Chinese Journal of Applied Chemistry ›› 2023, Vol. 40 ›› Issue (8): 1109-1125.DOI: 10.19894/j.issn.1000-0518.230126
• Review • Previous Articles Next Articles
Research Progress on Superhydrophilic/Superaerophobic Electrocatalysts for Water Splitting
Cui-Ying TAN1,2, Wei-Chao DING1, Ting-Ting MA1, Yao XIAO1( ), Jian LIU2(
), Jian LIU2( )
)
- 1.College of Materials Science and Engineering,Qingdao University of Science and Technology,Qingdao 266042,China
2.Qingdao Institute of Bioenergy and Bioprocess Technology,Chinese Academy of Science,Qingdao 266101,China
-
Received:2023-04-29Accepted:2023-07-06Published:2023-08-01Online:2023-08-24 -
Contact:Yao XIAO,Jian LIU -
About author:liujian@qibebt.ac.cn
xiaoyao@qust.edu.cn
-
Supported by:the National Natural Science Foundation of China(22109081);the National Undergraduate Innovation and Entrepreneurship Training Program(202210426071)
CLC Number:
Cite this article
Cui-Ying TAN, Wei-Chao DING, Ting-Ting MA, Yao XIAO, Jian LIU. Research Progress on Superhydrophilic/Superaerophobic Electrocatalysts for Water Splitting[J]. Chinese Journal of Applied Chemistry, 2023, 40(8): 1109-1125.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: http://yyhx.ciac.jl.cn/EN/10.19894/j.issn.1000-0518.230126
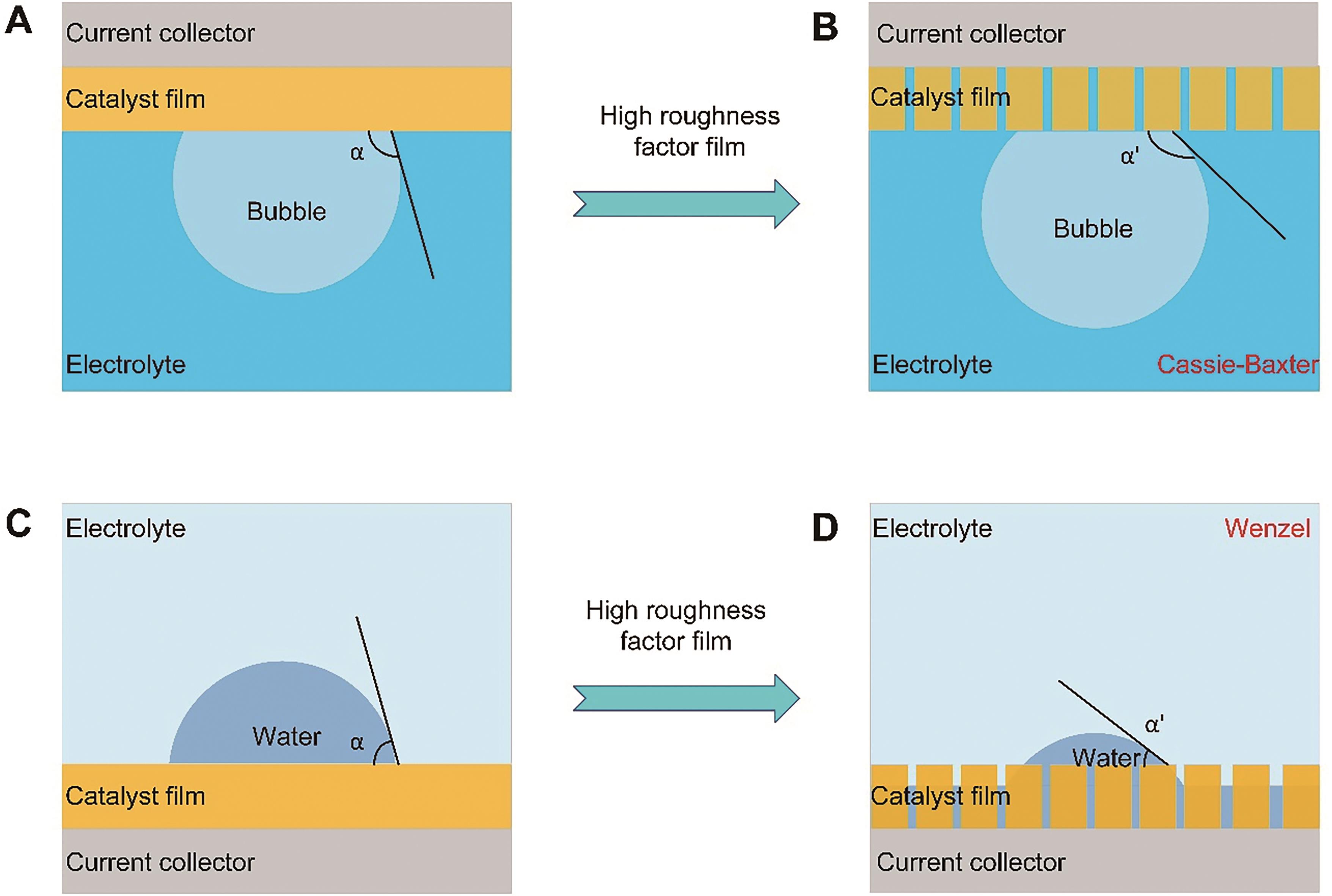
Fig.1 Schematic illustration of how the surface roughness affecting the bubble contact angle: (A) flat aerophobic surface, (B) rough superaerophobic surface, (C) flat hydrophilic surface, (D) rough superhydrophilic surface
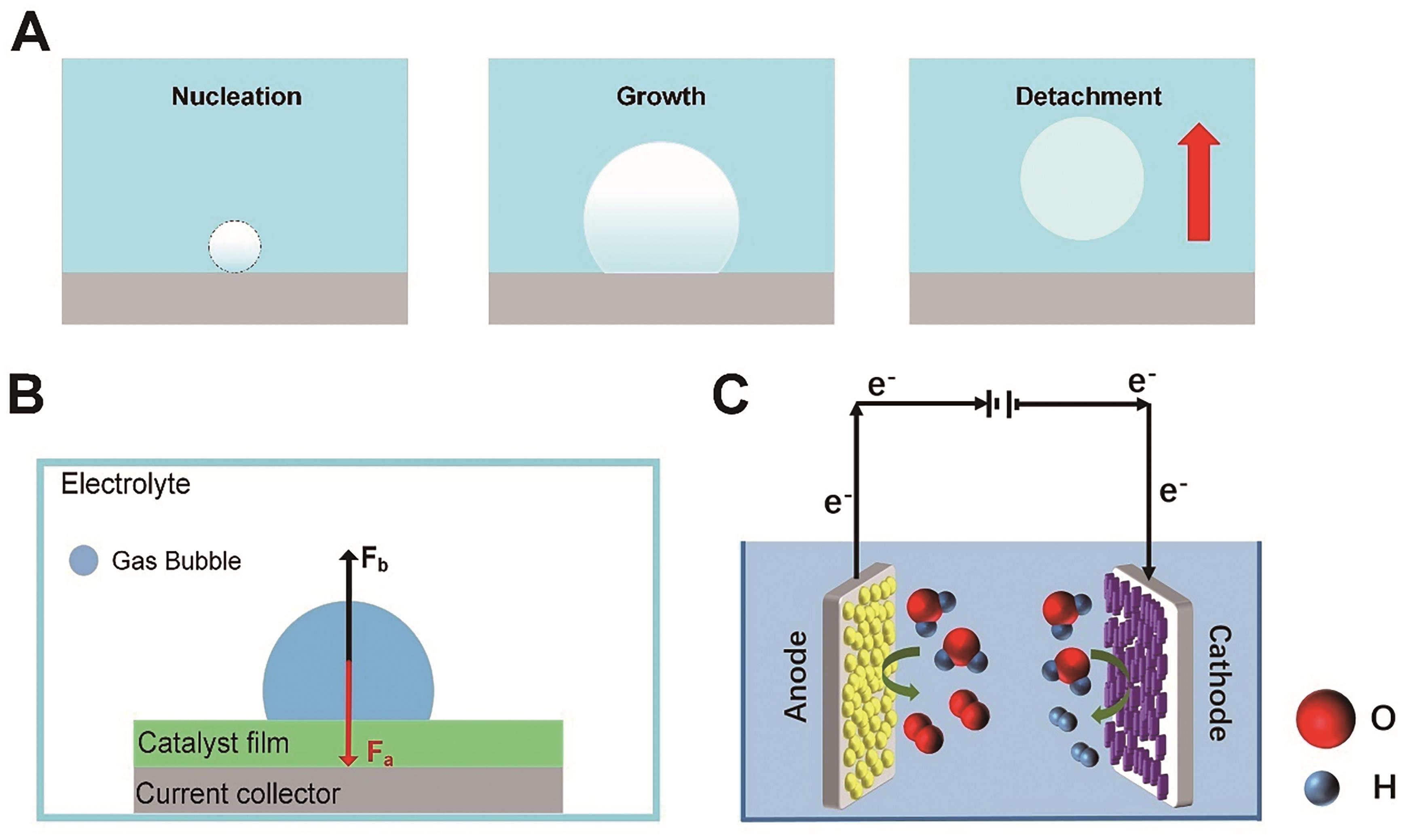
Fig.2 (A) Bubble evolution process; (B) Stress analysis of one single bubble on the electrode surface; (C) Illustration of electrodes for water splitting
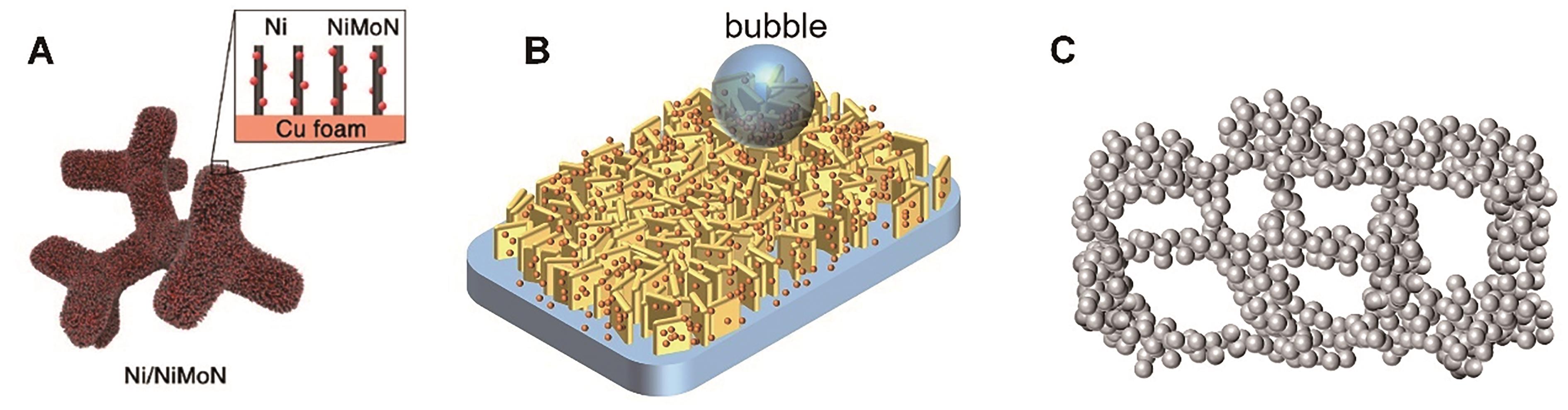
Fig.3 Nanocomposite catalyst: (A) Ni/NiMoN nanowire array[99]; (B) Nanoparticles distributed on the nanoparticle; (C) Interconnected nanoparticle structures
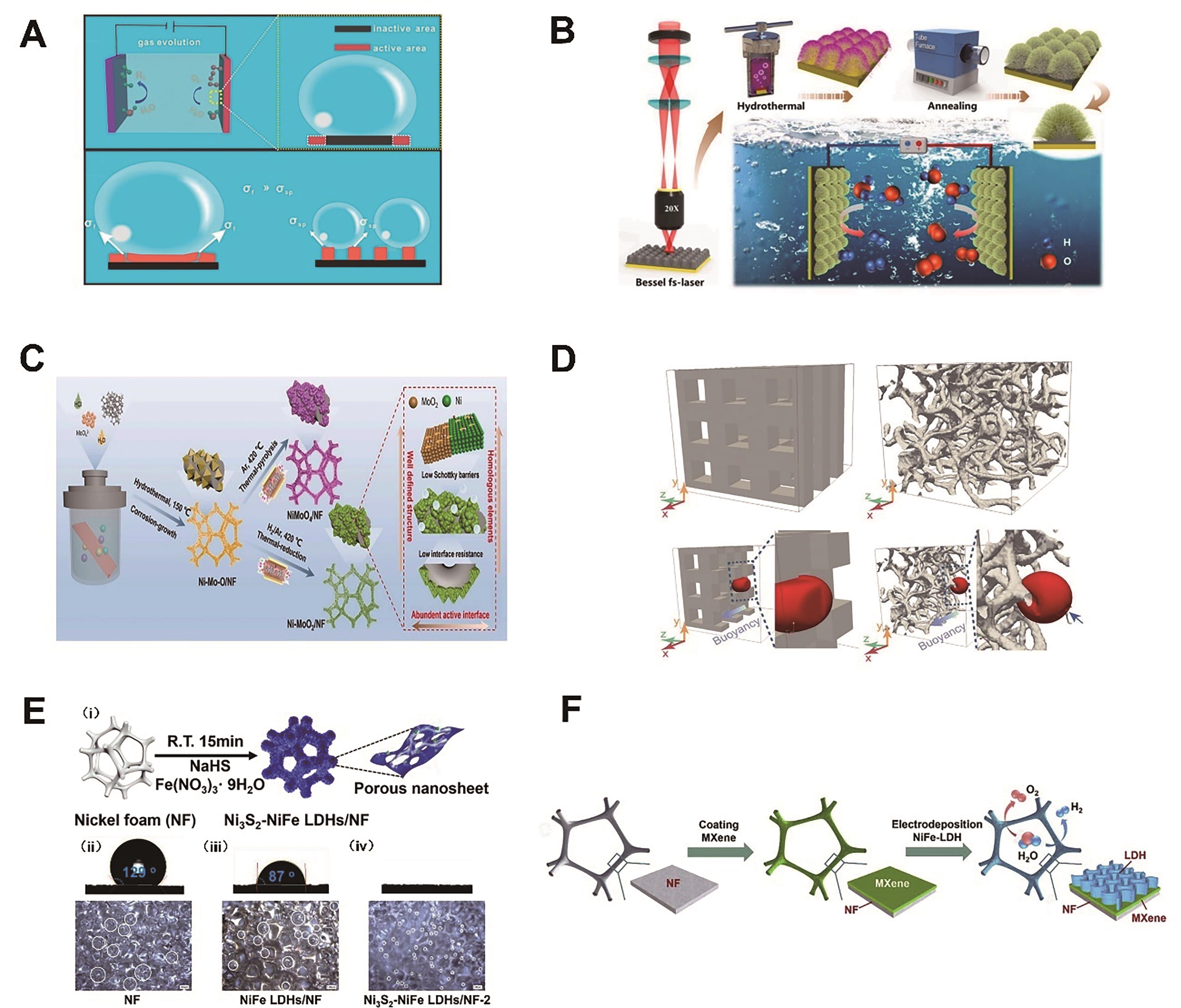
Fig.4 (A) Schematic illustration of the growth of gas bubbles on a flat film electrode and SP assemblies[66]; (B) The preparation process of the self-supported electrocatalysts with hierarchical chestnut burr-like structures and schematic diagram for overall water splitting application[69]; (C) Schematic illustration of the fabrication of Ni-MoO2/NF and NiMoO4/NF[70]; (D) Simulation frames showing bubble shape during transport in 3DPNi and NF[71]; (E) (i) Schemic of the synthesis of Ni3S2-NiFe LDHs/NF and surface wettability and bubble releasing behavior of the (ii) NF, (iii) NiFe LDHs/NF and (iv) Ni3S2-NiFe LDHs/NF-2[77]; (F) Schematic illustration of the fabrication of hierarchically structured 3D electrocatalytic electrode by growing me-soporous network of NiFe-LDH nanosheets onto macroporous MXene/NF frame[79]
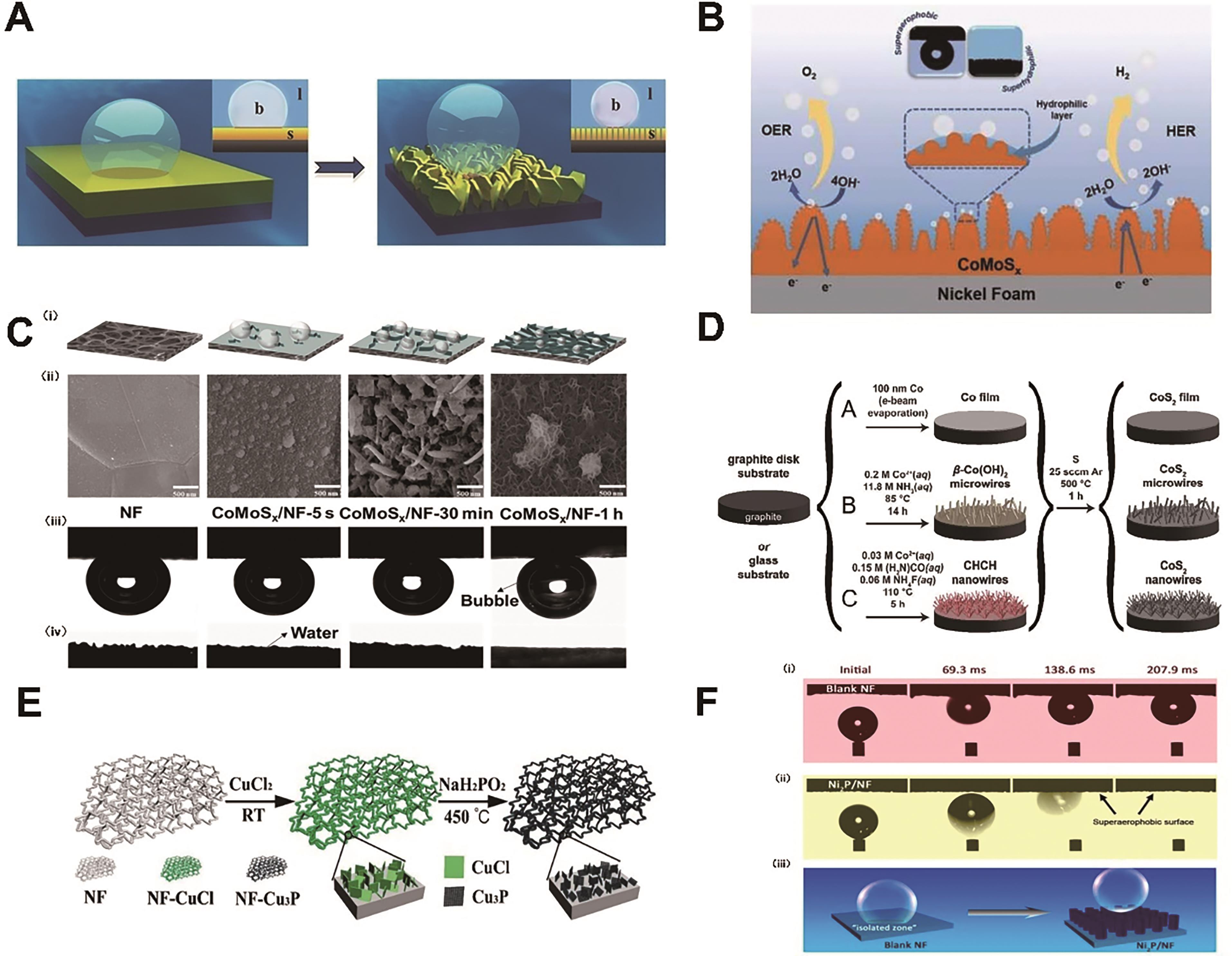
Fig.5 (A) Schematic illustration of adhesion behaviors of gas bubbles on flat film (left) and nanostructured film (right)?[9]; (B) Design of the superhydrophilic/superaerophobic CoMoS x /NF electrocatalysts for overall water splitting[84]; (C) (i)Schematic illustration of CoMoS x supported on the NF with three distinct geometries, (ii) SEM images of CoMoS x /NF at different reaction times, (iii) air-bubble contact angles under water and (iv) static water-droplet contact angles[83]; (D) Schematic depictions of the preparation of a cobalt pyrite (CoS2) film, microwire array, or nanowire array on a graphite disk (or glass) substrate[15]; (E) Schematic illustration of the preparation of Cu3P microsheets[92]; (F) Schematic illustration of the synthetic process for Ni2P nanoarrays[93]
| Catalyst | ηa (HER/OER)/mV | Bubble contact angle/(°) | Stability/h | Ref. |
|---|---|---|---|---|
| Pt nanoarray | -/- | 161.3±3.4 | 36(-0.5 V) | [ |
| Pt SP5 | η14.3=15 mV/- | — | 11(30 mA/cm2) | [ |
| Ni/NiO@MoO3-x | η10=7 mV/- | 149.4 | 40(100 mA/cm2) | [ |
| Ni-MoO2/NF | η20=1.53 V/- | — | 120(1.53 V) | [ |
| CoO/Co3O4 | η10=105 mV/η10=235 mV | — | 72 | [ |
| C-Ni1-x O/3DPNi | η1000=245 mV/- | — | 16(2.2 V) | [ |
| Ru/Co(OH)2 | η10=35 mV/- | 141 | 14(500 mA/cm2) | [ |
| P-Ni(OH)2/NiMoO4 | η10=60 mV/- | 153.8 | 30 | [ |
| NiFe-LDHs/NF | -/η1000=303 mV | — | 240 | [ |
| Pt@S-NiFe LDHs | η10=60 mV/- | 163.6 | 200 (100 mA/cm2) | [ |
| 3D NiFe/MXene | η500=205 mV/η500=30 200 mV | — | 280(10 mA/cm)2 | [ |
| CoS2 | η10=145 mV/- | — | 40 (10 mA) | [ |
| MoS2 | — | 153.6±2.4 | — | [ |
| CoMoS x /NF | η10=89 mV/- | — | 100 (500 mA/cm2) | [ |
| MoS2/Mo2C | η1000=220 mV/- | — | 24 | [ |
| FeCoNi-HNTAs | η10=58 mV/η10=184 mV | 171.0 | 100(50 mA/cm2) | [ |
| FeS/IF | η1000=336 mV/- | 151.7 | 30 | [ |
| Fe-Ni-P-S | — | 142.3 | 300(2 500 mA/cm2) | [ |
| CuMo6S8/Cu | η2500=321 mV/- | — | 100(2 500 mA/cm2) | [ |
| Cu3P | η10=130 mV/η10=290 mV | 155.7 | 24 | [ |
| Ni2P/NF | η1000=306 mV/- | — | 10(25000 mA/cm2) | [ |
| Ni2P NV/CF | η10=1.48 V/- | — | 50 | [ |
| NF@Co x P | η10=185 mV/- | — | 12 (800 mA/cm2) | [ |
Table 1 The performance of electrolytic water catalysts with superhydrophilic/superaerophobic interface structure in 2014-2023
| Catalyst | ηa (HER/OER)/mV | Bubble contact angle/(°) | Stability/h | Ref. |
|---|---|---|---|---|
| Pt nanoarray | -/- | 161.3±3.4 | 36(-0.5 V) | [ |
| Pt SP5 | η14.3=15 mV/- | — | 11(30 mA/cm2) | [ |
| Ni/NiO@MoO3-x | η10=7 mV/- | 149.4 | 40(100 mA/cm2) | [ |
| Ni-MoO2/NF | η20=1.53 V/- | — | 120(1.53 V) | [ |
| CoO/Co3O4 | η10=105 mV/η10=235 mV | — | 72 | [ |
| C-Ni1-x O/3DPNi | η1000=245 mV/- | — | 16(2.2 V) | [ |
| Ru/Co(OH)2 | η10=35 mV/- | 141 | 14(500 mA/cm2) | [ |
| P-Ni(OH)2/NiMoO4 | η10=60 mV/- | 153.8 | 30 | [ |
| NiFe-LDHs/NF | -/η1000=303 mV | — | 240 | [ |
| Pt@S-NiFe LDHs | η10=60 mV/- | 163.6 | 200 (100 mA/cm2) | [ |
| 3D NiFe/MXene | η500=205 mV/η500=30 200 mV | — | 280(10 mA/cm)2 | [ |
| CoS2 | η10=145 mV/- | — | 40 (10 mA) | [ |
| MoS2 | — | 153.6±2.4 | — | [ |
| CoMoS x /NF | η10=89 mV/- | — | 100 (500 mA/cm2) | [ |
| MoS2/Mo2C | η1000=220 mV/- | — | 24 | [ |
| FeCoNi-HNTAs | η10=58 mV/η10=184 mV | 171.0 | 100(50 mA/cm2) | [ |
| FeS/IF | η1000=336 mV/- | 151.7 | 30 | [ |
| Fe-Ni-P-S | — | 142.3 | 300(2 500 mA/cm2) | [ |
| CuMo6S8/Cu | η2500=321 mV/- | — | 100(2 500 mA/cm2) | [ |
| Cu3P | η10=130 mV/η10=290 mV | 155.7 | 24 | [ |
| Ni2P/NF | η1000=306 mV/- | — | 10(25000 mA/cm2) | [ |
| Ni2P NV/CF | η10=1.48 V/- | — | 50 | [ |
| NF@Co x P | η10=185 mV/- | — | 12 (800 mA/cm2) | [ |
| 1 | YAN D, LI Y, HUO J, et al. Defect chemistry of nonprecious-metal electrocatalysts for oxygen reactions[J]. Adv Mater, 2017, 29(48): 1606459. |
| 2 | SCHLAPBACH L. Hydrogen-fuelled vehicles[J]. Nature, 2009, 460(7257): 809-811. |
| 3 | MORALES-GUIO C G, STERN L A, HU X. Nanostructured hydrotreating catalysts for electrochemical hydrogen evolution[J]. Chem Soc Rev, 2014, 43(18): 6555-6569. |
| 4 | GAO N, CHENG M, QUAN C, et al. Syngas production via combined dry and steam reforming of methane over Ni-Ce/ZSM-5 catalyst[J]. Fuel, 2020, 273: 117702. |
| 5 | CHI J, YU H. Water electrolysis based on renewable energy for hydrogen production[J]. Chin J Catal, 2018, 39(3): 390-394. |
| 6 | WANG J, YUE X, YANG Y, et al. Earth-abundant transition-metal-based bifunctional catalysts for overall electrochemical water splitting: a review[J]. J Alloys Compd, 2020, 819: 153346. |
| 7 | HU S, GE S, LIU H, et al. Low-dimensional electrocatalysts for acidic oxygen evolution: intrinsic activity, high current density operation, and long‐term stability[J]. Adv Funct Mater, 2022, 32(23): 2201726. |
| 8 | WU Z, LU X, ZANG S, et al. Non‐noble‐metal‐based electrocatalysts toward the oxygen evolution reaction[J]. Adv Funct Mater, 2020, 30(15): 1910274. |
| 9 | LU Z, ZHU W, YU X, et al. Ultrahigh hydrogen evolution performance of under-water “superaerophobic” MoS2 nanostructured electrodes[J]. Adv Mater, 2014, 26(17): 2683-2687, 2615. |
| 10 | CHU S, MAJUMDAR A. Opportunities and challenges for a sustainable energy future[J]. Nature, 2012, 488(7411): 294-303. |
| 11 | JUNG W B, YUN G T, KIM Y, et al. Relationship between hydrogen evolution and wettability for multiscale hierarchical wrinkles[J]. ACS Appl Mater Interfaces, 2019, 11(7): 7546-7552. |
| 12 | ZHANG C, XU Z, HAN N, et al. Superaerophilic/superaerophobic cooperative electrode for efficient hydrogen evolution reaction via enhanced mass transfer[J]. Sci Adv, 2023, 9(3): eadd6978. |
| 13 | MILLER H A, BOUZEK K, HNAT J, et al. Green hydrogen from anion exchange membrane water electrolysis: a review of recent developments in critical materials and operating conditions[J]. Sustainable Energy Fuels, 2020, 4(5): 2114-2133. |
| 14 | LUO Y, ZHANG Z, CHHOWALLA M, et al. Recent advances in design of electrocatalysts for high-current-density water splitting[J]. Adv Mater, 2022, 34(16): e2108133. |
| 15 | FABER M S, DZIEDZIC R, LUKOWSKI M A, et al. High-performance electrocatalysis using metallic cobalt pyrite (CoS2) micro- and nanostructures[J]. J Am Chem Soc, 2014, 136(28): 10053-10061. |
| 16 | QU M, MA L, WANG J, et al. Multifunctional superwettable material with smart pH responsiveness for efficient and controllable oil/water separation and emulsified wastewater purification[J]. ACS Appl Mater Interfaces, 2019, 11(27): 24668-24682. |
| 17 | YU S Q, LING Y H, WANG R G, et al. Constructing superhydrophobic WO3@TiO2 nanoflake surface beyond amorphous alloy against electrochemical corrosion on iron steel[J]. Appl Surf Sci, 2018, 436: 527-535. |
| 18 | PAN R, ZHANG H, ZHONG M. Triple-scale superhydrophobic surface with excellent anti-icing and icephobic performance via ultrafast laser hybrid fabrication[J]. ACS Appl Mater Interfaces, 2021, 13(1): 1743-1753. |
| 19 | ZHAO Q, AN J, WANG S, et al. Superhydrophobic air-breathing cathode for efficient hydrogen peroxide generation through two-electron pathway oxygen reduction reaction[J]. ACS Appl Mater Interfaces, 2019, 11(38): 35410-35419. |
| 20 | SU B, TIAN Y, JIANG L. Bioinspired Interfaces with superwettability: from materials to chemistry[J]. J Am Chem Soc, 2016, 138(6): 1727-1748. |
| 21 | LI Y, ZHANG H, XU T, et al. Under-water superaerophobic pine-shaped Pt nanoarray electrode for ultrahigh-performance hydrogen evolution[J]. Adv Funct Mater, 2015, 25(11): 1737-1744. |
| 22 | AKBAR K, HUSSAIN S, TRUONG L, et al. Induced superaerophobicity onto a non-superaerophobic catalytic surface for enhanced hydrogen evolution reaction[J]. ACS Appl Mater Interfaces, 2017, 9(50): 43674-43680. |
| 23 | ZHANG J, ZHANG Q, FENG X. Support and interface effects in water-splitting electrocatalysts[J]. Adv Mater, 2019, 31(31): e1808167. |
| 24 | THOMAS Y. An essay on the cohesion of fluids[J]. Proc R Soc, 1805, 95: 65-87. |
| 25 | LIU M, WANG S, JIANG L. Nature-inspired superwettability systems[J]. Nat Rev Mater, 2017, 2(7): 17036. |
| 26 | WENZEL P, ROBERT N. Surface roughness and contact angle[J]. J Phys Chem C, 1949, 53(9): 1466-1467. |
| 27 | CASSIE A B D, BAXTER S. Wettability of porous surfaces[J]. Trans Faraday Soc, 1944, 40: 546-551. |
| 28 | WENZEL P, ROBERT N. Resistance of solid surfaces to wetting by water[J]. Trans Faraday Soc, 1936, 28(8): 988-994. |
| 29 | WANG S, JIANG L. Definition of superhydrophobic states [J]. Adv Mater, 2007, 19(21): 3423-3424. |
| 30 | FENG L, ZHANG Z, MAI Z, et al. A super-hydrophobic and super-oleophilic coating mesh film for the separation of oil and water[J]. Angew Chem Int Ed Engl, 2004, 43(15): 2012-2014. |
| 31 | WANG J, ZHENG Y, NIE F Q, et al. Air bubble bursting effect of lotus leaf[J]. Langmuir, 2009, 25(24): 14129-14134. |
| 32 | DE MALEPRADE H, CLANET C, QUERE D. Spreading of bubbles after contacting the lower side of an aerophilic slide immersed in water[J]. Phys Rev Lett, 2016, 117(9): 094501. |
| 33 | LI Z, HU R, SONG J, et al. Gas-liquid-solid triphase interfacial chemical reactions associated with gas wettability[J]. Adv Mater Interfaces, 2021, 8(6): 2001636. |
| 34 | ZOU X, ZHANG Y. Noble metal-free hydrogen evolution catalysts for water splitting[J]. Chem Soc Rev, 2015, 44(15): 5148-5180. |
| 35 | GUO Y, PARK T, YI J W, et al. Nanoarchitectonics for transition-metal-sulfide-based electrocatalysts for water splitting[J]. Adv Mater, 2019, 31(17): e1807134. |
| 36 | MAN I C, SU H Y, CALLE‐VALLEJO F, et al. Universality in oxygen evolution electrocatalysis on oxide surfaces[J]. ChemCatChem, 2011, 3(7): 1159-1165. |
| 37 | FABBRI E, SCHMIDT T J. Oxygen evolution reaction-the enigma in water electrolysis[J]. ACS Catal, 2018, 8(10): 9765-9774. |
| 38 | HE Y, CUI Y, SHANG W, et al. Insight into the bubble-induced overpotential towards high-rate charging of Zn-air batteries[J]. Chem Eng J, 2022, 448: 137782. |
| 39 | YANG L, LI H, YU Y, et al. Assembled 3D MOF on 2D nanosheets for self-boosting catalytic synthesis of N-doped carbon nanotube encapsulated metallic Co electrocatalysts for overall water splitting[J]. Appl Catal B: Environ, 2020, 271: 118939. |
| 40 | EIGELDINGER J, VOGT H. The bubble coverage of gas-evolving electrodes in a flowing electrolyte[J]. Electrochim Acta, 2000, 45(27): 4449-4456. |
| 41 | ZHANG L, XIONG K, CHEN S, et al. In situ growth of ruthenium oxide-nickel oxide nanorod arrays on nickel foam as a binder-free integrated cathode for hydrogen evolution[J]. J Power Sources, 2015, 274: 114-120. |
| 42 | MANI-LATA C, HUSSAKAN C, PANOMSUWAN G. Fast and facile synthesis of Pt nanoparticles supported on ketjen black by solution plasma sputtering as bifunctional HER/ORR catalysts[J]. J Compos Sci, 2020, 4(3): 121. |
| 43 | DERJAGUIN B V, MULLER V M, TOPOROV Y P. Effect of contact deformations on the adhesion of particles[J]. J Colloid Interface Sci, 1975, 53(2): 314-326. |
| 44 | WANG M, WANG Z, GUO Z. Water electrolysis enhanced by super gravity field for hydrogen production[J]. Int J Hydrogen Energy, 2010, 35(8): 3198-3205. |
| 45 | LI S, WANG C, CHEN C. Water electrolysis in the presence of an ultrasonic field[J]. Electrochim Acta, 2009, 54(15): 3877-3883. |
| 46 | WANG K, LIU X, PEI P, et al. Guiding bubble motion of rechargeable zinc-air battery with electromagnetic force[J]. Chem Eng J, 2018, 352: 182-187. |
| 47 | DARBAND G B, ALIOFKHAZRAEI M, SHANMUGAM S. Recent advances in methods and technologies for enhancing bubble detachment during electrochemical water splitting[J]. Renew Sustainable Energy Rev, 2019, 114: 109300. |
| 48 | SWIEGERS G F, TERRETT R N L, TSEKOURAS G, et al. The prospects of developing a highly energy-efficient water electrolyser by eliminating or mitigating bubble effects[J]. Sustainable Energy Fuels, 2021, 5(5): 1280-1310. |
| 49 | KANG E, LEE D H, KIM C B, et al. A hemispherical microfluidic channel for the trapping and passive dissipation of microbubbles[J]. J Micromech Microeng, 2010, 20(4): 045009. |
| 50 | BAE M, KANG Y, LEE D W, et al. Superaerophobic polyethyleneimine hydrogels for improving electrochemical hydrogen production by promoting bubble detachment[J]. Adv Energy Mater, 2022, 12(29): 2201452. |
| 51 | ZHANG J, DONG F, WANG C, et al. Integrated bundle electrode with wettability-gradient copper cones inducing continuous generation, directional transport, and efficient collection of H2 bubbles[J]. ACS Appl Mater Interfaces, 2021, 13(27): 32435-32441. |
| 52 | KIM M, ANJUM M A R, CHOI M, et al. Covalent 0D-2D heterostructuring of Co9S8-MoS2 for enhanced hydrogen evolution in all pH electrolytes[J]. Adv Funct Mater, 2020, 30(40): 2002536. |
| 53 | ANGULO A, VAN DER LINDE P, GARDENIERS H, et al. Influence of bubbles on the energy conversion efficiency of electrochemical reactors[J]. Joule, 2020, 4(3): 555-579. |
| 54 | ANDAVEH R, BARATI DARBAND G, MALEKI M, et al. Superaerophobic/superhydrophilic surfaces as advanced electrocatalysts for the hydrogen evolution reaction: a comprehensive review[J]. J Mater Chem A, 2022, 10(10): 5147-5173. |
| 55 | LIU W, HU E, JIANG H, et al. A highly active and stable hydrogen evolution catalyst based on pyrite-structured cobalt phosphosulfide[J]. Nat Commun, 2016, 7: 10771. |
| 56 | LIU R, GU S, DU H, et al. Controlled synthesis of FeP nanorod arrays as highly efficient hydrogen evolution cathode[J]. J Mater Chem A, 2014, 2(41): 17263-17267. |
| 57 | XIE J, QU H, XIN J, et al. Defect-rich MoS2 nanowall catalyst for efficient hydrogen evolution reaction[J]. Nano Res, 2017, 10(4): 1178-1188. |
| 58 | LIU Y, ZHOU X, DING T, et al. 3D architecture constructed via the confined growth of MoS2 nanosheets in nanoporous carbon derived from metal-organic frameworks for efficient hydrogen production[J]. Nanoscale, 2015, 7(43): 18004-18009. |
| 59 | XU W, LU Z, SUN X, et al. Superwetting electrodes for gas-involving electrocatalysis[J]. Acc Chem Res, 2018, 51(7): 1590-1598. |
| 60 | JIA Y, ZHANG L, GAO G, et al. A heterostructure coupling of exfoliated Ni-Fe hydroxide nanosheet and defective graphene as a bifunctional electrocatalyst for overall water splitting[J]. Adv Mater, 2017, 29(17): 201700017. |
| 61 | TANG T, JIANG W J, NIU S, et al. Electronic and morphological dual modulation of cobalt carbonate hydroxides by Mn doping toward highly efficient and stable bifunctional electrocatalysts for overall water splitting[J]. J Am Chem Soc, 2017, 139(24): 8320-8328. |
| 62 | LI Y, YIN K, WANG L, et al. Engineering MoS2 nanomesh with holes and lattice defects for highly active hydrogen evolution reaction[J]. Appl Catal B: Environ, 2018, 239: 537-544. |
| 63 | LING W, LU G, NG T. Increased stability and size of a bubble on a superhydrophobic surface[J]. Langmuir, 2011, 27(7): 3233-3237. |
| 64 | ZHANG P, WANG S, WANG S, et al. Superwetting surfaces under different media: effects of surface topography on wettability[J]. Small, 2015, 11(16): 1939-1946. |
| 65 | TAN Y, XIE R, ZHAO S, et al. Facile fabrication of robust hydrogen evolution electrodes under high current densities via Pt@Cu interactions[J]. Adv Funct Mater, 2021, 31(45): 2105579. |
| 66 | SONG Q, XUE Z, LIU C, et al. General strategy to optimize gas evolution reaction via assembled striped-pattern superlattices[J]. J Am Chem Soc, 2020, 142(4): 1857-1863. |
| 67 | ZHANG J, LIANG J, MEI B, et al. Synthesis of Ni/NiO@MoO3- x composite nanoarrays for high current density hydrogen evolution reaction[J]. Adv Energy Mater, 2022, 12(22): 2200001. |
| 68 | MA T Y, DAI S, JARONIEC M, et al. Metal-organic framework derived hybrid Co3O4-carbon porous nanowire arrays as reversible oxygen evolution electrodes[J]. J Am Chem Soc, 2014, 136(39): 13925-13931. |
| 69 | LIU H, LI Z, HU J, et al. Self-supported cobalt oxide electrocatalysts with hierarchical chestnut burr-like nanostructure for efficient overall water splitting[J]. Chem Eng J, 2022, 435: 134995. |
| 70 | REN J, WU X, LIU T, et al. Interfacing nickel with molybdenum oxides as monolithic catalyst to accelerate alkaline hydrogen electrocatalysis with robust stability[J]. Appl Catal B: Environ, 2022, 317: 121786. |
| 71 | KOU T, WANG S, SHI R, et al. Periodic porous 3D electrodes mitigate gas bubble traffic during alkaline water electrolysis at high current densities[J]. Adv Energy Mater, 2020, 10(46): 2002955. |
| 72 | GAO X, ZHANG H, LI Q, et al. Hierarchical NiCo2O4 hollow microcuboids as bifunctional electrocatalysts for overall water-splitting[J]. Angew Chem Int Ed Engl, 2016, 55(21): 6290-6294. |
| 73 | GUAN C, LIU X, REN W, et al. Rational design of metal-organic framework derived hollow NiCo2O4 arrays for flexible supercapacitor and electrocatalysis[J]. Adv Energy Mater, 2017, 7(12): 1602391. |
| 74 | XIAO C, LI Y, LU X, et al. Bifunctional porous NiFe/NiCo2O4/Ni foam electrodes with triple hierarchy and double synergies for efficient whole cell water splitting[J]. Adv Funct Mater, 2016, 26(20): 3515-3523. |
| 75 | SHEN J, LI B, ZHENG Y, et al. Engineering the composition and structure of superaerophobic nanosheet array for efficient hydrogen evolution[J]. Chem Eng J, 2022, 433: 133517. |
| 76 | XI W, YAN G, TAN H, et al. Superaerophobic P-doped Ni(OH)2/NiMoO4 hierarchical nanosheet arrays grown on Ni foam for electrocatalytic overall water splitting[J]. Dalton Trans, 2018, 47(26): 8787-8793. |
| 77 | WU S, LIU S, TAN X, et al. Ni3S2-embedded NiFe LDH porous nanosheets with abundant heterointerfaces for high-current water electrolysis[J]. Chem Eng J, 2022, 442: 136105. |
| 78 | LEI H, WAN Q, TAN S, et al. Pt quantum dots modified S-NiFe layered double hydroxide for high-current-density alkaline water splitting at industrial temperature[J]. Adv Mater, 2023: e2208209. |
| 79 | YU M, WANG Z, LIU J, et al. A hierarchically porous and hydrophilic 3D nickel-iron/MXene electrode for accelerating oxygen and hydrogen evolution at high current densities[J]. Nano Energy, 2019, 63: 103880. |
| 80 | YE Y, ZHANG N, LIU X. Amorphous NiFe(oxy)hydroxide nanosheet integrated partially exfoliated graphite foil for high efficiency oxygen evolution reaction[J]. J Mater Chem A, 2017, 5(46): 24208-24216. |
| 81 | LUO Y, TANG L, KHAN U, et al. Morphology and surface chemistry engineering toward pH-universal catalysts for hydrogen evolution at high current density[J]. Nat Commun, 2019, 10(1): 269. |
| 82 | LIU H, XIE R, LUO Y, et al. Dual interfacial engineering of a chevrel phase electrode material for stable hydrogen evolution at 2500 mA·cm-2[J]. Nat Commun, 2022, 13(1): 6382. |
| 83 | MU H, LIN G, ZHANG Y, et al. Rational engineering of superaerophobic CoMoSx electrocatalysts for overall water splitting[J]. Colloids Surf A Physicochem Eng Asp, 2021, 623: 126734. |
| 84 | SHAN X, LIU J, MU H, et al. An engineered superhydrophilic/superaerophobic electrocatalyst composed of the supported CoMoSx chalcogel for overall water splitting[J]. Angew Chem Int Ed Engl, 2020, 59(4): 1659-1665. |
| 85 | WAN L, XU Z, WANG P, et al. Dual regulation both intrinsic activity and mass transport for self-supported electrodes using in anion exchange membrane water electrolysis[J]. Chem Eng J, 2022, 431: 133942. |
| 86 | LI H, CHEN S, ZHANG Y, et al. Systematic design of superaerophobic nanotube-array electrode comprised of transition-metal sulfides for overall water splitting[J]. Nat Commun, 2018, 9(1): 2452. |
| 87 | LONG X, LI G, WANG Z, et al. Metallic iron-nickel sulfide ultrathin nanosheets as a highly active electrocatalyst for hydrogen evolution reaction in acidic media[J]. J Am Chem Soc, 2015, 137(37): 11900-11903. |
| 88 | YANG Y, YAO H, YU Z, et al. Hierarchical nanoassembly of MoS2/Co9S8/Ni3S2/Ni as a highly efficient electrocatalyst for overall water splitting in a wide pH range[J]. J Am Chem Soc, 2019, 141(26): 10417-10430. |
| 89 | YU Q, ZHANG Z, QIU S, et al. A Ta-TaS2 monolith catalyst with robust and metallic interface for superior hydrogen evolution[J]. Nat Commun, 2021, 12(1): 6051. |
| 90 | ZHANG C, LUO Y, TAN J, et al. High-throughput production of cheap mineral-based two-dimensional electrocatalysts for high-current-density hydrogen evolution[J]. Nat Commun, 2020, 11(1): 3724. |
| 91 | ZOU X, WU Y, LIU Y, et al. In situ generation of bifunctional, efficient Fe-based catalysts from mackinawite iron sulfide for water splitting[J]. Chem, 2018, 4(5): 1139-1152. |
| 92 | HAO J, YANG W, HUANG Z, et al. Superhydrophilic and superaerophobic copper phosphide microsheets for efficient electrocatalytic hydrogen and oxygen evolution[J]. Adv Mater Interfaces, 2016, 3(16): 1600236. |
| 93 | YU X, YU Z, ZHANG X, et al. “Superaerophobic” nickel phosphide nanoarray catalyst for efficient hydrogen evolution at ultrahigh current densities[J]. J Am Chem Soc, 2019, 141(18): 7537-7543. |
| 94 | YIN Y, TAN Y, WEI Q, et al. Nanovilli electrode boosts hydrogen evolution: a surface with superaerophobicity and superhydrophilicity[J]. Nano Res, 2020, 14(4): 961-968. |
| 95 | CHEN X, SHENG L, LI S, et al. Facile syntheses and in-situ study on electrocatalytic properties of superaerophobic CoxP-nanoarray in hydrogen evolution reaction[J]. Chem Eng J, 2021, 426: 131029. |
| 96 | LI Y, ZHANG H, JIANG M, et al. 3D self-supported Fe-doped Ni2P nanosheet arrays as bifunctional catalysts for overall water splitting [J]. Adv Funct Mater, 2017, 27(37): 1702513. |
| 97 | YU X, WANG M, GONG X, et al. Self-supporting porous CoP-based films with phase-separation structure for ultrastable overall water electrolysis at large current density[J]. Adv Energy Mater, 2018, 8(34): 1802445. |
| 98 | ZHANG X, LI J, YANG Y, et al. Co3O4/Fe0.33Co0.66P interface nanowire for enhancing water oxidation catalysis at high current density[J]. Adv Mater, 2018, 30(45): e1803551. |
| 99 | SHANG L, ZHAO Y, KONG X Y, et al. Underwater superaerophobic Ni nanoparticle-decorated nickel-molybdenum nitride nanowire arrays for hydrogen evolution in neutral media[J]. Nano Energy, 2020, 78: 105375. |
| 100 | SHI Y, ZHANG B. Recent advances in transition metal phosphide nanomaterials: synthesis and applications in hydrogen evolution reaction[J]. Chem Soc Rev, 2016, 45(6): 1529-1541. |
| 101 | NøRSKOV J K, BLIGAARD T, LOGADOTTIR A, et al. Trends in the exchange current for hydrogen evolution[J]. J Electrochem Soc, 2005, 152(3): J23-J26. |
| 102 | MAHMOOD J, LI F, JUNG S M, et al. An efficient and pH-universal ruthenium-based catalyst for the hydrogen evolution reaction[J]. Nat Nanotechnol, 2017, 12(5): 441-446. |
| 103 | YAO H, WANG X, LI K, et al. Strong electronic coupling between ruthenium single atoms and ultrafine nanoclusters enables economical and effective hydrogen production[J]. Appl Catal B: Environ, 2022, 312: 121378. |
| 104 | ZHAO Q, YAN Z, CHEN C, et al. Spinels: controlled preparation, oxygen reduction/evolution reaction application, and beyond[J]. Chem Rev, 2017, 117(15): 10121-10211. |
| 105 | BAO J, ZHANG X, FAN B, et al. Ultrathin spinel-structured nanosheets rich in oxygen deficiencies for enhanced electrocatalytic water oxidation[J]. Angew Chem Int Ed Engl, 2015, 54(25): 7399-7404. |
| 106 | JIN Y, WANG H, LI J, et al. Porous MoO2 nanosheets as non-noble bifunctional electrocatalysts for overall water splitting[J]. Adv Mater, 2016, 28(19): 3785-3790. |
| 107 | LI C, HAN X, CHENG F, et al. Phase and composition controllable synthesis of cobalt manganese spinel nanoparticles towards efficient oxygen electrocatalysis[J]. Nat Commun, 2015, 6: 7345. |
| 108 | PAN Y, REN H, DU H, et al. Active site engineering by surface sulfurization for a highly efficient oxygen evolution reaction: a case study of Co3O4 electrocatalysts[J]. J Mater Chem A, 2018, 6(45): 22497-22502. |
| 109 | HE Y, CUI Y, ZHAO Z, et al. Strategies for bubble removal in electrochemical systems[J]. Energy Rev, 2023, 2(1): 100015. |
| 110 | INAMDAR A I, CHAVAN H S, SEOK J H, et al. Optimal rule-of-thumb design of NiFeMo layered double hydroxide nanoflakes for highly efficient and durable overall water-splitting at large currents[J]. J Mater Chem A, 2022, 10(38): 20497-20508. |
| 111 | NIU S, JIANG W, WEI Z, et al. Se-doping activates FeOOH for cost-effective and efficient electrochemical water oxidation[J]. J Am Chem Soc, 2019, 141(17): 7005-7013. |
| 112 | ZHOU J, YU L, ZHU Q, et al. Defective and ultrathin NiFe LDH nanosheets decorated on V-doped Ni3S2 nanorod arrays: a 3D core-shell electrocatalyst for efficient water oxidation[J]. J Mater Chem A, 2019, 7(30): 18118-18125. |
| 113 | ZHANG L, KAN X, HUANG T, et al. Electric field modulated water permeation through laminar Ti3C2Tx MXene membrane[J]. Water Res, 2022, 219: 118598. |
| 114 | CAI P, HUANG J, CHEN J, et al. Oxygen-containing amorphous cobalt sulfide porous nanocubes as high-activity electrocatalysts for the oxygen evolution reaction in an alkaline/neutral medium[J]. Angew Chem Int Ed Engl, 2017, 56(17): 4858-4861. |
| 115 | CHENG Y, GUO H, ZHANG L, et al. Mo‐mediated transition of the lattice to long‐range disorder enables ultra-high current density hydrogen production at low potentials[J]. Adv Funct Mater, 2023, 33(12): 2208718. |
| 116 | FENG L L, YU G, WU Y, et al. High-index faceted Ni3S2 nanosheet arrays as highly active and ultrastable electrocatalysts for water splitting[J]. J Am Chem Soc, 2015, 137(44): 14023-14026. |
| 117 | LI H, CHEN S, JIA X, et al. Amorphous nickel-cobalt complexes hybridized with 1T-phase molybdenum disulfide via hydrazine-induced phase transformation for water splitting[J]. Nat Commun, 2017, 8: 15377. |
| 118 | LYU F, ZENG S, JIA Z, et al. Two-dimensional mineral hydrogel-derived single atoms-anchored heterostructures for ultrastable hydrogen evolution[J]. Nat Commun, 2022, 13(1): 6249. |
| 119 | SINGH S, NGUYEN D C, KIM N H, et al. Interface engineering induced electrocatalytic behavior in core-shelled CNTs@NiP2/NbP heterostructure for highly efficient overall water splitting[J]. Chem Eng J, 2022, 442: 136120. |
| 120 | CHENG X, PAN Z, LEI C, et al. A strongly coupled 3D ternary Fe2O3@Ni2P/Ni(PO3)2 hybrid for enhanced electrocatalytic oxygen evolution at ultra-high current densities[J]. J Mater Chem A, 2019, 7(3): 965-971. |
| 121 | KUANG Y, FENG G, LI P, et al. Single-crystalline ultrathin nickel nanosheets array from in situ topotactic reduction for active and stable electrocatalysis[J]. Angew Chem Int Ed Engl, 2016, 55(2): 693-697. |
| 122 | JIANG M, WANG H, LI Y, et al. Superaerophobic RuO2-based nanostructured electrode for high-performance chlorine evolution reaction[J]. Small, 2017, 13(4): 1602240. |
| 123 | LU Z, XU W, MA J, et al. Superaerophilic carbon-nanotube-array electrode for high-performance oxygen reduction reaction[J]. Adv Mater, 2016, 28(33): 7155-7161. |
| 124 | LONG C, WANG K, SHI Y, et al. Tuning the electronic structure of PtRu bimetallic nanoparticles for promoting the hydrogen oxidation reaction in alkaline media[J]. Inorg Chem Front, 2019, 6(10): 2900-2905. |
| 125 | LIN G, ZHANG Y, HUA Y, et al. Bioinspired metalation of the metal-organic framework MIL-125-NH2 for photocatalytic NADH regeneration and gas-liquid-solid three-phase enzymatic CO2 reduction[J]. Angew Chem Int Ed Engl, 2022, 61(31): e202206283. |
| [1] | Hui-Hui LI, Kai-Sheng YAO, Ya-Nan ZHAO, Li-Na FAN, Yu-Lin TIAN, Wei-Wei LU. Ionic Liquid-Modulated Synthesis of Pt-Pd Bimetallic Nanomaterials and Their Catalytic Performance for Ammonia Borane Hydrolysis to Generate Hydrogen [J]. Chinese Journal of Applied Chemistry, 2023, 40(4): 597-609. |
| [2] | Xiao-Hu LIU, Xiao-Juan LAI, Hong-Yan CAO, Ting-Ting WANG, Zhi-Qiang DANG. Synergistic Performance of Foaming Agent/Stabilizer/SiO2 Composite Foam Retarded Acid System [J]. Chinese Journal of Applied Chemistry, 2023, 40(1): 91-99. |
| [3] | Yu MENG, Qing ZHANG, Wen-Hao PENG, Xiao-Fei ZHU, De-Feng ZHOU. Preparation and Electrochemical Performance of Pr0.8Sr0.2Fe0.7Ni0.3O3-δ ⁃Pr1.2Sr0.8Ni0.6Fe0.4O4+δ Composite Cathode [J]. Chinese Journal of Applied Chemistry, 2022, 39(5): 797-808. |
| [4] | Hui-Bing TAO, Zhen TIAN, Yong XIE, Yu SUN, Li WANG, Zhuo KANG, Yue ZHANG. Progress of In situ Raman Study on the Dynamic Structure Performance Correlation of Water Splitting Catalysts [J]. Chinese Journal of Applied Chemistry, 2022, 39(4): 528-539. |
| [5] | Xiao-Feng WU, De-Shun CHEN, Wei MA, Ke-Ke HUANG. WO3/Fe2TiO5 Composite Photoanode Deposited via Electrospray for Enhanced Photoelectrochemical Water Splitting [J]. Chinese Journal of Applied Chemistry, 2022, 39(4): 694-696. |
| [6] | Hui DU, Chen-Yang YAO, Hao PENG, Bo JIANG, Shun-Xiang LI, Jun-Lie YAO, Fang ZHENG, Fang YANG, Ai-Guo WU. Applications of Transition Metal⁃doped Iron⁃based Nanoparticles in Biomedicine [J]. Chinese Journal of Applied Chemistry, 2022, 39(3): 391-406. |
| [7] | FAN Yue, TIAN Xue-Lin . Liquid⁃like Dynamic Interfacial Materials: Recent Progress on Their Applications [J]. Chinese Journal of Applied Chemistry, 2022, 39(1): 131-141. |
| [8] | HUANG Xiao-Tong, CHEN Ying-Xin, ZHU Ze-Bin, ZHOU Li-Hua. Research Progress on Detection of Ascorbic Acid by Nanomaterial-Based Spectral Analysis Method [J]. Chinese Journal of Applied Chemistry, 2021, 38(6): 637-650. |
| [9] | LIU Jiao, ZOU Peng-Fei, LI Ping, ZHANG Xiao, WANG Xin-Xin, GAO Yuan-Yuan, LI Li-Li. Research Progress of the Peptide-Based Self-assembled Nanomaterials Against Microbial Resistance [J]. Chinese Journal of Applied Chemistry, 2021, 38(5): 546-558. |
| [10] | LIU Lin-Chang, GUO Ya-Jun, ZHU Hong-Lin, MA Jing-Jing, LI Zhong-Yi, SHUI Miao, ZHENG Yue-Qing. Research Progress on Supported Ultrafine Nano-catalysts for Hydrolytic Dehydrogenation of Ammonia Borane [J]. Chinese Journal of Applied Chemistry, 2021, 38(11): 1405-1422. |
| [11] | MENG Yang, YANG Chan, PENG Juan. Progress in Iron, Cobalt and Nickel-Based Metal Phosphide Nano-catalysts for Hydrogen Production under Alkaline Conditions [J]. Chinese Journal of Applied Chemistry, 2020, 37(7): 733-745. |
| [12] | FAN Zhe,ZHANG Shengsheng,TANG Jiahao,FAN Ping. Structure, Preparation and Application of Graded Nanomaterials [J]. Chinese Journal of Applied Chemistry, 2020, 37(5): 489-501. |
| [13] | WANG Chunli,SUN Lianshan,ZHONG Ming,WANG Limin,CHENG Yong. Research Progress of Transition Metal and Compounds for Lithium-Sulfur Batteries [J]. Chinese Journal of Applied Chemistry, 2020, 37(4): 387-404. |
| [14] | WANG Qiushi, HE Junhui. Synthesis of Magnetic CuS Composite Nanomaterial and Its Specific Adsorption of Hg(II) in Water [J]. Chinese Journal of Applied Chemistry, 2020, 37(11): 1316-1323. |
| [15] | LIU Bing, GONG Huili, LIU Rui, HU Changwen. One-Step Synthesis of TiO2-Au Composite and Its Performance for Photocatalytic Hydrogen Evolution [J]. Chinese Journal of Applied Chemistry, 2019, 36(9): 1076-1084. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||