
应用化学 ›› 2022, Vol. 39 ›› Issue (4): 585-598.DOI: 10.19894/j.issn.1000-0518.210448
稀土溴化物固态电解质材料在全固态电池中的应用研究进展
张琦1, 张乾1( ), 师晓梦2, 孔娅淇1, 高可心1, 杜亚平2(
), 师晓梦2, 孔娅淇1, 高可心1, 杜亚平2( )
)
- 1.西安理工大学理学院应用化学系,西北旱区生态水利国家重点实验室,西安 710048
2.南开大学材料科学与工程学院&国家新材料研究院,天津市稀土材料及应用重点实验室,南开大学稀土与无机功能材料研究中心,天津 300350
-
收稿日期:2021-09-01接受日期:2021-11-22出版日期:2022-04-01发布日期:2022-04-19 -
通讯作者:张乾,杜亚平 -
作者简介:E-mail:qzh@xaut.edu.cn
E-mail:ypdu@nankai.edu.cn;
第一联系人:共同第一作者 -
基金资助:西北旱区生态水利工程国家重点实验室“节水再利用创新团队”项目(2019KJCXTD-8);国家自然科学基金(21971117);南开大学中央高校研究基金(63186005);天津市稀土材料与应用重点实验室(ZB19500202);稀土资源利用国家重点实验室开放基金(RERU2019001);111项目(B18030);京津冀协同创新项目(19YFSLQY00030);天津市自然科学基金杰出青年项目(20JCJQJC00130);天津市自然科学基金重点项目(20JCZDJC00650)
Research Progress of Rare Earth Bromides Based Solid Electrolytes for All⁃Solid⁃State Batteries
Qi ZHANG1, Qian ZHANG1( ), Xiao-Meng SHI2, Ya-Qi KONG1, Ke-Xin GAO1, Ya-Ping DU2(
), Xiao-Meng SHI2, Ya-Qi KONG1, Ke-Xin GAO1, Ya-Ping DU2( )
)
- 1.State Key Laboratory of Eco-Hydraulics in Northwest Arid Region,Department of Applied Chemistry,Xi'an University of Technology,Xi'an 710048,China
2.Tianjin Key Lab for Rare Earth Materials and Applications,Center for Rare Earth and Inorganic Functional Materials,School of Materials Science and Engineering & National Institute for Advanced Materials,Nankai University,Tianjin 300350,China
-
Received:2021-09-01Accepted:2021-11-22Published:2022-04-01Online:2022-04-19 -
Contact:Qian ZHANG,Ya-Ping DU -
Supported by:the “Water Saving and Reuse Innovation Team” Program of State Key Laboratory of Eco?Hydraulics in Northwest Arid Region(2019KJCXTD?8);from the National Natural Science Foundation of China(21971117);Functional Research Funds for the Central Universities, Nankai University(63186005);Tianjin Key Lab for Rare Earth Materials and Applications(ZB19500202);the Open Funds of the State Key Laboratory of Rare Earth Resource Utilization(RERU2019001);111 Project from China(B18030);Beijing?Tianjin?Hebei Collaborative Innovation Project(19YFSLQY00030);the Outstanding Youth Project of Tianjin Natural Science Foundation(20JCJQJC00130);the Key Project of Tianjin Natural Science Foundation(20JCZDJC00650)
摘要:
全固态锂电池具有优异的安全性能和能量密度,有望成为替代传统有机液态锂离子电池的下一代储能产品。固态电解质是决定全固态电池性能的关键材料,其中卤化物固态电解质,尤其是稀土溴化物固态电解质(RE-BSEs)材料近年来在离子电导率(高达mS/cm数量级)和电化学稳定性(1.5~3.4 V vs.Li+/Li)等方面取得了一系列重要的研究进展,具有可期待的应用前景。本文通过对RE-BSEs的发展历程、研究进展、技术瓶颈、发展方向和应用前景等方面进行综述和回顾,给予研究人员在RE-BSEs的合成方法、锂离子传输机理、构效关系和材料设计等方面的参考和启发。稀土是我国乃至世界的重要战略资源,RE-BSEs材料的研究和重要成果明示了稀土元素在固态离子导体和新能源领域的重大价值,因此,做好稀土在该方面的研究工作对能源结构调整、节能减排、碳达峰和碳中和具有重大意义。
中图分类号:
引用本文
张琦, 张乾, 师晓梦, 孔娅淇, 高可心, 杜亚平. 稀土溴化物固态电解质材料在全固态电池中的应用研究进展[J]. 应用化学, 2022, 39(4): 585-598.
Qi ZHANG, Qian ZHANG, Xiao-Meng SHI, Ya-Qi KONG, Ke-Xin GAO, Ya-Ping DU. Research Progress of Rare Earth Bromides Based Solid Electrolytes for All⁃Solid⁃State Batteries[J]. Chinese Journal of Applied Chemistry, 2022, 39(4): 585-598.
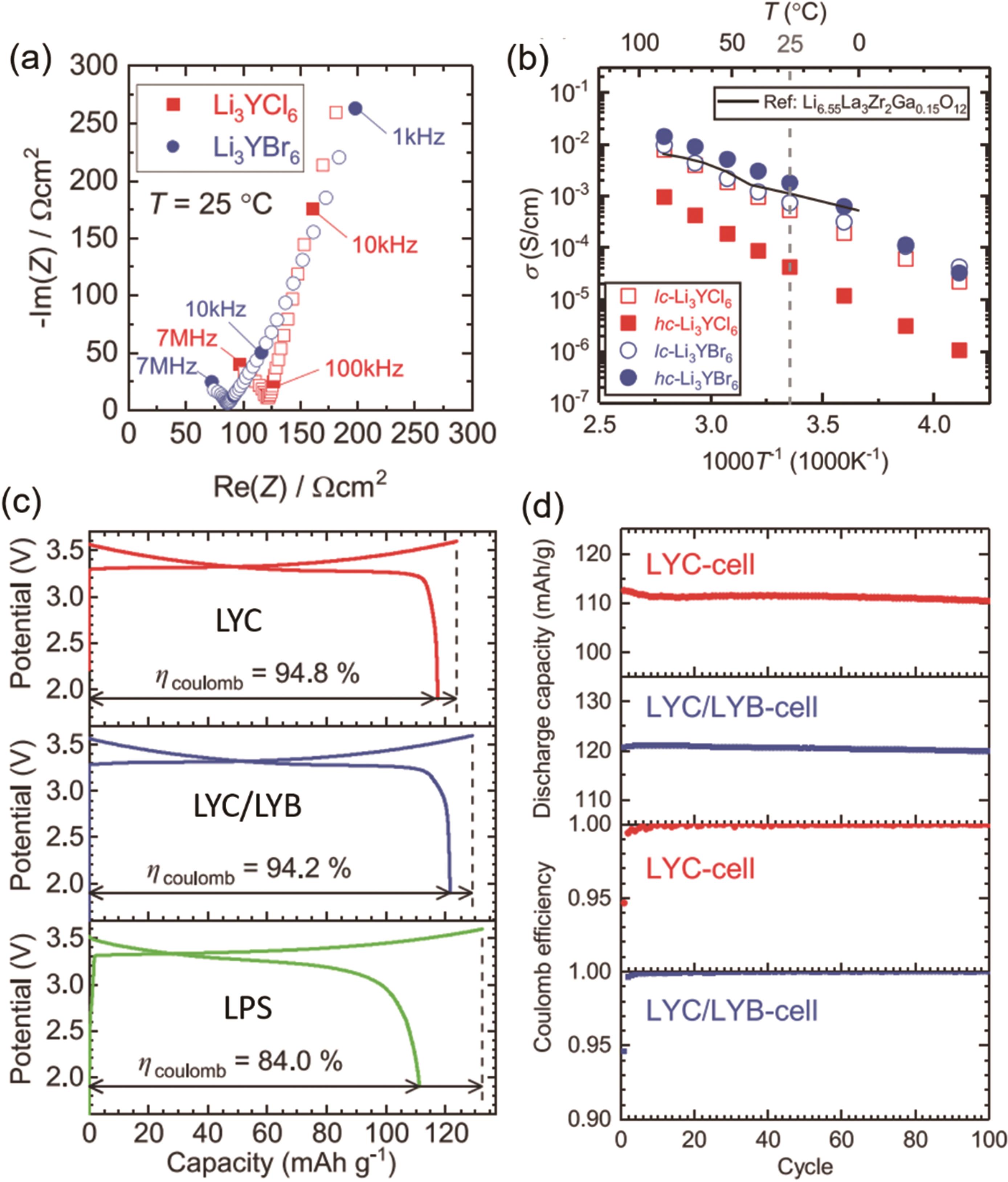
图2 (a) LYC和LYB的阻抗图谱;(b) LYC和LYB的Arrhenius电导率图;(c) 基于LYC、LYC/LYB和Li2S-P2S5 (LPS)的全固态电池的初始充放电曲线;(d) 在100次循环过程中,LYC电池和LYC/ LYB电池的放电容量和库仑效率[35]
Fig.2 (a) The Nyquist plots of the EIS measurement results of LYC and LYB. (b) Arrhenius conductivity plots of LYC and LYB. (c) Initial charge/discharge curves of all-solid-state cells (LYC, LYC/LYB, Li2S-P2S5 (LPS)). (d) The discharge capacity retention and coulombic efficiency of the LYC-cell and the LYC/LYB-cell for 100 cycles[35]
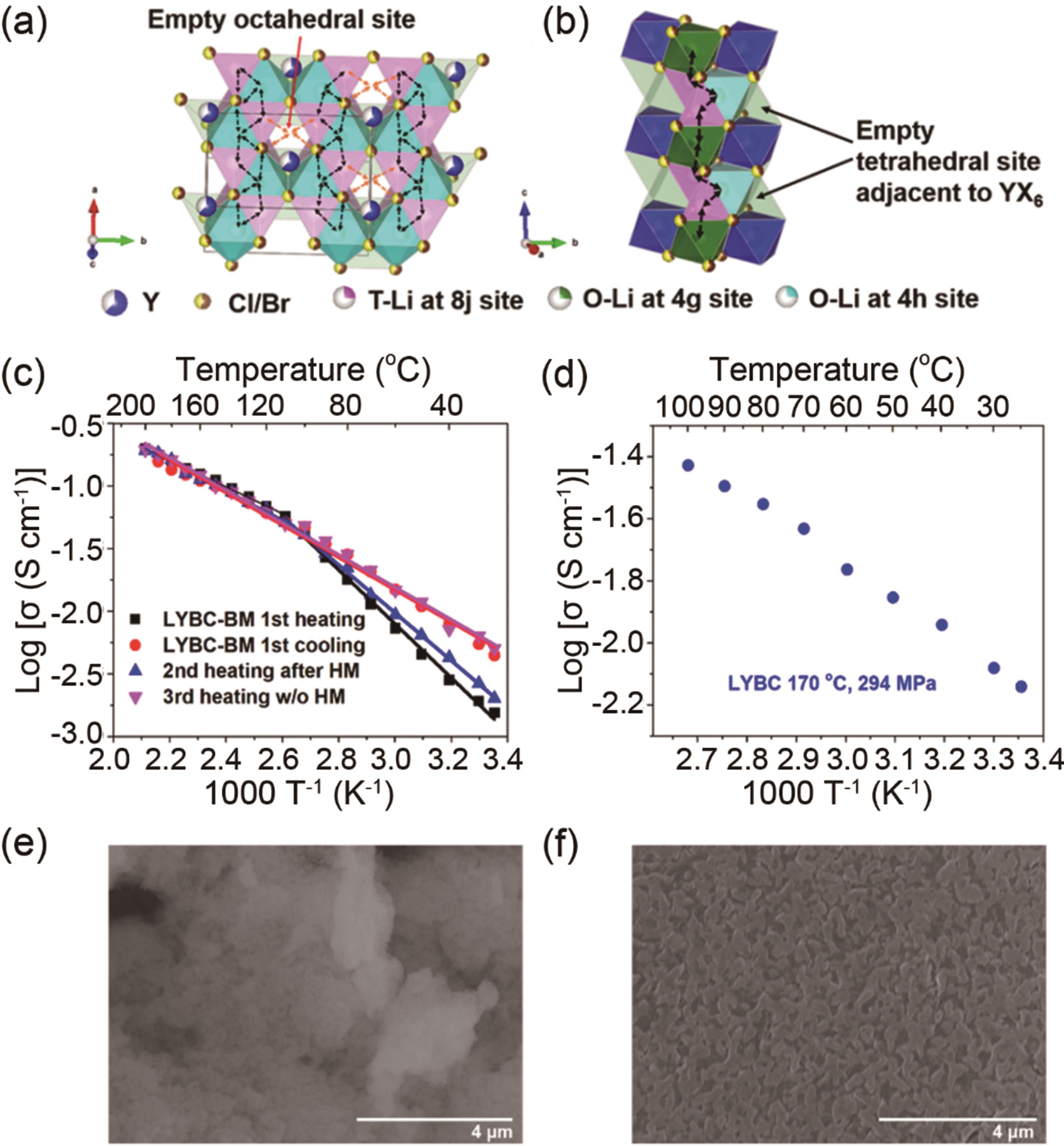
图3 (a, b) LYBC-HP(热压)在不同方向的锂离子扩散路径;(c) LYBC-BM(球磨)样品的Arrhenius图; (d) 在170 ℃、294 MPa条件下热压后,LYBC-BM 的Arrhenius图;(e) 冷压LYBC-BM和 (f) 热压LYBC-HP样品的扫描电子显微镜(SEM)图[43]
Fig. 3 (a, b) Proposed Li+ diffusion pathways along different orientations of LYBC-HP. (c) Arrhenius plots of conductivities of LYBC-BM samples. (d) LYBC-BM after hot-pressed at 170 ℃, 294 MPa. SEM images of (e) cold-pressed LYBC-BM and (f) hot-pressed LYBC-HP[43]
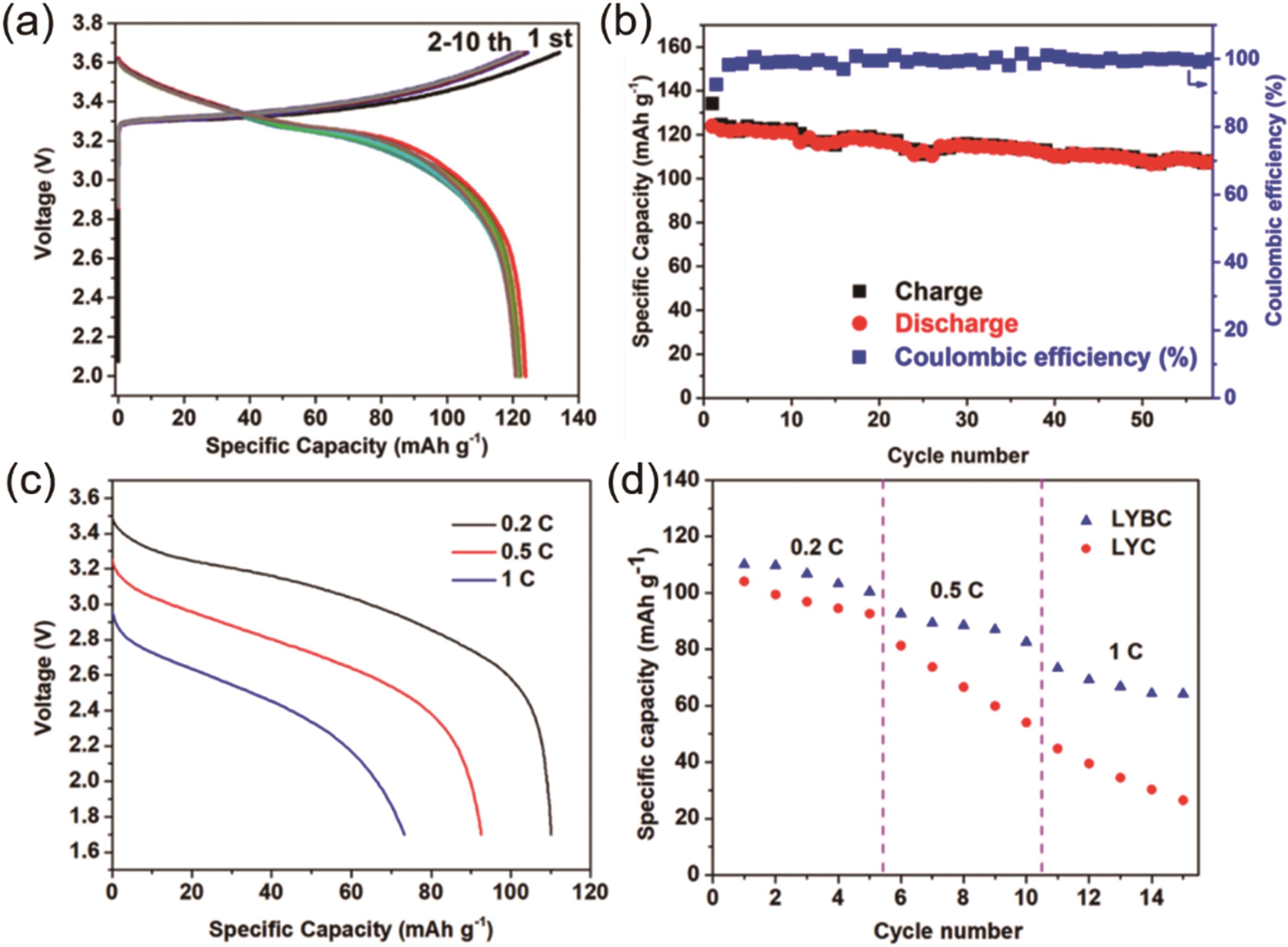
图4 LCO/LYBC/In-Li电池的电化学性能(a) 0.1 C下的充放电曲线;(b) 0.1 C下的循环性能;(c) 不同倍率下的放电曲线;(d)基于LYBC和LYC的全固态电池的倍率性能[43]
Fig. 4 Electrochemical performance of LCO/LYBC/In-Li battery(a) Charge-discharge curves at 0.1 C. (b) Cycling performance at 0.1 C. (c) Discharge curves at different rates. (d) Rate performances of ASSB cells with LYBC and LYC, respectively[43]
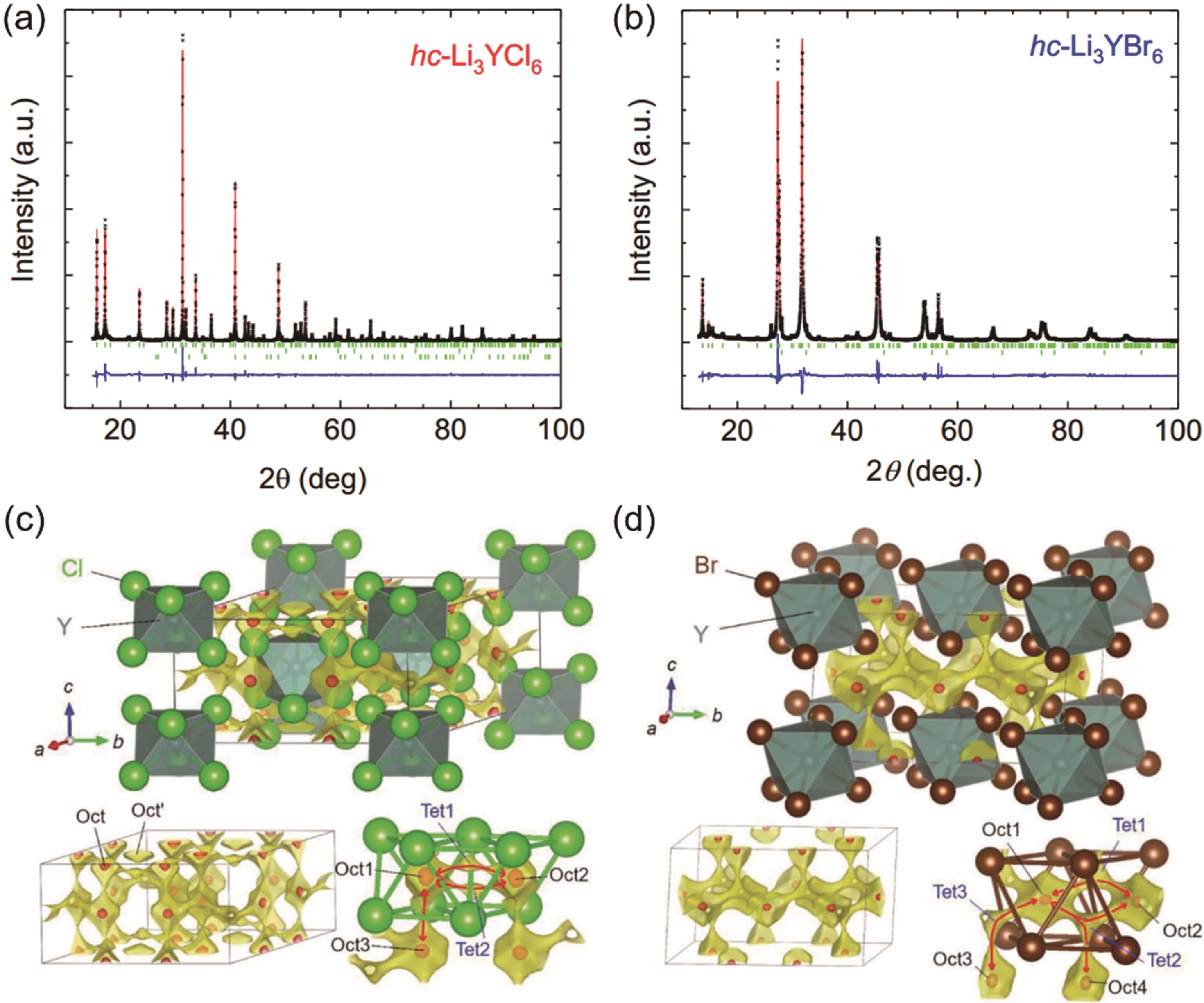
图5 (a,b)高结晶度hc-LYC和hc-LYB的XRD精修结果;(c,d) 晶体结构精修得到LYC和LYB的晶体结构,并叠加了基于BVSE的锂离子电势图。黄色表面对应离子传导路径,红色表面包围的区域对应稳定的锂离子位置[35]
Fig. 5 The XRD Rietveld refinement results of (a) hc-LYC and (b) hc-LYB. (c,d) The crystal structures of LYC and LYB obtained from Rietveld refinement, superimposed with a calculated BVSE-based lithium-ion potential map. The yellow surface represents the ionic conduction path, and the regions enclosed with red surfaces represent the stable lithium-ion positions[35]
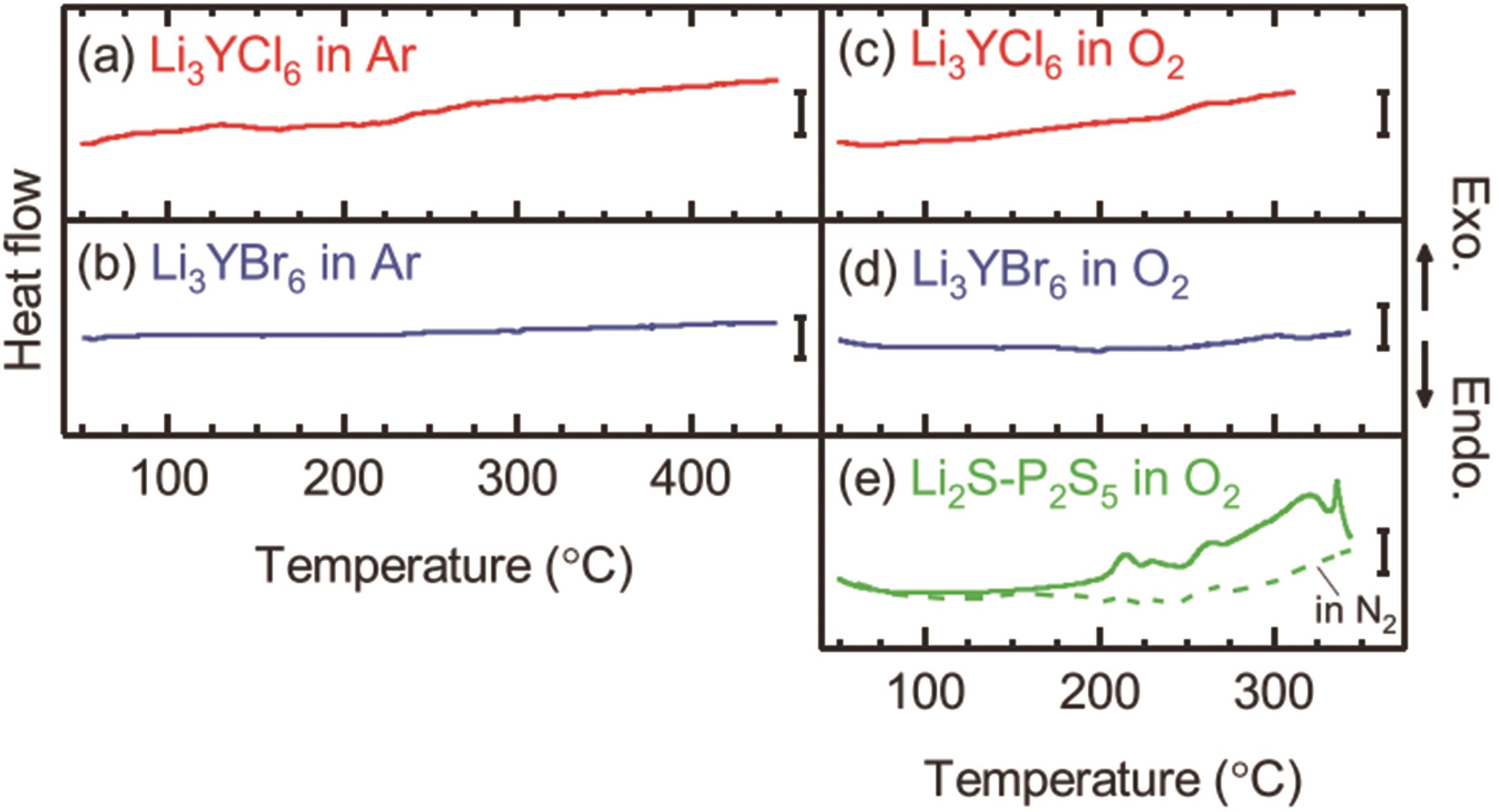
图6 (a-d) LYB和LYC在氩气和氧气中的DSC表征;(e) 硫化物电解质在氧气中的DSC表征[35]
Fig.6 (a-d) DSC characterization of LYB and LYC in argon and oxygen. (e) DSC characterization of sulfide electrolyte in oxygen[35]
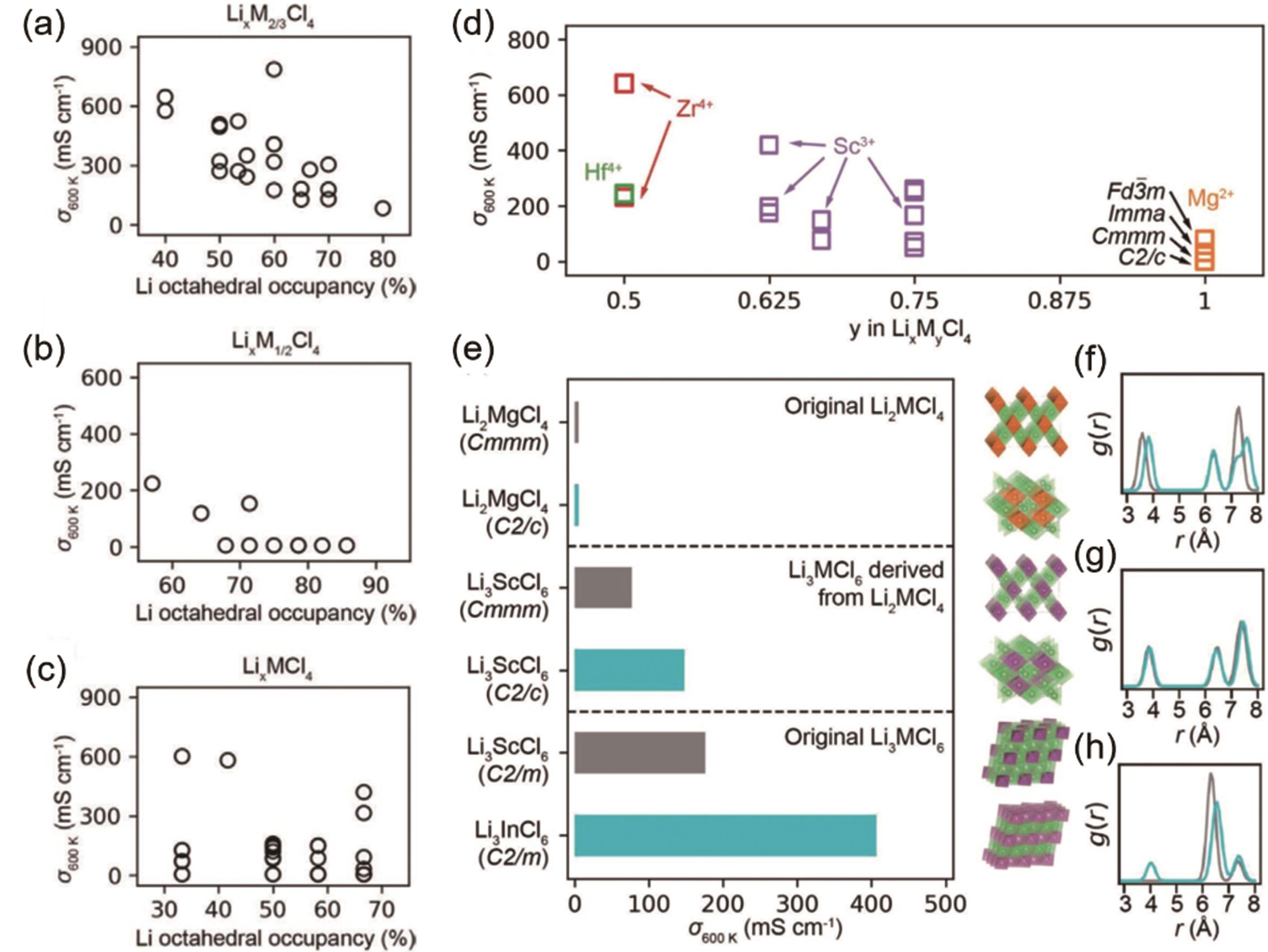
图7 在 (a) LixM2/3Cl4、(b) LixM1/2Cl4和 (c) LixMCl4中锂离子导率与锂八面体占有率的关系;(d) 由原始Li2MgCl4材料中的M离子取代衍生的假设的锂离子导体LixMyCl4(M = Sc3+,Zr4+和Hf4+),在600 K时的锂离子电导率与M离子浓度y的函数;(e) 原始Li2MgCl4、阳离子浓度降低的Li2MgCl4衍生的假设锂离子导体和原始Li3MCl6材料在600 K时的锂离子导率及其晶体结构(右)和 (f-h) M阳离子的径向分布函数图[59]
Fig. 7 Li-ion conductivities versus Li octahedral occupancy in (a) Li x M2/3Cl4, (b) Li x M1/2Cl4 and (c) Li x MCl4. (d) Li-ion conductivities as a function of M cation concentration y in hypothetical Li-ion conductors Li x M y Cl4, M = Sc3+, Zr4+, and Hf4+ at 600 K, substituted with reduced M cation concentration from original Li2MgCl4 materials. (e) Li-ion conductivities at 600 K of original Li2MgCl4, hypothetical Li-ion conductors derived from Li2MgCl4 with reduced cation concentrations, and original Li3MCl6 materials, along with their crystal structures (right) and (f-h) radial distribution function (RDF) g(r) of M cations[59]
| 1 | LARCHER D, TARASCON J M. Towards greener and more sustainable batteries for electrical energy storage[J]. Nat Chem, 2015, 7(1): 19-29. |
| 2 | ALBERTUS P, BABINEC S, LITZELMAN S, et al. Status and challenges in enabling the lithium metal electrode for high-energy and low-cost rechargeable batteries[J]. Nat Energy, 2018, 3(1): 16-21. |
| 3 | GOODENOUGH J B, KIM Y. Challenges for rechargeable Li batteries[J]. Chem Mater, 2010, 22(3): 587-603. |
| 4 | GOODENOUGH J B, PARK K S. The Li-ion rechargeable battery: a perspective[J]. J Am Chem Soc, 2013, 135(4): 1167-1176. |
| 5 | TARASCON J M, ARMAND M. Issues and challenges facing rechargeable lithium batteries[J]. Nature, 2001, 414(6861): 359-367. |
| 6 | ARMAND M, TARASCON J M. Building better batteries[J]. Nature, 2008, 451(7179): 652-657. |
| 7 | KATO Y, HORI S, SAITO T, et al. High-power all-solid-state batteries using sulfide superionic conductors[J]. Nat Energy, 2016, 1: 16030. |
| 8 | NAM Y J, CHO S J, OH D Y, et al. Bendable and thin sulfide solid electrolyte film: a new electrolyte opportunity for free-standing and stackable high-energy all-solid-state lithium-ion batteries[J]. Nano Lett, 2015, 15(5): 3317-3323. |
| 9 | SCHNELL J, GUNTHER T, KNOCHE T, et al. All-solid-state lithium-ion and lithium metal batteries-paving the way to large-scale production[J]. J Power Sources, 2018, 382: 160-175. |
| 10 | MA C, CHENG Y Q, CHEN K, et al. Mesoscopic framework enables facile ionic transport in solid electrolytes for Li batteries[J]. Adv Energy Mater, 2016, 6(11): 1600053. |
| 11 | BERNUY-LOPEZ C, MANALASTAS W, DEL AMO J M L, et al. Atmosphere controlled processing of Ga-substituted garnets for high Li-ion conductivity ceramics[J]. Chem Mater, 2014, 26(12): 3610-3617. |
| 12 | GEIGER C A, ALEKSEEV E, LAZIC B, et al. Crystal chemistry and stability of “Li7La3Zr2O12” garnet: a fast lithium-ion conductor[J]. Inorg Chem, 2011, 50(3): 1089-1097. |
| 13 | KAMAYA N, HOMMA K, YAMAKAWA Y, et al. A lithium superionic conductor[J]. Nat Mater, 2011, 10(9): 682-686. |
| 14 | SEINO Y, OTA T, TAKADA K, et al. A sulphide lithium super ion conductor is superior to liquid ion conductors for use in rechargeable batteries[J]. Energy Environ Sci, 2014, 7(2): 627-631. |
| 15 | ZHANG Q, GAO Z Q, SHI X M, et al. Recent advances on rare earths in solid lithium ion conductors[J]. J Rare Earths, 2021, 39(1): 1-10. |
| 16 | LI X, LIANG J, YANG X, et al. Progress and perspectives on halide lithium conductors for all-solid-state lithium batteries[J]. Energy Environ Sci, 2020, 13(5): 1429-1461. |
| 17 | LAU J, DEBLOCK R H, BUTTS D M, et al. Sulfide solid electrolytes for lithium battery applications[J]. Adv Energy Mater, 2018, 8(27): 1800933. |
| 18 | 惠康龙, 傅继澎, 高湉, 等. 金属硫化物在可充电电池中的研究进展[J]. 应用化学, 2020, 37(12): 1384-1402. |
| HUI K L, FU J P, GAO T, et al. Research progress of metal sulfides in rechargeable batteries[J]. Chinese J Appl Chem, 2020, 37(12): 1384-1402. | |
| 19 | SAKUDA A, HAYASHI A, TATSUMISAGO M, et al. Sulfide solid electrolyte with favorable mechanical property for all-solid-state lithium battery[J]. Sci Rep, 2013, 3: 2261. |
| 20 | PARK H, YU S, SIEGEL D J. Predicting charge transfer stability between sulfide solid electrolytes and Li metal anodes[J]. ACS Energy Lett, 2021, 6(1): 150-157. |
| 21 | MURAMATSU H, HAYASHI A, OHTOMO T, et al. Structural change of Li2S-P2S5 sulfide solid electrolytes in the atmosphere[J]. Solid State Ionics, 2011, 182(1): 116-119. |
| 22 | THOMPSON T, YU S H, WILLIAMS L, et al. Electrochemical window of the Li-ion solid electrolyte Li7La3Zr2O12[J]. ACS Energy Lett, 2017, 2(2): 462-468. |
| 23 | WANG D W, SUN Q, LUO J, et al. Mitigating the interfacial degradation in cathodes for high-performance oxide-based solid-state lithium batteries[J]. ACS Appl Mater Interfaces, 2019, 11(5): 4954-4961. |
| 24 | MANTHIRAM A, YU X W, WANG S F. Lithium battery chemistries enabled by solid-state electrolytes[J]. Nat Rev Mater, 2017, 2(4): 16103. |
| 25 | TYAGI A K, KOHLER J, BALOG P, et al. Syntheses and structures of Li3ScF6 and high pressure LiScF4-, luminescence properties of LiScF4, a new phase in the system LiF-ScF3[J]. J Solid State Chem, 2005, 178(9): 2620-2625. |
| 26 | RAKHMATULLIN A, POLOVOV I B, MALTSEV D, et al. Combined approach for the structural characterization of Alkali fluoroscandates: solid-state NMR, powder X-ray diffraction, and density functional theory calculations[J]. Inorg Chem, 2018, 57(3): 1184-1195. |
| 27 | SOROKIN N I, KARIMOV D N, KOMAR'KOVA O N. Electrophysical properties of LiYbF4 crystals[J]. Crystallogr Rep, 2010, 55(3): 448-449. |
| 28 | OI T. Ionic-conductivity of amorphous mLiFnMF3 thin films(M=Al,Cr,Sc or Al+Sc)[J]. Mater Res Bull, 1984, 19(10): 1343-1348. |
| 29 | OI T. Ionic-conductivity of LiF thin-films containing diavalent or trivalent metal fluorides[J]. Mater Res Bull, 1984, 19(4): 451-457. |
| 30 | 陈昆峰, 李宫, 梁晰童, 等. 稀土改性电化学储能电极材料的研究进展[J]. 硅酸盐学报, 2016, 44(8): 1241-1247. |
| CHEN K F,Li G,LIANG X T, et al. Rare earth element ion modified electrochemical energy storage electrode materials- a short review[J]. J Chinese Ceram Soc, 2016, 44(8): 1241-1247. | |
| 31 | MUY S, VOSS J, SCHLEM R, et al. High-throughput screening of solid-state Li-ion conductors using lattice-dynamics descriptors[J]. iScience, 2019, 16: 270-282. |
| 32 | PARK K H, KAUP K, ASSOUD A, et al. High-voltage superionic halide solid electrolytes for all-solid-state Li-ion batteries[J]. ACS Energy Lett, 2020, 5(2): 533-539. |
| 33 | LIANG J, LI X, WANG S, et al. Site-occupation-tuned superionic LixScCl3+x halide solid electrolytes for all-solid-state batteries[J]. J Am Chem Soc, 2020, 142(15): 7012-7022. |
| 34 | BOHNSACK A, STENZEL F, ZAJONC A, et al. Ternary halides of the A3MX6 type.VII. The bromides Li3MBr6 (M = Sm-Lu, Y): synthesis, crystal structure, and ionic mobility [J]. Z Anorg Allg Chem, 1997, 623(7): 1352-1356. |
| 35 | ASANO T, SAKAI A, OUCHI S, et al. Solid halide electrolytes with high lithium-ion conductivity for application in 4 V class bulk-type all-solid-state batteries[J]. Adv Mater, 2018, 30(44): 1803075. |
| 36 | NEUDECKER B J, WEPPNER W. Li9SiAlO8: a lithium ion electrolyte for voltages above 5.4 V[J]. J Electrochem Soc, 1996, 143(7): 2198-2203. |
| 37 | IWAMOTO K, AOTANI N, TAKADA K, et al. Application of Li3PO4-Li2S-SiS2 glass to the solid-state secondary batteries[J]. Solid State Ionics, 1995, 79: 288-291. |
| 38 | WANG S, BAI Q, NOLAN A M, et al. Lithium chlorides and bromides as promising solid-state chemistries for fast ion conductors with good electrochemical stability[J]. Angew Chem Int Ed, 2019, 58(24): 8039-8043. |
| 39 | YU C, LI Y, ADAIR K R, et al. Tuning ionic conductivity and electrode compatibility of Li3YBr6 for high-performance all solid-state Li batteries[J]. Nano Energy, 2020, 77: 105097. |
| 40 | YU C, VAN EIJCK L, GANAPATHY S, et al. Synthesis, structure and electrochemical performance of the argyrodite Li6PS5Cl solid electrolyte for Li-ion solid state batteries[J]. Electrochim Acta, 2016, 215: 93-99. |
| 41 | YU C, GANAPATHY S, HAGEMAN J, et al. Facile synthesis toward the optimal structure-conductivity characteristics of the argyrodite Li6PS5Cl solid-state electrolyte[J]. ACS Appl Mater Interfaces, 2018, 10(39): 33296-33306. |
| 42 | YU C, GANAPATHY S, VAN ECK E R H, et al. Investigation of Li-ion transport in Li7P3S11 and solid-state lithium batteries[J]. J Energy Chem, 2019, 38: 1-7. |
| 43 | LIU Z T, MA S, LIU J, et al. High ionic conductivity achieved in Li3Y(Br3Cl3) mixed halide solid electrolyte via promoted diffusion pathways and enhanced grain boundary[J]. ACS Energy Lett, 2021, 6(1): 298-304. |
| 44 | SHI X M, ZENG Z Z, SUN M Z, et al. Fast Li-ion conductor of Li3HoBr6 for stable all-solid-state Li-S battery[J]. Nano Lett, 2021, 21(21): 9325-9331. |
| 45 | SHI X M, ZENG Z Z, ZHANG H T, et al. Gram-scale synthesis of nanosized Li3HoBr6 solid electrolyte for all-solid-state Li-Se battery[J]. Small Methods, 2021, 5(11): 2101002. |
| 46 | NAGEL R, GROSS T W, GUNTHER H, et al. Li-6 and Li-7 MAS NMR studies on fast ionic conducting spinel-type Li2MgCl4, Li2- xCuxMgCl4, Li2- xNaxMgCl4, and Li2ZnCl4[J]. J Solid State Chem, 2002, 165(2): 303-311. |
| 47 | LI X, LIANG J, CHEN N, et al. Water-mediated synthesis of a superionic halide solid electrolyte[J]. Angew Chem Int Ed, 2019, 58(46): 16427-16432. |
| 48 | LI X, LIANG J, LUO J, et al. Air-stable Li3InCl6 electrolyte with high voltage compatibility for all-solid-state batteries[J]. Energy Environ Sci, 2019, 12(9): 2665-2671. |
| 49 | WANG C H, LIANG J W, LUO J, et al. A universal wet-chemistry synthesis of solid-state halide electrolytes for all-solid-state lithium-metal batteries[J]. Sci Adv, 2021, 7(37). |
| 50 | WANG Y, RICHARDS W D, ONG S P, et al. Design principles for solid-state lithium superionic conductors[J]. Nat Mater, 2015, 14(10): 1026-1031. |
| 51 | RAO R P, ADAMS S. Studies of lithium argyrodite solid electrolytes for all-solid-state batteries[J]. Phys Status Solid A, 2011, 208(8): 1804-1807. |
| 52 | LI W, LIANG J, LI M, et al. Unraveling the origin of moisture stability of halide solid-state electrolytes by in situ and operando synchrotron X-ray analytical techniques[J]. Chem Mater, 2020, 32(16): 7019-7027. |
| 53 | LI X, LIANG J, ADAIR K R, et al. Origin of superionic Li3Y1- xInxCl6 halide solid electrolytes with high humidity tolerance[J]. Nano Lett, 2020, 20(6): 4384-4392. |
| 54 | WANG S H, XU X W, CUI C, et al. Air sensitivity and degradation evolution of halide solid state electrolytes upon exposure[J]. Adv Funct Mater, 2021, 32(7): 2108805. |
| 55 | RIEGGER L, SCHLEM R, SANN J, et al. Lithium-metal anode instability of the superionic halide solid electrolytes and the implications for solid-state batteries[J]. Angew Chem Int Ed, 2020, 60(12): 6718-6723. |
| 56 | CHOI S, JEON M, KIM B K, et al. Electrochemical behaviors of Li-argyrodite-based all-solid-state batteries under deep-freezing conditions[J]. Chem Commun, 2018, 54(100): 14116-14119. |
| 57 | TAKADA K, AOTANI N, IWAMOTO K, et al. Solid state lithium battery with oxysulfide glass[J]. Solid State Ionics, 1996, 86/88: 877-882. |
| 58 | YU T W, LIANG J W, LUO L, et al. Superionic fluorinated halide solid electrolytes for highly stable Li-metal in all-solid-state Li batteries[J]. Adv Energy Mater, 2021, 11(36): 2101915. |
| 59 | LIU Y, WANG S, NOLAN A M, et al. Tailoring the cation lattice for chloride lithium-ion conductors[J]. Adv Energy Mater, 2020, 10(40): 2002356. |
| 60 | SCHLEM R, MUY S, PRINZ N, et al. Mechanochemical synthesis: a tool to tune cation site disorder and ionic transport properties of Li3MCl6 (M = Y, Er) superionic conductors[J]. Adv Energy Mater, 2020, 10(6): 1903719. |
| 61 | SCHLEM R, BERNGES T, LI C, et al. Lattice dynamical approach for finding the lithium superionic conductor Li3ErI6[J]. ACS Appl Energy Mater, 2020, 3(4): 3684-3691. |
| 62 | XU Z, CHEN X, LIU K, et al. Influence of anion charge on Li ion diffusion in a new solid-state electrolyte, Li3LaI6[J]. Chem Mater, 2019, 31(18): 7425-7433. |
| 63 | KIM K, PARK D, JUNG H G, et al. Material design strategy for halide solid electrolytes Li3MX6 (X = Cl, Br, and I) for all-solid-state high-voltage Li-ion batteries[J]. Chem Mater, 2021, 33(10): 3669-3677. |
| 64 | KRESSE G, FURTHMULLER J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set[J]. Comput Mater Sci, 1996, 6(1): 15-50. |
| 65 | KRESSE G, JOUBERT D. From ultrasoft pseudopotentials to the projector augmented-wave method[J]. Phys Rev B, 1999, 59(3): 1758-1775. |
| 66 | BLOCHL P E, FORST C J, SCHIMPL J. Projector augmented wave method: ab initio molecular dynamics with full wave functions[J]. Bull Mater Sci, 2003, 26(1): 33-41. |
| 67 | PERDEW J P, BURKE K, ERNZERHOF M. Generalized gradient approximation made simple[J]. Phys Rev Lett, 1997, 77(18): 3865-3868. |
| 68 | KLIMES J, BOWLER D R, MICHAELIDES A. Chemical accuracy for the van der Waals density functional[J]. J Phys-Condens Matter, 2010, 22(2): 22201. |
| 69 | WAN T H, CIUCCI F. Ab initio study of the defect chemistry and substitutional strategies for highly conductive Li3YX6 (X = F, Cl, Br, and I) electrolyte for the application of solid-state batteries[J]. ACS Appl Energy Mater, 2021, 4(8): 7930-7941. |
| [1] | 陈兵帅, 卓海涛, 黄书, 陈少军. 高性能硅基负极聚合物粘结剂的研究进展[J]. 应用化学, 2023, 40(5): 625-639. |
| [2] | 胡方正, 高兴, 刘雷, 袁天恒, 曹宁, 李凯, 王亚涛, 李建华, 连慧琴, 汪晓东, 崔秀国. 锂离子电池黑磷负极的储能优势及其优化的研究进展[J]. 应用化学, 2023, 40(4): 571-582. |
| [3] | 卜芃, 李宏亮. 稀土Yb3+/Tm3+掺杂NaGd(MO4)2荧光粉的制备及其光致发光[J]. 应用化学, 2023, 40(3): 374-379. |
| [4] | 王恩通, 杨林芳. 高比容量锂离子电池正极材料LiNi0.6Co0.2Mn0.2O2的制备及性能[J]. 应用化学, 2022, 39(8): 1209-1215. |
| [5] | 宋林虎, 李世友, 王洁, 张晶晶, 张宁霜, 赵冬妮, 徐菲. 锂离子电池电解液除酸除水添加剂的研究进展[J]. 应用化学, 2022, 39(5): 697-706. |
| [6] | 陈洪霞, 李宝霞, 姚英明, 刘朋. 苯胺基桥联双芳氧基稀土金属配合物的合成、表征及催化性能[J]. 应用化学, 2022, 39(5): 843-851. |
| [7] | 张松涛, 王樱蕙, 张洪杰. Nd3+离子敏化的荧光纳米探针用于近红外二区血管成像[J]. 应用化学, 2022, 39(4): 685-693. |
| [8] | 王玉乐, 杨柯利, 高艳芳. 碳化钼改性二氧化硅的制备及其电化学性能[J]. 应用化学, 2022, 39(11): 1716-1725. |
| [9] | 赵莹, 邵奕嘉, 李罗钱, 任建伟, 廖世军. 富锂正极材料的衰减机理及循环稳定性提升的研究进展[J]. 应用化学, 2022, 39(02): 205-222. |
| [10] | 王旭尧, 方应军, 张灵志. 氰基功能化聚乙烯亚胺交联固态聚合物电解质的制备及理化性能[J]. 应用化学, 2021, 38(8): 946-953. |
| [11] | 陶承业, 杨占旭, 李玥. 红磷/石墨复合材料的制备及电化学性能[J]. 应用化学, 2020, 37(9): 1056-1061. |
| [12] | 刘学, 马华, 徐恒, 计海聪, 王栋. 无纺布基聚吡咯柔性电极的储锂性能[J]. 应用化学, 2020, 37(5): 555-561. |
| [13] | 王金莹, 曲江英, 李杰兰, 汤占磊, 臧云浩, 王涛, 顾建峰, 周钢, 高峰. 二次包覆法制备煤沥青基硅/碳复合物及其锂离子电池性能[J]. 应用化学, 2020, 37(5): 562-569. |
| [14] | 胡晨,金翼,朱少青,徐晔,水江澜. 磷酸铁锂电池低温性能的改性方法简述[J]. 应用化学, 2020, 37(4): 380-386. |
| [15] | 胡家乐, 薛冬峰. 稀土离子特性与稀土功能材料研究进展[J]. 应用化学, 2020, 37(3): 245-255. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
