et al. High-entropy phosphate/C hybrid nanosheets for efficient acidic hydrogen evolution reaction[J]. Chem Eng J, 2022, 437: 135375. |
| 48 | GUO T, ZHANG L, YUN S, et al. One-step synthesis of bimetallic Ni-Fe phosphates and their highly electrocatalytic performance for water oxidation[J]. Mater Res Bull, 2019, 114: 80-84. |
| 49 | WANG X, FENG Z, HUANG J, et al. Graphene-decorated carbon-coated LiFePO4 nanospheres as a high-performance cathode material for lithium-ion batteries[J]. Carbon, 2018, 127: 149-157. |
| 50 | WEI S, YAO J, SHI B. 1D highly porous Li3V2(PO4)3/C nanofibers as superior high-rate and ultralong cycle-life cathode material for electrochemical energy storage[J]. Solid State Ionics, 2017, 305: 36-42. |
| 51 | YUAN C Z, JIANG Y F, WANG Z, et al. Cobalt phosphate nanoparticles decorated with nitrogen-doped carbon layers as highly active and stable electrocatalysts for the oxygen evolution reaction[J]. J Mater Chem A, 2016, 4(21): 8155-8160. |
| 52 | KIM H, PARK J, PARK I, et al. Coordination tuning of cobalt phosphates towards efficient water oxidation catalyst[J]. Nat Commun, 2015, 6: 8253. |
| 53 | CHENG Q, ZHAO X, YANG G, et al. Recent advances of metal phosphates-based electrodes for high-performance metal ion batteries[J]. Energy Storage Mater, 2021, 41: 842-882. |
| 54 | JIANG S, ZHU L, YANG Z, et al. Enhanced electrocatalytic performance of FeNiCoP amorphous alloys as oxygen-evolving catalysts for electrolytic water splitting application[J]. Electrochim Acta, 2021, 368: 137618. |
| 55 | GUO X, LI N, CHENG Y, et al. General synthesis of nitrogen-doped metal (M=Co2+, Mn2+, Ni2+, or Cu2+) phosphates[J]. Chem Eng J, 2021, 411: 128544. |
| 56 | YUE Q, GAO T, WU Y, et al. S-doped Co-Fe-Pi nanosheets as highly efficient oxygen evolution electrocatalysts in alkaline media[J]. Electrochim Acta, 2020, 362: 137123. |
| 57 | SONG Z, WANG K C, SUN Q, et al. High-performance ammonium cobalt phosphate nanosheet electrocatalyst for alkaline saline water oxidation[J]. Adv Sci, 2021, 8(14): 2100498. |
| 58 | THAKUR N, KUMAR M, MANDAL D, et al. Nickel iron phosphide/phosphate as an oxygen bifunctional electrocatalyst for high-power-density rechargeable Zn-air batteries[J]. ACS Appl Mater Interfaces, 2021,19(44):52487-52497. |
| 59 | RAMOS-GARC S M V, SANCHEZ J, DEL TORO-PEDROSA D E, et al. Transition metal-modified exfoliated zirconium phosphate as an electrocatalyst for the oxygen evolution reaction[J]. ACS Appl Energ Mater, 2019, 2(5): 3561-3567. |
| 60 | QIAO H, WANG X, DONG Q, et al. A high-entropy phosphate catalyst for oxygen evolution reaction[J]. Nano Energy, 2021, 86: 106029. |
| 61 | HUANG Y, HU L, LIU R, et al. Nitrogen treatment generates tunable nanohybridization of Ni5P4 nanosheets with nickel hydr(oxy)oxides for efficient hydrogen production in alkaline, seawater and acidic media[J]. Appl Catal B: Environ, 2019, 251: 181-194. |
| 62 | MAJHI K C, YADAV M. Neodymium oxide doped neodymium phosphate as efficient electrocatalyst towards hydrogen evolution reaction in acidic medium[J]. J Environ Chem Eng, 2022, 10(3): 107416. |
| 63 | XIONG D, LU C, CHEN C, et al. CoFeP nanocube-arrays based on Prussian blue analogues for accelerated oxygen evolution electrocatalysis[J]. J Power Sources, 2022, 520: 230884. |
| 64 | ZOU Y, LIU Z, LIU R, et al. Disordered CoFePi nanosheets with rich vacancies as oxygen evolving electrocatalysts: insight into the local atomic environment[J]. J Power Sources, 2019, 427: 215-222. |
| 65 | ZHAO D, SHAO Q, ZHANG Y, et al. N-doped carbon shelled bimetallic phosphates for efficient electrochemical overall water splitting[J]. Nanoscale, 2018, 10(48): 22787-22791. |
| 66 | HUANG L, YAO R, WANG X, et al. In situ phosphating of Zn-doped bimetallic skeletons as a versatile electrocatalyst for water splitting[J]. Energy Environ Sci, 2022, 15(6): 2425-2434. |
| 67 | YANG B, XU J, BIN D, et al. Amorphous phosphatized ruthenium-iron bimetallic nanoclusters with Pt-like activity for hydrogen evolution reaction[J]. Appl Catal B: Environ, 2021, 283: 119583. |
| 68 | SEPTIANI N L W, KANETI Y V, FATHONI K B, et al. Tailorable nanoarchitecturing of bimetallic nickel-cobalt hydrogen phosphate via the self-weaving of nanotubes for efficient oxygen evolution[J]. J Mater Chem A, 2020, 8(6): 3035-3047. |
| 69 | PENG Z, HU L, SHI J, et al. Gap channel carbon layer coated bimetallic CoFe phosphate as a bifunctional electrocatalyst for overall water splitting[J]. J Power Sources, 2022, 538: 231571. |
| 70 | SEPTIANI N L W, KANETI Y V, FATHONI K B, et al. Self-assembly of two-dimensional bimetallic nickel-cobalt phosphate nanoplates into one-dimensional porous chainlike architecture for efficient oxygen evolution reaction[J]. Chem Mater, 2020, 32(16): 7005-7018. |
| 71 | ZHU C, YU Z, LIN T, et al. Structural design of cobalt phosphate on nickel foam for electrocatalytic oxygen evolution[J]. Nanotechnology, 2021, 32(30): 305702. |
| 72 | GAO H, WANG Y, ZHOU S, et al. Nickel-cobalt phosphate nanoparticles wrapped in nitrogen-doped carbon loading on partially phosphatized foamed nickel as efficient electrocatalyst for water splitting[J]. Chem Eng J, 2021, 426: 130854. |
| 73 | WANG D, XU Y, GUO X, et al. Nickel foam as conductive substrate enhanced low-crystallinity two-dimensional iron hydrogen phosphate for oxygen evolution reaction[J]. J Alloys Compd, 2021, 870: 159472. |
| 74 | WAN H, MA R, LIU X, et al. Rare cobalt-based phosphate nanoribbons with unique 5-coordination for electrocatalytic water oxidation[J]. ACS Energy Lett, 2018, 3(6): 1254-1260. |
| 75 | LI C, MEI X, LAM F L Y, et al. Hybridizing amorphous nickel cobalt phosphate and nickel phosphide as an efficient bifunctional nanocatalyst towards overall water splitting[J]. Catal Today, 2020, 358: 215-220. |
| 76 | LI Y, ZHAO C. Iron-doped nickel phosphate as synergistic electrocatalyst for water oxidation[J]. Chem Mater, 2016, 28(16): 5659-5666. |
| 77 | QIAN L, MIAO Y. Nanosheet organized flower-like Co/Zn phosphate on nickel foam for efficient water splitting in both acid and basic solutions[J]. Polyhedron, 2019, 160: 213-218. |
| 78 | DUAN R, LI Y J, WANG S, et al. Effects of phosphate precursors on morphology and oxygen evolution reaction activity of NiFe (oxy)hydroxide on nickel foams[J]. T Nonferr Metal Soc, 2022, 32(12): 4050-4061. |
| 79 | LI T, ZHAO X, GETAYE SENDEKU M, et al. Phosphate-decorated Ni3Fe-LDHs@CoPx nanoarray for near-neutral seawater splitting[J]. Chem Eng J, 2023, 460: 141413. |
| 80 | SIAL M, LIN H, WANG X. Microporous 2D NiCoFe phosphate nanosheets supported on Ni foam for efficient overall water splitting in alkaline media[J]. Nanoscale, 2018, 10(27): 12975-12980. |
| 81 | YANG M, ZHANG S, WANG T, et al. Multiple interface Ni(PO3)2-CoP4/CoMoO4 nanorods for highly efficient hydrogen evolution in alkaline water/seawater electrolysis[J]. ACS Sustainable Chem Eng, 2022, 10(37): 12423-12432. |
| 82 | LIU Y, WANG H, LIN D, et al. Electrochemical tuning of olivine-type lithium transition-metal phosphates as efficient water oxidation catalysts[J]. Energy Environ Sci, 2015, 8(6): 1719-1724. |
| 83 | ZHOU X, YANG T, ZI Y, et al. Self-supporting NiMo-Fe-P nanowire arrays as bifunctional catalysts for efficient overall water splitting[J]. Dalton trans, 2023, 52(11): 3508-3516. |
| 84 | LIU H, HUANG R, CHEN W, et al. Porous 2D cobalt-nickel phosphide triangular nanowall architecture assembled by 3D microsphere for enhanced overall water splitting[J]. Appl Surf Sci, 2021, 569: 150762. |
| 85 | AI G, MO R, LI H, et al. Cobalt phosphate modified TiO2 nanowire arrays as co-catalysts for solar water splitting[J]. Nanoscale, 2015, 7(15): 6722-6728. |
| 86 | LI X, ZHA Q, NI Y. Ni-Fe phosphate/Ni foam electrode: facile hydrothermal synthesis and ultralong oxygen evolution reaction durability[J]. ACS Sustainable Chem Eng, 2019, 7(22): 18332-18340. |
| 87 | LIU Z, HE H, LIU Y, et al. Soft-template derived Ni/Mo2C hetero-sheet arrays for large current density hydrogen evolution reaction[J]. J Colloid Interface Sci, 2023, 635: 23-31. |
| 88 | ZHOU S, LIU Y, LI J, et al. Surface-neutralization engineered NiCo-LDH/phosphate hetero-sheets toward robust oxygen evolution reaction[J]. Green Energy Environ, 2022. doi.org/10.1016/j.gee.2022.12.003. |
| 89 | ZHANG D, HU L L, SUN Y G, et al. Construction of uniform transition-metal phosphate nanoshells and their potential for improving Li-ion battery performance[J]. J Mater Chem A, 2018, 6(19): 8992-8999. |
| 90 | ZHANG P, HE M, LI F, et al. Engineering bimetallic capture sites on hierarchically porous carbon electrode for efficient phosphate electrosorption: multiple active centers and excellent electrochemical properties[J]. J Mater Chem A, 2023, 11(2): 579-588. |
| 91 | BAU J A, TAKANABE K. Ultrathin microporous SiO2 membranes photodeposited on hydrogen evolving catalysts enabling overall water splitting[J]. ACS Catal, 2017, 7(11): 7931-7940. |
| 92 | AI J, JIN R, LIU Z, et al. Three-dimensionally ordered macroporous FeP self-supported structure for high-efficiency hydrogen evolution reaction[J]. Int J Hydrogen Energy, 2019, 44(12): 5854-5862. |
| 93 | HE M, ZHANG P, HUO S, et al. Remarkable phosphate electrosorption/desorption by bimetallic MOF-derived hierarchically porous carbon electrode: in-situ creation of multiple active centers and boosting electrochemical activities[J]. Chem Eng J, 2022, 446: 137396. |
| 94 | HUANG Z Q, LU W X, WANG B, et al. A mesoporous C,N-co doped Co-based phosphate ultrathin nanosheet derived from a phosphonate-based-MOF as an efficient electrocatalyst for water oxidation[J]. Catal Sci Technol, 2019, 9(17): 4718-4724. |
| 95 | AL-SHARIF M S, ARUNACHALAM P, ABITI T, et al. Mesoporous cobalt phosphate electrocatalyst prepared using liquid crystal template for methanol oxidation reaction in alkaline solution[J]. Arab J Chem, 2020, 13(1): 2873-2882. |
| 96 | CHE Q, XIE X, MA Q, et al. In-situ transformation of Co(OH)2 into NH4CoPO4·H2O on Co foil: 3D self-supported electrocatalyst with asymmetric local atomic and electronic structure for enhanced oxygen evolution reaction[J]. J Energy Chem, 2020, 51: 167-174. |
| 97 | WANG Y, ZHANG C, DU X, et al. Transition metal atom M (M=Fe, Co, Cu, Cr) doping and oxygen vacancy modulated M-Ni5P4-NiMOH nanosheets as multifunctional electrocatalysts for efficient overall water splitting and urea electrolysis reaction[J]. Dalton Trans, 2022, 51(39): 14937-14944. |
| 98 | XIANG C, JI Q, ZHANG G, et al. In situ creation of oxygen vacancies in porous bimetallic La/Zr sorbent for aqueous phosphate: hierarchical pores control mass transport and vacancy sites determine interaction[J]. Environ Sci Technol, 2020, 54(1): 437-445. |
| 99 | XIONG J, LI J, SHI J, et al. Metallic 1T-MoS2 nanosheets in-situ entrenched on N,P,S-codoped hierarchical carbon microflower as an efficient and robust electro-catalyst for hydrogen evolution[J]. Appl Catal B: Environ, 2019, 243: 614-620. |
| 100 | WANG J, TRAN D T, CHANG K, et al. Bifunctional catalyst derived from sulfur-doped VMoOx nanolayer shelled Co nanosheets for efficient water splitting[J]. ACS Appl Mater Interfaces, 2021, 13(36): 42944-42956. |
| 101 | SONG L, FAN H, FAN X, et al. A simultaneous phosphorization and carbonization strategy to synthesize a defective Co2P/doped-CNTs composite for bifunctional oxygen electrocatalysis[J]. Chem Eng J, 2022, 435: 134612. |
| 102 | LIU Z, XUE S, ZHOU S, et al. Mutual promotion effect of Ni and Mo2C encapsulated in N-doped porous carbon on bifunctional overall urea oxidation catalysis[J]. J Catal, 2022, 405: 606-613. |
| 103 | TROTOCHAUD L, YOUNG S L, RANNEY J K, et al. Nickel-iron oxyhydroxide oxygen-evolution electrocatalysts: the role of intentional and incidental iron incorporation[J]. J Am Chem Soc, 2014, 136(18): 6744-6753. |
| 104 | XIONG J, LI J, SHI J, et al. In situ engineering of double-phase interface in Mo/Mo2C heteronanosheets for boosted hydrogen evolution reaction[J]. ACS Energy Lett, 2018, 3(2): 341-348. |
| 105 | KHALATE S A, KADAM S A, MA Y R, et al. Cobalt doped iron phosphate thin film: an effective catalyst for electrochemical water splitting[J]. J Alloys Compd, 2021, 885: 160914. |
| 106 | LIU Z, LIU Y, HE H, et al. Valence regulation of Ru/Mo2C heterojunction for efficient acidic overall water splitting[J]. Electrochim Acta, 2023, 443: 141920. |
| 107 | SUN S, LIU Y, XU G, et al. Controllably constructed carbide/oxide heterointerfaces of molybdenum for efficient hydrogen evolution[J]. Fuel, 2023, 335: 127084. |
| 108 | CHENG X, PAN Z, LEI C, et al. A strongly coupled 3D ternary Fe2O3@Ni2P/Ni(PO3)2 hybrid for enhanced electrocatalytic oxygen evolution at ultra-high current densities[J]. J Mater Chem A, 2019, 7(3): 965-971. |
| 109 | ZENG F, LI J, HOFMANN J P, et al. Phosphate-assisted efficient oxygen evolution over finely dispersed cobalt particles supported on graphene[J]. Catal Sci Technol, 2021, 11(3): 1039-1048. |
| 110 | ZHAO H, WENG C C, REN J T, et al. Phosphonate-derived nitrogen-doped cobalt phosphate/carbon nanotube hybrids as highly active oxygen reduction reaction electrocatalysts[J]. Chin J Catal, 2020, 41(2): 259-267. |
| 111 | MIRGHNI A A, OYEDOTUN K O, FASAKIN O, et al. High-performance bimetallic Ni-Mn phosphate hybridized with 3-D graphene foam for novel hybrid supercapacitors[J]. J Energy Storage, 2020, 31: 101584. |
| 112 | ZHANG L, WANG Z, XU X, et al. Insights into the phosphate adsorption behavior onto 3D self-assembled cellulose/graphene hybrid nanomaterials embedded with bimetallic hydroxides[J]. Sci Total Environ, 2019, 653: 897-907. |
| 113 | MAHMOUD B A, MIRGHNI A A, FASAKIN O, et al. Bullet-like microstructured nickel ammonium phosphate/graphene foam composite as positive electrode for asymmetric supercapacitors[J]. RSC Adv, 2020, 10(28): 16349-16360. |
| 114 | LI D, BAYDOUN H, VERANI C N, et al. Efficient water oxidation using CoMnP nanoparticles[J]. J Am Chem Soc, 2016, 138(12): 4006-4009. |
| 115 | LIU P F, LI X, YANG S, et al. Ni2P(O)/Fe2P(O) interface can boost oxygen evolution electrocatalysis[J]. ACS Energy Lett, 2017, 2(10): 2257-2263. |
| 116 | ZHOU S, WANG J, LI J, et al. Surface-growing organophosphorus layer on layered double hydroxides enables boosted and durable electrochemical freshwater/seawater oxidatio[J]. Appl Catal B: Environ, 2023, 332: 122749. |
| 117 | JIA H, STARK J, ZHOU L Q, et al. Different catalytic behavior of amorphous and crystalline cobalt tungstate for electrochemical water oxidation[J]. RSC Adv, 2012, 2(29): 10874. |
| 118 | GE M, ZHANG X, XIA S, et al. Uniform formation of amorphous cobalt phosphate on carbon nanotubes for hydrogen evolution reaction†[J]. Chin J Chem, 2021, 39(8): 2113-2118. |
| 119 | EOM C J, SUNTIVICH J. In situ stimulated raman spectroscopy reveals the phosphate network in the amorphous cobalt oxide catalyst and its role in the catalyst formation[J]. J Phys Chem C, 2019, 123(48): 29284-29290. |
| 120 | YANG L, GUO Z, HUANG J, et al. Vertical growth of 2D amorphous FePO4 nanosheet on Ni foam: outer and inner structural design for superior water splitting[J]. Adv Mater, 2017, 29(46): 1704574. |
| 121 | OSTOVARI MOGHADDAM A, TROFIMOV E A. Toward expanding the realm of high entropy materials to platinum group metals: a review[J]. J Alloys Compd, 2021, 851: 156838. |
| 122 | XIE L, ZHANG R, CUI L, et al. High-performance electrolytic oxygen evolution in neutral media catalyzed by a cobalt phosphate nanoarray[J]. Angew Chem, 2017, 56(4): 1064-1068. |
| 123 | QI J, XIE J, WEI Z, et al. Modulation of crystal water in cobalt phosphate for promoted water oxidation[J]. Chem Commun, 2020, 56(33): 4575-4578. |
| [1] | 罗二桂, 唐涛, 王艺, 张俊明, 常宇虹, 胡天军, 贾建峰. 两电子氧还原制备过氧化氢:贵金属催化剂的几何与电子结构调控的研究进展[J]. 应用化学, 2023, 40(8): 1063-1076. |
| [2] | 董以宁, 李赫, 宫雪, 韩策, 宋平, 徐维林. 非Pt基催化剂在质子交换膜燃料电池阴极氧还原反应中的研究进展[J]. 应用化学, 2023, 40(8): 1077-1093. |
| [3] | 钱音男, 石钏, 张卫, 罗兆艳. 酸性环境下析氧反应Ir、Ru贵金属电催化剂的研究进展[J]. 应用化学, 2023, 40(8): 1126-1139. |
| [4] | 王伟, 李家源. 电解水析氢反应磷化钴异质结催化剂的研究进展[J]. 应用化学, 2023, 40(8): 1175-1186. |
| [5] | 郑锐雪, 孟庆磊, 张丽, 刘长鹏, 邢巍, 肖梅玲. 分级孔结构的Fe-N-C催化剂用于高效电催化氧还原[J]. 应用化学, 2023, 40(8): 1187-1194. |
| [6] | 刘嘉欣, 范家禾, 李曙辉, 马亮. Rh@Pt内凹立方体核壳催化剂的制备及其乙醇电氧化性能[J]. 应用化学, 2023, 40(8): 1195-1204. |
| [7] | 张毅城, 查飞, 唐小华, 常玥, 田海锋, 郭效军. 非均相催化制备有机过氧化物的研究进展[J]. 应用化学, 2023, 40(6): 769-788. |
| [8] | 于宜辰, 张雨宸, 张耀远, 吴芹, 史大昕, 陈康成, 黎汉生. 本体金属氧化物在丙烷无氧脱氢中的研究进展[J]. 应用化学, 2023, 40(6): 789-805. |
| [9] | 李冰, 刘军辉, 宋亚坤, 李想, 郭旭明, 熊健. 金属-有机骨架材料在催化氨硼烷水解释氢中的研究进展[J]. 应用化学, 2023, 40(3): 329-340. |
| [10] | 王路飞, 甄蒙蒙, 沈伯雄. 贫电解液下电催化剂对调控锂硫电池性能的研究进展[J]. 应用化学, 2023, 40(2): 188-209. |
| [11] | 曹蓉, 夏杰桢, 廖漫华, 赵路超, 赵晨, 吴琪. 单原子催化剂在电化学合成氨中的理论研究进展[J]. 应用化学, 2023, 40(1): 9-23. |
| [12] | 张丹, 尚润梅, 赵振涛, 李君华, 邢锦娟. V/Ce-Al2O3催化甲醇选择性氧化制备二甲氧基甲烷[J]. 应用化学, 2022, 39(9): 1429-1436. |
| [13] | 王显, 杨小龙, 马荣鹏, 刘长鹏, 葛君杰, 邢巍. 单原子分散的Ir-N-C燃料电池阳极抗中毒催化剂[J]. 应用化学, 2022, 39(8): 1202-1208. |
| [14] | 刘也, 郭少波, 梁艳莉, 葛红光, 马剑琪, 刘智峰, 刘波. 核壳型纳米复合材料CuFe2O4@NH2@Pt的制备及催化性能[J]. 应用化学, 2022, 39(8): 1237-1245. |
| [15] | 杜卫民, 刘欣, 朱琳, 付佳敏, 郭文山, 杨晓晴, 双培硕. 三元镍基硫属化物纳米棒阵列的简单合成及其高效的电催化析氧性能[J]. 应用化学, 2022, 39(8): 1252-1261. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||

