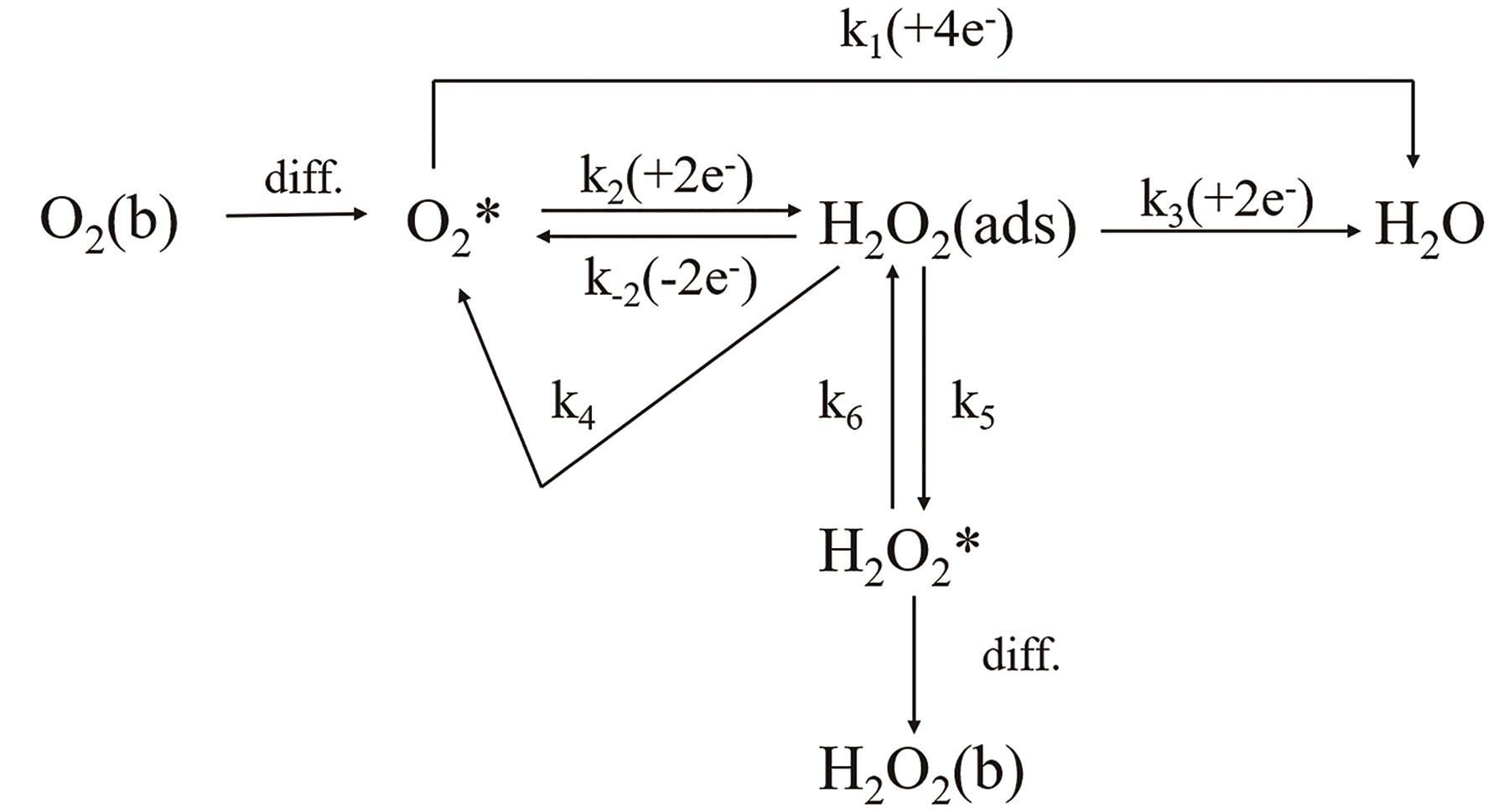
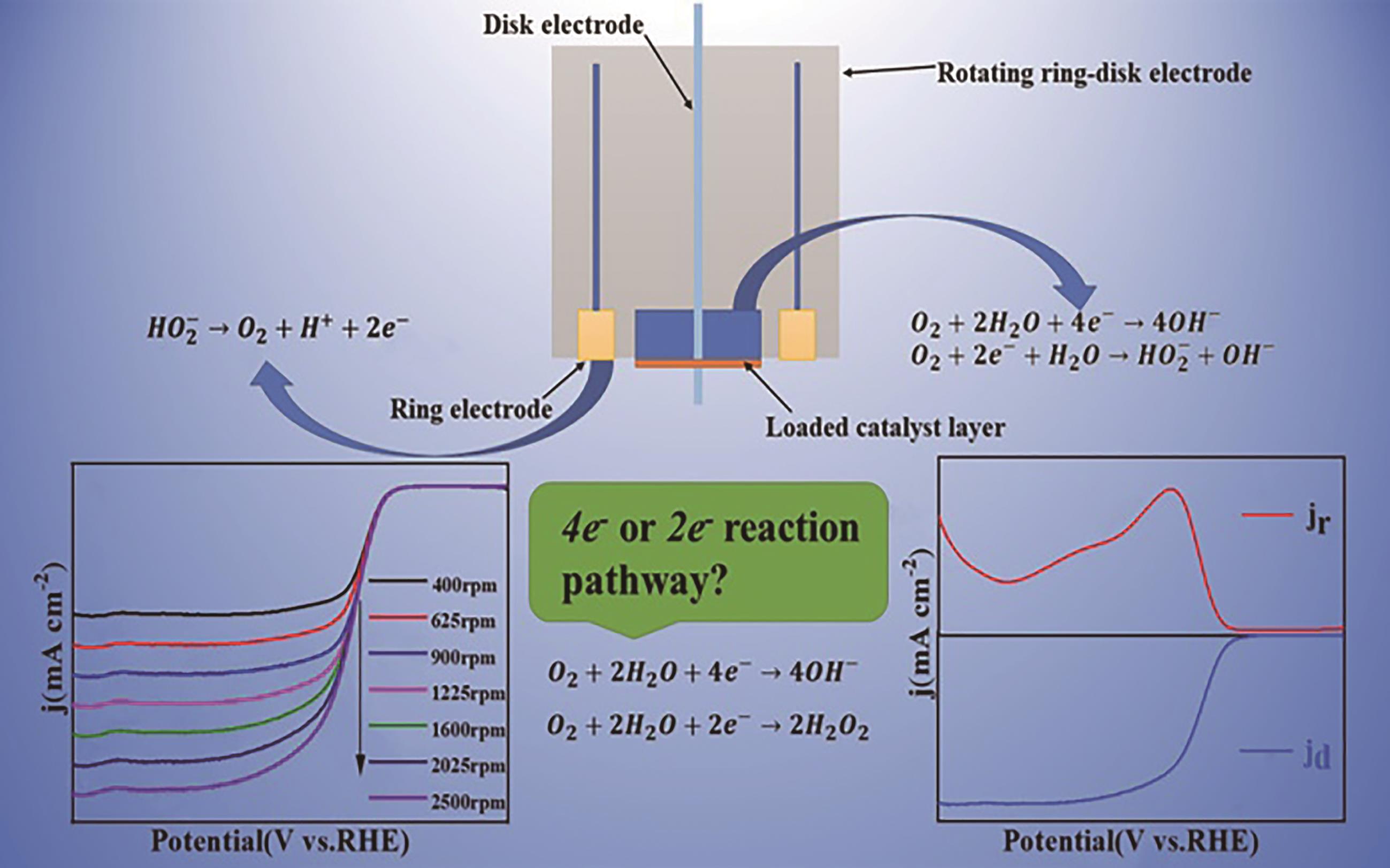
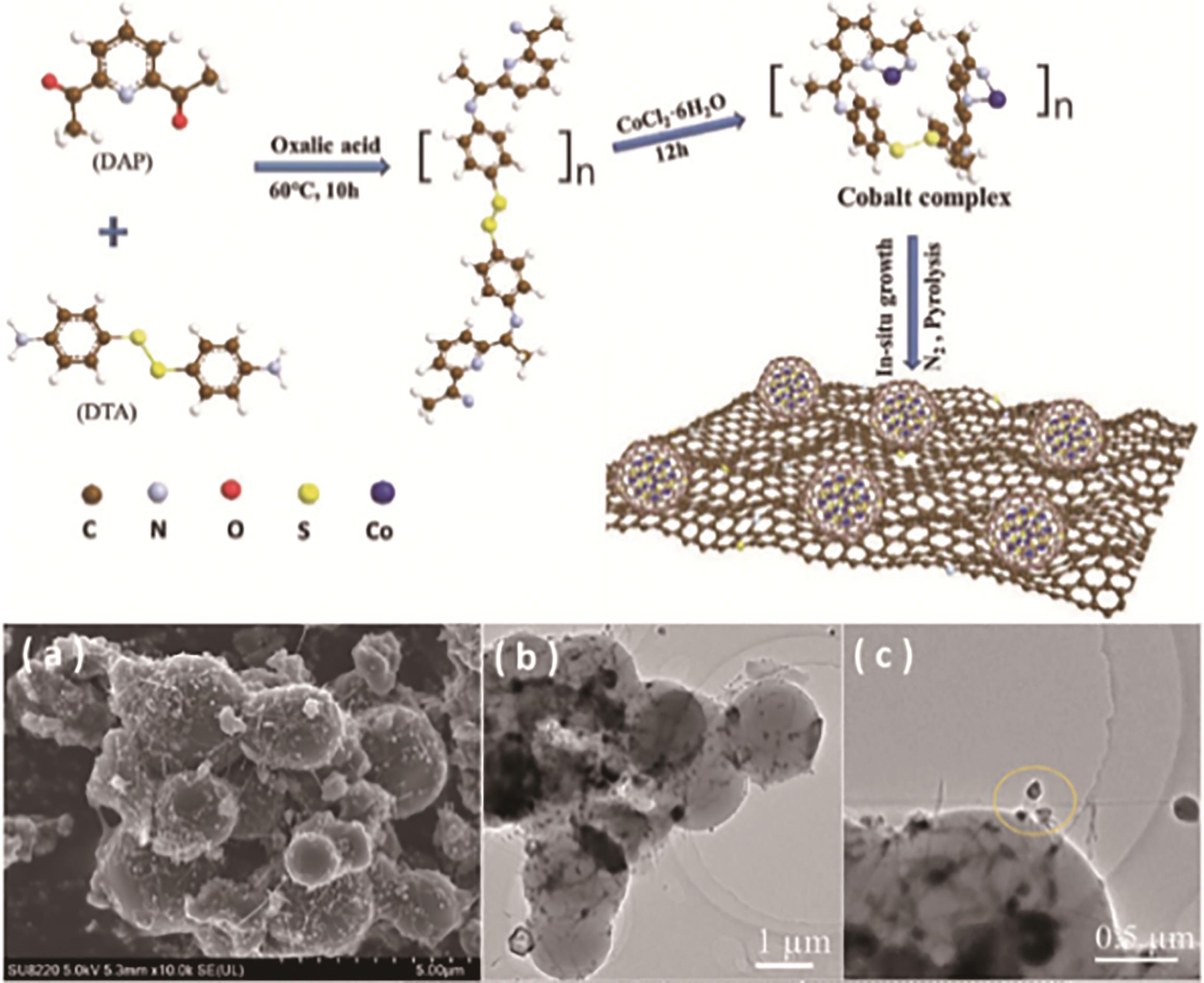
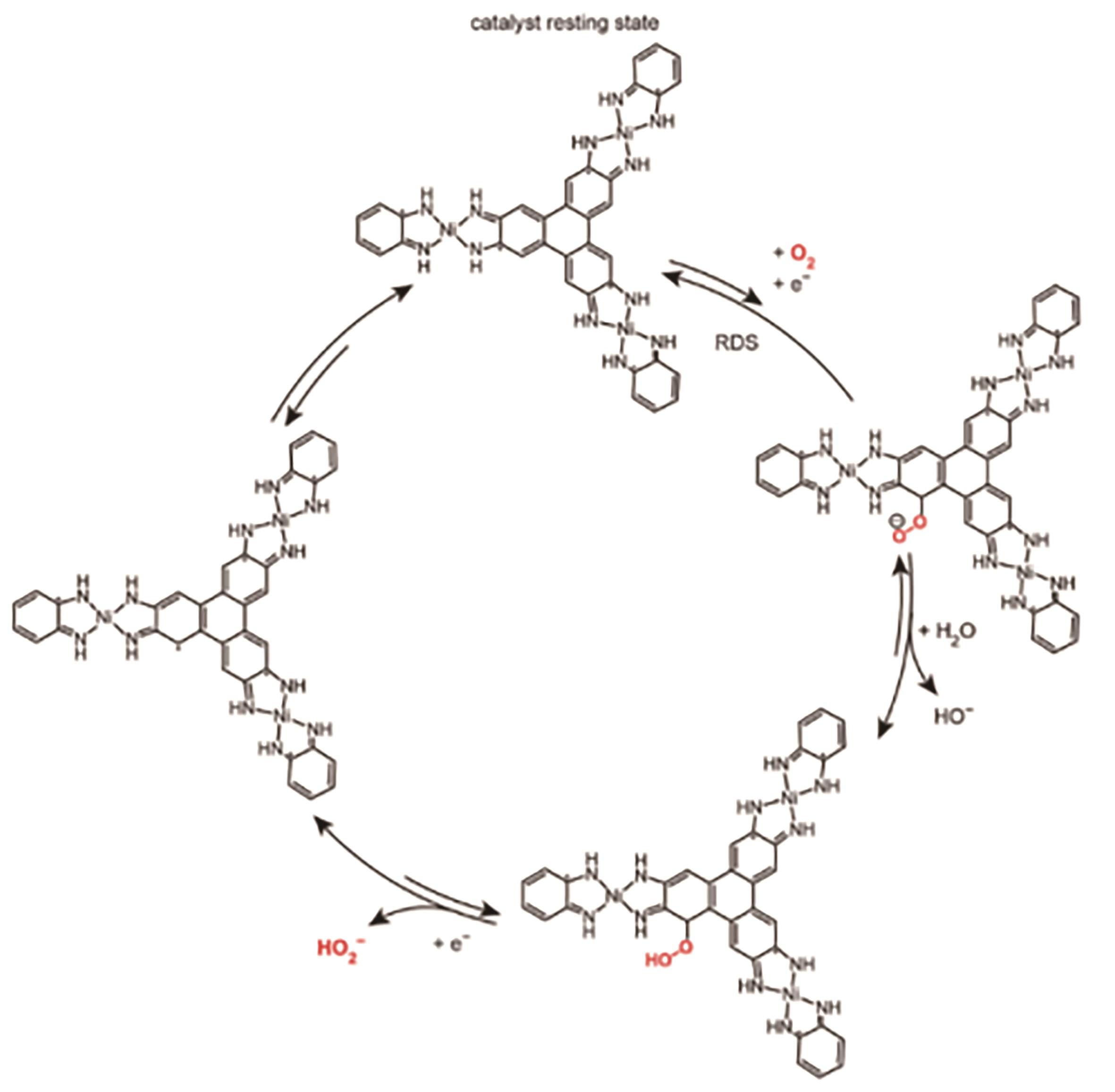
应用化学 ›› 2023, Vol. 40 ›› Issue (8): 1077-1093.DOI: 10.19894/j.issn.1000-0518.230075
非Pt基催化剂在质子交换膜燃料电池阴极氧还原反应中的研究进展
董以宁1,2, 李赫1,2, 宫雪1, 韩策1, 宋平1( ), 徐维林1,2(
), 徐维林1,2( )
)
- 1.中国科学院长春应用化学研究所,电分析化学国家重点实验室&吉林省低碳化学电源重点实验室,长春 130022
2.中国科学技术大学应用化学与工程学院,合肥 230026
-
收稿日期:2023-03-27接受日期:2023-06-02出版日期:2023-08-01发布日期:2023-08-24 -
通讯作者:宋平,徐维林 -
基金资助:科技部重点研发项目(2022YFA1203400);国家自然科学基金(21925205)
Research Progress of Non-Pt-Based Catalysts in Cathode Oxygen Reduction Reaction of Proton Exchange Membrane Fuel Cells
Yi-Ning DONG1,2, He LI1,2, Xue GONG1, Ce HAN1, Ping SONG1( ), Wei-Lin XU1,2(
), Wei-Lin XU1,2( )
)
- 1.State Key Laboratory of Electroanalytical Chemistry & Jilin Province Key Laboratory of Low Carbon Chemical Power,Changchun Institute of Applied Chemistry,Chinese Academy of Sciences,Changchun 130022,China
2.School of Applied Chemistry and Engineering,University of Science and Technology of China,Hefei 230026,China
-
Received:2023-03-27Accepted:2023-06-02Published:2023-08-01Online:2023-08-24 -
Contact:Ping SONG,Wei-Lin XU -
About author:songping@ciac.ac.cn
weilinxu@ciac.ac.cn
-
Supported by:the Key Research and Development Program Sponsored by the Ministry of Science and Technology (MOST)(2022YFA1203400);the National Natural Science Foundation of China(22072145)
摘要:
对绿色、高效能源储存装置日趋强烈的需求,使得用于清洁能源转换的先进技术获得了研究者的密切关注。具有环境友好、高能量转换效率等优势的燃料电池是传统能源转换装置极具希望的替代品。然而,工业催化界中商业化程度高的Pt体系催化剂存在成本高、稳定性差和抗毒化能力弱等问题,限制了燃料电池的进一步发展。开发储量丰富、成本低廉且性能优异的非Pt体系氧还原(ORR)催化剂是降低燃料电池成本,促进其大规模应用的有效途径。对此,结合近10年来国内外研究成果,系统介绍了当前各类非Pt体系ORR催化剂的研究进展,包括非贵金属基以及非金属基催化剂。同时,针对各类催化剂的优点、不足及改性策略进行了归纳与总结,并对未来ORR电催化剂的发展提出挑战、做出展望。
中图分类号:
引用本文
董以宁, 李赫, 宫雪, 韩策, 宋平, 徐维林. 非Pt基催化剂在质子交换膜燃料电池阴极氧还原反应中的研究进展[J]. 应用化学, 2023, 40(8): 1077-1093.
Yi-Ning DONG, He LI, Xue GONG, Ce HAN, Ping SONG, Wei-Lin XU. Research Progress of Non-Pt-Based Catalysts in Cathode Oxygen Reduction Reaction of Proton Exchange Membrane Fuel Cells[J]. Chinese Journal of Applied Chemistry, 2023, 40(8): 1077-1093.
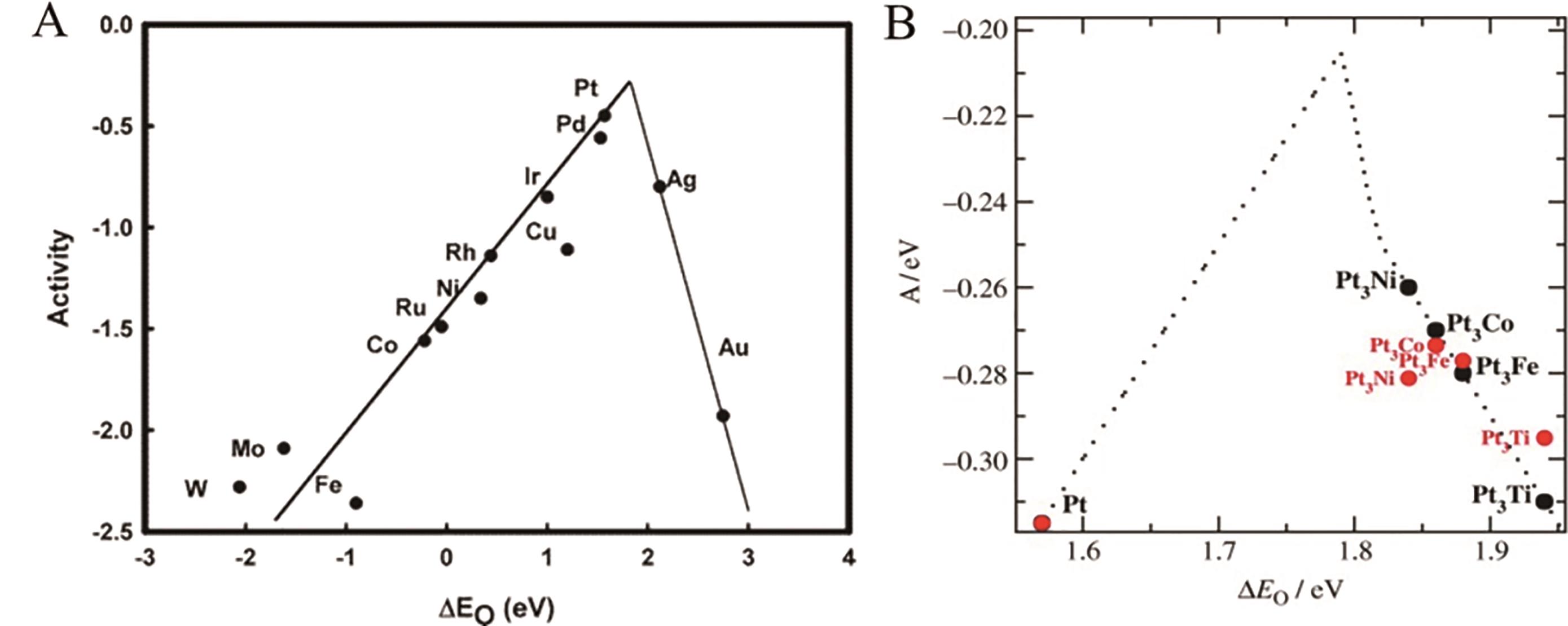
图3 (A) 氧中间体吸附能与ORR活性的火山关系图[20]; (B) 电极电位为0.9 V时,建立的活性A与氧的吸附能ΔEO的函数关系模型。(A=kBTln (r)),r为每秒钟表面原子的产生速率; 红色部分表示其它合金催化剂相对于Pt的活性测定结果; 假定对于Pt和所有合金单位表面积的活性位点数相同[18],则实验活性表达为A=kBTln (i/iPt)+Apt,APt为Pt活性的理论值,式中i/iPt为相对于Pt的电流密度
Fig.3 (A) Volcanic relationship between adsorption energy of oxygen intermediates and ORR activity[20]. Copyright?2004, American Chemical Society. (B) The model of the activity as a function of the adsorption energy of oxygen at a cell potential of 0.9 V. (A=kBTln (r)), where r is the rate per surface atom per second; Those shown in red are the measured activities relative to that of Pt. The activity of the experiment is A=kBTln (i/iPt)+Apt, where i/iPt is the current density relative to Pt, and APt is the theoretical value for the activity of Pt. It is assumed that the number of active sites per surface area is the same for Pt and all the alloys[18]. Copyright?2006 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim

图4 (A) +1.2 V时不同M-N-C类催化剂及N-C催化剂旋转环盘电极线性扫描曲线[31]; (B) 0.1 mol/L NaOH中,在普通热解石墨上吸附不同MN4催化剂的ORR活性火山型曲线[32]; (C-H) ΔG*OH与d-带中心位置的关系[33]
Fig.4 (A) Linear sweep voltammetry (LSV) in rotating ring-disk electrode (RRDE) for different M-N-C catalysts at +1.2 V[31]; Copyright?2019 American Chemical Society. (B) Activity volcano correlation for the reduction of O2 in 0.1 mol/L NaOH on different molecular MN4 catalysts adsorbed on ordinary pyrolytic graphite (OPG) [32].Copyright?2016 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. (C-H) The relationship between gibbs free energy of *OH and the position of d-band[33]. Copyright? 2021 Elsevier B.V.
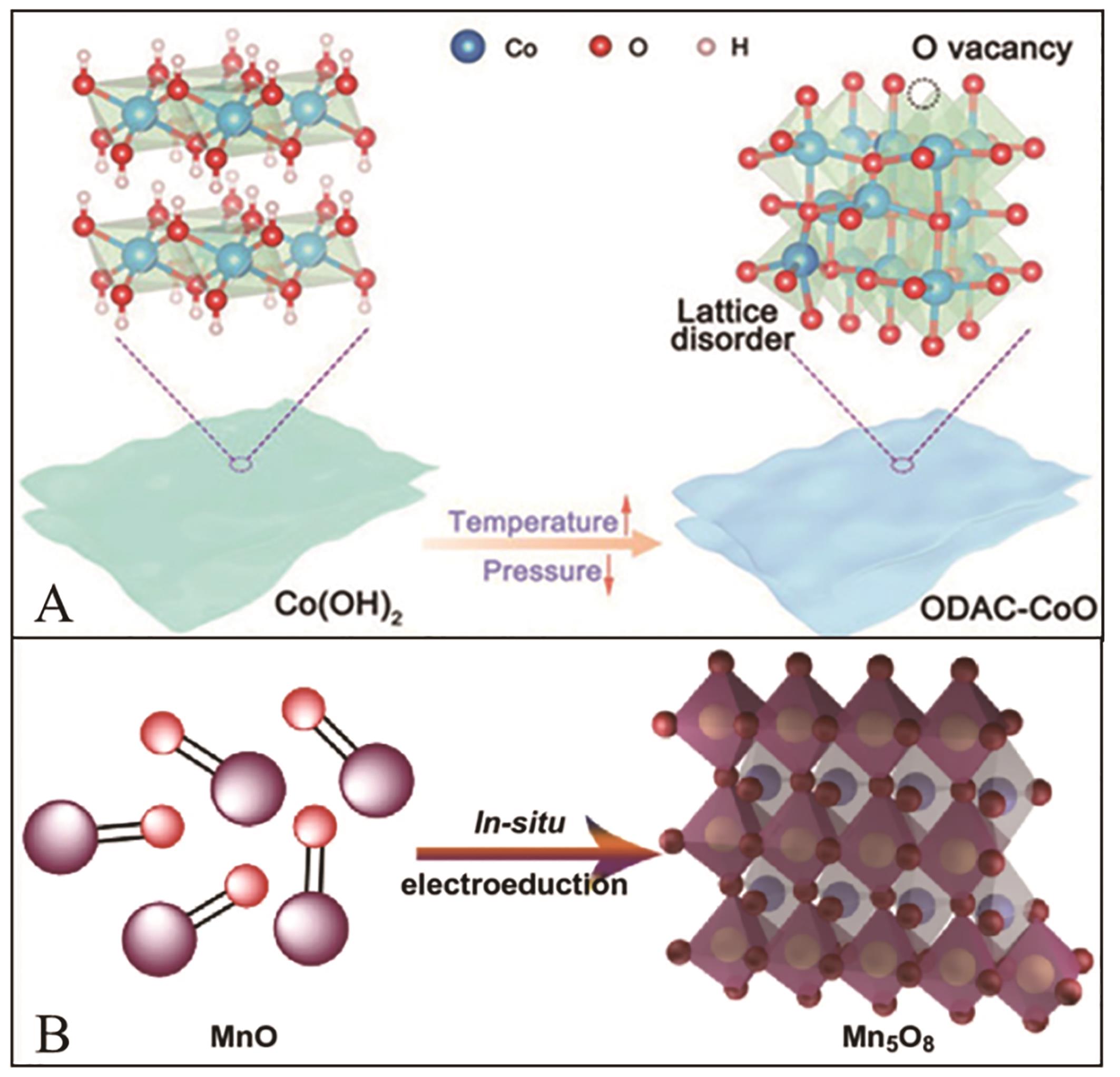
图5 (A) MnO进一步结构转换示意图[44]; (B)制备氧缺陷型CoO纳米片示意图[45]
Fig.5 (A) Structural evolution of MnO[44]. Copyright?2020; (B) The schematic diagram of CoO nanosheet with oxygen defect[45]. Copyright?2021 Wiley-VCH GmbH
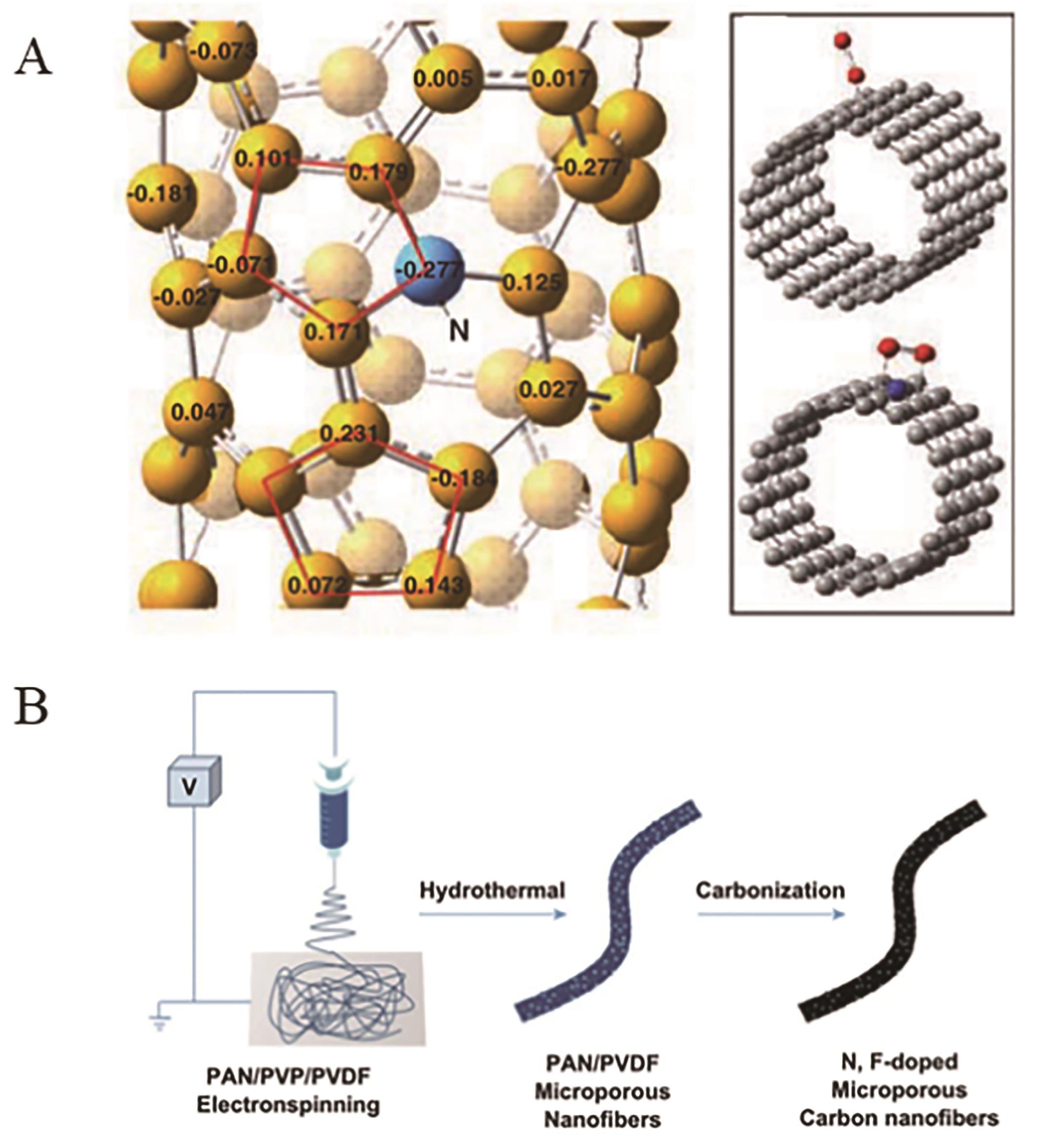
图8 (A) 碳纳米管一元N掺杂示意图[78]; (B)静电纺丝法制备N,F双掺杂催化剂[80]
Fig.8 (A) The structure model for N-doped carbon nanotube[78]; (B) The N,?F-codoping catalyst preparing by electrospinning[80]. Copyright@2020, Royal Society of Chemistry
| 1 | HAO L N, UMAR M, KHAN Z, et al. Green growth and low carbon emission in G7 countries: how critical the network of environmental taxes, renewable energy and human capital is?[J]. Sci Total Environ, 2021, 752: 141853. |
| 2 | QIU S, WANG Z, GENG S. How do environmental regulation and foreign investment behavior affect green productivity growth in the industrial sector? an empirical test based on Chinese provincial panel data[J]. J Environ Manage, 2021, 287: 112282. |
| 3 | KATSOUNAROS I, SCHNEIDER W B, MEIER J C, et al. Hydrogen peroxide electrochemistry on platinum: towards understanding the oxygen reduction reaction mechanism[J]. Phys Chem Chem Phys, 2012, 14(20): 7384-7391. |
| 4 | BOCKRIS J O, OLDFIELD L F. The oxidation-reduction reactions of hydrogen peroxide at inert metal electrodes and mercury cathodes[J]. Transact Faraday Soc, 1955, 51: 249-259. |
| 5 | LIVINGSTON R J. The oxidation states of the elements and their potentials in aqueous solutions (Latimer, Wendell M.)[J]. J Chem Educ, 1940, 17(7): 350. |
| 6 | HALINA S, WRIBLOWA, YEN C,et al, Electroreduction of oxygen: a new mechanistic criterion[J]. J Electroanal Chem Interfacial Electrochem, 1976, 69(2): 195-201. |
| 7 | JIAO M, SONG W, LI K, et al. First-principles study on nitrobenzene-doped graphene as a metal-free electrocatalyst for oxygen reduction reaction[J]. J Phys Chem C, 2016, 120(16): 8804-8812. |
| 8 | ZHI W S, KIBSGARRD J, DICKENS C F, et al. Combining theory and experiment in electrocatalysis: insights into materials design[J]. Science, 2017, 355(6321): eaad4998. |
| 9 | JIAO Y, ZHENG Y, J M, et al. Design of electrocatalysts for oxygen- and hydrogen-involving energy conversion reactions[J]. Chem Soc Rev, 2015, 44(8): 2060-2086. |
| 10 | XIA Y F, GUO P, LI J Z, et al. How to appropriately assess the oxygen reduction reaction activity of platinum group metal catalysts with rotating disk electrode[J]. iScience, 2021, 24(9): 103024. |
| 11 | LI C, ZHENG Q, XIANG Q, et al. Multielectron electrode reaction kinetics with RDE and RRDE: an advanced electrochemical laboratory experiment[J]. J Chem Educ, 2021, 98(9): 3026-3031. |
| 12 | TIAN X, LU X F, XIA B Y, et al. Advanced electrocatalysts for the oxygen reduction reaction in energy conversion technologies[J]. Joule, 2020, 4(1): 45-68. |
| 13 | SHAO M, CHANG Q, DOBELET J P, et al. Recent advances in electrocatalysts for oxygen reduction reaction[J]. Chem Rev, 2016, 116(6): 3594-3657. |
| 14 | 廖玲丈, 陈栋, 郑勇力, 等, 气体电极反应动力学的薄膜旋转圆盘电极方法研究[J]. 中国科学, 2013, 43(2): 178-184. |
| LIAO L Z, CHEN D, ZHENG Y L, et al. Effect of catalyst loading on the evaluation of kinetic parameters of gas electrode reactions by using thin film rotating disk electrode method[J]. Sci Sin Chim, 2013, 43(2): 178-184. | |
| 15 | 陈微, 廖玲文, 何政达, 等. 利用K-L方程估算旋转圆盘电极体系反应动力学电流的误差来源分析[J]. 电化学, 2014, 5: 444-451. |
| CHEN W, LIAO L W, HE Z D, et al. On the origin of the errors of ik as estimated from K-L equation in rotating disk electrode system[J]. J Electrochem, 2014, 5: 444-451. | |
| 16 | WIECKOWSKI A. Fuel cell catalysis[M]. New Jersey: Wiley-Blackwell, 2009: 459-630. |
| 17 | GOTTESFELD S, RAISTRICK I D, SRINIVASAN J C. Oxygen reduction kinetics on a platinum RDE coated with a recast Nafion film[J]. J Electrochem Soc, 1987, 18: 1455. |
| 18 | STAMENKOVIC V, MUN B S, MAYRHOFER K J, et al. Changing the activity of electrocatalysts for oxygen reduction by tuning the surface electronic structure[J]. Angew Chem Int Ed Engl, 2006, 45(18): 2897-2901. |
| 19 | NIE Y, LI L, WEI Z. Recent advancements in Pt and Pt-free catalysts for oxygen reduction reaction[J]. Chem Soc Rev, 2015, 44(8): 2168-2201. |
| 20 | NØRSKOV J K, ROSSMEISL J, LOGADOTTIR A, et al. Origin of the overpotential for oxygen reduction at a fuel-cell cathode[J]. J Phys Chem B, 2004, 108(46): 17886-17892. |
| 21 | VISWANATHAN V, HANSEN H A, ROSSMEISL J, et al. Universality in oxygen reduction electrocatalysis on metal surfaces[J]. ACS Catal, 2012, 2(8): 1654-1660. |
| 22 | HANSEN H A, VISWANATHAN V, NØRSKOV J K. Unifying kinetic and thermodynamic analysis of 2e- and 4e- reduction of oxygen on metal surfaces[J]. J Phys Chem C, 2014, 118(13): 6706-6718. |
| 23 | GREELEY J, NØRSKOV J K. Combinatorial density functional theory-based screening of surface alloys for the oxygen reduction reaction[J]. J Phys Chem C, 2009, 113(12): 4932-4939. |
| 24 | FAN X J, PENG Z W, YE R Q, et al. M3C (M: Fe, Co, Ni) nanocrystals encased in graphene nanoribbons: an active and stable bifunctional electrocatalyst for oxygen reduction and hydrogen evolution reactions[J]. ACS Nano, 2015, 9(7): 7407-7418. |
| 25 | JASINSKI R J N. A new fuel cell cathode catalyst[J]. Nature, 1964, 201: 1212-1213. |
| 26 | KRAMM U I, HERRANZ J, LAROUCHE N, et al. Structure of the catalytic sites in Fe/N/C-catalysts for O 2 - reduction in PEM fuel cells[J]. Phys Chem Chem Phys, 2012, 14(33): 11673-11688. |
| 27 | CHUNG H T, CULLEN D A, HIGGINS D, et al. Direct atomic-level insight into the active sites of a high-performance PGM-free ORR catalyst[J]. Science, 2017, 357(6350): 479-484. |
| 28 | KRAMM U I, HERRMANN G I, BEHRENDS J, et al. On an easy way to prepare metal-nitrogen doped carbon with exclusive presence of MeN4-type sites active for the ORR[J]. J Am Chem Soc, 2016, 138(138): 635-640. |
| 29 | SA Y J, SEO D J, WOO J, et al. A general approach to preferential formation of active Fe-Nx sites in Fe-N/C electrocatalysts for efficient oxygen reduction reaction[J]. J Am Chem Soc, 2016, 138(45): 15046-15056. |
| 30 | CHEN M X, ZHU M, ZUO M, et al. Identification of catalytic sites for oxygen reduction in metal/nitrogen-doped carbons with encapsulated metal nanoparticles[J]. Angew Chem Int Ed Engl, 2020, 59(4): 1627-1633. |
| 31 | YAN Y, SILVIOLI, LU C A, et al. Activity-selectivity trends in the electrochemical production of hydrogen peroxide over single-site metal-nitrogen-carbon catalysts[J]. J Am Chem Soc, 2019, 141(31): 12372-12381. |
| 32 | ZAGAL J H, KOPER M T. Reactivity descriptors for the activity of molecular MN4 catalysts for the oxygen reduction reaction[J]. Angew Chem Int Ed Engl, 2016, 55(47): 14510-14521. |
| 33 | LIU J, XIAO J, LUO B, et al. Central metal and ligand effects on oxygen electrocatalysis over 3D transition metal single-atom catalysts: a theoretical investigation[J]. Chem Eng J, 2022, 427:132038. |
| 34 | HE Y, YANG X, LI Y, et al. Atomically dispersed Fe-Co dual metal sites as bifunctional oxygen electrocatalysts for rechargeable and flexible Zn-air batteries[J]. ACS Catal, 2022, 12(2): 1216-1227. |
| 35 | YE C W, XU L. Recent advances in the design of a high performance metal-nitrogen-carbon catalyst for the oxygen reduction reaction[J]. J Mater Chem A, 2021, 9: 22218-22247. |
| 36 | WANG D, YANG P, LIU L, et al. Atomically dispersed metal-nitrogen-carbon electrocatalysts for oxygen reduction reaction: from synthesis strategies to activity engineering[J]. Mater Today Energy, 2022, 26: 101017. |
| 37 | PALANISELVAM T, KASHYAP V, BHANGE S N, et al. Nanoporous graphene enriched with Fe/Co-N active sites as a promising oxygen reduction electrocatalyst for anion exchange membrane fuel cells[J]. Adv Funct Mater, 2016, 26(13): 2150-2162. |
| 38 | ZHANG G, CHENITZ R, LEFÈVRE M, et al. Is iron involved in the lack of stability of Fe/N/C electrocatalysts used to reduce oxygen at the cathode of PEM fuel cells?[J]. Nano Energy, 2016, 29: 111-125. |
| 39 | WANG R, ZHANG P, WANG Y, et al. ZIF-derived Co-N-C ORR catalyst with high performance in proton exchange membrane fuel cells[J]. Science, 2020, 30(6): 855-860. |
| 40 | XU H, CHENG D, CAO D, et al. A universal principle for a rational design of single-atom electrocatalysts[J]. Nat Catal, 2018, 1(8): 339-348. |
| 41 | ISHIHARA A, OHGI Y, MATSUZAWA K, et al. Progress in non-precious metal oxide-based cathode for polymer electrolyte fuel cells[J]. Electrochim Acta, 2010, 55(27): 8005-8012. |
| 42 | LEE J S, PARK G S, LEE H I, et al. Black carbon supported amorphous manganese oxides nanowires as highly efficient electrocatalyst for oxygen reduction reaction in alkaline solutions[J]. Nano Lett, 2011, 11(12): 5362-5366. |
| 43 | CHENG F, ZHANG T, ZHANG Y, et al. Enhancing electrocatalytic oxygen reduction on MnO2 with vacancies[J]. Angew Chem Int Ed Engl, 2013, 52(9): 2474-2477. |
| 44 | TIAN H, ZENG L, HUANG Y, et al. In situ electrochemical Mn(Ⅲ)/Mn(Ⅳ) generation of Mn(Ⅱ)O electrocatalysts for high-performance oxygen reduction[J]. Nanomicro Lett, 2020, 12: 161. |
| 45 | TIAN Y, LIU X, XU L, et al. Engineering crystallinity and oxygen vacancies of Co(Ⅱ) oxide nanosheets for high performance and robust rechargeable Zn-air batteries[J]. Adv Funct Mater, 2021, 31(20): 210-239. |
| 46 | LIU Y, ISHIHARA A, MITSUSHIMA S, et al. Transition metal oxides as DMFC cathodes without platinum[J]. Nano Energy, 2007, 154: B664-B669. |
| 47 | LIU Y, ISHIHARA A, MITSUSHIMA S, et al. Influence of sputtering power on oxygen reduction reaction activity of zirconium oxides prepared by radio frequency reactive sputtering[J]. Electrochim Acta, 2010, 55(3): 1239-1244. |
| 48 | KIM J, KO W, YOO J M, et al. Structural insights into multi-metal spinel oxide nanoparticles for boosting oxygen reduction electrocatalysis[J]. Adv Mater, 2022, 34(8): e2107868. |
| 49 | VANTE N A, TRIBUTSCH H J N. Energy conversion catalysis using semiconducting transition metal cluster compounds[J]. Nature, 1986, 323: 431-432. |
| 50 | HIGGINS D C, HASSAN F M, SEO M H, et al. Shape-controlled octahedral cobalt disulfide nanoparticles supported on nitrogen and sulfur-doped graphene/carbon nanotube composites for oxygen reduction in acidic electrolyte[J]. J Mater Chem A, 2015, 3: 6340-6350. |
| 51 | CHAO Y S, TSAI D S, WU A P, et al. Cobalt selenide electrocatalyst supported by nitrogen-doped carbon and its stable activity toward oxygen reduction reaction[J]. Int J Hydrogen Energ, 2013, 38(14): 5655-5664. |
| 52 | WU G, CHUNG H T, NELSON M, et al. Graphene-riched Co9S8-N-C non-precious metal catalyst for oxygen reduction in alkaline media[J]. ECS Trans, 2011, 1(1):1709-1717. |
| 53 | WANG J, LI L, CHEN X, et al. Monodisperse cobalt sulfides embedded within nitrogen-doped carbon nanoflakes: an efficient and stable electrocatalyst for the oxygen reduction reaction[J]. J Mater Chem A, 2016, 4: 11342-11350. |
| 54 | SIDIK R A, ANDERSON A B. Co9S8 as a catalyst for electroreduction of O2: quantum chemistry predictions[J]. J Phys Chem, 2006, 110(2): 936-941. |
| 55 | LYU D, YAO S, ALI A, et al. N, S Codoped carbon matrix-encapsulated Co9S8 nanoparticles as a highly efficient and durable bifunctional oxygen redox electrocatalyst for rechargeable Zn-air batteries[J]. Adv Energy Mater, 2021, 11(28): 2101249. |
| 56 | XIE Q, LAN M, LI B, et al. Interface engineering of iron sulfide/tungsten nitride heterostructure catalyst for boosting oxygen reduction activity[J]. Chem Eng J, 2022, 431(4): 133274. |
| 57 | LUO J, TIAN X, ZENG J, et al. Limitations and improvement strategies for early-transition-metal nitrides as competitive catalysts toward the oxygen reduction reaction[J]. ACS Catal, 2016, 6(9): 6165-6174. |
| 58 | HAM D, LEE J. Transition metal carbides and nitrides as electrode materials for low temperature fuel cells[J]. Energies, 2009, 2(4): 873-899. |
| 59 | YANG Y, ZENG R, XIONG Y, et al. Cobalt-based nitride-core oxide-shell oxygen reduction electrocatalysts[J]. J Am Chem Soc, 2019, 141(49): 19241-19245. |
| 60 | YUAN Y, WANG J, ADIMI S, et al. Zirconium nitride catalysts surpass platinum for oxygen reduction[J]. Nat Mater, 2020, 19: 282-286. |
| 61 | UKITA K, ISHIHARA A, OHGI Y, et al. Zirconium oxide-based compounds as non-Pt cathode for polymer electrolyte fuel cell[J]. Electrochem, 2011(5): 340-342. |
| 62 | SHIBATA Y, ISHIHARA A, MITSUSHIMA S, et al. Effect of heat treatment on catalytic activity for oxygen reduction reaction of TaOxNy/Ti prepared by electrophoretic deposition[J]. Electrochem Solid ST, 2007, 10(2): B43-B46. |
| 63 | ANDO T, IZHAR S, TOMINAGA H, et al. Ammonia-treated carbon-supported cobalt tungsten as fuel cell cathode catalyst[J]. Electrochim Acta, 2010, 55: 2614-2621. |
| 64 | YUAN Y, ADIMI S, THOMAS T, et al. Co3Mo3N, an efficient multifunctional electrocatalyst[J]. Innovation, 2021, 2: 100096. |
| 65 | LV X L, YUAN S, XIE L H, et al. Ligand rigidification for enhancing the stability of metal-organic frameworks[J]. J Am Chem Soc, 2019, 141(26): 10283-10293. |
| 66 | LU X F, XIA B Y, ZANG S Q, et al. Metal-organic frameworks based electrocatalysts for the oxygen reduction reaction[J]. Angew Chem Int Ed Engl, 2020, 59(12): 4634-4650. |
| 67 | LIAO P Q, SHEN J Q, ZHANG J P. Metal-organic frameworks for electrocatalysis[J]. Coordin Chem Rev, 2018, 373: 22-48. |
| 68 | JIA Y, XUE Z, YANG J, et al. Tailoring the electronic structure of an atomically dispersed zinc electrocatalyst: coordination environment regulation for high selectivity oxygen reduction[J]. Angew Chem Int Ed Engl, 2022, 61(2): e202110838. |
| 69 | XU Y, HUANG Z, WANG B, et al. A two-dimensional multi-shelled metal-organic framework and its derived bimetallic N-doped porous carbon for electrocatalytic oxygen reduction[J]. Chem Commun, 2019, 55: 14805-14808. |
| 70 | MINER E M, GUL S, RICKE N D, et al. Mechanistic evidence for ligand-centered electrocatalytic oxygen reduction with the conductive MOF Ni3(hexaiminotriphenylene)2[J]. ACS Catal, 2017, 7(11): 7726-7731. |
| 71 | SUN L, CAMPBELL M G, DINCA M. Electrically conductive porous metal-organic frameworks[J]. Angew Chem Int Ed Engl, 2016, 55(11): 3566-3579. |
| 72 | LIU X, DAI L. Carbon-based metal-free catalysts[J]. Nat Rev Mater, 2016, 1: 16064. |
| 73 | SHUANG Y, WANG, DING S, et al. Polyelectrolyte functionalized carbon nanotubes as efficient metal-free electrocatalysts for oxygen reduction[J]. J Am Chem Soc, 2011, 133(14): 5182-5185. |
| 74 | WANG S, YU D, DAI L, et al. Polyelectrolyte-functionalized graphene as metal-free electrocatalysts for oxygen reduction[J]. ACS Nano, 2011, 5(8): 6202-6209. |
| 75 | JIN H, HUANG H, HE Y, et al. Graphene quantum dots supported by graphene nanoribbons with ultrahigh electrocatalytic performance for oxygen reduction[J]. J Am Chem Soc, 2015, 137(24): 7588-7591. |
| 76 | CHEN S, ZHAO L, MA J, et al. Edge-doping modulation of N,P-codoped porous carbon spheres for high-performance rechargeable Zn-air batteries[J]. Nano Energy, 2019, 60: 536-544. |
| 77 | YAN Z, ZHANG Y, JIANG Z, et al. Nitrogen-doped bimetallic carbide-graphite composite as highly active and extremely stable electrocatalyst for oxygen reduction reaction in alkaline media[J]. Adv Funct Mater, 2022, 32(30): 2204031. |
| 78 | HUANG N B, ZHANG J J, SUN Y, et al. A non-traditional biomass-derived N, P, and S ternary self-doped 3D multichannel carbon ORR electrocatalyst[J]. New J Chem, 2020, 44: 14604-14614. |
| 79 | GONG K P, DU F, XIA Z M, et al. Nitrogen-doped carbon nanotube arrays with high electrocatalytic activity for oxygen reduction[J]. Science, 2009, 323(5915): 760-764. |
| 80 | GONG T, QI R, LIU X, et al. N,F-codoped microporous carbon nanofibers as efficient metal-free electrocatalysts for ORR[J]. Nanomicro Lett, 2019, 11(1): 9. |
| 81 | SUN X, SONG P, ZHANG Y, et al. A class of high performance metal-free oxygen reduction electrocatalysts based on cheap carbon blacks[J]. Sci Rep, 2013, 3: 2505. |
| 82 | ZHENG Y, CHEN S, YU X, et al. Nitrogen-doped carbon spheres with precisely-constructed pyridinic-N active sites for efficient oxygen reduction[J]. Appl Surface Sci, 2022, 598: 153786. |
| 83 | KONG F, CUI X, HUANG Y, et al. N-Doped carbon electrocatalyst: marked ORR activity in acidic media without the contribution from metal sites?[J]. Angew Chem Int Ed Engl, 2022, 61(15): e202116290. |
| 84 | LIU J, SONG P, XU W L. Structure-activity relationship of doped-nitrogen (N)-based metal-free active sites on carbon for oxygen reduction reaction[J]. Carbon, 2017, 115: 763-772. |
| 85 | DAI L, XUE Y, QU L, et al. Metal-free catalysts for oxygen reduction reaction[J]. Chem Rev, 2015,115(11): 4823-4892. |
| 86 | YANG H B, MIAO J, HUNG S F, et al. Identification of catalytic sites for oxygen reduction and oxygen evolution in N-doped graphene materials: development of highly efficient metal-free bifunctional electrocatalyst[J]. Sci Adv, 2016, 2(4): e1501122. |
| 87 | SUN J, LOWE S E, ZHANG L, et al. Ultrathin nitrogen-doped holey carbon@graphene bifunctional electrocatalyst for oxygen reduction and evolution reactions in alkaline and acidic media[J]. Angew Chem Int Ed Engl, 2018, 57(50): 16511-16515. |
| [1] | 罗二桂, 唐涛, 王艺, 张俊明, 常宇虹, 胡天军, 贾建峰. 两电子氧还原制备过氧化氢:贵金属催化剂的几何与电子结构调控的研究进展[J]. 应用化学, 2023, 40(8): 1063-1076. |
| [2] | 惠连成, 庄鉴行, 肖顺, 李美平, 靳梦圆, 吕青. 镍氮掺杂石墨炔用作高效氧还原电催化剂[J]. 应用化学, 2023, 40(8): 1205-1213. |
| [3] | 张艺潆, 李翠艳, 赵杰, 余笑明, 方千荣. 卟啉-硫醚基共价有机框架材料用于氧还原反应电催化剂[J]. 应用化学, 2022, 39(4): 647-656. |
| [4] | 王丹, 侯现飚, 汪兴坤, 刘志承, 王焕磊, 黄明华. 应用于锌空气电池的碳包覆铁基纳米颗粒电催化剂研究进展[J]. 应用化学, 2022, 39(10): 1488-1500. |
| [5] | 李赫, 李宫, 宫雪, 阮明波, 韩策, 宋平, 徐维林. Pt/C催化剂长时间ORR过程性能衰减的机理[J]. 应用化学, 2022, 39(10): 1564-1571. |
| [6] | 李宫, 金龙一, 姚鹏飞, 刘聪, 徐维林. 介孔炭负载铂纳米粒子的高效氧还原催化剂的可控设计[J]. 应用化学, 2021, 38(12): 1639-1646. |
| [7] | 魏振业, 孟君玲, 王浩聪, 张文文, 刘孝娟, 孟健. 同型异质表面修饰提高La2NiO4+δ阴极的电催化活性[J]. 应用化学, 2020, 37(8): 939-951. |
| [8] | 付凤艳,程敬泉. 静电纺丝纳米纤维在燃料电池质子交换膜中应用的研究进展[J]. 应用化学, 2020, 37(4): 405-415. |
| [9] | 李新杰,徐鹤,于美,张超,郭安儒,刘畅. 氮掺杂石墨炭包覆的钴纳米催化剂作高效、高稳定性电催化制氢[J]. 应用化学, 2019, 36(5): 571-577. |
| [10] | 李新杰, 徐鹤, 于美, 张超, 郭安儒, 刘畅. 氮掺杂石墨炭包覆的钴纳米催化剂作高效、高稳定性电催化制氢[J]. 应用化学, 2019, 36(5): 0-0. |
| [11] | 陈凤英, 李克智, 胡广志. 碳纳米管负载四硝基金属酞菁-MnO2双催化剂催化氧还原性能[J]. 应用化学, 2019, 36(1): 97-106. |
| [12] | 陈思,孙立臻,舒欣欣,张进涛. 石墨烯基催化剂的设计合成与电催化应用[J]. 应用化学, 2018, 35(3): 272-285. |
| [13] | 马娟, 隋琪, 陆天虹. 配位自还原法制备纳米银及其电催化活性[J]. 应用化学, 2014, 31(11): 1330-1335. |
| [14] | 库里松.哈衣尔别克, 曾涵. 介孔碳掺杂氮材料-壳聚糖固定漆酶电极的直接电化学行为及化学传感性能[J]. 应用化学, 2013, 30(10): 1194-1201. |
| [15] | 杨勇, 赵文文, 施庆乐, 张华. 电化学还原制备铂-氢钨青铜阳极[J]. 应用化学, 2011, 28(12): 1408-1414. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||