Chinese Journal of Applied Chemistry ›› 2023, Vol. 40 ›› Issue (5): 666-680.DOI: 10.19894/j.issn.1000-0518.220316
• Review • Previous Articles Next Articles
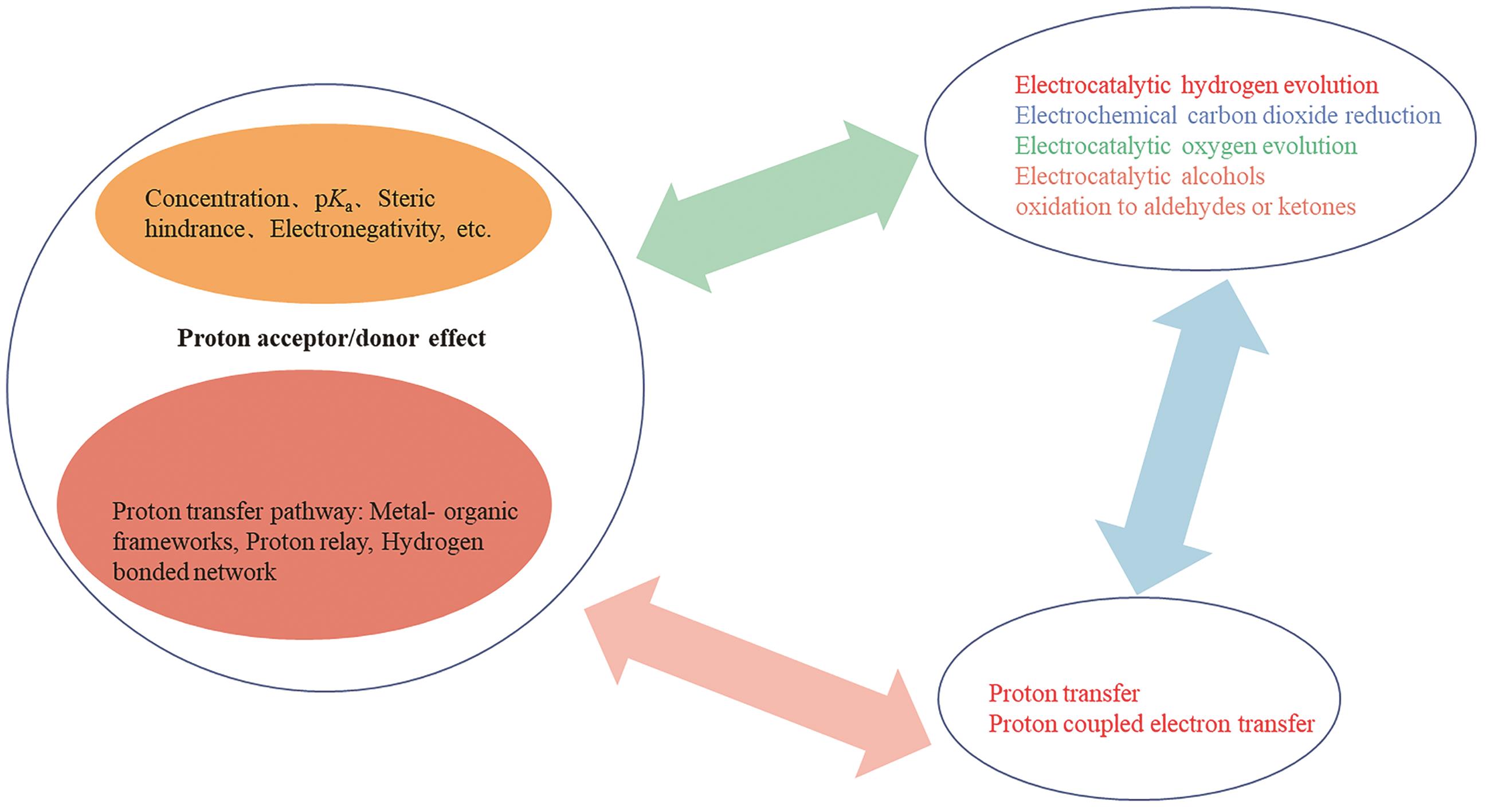
Research Progress in the Effects of Proton Acceptor/Donor on Electrocatalytic Reactions
Feng ZHU1,2, Xiao-Lian PENG1, Wen-Bin ZHANG1,2( )
)
- 1.College of Chemistry and Bio-engineering,Yichun University,Yichun 336000,China
2.Key Laboratory of Jiangxi University of Applied Chemistry and Chemical Biology,Yichun 336000,China
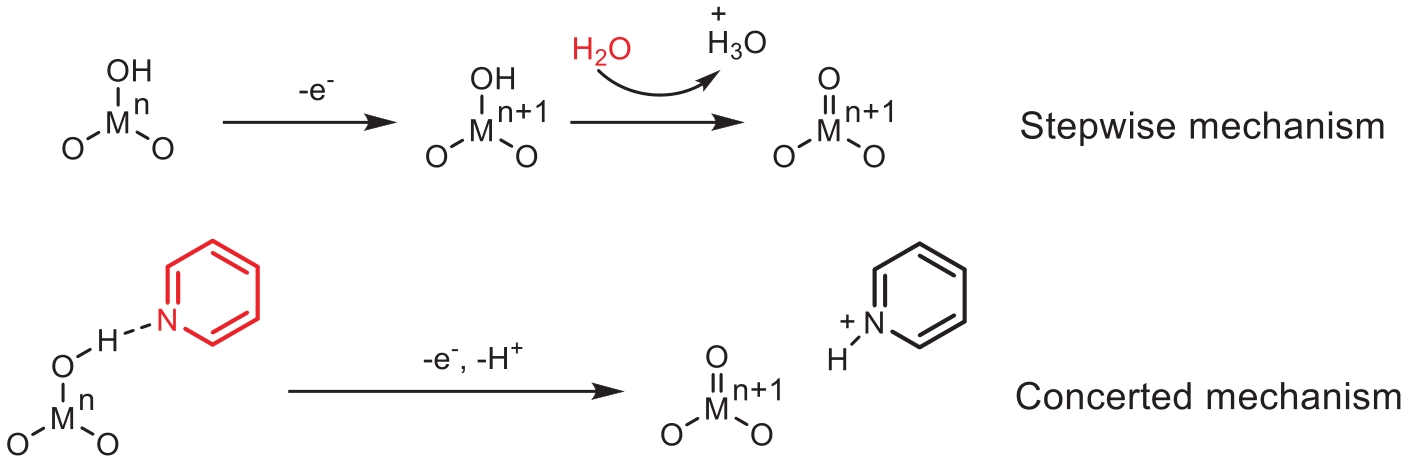
-
Received:2022-09-27Accepted:2023-02-18Published:2023-05-01Online:2023-05-26 -
Contact:Wen-Bin ZHANG -
About author:zhangwbycu@163.com
-
Supported by:the National Natural Science Foundation of China(21962019)
CLC Number:
Cite this article
Feng ZHU, Xiao-Lian PENG, Wen-Bin ZHANG. Research Progress in the Effects of Proton Acceptor/Donor on Electrocatalytic Reactions[J]. Chinese Journal of Applied Chemistry, 2023, 40(5): 666-680.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: http://yyhx.ciac.jl.cn/EN/10.19894/j.issn.1000-0518.220316
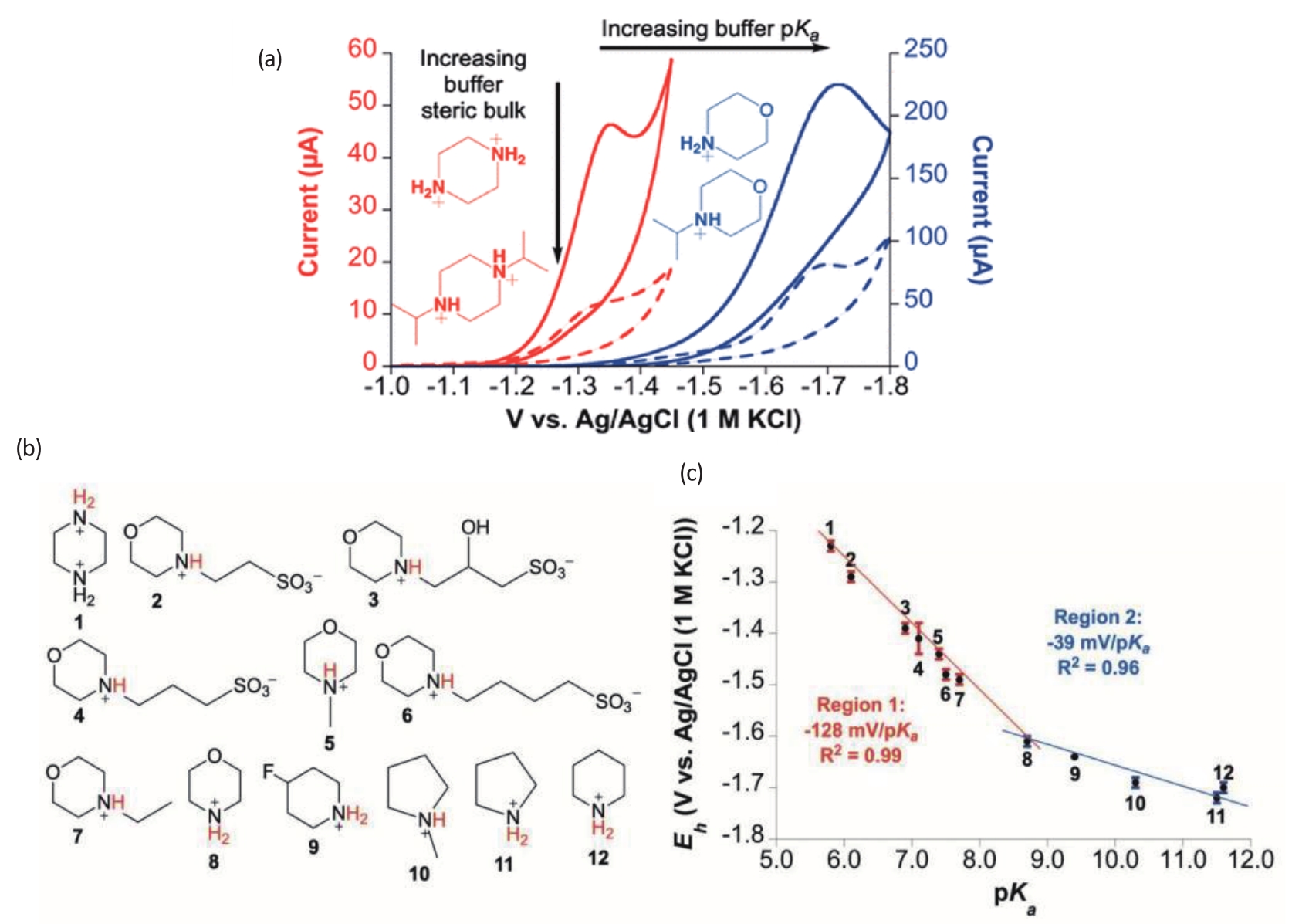
Fig.3 (a) Cyclic voltammograms for hydrogen evolution with the addition of different buffer species, measurement system: 1.0 μmol/L CoMC6*a, 50 mmol/L of the buffer, 100 mmol/L KCl, and a pH of 6.5, A glassy carbon counter electrode, Ag/AgCl (1 mol/L KCl) reference electrode, and hanging drop mercury electrode; (b) Molecular structure of different buffer species; (c) A plot of catalytic potential values as a function of buffer acid pKa, Region 1 has a slope of -128 mV/pKa unit (pKa: 1~8), and region 2 has a slope of -39 mV/pKa unit (pKa: 8~12)[15]
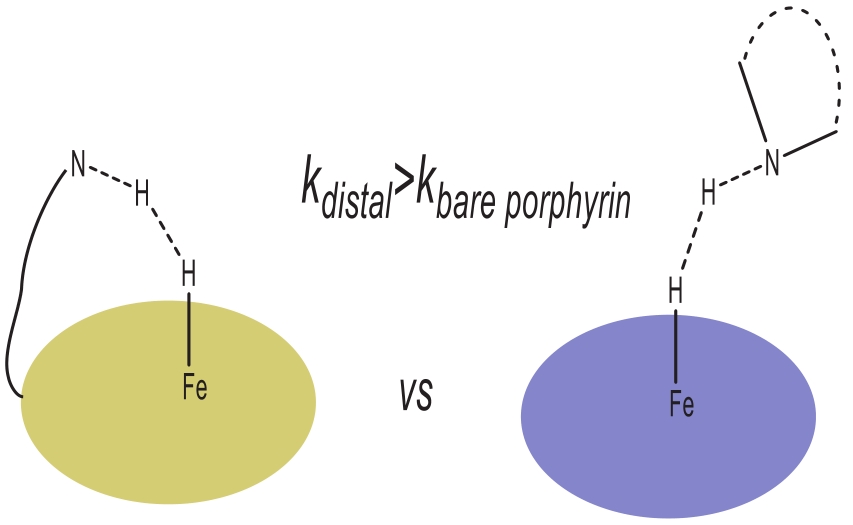
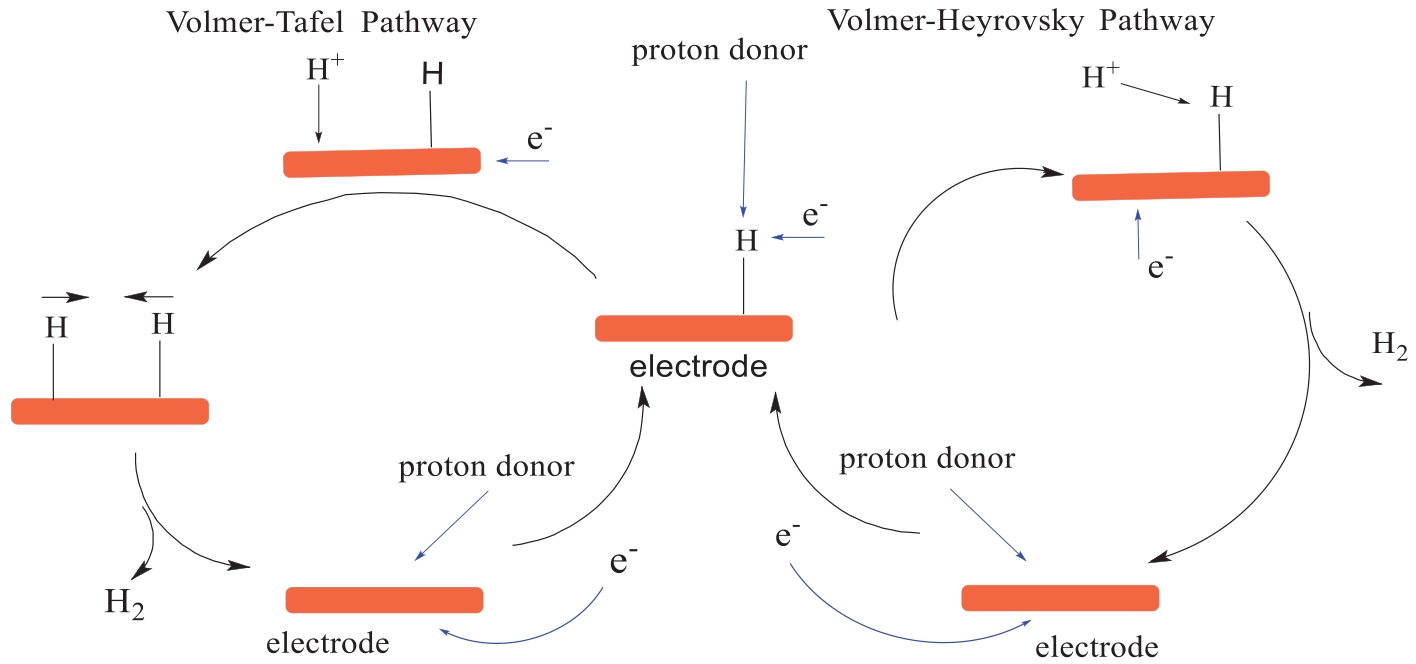
Fig.5 Schematic view of proton sources (intermolecular or intramolecular) comparison in electrochemical hydrogen evolution for iron porphyrin catalyst[25]

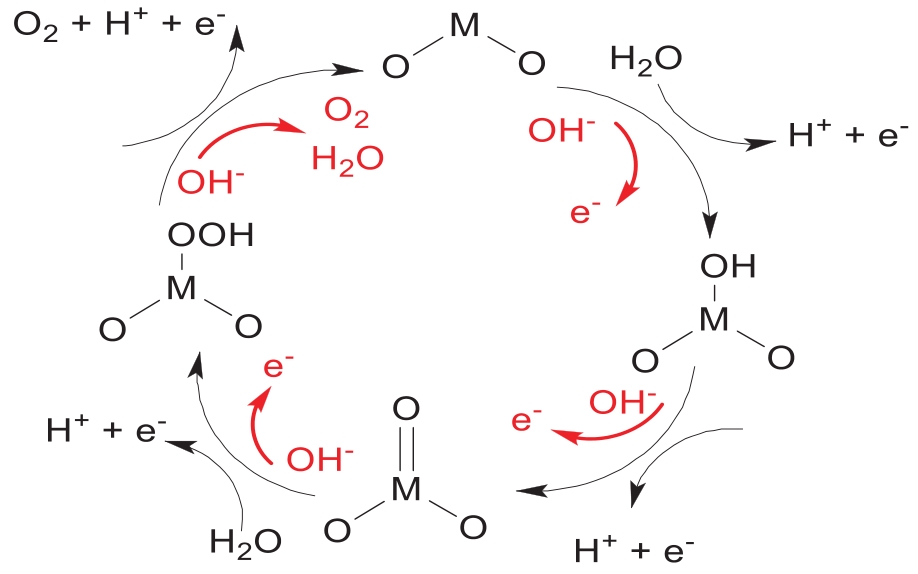
Fig.15 Schematic diagram of oxygen evolution mechanism of perovskite oxide type catalyst with (a) a traditional proton acceptor and (b) a molecular-level proton acceptor[65-66]
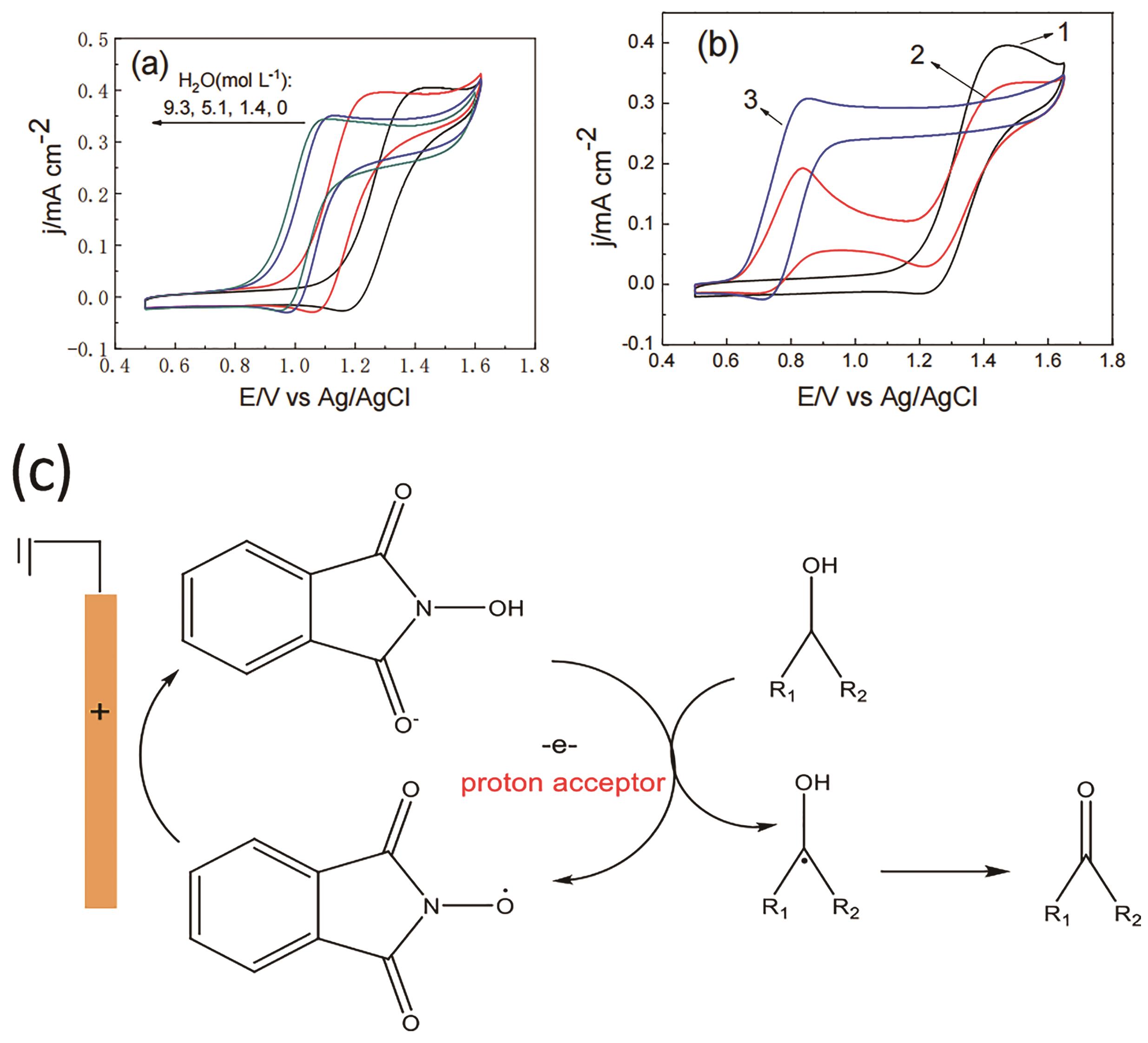
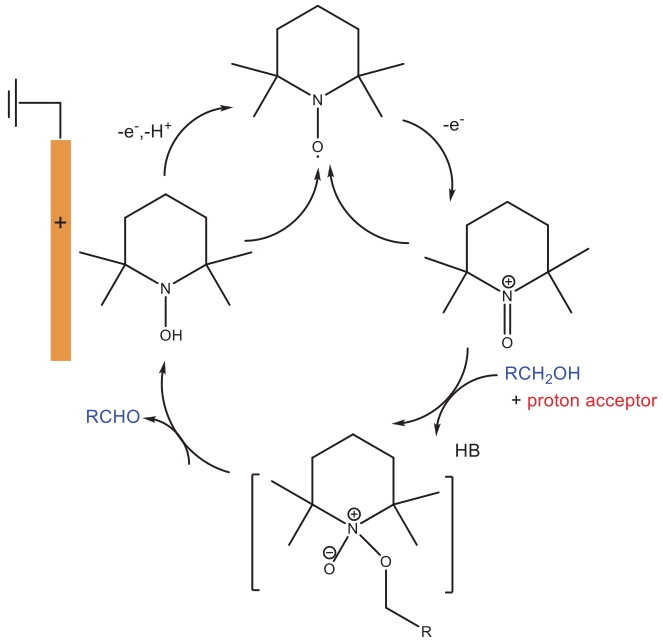
Fig.17 (a) CVs of 2.0 mmol/L NHPI in the presence of 10.0 mmol /L benzyl alcohol (a) with the addition of different amounts of water, (b) in the presence of different concentration of 2,6-lutidine, (c) suggested mechanism of NHPI-mediated electrooxidation of alcohols[72]
| 1 | HOLADE Y, SERVAT K, TINGRY S. Advances in electrocatalysis for energy conversion and synthesis of organic molecules[J]. ChemPhysChem, 2017, 18: 2573-2605. |
| 2 | AKHADE S A, SINGH N, GUTIERREZ O Y, et al. Electrocatalytic hydrogenation of biomass-derived organics: a review[J]. Chem Rev, 2020, 120(20): 11370-11419. |
| 3 | HOLADE Y, TULEUSHOVA N, TINGRY S, et al. Recent advances in the electrooxidation of biomass-based organic molecules for energy, chemicals and hydrogen production[J]. Catal Sci Tech, 2020, 10(10): 3071-3112. |
| 4 | CHENG W, SUN L, HE X, et al. Recent advances in fuel cell reaction electrocatalysis based on porous noble metal nanocatalysts[J]. Dalton Trans, 2022, 51: 7763-7774. |
| 5 | LIU Y, LIANG X, GU L, et al. Corrosion engineering towards efficient oxygen evolution electrodes with stable catalytic activity for over 6000 hours[J]. Nat Commun, 2018, 9: 2609. |
| 6 | DATTILA F, SEEMAKURTHI R R, ZHOU Y. Modeling operando electrochemical CO2 reduction[J]. Chem Rev, 2022, 122(12): 11085-11130. |
| 7 | WARBURTON R E, SOUDACKOV A V, HAMMES-SCHIFFER S. Theoretical modeling of electrochemical proton-coupled electron transfer[J]. Chem Rev, 2022, 122(12): 10599-10650. |
| 8 | AGARWAL R G, COSTE S C, GROFF B D, et al. Free energies of proton-coupled electron transfer reagents and their applications[J]. Chem Rev, 2022, 122(1): 1-49. |
| 9 | ZHANG X, LI Y, GUO P. Theory on optimizing the activity of electrocatalytic proton coupled electron transfer reactions[J]. J Catal, 2019, 376: 17-24. |
| 10 | 孙世刚. 电催化纳米材料[M]. 北京: 化学工业出版社, 2018. |
| SUN S G. Nanostructured electrocatalysts[M]. Beijing: Chemical Industry Press, 2018. | |
| 11 | ZHANG X, LI Y, GUO P, et al. Theory on optimizing the activity of electrocatalytic proton coupled electron transfer reactions[J]. J Catal, 2019, 376: 17-24. |
| 12 | ZHU J, HU L, ZHAO P, et al. Recent advances in electrocatalytic hydrogen evolution using nanoparticles[J]. Chem Rev, 2019, 120(2): 851-918. |
| 13 | LAMOUREUX P S, SINGH A R, CHAN K. pH effects on hydrogen evolution and oxidation over Pt(111): insights from first-principles[J]. ACS Catal, 2019, 9(7): 6194-6201. |
| 14 | ALVAREZ-HERNANDEZ J, SOPCHAK A, BREN K. Buffer pKa impacts the mechanism of hydrogen evolution catalyzed by a cobalt porphyrin-peptide[J]. Inorg Chem, 2020, 59(12): 8061-8069. |
| 15 | LE J, ALACHOUZOS G, FRONTIER A, et al. Tuning mechanism through buffer dependence of hydrogen evolution catalyzed by a cobalt mini-enzyme[J]. Biochemistry, 2020, 59(12): 1289-1297. |
| 16 | JACKSON M N, SURENDRANATH Y. Donor-dependent kinetics of interfacial proton-coupled electron transfer[J]. J Am Chem Soc, 2016, 138(9): 3228-3234. |
| 17 | CLARY K E, KARAYILAN M, MCCLEARY-PETERSEN K C, et al. Increasing the rate of the hydrogen evolution reaction in neutral water with protic buffer electrolytes[J]. Proc Nat Acad Sci USA, 2020(52): 3737-3743. |
| 18 | JACKSON M N, JUNG O, LAMOTTE H C, et al. Donor-dependent promotion of interfacial proton-coupled electron transfer in aqueous electrocatalysis[J]. ACS Catal, 2019, 9(4): 3737-3743. |
| 19 | HOD I, DERIA P, BURY W, et al. A porous proton-relaying metal-organic framework material that accelerates electrochemical hydrogen evolution[J]. Nat Commun, 2015, 6: 8304. |
| 20 | DOLUI D, GHORAI S, DUTTA A. Tuning the reactivity of cobalt-based H2 production electrocatalysts via the incorporation of the peripheral basic functionalities[J]. Coordin Chem Rev, 2020, 416: 213335. |
| 21 | 刘涛, 张清鑫, 郭鸿波, 等. 金属卟啉类化合物在电催化氧还原反应的应用[J]. 中国科学: 化学, 2022, 52(8): 1306-1320. |
| LIU T, ZHANG Q X, GUO H B, et al. Electrocatalytic oxygen reduction reaction with metalloporphyrins[J]. Sci China-Chem, 2022, 52(8): 1306-1320. | |
| 22 | WIEDNER E S, APPEL A M, RAUGEI S, et al. Molecular catalysts with diphosphine ligands containing pendant amines[J]. Chem Rev, 2022, 122(14): 12427-12474. |
| 23 | ROUBELAKIS M M, BEDIAKO D K, DOGUTAN D K, et al. Proton-coupled electron transfer kinetics for the hydrogen evolution reaction of hangman porphyrins[J]. Energy Environ Sci, 2012, 5: 7737-7740. |
| 24 | THAMMAVONGSY Z, MERCER I P, YANG J Y. Promoting proton coupled electron transfer in redox catalysts through molecular design[J]. Chem Commun, 2019, 55(70): 10342-10358. |
| 25 | BHUNIA S, RANA A, HEMATIAN S, et al. Proton relay in iron porphyrins for hydrogen evolution reaction[J]. Inorg Chem, 2021, 60(18): 13876-13887. |
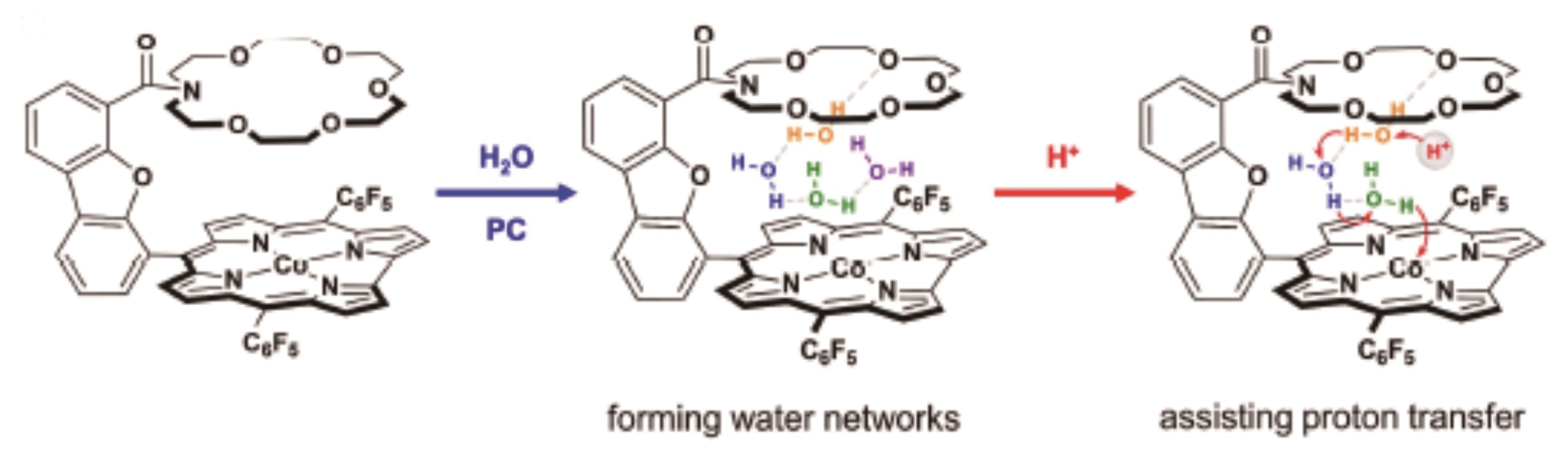
| 26 | LI X L, LV B, ZHANG X P, et al. Introducing water-network-assisted proton transfer for boosted electrocatalytic hydrogen evolution with cobalt corrole[J]. Angew Chem Int Ed, 2022, 61(9): 202114310. |
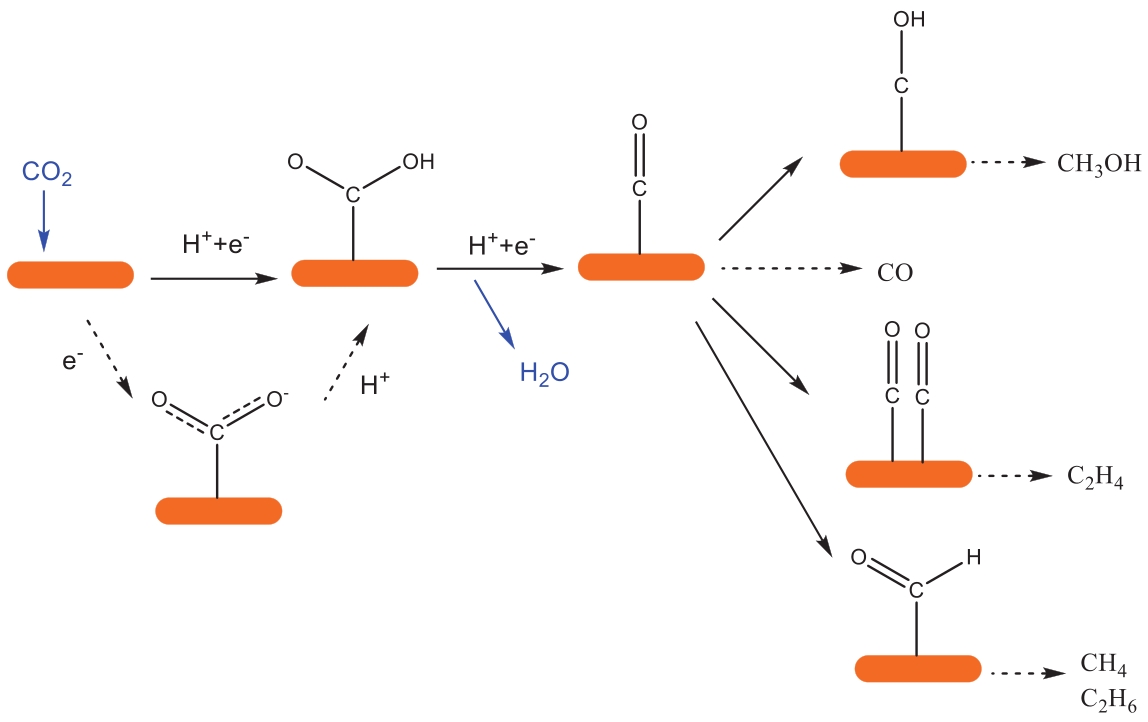
| 27 | FRANCKE R, SCHILLE B, ROEMELT M, et al. Homogeneously catalyzed electroreduction of carbon dioxide-methods, mechanisms, and catalysts[J]. Chem Rev, 2018, 118(9): 4631-4701. |
| 28 | ZHANG R, WU B, LI Q, et al. Design strategies and mechanism studies of CO2 electroreduction catalysts based on coordination chemistry[J]. Coord Chem Rev, 2020, 422: 213436. |
| 29 | ANGAMUTHU R, BYERS P, LUTZ M, et al. Electrocatalytic CO2 conversion to oxalate by a copper complex[J]. Science, 2010, 327(5963): 313-315. |
| 30 | CHU A T, SURENDRANATH Y. Aprotic solvent exposes an altered mechanism for copper-catalyzed ethylene electrosynthesis[J]. J Am Chem Soc, 2022, 144(12): 5359-5365. |
| 31 | RUDNEV A V, ZHUMAEV U E, KUZUME A, et al. The promoting effect of water on the electroreduction of CO2 in acetonitrile[J]. Electrochim Acta, 2016, 189: 38-44. |
| 32 | FIGUEIREDO M C, LEDEZMA-YANEZ I, KOPER M. In situ spectroscopic study of CO2 electroreduction at copper electrodes in acetonitrile[J]. ACS Catal, 2016, 6(4): 2382-2392. |
| 33 | SAMPSON M D, NGUYEN A D, GRICE K A. et al. Manganese catalysts with bulky bipyridine ligands for the electrocatalytic reduction of carbon dioxide: eliminating dimerization and altering catalysis[J]. J Am Chem Soc, 2014, 136(14): 5460-5471. |
| 34 | BHUGUN I, LEXA D, SAVÉANT J M, et al. Catalysis of the electrochemical reduction of carbon dioxide by iron(0) porphyrins. synergistic effect of lewis acid cations[J]. J Phys Chem B, 1996, 100(51): 19981-19985. |
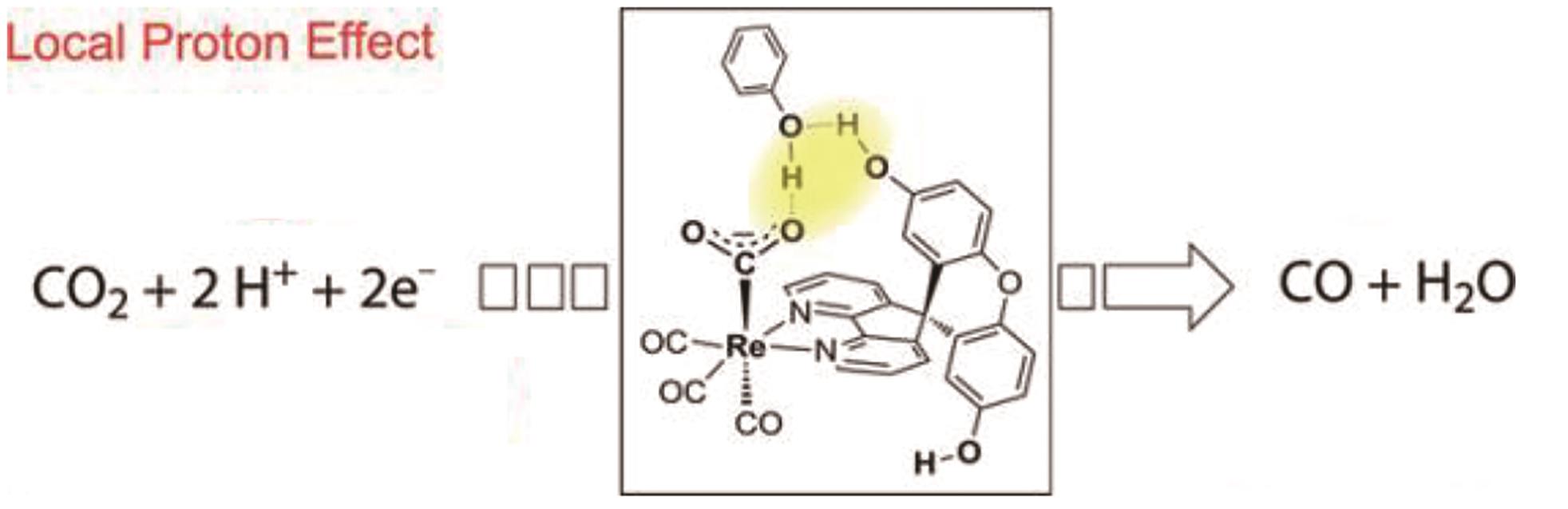
| 35 | COSTENTIN C, DROUET S, ROBERT M, et al. A local proton source enhances CO2 electroreduction to CO by a molecular Fe catalyst[J]. Science, 2012, 338(6103): 90-94. |
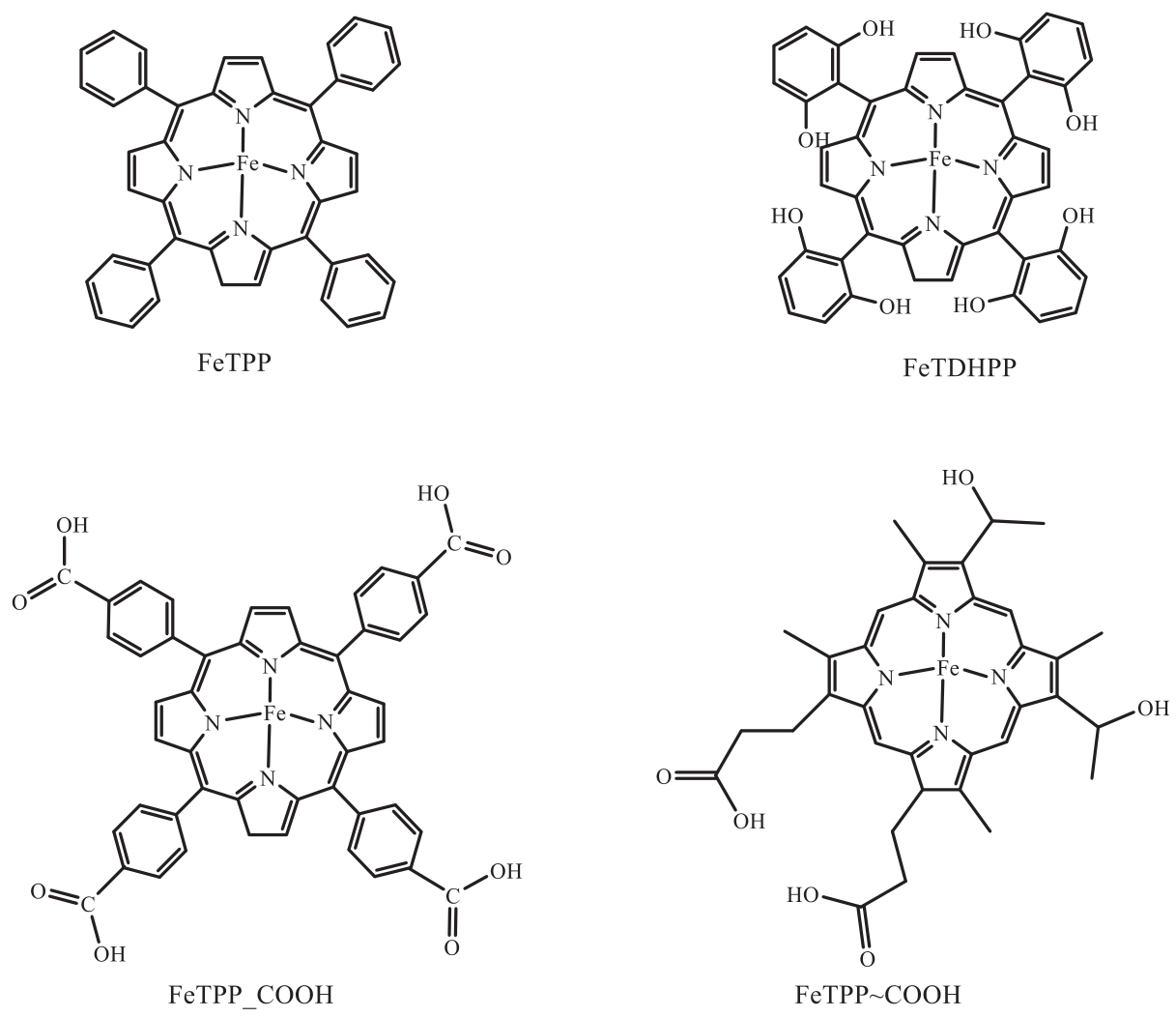
| 36 | ZHOU Y, XIAO Y, ZHAO J. A local proton source from carboxylic acid functionalized metal porphyrins for enhanced electrocatalytic CO2 reduction[J]. New J Chem, 2020, 44: 16062-16068. |
| 37 | ZHAO Y J, ZHENG L L, JIANG D, et al. Nanoengineering metal-organic framework-based materials for use in electrochemical CO2 reduction reactions[J]. Small, 2021, 17(16): 2006590. |
| 38 | HUI S R, LUNA P D. How increasing proton and electron conduction benefits electrocatalytic CO2 reduction[J]. Matter, 2021, 4(5): 1555-1577. |
| 39 | NICHOLS A W, HOOE S L, KUEHNER J S, et al. Electrocatalytic CO2 reduction to formate with molecular Fe(III) complexes containing pendent proton relays[J]. Inorg Chem, 2020, 59(9): 5854-5864. |
| 40 | YANG Y, ZHANG Z Y, ZHANG Z Y, et al. Electrocatalytic CO2 reduction with Re-based spiro bipyridine complexes: effects of the local proton in the second coordination sphere[J]. Chin J Chem, 2021, 39(5): 1281-1287. |
| 41 | CHAPOVETSKY A, WELBORN M, LUNA J M, et al. Pendant hydrogen-bond donors in cobalt catalysts independently enhance CO 2 reduction[J]. ACS Central Sci, 2018, 4(3): 397-404. |
| 42 | DERRICK J S, LOIPERSBERGER M, NISTANAKI S K, et al. Templating bicarbonate in the second coordination sphere enhances electrochemical CO2 reduction catalyzed by iron porphyrins[J]. J Am Chem Soc, 2022, 144(26): 11656-11663. |
| 43 | SIEWERT I. Electrochemical CO2 reduction catalyzed by binuclear LRe2(CO)6Cl2 and LMn2(CO)6Br2 complexes with an internal proton source[J]. Acc Chem Res, 2022, 55(4): 473-483. |
| 44 | WILTING A, STOLPER T, MATA R A, et al. Dinuclear rhenium complex with a proton responsive ligand as a redox catalyst for the electrochemical CO2 reduction[J]. Inorg Chem, 2017, 56(7): 4176-4185. |
| 45 | FOKIN I, DENISIUK A, WÜRTELE C, et al. The impact of a proton relay in binuclear α-diimine-Mn(CO)3 complexes on the CO2 reduction catalysis[J]. Inorg Chem, 2019, 58(16): 10444-10453. |
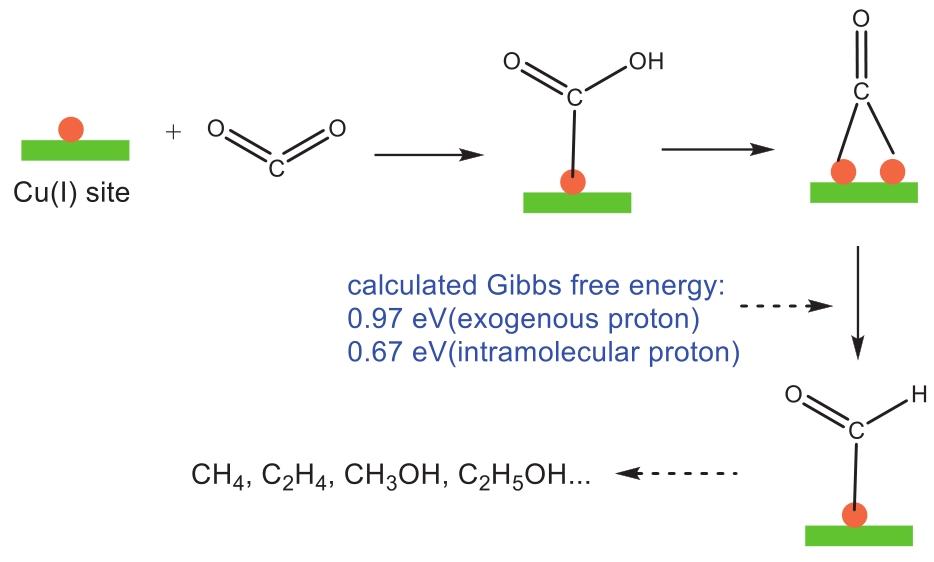
| 46 | ZHU H L, CHEN H Y, HAN Y X, et al. A porous π-π stacking framework with dicopper(I) sites and adjacent proton relays for electroreduction of CO2 to C2+ products[J]. J Am Chem Soc, 2022, 144(29): 13319-13326. |
| 47 | MIYAMOTO K, ASAHI R. Water facilitated electrochemical reduction of CO2 on cobalt-porphyrin catalysts[J]. J Phys Chem C, 2019, 123(15): 9944-9948. |
| 48 | BLAKEMORE J D, CRABTREE R H, BRUDVIG G W. Molecular catalysts for water oxidation[J]. Chem Rev, 2015, 115(23): 12974-13005. |
| 49 | MAN I C, SU H Y, CALLE-VALLEJO F, et al. Universality in oxygen evolution electro-catalysis on oxide surfaces[J]. ChemCatChem, 2011, 3(7): 1159-1165. |
| 50 | GIORDANO L, HAN B, RISCH M, et al. pH dependence of OER activity of oxides: current and future perspectives[J]. Catal Today, 2016, 262(15): 2-10. |
| 51 | COGGINS M K, ZHANG M, CHEN Z, et al. Single-site copper(II) water oxidation electrocatalysis: rate enhancements with HPO 4 2 - as a proton acceptor at pH 8[J]. Angew Chem Int Ed, 2014, 53(45): 12226-12230. |
| 52 | ZHANG Z, ZHANG T, LEE J Y. The enhancement effect of borate doping on the oxygen evolution activity of α-nickel hydroxide[J]. ACS Appl Nano Mater, 2018, 1(2): 751-758 |
| 53 | MAVROS M G, TSUCHIMOCHI T, KOWALCZYK T, et al. What can density functional theory tell us about artificial catalytic water splitting?[J]. Inorg Chem, 2014, 53(13): 6386-6397. |
| 54 | GERKEN J B, MCALPIN J G, CHEN J Y C, et al. Electrochemical water oxidation with cobalt-based electrocatalysts from pH 0~14: the thermodynamic basis for catalyst structure, stability, and activity[J]. J Am Chem Soc, 2011, 133(36): 14431-14442. |
| 55 | SURENDRANATH Y, KANAN M W, NOCERA D G. Mechanistic studies of the oxygen evolution reaction by a cobalt-phosphate catalyst at neutral pH[J]. J Am Chem Soc, 2010, 132(46): 16501-16509. |
| 56 | BEDIAKO D K, SURENDRANATH Y, NOCERA D G. Mechanistic studies of the oxygen evolution reaction mediated by a nickel-borate thin film electrocatalyst[J]. J Am Chem Soc, 2013, 135(9): 3662-3674. |
| 57 | YAMAGUCHI A, INUZUKA R, TAKASHIMA T, et al. Regulating proton-coupled electron transfer for efficient water splitting by manganese oxides at neutral pH[J]. Nat Comm, 2014, 5: 4256. |
| 58 | TAKASHIMA T, ISHIKAWA K, IRIE H. Efficient oxygen evolution on hematite at neutral pH enabled by proton-coupled electron transfer[J]. Chem Comm, 2016, 52(97): 14015-14018. |
| 59 | TAKASHIMA T, ISHIKAWA K, IRIE H. Induction of concerted proton-coupled electron transfer during oxygen evolution on hematite using lanthanum oxide as a solid proton acceptor[J]. ACS Catal, 2019, 9(10): 9212-9215. |
| 60 | DOGUTAN D K, MCGUIREJR R, NOCERA D G. Electrocatalytic water oxidation by cobalt(III) hangman β-octafluoro corroles[J]. J Am Chem Soc, 2011, 133(24): 9178-9180. |
| 61 | CHEN F, WANG N, LEI H, et al. Electrocatalytic water oxidation by a water-soluble copper(II) complex with a copper-bound carbonate group acting as a potential proton shuttle[J]. Inorg Chem, 2017, 56(21): 13368-13375. |
| 62 | LIU Y Z, LI X T, SUN Q D, et al. Freestanding 2D NiFe metal-organic framework nanosheets: facilitating proton transfer via organic ligands for efficient oxygen evolution reaction[J]. Small, 2022, 18(26): 2201076. |
| 63 | SU X, GAO M, JIAO L, et al. Electrocatalytic water oxidation by a dinuclear copper complex in a neutral aqueous solution[J]. Angew Chem Int Ed, 2015, 54(16): 4909-4914. |
| 64 | ZHENG H, YE H, XU, T, et al. Electrochemical water oxidation catalyzed by a mononuclear cobalt complex of a pentadentate ligand: the critical effect of the borate anion[J]. New J Chem, 2022, 46 (16): 7522-7527. |
| 65 | SHE S, ZHU Y, CHEN Y, et al. Realizing ultrafast oxygen evolution by introducing proton acceptor into perovskites[J]. Adv Energy Mater, 2019, 9(20):1900429. |
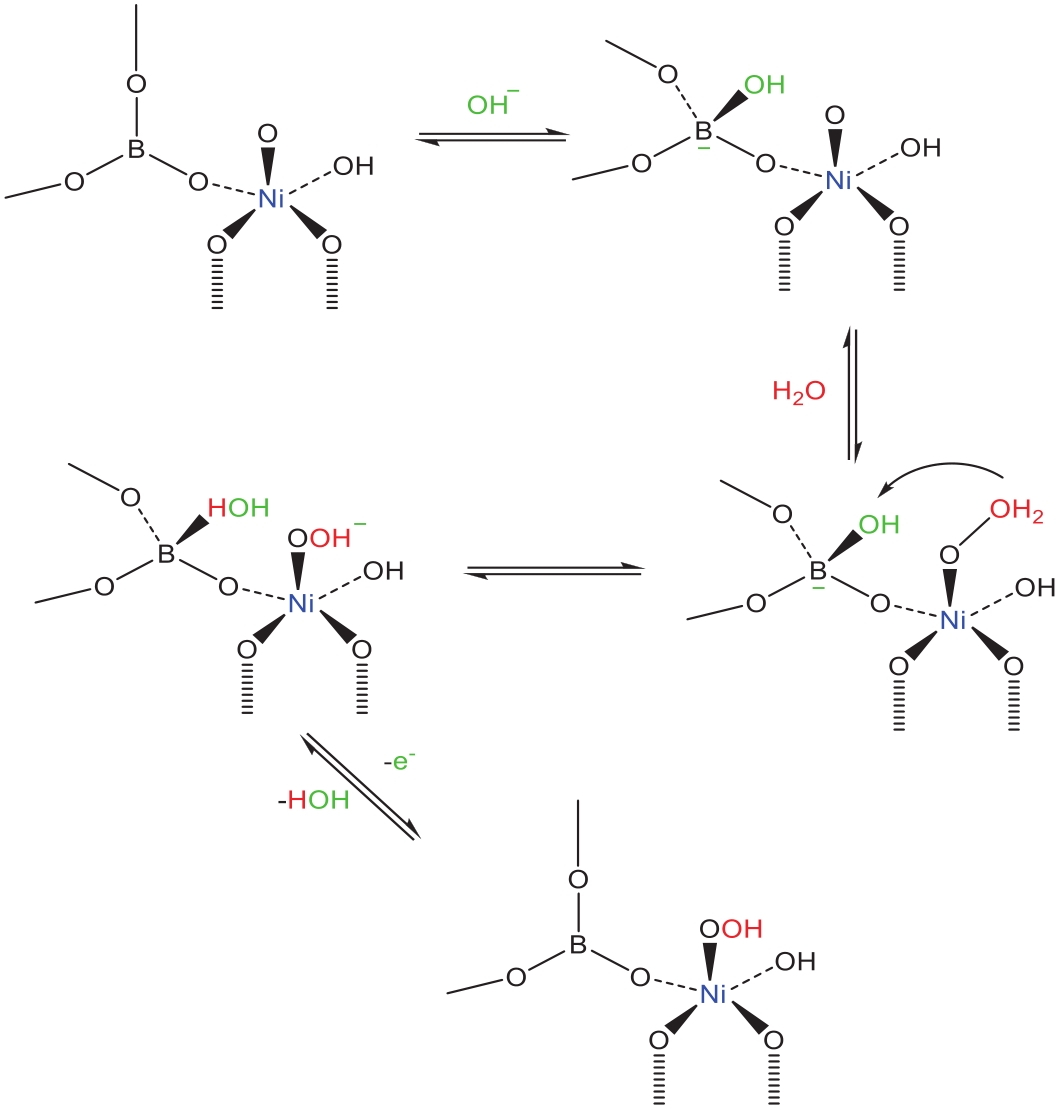
| 66 | WANG Y B, LU Q, GE X L. Molecular-level proton acceptor boosts oxygen evolution catalysis to enable efficient industrial-scale water splitting[J] Green Energy Environ, 2022, DOI:10.1016/j.gee.2022.07.001. |
| 67 | NUTTING J, RAFIEE M, STAHL S. Tetramethylpiperidine N-oxyl (TEMPO), phthalimide N-oxyl (PINO), and related N-oxyl species: electrochemical properties and their use in electrocatalytic reactions[J]. Chem Rev, 2018, 118(9): 4834-4885. |
| 68 | RAFIEE M, KAEIMI B, ALIZADEH S. Mechanistic study of the electrocatalytic oxidation of alcohols by TEMPO and NHPI[J]. ChemElectroChem, 2014, 1(2): 455-462. |
| 69 | COMMINGES C, BARHDAI R, DOHERTY A P, et al. Mechanism of 2′6,6′-tetramethylpiperidin-N-oxyl mediated oxidation of alcohols in ionic liquids[J]. J Phys Chem A, 2008, 112(34): 7848-7855. |
| 70 | ZHANG W, XIONG Z, GAO Y, et al. Roles of the base in the electrocatalytic reactions: a case study of glycine electrooxidation[J]. J Electroanal Chem, 2017, 785: 216-219. |
| 71 | BADALYAN A, STAHL S. Cooperative electrocatalytic alcohol oxidation with electron-proton-transfer mediators[J]. Nature, 2016, 535: 406-410. |
| 72 | LI Y, WEI Y, ZHANG W. Oxidation behavior of N-hydroxyphthalimide (NHPI) and its electrocatalytic ability toward benzyl alcohol: proton acceptor effect[J]. J Electroanal Chem, 2020, 870: 114251. |
| 73 | RAFIEE M, WANG F, HRUSZKEWYCZ D, et al. N-Hydroxyphthalimide-mediated electrochemical iodination of methylarenes and comparison to electron-transfer-initiated C—H functionalization[J]. J Am Chem Soc, 2018, 140(1): 22-25. |
| 74 | YANG C, FARMER L, PRATT D, et al. Mechanism of electrochemical generation and decomposition of phthalimide-N-oxyl[J]. J Am Chem Soc, 2021, 143(27):10324-10332. |
| [1] | Wei-Min DU, Xin LIU, Lin ZHU, Jia-Min FU, Wen-Shan GUO, Xiao-Qing YANG, Pei-Shuo SHUANG. Facile Synthesis and High⁃Efficiency Electrocatalytic Oxygen Evolution Performance of Ternary Nickel⁃Based Chalcogenide Nanorod Arrays [J]. Chinese Journal of Applied Chemistry, 2022, 39(8): 1252-1261. |
| [2] | Yan WANG, Shu-Cong ZHANG, Xing-Kun WANG, Zhi-Cheng LIU, Huan-Lei WANG, Ming-Hua HUANG. Research Progress on Transition Metal⁃Based Catalysts for Hydrogen Evolution Reaction via Seawater Electrolysis [J]. Chinese Journal of Applied Chemistry, 2022, 39(6): 927-940. |
| [3] | Wei-Jin CAO, Lu BAI, Lan-Lan WU, Jing-De LI, Shu-Yan SONG. Multi⁃Shell Hollow Nickel⁃Cobalt Bimetallic Phosphide Nanospheres for Highly Efficient Oxygen Evolution Reaction [J]. Chinese Journal of Applied Chemistry, 2022, 39(4): 666-672. |
| [4] | Xue WANG, Yi-Bo WANG, Xian WANG, Jian-Bing ZHU, Jun-Jie GE, Chang-Peng LIU, Wei XING. Research Progress of Mechanism of Acidic Oxygen Evolution Reaction and Development of Ir⁃based Catalysts [J]. Chinese Journal of Applied Chemistry, 2022, 39(4): 616-628. |
| [5] | Dan WANG, Xian-Biao HOU, Xing-Kun WANG, Zhi-Cheng LIU, Huan-Lei WANG, Ming-Hua HUANG. Research Progress of Carbon‑Encapsulated Iron‑Based Nanoparticles Electrocatalysts for Zinc‑Air Batteries [J]. Chinese Journal of Applied Chemistry, 2022, 39(10): 1488-1500. |
| [6] | MENG Yang, YANG Chan, PENG Juan. Progress in Iron, Cobalt and Nickel-Based Metal Phosphide Nano-catalysts for Hydrogen Production under Alkaline Conditions [J]. Chinese Journal of Applied Chemistry, 2020, 37(7): 733-745. |
| [7] | LI Xinjie,XU He,YU Mei,ZHANG Chao,GUO Anru,LIU Chang. Nitrogen-Doped Graphitic Carbon Coated Cobalt Nanocatalysts for Highly Efficient and Durable Hydrogen Evolution Reaction [J]. Chinese Journal of Applied Chemistry, 2019, 36(5): 571-577. |
| [8] | LI Xinjie, XU He, YU Mei, ZHANG Chao, GUO Anru, LIU Chang. Nitrogen-Doped Graphitic Carbon Coated Cobalt Nanocatalysts for Highly Efficient and Durable Hydrogen Evolution Reaction [J]. Chinese Journal of Applied Chemistry, 2019, 36(5): 0-0. |
| [9] | YU Peng,LI Jinghong. Metal Sulfide(Phosphide) for Electrocatalytic Hydrogen Evolution Reaction [J]. Chinese Journal of Applied Chemistry, 2018, 35(9): 1093-1101. |
| [10] | CHEN Si,SUN Lizhen,SHU Xinxin,ZHANG Jintao. Graphene-based Catalysts for Efficient Electrocatalytic Applications [J]. Chinese Journal of Applied Chemistry, 2018, 35(3): 272-285. |
| [11] | YAO Huiying, YANG Tao, HUANG Xing, ZHU Jia, LI Qing, XU Wei, CHI Lifeng. Coordination Complexes Based on MX4 Structure as Catalyst for Hydrogen Evolution Reaction [J]. Chinese Journal of Applied Chemistry, 2018, 35(3): 328-341. |
| [12] | SONG Hongwei, HUANG Hui, TAN Ning, CHENG Buming, GUO Zhongcheng. Electrochemical Behavior of Al/Pb-0.2%Ag Anode in Fluoride Ion and Sulfuric Acid [J]. Chinese Journal of Applied Chemistry, 2016, 33(12): 1455-1461. |
| [13] | MA Nan, GAO Guofeng, HAO Genyan, ZHAO Qiang, LI Jinping. Cobalt Oxygen-evolving Catalysts Generated in Situ and Interfaced with Silicon-based Semiconductors for Solar Water Splitting [J]. Chinese Journal of Applied Chemistry, 2015, 32(5): 576-582. |
| [14] | JIANG Wei1,3, WU Yaoming1,2*, CHENG Yong1, WANG Limin1,2. Industrial Application of Nickel-Iron Battery and Its Recent Research Progress [J]. Chinese Journal of Applied Chemistry, 2014, 31(07): 749-756. |
| [15] | WANG Lipin, WANG Senlin*, DUAN Qianhua. Ni/NiFe2O4 Composite Electrode Prepared by Electro-deposition and Its Electro-catalytic Performance Towards Oxygen Evolution Reaction [J]. Chinese Journal of Applied Chemistry, 2013, 30(06): 690-697. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||