Chinese Journal of Applied Chemistry ›› 2023, Vol. 40 ›› Issue (8): 1158-1174.DOI: 10.19894/j.issn.1000-0518.230131
• Review • Previous Articles Next Articles
Basics of Research Progress for Urea Electrolysis for Hydrogen Generation and Urea Fuel Cells
Chun YIN, Jia-Xin LI, Li-Gang FENG( )
)
- School of Chemistry and Chemical Engineering,Yangzhou University,Yangzhou 225002,China
-
Received:2023-05-04Accepted:2023-07-06Published:2023-08-01Online:2023-08-24 -
Contact:Li-Gang FENG -
About author:ligang.feng@yzu.edu.cn
-
Supported by:the National Natural Science Foundation of China(22272148)
CLC Number:
Cite this article
Chun YIN, Jia-Xin LI, Li-Gang FENG. Basics of Research Progress for Urea Electrolysis for Hydrogen Generation and Urea Fuel Cells[J]. Chinese Journal of Applied Chemistry, 2023, 40(8): 1158-1174.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: http://yyhx.ciac.jl.cn/EN/10.19894/j.issn.1000-0518.230131
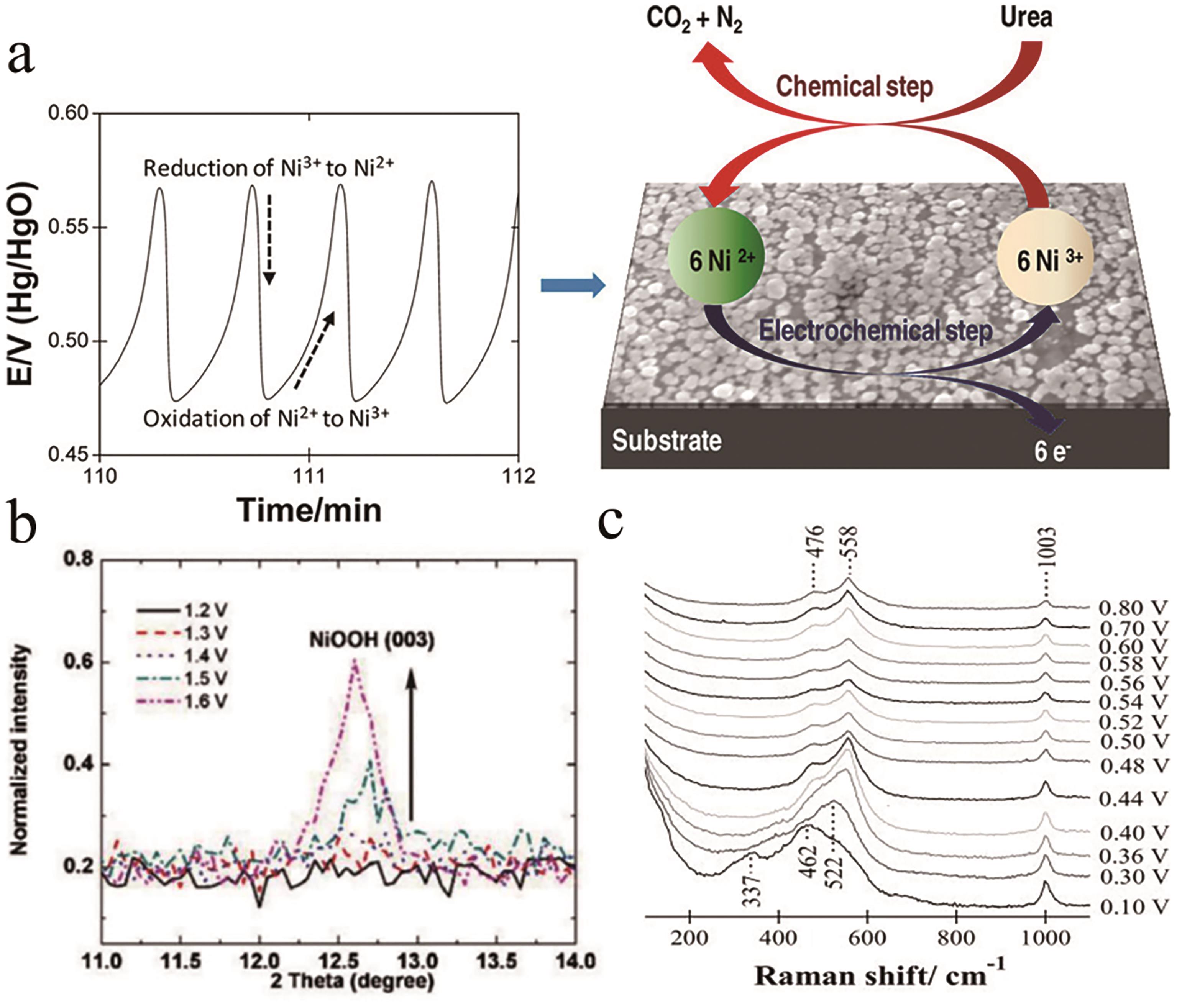
Fig. 2 (a) Schematic illustration of the EC mechanism[26]; (b) XRD reflections of NiOOH for Ni(OH)2 electrode in 5 mol/L KOH[27]; (c) In situ SERS spectra of Ni(OH)2 catalyst in 1 mol/L urea+5 mol/L KOH[28]
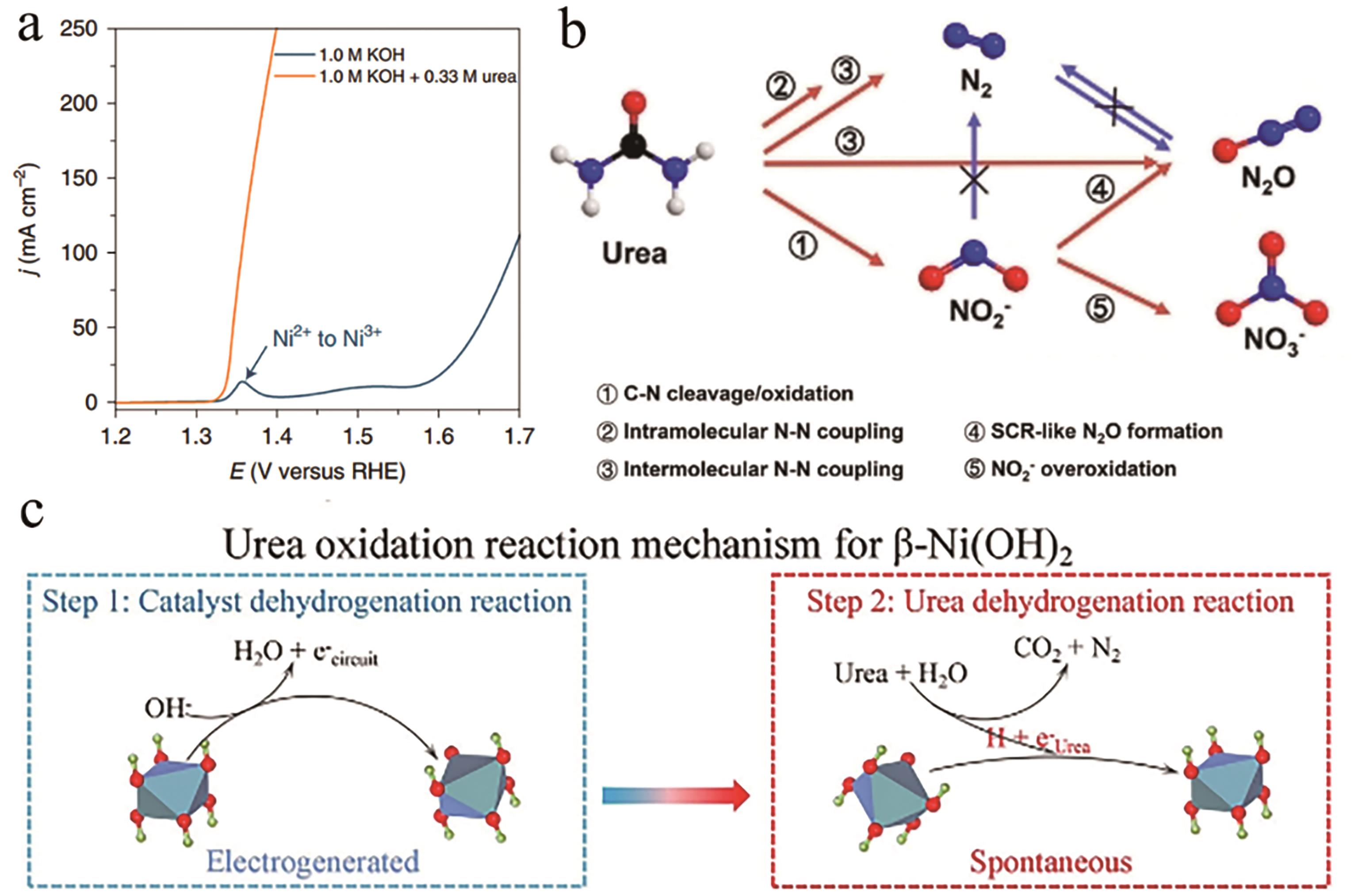
Fig.3 (a) LSV curves of the Ni2Fe(CN)6[30]; (b) Schematic illustration of the correlation among N2, N2O, NO2- and NO3- products during UOR[31]; (c) The UOR mechanism on the β-Ni(OH)2 electrode[32]
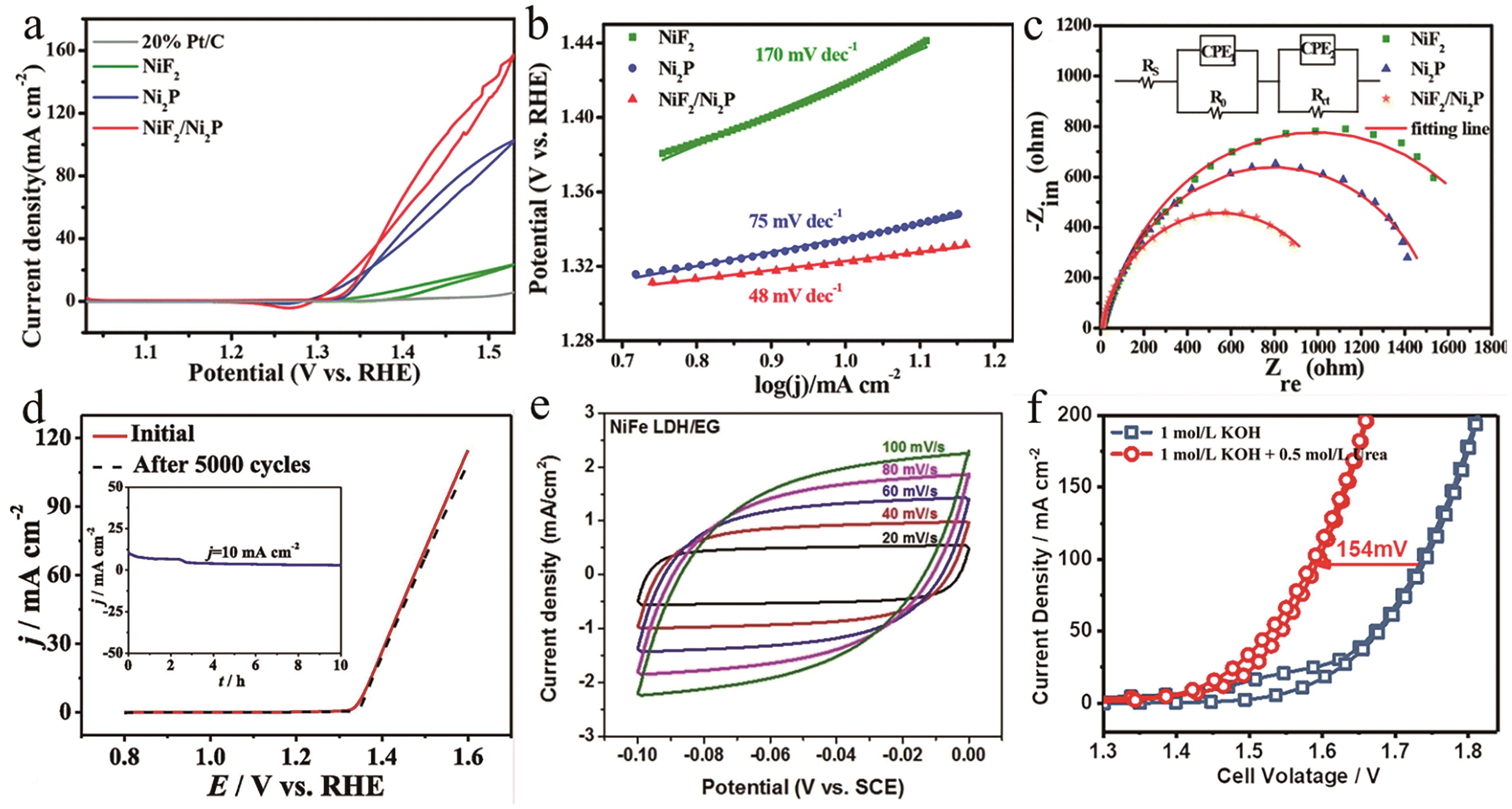
Fig.4 (a) CV curves of the catalysts, (b) Tafel slopes of the catalysts and (c) Nyquist plots with a fitting equivalent circuit[33]. (d) The polarization curves of NiF3/Ni2P@CC-2 before and after 5000 CV cycles (insert: the chronoamperometric for 10 h)[37]. (e) CV curves at different scan rates for Ni-Fe LDH/EG[41]. (f) Polarization curves of Ni-S-Se/NF||Ni-S-Se/NF electrolyzer[42]
| Hydrogen carrier | w(H element)/% | Melting point/℃, boiling point/℃ | Lower/upper explosive limit | Toxicity threshold limit value/(mg·L-1) | Energy density/(MJ·kg-1) | Cost/($·kg-1) |
|---|---|---|---|---|---|---|
| Hydrogen | 100 | -259, -253 | 4%/75% | Nontoxic | 142.0 | 0.79 |
| Ammonia | 17.6 | -78, -33 | 15%/28% | 25/35 | 22.5 | 0.39 |
| Ethanol | 13.1 | -117, 79 | 3.3%/19% | 1 000 | 26.8 | 0.64 |
| Methanol | 12.6 | -98, 65 | 6.7%/36% | 200/250 | 15.2 | 0.49 |
| Urea | 6.7 | 132.7, 196.6 | Low-vapor-pressure solid | Nontoxic | 15.66 | 0.24 |
Table 1 Physicochemical and toxicological characteristics of various hydrogen carriers[1]
| Hydrogen carrier | w(H element)/% | Melting point/℃, boiling point/℃ | Lower/upper explosive limit | Toxicity threshold limit value/(mg·L-1) | Energy density/(MJ·kg-1) | Cost/($·kg-1) |
|---|---|---|---|---|---|---|
| Hydrogen | 100 | -259, -253 | 4%/75% | Nontoxic | 142.0 | 0.79 |
| Ammonia | 17.6 | -78, -33 | 15%/28% | 25/35 | 22.5 | 0.39 |
| Ethanol | 13.1 | -117, 79 | 3.3%/19% | 1 000 | 26.8 | 0.64 |
| Methanol | 12.6 | -98, 65 | 6.7%/36% | 200/250 | 15.2 | 0.49 |
| Urea | 6.7 | 132.7, 196.6 | Low-vapor-pressure solid | Nontoxic | 15.66 | 0.24 |
| Catalyst | Electrolyte | Scan rate/(mV·s-1) | Peak current density (mA·cm-2) at (@) specific potential | Potential/V (i=10 mA/cm2) | Ref. |
|---|---|---|---|---|---|
| Ni-MOF-0.5 | 1 mol/L KOH+0.5 mol/L urea | 5 | — | 1.38 V(vs.RHE) | [ |
| C@NiO | 1 mol/L KOH+0.33 mol/L urea | 10 | 25@1.46 V(vs.RHE) | 1.36 V(vs.RHE) | [ |
| Ni(OH)2@NF | 1 mol/L KOH+0.33 mol/L urea | 5 | — | 1.35 V(vs.RHE) | [ |
| V-Ni3N/NF | 1 mol/L KOH+0.5 mol/L urea | 2 | — | 1.361 V(vs.RHE) | [ |
| Ni@NCNT-3 | 1 mol/L KOH+0.5 mol/L urea | 10 | 45.8@1.5 V(vs.RHE) | 1.38 V(vs.RHE) | [ |
| Ni/SiO x /N-C | 1 mol/L KOH+0.33 mol/L urea | 5 | — | 1.384 V(vs.RHE) | [ |
| NiFe/N-C | 1 mol/L KOH+1 mol/L urea | 5 | 100@1.37 V(vs.RHE) | 1.37 V(vs.RHE) (at 100 mA/cm2) | [ |
| NP-Ni0.7Fe0.3 NF | 1 mol/L KOH+0.33 mol/L urea | 5 | — | 1.33 V(vs.RHE) | [ |
| NiFeMo | 1 mol/L KOH+0.33 mol/L urea | 5 | 152@1.5 V(vs.RHE) | 1.38 V(vs.RHE) | [ |
| Ni3N-350/NF | 1 mol/L KOH+0.5 mol/L urea | 5 | — | 1.34 V(vs.RHE) | [ |
| a-Ni2P/G | 1 mol/L KOH+0.5 mol/L urea | 5 | 209.1@1.7 V(vs.RHE) | 1.28 V(vs.RHE) | [ |
| Ni3S2/MWCNTs/NF | 1 mol/L KOH+0.5 mol/L urea | 5 | — | 1.338 V(vs.RHE) | [ |
| β-NiS | 1 mol/L KOH+0.33 mol/L urea | 5 | — | 1.4 V(vs.RHE) | [ |
| Ni0.85Se/rGO | 1 mol/L KOH+0.5 mol/L urea | 5 | — | 1.36 V(vs.RHE) | [ |
| Ni/NiO@NC | 1 mol/L KOH+0.33 mol/L urea | 5 | — | 1.35 V(vs.RHE) | [ |
| Ni@C-V2O3/NF | 1 mol/L KOH+0.5 mol/L urea | 5 | — | 1.32 V(vs.RHE) | [ |
| NiSe2-NiO 350 | 1 mol/L KOH+0.33 mol/L urea | 10 | 1.33 V(vs.RHE) | [ | |
| NiF3/Ni2P@CC-2 | 1 mol/L KOH+0.33 mol/L urea | 10 | 122@1.6 V(vs.RHE) | 1.36 V(vs.RHE) | [ |
| MNPBA-P | 1 mol/L KOH+0.5 mol/L urea | 20 | — | 1.344 V(vs.RHE) | [ |
| Rh-Ni | 1 mol/L KOH+0.33 mol/L urea | 10 | 82@0.7 V(vs.Hg/HgO) | 1.4 V(vs.RHE) (at 50 mA/cm2) | [ |
| Ni/Rh | 5 mol/L KOH+0.33 mol/L urea | 5 | 45@0.65 V(vs. Ag/AgCl) | — | [ |
| PtIr | 5 mol/L KOH+0.33 mol/L urea | 10 | 125@0.8 V(vs.Hg/HgO) | — | [ |
| Rh-NCs/NiO-NSs | 1 mol/L KOH+0.33 mol/L urea | 50 | 616@1.55V(vs.RHE) | — | [ |
Table 2 The UOR performance reported in the years of 2009-2022
| Catalyst | Electrolyte | Scan rate/(mV·s-1) | Peak current density (mA·cm-2) at (@) specific potential | Potential/V (i=10 mA/cm2) | Ref. |
|---|---|---|---|---|---|
| Ni-MOF-0.5 | 1 mol/L KOH+0.5 mol/L urea | 5 | — | 1.38 V(vs.RHE) | [ |
| C@NiO | 1 mol/L KOH+0.33 mol/L urea | 10 | 25@1.46 V(vs.RHE) | 1.36 V(vs.RHE) | [ |
| Ni(OH)2@NF | 1 mol/L KOH+0.33 mol/L urea | 5 | — | 1.35 V(vs.RHE) | [ |
| V-Ni3N/NF | 1 mol/L KOH+0.5 mol/L urea | 2 | — | 1.361 V(vs.RHE) | [ |
| Ni@NCNT-3 | 1 mol/L KOH+0.5 mol/L urea | 10 | 45.8@1.5 V(vs.RHE) | 1.38 V(vs.RHE) | [ |
| Ni/SiO x /N-C | 1 mol/L KOH+0.33 mol/L urea | 5 | — | 1.384 V(vs.RHE) | [ |
| NiFe/N-C | 1 mol/L KOH+1 mol/L urea | 5 | 100@1.37 V(vs.RHE) | 1.37 V(vs.RHE) (at 100 mA/cm2) | [ |
| NP-Ni0.7Fe0.3 NF | 1 mol/L KOH+0.33 mol/L urea | 5 | — | 1.33 V(vs.RHE) | [ |
| NiFeMo | 1 mol/L KOH+0.33 mol/L urea | 5 | 152@1.5 V(vs.RHE) | 1.38 V(vs.RHE) | [ |
| Ni3N-350/NF | 1 mol/L KOH+0.5 mol/L urea | 5 | — | 1.34 V(vs.RHE) | [ |
| a-Ni2P/G | 1 mol/L KOH+0.5 mol/L urea | 5 | 209.1@1.7 V(vs.RHE) | 1.28 V(vs.RHE) | [ |
| Ni3S2/MWCNTs/NF | 1 mol/L KOH+0.5 mol/L urea | 5 | — | 1.338 V(vs.RHE) | [ |
| β-NiS | 1 mol/L KOH+0.33 mol/L urea | 5 | — | 1.4 V(vs.RHE) | [ |
| Ni0.85Se/rGO | 1 mol/L KOH+0.5 mol/L urea | 5 | — | 1.36 V(vs.RHE) | [ |
| Ni/NiO@NC | 1 mol/L KOH+0.33 mol/L urea | 5 | — | 1.35 V(vs.RHE) | [ |
| Ni@C-V2O3/NF | 1 mol/L KOH+0.5 mol/L urea | 5 | — | 1.32 V(vs.RHE) | [ |
| NiSe2-NiO 350 | 1 mol/L KOH+0.33 mol/L urea | 10 | 1.33 V(vs.RHE) | [ | |
| NiF3/Ni2P@CC-2 | 1 mol/L KOH+0.33 mol/L urea | 10 | 122@1.6 V(vs.RHE) | 1.36 V(vs.RHE) | [ |
| MNPBA-P | 1 mol/L KOH+0.5 mol/L urea | 20 | — | 1.344 V(vs.RHE) | [ |
| Rh-Ni | 1 mol/L KOH+0.33 mol/L urea | 10 | 82@0.7 V(vs.Hg/HgO) | 1.4 V(vs.RHE) (at 50 mA/cm2) | [ |
| Ni/Rh | 5 mol/L KOH+0.33 mol/L urea | 5 | 45@0.65 V(vs. Ag/AgCl) | — | [ |
| PtIr | 5 mol/L KOH+0.33 mol/L urea | 10 | 125@0.8 V(vs.Hg/HgO) | — | [ |
| Rh-NCs/NiO-NSs | 1 mol/L KOH+0.33 mol/L urea | 50 | 616@1.55V(vs.RHE) | — | [ |
| Anode catalyst | Cathode catalyst | Electrolyte | Cell voltage (V) at (@)specific current density (mA/cm2) for UE | Cell voltage (V) at (@) specific current density (mA/cm2) for WS/V | Ref. |
|---|---|---|---|---|---|
| P-CoNi2S4 | P-CoNi2S4 | 1 mol/L KOH+0.33 mol/L urea | 1.402@10 | 1.544@10 | [ |
| NiFeRh-LDH | NiFeRh-LDH | 1 mol/L KOH+0.33 mol/L urea | 1.346@10 | 1.455@10 | [ |
| Ni-S-Se/NF | Ni-S-Se/NF | 1 mol/L KOH+0.5 mol/L urea | 1.47@10 | 1.57@10 | [ |
| Ni-NiO-Mo0.84Ni0.16/NF | Ni-NiO-Mo0.84Ni0.16/NF | 1 mol/L KOH+0.5 mol/L urea | 1.37@10 | 1.52@10 | [ |
| MoP@NiCo-LDH/NF-20 | MoP@NiCo-LDH/NF-20 | 1 mol/L KOH+0.5 mol/L urea | 1.405@100 | 1.697@100 | [ |
| Fe2P@Ni x P/NF | Fe2P@Ni x P/NF | 1 mol/L KOH+0.5 mol/L urea | 1.538@100 | 1.705@100 | [ |
| Ni3N/Ni0.2Mo0.8N/NF | Ni3N/Ni0.2Mo0.8N/NF | 1 mol/L KOH+0.5 mol/L urea | 1.348@10 | 1.487@10 | [ |
| NiFeMo | NiFeMo | 1 mol/L KOH+0.33 mol/L urea | 1.46@10 | 1.60@10 | [ |
| NiCoFe-LTH | NiCoFe-LTH | 1 mol/L KOH+0.33 mol/L urea | 1.49@10 | 1.65@10 | [ |
| NC-FNCP | NC-FNCP | 1 mol/L KOH+0.5 mol/L urea | 1.52@10 | 1.63@10 | [ |
| Ni-CoP/HPFs | Ni-CoP/HPFs | 1 mol/L KOH+0.5 mol/L urea | 1.43@10 | 1.68@10 | [ |
| Ni-Mo | Ni-Mo | 1 mol/L KOH+0.1 mol/L urea | 1.43@10 | 1.59@10 | [ |
| Co-Z/Se-2 | Co-Z/Se-2 | 1 mol/L KOH+0.5 mol/L urea | 1.49@10 | 1.678@10 | [ |
| NF/CoMoS/NiFeOOH | NF/CoMoS/NiFeOOH | 1 mol/L KOH+0.5 mol/L urea | 1.66@100 | 1.732@100 | [ |
| V-FeNi3N/Ni3N | V-FeNi3N/Ni3N | 1 mol/L KOH+0.33 mol/L urea | 1.46@10 | 1.54@10 | [ |
| Ru-NiFe-③/NF | Ru-NiFe-③/NF | 1 mol/L KOH+0.33 mol/L urea | 1.47@100 | 1.63@100 | [ |
| Co(OH)F/NF | Co–P/NF | 1 mol/L KOH+0.7 mol/L urea | 1.42@10 | 1.65@10 | [ |
| NiO-NiPi | NiP/NiO-NiPi | 1 mol/L KOH+0.5 mol/L urea | 1.585@100 | 1.876@100 | [ |
| Ni/Ni2P/CNF | Pt/C | 1 mol/L KOH+0.33 mol/L urea | 1.40@10 | 1.58@10 | [ |
Table 3 The UE performance reported in the years of 2019-2022
| Anode catalyst | Cathode catalyst | Electrolyte | Cell voltage (V) at (@)specific current density (mA/cm2) for UE | Cell voltage (V) at (@) specific current density (mA/cm2) for WS/V | Ref. |
|---|---|---|---|---|---|
| P-CoNi2S4 | P-CoNi2S4 | 1 mol/L KOH+0.33 mol/L urea | 1.402@10 | 1.544@10 | [ |
| NiFeRh-LDH | NiFeRh-LDH | 1 mol/L KOH+0.33 mol/L urea | 1.346@10 | 1.455@10 | [ |
| Ni-S-Se/NF | Ni-S-Se/NF | 1 mol/L KOH+0.5 mol/L urea | 1.47@10 | 1.57@10 | [ |
| Ni-NiO-Mo0.84Ni0.16/NF | Ni-NiO-Mo0.84Ni0.16/NF | 1 mol/L KOH+0.5 mol/L urea | 1.37@10 | 1.52@10 | [ |
| MoP@NiCo-LDH/NF-20 | MoP@NiCo-LDH/NF-20 | 1 mol/L KOH+0.5 mol/L urea | 1.405@100 | 1.697@100 | [ |
| Fe2P@Ni x P/NF | Fe2P@Ni x P/NF | 1 mol/L KOH+0.5 mol/L urea | 1.538@100 | 1.705@100 | [ |
| Ni3N/Ni0.2Mo0.8N/NF | Ni3N/Ni0.2Mo0.8N/NF | 1 mol/L KOH+0.5 mol/L urea | 1.348@10 | 1.487@10 | [ |
| NiFeMo | NiFeMo | 1 mol/L KOH+0.33 mol/L urea | 1.46@10 | 1.60@10 | [ |
| NiCoFe-LTH | NiCoFe-LTH | 1 mol/L KOH+0.33 mol/L urea | 1.49@10 | 1.65@10 | [ |
| NC-FNCP | NC-FNCP | 1 mol/L KOH+0.5 mol/L urea | 1.52@10 | 1.63@10 | [ |
| Ni-CoP/HPFs | Ni-CoP/HPFs | 1 mol/L KOH+0.5 mol/L urea | 1.43@10 | 1.68@10 | [ |
| Ni-Mo | Ni-Mo | 1 mol/L KOH+0.1 mol/L urea | 1.43@10 | 1.59@10 | [ |
| Co-Z/Se-2 | Co-Z/Se-2 | 1 mol/L KOH+0.5 mol/L urea | 1.49@10 | 1.678@10 | [ |
| NF/CoMoS/NiFeOOH | NF/CoMoS/NiFeOOH | 1 mol/L KOH+0.5 mol/L urea | 1.66@100 | 1.732@100 | [ |
| V-FeNi3N/Ni3N | V-FeNi3N/Ni3N | 1 mol/L KOH+0.33 mol/L urea | 1.46@10 | 1.54@10 | [ |
| Ru-NiFe-③/NF | Ru-NiFe-③/NF | 1 mol/L KOH+0.33 mol/L urea | 1.47@100 | 1.63@100 | [ |
| Co(OH)F/NF | Co–P/NF | 1 mol/L KOH+0.7 mol/L urea | 1.42@10 | 1.65@10 | [ |
| NiO-NiPi | NiP/NiO-NiPi | 1 mol/L KOH+0.5 mol/L urea | 1.585@100 | 1.876@100 | [ |
| Ni/Ni2P/CNF | Pt/C | 1 mol/L KOH+0.33 mol/L urea | 1.40@10 | 1.58@10 | [ |
| Anode | Cathode | Anolyte | Oxidant | Peak power density/ (mW·cm-2) | Temperature/℃ | Ref. |
|---|---|---|---|---|---|---|
| Pt/C | Pt/C | 1 mol/L urea | Air | 0.55 | Room temperature | [ |
| Ni/C | Ag/C | 1 mol/L urea | Air | 0.14 | Room temperature | |
| Ni/C | MnO2/C | 1 mol/L urea | Air | 1.7 | 50 | |
| Ni@C | Pt/C | 1 mol/L KOH+0.33 mol/L urea | Air | 13.8 | 50 | [ |
| Ni-Cu/ZnO@MWCNT | Pt/C | 3 mol/L KOH+0.7 mol/L urea | Air | 44.36 | 50 | [ |
| h-NiWO4NPs/rGO | Pt/C | 1 mol/L KOH+0.33 mol/L urea | Air | 5 | Room temperature | [ |
| NiCo2O4/CC-12 | Pt/C | 1 mol/L KOH+0.05 mol/L urea | Air | 38 | 80 | [ |
| Co3O4/NiO | Co3O4@MnO2 | 0.1 mol/L KOH+0.05 mol/L urea | Air | 33.8 | 60 | [ |
| Ni-Co/MWCNT | Pt/C | 3 mol/L KOH+1 mol/L urea | O2 | 17.5 | 60 | [ |
| Ni/rGO-300 | Graphite/Filter paper | 6 mol/L KOH+0.33 mol/L urea | 30% H2O2 | 0.18 | 19 | [ |
| CoNi/rGO@NF | Pd/CFC | 5 mol/L KOH+0.33 mol/L urea | 0.9 mol/L H2O2+2 mol/L H2SO4 | 12.58 | 60 | [ |
| NiCo2S4@CS | Pd/Ti | 5 mol/L KOH+0.2 mol/L urea | 1 mol/L H2O2+2 mol/L H2SO4 | 10.36 | 60 | [ |
| Ni(OH)2/NF | Pd/C@TiC | 5 mol/L KOH+0.6 mol/L urea | 2 mol/L H2O2+2 mol/L H2SO4 | 19.7 | 20 | [ |
| NiCo/NF | Pd/CFC | 7 mol/L KOH+0.5 mol/L urea | 2 mol/L H2O2+2 mol/L H2SO4 | 17.4 | 20 | [ |
| Ni-Se/NF | Prussian blue | 1 mol/L KOH+0.5 mol/L urea | 2 mol/L H2O2+2 mol/L H2SO4 | 33 | 20 | [ |
| Ni-120/VC | Pt/C | 7 mol/L KOH+0.33 mol/L urea | 2 mol/L H2O2+2 mol/L H2SO4 | 36.4 | 70 | [ |
| Ni0.8Co0.2(OH)2 | Pt/C | 5 mol/L KOH+0.5 mol/L urea | 2 mol/L H2O2+2 mol/L H2SO4 | 11.2 | 20 | [ |
| Ni6Cr4-CNT@C | Pt/C | 5 mol/L KOH+1 mol/L urea | 2 mol/L H2O2+2 mol/L H2SO4 | 48.1 | 80 | [ |
| Pt | Graphite | 1 mol/L KOH+0.3 mol/L urea | 0.65 mol/L AgNO3 | 0.91 | Room temperature | [ |
| Ni/CC | CC | Fresh urine | 50 mg/L Cr(Ⅵ)+0.25 mol/L H2SO4 | 0.34 | 20 | [ |
| Pd-Ni/C | Pd/C | 1 mol/L KOH+0.33 mol/L urea | O2 | 1.12 | Room temperature | [ |
| Ni/CNT@sponge | Pt/C | 3 mol/L KOH+3 mol/L urea | 1.5 mol/L H2SO4 | 6.6 | 45 | [ |
| Ni nanorods/NF | Prussian blue | 1 mol/L NaOH+0.33 mol/L urea | 2 mol/L H2O2+2 mol/L KCl+ 2 mol/L H2SO4 | 10.6 | 20 | [ |
| Ni-Co NWAs | Pd/CFC | 5 mol/L KOH+0.33 mol/L urea | 2 mol/L H2O2+2 mol/L H2SO4 | 5.03 | 25 | [ |
| Ni-P HPNG-40 | Pt net | 9 mol/L KOH+0.5 mol/L urea | 2 mol/L H2O2+2 mol/L H2SO4 | 38.15 | 60 | [ |
Table 4 The DUFC performance reported in the years of 2010-2023
| Anode | Cathode | Anolyte | Oxidant | Peak power density/ (mW·cm-2) | Temperature/℃ | Ref. |
|---|---|---|---|---|---|---|
| Pt/C | Pt/C | 1 mol/L urea | Air | 0.55 | Room temperature | [ |
| Ni/C | Ag/C | 1 mol/L urea | Air | 0.14 | Room temperature | |
| Ni/C | MnO2/C | 1 mol/L urea | Air | 1.7 | 50 | |
| Ni@C | Pt/C | 1 mol/L KOH+0.33 mol/L urea | Air | 13.8 | 50 | [ |
| Ni-Cu/ZnO@MWCNT | Pt/C | 3 mol/L KOH+0.7 mol/L urea | Air | 44.36 | 50 | [ |
| h-NiWO4NPs/rGO | Pt/C | 1 mol/L KOH+0.33 mol/L urea | Air | 5 | Room temperature | [ |
| NiCo2O4/CC-12 | Pt/C | 1 mol/L KOH+0.05 mol/L urea | Air | 38 | 80 | [ |
| Co3O4/NiO | Co3O4@MnO2 | 0.1 mol/L KOH+0.05 mol/L urea | Air | 33.8 | 60 | [ |
| Ni-Co/MWCNT | Pt/C | 3 mol/L KOH+1 mol/L urea | O2 | 17.5 | 60 | [ |
| Ni/rGO-300 | Graphite/Filter paper | 6 mol/L KOH+0.33 mol/L urea | 30% H2O2 | 0.18 | 19 | [ |
| CoNi/rGO@NF | Pd/CFC | 5 mol/L KOH+0.33 mol/L urea | 0.9 mol/L H2O2+2 mol/L H2SO4 | 12.58 | 60 | [ |
| NiCo2S4@CS | Pd/Ti | 5 mol/L KOH+0.2 mol/L urea | 1 mol/L H2O2+2 mol/L H2SO4 | 10.36 | 60 | [ |
| Ni(OH)2/NF | Pd/C@TiC | 5 mol/L KOH+0.6 mol/L urea | 2 mol/L H2O2+2 mol/L H2SO4 | 19.7 | 20 | [ |
| NiCo/NF | Pd/CFC | 7 mol/L KOH+0.5 mol/L urea | 2 mol/L H2O2+2 mol/L H2SO4 | 17.4 | 20 | [ |
| Ni-Se/NF | Prussian blue | 1 mol/L KOH+0.5 mol/L urea | 2 mol/L H2O2+2 mol/L H2SO4 | 33 | 20 | [ |
| Ni-120/VC | Pt/C | 7 mol/L KOH+0.33 mol/L urea | 2 mol/L H2O2+2 mol/L H2SO4 | 36.4 | 70 | [ |
| Ni0.8Co0.2(OH)2 | Pt/C | 5 mol/L KOH+0.5 mol/L urea | 2 mol/L H2O2+2 mol/L H2SO4 | 11.2 | 20 | [ |
| Ni6Cr4-CNT@C | Pt/C | 5 mol/L KOH+1 mol/L urea | 2 mol/L H2O2+2 mol/L H2SO4 | 48.1 | 80 | [ |
| Pt | Graphite | 1 mol/L KOH+0.3 mol/L urea | 0.65 mol/L AgNO3 | 0.91 | Room temperature | [ |
| Ni/CC | CC | Fresh urine | 50 mg/L Cr(Ⅵ)+0.25 mol/L H2SO4 | 0.34 | 20 | [ |
| Pd-Ni/C | Pd/C | 1 mol/L KOH+0.33 mol/L urea | O2 | 1.12 | Room temperature | [ |
| Ni/CNT@sponge | Pt/C | 3 mol/L KOH+3 mol/L urea | 1.5 mol/L H2SO4 | 6.6 | 45 | [ |
| Ni nanorods/NF | Prussian blue | 1 mol/L NaOH+0.33 mol/L urea | 2 mol/L H2O2+2 mol/L KCl+ 2 mol/L H2SO4 | 10.6 | 20 | [ |
| Ni-Co NWAs | Pd/CFC | 5 mol/L KOH+0.33 mol/L urea | 2 mol/L H2O2+2 mol/L H2SO4 | 5.03 | 25 | [ |
| Ni-P HPNG-40 | Pt net | 9 mol/L KOH+0.5 mol/L urea | 2 mol/L H2O2+2 mol/L H2SO4 | 38.15 | 60 | [ |
| 1 | SINGH R K, RAJAVELU K, MONTAG M, et al. Advances in catalytic electrooxidation of urea: a review[J]. Energy Technol, 2021, 9(8): 2100017. |
| 2 | KAMEDA T, ITO S, YOSHIOKA T. Kinetic and equilibrium studies of urea adsorption onto activated carbon: adsorption mechanism[J]. J Dispersion Sci Technol, 2017, 38(7): 1063-1066. |
| 3 | LEI T, GUO X, MA J, et al. Kinetics and thermodynamics of urea hydrolysis under the coupling of nitrogen application rate and temperature[J]. Environ Sci Pollut Res, 2018, 25(25): 25413-25419. |
| 4 | VON AHNEN M, PEDERSEN L F, PEDERSEN P B, et al. Degradation of urea, ammonia and nitrite in moving bed biofilters operated at different feed loadings[J]. Aquac Eng, 2015, 69: 50-59. |
| 5 | URBAŃCZYK E, SOWA M, SIMKA W. Urea removal from aqueous solutions-a review[J]. J Appl Electrochem, 2016, 46(10): 1011-1029. |
| 6 | ZHENG S, ZHENG Y, XUE H, et al. Ultrathin nickel terephthalate nanosheet three-dimensional aggregates with disordered layers for highly efficient overall urea electrolysis[J]. Chem Eng J, 2020, 395: 125166. |
| 7 | CAO J, JIAO Z, ZHU R, et al. Enhancing hydrogen evolution through urea electrolysis over Co-doped Ni-P-O film on nickel foam[J]. J Alloys Compd, 2022, 914: 165362. |
| 8 | BOGGS B K, KING R L, BOTTE G G. Urea electrolysis: direct hydrogen production from urine[J]. Chem Commun, 2009, (32): 4859-4861. |
| 9 | NGUYEN P K T, KIM J, YOON Y S, et al. Mathematical modeling of a direct urea fuel cell[J]. Int J Hydrogen Energy, 2023, 48(6): 2314-2327. |
| 10 | LAN R, TAO S, IRVINE J T S. A direct urea fuel cell-power from fertiliser and waste[J]. Energy Environ Sci, 2010, 3(4): 438-441. |
| 11 | SUN H, LIU J, KIM H, et al. Ni-doped CuO nanoarrays activate urea adsorption and stabilizes reaction intermediates to achieve high-performance urea oxidation catalysts[J]. Adv Sci, 2022, 9(34): 2204800. |
| 12 | SIMKA W, PIOTROWSKI J, NAWRAT G. Influence of anode material on electrochemical decomposition of urea[J]. Electrochim Acta, 2007, 52(18): 5696-5703. |
| 13 | TONG Y, CHEN P, ZHANG M, et al. Oxygen vacancies confined in nickel molybdenum oxide porous nanosheets for promoted electrocatalytic urea oxidation[J]. ACS Catal, 2018, 8(1): 1-7. |
| 14 | LU S, HUMMEL M, GU Z, et al. Highly efficient urea oxidation via nesting nano-nickel oxide in eggshell membrane-derived carbon[J]. ACS Sustainable Chem Eng, 2021, 9(4): 1703-1713. |
| 15 | LU X F, ZHANG S L, SIM W L, et al. Phosphorized CoNi2S4 yolk-shell spheres for highly efficient hydrogen production via water and urea electrolysis[J]. Angew Chem Int Ed, 2021, 60(42): 22885-22891. |
| 16 | MAI W, CUI Q, ZHANG Z, et al. Coaxial Ni3S2@CoMoS4/NiFeOOH nanorods for energy-saving water splitting and urea electrolysis[J]. Int J Hydrogen Energy, 2021, 46(47): 24078-24093. |
| 17 | SUÁREZ D, DÍAZ N, MERZ K M. Ureases: Quantum chemical calculations on cluster models[J]. J Am Chem Soc, 2003, 125(50): 15324-15337. |
| 18 | ESTIU G, MERZ K M. Enzymatic catalysis of urea decomposition: elimination or hydrolysis?[J]. J Am Chem Soc, 2004, 126(38): 11832-11842. |
| 19 | 王留留, 任洁, 卢星宇, 等. 尿素分解制氢催化剂研究进展[J]. 材料导报, 2023(12): 1-24. |
| WANG L L, REN J, LU X Y, et al. Research progress of urea splitting catalysts for hydrogen generation[J]. Mater Rep, 2023(12): 1-24. | |
| 20 | 徐秀娟, 张清硕, 国通, 等. 过渡金属磷化物纳米材料催化尿素电解制氢研究进展[J]. 化学研究, 2022, 33(1): 1-8. |
| XU X, ZHANG Q, GUO T, et al. Recent advances in transition metal phosphide nanomaterials for hydrogen production by urea electrolysis[J]. Chem Res, 2022, 33(1): 1-8. | |
| 21 | 向阳, 熊昆, 张海东, 等. 电催化尿素氧化的镍基催化剂表界面调控[J]. 材料导报, 2022, 36(10): 20080297-20080298. |
| XIANG Y, XIONG K, ZHANG H, et al. Surface interface regulation of nickel-based catalysts for electrocatalytic urea oxidation[J]. Mater Rep, 2022, 36(10): 20080297-20080298. | |
| 22 | HU S, WU H, FENG C, et al. Synthesis of non-noble NiMoO4-Ni(OH)2/NF bifunctional electrocatalyst and its application in water-urea electrolysis[J]. Int J Hydrogen Energy, 2020, 45(41): 21040-21050. |
| 23 | KAPAŁKA A, CALLY A, NEODO S, et al. Electrochemical behavior of ammonia at Ni/Ni(OH)2 electrode[J]. Electrochem Commun, 2010, 12(1): 18-21. |
| 24 | VEDHARATHINAM V, BOTTE G G. Understanding the electro-catalytic oxidation mechanism of urea on nickel electrodes in alkaline medium[J]. Electrochim Acta, 2012, 81: 292-300. |
| 25 | PUTRI Y M T A, GUNLAZUARDI J, YULIZAR Y, et al. Recent progress in direct urea fuel cell[J]. Open Chem, 2021, 19(1): 1116-1133. |
| 26 | VEDHARATHINAM V, BOTTE G G. Experimental investigation of potential oscillations during the electrocatalytic oxidation of urea on Ni catalyst in alkaline medium[J]. J Phys Chem C, 2014, 118(38): 21806-21812. |
| 27 | WANG D, BOTTE G G. In situ X-ray diffraction study of urea electrolysis on nickel catalysts[J]. ECS Electrochem Lett, 2014, 3(9): H29. |
| 28 | VEDHARATHINAM V, BOTTE G G. Direct evidence of the mechanism for the electro-oxidation of urea on Ni(OH)2 catalyst in alkaline medium[J]. Electrochim Acta, 2013, 108: 660-665. |
| 29 | GUO F, YE K, DU M, et al. Electrochemical impedance analysis of urea electro-oxidation mechanism on nickel catalyst in alkaline medium[J]. Electrochim Acta, 2016, 210: 474-482. |
| 30 | GENG S, ZHENG Y, LI S, et al. Nickel ferrocyanide as a high-performance urea oxidation electrocatalyst[J]. Nat Energy, 2021, 6(9): 904-912. |
| 31 | LI J, LI J, LIU T, et al. Deciphering and suppressing over-oxidized nitrogen in nickel-catalyzed urea electrolysis[J]. Angew Chem Int Ed, 2021, 60(51): 26656-26662. |
| 32 | CHEN W, XU L, ZHU X, et al. Unveiling the electrooxidation of urea: intramolecular coupling of the N—N bond[J]. Angew Chem Int Ed, 2021, 60(13): 7297-7307. |
| 33 | LIU H, LIU Z, FENG L. Bonding state synergy of the NiF2/Ni2P hybrid with the co-existence of covalent and ionic bonds and the application of this hybrid as a robust catalyst for the energy-relevant electrooxidation of water and urea[J]. Nanoscale, 2019, 11(34): 16017-16025. |
| 34 | FANG Y, LIU Z. Tafel kinetics of electrocatalytic reactions: from experiment to first-principles[J]. ACS Catal, 2014, 4(12): 4364-4376. |
| 35 | MEDDINGS N, HEINRICH M, OVERNEY F, et al. Application of electrochemical impedance spectroscopy to commercial Li-ion cells: a review[J]. J Power Sources, 2020, 480: 228742. |
| 36 | LI J, CHANG Y, LI D, et al. Efficient synergism of V2O5 and Pd for alkaline methanol electrooxidation[J]. Chem Commun, 2021, 57(57): 7035-7038. |
| 37 | WANG K, HUANG W, CAO Q, et al. Engineering NiF3/Ni2P heterojunction as efficient electrocatalysts for urea oxidation and splitting[J]. Chem Eng J, 2022, 427: 130865. |
| 38 | BAO Y, LIU H, LIU Z, et al. Pd/FeP catalyst engineering via thermal annealing for improved formic acid electrochemical oxidation[J]. Appl Catal B: Environ, 2020, 274: 119106. |
| 39 | ESCUDERO-ESCRIBANO M, VERDAGUER-CASADEVALL A, MALACRIDA P, et al. Pt5Gd as a highly active and stable catalyst for oxygen electroreduction[J]. J Am Chem Soc, 2012, 134(40): 16476-16479. |
| 40 | ZHENG J, SHENG W, ZHUANG Z, et al. Universal dependence of hydrogen oxidation and evolution reaction activity of platinum-group metals on pH and hydrogen binding energy[J]. Sci Adv, 2016, 2(3): e1501602. |
| 41 | ZHAO X, WANG Y, ZHANG Y, et al. Ni-Fe layered double hydroxide nanosheets supported on exfoliated graphite for efficient urea oxidation in direct urea fuel cells[J]. ChemSusChem, 2022, 15(7): e202102614. |
| 42 | CHEN N, DU Y X, ZHANG G, et al. Amorphous nickel sulfoselenide for efficient electrochemical urea-assisted hydrogen production in alkaline media[J]. Nano Energy, 2021, 81: 105605. |
| 43 | ZHANG Y, GAO L, HENSEN E J M, et al. Evaluating the stability of Co2P electrocatalysts in the hydrogen evolution reaction for both acidic and alkaline electrolytes[J]. ACS Energy Lett, 2018, 3(6): 1360-1365. |
| 44 | MCCRORY C C L, JUNG S, PETERS J C, et al. Benchmarking heterogeneous electrocatalysts for the oxygen evolution reaction[J]. J Am Chem Soc, 2013, 135(45): 16977-16987. |
| 45 | CHEN J, LI H, PEI Z, et al. Catalytic activity atlas of ternary Co-Fe-V metal oxides for the oxygen evolution reaction[J]. J Mater Chem A, 2020, 8(31): 15951-15961. |
| 46 | WANG L, LI M, HUANG Z, et al. Ni-WC/C nanocluster catalysts for urea electrooxidation[J]. J Power Sources, 2014, 264: 282-289. |
| 47 | WANG D, YAN W, VIJAPUR S H, et al. Enhanced electrocatalytic oxidation of urea based on nickel hydroxide nanoribbons[J]. J Power Sources, 2012, 217: 498-502. |
| 48 | ZUBAIR M, ULHASSAN M M, MEHRAN M T, et al. 2D MXenes and their heterostructures for HER, OER and overall water splitting: a review[J]. Int J Hydrogen Energy, 2022, 47(5): 2794-2818. |
| 49 | ZHU W, YUE Z, ZHANG W, et al. Wet-chemistry topotactic synthesis of bimetallic iron-nickel sulfide nanoarrays: an advanced and versatile catalyst for energy efficient overall water and urea electrolysis[J]. J Mater Chem A, 2018, 6(10): 4346-4353. |
| 50 | CAO Q, HUANG W, SHOU J, et al. Coupling dual-phased nickel selenides with N-doped carbon enables efficient urea electrocatalytic oxidation[J]. J Colloid Interface Sci, 2023, 629: 33-43. |
| 51 | UDERT K M, LARSEN T A, BIEBOW M, et al. Urea hydrolysis and precipitation dynamics in a urine-collecting system[J]. Water Res, 2003, 37(11): 2571-2582. |
| 52 | LI J, WANG S, CHANG J, et al. A review of Ni based powder catalyst for urea oxidation in assisting water splitting reaction[J]. Adv Powder Mater, 2022, 1(3): 100030. |
| 53 | MA G, XUE Q, ZHU J, et al. Ultrafine Rh nanocrystals decorated ultrathin NiO nanosheets for urea electro-oxidation[J]. Appl Catal B, 2020, 265: 118567. |
| 54 | SHA L, YE K, WANG G, et al. Rational design of NiCo2S4 nanowire arrays on nickle foam as highly efficient and durable electrocatalysts toward urea electrooxidation[J]. Chem Eng J, 2019, 359: 1652-1658. |
| 55 | YANG D, GU Y, YU X, et al. Nanostructured Ni2P-C as an efficient catalyst for urea electrooxidation[J]. ChemElectroChem, 2018, 5(4): 659-664. |
| 56 | LI Y, JIANG H, CUI Z, et al. Spin state tuning of the octahedral sites in Ni-Co-based spinel toward highly efficient urea oxidation reaction[J]. J Phys Chem C, 2021, 125(17): 9190-9199. |
| 57 | YANG X, LV Y, HU J, et al. A three-dimensional nanostructure of NiFe(OH) x nanoparticles/nickel foam as an efficient electrocatalyst for urea oxidation[J]. RSC Adv, 2021, 11(28): 17352-17359. |
| 58 | LI J, WANG S, SUN S, et al. A review of hetero-structured Ni-based active catalysts for urea electrolysis[J]. J Mater Chem A, 2022, 10(17): 9308-9326. |
| 59 | LI J, ZHANG J, YANG J H. Research progress and applications of nickel-based catalysts for electrooxidation of urea[J]. Int J Hydrogen Energy, 2022, 47(12): 7693-7712. |
| 60 | XIA L, LIAO Y, QING Y, et al. In situ growth of porous ultrathin Ni(OH)2 nanostructures on nickel foam: an efficient and durable catalysts for urea electrolysis[J]. ACS Appl Energy Mater, 2020, 3(3): 2996-3004. |
| 61 | LI R, LIU Q, ZHOU Y, et al. 3D self-supported porous vanadium-doped nickel nitride nanosheet arrays as efficient bifunctional electrocatalysts for urea electrolysis[J]. J Mater Chem A, 2021, 9(7): 4159-4166. |
| 62 | ZHANG Q, KAZIM F M D, MA S, et al. Nitrogen dopants in nickel nanoparticles embedded carbon nanotubes promote overall urea oxidation[J]. Appl Catal B: Environ, 2021, 280: 119436. |
| 63 | YUAN M, GUO X, LI N, et al. Silicon oxide-protected nickel nanoparticles as biomass-derived catalysts for urea electro-oxidation[J]. J Colloid Interface Sci, 2021, 589: 56-64. |
| 64 | ZHANG J, XING F, ZHANG H, et al. Ultrafine NiFe clusters anchored on N-doped carbon as bifunctional electrocatalysts for efficient water and urea oxidation[J]. Dalton Trans, 2020, 49(40): 13962-13969. |
| 65 | CAO Z, ZHOU T, MA X, et al. Hydrogen production from urea sewage on nife-based porous electrocatalysts[J]. ACS Sustainable Chem Eng, 2020, 8(29): 11007-11015. |
| 66 | LV Z, LI Z, TAN X, et al. One-step electrodeposited NiFeMo hybrid film for efficient hydrogen production via urea electrolysis and water splitting[J]. Appl Surf Sci, 2021, 552: 149514. |
| 67 | ZHAO Z, ZHAO J, WANG H, et al. Porous flower-like nickel nitride as highly efficient bifunctional electrocatalysts for less energy-intensive hydrogen evolution and urea oxidation[J]. Int J Hydrogen Energy, 2020, 45(28): 14199-14207. |
| 68 | TONG Y, CHEN L, DYSON P J, et al. Boosting hydrogen production via urea electrolysis on an amorphous nickel phosphide/graphene hybrid structure[J]. J Mater Sci, 2021, 56(31): 17709-17720. |
| 69 | ZHAO Z, YANG L, WANG Z, et al. Ni3S2/MWCNTs/NF hybrid nanostructure as effective bifunctional electrocatalysts for urea electrolysis assisted hydrogen evolution[J]. J Electrochem Soc, 2020, 167(12): 126514. |
| 70 | WU T H, LIN Y C, HOU B W, et al. Nanostructured β-NiS catalyst for enhanced and stable electro-oxidation of urea[J]. Catalysts, 2020, 10(11): 1280. |
| 71 | ZHAO L, CHANG Y, JIA M, et al. Monodisperse Ni0·85Se nanocrystals on rGO for high-performance urea electrooxidation[J]. J Alloys Compd, 2021, 852: 156751. |
| 72 | JI X, ZHANG Y, MA Z, et al. Oxygen vacancy-rich Ni/NiO@NC nanosheets with schottky heterointerface for efficient urea oxidation reaction[J]. ChemSusChem, 2020, 13(18): 5004-5014. |
| 73 | QIAN G, CHEN J, LUO L, et al. Novel bifunctional V2O3 nanosheets coupled with N-doped-carbon encapsulated Ni heterostructure for enhanced electrocatalytic oxidation of urea-rich wastewater[J]. ACS Appl Mater Interfaces, 2020, 12(34): 38061-38069. |
| 74 | LIU Z, ZHANG C, LIU H, et al. Efficient synergism of NiSe2 nanoparticle/NiO nanosheet for energy-relevant water and urea electrocatalysis[J]. Appl Catal B: Environ, 2020, 276: 119165. |
| 75 | XU H, YE K, ZHU K, et al. Transforming carnation-shaped MOF-Ni to Ni-Fe prussian blue analogue derived efficient bifunctional electrocatalyst for urea electrolysis[J]. ACS Sustainable Chem Eng, 2020, 8(42): 16037-16045. |
| 76 | KING R L, BOTTE G G. Investigation of multi-metal catalysts for stable hydrogen production via urea electrolysis[J]. J Power Sources, 2011, 196(22): 9579-9584. |
| 77 | MILLER A T, HASSLER B L, BOTTE G G. Rhodium electrodeposition on nickel electrodes used for urea electrolysis[J]. J Appl Electrochem, 2012, 42(11): 925-934. |
| 78 | SUN H, ZHANG W, LI J, et al. Rh-engineered ultrathin NiFe-LDH nanosheets enable highly-efficient overall water splitting and urea electrolysis[J]. Appl Catal B: Environ, 2021, 284: 119740. |
| 79 | XU Q, QIAN G, YIN S, et al. Design and synthesis of highly performing bifunctional Ni-NiO-MoNi hybrid catalysts for enhanced urea oxidation and hydrogen evolution reactions[J]. ACS Sustain Chem Eng, 2020, 8(18): 7174-7181. |
| 80 | WANG T, WU H, FENG C, et al. MoP@NiCo-LDH on nickel foam as bifunctional electrocatalyst for high efficiency water and urea-water electrolysis[J]. J Mater Chem A, 2020, 8(35): 18106-18116. |
| 81 | GUO T, XU X, WANG X, et al. Enabling the full exposure of Fe2P@NixP heterostructures in tree-branch-like nanoarrays for promoted urea electrolysis at high current densities[J]. Chem Eng J, 2021, 417: 128067. |
| 82 | LI R, WAN X, CHEN B, et al. Hierarchical Ni3N/Ni0.2Mo0.8N heterostructure nanorods arrays as efficient electrocatalysts for overall water and urea electrolysis[J]. Chem Eng J, 2021, 409: 128240. |
| 83 | PATIL K, BABAR P, BAE H, et al. Enhanced electrocatalytic activity of a layered triple hydroxide (LTH) by modulating the electronic structure and active sites for efficient and stable urea electrolysis[J]. Sustain Energy Fuels, 2022, 6(2): 474-483. |
| 84 | ZHANG J, HUANG S, NING P, et al. Nested hollow architectures of nitrogen-doped carbon-decorated Fe, Co, Ni-based phosphides for boosting water and urea electrolysis[J]. Nano Res, 2022, 15(3): 1916-1925. |
| 85 | PAN Y, SUN K, LIN Y, et al. Electronic structure and D-band center control engineering over M-doped CoP (M=Ni, Mn, Fe) hollow polyhedron frames for boosting hydrogen production[J]. Nano Energy, 2019, 56: 411-419. |
| 86 | ZHANG J Y, HE T, WANG M, et al. Energy-saving hydrogen production coupling urea oxidation over a bifunctional nickel-molybdenum nanotube array[J]. Nano Energy, 2019, 60: 894-902. |
| 87 | XU H, YE K, YIN J, et al. In situ growth of ZIF67 at the edge of nanosheet transformed into yolk-shell CoSe2 for high efficiency urea electrolysis[J]. J Power Sources, 2021, 491: 229592. |
| 88 | WANG J, SUN Y, QI Y, et al. Vanadium-doping and interface engineering for synergistically enhanced electrochemical overall water splitting and urea electrolysis[J]. ACS Appl Mater Interfaces, 2021, 13(48): 57392-57402. |
| 89 | WANG Y, WANG C, SHANG H, et al. Self-driven Ru-modified NiFe MOF nanosheet as multifunctional electrocatalyst for boosting water and urea electrolysis[J]. J Colloid Interface Sci, 2022, 605: 779-789. |
| 90 | SONG M, ZHANG Z, LI Q, et al. Ni-foam supported Co(OH)F and Co-P nanoarrays for energy-efficient hydrogen production via urea electrolysis[J]. J Mater Chem A, 2019, 7(8): 3697-3703. |
| 91 | XU X, GUO T, XIA J, et al. Modulation of the crystalline/amorphous interface engineering on Ni-P-O-based catalysts for boosting urea electrolysis at large current densities[J]. Chem Eng J, 2021, 425: 130514. |
| 92 | LI J, SUN S, YANG Y, et al. An efficient heterogeneous Ni/Ni2P catalyst for urea-assisted water electrolysis[J]. Chem Commun, 2022, 58(68): 9552-9555. |
| 93 | TABASSUM H, MAHMOOD A, ZHU B, et al. Recent advances in confining metal-based nanoparticles into carbon nanotubes for electrochemical energy conversion and storage devices[J]. Energy Environ Sci, 2019, 12(10): 2924-2956. |
| 94 | BASUMATARY P, KONWAR D, YOON Y S. A novel NiCu/ZnO@MWCNT anode employed in urea fuel cell to attain superior performances[J]. Electrochim Acta, 2018, 261: 78-85. |
| 95 | XU W, WU Z, TAO S. Urea-based fuel cells and electrocatalysts for urea oxidation[J]. Energy Technol, 2016, 4(11): 1329-1337. |
| 96 | SAYED E T, EISA T, MOHAMED H O, et al. Direct urea fuel cells: challenges and opportunities[J]. J Power Sources, 2019, 417: 159-175. |
| 97 | TRAN M H, PARK B J, KIM B H, et al. Mesoporous silica template-derived nickel-cobalt bimetallic catalyst for urea oxidation and its application in a direct urea/H2O2 fuel cell[J]. Int J Hydrogen Energy, 2020, 45(3): 1784-1792. |
| 98 | SAFEER N. K M, ALEX C, JANA R, et al. Remarkable COx tolerance of Ni3+ active species in a Ni2O3 catalyst for sustained electrochemical urea oxidation[J]. J Mater Chem A, 2022, 10(8): 4209-4221. |
| 99 | ZHANG C, LIANG P, YANG X, et al. Binder-free graphene and manganese oxide coated carbon felt anode for high-performance microbial fuel cell[J]. Biosens Bioelectron, 2016, 81: 32-38. |
| 100 | SAYED E T, ABDELKAREEM M A, BAHAA A, et al. Synthesis and performance evaluation of various metal chalcogenides as active anodes for direct urea fuel cells[J]. Renew Sustainable Energy Rev, 2021, 150: 111470. |
| 101 | ASAHI R, MORIKAWA T, OHWAKI T, et al. Visible-light photocatalysis in nitrogen-doped titanium oxides[J]. Science, 2001, 293(5528): 269-271. |
| 102 | ZHU X, DOU X, DAI J, et al. Metallic nickel hydroxide nanosheets give superior electrocatalytic oxidation of urea for fuel cells[J]. Angew Chem Int Ed, 2016, 55(40): 12465-12469. |
| 103 | ABDELKAREEM M A, SAYED E T, MOHAMED H O, et al. Nonprecious anodic catalysts for low-molecular-hydrocarbon fuel cells: theoretical consideration and current progress[J]. Prog Energy Combust Sci, 2020, 77: 100805. |
| 104 | TRAN T Q N, PARK B J, YUN W H, et al. Metal-organic framework-derived Ni@C and NiO@C as anode catalysts for urea fuel cells[J]. Sci Rep, 2020, 10(1): 278. |
| 105 | WANG Y, LIU G. Reduced graphene oxide supported nickel tungstate nano-composite electrocatalyst for anodic urea oxidation reaction in direct urea fuel cell[J]. Int J Hydrogen Energy, 2020, 45(58): 33500-33511. |
| 106 | RANJANI M, SENTHILKUMAR N, GNANA KUMAR G, et al. 3D flower-like hierarchical NiCo2O4 architecture on carbon cloth fibers as an anode catalyst for high-performance, durable direct urea fuel cells[J]. J Mater Chem A, 2018, 6(45): 23019-23027. |
| 107 | SENTHILKUMAR N, GNANA KUMAR G, MANTHIRAM A. 3D hierarchical core-shell nanostructured arrays on carbon fibers as catalysts for direct urea fuel cells[J]. Adv Energy Mater, 2018, 8(6): 1702207. |
| 108 | TESFAYE R M, DAS G, PARK B J, et al. Ni-Co bimetal decorated carbon nanotube aerogel as an efficient anode catalyst in urea fuel cells[J]. Sci Rep, 2019, 9(1): 479. |
| 109 | GLASS D E, GALVAN V, PRAKASH G K S. The effect of annealing temperature on nickel on reduced graphene oxide catalysts on urea electrooxidation[J]. Electrochim Acta, 2017, 253: 489-497. |
| 110 | LI B, SONG C, YIN J, et al. Effect of graphene on the performance of nickel foam-based CoNi nanosheet anode catalyzed direct urea-hydrogen peroxide fuel cell[J]. Int J Hydrogen Energy, 2020, 45(17): 10569-10579. |
| 111 | LI B, SONG C, RONG J, et al. A new catalyst for urea oxidation: NiCo2S4 nanowires modified 3D carbon sponge[J]. J Energy Chem, 2020, 50: 195-205. |
| 112 | YE K, ZHANG H, ZHAO L, et al. Facile preparation of three-dimensional Ni(OH)2/Ni foam anode with low cost and its application in a direct urea fuel cell[J]. New J Chem, 2016, 40(10): 8673-8680. |
| 113 | GUO F, CAO D, DU M, et al. Enhancement of direct urea-hydrogen peroxide fuel cell performance by three-dimensional porous nickel-cobalt anode[J]. J Power Sources, 2016, 307: 697-704. |
| 114 | TRAN M H, PARK B J, YOON H H. A highly active Ni-based anode material for urea electrocatalysis by a modified sol-gel method[J]. J Colloid Interface Sci, 2020, 578: 641-649. |
| 115 | KIM B, DAS G, PARK B J, et al. A free-standing NiCr-CNT@C anode mat by electrospinning for a high-performance urea/H2O2 fuel cell[J]. Electrochim Acta, 2020, 354: 136657. |
| 116 | CHINO I, MUNEEB O, DO E, et al. A paper microfluidic fuel cell powered by urea[J]. J Power Sources, 2018, 396: 710-714. |
| 117 | XU W, ZHANG H, LI G, et al. A urine/Cr(VI) fuel cell-electrical power from processing heavy metal and human urine[J]. J Electroanal Chem, 2016, 764: 38-44. |
| 118 | YOON J, LEE D, LEE Y N, et al. Solid solution palladium-nickel bimetallic anode catalysts by co-sputtering for direct urea fuel cells (DUFC)[J]. J Power Sources, 2019, 431: 259-264. |
| 119 | ZHANG H, WANG Y, WU Z, et al. A direct urea microfluidic fuel cell with flow-through Ni-supported-carbon- nanotube-coated sponge as porous electrode[J]. J Power Sources, 2017, 363: 61-69. |
| 120 | EISA T, PARK S G, MOHAMED H O, et al. Outstanding performance of direct urea/hydrogen peroxide fuel cell based on precious metal-free catalyst electrodes[J]. Energy, 2021, 228: 120584. |
| 121 | GUO F, CHENG K, YE K, et al. Preparation of nickel-cobalt nanowire arrays anode electro-catalyst and its application in direct urea/hydrogen peroxide fuel cell[J]. Electrochim Acta, 2016, 199: 290-296. |
| 122 | PEI C, CHEN S, ZHOU M, et al. Direct urea/H2O2 fuel cell with a hierarchical porous nanoglass anode for high-efficiency energy conversion[J]. ACS Appl Mater Interfaces, 2023, 15(20): 24319-24328. |
| [1] | Er-Gui LUO, Tao TANG, Yi WANG, Jun-Ming ZHANG, Yu-Hong CHANG, Tian-Jun HU, Jian-Feng JIA. Progress on Tuning the Geometric and Electronic Structure of Precious Metal Catalysts for Hydrogen Peroxide Production via Two-Electron Oxygen Reduction [J]. Chinese Journal of Applied Chemistry, 2023, 40(8): 1063-1076. |
| [2] | Yi-Ning DONG, He LI, Xue GONG, Ce HAN, Ping SONG, Wei-Lin XU. Research Progress of Non-Pt-Based Catalysts in Cathode Oxygen Reduction Reaction of Proton Exchange Membrane Fuel Cells [J]. Chinese Journal of Applied Chemistry, 2023, 40(8): 1077-1093. |
| [3] | Jin-Hui LIANG, Le-Cheng LIANG, Zhi-Ming CUI. Research Progress on Intermetallic Compound Electrocatalysts Applied in the Interconversion Between Hydrogen and Electric Power [J]. Chinese Journal of Applied Chemistry, 2023, 40(8): 1140-1157. |
| [4] | Wei WANG, Jia-Yuan LI. Research Progress of Cobalt Phosphide Heterojunction Catalysts for Electrolytic Hydrogen Evolution Reaction [J]. Chinese Journal of Applied Chemistry, 2023, 40(8): 1175-1186. |
| [5] | Cong-Hui MIAO, Dilinuer AILI, Zheng-Kun LIAO, Jing-Shan LU, Qing-Hua LIU, Jiang-Yuan LI, Nulahong AISHA. Preparation of Fly Ash-based ZSM-5 Zeolite Molecular Sieve and Hydrogen Storage Properties [J]. Chinese Journal of Applied Chemistry, 2023, 40(7): 995-1003. |
| [6] | Yi-Chen YU, Yu-Chen ZHANG, Yao-Yuan ZHANG, Qin WU, Da-Xin SHI, Kang-Cheng CHEN, Han-Sheng LI. Research Progress of Bulk Metal Oxides for Non-oxidative Propane Dehydrogenation [J]. Chinese Journal of Applied Chemistry, 2023, 40(6): 789-805. |
| [7] | Hai-Xiang XIU, Wan-Qiang LIU, Dong-Ming YIN, Yong CHENG, Chun-Li WANG, Li-Min WANG. Research Progress of AB2 Laves Phase Hydrogen Storage Alloys [J]. Chinese Journal of Applied Chemistry, 2023, 40(5): 640-652. |
| [8] | Feng ZHU, Xiao-Lian PENG, Wen-Bin ZHANG. Research Progress in the Effects of Proton Acceptor/Donor on Electrocatalytic Reactions [J]. Chinese Journal of Applied Chemistry, 2023, 40(5): 666-680. |
| [9] | Hui-Hui LI, Kai-Sheng YAO, Ya-Nan ZHAO, Li-Na FAN, Yu-Lin TIAN, Wei-Wei LU. Ionic Liquid-Modulated Synthesis of Pt-Pd Bimetallic Nanomaterials and Their Catalytic Performance for Ammonia Borane Hydrolysis to Generate Hydrogen [J]. Chinese Journal of Applied Chemistry, 2023, 40(4): 597-609. |
| [10] | Xiao-Ping ZHANG, Si-Yue ZHANG, Ming-Chang WANG, Yu-Tong ZHANG, Sha-Lin MIAO, Yu WANG, Wei SUN. A New Hyphenated Technique of in Situ Electrochemical NMR and the Application Progress [J]. Chinese Journal of Applied Chemistry, 2023, 40(3): 317-328. |
| [11] | Bing LI, Jun-Hui LIU, Ya-Kun SONG, Xiang LI, Xu-Ming GUO, Jian XIONG. Recent Advances in Application of Metal-Organic Frameworks for Hydrogen Generation by Catalytic Hydrolysis of Ammonia Borane [J]. Chinese Journal of Applied Chemistry, 2023, 40(3): 329-340. |
| [12] | Jin LIN, Fang-Zhu WANG, Ling-Ling LYU. Preparation of Pseudo-boehamite from Industrial Materials and Its Application in Selective Hydrogenation of Isophorone [J]. Chinese Journal of Applied Chemistry, 2023, 40(1): 79-90. |
| [13] | Wei-Qiang ZHANG, Chen WANG, Yu-Rong ZHAO, Dong WANG, Ji-Qian WANG, Hai XU. Research Progress of Regulation of Driving Forces in Short Peptide Supramolecular Self‑Assembly [J]. Chinese Journal of Applied Chemistry, 2022, 39(8): 1190-1201. |
| [14] | Xian WANG, Xiao-Long YANG, Rong-Peng MA, Chang-Peng LIU, Jun-Jie GE, Wei XING. Atomic Dispersion Ir‑N‑C Catalysts for Anode Anti‑poisoning Electrolysis in Fuel Cell [J]. Chinese Journal of Applied Chemistry, 2022, 39(8): 1202-1208. |
| [15] | Wei-Min DU, Xin LIU, Lin ZHU, Jia-Min FU, Wen-Shan GUO, Xiao-Qing YANG, Pei-Shuo SHUANG. Facile Synthesis and High⁃Efficiency Electrocatalytic Oxygen Evolution Performance of Ternary Nickel⁃Based Chalcogenide Nanorod Arrays [J]. Chinese Journal of Applied Chemistry, 2022, 39(8): 1252-1261. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||