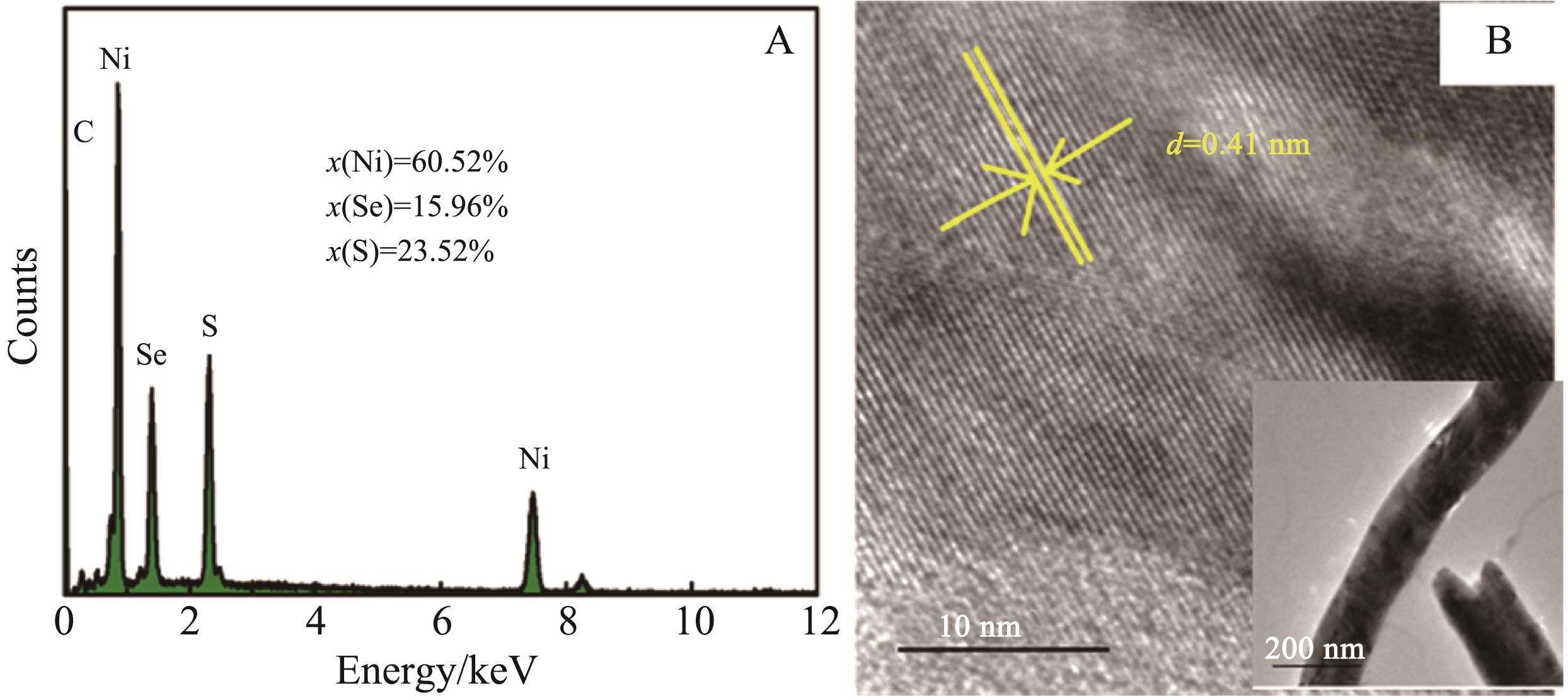
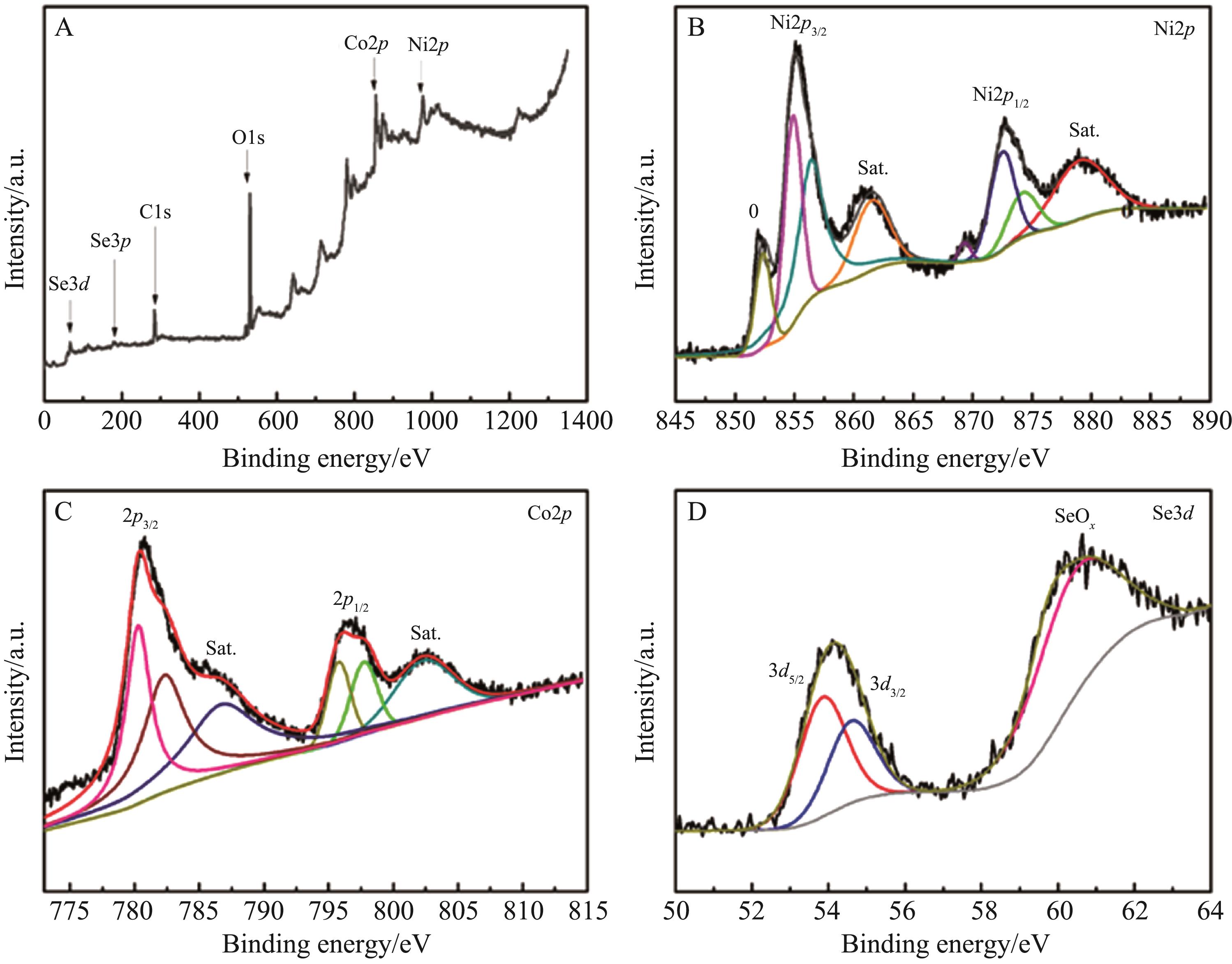
Chinese Journal of Applied Chemistry ›› 2022, Vol. 39 ›› Issue (8): 1252-1261.DOI: 10.19894/j.issn.1000-0518.210458
• Full Papers • Previous Articles Next Articles
Facile Synthesis and High⁃Efficiency Electrocatalytic Oxygen Evolution Performance of Ternary Nickel⁃Based Chalcogenide Nanorod Arrays
Wei-Min DU1( ), Xin LIU1,2, Lin ZHU1, Jia-Min FU1, Wen-Shan GUO1, Xiao-Qing YANG1, Pei-Shuo SHUANG1
), Xin LIU1,2, Lin ZHU1, Jia-Min FU1, Wen-Shan GUO1, Xiao-Qing YANG1, Pei-Shuo SHUANG1
- 1.College of Chemistry and Chemical Engineering,Anyang Normal University,Anyang 455000,China
2.School of Chemistry,Zhengzhou University,Zhengzhou 450001,China
-
Received:2021-09-08Accepted:2022-02-17Published:2022-08-01Online:2022-08-04 -
Contact:Wei-Min DU -
About author:dwmchem@163.com
-
Supported by:the Natural Science Foundation of China(U1404203);the Natural Science Foundation of Henan Province(212300410324);the Basic Research Special of Key Scientific Research Project Plan in the Universities of Henan Province(20ZX007);the Science and Technology Research Project of Henan Province(212102210037)
CLC Number:
Cite this article
Wei-Min DU, Xin LIU, Lin ZHU, Jia-Min FU, Wen-Shan GUO, Xiao-Qing YANG, Pei-Shuo SHUANG. Facile Synthesis and High⁃Efficiency Electrocatalytic Oxygen Evolution Performance of Ternary Nickel⁃Based Chalcogenide Nanorod Arrays[J]. Chinese Journal of Applied Chemistry, 2022, 39(8): 1252-1261.
share this article
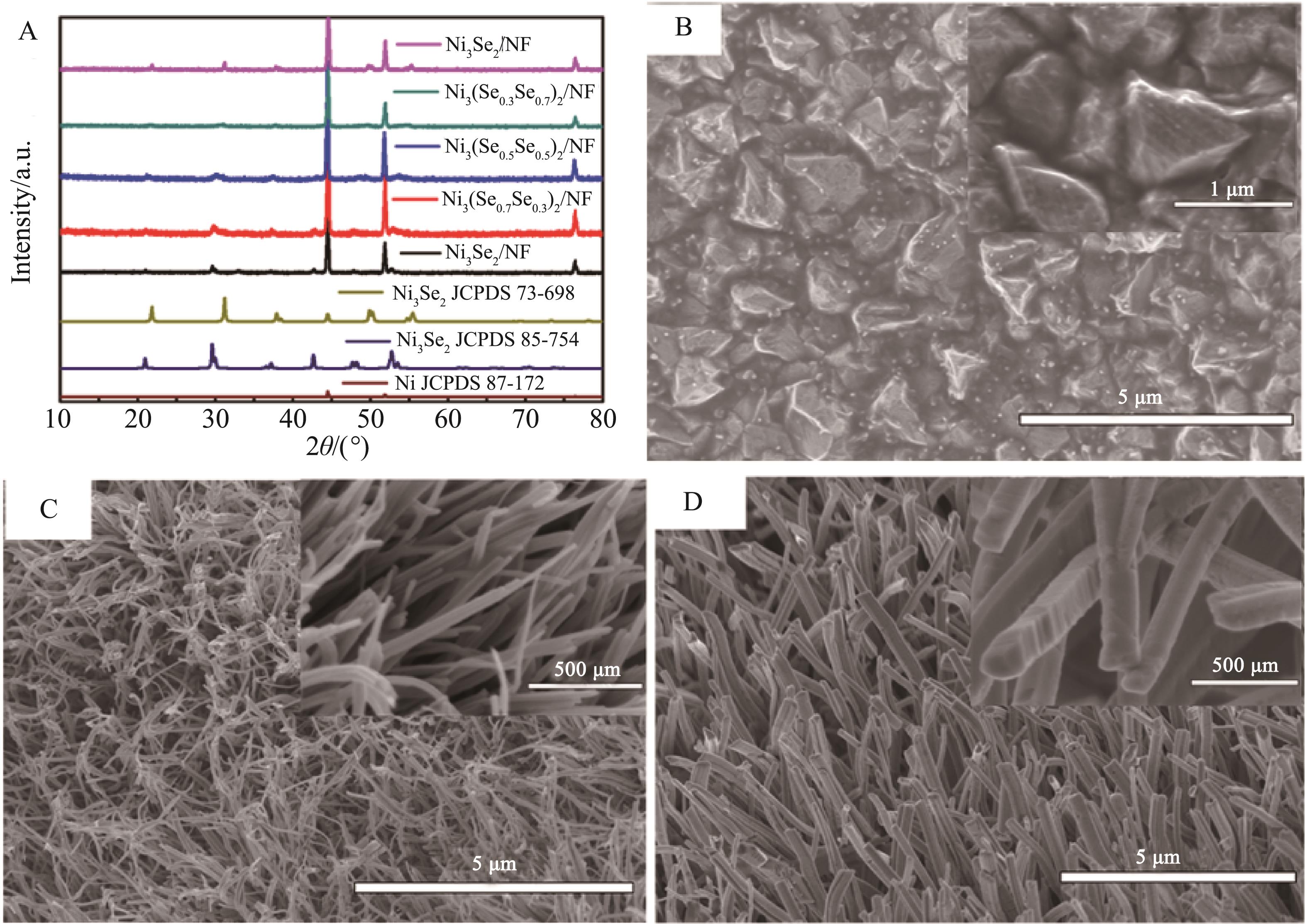
Fig.1 (A) XRD characterization of Ni3(Se x S1-x )2/NF materials;SEM images of the synthesized products:Ni3S2/NF (B),Ni3Se2/NF (C) and Ni3(Se0.3S0.7)2/NF (D) (the inserts are the high-magnification ones)
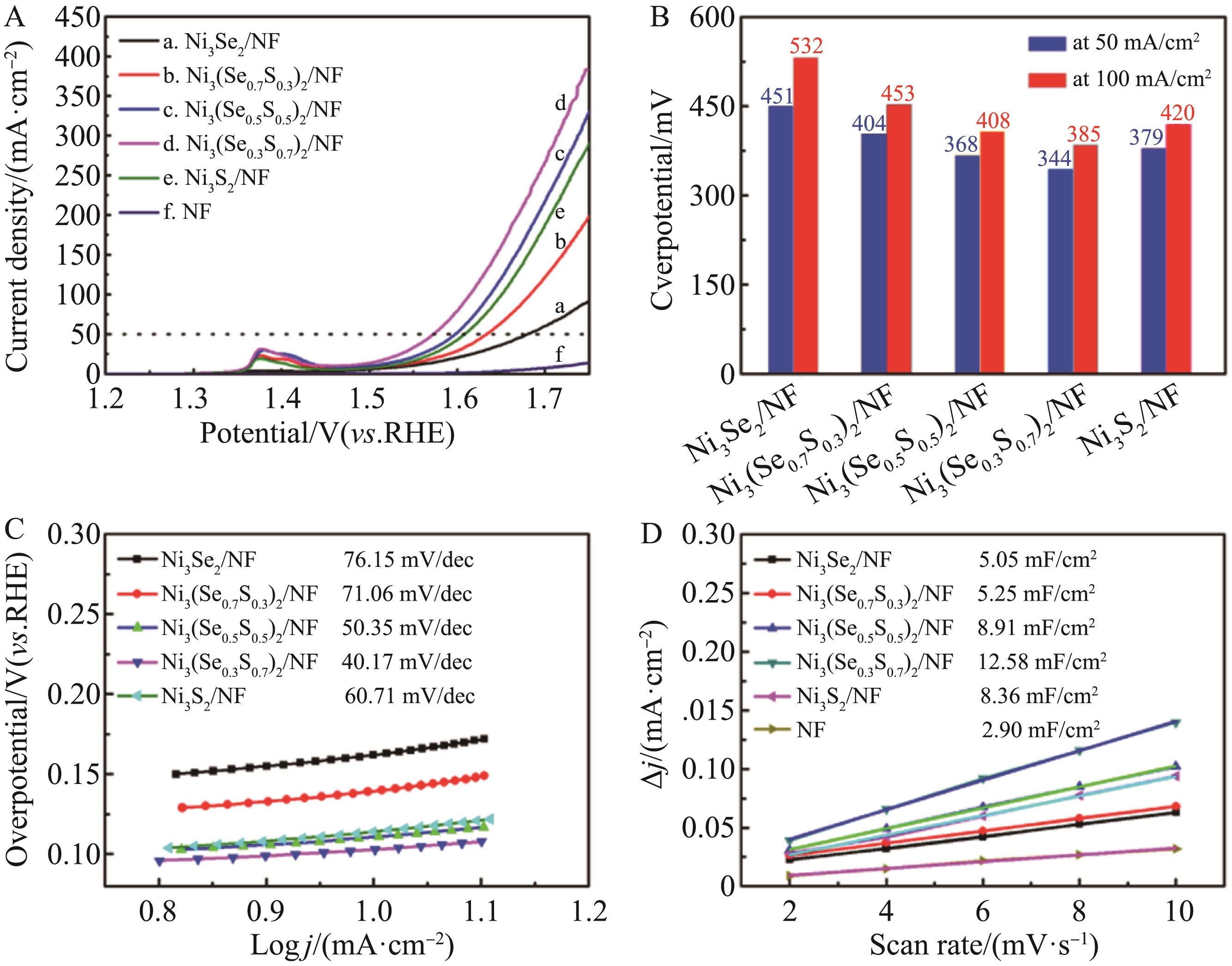
Fig.4 (A) LSV curves of the obtained electrode materials;(B) Compared overpotentials of five electrodes at current densities of 50 and 100 mA/cm2;(C) Tafel curves of the obtained electrode materials;(D) The relationship between the current density and the scanning rate

Fig.5 (A,B) EIS diagrams at different magnifications of the obtained electrodes;(C) Overpotential comparison between Ni3(Se0.3S0.7)2/NF electrodes and other nickel-based catalysts;(D) Compared LSV curves of Ni3(Se0.3S0.7)2/NF electrode after 1000 cycles of cyclic voltammetry(the insert is the amperometric i-t curve of Ni3(Se0.3S0.7)2/NF electrodes)
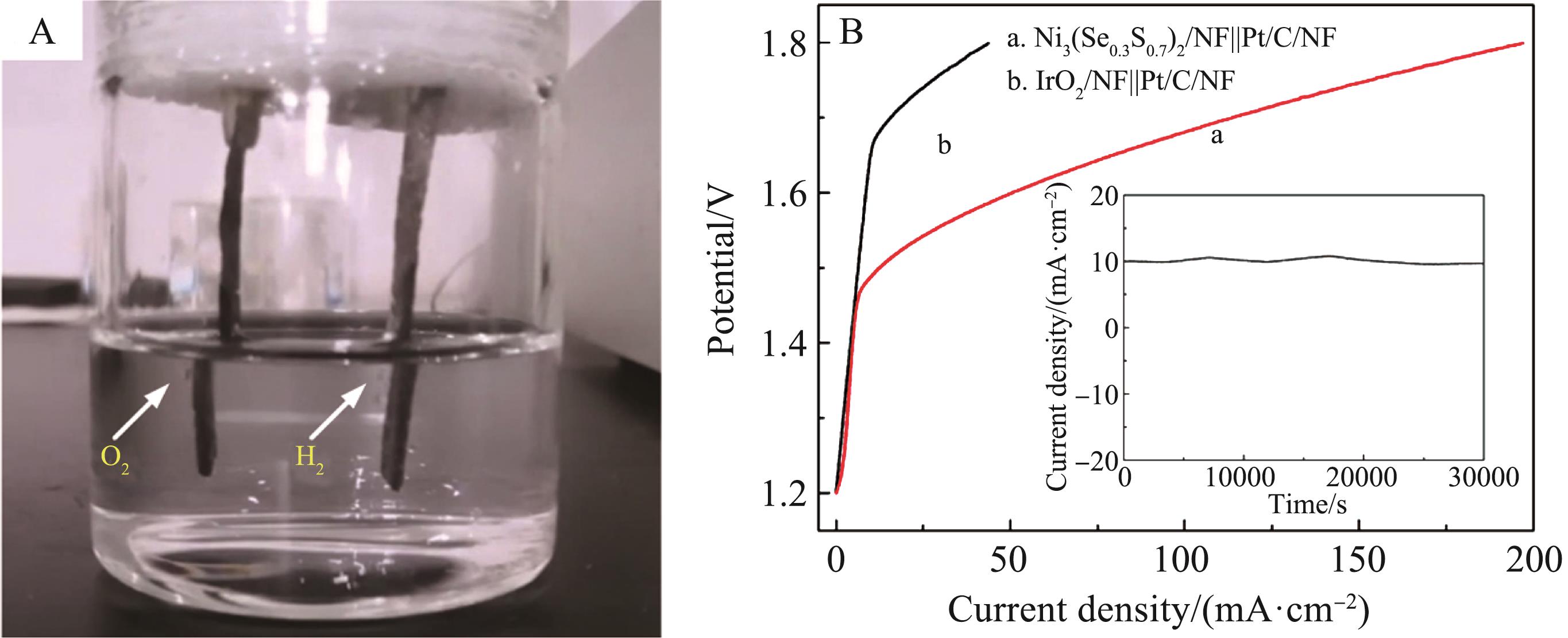
Fig.6 (A) Overall water splitting photographs of Ni3(Se0.3S0.7)2/NF Pt/C/NF electrolysis cell;(B) Compared LSV curves of Ni3(Se0.3S0.7)2/NF Pt/C/NF electrolysis cell in 1.0 mol/L KOH electrolyte for overall water splitting to produce hydrogen (the insert is the amperometric i-t curve of the electrolysis cell)
| 1 | DEBE M K. Electrocatalyst approaches and challenges for automotive fuel cells[J]. Nature, 2012, 486(7401): 43-51. |
| 2 | ZHENG Y, JIAO Y, JARONIEC M, et al. Advancing the electrochemistry of the hydrogen-evolution reaction through combining experiment and theory[J]. Angew Chem Int Ed, 2015, 54(1): 52-65. |
| 3 | CUI B, LIN H, LI J B, et al. Core-ring structured NiCo2O4 nanoplatelets: synthesis, characterization, and electrocatalytic applications[J]. Adv Funct Mater, 2008, 18(9): 1440-1447. |
| 4 | ASLAM S, SAGAR R U R, KUMAR H, et al. Mixed-dimensional heterostructures of hydrophobic/hydrophilic graphene foam for tunable hydrogen evolution reaction[J]. Chemosphere, 2020, 245(4): 125607.125601-125607.125608. |
| 5 | HONG W T, RISCH M, STOERZINGER K A, et al. Toward the rational design of non-precious transition metal oxides for oxygen electrocatalysis[J]. Energ Environ Sci, 2015, 8(5): 1404-1427. |
| 6 | YAO R Q, SHI H, WAN W B, et al. Flexible Co-Mo-N/Au electrodes with a hierarchical nanoporous architecture as highly efficient electrocatalysts for oxygen evolution reaction[J]. Adv Mater, 2020, 32(10): 1907214. |
| 7 | BUVAT G, ESLAMIBIDGOLI M J, YOUSSEF A H, et al. Effect of IrO6 octahedron distortion on the OER activity at (100) IrO2 thin film[J]. ACS Catal, 2020, 10(1): 806-817. |
| 8 | CHEN S, HUANG H, JIANG P, et al. Mn-doped RuO2 nanocrystals as highly active electrocatalysts for enhanced oxygen evolution in acidic media[J]. ACS Catal, 2020, 10(2): 1152-1160. |
| 9 | CHO K H, SEO H, PARK S, et al. Uniform, assembled 4 nm Mn3O4 nanoparticles as efficient water oxidation electrocatalysts at neutral pH[J]. Adv Funct Mater, 2020, 30(10): 1910424. |
| 10 | XIAO Z H, WANG Y, HUANG Y C, et al. Filling the oxygen vacancies in Co3O4 with phosphorus: an ultra-efficient electrocatalyst for overall water splitting[J]. Energ Environ Sci, 2017, 10(12): 2563-2569. |
| 11 | YUAN G, HU Y J, WANG Z H, et al. Facile synthesis of self-supported amorphous phosphorus-doped Ni(OH)2 composite anodes for efficient water oxidation[J]. Catal Sci Technol, 2020, 10(1): 263-267. |
| 12 | CHU W J, SHI Z J, HOU Y D, et al. Trifunctional of phosphorus-doped NiCo2O4 nanowire materials for asymmetric supercapacitor, oxygen evolution reaction, and hydrogen evolution reaction[J]. ACS Appl Mater Interfaces, 2020, 12(2): 2763-2772. |
| 13 | ZHANG K, MIN X, ZHANG T, et al. Biodeposited nano-CdS drives the in situ growth of highly dispersed sulfide nanoparticles during pyrolysis for enhanced oxygen evolution reaction[J]. ACS Appl Mater Interfaces, 2020, 12(49): 54553-54562. |
| 14 | YOU B, SUN Y J. Innovative strategies for electrocatalytic water splitting[J]. Acc Chem Res, 2018, 51(7): 1571-1580. |
| 15 | WANG H F, TANG C, ZHANG Q. A review of precious-metal-free bifunctional oxygen electrocatalysts: rational design and applications in Zn-air batteries[J]. Adv Funct Mater, 2018, 28(46): 1803329. |
| 16 | ZENG L, SUN K, CHEN Y, et al. Neutral-pH overall water splitting catalyzed efficiently by a hollow and porous structured ternary nickel sulfoselenide electrocatalyst[J]. J Mater Chem A, 2019, 7(28): 16793-16802. |
| 17 | ACEDERA R A E, GUPTA G, MAMLOUK M, et al. Solution combustion synthesis of porous Co3O4 nanoparticles as oxygen evolution reaction (OER) electrocatalysts in alkaline medium[J]. J Alloys Compd, 2020, 836: 154919. |
| 18 | SHI X, WANG H, KANNAN P, et al. Rich-grain-boundary of Ni3Se2 nanowire arrays as multifunctional electrode for electrochemical energy storage and conversion applications[J]. J Mater Chem A, 2019, 7(7): 3344-3352. |
| 19 | DENG S, SHEN Y, XIE D, et al. Directional construction of Cu2S branch arrays for advanced oxygen evolution reaction[J]. J Energy Chem, 2019, 39: 61-67. |
| 20 | XU R, WU R, SHI Y, et al. Ni3Se2 nanoforest/Ni foam as a hydrophilic, metallic, and self-supported bifunctional electrocatalyst for both H2 and O2 generations[J]. Nano Energy, 2016, 24: 103-110. |
| 21 | HUANG Z F, SONG J J, DU Y H, et al. Chemical and structural origin of lattice oxygen oxidation in Co-Zn oxyhydroxide oxygen evolution electrocatalysts[J]. Nat Energy, 2019, 4(4): 329-338. |
| 22 | LIN J, WANG H, YAN Y, et al. Sandwich-like structured NiSe2/Ni2P@FeP interface nanosheets with rich defects for efficient electrocatalytic water splitting[J]. J Power Sources, 2020, 445: 227294. |
| 23 | MA F X, YU L, XU C Y, et al. Self-supported formation of hierarchical NiCo2O4 tetragonal microtubes with enhanced electrochemical properties[J]. Energ Environ Sci, 2016, 9(3): 862-866. |
| 24 | ZHANG Q, ZHONG H X, MENG F, et al. Three-dimensional interconnected Ni(Fe)OxHy nanosheets on stainless steel mesh as a robust integrated oxygen evolution electrode[J]. Nano Res, 2018, 11(3): 1294-1300. |
| 25 | PAN J, HAO S, ZHANG X, et al. In situ growth of Fe and Nb co-doped β-Ni(OH)2 nanosheet arrays on nickel foam for an efficient oxygen evolution reaction[J]. Inorg Chem Front, 2020, 7(18): 3465-3474. |
| 26 | FANG L, LI W, GUAN Y, et al. Tuning unique peapod-like Co(SxSe1- x)2 nanoparticles for efficient overall water splitting[J]. Adv Funct Mater, 2017, 27(24): 1701008. |
| 27 | CHEN H, GAO Y, YE L, et al. A Cu2Se-Cu2O film electrodeposited on titanium foil as a highly active and stable electrocatalyst for the oxygen evolution reaction[J]. Chem Commun, 2018, 54(39): 4979-4982. |
| 28 | KRISHNAMOORTHY K, VEERASUBRAMANI G K, RADHAKRISHNAN S, et al. One pot hydrothermal growth of hierarchical nanostructured Ni3S2 on Ni foam for supercapacitor application[J]. Chem Eng J, 2014, 251: 116-122. |
| 29 | GU Y, DU W, LIU X, et al. Matching design of high-performance electrode materials with different energy-storage mechanism suitable for flexible hybrid supercapacitors[J]. J Alloy Compd, 2020, 844: 156196. |
| 30 | GU Y, DU W, DARRAT Y, et al. In situ growth of novel nickel diselenide nanoarrays with high specific capacity as the electrode material of flexible hybrid supercapacitors[J]. Appl Nanosci, 2019, 10(5): 1591-1601. |
| 31 | ZHENG J, CHENG K, ZHANG R, et al. Hydrothermal preparation of CQDs/MoS2/NiSe2 composite as electrode material for supercapacitor[J]. J Mater Sci-Mater El, 2020, 31(12): 9366-9376. |
| 32 | DAI Z, XUE L, ZHANG Z, et al. Construction of single-phase nickel disulfide microflowers as high-performance electrodes for hybrid supercapacitors[J]. Energ Fuel, 2020, 34(8): 10178-10187. |
| 33 | MIAO Y, ZHANG X, ZHAN J, et al. Hierarchical NiS@CoS with controllable core-shell structure by two-step strategy for supercapacitor electrodes[J]. Adv Mater Interfaces, 2019, 7(3): 1901618. |
| 34 | DU L, DU W, REN H, et al. Honeycomb-like metallic nickel selenide nanosheet arrays as binder-free electrodes for high-performance hybrid asymmetric supercapacitors[J]. J Mater Chem A, 2017, 5(43): 22527-22535. |
| 35 | HOU L, SHI Y, WU C, et al. Monodisperse metallic NiCoSe2 hollow sub-microspheres: formation process, intrinsic charge-storage mechanism, and appealing pseudocapacitance as highly conductive electrode for electrochemical supercapacitors[J]. Adv Funct Mater, 2018, 28(13): 1705921. |
| 36 | VRUBEL H, MOEHL T, GRAETZEL M, et al. Revealing and accelerating slow electron transport in amorphous molybdenum sulphide particles for hydrogen evolution reaction[J]. Chem Commun, 2013, 49(79): 8985-8987. |
| 37 | CUI W, CHENG N, LIU Q, et al. Mo2C nanoparticles decorated graphitic carbon sheets: biopolymer-derived solid-state synthesis and application as an efficient electrocatalyst for hydrogen generation[J]. ACS Catal, 2014, 4(8): 2658-2661. |
| 38 | YANG M, LU W, JIN R, et al. Superior oxygen evolution reaction performance of Co3O4/NiCo2O4/Ni foam composite with hierarchical structure[J]. ACS Sustain Chem Eng, 2019, 7(14): 12214-12221. |
| 39 | ELIZABETH I, NAIR A K, SINGH B P, et al. Multifunctional Ni-NiO-CNT composite as high performing free standing anode for Li ion batteries and advanced electro catalyst for oxygen evolution reaction[J]. Electrochim Acta, 2017, 230: 98-105. |
| 40 | LIANG H, MENG F, CAB N-ACEVEDO M, et al. Hydrothermal continuous flow synthesis and exfoliation of NiCo layered double hydroxide nanosheets for enhanced oxygen evolution catalysis[J]. Nano Lett, 2015, 15(2): 1421-1427. |
| 41 | ZHOU W, WU X J, CAO X, et al. Ni3S2 nanorods/Ni foam composite electrode with low overpotential for electrocatalytic oxygen evolution[J]. Energ Environ Sci, 2013, 6(10): 2921-2924. |
| 42 | AIJAZ A, MASA J, R SLER C, et al. Metal-organic framework derived carbon nanotube grafted cobalt/carbon polyhedra grown on nickel foam: an efficient 3D electrode for full water splitting[J]. Chemelectrochem, 2016, 4(1): 188-193. |
| 43 | LIANG H, GANDI A N, ANJUM D H, et al. Plasma-assisted synthesis of NiCoP for efficient overall water splitting[J]. Nano Lett, 2016, 16(12): 7718-7725. |
| 44 | TAHIRA M, PANA L, IDREESD F, et al. Electrocatalytic oxygen evolution reaction for energy conversion and storage: a comprehensive review[J]. Nano Energy 2017, 37: 136-157. |
| [1] | Ying LI, Yun ZHANG, Liang-Liang LIN, Hu-Jun XU. Synergistic Effect of Ternary Compound System of Sodium N‑Lauroyl Methylalanine [J]. Chinese Journal of Applied Chemistry, 2022, 39(8): 1262-1273. |
| [2] | Wei-Jin CAO, Lu BAI, Lan-Lan WU, Jing-De LI, Shu-Yan SONG. Multi⁃Shell Hollow Nickel⁃Cobalt Bimetallic Phosphide Nanospheres for Highly Efficient Oxygen Evolution Reaction [J]. Chinese Journal of Applied Chemistry, 2022, 39(4): 666-672. |
| [3] | Xue WANG, Yi-Bo WANG, Xian WANG, Jian-Bing ZHU, Jun-Jie GE, Chang-Peng LIU, Wei XING. Research Progress of Mechanism of Acidic Oxygen Evolution Reaction and Development of Ir⁃based Catalysts [J]. Chinese Journal of Applied Chemistry, 2022, 39(4): 616-628. |
| [4] | Dan WANG, Xian-Biao HOU, Xing-Kun WANG, Zhi-Cheng LIU, Huan-Lei WANG, Ming-Hua HUANG. Research Progress of Carbon‑Encapsulated Iron‑Based Nanoparticles Electrocatalysts for Zinc‑Air Batteries [J]. Chinese Journal of Applied Chemistry, 2022, 39(10): 1488-1500. |
| [5] | ZHANG Shuai, TAO You-Hua. Synthesis of Two Functional Cyclic Lysine Monomers [J]. Chinese Journal of Applied Chemistry, 2021, 38(12): 1676-1678. |
| [6] | SUN Xiaotong,CHEN Nan,LIANG Hanxue,LI Zengling,LIU Qianwen,QU Liangti. Progress of Fabrication of One-Dimensional Hybrid Nanomaterials by Template-Confined Growth and Their Diverse Applications [J]. Chinese Journal of Applied Chemistry, 2020, 37(2): 123-133. |
| [7] | YANG Tao, LIU Wenfeng, MA Mengyue, DONG Hongyu, YANG Shuting. Fade Mechanism of Ternary Lithium Ion Power Battery [J]. Chinese Journal of Applied Chemistry, 2020, 37(10): 1181-1186. |
| [8] | ZHANG Tong, OUYANG Jinyang, ZHAO Xiaoli, YANG Xiaoniu. High Efficiency Ternary Polymer Solar Cells Fabricated by Spray Coating [J]. Chinese Journal of Applied Chemistry, 2020, 37(1): 24-31. |
| [9] | HU Kuan, JIANG Hai, HUANG Dong, LIU Chang, ZHANG Kunyu, PAN Li. Synergetic Modification of Polybutylene Succinate and Poly(epichlorohydrin-co-ethylene oxide) Elastomer in Toughening Poly(lactic acid) [J]. Chinese Journal of Applied Chemistry, 2019, 36(9): 996-1002. |
| [10] | WANG Renliang, ZHU Yanmei, JI Haiwei. Synthesis of Ordered Supermicroporous Silica Using Short-Chain Quaternary Ammonium/Fatty Acid Salts as Template [J]. Chinese Journal of Applied Chemistry, 2019, 36(1): 51-57. |
| [11] | CHEN Si,SUN Lizhen,SHU Xinxin,ZHANG Jintao. Graphene-based Catalysts for Efficient Electrocatalytic Applications [J]. Chinese Journal of Applied Chemistry, 2018, 35(3): 272-285. |
| [12] | ZHANG He,ZHANG Mengshi,LIAO Shijun. Recent Progress in the Lithium-Rich Ternary Layered Cathode Materials [J]. Chinese Journal of Applied Chemistry, 2018, 35(11): 1277-1288. |
| [13] | ZHU Yan, YUAN Guang, YUAN Shuai, CHEN Shaojie, YAO Weiqin, TIAN Longzhao, QIN Fan, LIU Linmei. Influence of Alkali and Salt on Phase Diagram of Sodium Dodecyl Sulfate/n-Pentanol- Cyclohexane-Water Pseudo Ternary Systems [J]. Chinese Journal of Applied Chemistry, 2017, 34(7): 833-838. |
| [14] | ZHU Zhaoqiang,DU Weimin,GUO Wei,ZHU Wenjuan. Research Progress of Preparation and Application of Transition Metal Ternary Compounds in Supercapacitors [J]. Chinese Journal of Applied Chemistry, 2016, 33(3): 267-276. |
| [15] | SONG Hongwei, HUANG Hui, TAN Ning, CHENG Buming, GUO Zhongcheng. Electrochemical Behavior of Al/Pb-0.2%Ag Anode in Fluoride Ion and Sulfuric Acid [J]. Chinese Journal of Applied Chemistry, 2016, 33(12): 1455-1461. |
| Viewed | ||||||||||||||||||||||||||||||||||||||||||||||||||
|
Full text 681
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||
|
Abstract 682
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||