应用化学 ›› 2022, Vol. 39 ›› Issue (4): 599-615.DOI: 10.19894/j.issn.1000-0518.210451
人工固碳技术—热催化还原CO2催化剂的研究进展
- 中国科学院长春应用化学研究所,稀土资源利用国家重点实验室,长春 130022
-
收稿日期:2021-09-05接受日期:2021-11-25出版日期:2022-04-01发布日期:2022-04-19 -
通讯作者:于洋,宋术岩 -
基金资助:国家自然科学基金(21771173);国家重点研发计划(2016YFA0203200)
Artificial Carbon Sequestration Technology—Research Progress on the Catalysts for Thermal Catalytic Reduction of CO2
Xue-Ting WU, Yang YU( ), Shu-Yan SONG(
), Shu-Yan SONG( ), Hong-Jie ZHANG
), Hong-Jie ZHANG
- State Key Laboratory of Rare Earth Resources Utilization,Changchun Institute of Applied Chemistry,Chinese Academy of Sciences,Changchun 130022,China
-
Received:2021-09-05Accepted:2021-11-25Published:2022-04-01Online:2022-04-19 -
Contact:Yang YU,Shu-Yan SONG -
About author:songsy@ciac.ac.cn
-
Supported by:the National Natural Science Foundation of China(21771173);the National Key R&D Program(2016YFA0203200)
摘要:
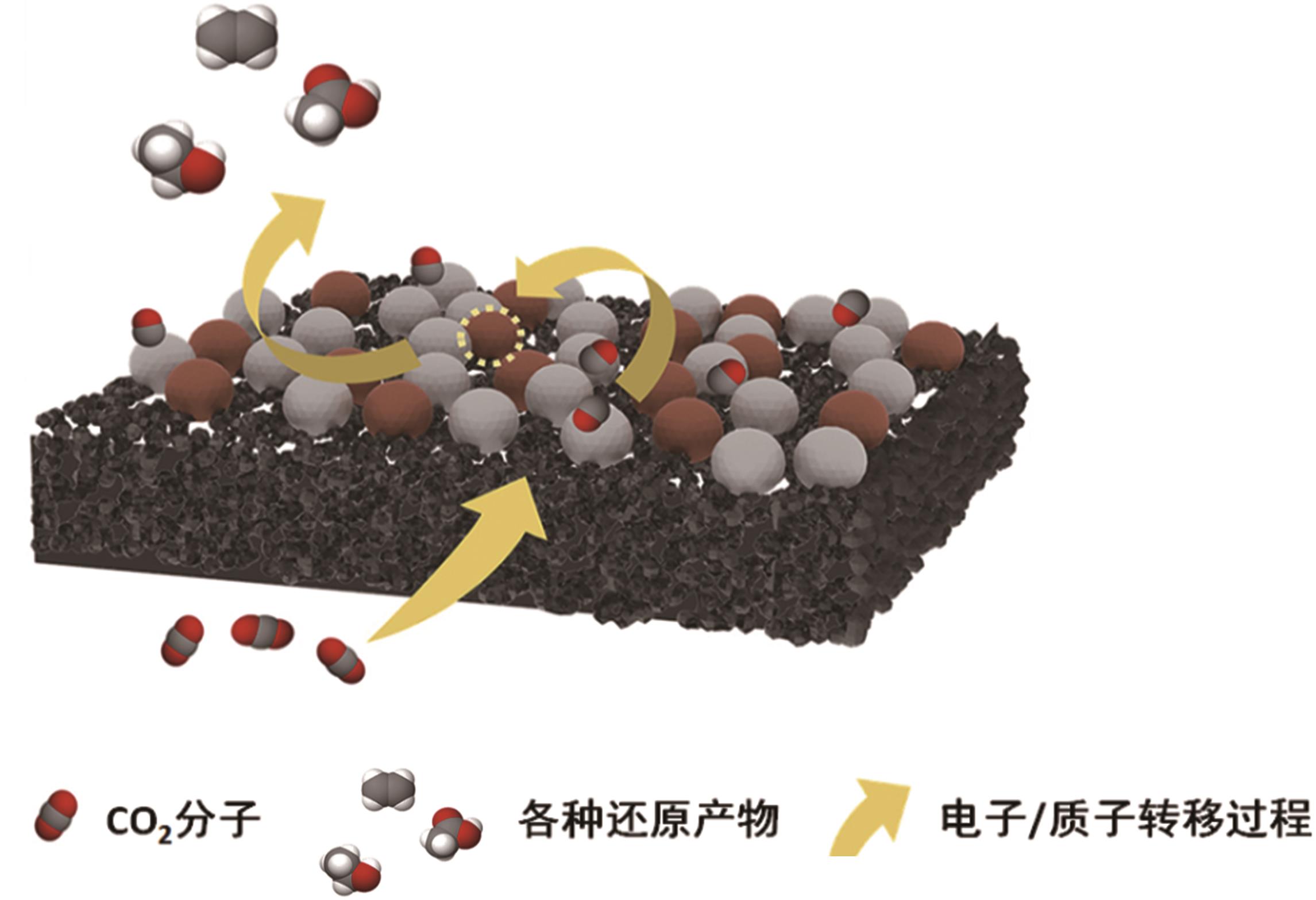
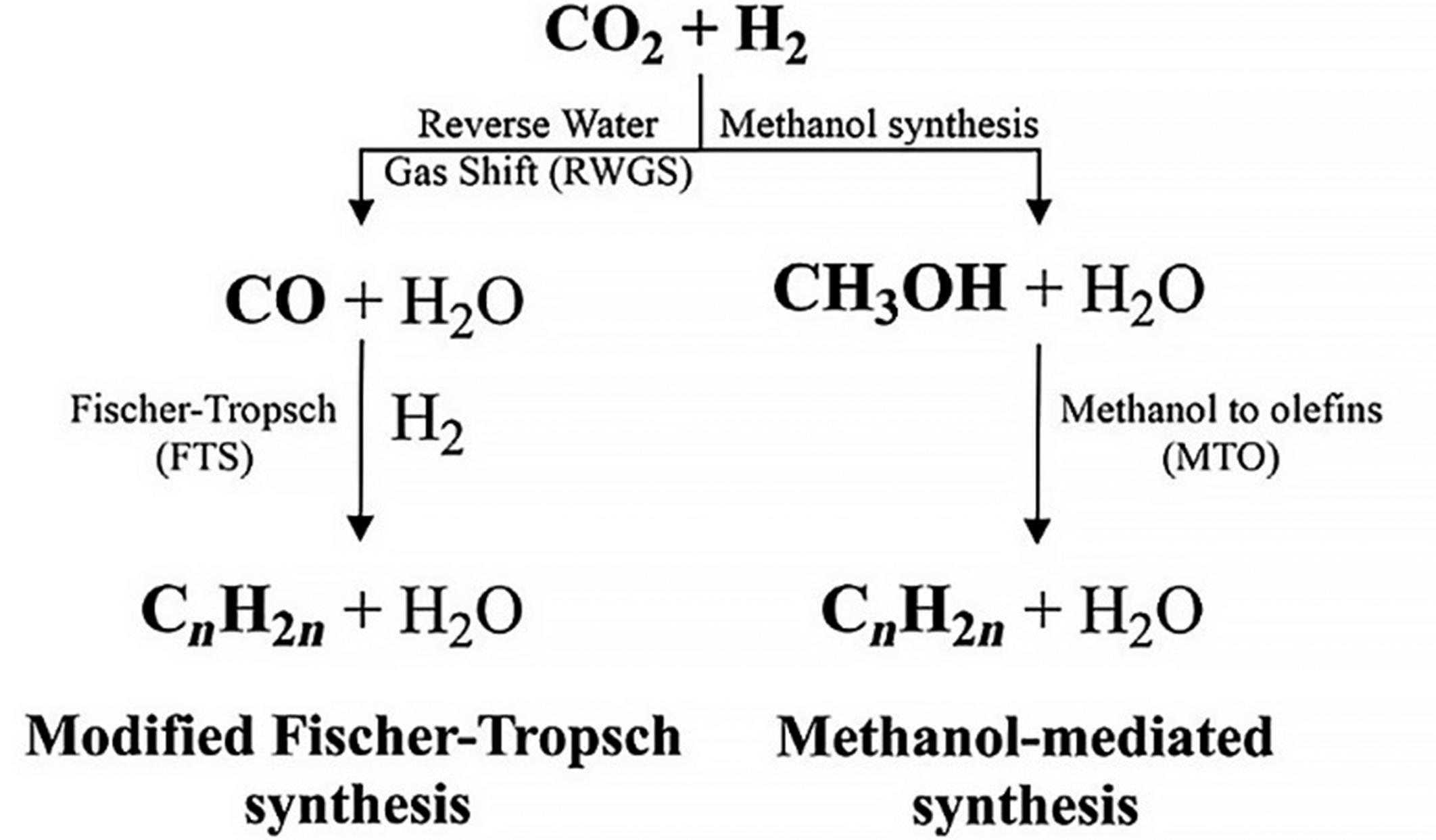
选择性加氢在功能材料合成和化学产品提纯等化工领域中有非常重要的应用,并且近年来为减少温室效应的影响,将CO2催化选择性加氢转化成其他有应用价值的物质成为研究热点之一。其中热催化是应用较为广泛、易得到多种目标产物并且获得产品收率较高的方法。目前,利用CO2多相热催化加氢制得甲烷、甲醇、轻烯烃等多种高价值的燃料和化学品已取得了一定进展,但仍存在一些难点问题,其中制备高效催化剂是催化加氢反应的关键问题之一。一直以来,研究人员致力于解决催化剂的活性和选择性问题,通过助剂掺杂和加入功能性载体对催化剂进行改性。针对这些问题,本文简要介绍了CO2催化加氢的研究背景,总结了近5年来热催化CO2加氢制得甲烷、甲醇、轻烯烃产品过程中使用催化剂的种类及对加氢反应的影响,期望为CO2多相催化加氢中新型催化剂的开发提供参考。
中图分类号:
引用本文
吴雪婷, 于洋, 宋术岩, 张洪杰. 人工固碳技术—热催化还原CO2催化剂的研究进展[J]. 应用化学, 2022, 39(4): 599-615.
Xue-Ting WU, Yang YU, Shu-Yan SONG, Hong-Jie ZHANG. Artificial Carbon Sequestration Technology—Research Progress on the Catalysts for Thermal Catalytic Reduction of CO2[J]. Chinese Journal of Applied Chemistry, 2022, 39(4): 599-615.
催化剂 Catalyst | n(H2)∶n(CO2) | 温度 Temperature/℃ | 压力 Pressure/MPa | GHSV/ (mL·g-1·h-1) | CO2转化率 Conversion/% | 选择性 Selectivity/% | 文献 Ref. | |
|---|---|---|---|---|---|---|---|---|
甲烷 Methane | 20%Ni?TiO2 | 4∶1 | 350 | -- | 48 000 | 52 | 98 | [ |
| Ni?5Mg/SBA?15?AE | 4∶1 | 350 | -- | 30 000 | 73 | 98 | [ | |
| Ni/Pr?Ce | 4∶1 | 350 | -- | 25 000 | 54.5 | 100 | [ | |
| NiO/Zn0.3Zr0.7 | 4∶1 | 500 | -- | 24 000 | 79 | 94 | [ | |
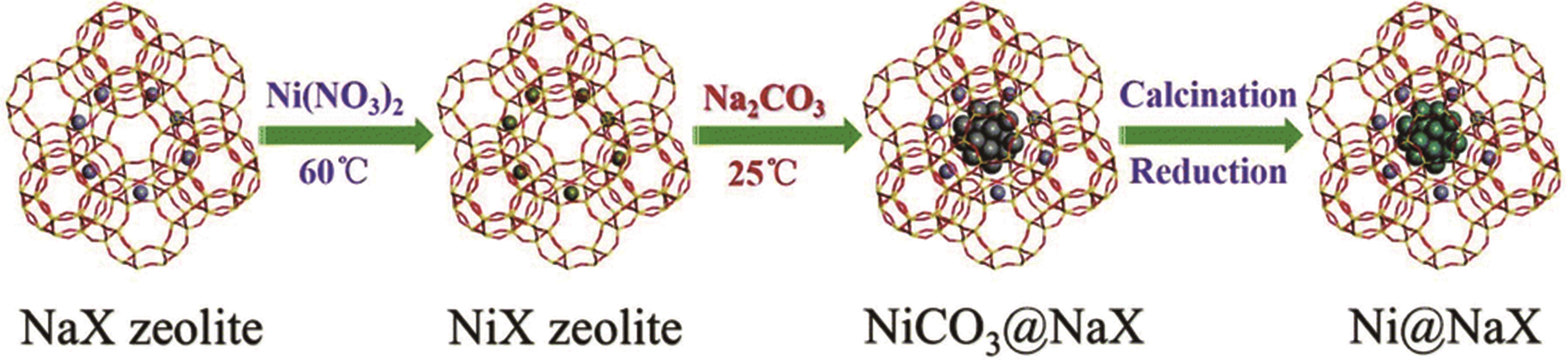
| 8%Ni@NaX | 4∶1 | 380 | 0.1 | 15 000 | 92.70 | ≈99.9 | [ | |
| RuSA/TiO2* | 4∶1 | 340 | 0.1 | 16 500 | 15.6 | 97.3 | [ | |
| 0.89%RuSA/CeO2 | 4∶1 | 260 | 0.1 | 4 800 | 28 | 100 | [ | |
甲醇 Methanol | ZrO2/Cu?0.1 | 3∶1 | 220 | 3 | 48 000 | <5 | 70 | [ |
| Cu1La0.2/SBA?15 | 3∶1 | 240 | 3 | 12 000 | 5.7 | 81.2 | [ | |
| Pd?CZ?0.01 | 3∶1 | 150 | 4.5 | 10 800 | 30 | 65 | [ | |
| 10Cu/CeO2 | 3∶1 | 190 | 3 | 3 000 | 1.8 | 84 | [ | |
| 1Pd?10Cu/CeO2 | 3∶1 | 270 | 3 | 3 000 | 17.8 | 23.7 | [ | |
| Pd@Zn | 3∶1 | 250 | 2 | 18 000 | 5 | 70.5 | [ | |
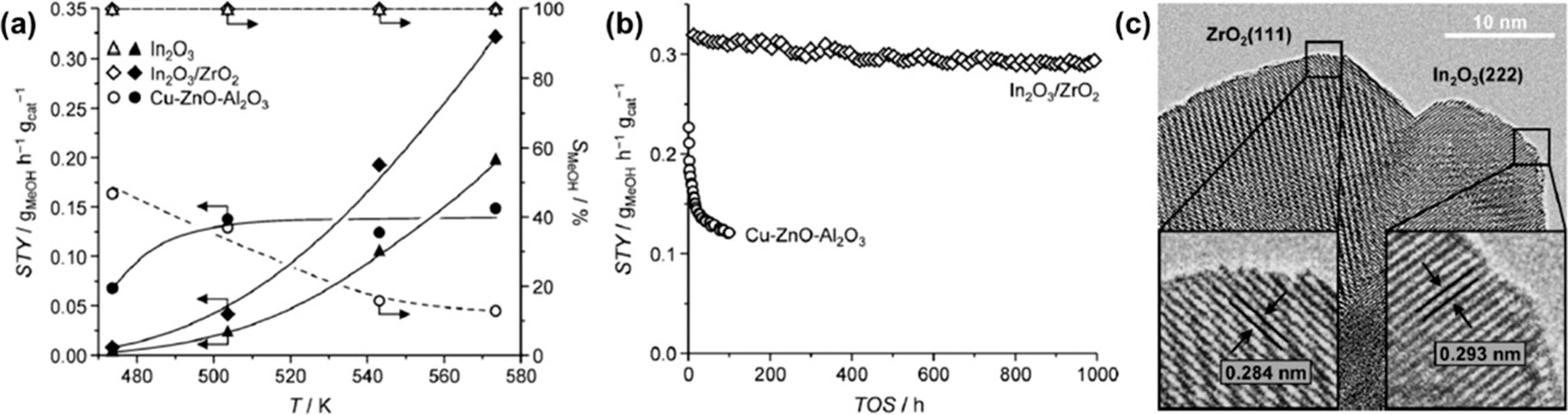
| In2O3/ZrO2 | 4∶1 | 300 | 5 | 16 000 | 5.2 | 99.8 | [ | |
轻烯烃 Light olefin | 35Fe?7Zr?1Ce?K | 3∶1 | 320 | 2 | 6 000 | 57.3 | 55.6 | [ |
Fe?Co/K?Al2O3 (Fe/K=2.5) | 3∶1 | 320 | 2 | 9 000 | 41.4 | 45 | [ | |
| C?Fe?Zn/K | 3∶1 | 320 | 2 | 3 000 | 54.8 | 57.4 | [ | |
| Na?Fe3O4/HZSM?5 | 3∶1 | 320 | 1.1 | 3 600 | 22 | 46.6 | [ | |
| Fe/C+K(0.75) | 3∶1 | 350 | 3 | 24 000 | 38.5 | 38.8 | [ | |
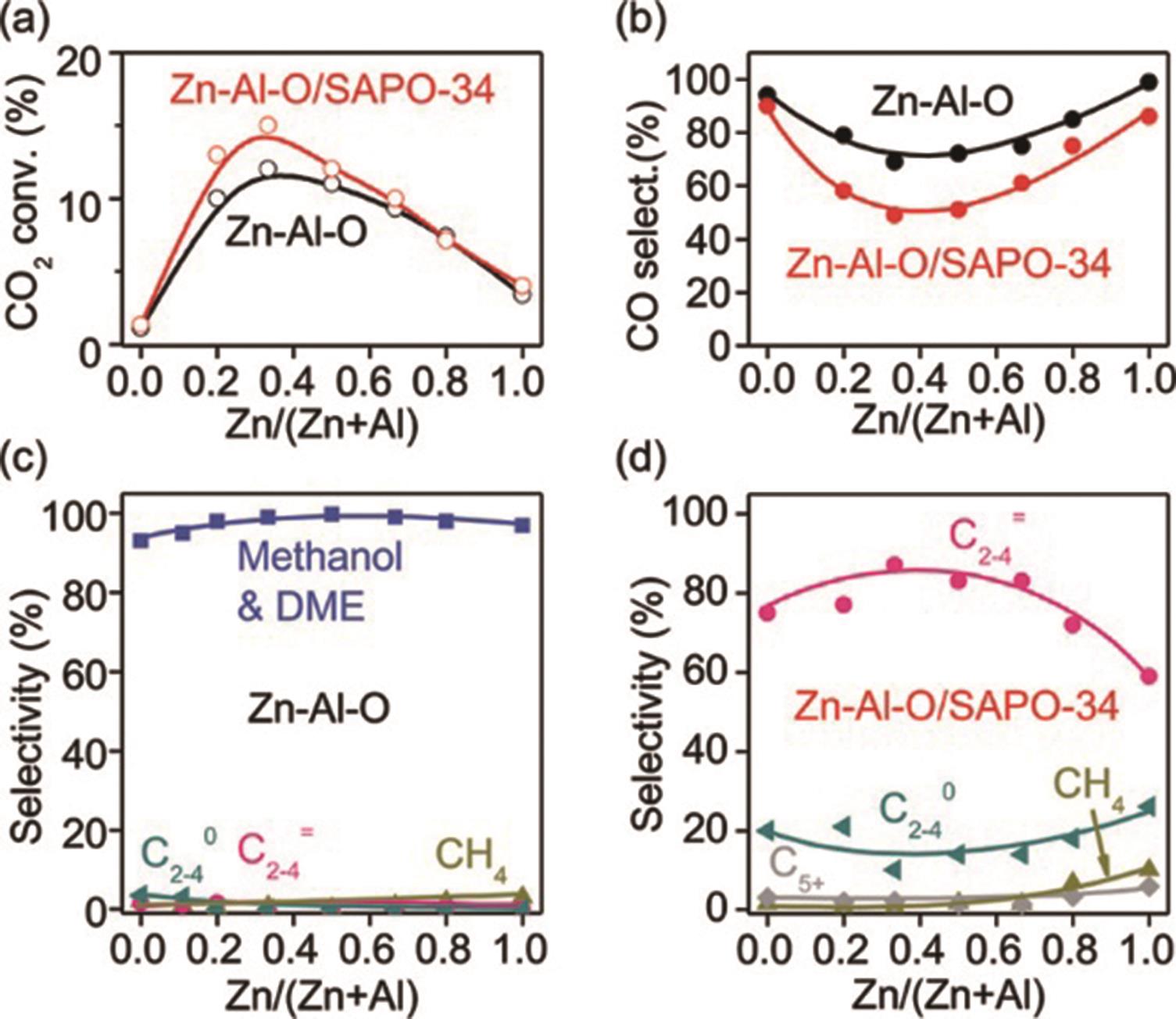
| In?Zr/SAPO?34 | 3∶1 | 400 | 3 | 9 000 | 30 | 84 | [ | |
| ZnZrO/SAPO | 3∶1 | 380 | 2 | 3 600 | 12.6 | 80 | [ | |
| ZnGa2O4/SAPO?34 | 3∶1 | 370 | 3 | 5 400 | 13 | 86 | [ | |
| ZnAl2O4/SAPO?34 | 3∶1 | 370 | 3 | 5 400 | 15 | 87 | [ |
表1 CO2加氢催化制备不同产物的性能总结
Table 1 Summary of the performance of catalysts for different products by CO2 hydrogenation
催化剂 Catalyst | n(H2)∶n(CO2) | 温度 Temperature/℃ | 压力 Pressure/MPa | GHSV/ (mL·g-1·h-1) | CO2转化率 Conversion/% | 选择性 Selectivity/% | 文献 Ref. | |
|---|---|---|---|---|---|---|---|---|
甲烷 Methane | 20%Ni?TiO2 | 4∶1 | 350 | -- | 48 000 | 52 | 98 | [ |
| Ni?5Mg/SBA?15?AE | 4∶1 | 350 | -- | 30 000 | 73 | 98 | [ | |
| Ni/Pr?Ce | 4∶1 | 350 | -- | 25 000 | 54.5 | 100 | [ | |
| NiO/Zn0.3Zr0.7 | 4∶1 | 500 | -- | 24 000 | 79 | 94 | [ | |
| 8%Ni@NaX | 4∶1 | 380 | 0.1 | 15 000 | 92.70 | ≈99.9 | [ | |
| RuSA/TiO2* | 4∶1 | 340 | 0.1 | 16 500 | 15.6 | 97.3 | [ | |
| 0.89%RuSA/CeO2 | 4∶1 | 260 | 0.1 | 4 800 | 28 | 100 | [ | |
甲醇 Methanol | ZrO2/Cu?0.1 | 3∶1 | 220 | 3 | 48 000 | <5 | 70 | [ |
| Cu1La0.2/SBA?15 | 3∶1 | 240 | 3 | 12 000 | 5.7 | 81.2 | [ | |
| Pd?CZ?0.01 | 3∶1 | 150 | 4.5 | 10 800 | 30 | 65 | [ | |
| 10Cu/CeO2 | 3∶1 | 190 | 3 | 3 000 | 1.8 | 84 | [ | |
| 1Pd?10Cu/CeO2 | 3∶1 | 270 | 3 | 3 000 | 17.8 | 23.7 | [ | |
| Pd@Zn | 3∶1 | 250 | 2 | 18 000 | 5 | 70.5 | [ | |
| In2O3/ZrO2 | 4∶1 | 300 | 5 | 16 000 | 5.2 | 99.8 | [ | |
轻烯烃 Light olefin | 35Fe?7Zr?1Ce?K | 3∶1 | 320 | 2 | 6 000 | 57.3 | 55.6 | [ |
Fe?Co/K?Al2O3 (Fe/K=2.5) | 3∶1 | 320 | 2 | 9 000 | 41.4 | 45 | [ | |
| C?Fe?Zn/K | 3∶1 | 320 | 2 | 3 000 | 54.8 | 57.4 | [ | |
| Na?Fe3O4/HZSM?5 | 3∶1 | 320 | 1.1 | 3 600 | 22 | 46.6 | [ | |
| Fe/C+K(0.75) | 3∶1 | 350 | 3 | 24 000 | 38.5 | 38.8 | [ | |
| In?Zr/SAPO?34 | 3∶1 | 400 | 3 | 9 000 | 30 | 84 | [ | |
| ZnZrO/SAPO | 3∶1 | 380 | 2 | 3 600 | 12.6 | 80 | [ | |
| ZnGa2O4/SAPO?34 | 3∶1 | 370 | 3 | 5 400 | 13 | 86 | [ | |
| ZnAl2O4/SAPO?34 | 3∶1 | 370 | 3 | 5 400 | 15 | 87 | [ |
催化剂 Catalyst | CO2转化率 CO2 conversion/% | CH4产率 CH4 yield/% | CH4选择性 CH4 selectivity/% |
|---|---|---|---|
| Ni/Ce | 39.4 | 39.4 | 100 |
| Ni/Sm?Ce | 44.9 | 44.9 | 100 |
| Ni/Pr?Ce | 54.5 | 54.5 | 100 |
| Ni/Mg?Ce | 43.2 | 43.2 | 100 |
表2 不同催化剂的催化性能[19]
Table 2 Reaction performance of different catalysts[19]
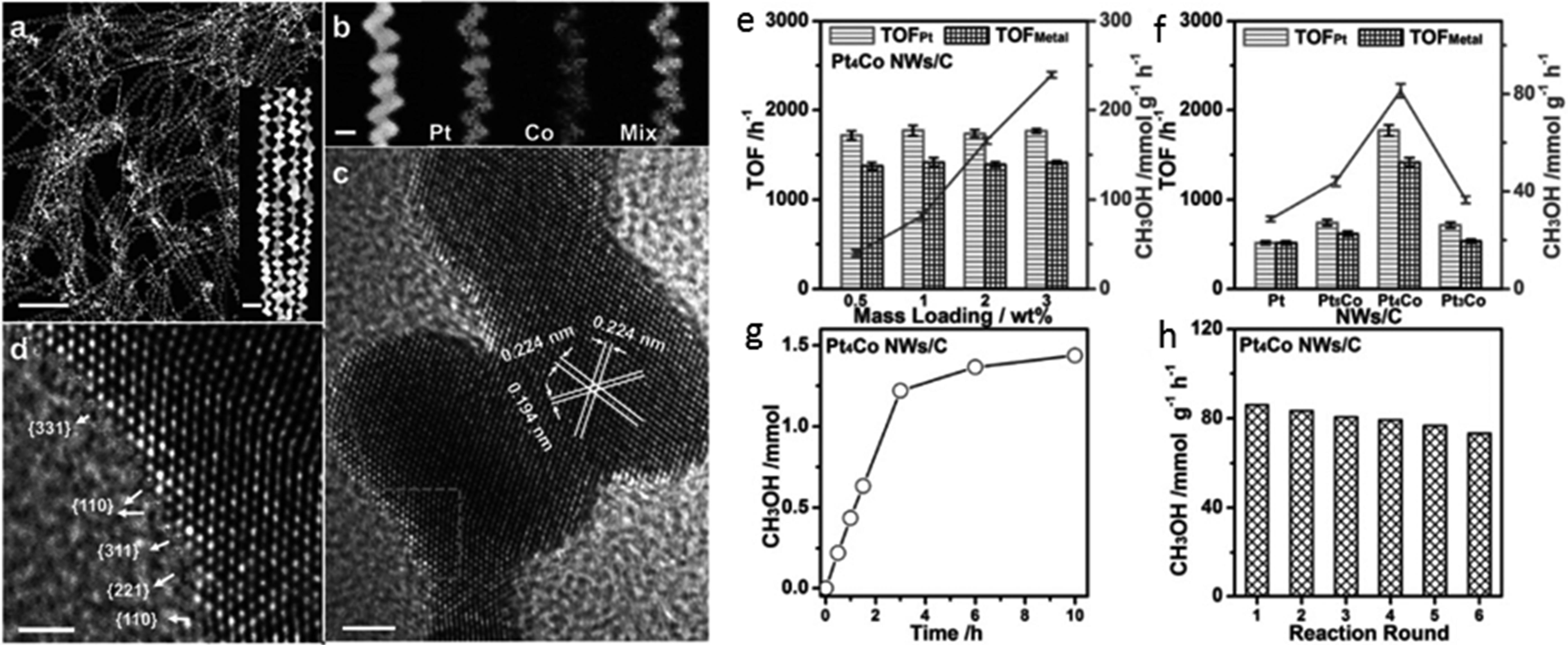
催化剂 Catalyst | CO2转化率 CO2 conversion/% | CH4产率 CH4 yield/% | CH4选择性 CH4 selectivity/% |
|---|---|---|---|
| Ni/Ce | 39.4 | 39.4 | 100 |
| Ni/Sm?Ce | 44.9 | 44.9 | 100 |
| Ni/Pr?Ce | 54.5 | 54.5 | 100 |
| Ni/Mg?Ce | 43.2 | 43.2 | 100 |
催化剂 Catalyst | CO2转化率 CO2 conversion/% | CO选择性/% CO selectivity/% | CH4选择性 CH4 selectivity/% |
|---|---|---|---|
| NiSA/CNTox-400 | 5.4 | 93.7 | 6.3 |
| NiSA?Na/CNTox-400 | 8.1 | 95.5 | 4.5 |
| RuSA/CNTox-400 | 8.4 | 56.0 | 44.0 |
| RuSA?Na/CNTox-400 | 20.2 | 79.5 | 20.5 |
| NiSA/TiO2* | 11.8 | 99.6 | 0.4 |
| Ni/TiO2 | 2.8 | 99.3 | 0.7 |
| RuSA/TiO2* | 15.6 | 2.7 | 97.3 |
| Ru/TiO2* | 11.5 | 3.6 | 96.4 |
表3 不同催化剂的催化性能[43]
Table 3 Reaction performance of different catalysts[43]
催化剂 Catalyst | CO2转化率 CO2 conversion/% | CO选择性/% CO selectivity/% | CH4选择性 CH4 selectivity/% |
|---|---|---|---|
| NiSA/CNTox-400 | 5.4 | 93.7 | 6.3 |
| NiSA?Na/CNTox-400 | 8.1 | 95.5 | 4.5 |
| RuSA/CNTox-400 | 8.4 | 56.0 | 44.0 |
| RuSA?Na/CNTox-400 | 20.2 | 79.5 | 20.5 |
| NiSA/TiO2* | 11.8 | 99.6 | 0.4 |
| Ni/TiO2 | 2.8 | 99.3 | 0.7 |
| RuSA/TiO2* | 15.6 | 2.7 | 97.3 |
| Ru/TiO2* | 11.5 | 3.6 | 96.4 |
催化剂 Catalyst | CO2转化率 CO2 conversion/% | C2-C4烯烃产率 C2-C4 olefin/% | 烯烃/烷烃摩尔比 n(olefin)∶n(paraffin) |
|---|---|---|---|
| Fe/K?Al2O3 | 34.0 | 45.5 | 6.7∶1 |
| Fe?Cu/K?Al2O3 | 36.0 | 42.2 | 5.4∶1 |
| Fe?Zn/K?Al2O3 | 34.2 | 42.6 | 5.6∶1 |
| Fe?Co/K?Al2O3 | 40.0 | 46.1 | 5.9∶1 |
| Fe?Mn/K?Al2O3 | 29.4 | 48.7 | 7.4∶1 |
| Fe?V/K?Al2O3 | 30.6 | 44.9 | 6.4∶1 |
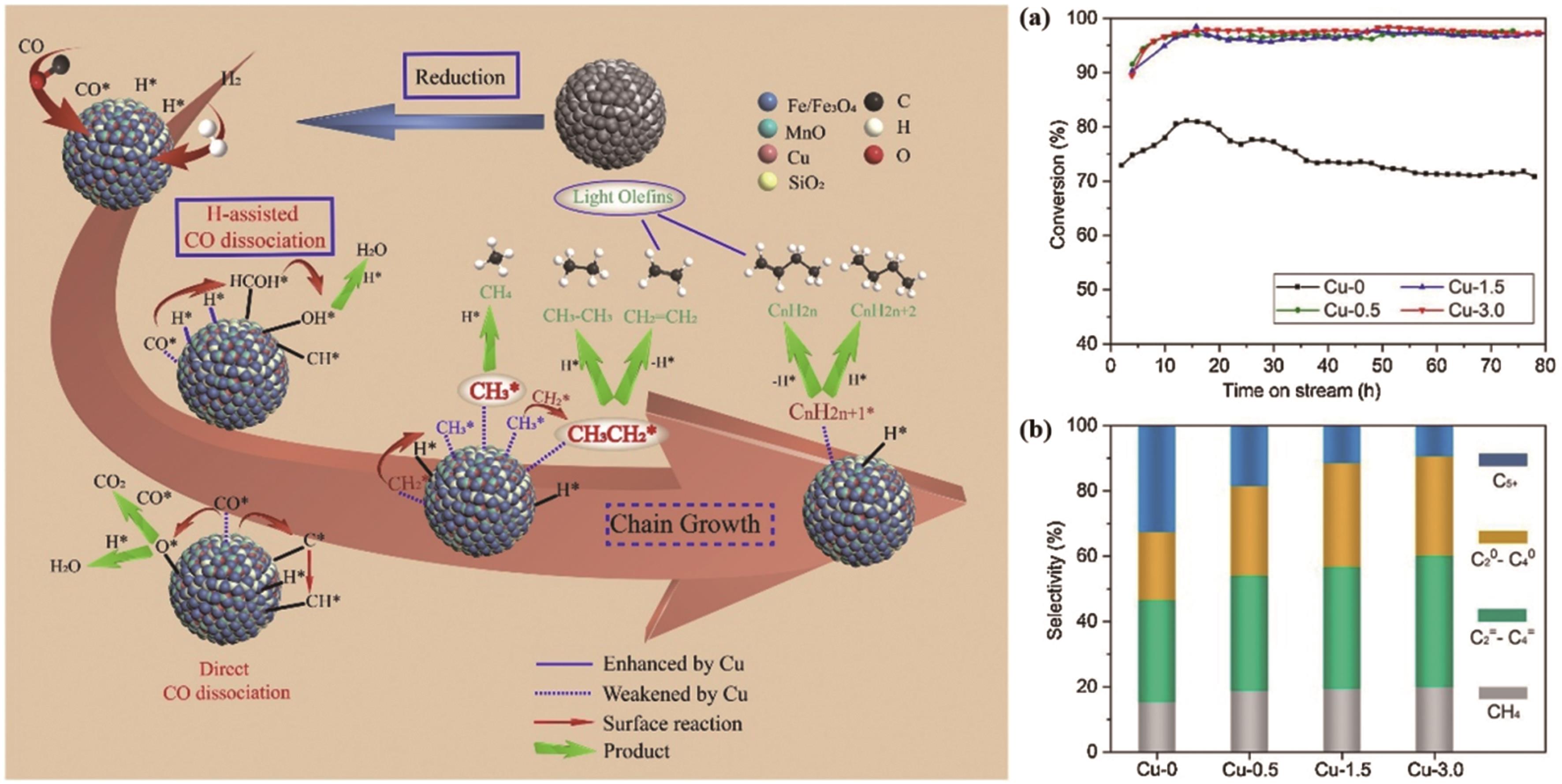
表4 改进后的Fe/K基催化剂用于CO2加氢各种产物分布统计[50]
Table 4 Distribution of various products of CO2 hydrogenation catalyzed by improved Fe/K?based catalyst [50]
催化剂 Catalyst | CO2转化率 CO2 conversion/% | C2-C4烯烃产率 C2-C4 olefin/% | 烯烃/烷烃摩尔比 n(olefin)∶n(paraffin) |
|---|---|---|---|
| Fe/K?Al2O3 | 34.0 | 45.5 | 6.7∶1 |
| Fe?Cu/K?Al2O3 | 36.0 | 42.2 | 5.4∶1 |
| Fe?Zn/K?Al2O3 | 34.2 | 42.6 | 5.6∶1 |
| Fe?Co/K?Al2O3 | 40.0 | 46.1 | 5.9∶1 |
| Fe?Mn/K?Al2O3 | 29.4 | 48.7 | 7.4∶1 |
| Fe?V/K?Al2O3 | 30.6 | 44.9 | 6.4∶1 |
| 1 | NIU Y, HUANG X, WANG Y, et al. Manipulating interstitial carbon atoms in the nickel octahedral site for highly efficient hydrogenation of alkyne[J]. Nat Commun, 2020, 11(1): 3324. |
| 2 | LIU S, NIU Y, WANG Y, et al. Unravelling the role of active-site isolation in reactivity and reaction pathway control for acetylene hydrogenation[J]. Chem Commun, 2020, 56(47): 6372-6375. |
| 3 | NIU Y, LIU X, WANG Y, et al. Visualizing formation of intermetallic PdZn in a palladium/zinc oxide catalyst: interfacial fertilization by PdHx[J]. Angew Chem Int Ed, 2019, 58(13): 4232-4237. |
| 4 | JIANG X, NIE X, GUO X, et al. Recent advances in carbon dioxide hydrogenation to methanol via heterogeneous catalysis[J]. Chem Rev, 2020, 120(15): 7984-8034. |
| 5 | LONG Y, LI J, WU L, et al. One-pot synthesis of cobalt-doped Pt-Au alloy nanoparticles supported on ultrathin alpha-Co(OH)2 nanosheets and their enhanced performance in the reduction of p-nitrophenol[J]. Eur J Inorg Chem, 2017, (1): 146-152. |
| 6 | PAN J, ZHANG L, ZHANG S, et al. Half-encapsulated Au nanorods@CeO2 core@shell nanostructures for near-infrared plasmon-enhanced catalysis[J]. ACS Appl Nano Mater, 2019, 2(3): 1516-1524. |
| 7 | LI J, SONG S, LONG Y, et al. Investigating the hybrid-structure-effect of CeO2-encapsulated Au nanostructures on the transfer coupling of nitrobenzene[J]. Adv Mater, 2018, 30(7): 1704416. |
| 8 | LI J, LONG Y, LIU Y, et al. Robust synthesis of gold-based multishell structures as plasmonic catalysts for selective hydrogenation of 4-nitrostyrene[J]. Angew Chem Int Ed, 2020, 59(3): 1103-1107. |
| 9 | LONG Y, LI J, WU L, et al. Construction of trace silver modified core@shell structured Pt-Ni nanoframe@CeO2 for semihydrogenation of phenylacetylene[J]. Nano Res, 2019, 12(4): 869-875. |
| 10 | SONG S, LI K, PAN J, et al. Achieving the trade-off between selectivity and activity in semihydrogenation of alkynes by fabrication of (asymmetrical Pd@Ag core)@(CeO2 shell) nanocatalysts via autoredox reaction[J]. Adv Mater, 2017, 29(8): 1605332. |
| 11 | LIU Y, WANG Q, WU L, et al. Tunable bimetallic Au-Pd@CeO2 for semihydrogenation of phenylacetylene by ammonia borane[J]. Nanoscale, 2019, 11(27): 12932-12937. |
| 12 | SONG S, LIU X, LI J, et al. Confining the nucleation of Pt to in situ form (Pt-enriched cage)@CeO2 core@shell nanostructure as excellent catalysts for hydrogenation reactions[J]. Adv Mater, 2017, 29(28): 1700495. |
| 13 | LONG Y, SONG S, LI J, et al. Pt/CeO2@MOF core@shell nanoreactor for selective hydrogenation of furfural via the channel screening effect[J]. ACS Catal, 2018, 8(9): 8506-8512. |
| 14 | XU J, ZHU J, LIU Y, et al. In situ construction of Pt-Ni NF@Ni-MOF-74 for selective hydrogenation of p-nitrostyrene by ammonia borane[J]. Chem Eur J, 2020, 26(55): 12539-12543. |
| 15 | WANG Q, LI J, WANG X, et al. Surfactant-guided synthesis of porous Pt shells with ordered tangential channels, coated on Pd nanostructures, and their enhanced catalytic activities[J]. Chem Eur J, 2018, 24(58): 15649-15655. |
| 16 | XU J, YANG W, SONG S, et al. Ultra-small noble metal ceria-based catalytic materials: from synthesis to application[J]. Eur J Inorg Chem, 2021, 2021(8): 689-701. |
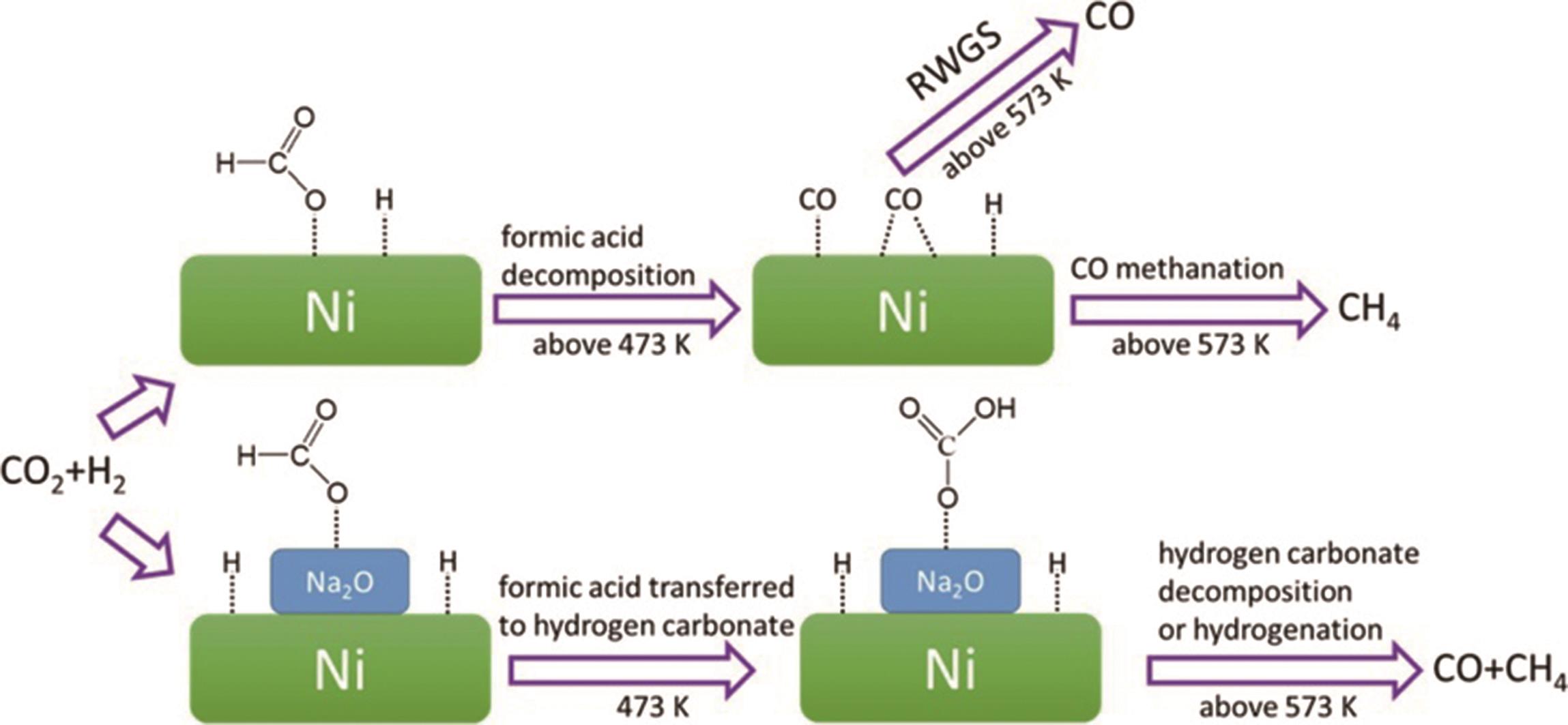
| 17 | WU H C, CHEN T C, WU J H, et al. Influence of sodium-modified Ni/SiO2 catalysts on the tunable selectivity of CO2 hydrogenation: effect of the CH4 selectivity, reaction pathway and mechanism on the catalytic reaction[J]. J Colloid Interf Sci, 2021, 586: 514-527. |
| 18 | HONGMANOROM P, ASHOK J, ZHANG G, et al. Enhanced performance and selectivity of CO2 methanation over phyllosilicate structure derived Ni-Mg/SBA-15 catalysts[J]. Appl Catal B, 2021, 282: 119564. |
| 19 | SIAKAVELAS G I, CHARISIOU N D, ALKHOORI S, et al. Highly selective and stable nickel catalysts supported on ceria promoted with Sm2O3, Pr2O3 and MgO for the CO2 methanation reaction[J]. Appl Catal B, 2021, 282: 119562. |
| 20 | WU C Y, LIN L L, LIU J J, et al. Inverse ZrO2/Cu as a highly efficient methanol synthesis catalyst from CO2 hydrogenation[J]. Nat Commun, 2020, 11(1): 5767. |
| 21 | CHEN K, FANG H, WU S, et al. CO2 hydrogenation to methanol over Cu catalysts supported on La-modified SBA-15: the crucial role of Cu-LaOx interfaces[J]. Appl Catal B, 2019, 251: 119-129. |
| 22 | HU B, YIN Y Z, LIU G L, et al. Hydrogen spillover enabled active Cu sites for methanol synthesis from CO2 hydrogenation over Pd doped CuZn catalysts[J]. J Catal, 2018, 359: 17-26. |
| 23 | NUMPILAI T, CHANLEK N, POO-ARPORN Y, et al. Pore size effects on physicochemical properties of Fe-Co/K-Al2O3 catalysts and their catalytic activity in CO2 hydrogenation to light olefins[J]. Appl Surf Sci, 2019, 483: 581-592. |
| 24 | SATTHAWONG R, KOIZUMI N, SONG C S, et al. Comparative study on CO2 hydrogenation to higher hydrocarbons over Fe-based bimetallic catalysts[J]. Top Catal, 2014, 57(6/7/8/9): 588-594. |
| 25 | RAMIREZ A, GEVERS L, BAVYKINA A, et al. Metal organic framework-derived iron catalysts for the direct hydrogenation of CO2 to short chain olefins[J]. ACS Catal, 2018, 8(10): 9174-9182. |
| 26 | RUI N, ZHANG X, ZHANG F, et al. Highly active Ni/CeO2 catalyst for CO2 methanation: preparation and characterization[J]. Appl Catal B, 2021, 282: 119581. |
| 27 | XIE Z H, TIAN D, XIE M, et al. Interfacial active sites for CO2 assisted selective cleavage of C—C/C—H bonds in ethane[J]. Chem, 2020, 6(10): 2703-2716. |
| 28 | YAN B H, YAO S Y, KATTEL S, et al. Active sites for tandem reactions of CO2 reduction and ethane dehydrogenation[J]. Proc Natl Acad Sci USA, 2018, 115(33): 8278-8283. |
| 29 | LI S W, XU Y, CHEN Y F, et al. Tuning the selectivity of catalytic carbon dioxide hydrogenation over iridium/cerium oxide catalysts with a strong metal-support interaction[J]. Angew Chem Int Ed, 2017, 56(36): 10761-10765. |
| 30 | YE R P, DING J, GONG W, et al. CO2 hydrogenation to high-value products via heterogeneous catalysis[J]. Nat Commun, 2019, 10: 5698. |
| 31 | MOTA F M, KIM D H. From CO2 methanation to ambitious long-chain hydrocarbons: alternative fuels paving the path to sustainability[J]. Chem Soc Rev, 2019, 48(1): 205-259. |
| 32 | ZHAO Y, GAO W, LI S, et al. Solar-versus thermal-driven catalysis for energy conversion[J]. Joule, 2019, 3(4): 920-937. |
| 33 | XIE S, ZHANG Q, LIU G, et al. Photocatalytic and photoelectrocatalytic reduction of CO2 using heterogeneous catalysts with controlled nanostructures[J]. Chem Commun, 2016, 52(1): 35-59. |
| 34 | YANG F, ELNABAWY A O, SCHIMMENTI R, et al. Bismuthene for highly efficient carbon dioxide electroreduction reaction[J]. Nat Commun, 2020, 11(1): 1088. |
| 35 | CHEN C, LI Y, YU S, et al. Cu-Ag tandem catalysts for high-rate CO2 electrolysis toward multicarbons[J]. Joule, 2020, 4(8): 1688-1699. |
| 36 | KWAK J H, KOVARIK L, SZANYI J. CO2 reduction on supported Ru/Al2O3 catalysts: cluster size dependence of product selectivity[J]. ACS Catal, 2013, 3(11): 2449-2455. |
| 37 | DUTTA A, RAHAMAN M, LUEDI N C, et al. Morphology matters: tuning the product distribution of CO2 electroreduction on oxide-derived Cu foam catalysts[J]. ACS Catal, 2016, 6(6): 3804-3814. |
| 38 | KWAK J H, KOVARIK L, SZANYI J. Heterogeneous catalysis on atomically dispersed supported metals: CO2 reduction on multifunctional Pd catalysts[J]. ACS Catal, 2013, 3(9): 2094-2100. |
| 39 | WU H C, CHANG Y C, WU J H, et al. Methanation of CO2 and reverse water gas shift reactions on Ni/SiO2 catalysts: the influence of particle size on selectivity and reaction pathway[J]. Catal Sci Technol, 2015, 5(8): 4154-4163. |
| 40 | LIU M H, CHEN H A, CHEN C S, et al. Tiny Ni particles dispersed in platelet SBA-15 materials induce high efficiency for CO2 methanation[J]. Nanoscale, 2019, 11(43): 20741-20753. |
| 41 | YE J Y, LIU C J, MEI D H, et al. Active oxygen vacancy site for methanol synthesis from CO2 hydrogenation on In2O3(110): A DFT study[J]. ACS Catal, 2013, 3(6): 1296-1306. |
| 42 | QIU R, DING Z L, XU Y M, et al. CuPd bimetallic catalyst with high Cu/Pd ratio and its application in CO2 hydrogenation[J]. Appl Surf Sci, 2021, 544(2): 148974. |
| 43 | RIVERA-CARCAMO C, SCARFIELLO C, GARCIA A B, et al. Stabilization of metal single atoms on carbon and TiO2 supports for CO2 hydrogenation: the importance of regulating charge transfer[J]. Adv Mater Interfaces, 2021, 8(8): 2001777. |
| 44 | ZHU J D, SU Y Q, CHAI J C, et al. Mechanism and nature of active sites for methanol synthesis from CO/CO2 on Cu/CeO2[J]. ACS Catal, 2020, 10(19): 11532-11544. |
| 45 | SARFRAZ S, GARCIA-ESPARZA A T, JEDIDI A, et al. Cu-Sn bimetallic catalyst for selective aqueous electroreduction of CO2 to CO[J]. ACS Catal, 2016, 6(5): 2842-2851. |
| 46 | GAO P, DANG S S, LI S G, et al. Direct production of lower olefins from CO2 conversion via bifunctional catalysis[J]. ACS Catal, 2018, 8(1): 571-578. |
| 47 | ZHU Y F, ZHENG J, YE J Y, et al. Copper-zirconia interfaces in UiO-66 enable selective catalytic hydrogenation of CO2 to methanol[J]. Nat Commun, 2020, 11(1): 5849. |
| 48 | YANG G, KUWAHARA Y, MORI K, et al. PdAg alloy nanoparticles encapsulated in N-doped microporous hollow carbon spheres for hydrogenation of CO2 to formate[J]. Appl Catal B, 2021, 283: 119628. |
| 49 | GUO Y, MEI S, YUAN K, et al. Low-temperature CO2 methanation over CeO2-supported Ru single atoms, nanoclusters, and nanoparticles competitively tuned by strong metal-support interactions and H-spillover effect[J]. ACS Catal, 2018, 8(7): 6203-6215. |
| 50 | CHAIPRADITGUL N, NUMPILAI T, KUI CHENG C, et al. Tuning interaction of surface-adsorbed species over Fe/K-Al2O3 modified with transition metals (Cu, Mn, V, Zn or Co) on light olefins production from CO2 hydrogenation[J]. Fuel, 2021, 283: 119248. |
| 51 | WANG L, WANG L, ZHANG J, et al. Selective hydrogenation of CO2 to ethanol over cobalt catalysts[J]. Angew Chem Int Ed, 2018, 57(21): 6104-6108. |
| 52 | WEI J, YAO R, GE Q, et al. Precisely regulating Brønsted acid sites to promote the synthesis of light aromatics via CO2 hydrogenation[J]. Appl Catal B, 2021, 283: 119648. |
| 53 | NI Y, CHEN Z, FU Y, et al. Selective conversion of CO2 and H2 into aromatics[J]. Nat Commun, 2018, 9: 3457. |
| 54 | CHANG S, MAO W S, NA W, et al. Study on the performance of NiO/ZnxZr1- x catalysts for CO2 hydrogenation[J]. Rsc Adv, 2020, 10(70): 42790-42798. |
| 55 | ZHANG Z Y, XIAO Q B, GU J. Effective synthesis of zeolite-encapsulated Ni nanoparticles with excellent catalytic performance for hydrogenation of CO2 to CH4[J]. Dalton Trans, 2020, 49(42): 14771-14775. |
| 56 | CHOI E J, LEE Y H, LEE D W, et al. Hydrogenation of CO2 to methanol over Pd-Cu/CeO2 catalysts[J]. Mol Catal, 2017, 434: 146-153. |
| 57 | LIAO F L, WU X P, ZHENG J W, et al. A promising low pressure methanol synthesis route from CO2 hydrogenation over Pd@Zn core-shell catalysts[J]. Green Chem, 2017, 19(1): 270-280. |
| 58 | MARTIN O, MARTIN A J, MONDELLI C, et al. Indium oxide as a superior catalyst for methanol synthesis by CO2 hydrogenation[J]. Angew Chem Int Ed, 2016, 55(21): 6261-6265. |
| 59 | ZHANG J, SU X, WANG X, et al. Promotion effects of Ce added Fe-Zr-K on CO2 hydrogenation to light olefins[J]. React Kinet Mech Catal, 2018, 124(2): 575-585. |
| 60 | NUMPILAI T, CHANLEK N, POO-ARPORN Y, et al. Tuning interactions of surface-adsorbed species over Fe-Co/K-Al2O3 catalyst by different K contents: selective CO2 hydrogenation to light olefins[J]. ChemCatChem, 2020, 12(12): 3306-3320. |
| 61 | WANG X, ZHANG J, CHEN J, et al. Effect of preparation methods on the structure and catalytic performance of Fe-Zn/K catalysts for CO2 hydrogenation to light olefins[J]. Chin J Chem Eng, 2018, 26(4): 761-767. |
| 62 | LI Z L, WANG J J, QU Y Z, et al. Highly selective conversion of carbon dioxide to lower olefins[J]. ACS Catal, 2017, 7(12): 8544-8548. |
| 63 | LIU X L, WANG M H, YIN H R, et al. Tandem catalysis for hydrogenation of CO and CO2 to lower olefins with bifunctional catalysts composed of spinel oxide and SAPO-34[J]. ACS Catal, 2020, 10(15): 8303-8314. |
| 64 | LIU X L, WANG M H, ZHOU C, et al. Selective transformation of carbon dioxide into lower olefins with a bifunctional catalyst composed of ZnGa2O4 and SAPO-34[J]. Chem Commun, 2018, 54(2): 140-143. |
| 65 | VOGT C, MONAI M, KRAMER G J, et al. The renaissance of the Sabatier reaction and its applications on earth and in space[J]. Nat Catal, 2019, 2(3): 188-197. |
| 66 | CHANG F W, KUO M S, TSAY M T, et al. Hydrogenation of CO2 over nickel catalysts on rice husk ash-alumina prepared by incipient wetness impregnation[J]. Appl Catal A-Gen, 2003, 247(2): 309-320. |
| 67 | UNWISET P, CHANAPATTHARAPOL K C, KIDKHUNTHOD P, et al. Catalytic activities of titania-supported nickel for carbon-dioxide methanation[J]. Chem Eng Sci, 2020, 228: 115955. |
| 68 | ZHANG L, PAN J, LONG Y, et al. CeO2-encapsulated hollow Ag-Au nanocage hybrid nanostructures as high-performance catalysts for cascade reactions[J]. Small, 2019, 15(43): 1903182. |
| 69 | LI L, LIU Y, WANG Q, et al. CeO2 supported low-loading Au as an enhanced catalyst for low temperature oxidation of carbon monoxide[J]. Crystengcomm, 2019, 21(46): 7108-7113. |
| 70 | DUYAR M S, TREVIÑO M A A, FARRAUTO R J. Dual function materials for CO2 capture and conversion using renewable H2[J]. Appl Catal B, 2015, 168/169: 370-376. |
| 71 | PROAÑO L, TELLO E, ARELLANO-TREVINO M A, et al. In-situ DRIFTS study of two-step CO2 capture and catalytic methanation over Ru, “Na2O”/Al2O3 dual functional material[J]. Appl Surf Sci, 2019, 479: 25-30. |
| 72 | PORTA A, VISCONTI C G, CASTOLDI L, et al. Ru-Ba synergistic effect in dual functioning materials for cyclic CO2 capture and methanation[J]. Appl Catal B, 2021, 283: 119654. |
| 73 | ZHAO M, FENG J, YANG W, et al. Recent advances in graphitic carbon nitride supported single-atom catalysts for energy conversion[J]. ChemCatChem, 2021, 13(5): 1250-1270. |
| 74 | ZHOU X, LI K, LIN Y, et al. A single-atom manipulation approach for synthesis of atomically mixed nanoalloys as efficient catalysts[J]. Angew Chem Int Ed, 2020, 59(32): 13568-13574. |
| 75 | BANSODE A, TIDONA B, VON ROHR P R, et al. Impact of K and Ba promoters on CO2 hydrogenation over Cu/Al2O3 catalysts at high pressure[J]. Catal Sci Technol, 2013, 3(3): 767-778. |
| 76 | BAN H, LI C, ASAMI K, et al. Influence of rare-earth elements (La, Ce, Nd and Pr) on the performance of Cu/Zn/Zr catalyst for CH3OH synthesis from CO2[J]. Catal Commun, 2014, 54: 50-54. |
| 77 | NOMURA N, TAGAWA T, GOTO S. Effect of acid-base properties on copper catalysts for hydrogenation of carbon dioxide[J]. React Kinet Catal Lett, 1998, 63(1): 21-25. |
| 78 | PHONGAMWONG T, CHANTAPRASERTPORN U, WITOON T, et al. CO2 hydrogenation to methanol over CuO-ZnO-ZrO2-SiO2 catalysts: effects of SiO2 contents[J]. Chem Eng J, 2017, 316: 692-703. |
| 79 | HU J, LI Y, ZHEN Y, et al. In situ FTIR and ex situ XPS/HS-LEIS study of supported Cu/Al2O3 and Cu/ZnO catalysts for CO2 hydrogenation[J]. Chinese J Catal, 2021, 42(3): 367-375. |
| 80 | FREI M S, MONDELLI C, CESARINI A, et al. Role of zirconia in indium oxide-catalyzed CO2 hydrogenation to methanol[J]. ACS Catal, 2020, 10(2): 1133-1145. |
| 81 | CHEN T Y, CAO C X, CHEN T B, et al. Unraveling highly tunable selectivity in CO2 hydrogenation over bimetallic In-Zr oxide catalysts[J]. ACS Catal, 2019, 9(9): 8785-8797. |
| 82 | GAO P, LI S G, BU X N, et al. Direct conversion of CO2 into liquid fuels with high selectivity over a bifunctional catalyst[J]. Nat Chem, 2017, 9(10): 1019-1024. |
| 83 | KATTEL S, YAN B H, CHEN J G G, et al. CO2 hydrogenation on Pt, Pt/SiO2 and Pt/TiO2: importance of synergy between Pt and oxide support[J]. J Catal, 2016, 343: 115-126. |
| 84 | BAI S X, SHAO Q, FENG Y G, et al. Highly efficient carbon dioxide hydrogenation to methanol catalyzed by zigzag platinum-cobalt nanowires[J]. Small, 2017, 13(22): 1604311. |
| 85 | LI X L, ZENG Z Y, HU B, et al. Surface-atom dependence of ZnO-supported Ag@Pd core@shell nanocatalysts in CO2 hydrogenation to CH3OH[J]. ChemCatChem, 2017, 9(6): 924-928. |
| 86 | SEN R, KOCH C J, GOEPPERT A, et al. Tertiary amine-ethylene glycol based tandem CO2 capture and hydrogenation to methanol: direct utilization of post-combustion CO2[J]. ChemSusChem, 2020, 13(23): 6318-6322. |
| 87 | VISCONTI C G, MARTINELLI M, FALBO L, et al. CO2 hydrogenation to lower olefins on a high surface area K-promoted bulk Fe-catalyst[J]. Appl Catal B, 2017, 200: 530-542. |
| 88 | GRABOW L C, MAVRIKAKIS M. Mechanism of methanol synthesis on Cu through CO2 and CO hydrogenation[J]. ACS Catal, 2011, 1(4): 365-384. |
| 89 | PRIETO G. Carbon dioxide hydrogenation into higher hydrocarbons and oxygenates: thermodynamic and kinetic bounds and progress with heterogeneous and homogeneous catalysis[J]. ChemSusChem, 2017, 10(6): 1056-1070. |
| 90 | YANG C, ZHAO H B, HOU Y L, et al. Fe5C2 nanoparticles: a facile bromide-induced synthesis and as an active phase for Fischer-Tropsch synthesis[J]. J Am Chem Soc, 2012, 134(38): 15814-15821. |
| 91 | WANG S W, WU T J, LIN J, et al. Iron-potassium on single-walled carbon nanotubes as efficient catalyst for CO2 hydrogenation to heavy olefins[J]. ACS Catal, 2020, 10(11): 6389-6401. |
| 92 | NUMPILAI T, WITOON T, CHANLEK N, et al. Structure activity relationships of Fe-Co/K-Al2O3 catalysts calcined at different temperatures for CO2 hydrogenation to light olefins[J]. Appl Catal A: Gen, 2017, 547: 219-229. |
| 93 | WEI J, GE Q, YAO R, et al. Directly converting CO2 into a gasoline fuel[J]. Nat Commun, 2017, 8: 15174.. |
| 94 | MEIRI N, DINBURG Y, AMOYAL M, et al. Novel process and catalytic materials for converting CO2 and H2 containing mixtures to liquid fuels and chemicals[J]. Faraday Discuss, 2015, 183: 197-215. |
| 95 | AL-DOSSARY M, ISMAIL A A, FIERRO J L G, et al. Effect of Mn loading onto MnFeO nanocomposites for the CO2 hydrogenation reaction[J]. Appl Catal B, 2015, 165: 651-660. |
| 96 | DORNER R W, HARDY D R, WILLIAMS F W, et al. K and Mn doped iron-based CO2 hydrogenation catalysts: detection of KAlH4 as part of the catalyst's active phase[J]. Appl Catal A: Gen, 2010, 373(1/2): 112-121. |
| 97 | YANG Y, XIANG H W, ZHANG R L, et al. K and Mn doped iron-based CO2 hydrogenation catalysts: detection of KAlH4 as part of the catalyst's active phase[J]. Appl Catal A: Gen, 2010, 373(1/2): 112-121. |
| 98 | GONG W B, YE R P, DING J, et al. Effect of copper on highly effective Fe-Mn based catalysts during production of light olefins via Fischer-Tropsch process with low CO2 emission[J]. Appl Catal B, 2020, 278: 119302. |
| 99 | GUPTA S, JAIN V K, JAGADEESAN D. Fine tuning the composition and nanostructure of Fe-based core-shell nanocatalyst for efficient CO2 hydrogenation[J]. ChemNanoMat, 2016, 2(10): 989-996. |
| 100 | VAN DER LAAN G P, BEENACKERS A. Kinetics and selectivity of the Fischer-Tropsch synthesis: a literature review[J]. Catal Rev Sci Eng, 1999, 41(3/4): 255-318. |
| 101 | JIAO F, LI J J, PAN X L, et al. Selective conversion of syngas to light olefins[J]. Science, 2016, 351(6277): 1065-1068. |
| 102 | CHENG K, GU B, LIU X L, et al. Direct and highly selective conversion of synthesis gas into lower olefins: design of a bifunctional catalyst combining methanol synthesis and carbon-carbon coupling[J]. Angew Chem Int Ed, 2016, 55(15): 4725-4728. |
| [1] | 张丹, 尚润梅, 赵振涛, 李君华, 邢锦娟. V/Ce-Al2O3催化甲醇选择性氧化制备二甲氧基甲烷[J]. 应用化学, 2022, 39(9): 1429-1436. |
| [2] | 王克, 汪啸, 宋术岩. 甲烷直接催化氧化制备甲醇近期研究进展[J]. 应用化学, 2022, 39(4): 540-558. |
| [3] | 孙立智, 吕浩, 闵晓文, 刘犇. 介孔钯-硼合金纳米颗粒的制备和甲醇氧化电催化性能[J]. 应用化学, 2022, 39(4): 673-684. |
| [4] | 吴之强, 韩新宁, 刘阳, 王刚, 詹海鹃, 刘万毅. 水相或无溶剂绿色催化合成双吲哚基甲烷研究进展[J]. 应用化学, 2021, 38(8): 881-896. |
| [5] | 田舒阳, 吕荣文. 氧化镁载金钯双金属无溶剂催化氧化苯甲醇制备苯甲醛[J]. 应用化学, 2021, 38(7): 789-799. |
| [6] | 罗策, 颜燕, 李剑, 刘婷, 白焕焕, 卢凡, 冯婧. 甲烷动态反应-电感耦合等离子体质谱标准加入测定高纯钛中痕量铁[J]. 应用化学, 2021, 38(7): 874-880. |
| [7] | 乔宗文, 陈涛. 具有高效长侧链的芘磺酸型磺化聚砜阳离子交换膜的性能[J]. 应用化学, 2021, 38(6): 668-674. |
| [8] | 黄婕颖, 刘建军, 左胜利, 韩文静, 于迎春. Ag3PO4量子点/石墨相氮化碳纳米片复合光催化剂的制备及其对苯甲醇选择性氧化活性[J]. 应用化学, 2020, 37(9): 1030-1037. |
| [9] | 苗中硕,门永锋. 快速扫描量热法研究聚对苯二甲酸-1,4-环己烷二甲醇酯的结晶和熔融行为[J]. 应用化学, 2020, 37(6): 642-649. |
| [10] | 高佳, 宋夫交, 程文强, 葛艳, 许琦. Pd-Cu/ZrO2催化CO2加氢制甲醇的性能[J]. 应用化学, 2020, 37(2): 160-167. |
| [11] | 姜涛, 杜连云, 朱爽, 王欢, 战宇, 俞萍, 张哲, 王恩鹏, 陈长宝. 实时直接分析电离质谱法快速检测化妆品中的3种成分[J]. 应用化学, 2020, 37(11): 1333-1339. |
| [12] | 邓光荣, 梁亮, 李晨阳, 刘长鹏, 葛君杰, 邢巍. 直接甲醇燃料电池甲醇传质过程分析及浓度控制策略[J]. 应用化学, 2019, 36(10): 1211-1220. |
| [13] | 孙墨杰, 吕涛, 徐维林. 微流控合成高活性甲醇氧化碳载铂钌电催化剂[J]. 应用化学, 2018, 35(5): 564-573. |
| [14] | 孙墨杰, 吕涛, 徐维林. 微流控合成高活性甲醇氧化碳载铂钌电催化剂[J]. 应用化学, 2018, 35(5): 0-0. |
| [15] | 杨程, 齐金山, 崔杏雨, 程文萍, 马静红, 李瑞丰. A型沸石/活性炭复合材料的制备及甲烷/氮气吸附分离性能[J]. 应用化学, 2018, 35(4): 462-468. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||