Chinese Journal of Applied Chemistry ›› 2023, Vol. 40 ›› Issue (1): 40-51.DOI: 10.19894/j.issn.1000-0518.220156
• Review • Previous Articles Next Articles
Research Progress of Assembly of Phospholipid Tube in Vitro and Its Potential Application in the Field of Biology and Chemistry
- College of Biological and Food Engineering,Guangdong University of Petrochemical Technology,Maoming 525000,China
-
Received:2022-04-27Accepted:2022-08-31Published:2023-01-01Online:2023-01-28 -
Contact:Hong-Mei BI -
About author:hongmei_bi@126.com
-
Supported by:the Natural Science Foundation of Guangdong Province(2020A1515010522);the Characteristic Innovation Projects of Universities in Guangdong Province(2019KTSCX109);the Projects of Talents Recruitment of GDUPT(2019rc114)
CLC Number:
Cite this article
Hong-Mei BI. Research Progress of Assembly of Phospholipid Tube in Vitro and Its Potential Application in the Field of Biology and Chemistry[J]. Chinese Journal of Applied Chemistry, 2023, 40(1): 40-51.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: http://yyhx.ciac.jl.cn/EN/10.19894/j.issn.1000-0518.220156
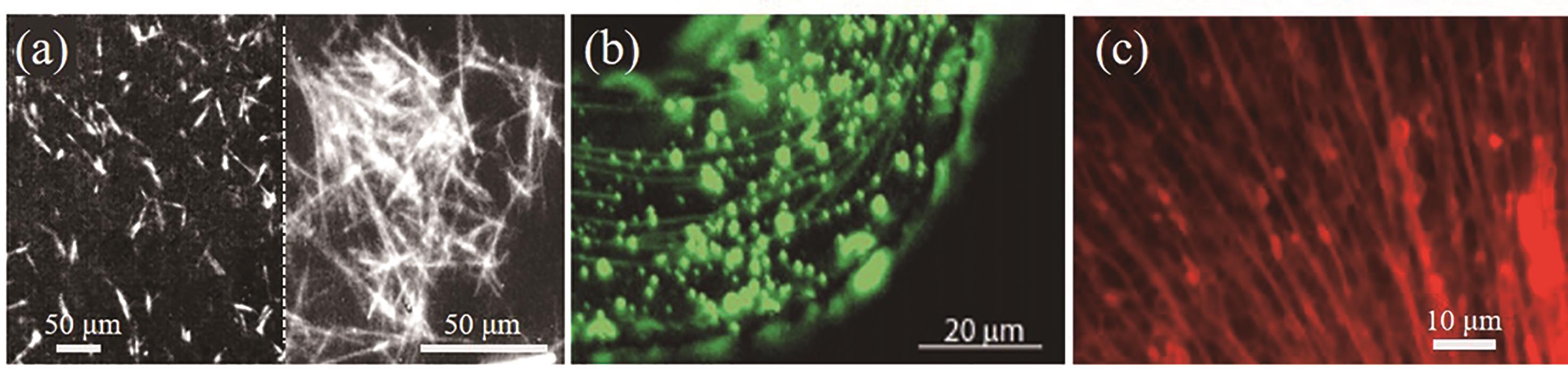
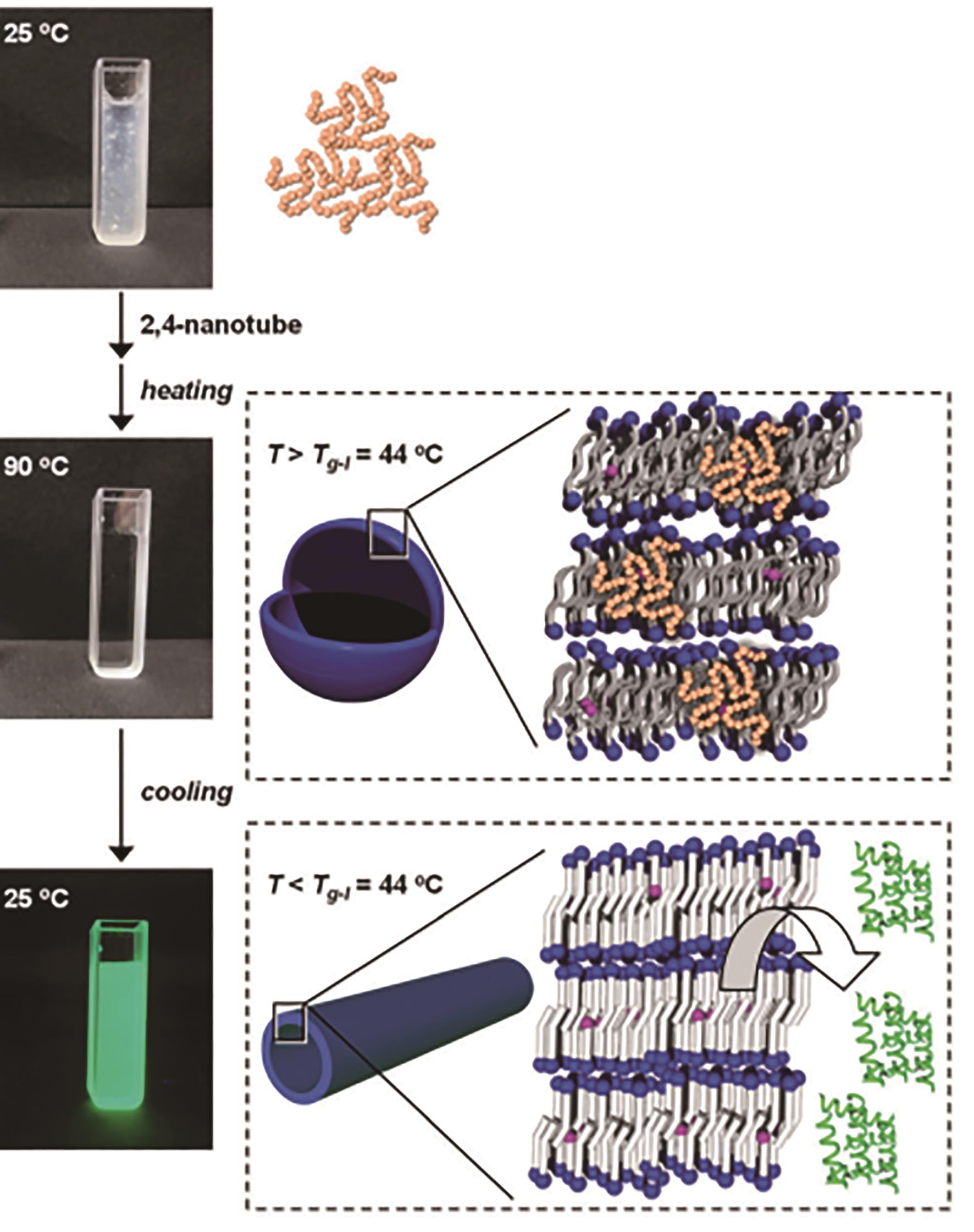
Fig.1 Assembly of phospholipids tube in vitro: (a) Self-assemble of lipid molecules[11]; (b) External force stretching[17]; (c) Actuation of electric field[23]
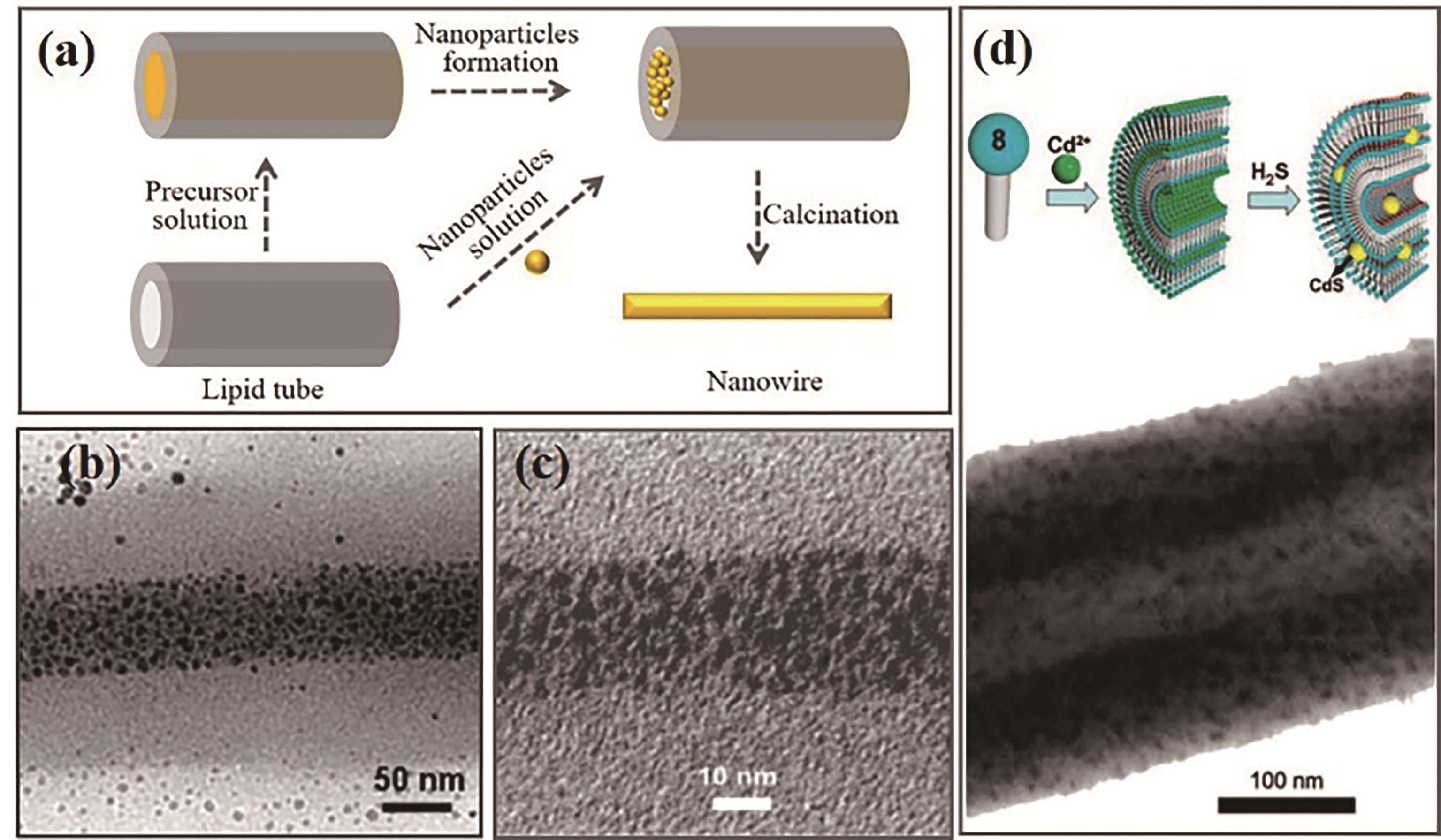
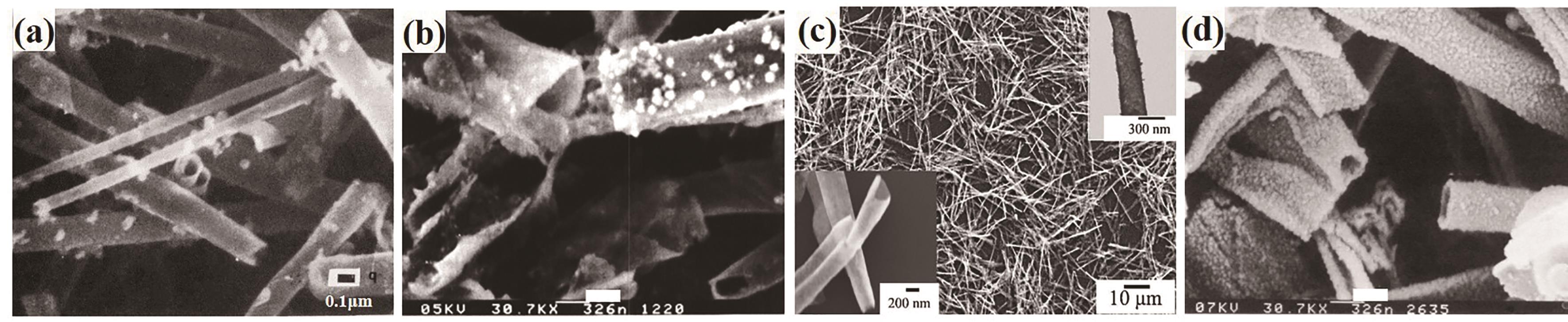
Fig.5 Formation of nanowire. (a) Schematic of nanowire formation; (b) Au nanoparticles synthesized in situ[76]; (c) Filled with Au nanoparticles[74]; (d) CdS nanopartices[76]
| 1 | RUSTOM A, SAFFRICH R, MARKOVIC I, et al. Nanotubular highways for intercellular organelle transport[J]. Science, 2004, 303(5660): 1007-1010. |
| 2 | HURTLEY S M. Tunneling nanotubes under the microscope[J]. Science, 2019, 363(6428): 704. |
| 3 | DUBEY G P, BEN Y S. Intercellular nanotubes mediate bacterial communication[J]. Cell, 2011, 144(4): 590-600. |
| 4 | REGEV R N, WILSON D W, CARVALHO T G, et al. Cell-cell communication between malaria-infected red blood cells via exosome-like vesicles[J]. Cell, 2013, 153(5): 1120-1133. |
| 5 | ROSTAMI J, HOLMQVIST S, LINDSTRÖM V, et al. Human astrocytes transfer aggregated alpha-synuclein via tunneling nanotubes[J]. J Neurosci, 2017, 37(49): 11835-11853. |
| 6 | SHIMIZU T, DING W, KAMETA N. Soft-Matter nanotubes: a platform for diverse functions and applications[J]. Chem Rev, 2020, 120(4): 2347-2407. |
| 7 | YAGER P, SCHOEN P E. Formation of tubules by a polymerizable surfactant[J]. Mol Cryst Liq Cryst, 1984, 106: 371-381. |
| 8 | HAN W B, KWAK R, KANG J Y, et al. Generation of solvent-free 3D lipid structure arrays on high aspect ratio Si microwell substrate[J]. Adv Mater Interfaces, 2019, 6(5): 1801554. |
| 9 | ZHOU Y. Lipid nanotubes as scaffold toward construction of one-dimensional nanostructures[J]. Sci Adv Mater, 2010, 2(3): 359-364. |
| 10 | BURKE T G, RUDOLPH A S, PRICE R R, et al. Differential scanning calorimetric study of the thermotropic phase-behavior of a polymerizable, tubule-forming lipid[J]. Chem Phys Lipids, 1988, 48(3/4): 215-230. |
| 11 | GEORGER J H, SINGH A, PRICE R R, et al. Helical and tubular microstructures formed by polymerizable phosphatidylcholines[J]. J Am Chem Soc, 1987, 109(20): 6169-6175. |
| 12 | CHAPPELL J S, YAGER P. Formation of mineral microstructures with a high aspect ratio from phospholipid-bilayer tubules[J]. J Mater Sci Lett, 1992, 11(10): 633-636. |
| 13 | BRAZHNIK K P, VREELAND W N, HUTCHISON J B, et al. Directed growth of pure phosphatidylcholine nanotubes in microfluidic channels[J]. Langmuir, 2005, 21(23): 10814-10817. |
| 14 | DITTRICH P S, HEULE M, RENAUD P, et al. On-chip extrusion of lipid vesicles and tubes through microsized apertures[J]. Lab Chip, 2006, 6(4): 488-493. |
| 15 | WEST J, MANZ A, DITTRICH P S. Lipid nanotubule fabrication by microfluidic tweezing[J]. Langmuir, 2008, 24(13): 6754-6758. |
| 16 | LIN Y C, HUANG K S, CHIANG J T, et al. Manipulating self-assembled phospholipid microtubes using microfluidic technology[J]. Sens Actuators B: Chem, 2006, 117(2): 464-471. |
| 17 | SUGIHARA K, CHAMI M, DERENYI I, et al. Directed self-assembly of lipid nanotubes from inverted hexagonal structures[J]. ACS Nano, 2012, 6(8): 6626-6632. |
| 18 | TAN Y C, SHEN A Q, LI Y, et al. Engineering lipid tubules using nano-sized building blocks: the combinatorial self-assembly of vesicles[J]. Lab Chip, 2008, 8(2): 339-345. |
| 19 | SEKINE Y, ABE K, SHIMIZU A, et al. Shear flow-induced nanotubulation of surface-immobilized liposomes[J]. RSC Adv, 2012, 2: 2682-2684. |
| 20 | ANTONOVA K, VITKOVA V, MEYER C. Membrane tubulation from giant lipid vesicles in alternating electric fields[J]. Phys Rev E, 2016, 93(1): 012413. |
| 21 | PRATHYUSHA K R, PAGONABARRAGA I, KUMAR P B S. Modification of lipid membrane compressibility induced by an electric field[J]. Phys Rev E, 2020, 102(6): 062413. |
| 22 | HAYES M A, PYSHER M D, CHEN K. Liposomes form nanotubules and long range networks in the presence of electric field[J]. J Nanosci Nanotechnol, 2007, 7(7): 2283-2286. |
| 23 | BI H M, FU D G, WANG L, et al. Lipid nanotube formation using space-regulated electric field above interdigitated electrodes[J]. ACS Nano, 2014, 8(4): 3961-3969. |
| 24 | ZHU C, ZHANG Y, WANG Y, et al. Point-to-Plane nonhomogeneous electric-field-induced simultaneous formation of giant unilamellar vesicles (GUVs) and lipid tubes[J]. Chem Eur J, 2016, 22(9): 2906-2909. |
| 25 | GHELLAB S E, HAN X J. Lipid tubes formation induced by electroosmotic flow[J]. Chem Phys Lett, 2018, 706:515-519. |
| 26 | CASTILLO J A, NARCISO D M, HAYES M A. Bionanotubule formation from surface-attached liposomes using electric fields[J]. Langmuir, 2009, 25(1): 391-396. |
| 27 | WANG Z, MAO X, WANG H, et al. Fabrication of lipid nanotubules by ultrasonic drag force[J]. Langmuir, 2021, 37(30): 8945-8952. |
| 28 | BAXTER A M, JORDAN L R, KULLAPPAN M, et al. Tubulation of supported lipid bilayer membranes induced by photosensitized lipid oxidation[J]. Langmuir, 2021, 37(19): 5753-5762. |
| 29 | JONES S, HUYNH A, GAO Y, et al. Calcium ion-assisted lipid tubule formation[J]. Mater Chem Front, 2018, 2(3): 603-608. |
| 30 | GHOSH M, NANDI S, LAYEK S, et al. Formation of lipid tubules induced by a sugar-like molecule myo-inositol[J]. Chem Commun, 2022, 58(3): 459-462. |
| 31 | WANG Q, QIN X, FANG J, et al. Nanomedicines for the treatment of rheumatoid arthritis: state of art and potential therapeutic strategies[J]. Acta Pharm Sin B, 2021, 11(5): 1158-1174. |
| 32 | CAO D Y, WANG Y Q. Wave dispersion in viscoelastic lipid nanotubes conveying viscous protein solution[J]. Eur Phys J Plus, 2020, 135(1): 24. |
| 33 | DAVOODI P, LEE L Y, XU Q, et al. Drug delivery systems for programmed and on-demand release[J]. Adv Drug Delivery Rev, 2018, 132:104-138. |
| 34 | QIN X, HE L, FAN D, et al. Targeting the resolution pathway of inflammation using Ac2-26 peptide-loaded PEGylated lipid nanoparticles for the remission of rheumatoid arthritis[J]. Asia J Pharma Sci, 2021, 16(4): 483-493. |
| 35 | KILIAN H I, PRADHAN A J, JAHAGIRDAR D, et al. Light-Triggered release of large biomacromolecules from porphyrin-phospholipid liposomes[J]. Langmuir, 2021, 37(36): 10859-10865. |
| 36 | BI H M, CHEN Z Q, QIU J Q, et al. Magnetic-field-triggered remote drug release and analysis of mechanism[J]. Sensor Mater, 2021, 33(9): 3221-3231. |
| 37 | BI H M, MA S H, LI Q C, et al. Magnetically triggered drug release from biocompatible microcapsules for potential cancer therapeutics[J]. J Mater Chem B, 2016, 4(19): 3269-3277. |
| 38 | LOMBARDO D, KISELEV M A. Methods of liposomes preparation: formation and control factors of versatile nanocarriers for biomedical and nanomedicine application[J]. Pharmaceutics, 2022, 14(3): 543. |
| 39 | SAGAR R, LOU J, WATSON A J, et al. Zinc triggered release of encapsulated cargo from liposomes via a synthetic lipid switch[J]. Bioconjugate Chem, 2021, 32(12): 2485-2496. |
| 40 | ODETTE W L, HENNECKER C D, MITTERMAIER A K, et al. EDTA-Gradient loading of doxorubicin into ferrocene-containing liposomes: effect of lipid composition and visualization of triggered release by Cryo-TEM[J]. Langmuir, 2021, 37(38): 11222-11232. |
| 41 | BI H M, CHEN Z Q, QIU J Q. Drug release and magneto-calorific analysis of magnetic lipid microcapsules for potential cancer therapeutics[J]. Des Monomers Polym, 2021, 24(1): 156-161. |
| 42 | BI H M, HAN X J. Magnetic field triggered drug release from lipid microcapsule containing lipid-coated magnetic nanoparticles[J]. Chem Phys Lett, 2018, 706:455-460. |
| 43 | JACOB S, NAIR A B, SHAH J, et al. Lipid nanoparticles as a promising drug delivery carrier for topical ocular therapy; an overview on recent advances[J]. Pharmaceutics, 2022, 14(3): 533. |
| 44 | RATHEL T, MANNELL H, PIRCHER J, et al. Magnetic stents retain nanoparticle-bound antirestenotic drugs transported by lipid microbubbles[J]. Pharm Res, 2012, 29(5): 1295-1307. |
| 45 | LEE K L, HUBBARD L C, HERN S, et al. Shape matters: the diffusion rates of TMV rods and CPMV icosahedrons in a spheroid model of extracellular matrix are distinct[J]. Biomater Sci, 2013, 1(6): 581. |
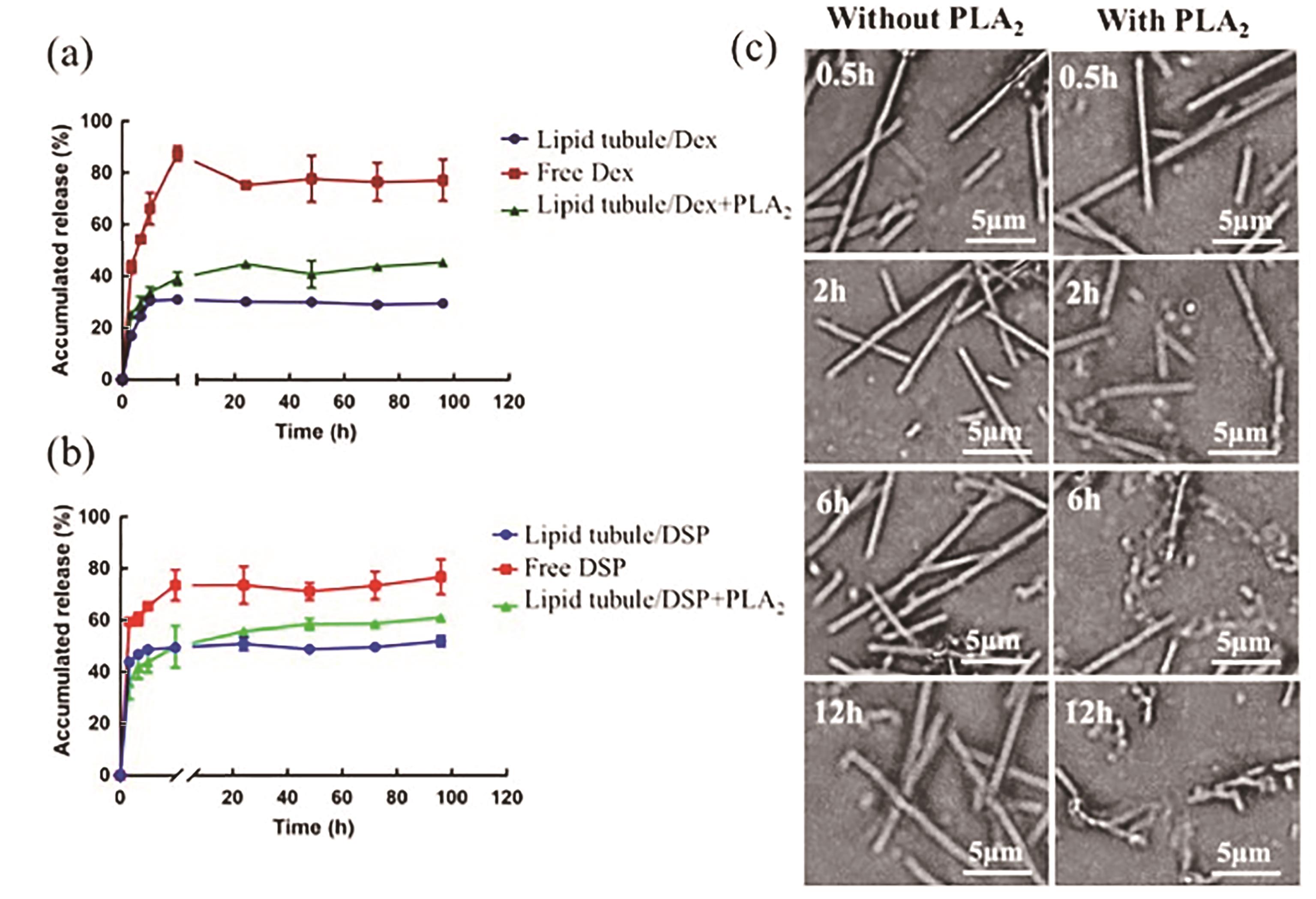
| 46 | WANG Q, HE L, FAN D, et al. PLA2-triggered release of drugs from self-assembled lipid tubules for arthritis treatments[J]. ACS Appl Bio Mater, 2020, 3(9): 6488-6496. |
| 47 | DING W, WADA M, MINAMIKAWA H, et al. Cisplatin-encapsulated organic nanotubes by endo-complexation in the hollow cylinder[J]. Chem Commun, 2012, 48(69): 8625-8627. |
| 48 | DING W, KAMETA N, MINAMIKAWA H, et al. Hybrid organic nanotubes with dual functionalities localized on cylindrical nanochannels control the release of doxorubicin[J]. Adv Healthc Mater, 2012, 1(6): 699-706. |
| 49 | GOLDSTEIN A S, GELB M H, YAGER P. Continuous and highly variable rate controlled release of model drugs from sphingolipid-based complex high axial ratio microstructures[J]. J Control Release, 2001, 70(1): 125-138. |
| 50 | SONG S, CHEN Y, YAN Z, et al. Self-assembled rosette nanotubes for incorporating hydrophobic drugs in physiological environments[J]. Int J Nanomed, 2011, 6: 101-107. |
| 51 | WAKASUGI A, ASAKAWA M, KOGISO M, et al. Organic nanotubes for drug loading and cellular delivery[J]. Int J Nanomed, 2011, 413(1): 271-278. |
| 52 | DING W X, CHECHETKA S A, MASUDA M, et al. Lipid nanotube tailored fabrication of uniquely shaped polydopamine nanofibers as photothermal converters[J]. Chem Eur J, 2016, 22(13): 4345-4350. |
| 53 | KAMETA N, MASUDA M, MINAMIKAWA H, et al. Functionalizable organic nanochannels based on lipid nanotubes: encapsulation and nanofluidic behavior of biomacromolecules[J]. Chem Mater, 2007, 19(14): 3553-3560. |
| 54 | KAMETA N, MINAMIKAWA H, SOMEYA Y, et al. Confinement effect of organic nanotubes toward green fluorescent protein (GFP) depending on the inner diameter size[J]. Chem Eur J, 2010, 16(14): 4217-4223. |
| 55 | KAMETA N, MASUDA M, MIZUNO G, et al. Supramolecular nanotube endo sensing for a guest protein[J]. Small, 2008, 4(5): 561-565. |
| 56 | KAMETA N, MINAMIKAWA H, MASUDA M, et al. Controllable biomolecule release from self-assembled organic nanotubes with asymmetric surfaces: pH and temperature dependence[J]. Soft Matter, 2008, 4(8): 1681-1687. |
| 57 | KAMETA N, MATSUZAWA T, YAOI K, et al. Glycolipid-based nanostructures with thermal-phase transition behavior functioning as solubilizers and refolding accelerators for protein aggregates[J]. Soft Matter, 2017, 13(17): 3084-3090. |
| 58 | MEILANDER N J, YU X, ZIATS N P, et al. Lipid-based microtubular drug delivery vehicles[J]. J Control Release, 2001, 71(1): 141-152. |
| 59 | MEILANDER N J, PASUMARTHY M K, KOWALCZYK T H, et al. Sustained release of plasmid DNA using lipid microtubules and agarose hydrogel[J]. J Control Release, 2003, 88(2): 321-331. |
| 60 | DING W, WADA M, KAMETA N, et al. Functionalized organic nanotubes as tubular nonviral gene transfer vector[J]. J Control Release, 2011, 156(1): 70-75. |
| 61 | HENRICUS M M, JOHNSON K T, BANERJEE I A. Investigation of insulin loaded self-assembled microtubules for drug release[J]. Bioconjugate Chem, 2008, 19(12): 2394-2400. |
| 62 | SCHNUR J M, PRICE R, SCHOEN P, et al. Lipid-based tubule microstructures[J]. Thin Solid Films, 1987, 152: 181-206. |
| 63 | SCHNUR J M. Lipid tubules-a paradigm for molecularly engineered structures[J]. Science, 1993, 262(5140): 1669-1676. |
| 64 | MARKOWITZ M, SINGH A. Self-assembling properties of 1,2-diacyl-sn-glycero-3-phosphohydroxyethanol: a headgroup-modified diacetylenic phospholipid[J]. Langmuir, 1991, 7(1): 16-18. |
| 65 | PRICE R, PATCHAN M. Controlled release from cylindrical microstructures[J]. J Microencapsulation, 1991, 8(3): 301-306. |
| 66 | MARKOWITZ M, BARAL S, BRANDOW S, et al. Palladium ion assisted formation and metallization of lipid tubules[J]. Thin Solid Films, 1993, 224(2): 242-247. |
| 67 | WANG Y, MA S, LI Q, et al. Hollow platinum nanospheres and nanotubes templated by shear flow-induced lipid vesicles and tubules and their applications on hydrogen evolution[J]. ACS Sustain Chem Eng, 2016, 4(7): 3773-3779. |
| 68 | WANG Y, LI Q, ZHANG P, et al. One-pot green synthesis of bimetallic hollow palladium-platinum nanotubes for enhanced catalytic reduction of p-nitrophenol[J]. J Colloid Int Sci, 2019, 539: 161-167. |
| 69 | CHATTOPADHYAY T, KOGISO M, AOYAGI M, et al. Single bilayered organic nanotubes: anchors for production of a reusable catalyst with nickel ions[J]. Green Chem, 2011, 13(5): 1138-1140. |
| 70 | CHATTOPADHYAY T, KOGISO M, ASAKAWA M, et al. Copper (II)-coordinated organic nanotube: a novel heterogeneous catalyst for various oxidation reactions[J]. Catal Commun, 2010, 12(1): 9-13. |
| 71 | BARAL S, SCHOEN P. Silica-deposited phospholipid tubules as a precursor to hollow submicron-diameter silica cylinders[J]. Chem Mater, 1993, 5(2): 145-147. |
| 72 | JI Q M, KAMIYA S, JUNG J H, et al. Self-assembly of glycolipids on silica nanotube templates yielding hybrid nanotubes with concentric organic and inorganic layers[J]. J Mater Chem, 2005, 15(7): 743-748. |
| 73 | JI Q M, IWAURA R, SHIMIZU T. Regulation of silica nanotube diameters: sol-gel transcription using solvent-sensitive morphological change of peptidic lipid nanotubes as templates[J]. Chem Mater, 2007, 19(6): 1329-1334. |
| 74 | YANG B, KAMIYA S, SHIMIZU Y, et al. Glycolipid nanotube hollow cylinders as substrates: fabrication of one-dimensional metallic-organic nanocomposites and metal nanowires[J]. Chem Mater, 2004, 16(14): 2826-2831. |
| 75 | SHIMIZU T. Self-assembled lipid nanotube hosts: the dimension control for encapsulation of nanometer-scale guest substances[J]. J Polym Sci Polym Chem, 2006, 44(17): 5137-5152. |
| 76 | ZHOU Y, SHIMIZU T. Lipid nanotubes: a unique template to create diverse one-dimensional nanostructures[J]. Chem Mater, 2008, 20(3): 625-633. |
| 77 | YANG B, KAMIYA S, YOSHIDA K, et al. Confined organization of Au nanocrystals in glycolipid nanotube hollow cylinders[J]. Chem Commun, 2004, (5): 500-501. |
| 78 | ZHOU Y, KOGISO M, ASAKAWA M, et al. Antimicrobial nanotubes consisting of Ag-embedded peptidic lipid-bilayer membranes as delivery vehicles[J]. Adv Mater, 2009, 21(17): 1742-1745. |
| 79 | ECKHARDT S, BRUNETTO P S, GAGNON J, et al. Nanobio silver: its interactions with peptides and bacteria, and its uses in medicine[J]. Chem Rev, 2013, 113(7): 4708-4754. |
| 80 | ZHOU Y, KOGISO M, HE C, et al. Fluorescent nanotubes consisting of CdS-embedded bilayer membranes of a peptide lipid[J]. Adv Mater, 2007, 19(8): 1055-1058. |
| 81 | ZHOU Y, JI Q, SHIMIZU Y, et al. One-dimensional confinement of CdS nanodots and subsequent formation of CdS nanowires by using a glycolipid nanotube as a ship-in-bottle scaffold[J]. J Phys Chem C, 2008, 112(47): 18412-18416. |
| 82 | KAMETA N, ISHIKAWA K, MASUDA M, et al. Soft nanotubes acting as a light-harvesting antenna system[J]. Chem Mater, 2012, 24(1): 209-214. |
| 83 | KAMETA N, AOYAGI M, ASAKAWA M. Enhancement of the photocatalytic activity of rhenium(I) complexes by encapsulation in light-harvesting soft nanotubes[J]. Chem Commun, 2017, 53(73): 10116-10119. |
| 84 | KAMETA N, MASUDA M, SHIMIZU T. Soft nanotubes acting as confinement effecters and chirality inducers for achiral polythiophenes[J]. Chem Commun, 2016, 52(7): 1346-1349. |
| 85 | 杨佳臻, 邹昊洋, 丁建勋, 等. 胱氨酸基聚氨基酸纳米材料的可控合成及其生物医学应用[J]. 高分子学报, 2021, 52(8): 960-977. |
| YANG J Z, ZOU H Y, DING J X, et al. Controlled synthesis and biomedical applications of cystine-based polypeptide nanomaterials[J]. Acta Polym Sin, 2021, 52(8): 960-977. |
| [1] | Yi-Cheng ZHANG, Fei ZHA, Xiao-Hua TANG, Yue CHANG, Hai-Feng TIAN, Xiao-Jun GUO. Research Progress of Heterogeneous Catalytic Preparation of Organic Peroxides [J]. Chinese Journal of Applied Chemistry, 2023, 40(6): 769-788. |
| [2] | Xing-Quan XIONG, Hui ZHANG, Li-Zhu GAO. Progress in Chemical Modification and Application of Lignin [J]. Chinese Journal of Applied Chemistry, 2023, 40(6): 806-819. |
| [3] | Xue-Bo LEI, Hui-Jing LIU, He-Yu DING, Guo-Dong SHEN, Run-Jun SUN. Research Progress on Photocatalysts for Degradation of Organic Pollutants in Printing and Dyeing Wastewater [J]. Chinese Journal of Applied Chemistry, 2023, 40(5): 681-696. |
| [4] | Zhen-Bang LIU, Shuo ZHANG, Yu BAO, Ying-Ming MA, Wei-Qi LIANG, Wei WANG, Ying HE, Li NIU. Progress of Application Research on Cheminformatics in Deep Learning [J]. Chinese Journal of Applied Chemistry, 2023, 40(3): 360-373. |
| [5] | Yu-Zhu CHEN, Si-Si LIU, Meng-Meng ZHANG, Xiang-De LIN, Dong-Dong ZENG. Polyurethane Dressing Based on Antibacterial Chitosan/Carboxymethyl Cellulose Composite Drug Coating [J]. Chinese Journal of Applied Chemistry, 2023, 40(2): 252-260. |
| [6] | Yan-Qin CHENG, Zhuo-Xi LI, You-Di WANG, Juan-Juan XU, Zheng BIAN. Structurally Simplified 4-Hydroxyprolinamide for Highly Efficient Asymmetric Michael Addition of Aldehydes to Nitroolefins [J]. Chinese Journal of Applied Chemistry, 2023, 40(1): 146-154. |
| [7] | Kai WANG, Hai-Kuan YANG, Hui-Lan LIU, Jia-Min LU, Chen ZHANG. Synthesis and Gelation Properties of Stigmasterol‑Based Supramolecular Gelators [J]. Chinese Journal of Applied Chemistry, 2022, 39(9): 1453-1463. |
| [8] | Xin HE, Cai-Yun JIANG, Tao DING, Yu-Ping WANG. Reserch Progress of Preparation of Ordered Surface Enhanced Raman Scattering Substrate [J]. Chinese Journal of Applied Chemistry, 2022, 39(8): 1167-1176. |
| [9] | Wei-Qiang ZHANG, Chen WANG, Yu-Rong ZHAO, Dong WANG, Ji-Qian WANG, Hai XU. Research Progress of Regulation of Driving Forces in Short Peptide Supramolecular Self‑Assembly [J]. Chinese Journal of Applied Chemistry, 2022, 39(8): 1190-1201. |
| [10] | Ye LIU, Shao-Bo GUO, Yan-Li LIANG, Hong-Guang GE, Jian-Qi MA, Zhi-Feng LIU, Bo LIU. Preparation and Catalytic Performance of Core‑Shell CuFe2O4@NH2@Pt Nanocomposites [J]. Chinese Journal of Applied Chemistry, 2022, 39(8): 1237-1245. |
| [11] | Xue-Xian YANG, Jian ZHANG, Zhi-Gang GU. Surface‑Coordinated Metal‑Organic Framework Thin Film HKUST‑1 for Optoelectronic Applications [J]. Chinese Journal of Applied Chemistry, 2022, 39(7): 1013-1025. |
| [12] | Wang LI. Morphology Control and Catalytic Dehydrogenation Performance of Zeolitic Imidazolate Frameworks⁃8 [J]. Chinese Journal of Applied Chemistry, 2022, 39(7): 1065-1072. |
| [13] | Zi-Li LI, Xing-Ran XU, Jiang-Hao ZHAN, Xiao-Hua HU, Zi-Ying ZHANG, Shi-Sheng XIONG. Advanced Materials for Lithography [J]. Chinese Journal of Applied Chemistry, 2022, 39(6): 859-870. |
| [14] | Chao ZHANG. Research Prospect of Single Atom Catalysts Towards Electrocatalytic Reduction of Carbon Dioxide [J]. Chinese Journal of Applied Chemistry, 2022, 39(6): 871-887. |
| [15] | Yan WANG, Shu-Cong ZHANG, Xing-Kun WANG, Zhi-Cheng LIU, Huan-Lei WANG, Ming-Hua HUANG. Research Progress on Transition Metal⁃Based Catalysts for Hydrogen Evolution Reaction via Seawater Electrolysis [J]. Chinese Journal of Applied Chemistry, 2022, 39(6): 927-940. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||