Chinese Journal of Applied Chemistry ›› 2023, Vol. 40 ›› Issue (8): 1175-1186.DOI: 10.19894/j.issn.1000-0518.230135
• Review • Previous Articles Next Articles
Research Progress of Cobalt Phosphide Heterojunction Catalysts for Electrolytic Hydrogen Evolution Reaction
- School of Chemistry and Chemical Engineering,Northwestern Polytechnical University,Xi′an 710072,China
-
Received:2023-05-06Accepted:2023-07-06Published:2023-08-01Online:2023-08-24 -
Contact:Jia-Yuan LI -
About author:jiayuanli@nwpu.edu.cn
-
Supported by:China Postdoctoral Science Foundation (No.?2021M702667)?, the Fundamental Research Funds for the Central Universities(D5000210829);Guangdong Basic and Applied Basic Research Foundation(2021A1515110643)
CLC Number:
Cite this article
Wei WANG, Jia-Yuan LI. Research Progress of Cobalt Phosphide Heterojunction Catalysts for Electrolytic Hydrogen Evolution Reaction[J]. Chinese Journal of Applied Chemistry, 2023, 40(8): 1175-1186.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: http://yyhx.ciac.jl.cn/EN/10.19894/j.issn.1000-0518.230135
| Catalysts | Electrolyte | η10/mV | Tafel slope/(mV·dec-1) | Durability/h | Ref. |
|---|---|---|---|---|---|
| CoP nanosheet | 1.0 mol/L KOH | 175 | 146.69 | — | [ |
| CoP | 1.0 mol/L KOH | 160 | 106 | — | [ |
| CoP nanwires | 1 mol/L KOH | 137 | 76.8 | — | [ |
| CoP nanoparticles | 0.5 mol/L H2SO4 | 137 | 67 | — | [ |
| CoP nanorods | 1 mol/L PBS | 122.4 | 81.1 | — | [ |
| CoP/CeO x | 1.0 mol/L KOH | 118 | 77.26 | 24 | [ |
| Pt2Ir1/CoP | 0.5 mol/L H2SO4 | η20=7 | 25.2 | 500 | [ |
| CoP/NiCoP | 1 mol/L KOH | 133 | 88 | 24 | [ |
| O,Cu-CoP | 1 mol/L KOH | 72 | 57.6 | 24 | [ |
| Ni2P/CoP | 0.5 mol/L H2SO4 | 105 | 64 | — | [ |
| CoP@CoOOH | 1 mol/L PBS | 89.6 | 64.4 | 40 | [ |
| CoP NS/CNTs | 1.0 mol/L KOH | 68 | 57 | 24 | [ |
| CoP/CoO PNTs | 1.0 mol/L KOH | 61 | 78 | 10 | [ |
| CoSe2/CoP | 0.5 mol/L H2SO4 | 65 | 54 | 50 | [ |
| CoP/CoMoP | 1.0 mol/L KOH | 34 | 33 | 12 | [ |
| NiFe-LDH@NiCoP/NF | 1 mol/L KOH | 120 | 88.2 | 100 | [ |
| CoP-Co x O y /CC | 1 mol/L KOH | 43 | 64.7 | 70 | [ |
| CoP-Mo2C@NC | 1 mol/L KOH | 94 | 79.7 | 15 | [ |
| R-Mn-CoP | 1 mol/L KOH | 117 | 54 | — | [ |
| CoP/Co-MOF | 0.5 mol/L H2SO4 | 27 | 43 | 17 | [ |
| 1 mol/L PBS | 49 | 63 | 17 | ||
| 1 mol/L KOH | 34 | 56 | 17 | ||
| N-CoP/CC | 0.5 mol/L H2SO4 | 25 | 49 | 30 | [ |
| 1 mol/L PBS | 74 | 69 | 30 | ||
| 1 mol/L KOH | 39 | 58 | 30 |
Table 1 Comparison of HER properties of CoP heterojunction electrocatalysts
| Catalysts | Electrolyte | η10/mV | Tafel slope/(mV·dec-1) | Durability/h | Ref. |
|---|---|---|---|---|---|
| CoP nanosheet | 1.0 mol/L KOH | 175 | 146.69 | — | [ |
| CoP | 1.0 mol/L KOH | 160 | 106 | — | [ |
| CoP nanwires | 1 mol/L KOH | 137 | 76.8 | — | [ |
| CoP nanoparticles | 0.5 mol/L H2SO4 | 137 | 67 | — | [ |
| CoP nanorods | 1 mol/L PBS | 122.4 | 81.1 | — | [ |
| CoP/CeO x | 1.0 mol/L KOH | 118 | 77.26 | 24 | [ |
| Pt2Ir1/CoP | 0.5 mol/L H2SO4 | η20=7 | 25.2 | 500 | [ |
| CoP/NiCoP | 1 mol/L KOH | 133 | 88 | 24 | [ |
| O,Cu-CoP | 1 mol/L KOH | 72 | 57.6 | 24 | [ |
| Ni2P/CoP | 0.5 mol/L H2SO4 | 105 | 64 | — | [ |
| CoP@CoOOH | 1 mol/L PBS | 89.6 | 64.4 | 40 | [ |
| CoP NS/CNTs | 1.0 mol/L KOH | 68 | 57 | 24 | [ |
| CoP/CoO PNTs | 1.0 mol/L KOH | 61 | 78 | 10 | [ |
| CoSe2/CoP | 0.5 mol/L H2SO4 | 65 | 54 | 50 | [ |
| CoP/CoMoP | 1.0 mol/L KOH | 34 | 33 | 12 | [ |
| NiFe-LDH@NiCoP/NF | 1 mol/L KOH | 120 | 88.2 | 100 | [ |
| CoP-Co x O y /CC | 1 mol/L KOH | 43 | 64.7 | 70 | [ |
| CoP-Mo2C@NC | 1 mol/L KOH | 94 | 79.7 | 15 | [ |
| R-Mn-CoP | 1 mol/L KOH | 117 | 54 | — | [ |
| CoP/Co-MOF | 0.5 mol/L H2SO4 | 27 | 43 | 17 | [ |
| 1 mol/L PBS | 49 | 63 | 17 | ||
| 1 mol/L KOH | 34 | 56 | 17 | ||
| N-CoP/CC | 0.5 mol/L H2SO4 | 25 | 49 | 30 | [ |
| 1 mol/L PBS | 74 | 69 | 30 | ||
| 1 mol/L KOH | 39 | 58 | 30 |
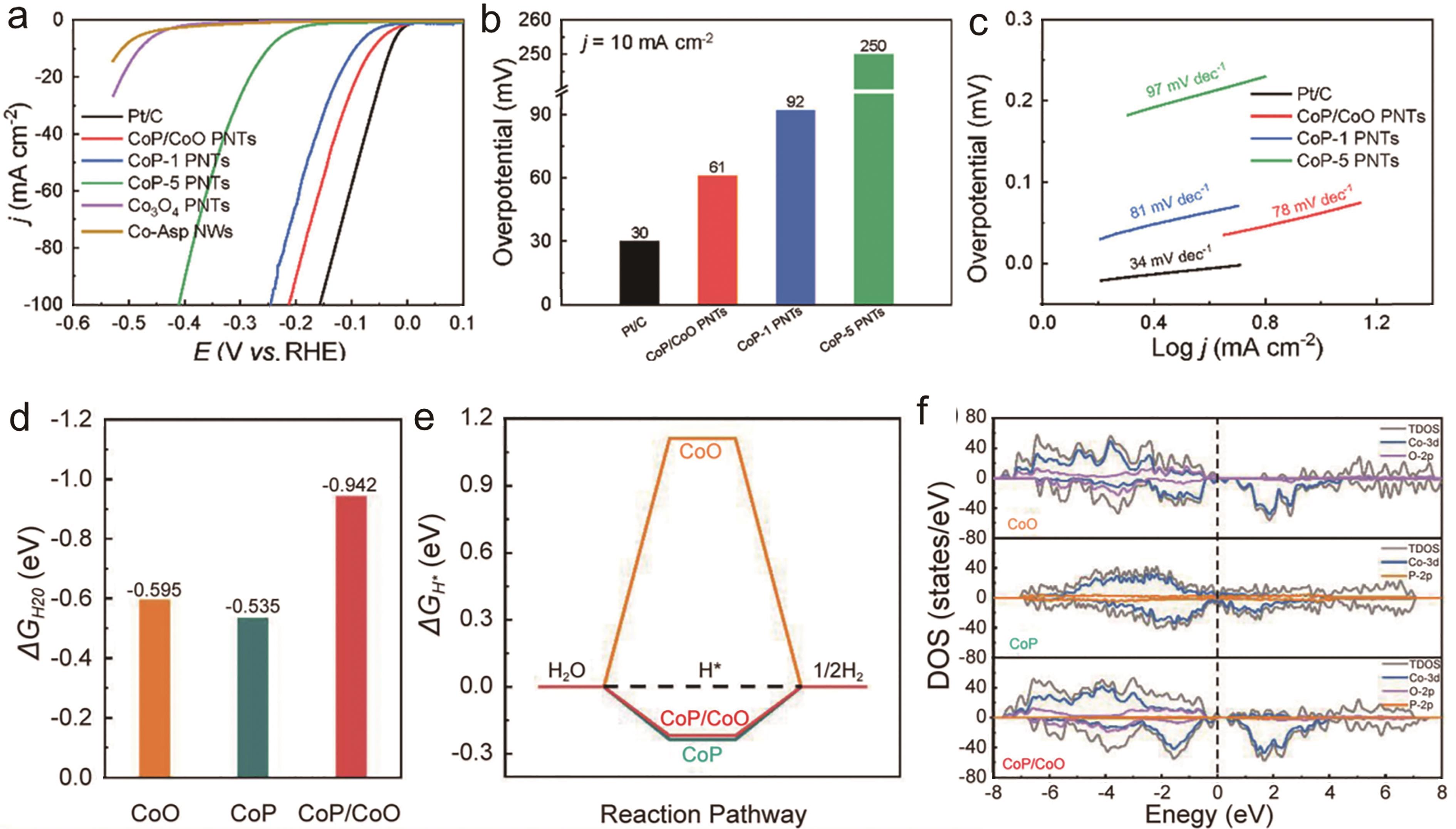
Fig.2 (a) HER polarization curves of different catalysts in 1.0 mol/L KOH; (b) Comparison of overpotentials at 10 mA/cm2; (c) Tafel plots of different catalysts; (d) Ealculated H2O adsorption energies on different surfaces; (e) Calculated H* adsorption energies on different surfaces; (f) DOSs on CoO, CoP and CoO/CoP heterostructures[34]
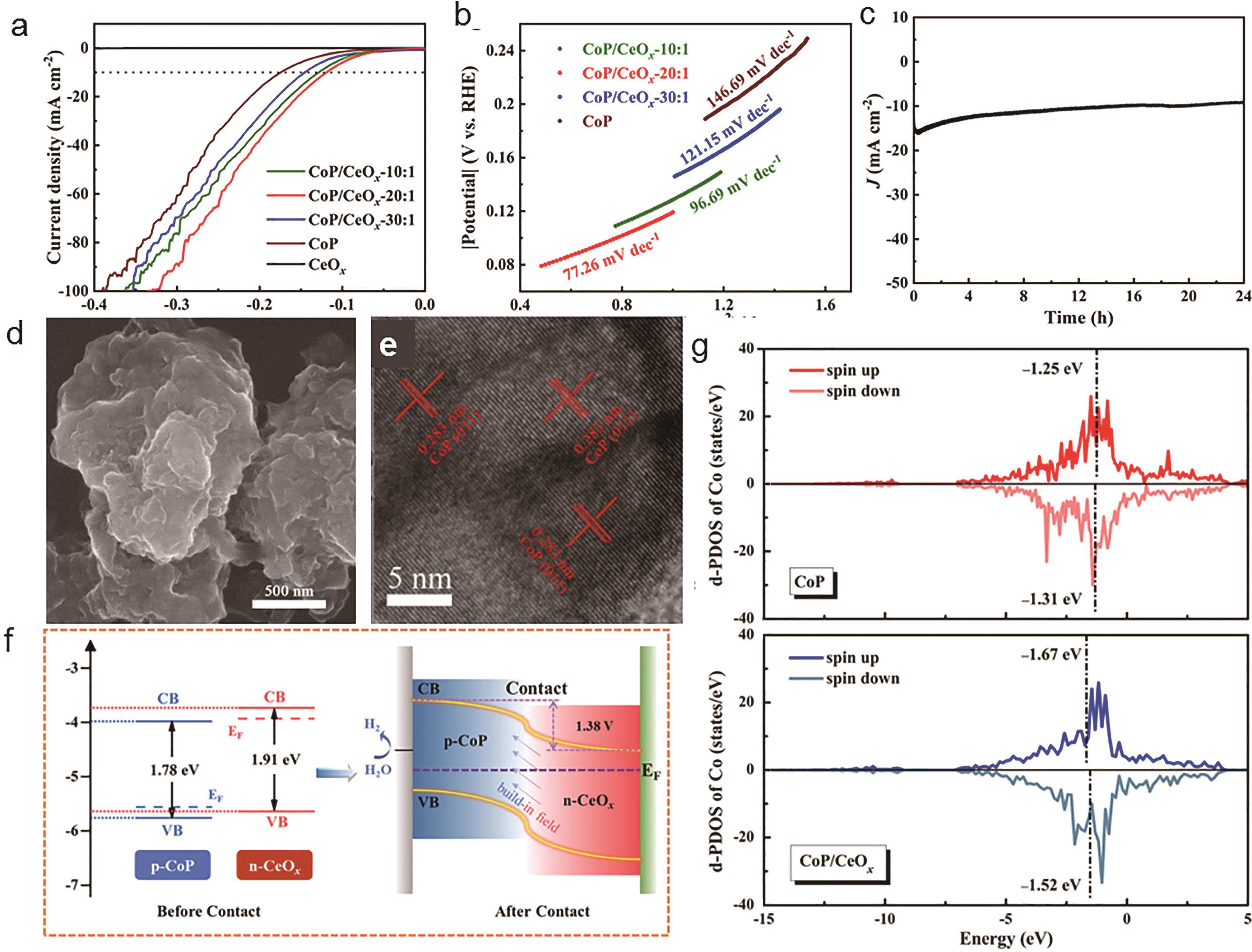
Fig.3 (a) HER polarization curves of CoP/CeO x -10∶?1, CoP/CeO x -20∶?1, CoP/CeO x -30∶?1, CoP, and CeO x in 1 mol/L KOH; (b) Tafel plots of CoP/CeO x -10∶?1, CoP/CeO x -20∶?1, CoP/CeO x -30∶?1, CoP, and CeO x in 1 mol/L KOH; (c) Chronoamperometry curve of CoP/CeO x -20∶?1; (d) SEM image of CoP/CeO x -20∶?1 after HER; (e) HAADF-STEM image of CoP/CeO x -20∶?1 after HER; (f) Energy diagrams of CeO x and CoP before and after contact and schematic diagram of the proposed mechanism for catalyzing HER in the CoP/CeO x p-n heterojunction; (g) Partial density of states (PDOS) of the Co3d band of CoP (211) (top panel) and CoP(211)/CeO2(111) heterojunction (bottom panel). The positions of d-band centers are marked by vertical dashed lines[7]
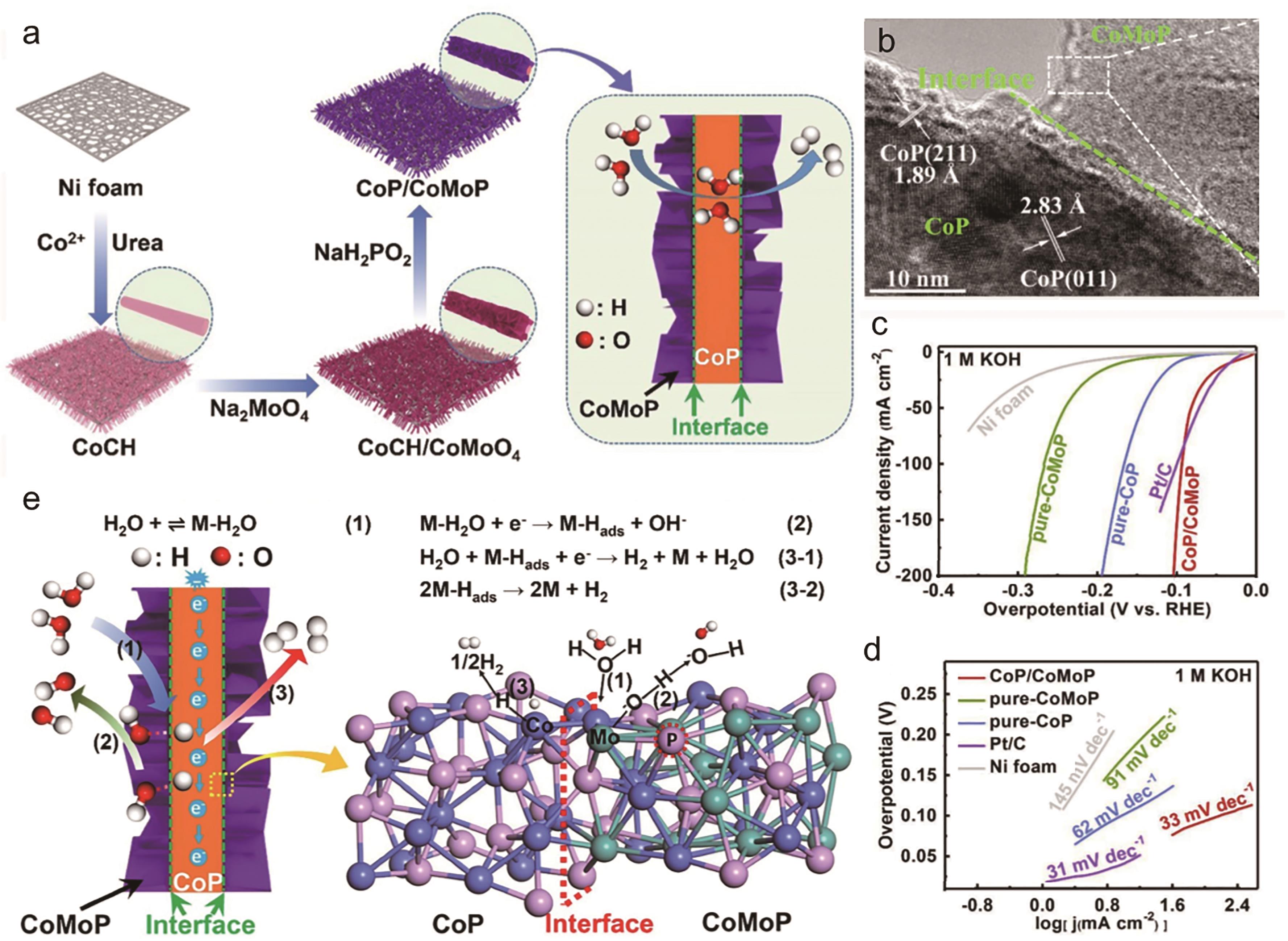
Fig.4 (a) The schematic diagram to illustrate the fabrication process of CoP/CoMoP via topotactic conversion from CoCH; (b) High-resolution TEM of CoP/CoMoP; (c) The HER iR-corrected polarization curves of CoP/CoMoP, pure-CoP, pure-CoMoP, Pt/C and bare Ni foam with a scan rate of 2 mV/s in 1 mol/L KOH; (d) The corresponding Tafel plots; (e) The mechanisms of the cooperative CoP/CoMoP interfaces acting on HER in alkaline electrolyte[36]

Fig.5 (a) LSV curves of various Pt2M1/CoP and Pt/CoP catalysts (mass percent 1.0%) and Pt/C (mass percent 20%) benchmarks; (b) LSV-derived Tafel plots for various catalysts; (c) Comparisons of the noble-metal utilization activity of Pt2Ir1/CoP (mass percent 1.0%) for HER with those of other well-known noble-metal based catalysts, especially the single-atom Pt catalysts; (d) Catalytic durability of Pt2Ir1/CoP (mass percent 1.0%) through a time-overpotential profile at 40 mA/cm2. Insets are cycle performance of LSV curves and Faradic efficiency; (e) Calculated free energy diagram for HER on Pt2Ir1/CoP paradigm and Pt/CoP benchmark; (f) The optimized H* adsorption structures at various sites; (g) Electron density difference map of interfaces, where a loss of electrons is indicated in blue and electron enrichment is indicated in red[10]
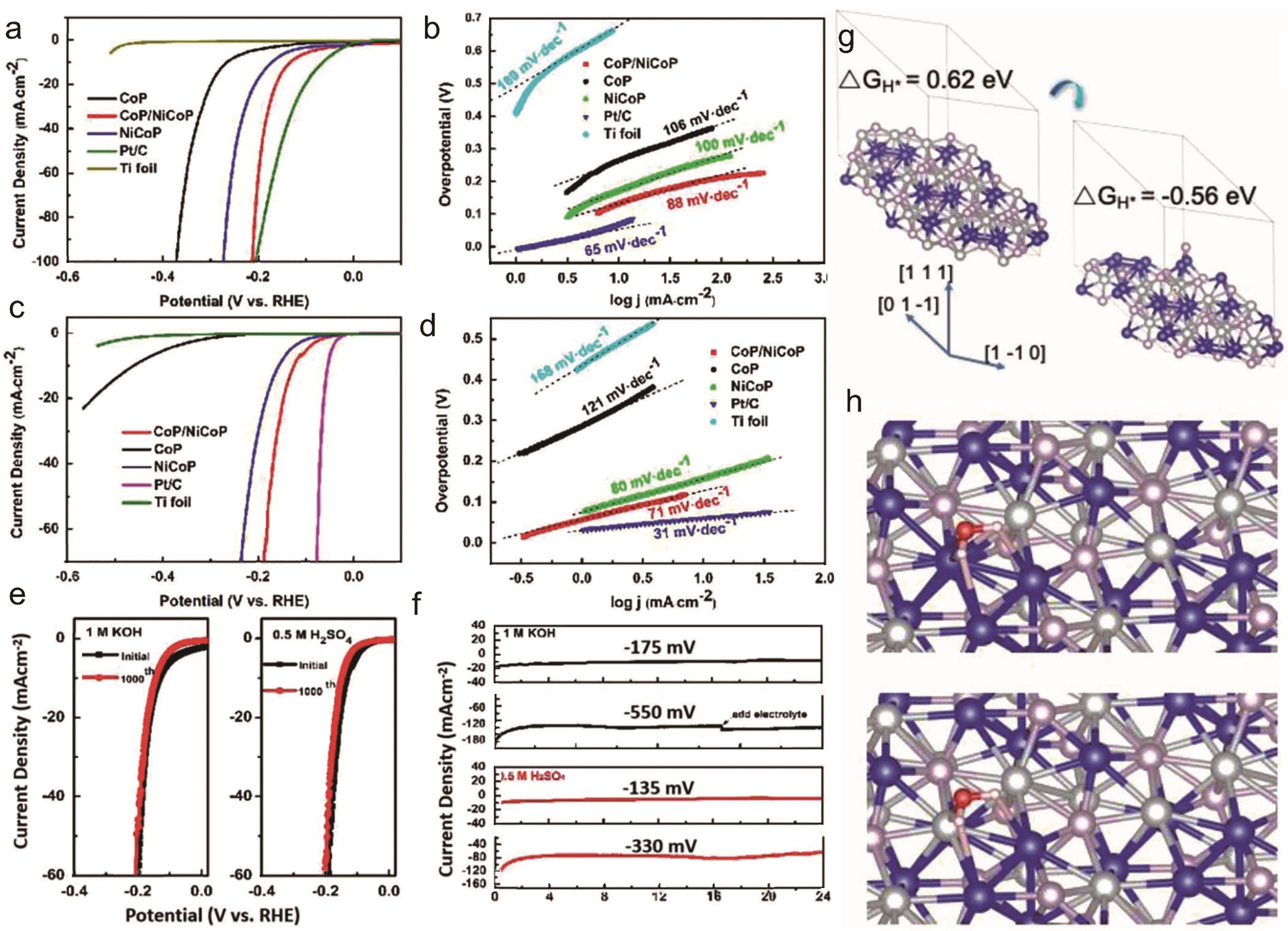
Fig.6 (a) Polarization curves in 1 mol/L KOH; (b) Tafel plots in 1 mol/L KOH; (c) Polarization curves in 0.5 mol/L H2SO4; (d) Tafel plots in 0.5 mol/L H2SO4; (e) Cycle performance of LSV curves of the samples in 1 mol/L KOH and 0.5 mol/L H2SO4; (f) Long-time chronoamperometry tests in 1 mol/L KOH and 0.5 mol/L H2SO4; (g) The optimized hydrogen atom adsorption structure; (h) The optimized hydrogen H2O adsorption structure[13]
| 1 | LUO Y T, ZHANG Z Y, CHHOWALLA M, et al. Recent advances in design of electrocatalysts for high-current-density water splitting[J]. Adv Mater, 2022, 34(16): 2108133. |
| 2 | FU H Q, ZHOU M, LIU P F, et al. Hydrogen spillover-bridged Volmer/Tafel processes enabling ampere-level current density alkaline hydrogen evolution reaction under low overpotential[J]. J Am Chem Soc, 2022, 144(13): 6028-6039. |
| 3 | ZHANG W, HAN N, LUO J S, et al. Critical role of phosphorus in hollow structures cobalt-based phosphides as bifunctional catalysts for water splitting[J]. Small, 2022, 18(4): 2103561. |
| 4 | BODHANKAR P M, SARAWADE P B, KUMAR P, et al. Nanostructured metal phosphide based catalysts for electrochemical water splitting: a review[J]. Small, 2022, 18(21): 2107572. |
| 5 | KUANG P Y, SAYED M, FAN J J, et al. 3D graphene-based H2-production photocatalyst and electrocatalyst[J]. Adv Energy Mater, 2020, 10(14): 1903802. |
| 6 | ZOU X X, ZHANG Y. Noble metal-free hydrogen evolution catalysts for water splitting[J]. Chem Soc Rev, 2015, 44(15): 5148-5180. |
| 7 | SONG X Z, ZHU W Y, NI J C, et al. Boosting hydrogen evolution electrocatalysis via regulating the electronic structure in a crystalline-amorphous CoP/CeOx p-n heterojunction[J]. ACS Appl Mater Interfaces, 2022, 14(29): 33151-33160. |
| 8 | HE J S, LIU P Y, RAN R, et al. Single-atom catalysts for high-efficiency photocatalytic and photoelectrochemical water splitting: distinctive roles, unique fabrication methods and specific design strategies[J]. J Mater Chem A, 2022, 10(13): 6835-6871. |
| 9 | XIE X Q, LIU J, GU C, et al. Hierarchical structured CoP nanosheets/carbon nanofibers bifunctional eletrocatalyst for high-efficient overall water splitting[J]. J Energy Chem, 2022, 64: 503-510. |
| 10 | LI J Y, HU J, ZHANG M K, et al. A fundamental viewpoint on the hydrogen spillover phenomenon of electrocatalytic hydrogen evolution[J]. Nat Commun, 2021, 12(1): 3502. |
| 11 | CHU S, MAJUMDAR A. Opportunities and challenges for a sustainable energy future[J]. Nature, 2012, 488: 294-303. |
| 12 | DAI J, ZHU Y L, CHEN Y, et al. Hydrogen spillover in complex oxide multifunctional sites improves acidic hydrogen evolution electrocatalysis[J]. Nat Commun, 2022, 13(1): 1189. |
| 13 | LIN Y, SUN K A, LIU S J, et al. Construction of CoP/NiCoP nanotadpoles heterojunction interface for wide pH hydrogen evolution electrocatalysis and supercapacitor[J]. Adv Energy Mater, 2019, 9(36): 1901213. |
| 14 | SEH Z W, KIBSGAARD J, DICKENS C F, et al. Combining theory and experiment in electrocatalysis: insights into materials design[J]. Science, 2017, 355(6321): eaad4998. |
| 15 | GREELEY J, JARAMILLO T F, BONDE J, et al. Computational high-throughput screening of electrocatalytic materials for hydrogen evolution[J]. Nat Mater, 2006, 5(11): 909-913. |
| 16 | BHANJA P, MOHANTY B, CHONGDAR S, et al. Novel microporous iron-embedded cobalt phosphonates feasible for electrochemical overall water splitting[J]. ACS Appl Energy Mater, 2022, 5(3): 3558-3567. |
| 17 | ARUNKUMAR P, GAYATHRI S, HAN J H. Impact of an incompatible atomic nickel-incorporated metal-organic framework on phase evolution and electrocatalytic activity of Ni-doped cobalt phosphide for the hydrogen evolution reaction[J]. ACS Appl Energy Mater, 2022, 5(3): 2975-2992. |
| 18 | LI Y, DONG Z H, JIAO L F. Multifunctional transition metal-based phosphides in energy-related electrocatalysis[J]. Adv Energy Mater, 2019, 10(11): 1902104. |
| 19 | ZHANG C, HUANG Y, YU Y F, et al. Sub-1.1 nm ultrathin porous CoP nanosheets with dominant reactive 200 facets: a high mass activity and efficient electrocatalyst for the hydrogen evolution reaction[J]. Chem Sci, 2017, 8(4): 2769-2775. |
| 20 | HUANG C Q, HUANG Y, LIU C B, et al. Integrating hydrogen production with aqueous selective semi-dehydrogenation of tetrahydroisoquinolines over a Ni2P bifunctional electrode[J]. Angew Chem Int Ed Engl, 2019, 58(35): 12014-12017. |
| 21 | WEI J M, ZHOU M, LONG A C, et al. Heterostructured electrocatalysts for hydrogen evolution reaction under alkaline conditions[J]. Nanomicro Lett, 2018, 10(4): 75. |
| 22 | JIAN K L, MA W S, LV Z P, et al. Tuning the electronic structure of the CoP/Ni2P nanostructure by nitrogen doping for an efficient hydrogen evolution reaction in alkaline media[J]. Inorg Chem, 2021, 60(23): 18544-18552. |
| 23 | LI X T, LIU Y Z, SUN Q D, et al. Surface engineered CoP/Co3O4 heterojunction for high-performance bi-functional water splitting electro-catalysis[J]. Nanoscale, 2021, 13(47): 20281-20288. |
| 24 | LU Z J, CAO Y L, XIE J, et al. Construction of Co2P/CoP@Co@NCNT rich-interface to synergistically promote overall water splitting[J]. Chem Eng J, 2022, 430: 132877. |
| 25 | XU W, WANG B B, NI X M, et al. Heterogeneous synergetic effect of metal-oxide interfaces for efficient hydrogen evolution in alkaline solutions[J]. ACS Appl Mater Interfaces, 2021, 13(11): 13838-13847. |
| 26 | ZHENG D, YU L H, LIU W X, et al. Structural advantages and enhancement strategies of heterostructure water-splitting electrocatalysts[J]. Cell Rep Phys Sci, 2021, 2(6): 100443. |
| 27 | MUTHUKUMAR P, MOON D, ANTHONY S P. Copper coordination polymer electrocatalyst for strong hydrogen evolution reaction activity in neutral medium: influence of coordination environment and network structure[J]. Catal Sci Technol, 2019, 9(16): 4347-4354. |
| 28 | LU Y, CHENG X, TIAN G, et al. Hierarchical CdS/m-TiO2/G ternary photocatalyst for highly active visible light-induced hydrogen production from water splitting with high stability[J]. Nano Energy, 2018, 47: 8-17. |
| 29 | GUO Y, TANG J, HENZIE J, et al. Mesoporous iron-doped MoS2/CoMo2S4 heterostructures through organic-metal cooperative interactions on spherical micelles for electrochemical water splitting[J]. ACS Nano, 2020, 14(4): 4141-4152. |
| 30 | XU K, SUN Y Q, SUN Y M, et al. Yin-yang harmony: metal and nonmetal dual-doping boosts electrocatalytic activity for alkaline hydrogen evolution[J]. ACS Energy Lett, 2018, 3(11): 2750-2756. |
| 31 | LIANG X, ZHENG B X, CHEN L G, et al. MOF-derived formation of Ni2P-CoP bimetallic phosphides with strong interfacial effect toward electrocatalytic water splitting[J]. ACS Appl Mater Interfaces, 2017, 9(27): 23222-23229. |
| 32 | ZHANG B, SHAN J W, WANG W L, et al. Oxygen vacancy and core-shell heterojunction engineering of anemone-like CoP@CoOOH bifunctional electrocatalyst for efficient overall water splitting[J]. Small, 2022, 18(12): 2106012. |
| 33 | ZHANG Y, WANG Y, WANG T T, et al. Heterostructure of 2D CoP nanosheets/1D carbon nanotubes to significantly boost the alkaline hydrogen evolution[J]. Adv Mater Interfaces, 2019, 7(2): 1901302. |
| 34 | ZHOU Q X, SUN R X, REN Y P, et al. Reactive template-derived interfacial engineering of CoP/CoO heterostructured porous nanotubes towards superior electrocatalytic hydrogen evolution[J]. Carbon Energy, 2022, 5(1): 273. |
| 35 | SHEN S J, WANG Z P, LIN Z P, et al. Crystalline-amorphous interfaces coupling of CoSe2/CoP with optimized d-band center and boosted electrocatalytic hydrogen evolution[J]. Adv Mater, 2022, 34(13): 2110631. |
| 36 | HUANG X K, XU X P, LUAN X X, et al. Cop nanowires coupled with CoMoP nanosheets as a highly efficient cooperative catalyst for hydrogen evolution reaction[J]. Nano Energy, 2020, 68: 104332. |
| 37 | ZHANG H J, LI X P, HäHNEL A, et al. Bifunctional heterostructure assembly of NiFe LDH nanosheets on NiCoP nanowires for highly efficient and stable overall water splitting[J]. Adv Funct Mater, 2018, 28(14): 1706847. |
| 38 | ALSABBAN M M, ESWARAN M K, PERAMAIAH K, et al. Unusual activity of rationally designed cobalt phosphide/oxide heterostructure composite for hydrogen production in alkaline medium[J]. ACS Nano, 2022, 16(3): 3906-3916. |
| 39 | DUTTA S, INDRA A, HAN H, et al. An intriguing pea-like nanostructure of cobalt phosphide on molybdenum carbide incorporated nitrogen-doped carbon nanosheets for efficient electrochemical water splitting[J]. ChemSusChem, 2018, 11(22): 3956-3964. |
| 40 | LI X M, HU Q Y, WANG H Y, et al. Charge induced crystal distortion and morphology remodeling: formation of Mn-CoP nanowire@Mn-CoOOH nanosheet electrocatalyst with rich edge dislocation defects[J]. Appl Catal B: Environ, 2021, 292: 120172. |
| 41 | LIU T, LI P, YAO N, et al. Cop-doped MOF-based electrocatalyst for pH-universal hydrogen evolution reaction[J]. Angew Chem Int Ed Engl, 2019, 58(14): 4679-4684. |
| 42 | MEN Y N, LI P, YANG F L, et al. Nitrogen-doped CoP as robust electrocatalyst for high-efficiency pH-universal hydrogen evolution reaction[J]. Appl Catal B: Environ, 2019, 253: 21-27. |
| 43 | EL-REFAEI S M, RUSSO P A, PINNA N. Recent advances in multimetal and doped transition-metal phosphides for the hydrogen evolution reaction at different pH values[J]. ACS Appl Mater Interfaces, 2021, 13(19): 22077-22097. |
| 44 | HE K, TSEGA T T, LIU X, et al. Utilizing the space-charge region of the FeNi-LDH/CoP p-n junction to promote performance in oxygen evolution electrocatalysis[J]. Angew Chem Int Ed Engl, 2019, 58(34): 11903-11909. |
| 45 | ZHUANG Z C, LI Y, LI Z L, et al. MoB/g-C3N4 interface materials as a schottky catalyst to boost hydrogen evolution[J]. Angew Chem Int Ed Engl, 2018, 57(2): 496-500. |
| 46 | LONG B, YANG H, LI M Y, et al. Interface charges redistribution enhanced monolithic etched copper foam-based Cu2O layer/TiO2 nanodots heterojunction with high hydrogen evolution electrocatalytic activity[J]. Appl Catal B: Environ, 2019, 243: 365-372. |
| 47 | ZHANG H J, MAIJENBURG A W, LI X P, et al. Bifunctional heterostructured transition metal phosphides for efficient electrochemical water splitting[J]. Adv Funct Mater, 2020, 30(34): 2003261. |
| 48 | YANG F L, CHEN Y T, CHENG G Z, et al. Ultrathin nitrogen-doped carbon coated with CoP for efficient hydrogen evolution[J]. ACS Catal, 2017, 7(6): 3824-3831. |
| 49 | LI Y, LI F M, ZHAO Y, et al. Iron doped cobalt phosphide ultrathin nanosheets on nickel foam for overall water splitting[J]. J Mater Chem A, 2019, 7(36): 20658-20666. |
| 50 | CALLEJAS J F, READ C G, POPCZUN E J, et al. Nanostructured Co2P electrocatalyst for the hydrogen evolution reaction and direct comparison with morphologically equivalent CoP[J]. Chem Mater, 2015, 27(10): 3769-3774. |
| 51 | PAN Y, LIN Y, CHEN Y J, et al. Cobalt phosphide-based electrocatalysts: synthesis and phase catalytic activity comparison for hydrogen evolution[J]. J Mater Chem A, 2016, 4(13): 4745-4754. |
| [1] | Jin-Hui LIANG, Le-Cheng LIANG, Zhi-Ming CUI. Research Progress on Intermetallic Compound Electrocatalysts Applied in the Interconversion Between Hydrogen and Electric Power [J]. Chinese Journal of Applied Chemistry, 2023, 40(8): 1140-1157. |
| [2] | Jia-Xin LIU, Jia-He FAN, Shu-Hui LI, Liang MA. Synthesis of Rh@Pt/C Concave Cubic Core-Shell Catalyst and Its Ethanol Electro-Oxidation Performance [J]. Chinese Journal of Applied Chemistry, 2023, 40(8): 1195-1204. |
| [3] | Lian-Cheng HUI, Jian-Xing ZHUANG, Shun XIAO, Mei-Ping LI, Meng-Yuan JIN, Qing LYU. Nickel-Nitrogen-Doped Graphdiyne as an Efficient Catalyst for Oxygen Reduction [J]. Chinese Journal of Applied Chemistry, 2023, 40(8): 1205-1213. |
| [4] | Chao ZHANG. Research Prospect of Single Atom Catalysts Towards Electrocatalytic Reduction of Carbon Dioxide [J]. Chinese Journal of Applied Chemistry, 2022, 39(6): 871-887. |
| [5] | Yan WANG, Shu-Cong ZHANG, Xing-Kun WANG, Zhi-Cheng LIU, Huan-Lei WANG, Ming-Hua HUANG. Research Progress on Transition Metal⁃Based Catalysts for Hydrogen Evolution Reaction via Seawater Electrolysis [J]. Chinese Journal of Applied Chemistry, 2022, 39(6): 927-940. |
| [6] | Lin-Jie SHANG, Jiang LIU, Ya-Qian LAN. Covalent Organic Framework Materials for Photo/ Electrocatalytic Carbon Dioxide Reduction [J]. Chinese Journal of Applied Chemistry, 2022, 39(4): 559-584. |
| [7] | Li-Zhi SUN, Hao LYU, Xiao-Wen MIN, Ben LIU. Mesoporous Palladium⁃Boron Alloy Nanocatalysts: Synthesis and Performance in Methanol Oxidation Electrocatalysis [J]. Chinese Journal of Applied Chemistry, 2022, 39(4): 673-684. |
| [8] | Xiao-Feng WU, De-Shun CHEN, Wei MA, Ke-Ke HUANG. WO3/Fe2TiO5 Composite Photoanode Deposited via Electrospray for Enhanced Photoelectrochemical Water Splitting [J]. Chinese Journal of Applied Chemistry, 2022, 39(4): 694-696. |
| [9] | Yu-Feng ZHOU, Chuan-Wei ZHOU, Tong-Ze HU, Zhan-Peng DUAN, Hao-Tong WANG, Shu-Yun SHI. Synthesis of Fe/V‑Sb2O3 Composites and UV‑light Catalytic Degradation of Pharmaceutical Wastewater [J]. Chinese Journal of Applied Chemistry, 2022, 39(10): 1572-1578. |
| [10] | ZHAO Xing-Peng, WANG Ya-Qiao, GAO Sheng-Wang, ZHU Jian-Chao, WANG Guo-Ying, XIA Xun-Feng, WANG Hong-Liang, WANG Shu-Ping. Synthesis of BiOBr/CeO2 Composites for Photocatalytic Degradation of Sulfisoxazole [J]. Chinese Journal of Applied Chemistry, 2021, 38(4): 422-430. |
| [11] | BI Yipiao, GONG Xue, YANG Fa, RUAN Mingbo, SONG Ping, XU Weilin. Polyvalent MnOx/C Electrocatalyst for Highly Efficient Nitrogen Reduction Reaction [J]. Chinese Journal of Applied Chemistry, 2020, 37(9): 1048-1055. |
| [12] | WEI Zhenye, MENG Junling, WANG Haocong, ZHANG Wenwen, LIU Xiaojuan, MENG Jian. Improving the Electrocatalytic Activity of La2NiO4+δ Cathode by Surface Modification with Conformal Heterojunction [J]. Chinese Journal of Applied Chemistry, 2020, 37(8): 939-951. |
| [13] | MENG Yang, YANG Chan, PENG Juan. Progress in Iron, Cobalt and Nickel-Based Metal Phosphide Nano-catalysts for Hydrogen Production under Alkaline Conditions [J]. Chinese Journal of Applied Chemistry, 2020, 37(7): 733-745. |
| [14] | CHEN Jiaqi, ZHOU Yan, SUN Jingwen, ZHU Junwu, WANG Xin, FU Yongsheng. Recent Progress of Metal Organic Frameworks-Based Hollow Materials [J]. Chinese Journal of Applied Chemistry, 2020, 37(11): 1221-1235. |
| [15] | CHE Tinghua, TAN Xiao, YAN Jiawei, SONG Fengdan, ZHANG Hongmei, QI Suitao. Synthesis of Copper Modified Porous Nickel Self-supported Electrode and Its Catalytic Oxidation of Glucose [J]. Chinese Journal of Applied Chemistry, 2019, 36(9): 1091-1098. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||