Chinese Journal of Applied Chemistry ›› 2023, Vol. 40 ›› Issue (3): 341-359.DOI: 10.19894/j.issn.1000-0518.220227
• Review • Previous Articles Next Articles
Research Progress of Deep Eutectic Solvent and Its Application Prospects as Antistatic Agents
Qi-Hang CHEN, Fei-Jian XU, Feng WANG, Shuang-Chan FU, Ying-Hao YU( )
)
- School of Chemistry and Chemical Engineering,South China University of Technology,Guangzhou 510641,China
-
Received:2022-06-30Accepted:2022-11-09Published:2023-03-01Online:2023-03-27 -
Contact:Ying-Hao YU -
About author:ceyhyu@scut.edu.cn
-
Supported by:the National Natural Science Foundation of China(21676099);the Natural Science Foundation of Guangdong Province(2021A1515010140)
CLC Number:
Cite this article
Qi-Hang CHEN, Fei-Jian XU, Feng WANG, Shuang-Chan FU, Ying-Hao YU. Research Progress of Deep Eutectic Solvent and Its Application Prospects as Antistatic Agents[J]. Chinese Journal of Applied Chemistry, 2023, 40(3): 341-359.
share this article
| Type | Composition | Formula | Example |
|---|---|---|---|
| Ⅰ | Metal salt+organic salt | Cat+X -zMCl x M=Zn,Sn,Fe,Al,Ga,In | ZnCl2+ChCl |
| Ⅱ | Metal salt hydrate+organic salt | Cat+X -zMCl x ·yH2OM=Cr,Co,Cu,Ni,Fe | 6H2O+ChCl |
| Ⅲ | Organic salt+HBD | Cat+X -zRZZ=CONH2,COOH,OH | ChCl+Urea |
| Ⅳ | Metal salt (hydrate)+HBD | MCl x +RZ=MCl x-1·RZ+MCl x+1M=Al,ZnZ=CONH2,OH | ZnCl2+Urea |
Table 1 The main types of deep eutectic solvents[11]
| Type | Composition | Formula | Example |
|---|---|---|---|
| Ⅰ | Metal salt+organic salt | Cat+X -zMCl x M=Zn,Sn,Fe,Al,Ga,In | ZnCl2+ChCl |
| Ⅱ | Metal salt hydrate+organic salt | Cat+X -zMCl x ·yH2OM=Cr,Co,Cu,Ni,Fe | 6H2O+ChCl |
| Ⅲ | Organic salt+HBD | Cat+X -zRZZ=CONH2,COOH,OH | ChCl+Urea |
| Ⅳ | Metal salt (hydrate)+HBD | MCl x +RZ=MCl x-1·RZ+MCl x+1M=Al,ZnZ=CONH2,OH | ZnCl2+Urea |
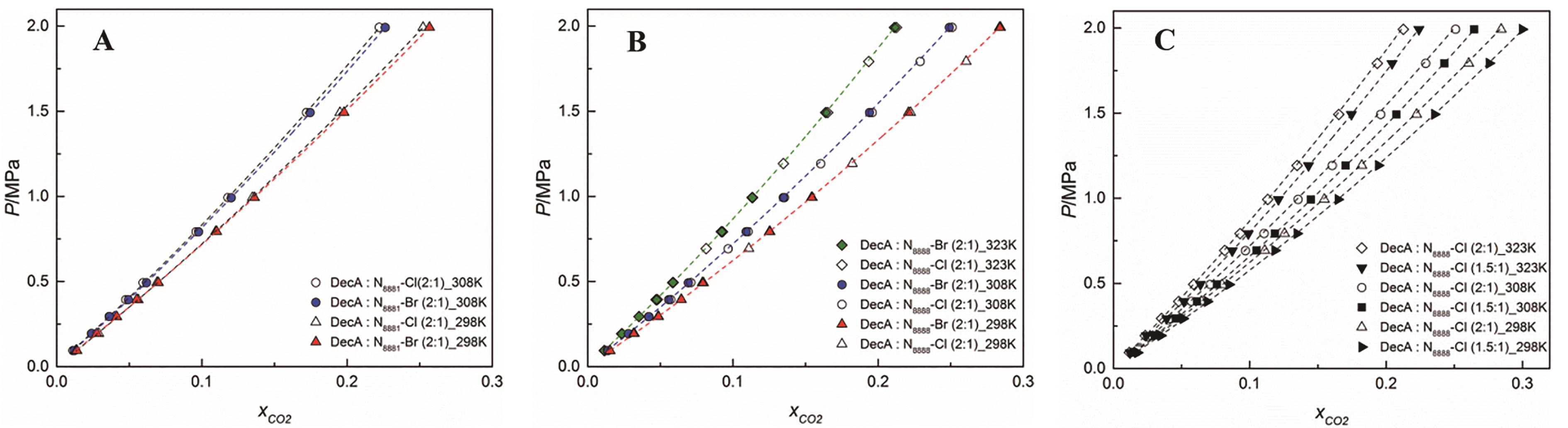
Fig.3 Solubility isotherms of CO2 in the prepared DESs as a function of pressure.The effects of (A) the halide ion, (B) the temperature and (C) the HBD∶HBA ratio on the CO2 solubility are presented[21]
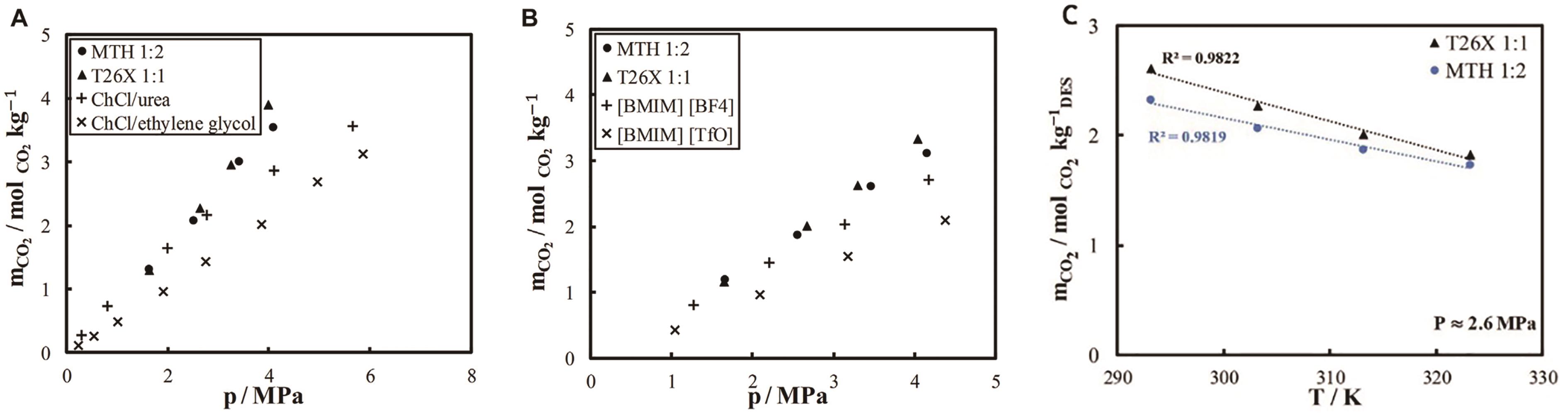
Fig.4 Carbon dioxide solubility (A) in L-menthol/thymol, thymol/2,6-xylenol, ChCl/urea, and ChCl/ethylene glycol at 303.15 K (B) and in MTH, T26X, [BIMI][BF4], and [BMIM][TfO] at 313.15 K; (C) Temperature dependence of carbon dioxide solubility at medium pressure ~2.6 MPa in L-menthol/thymol and thymol/2,6-xylenol[23]
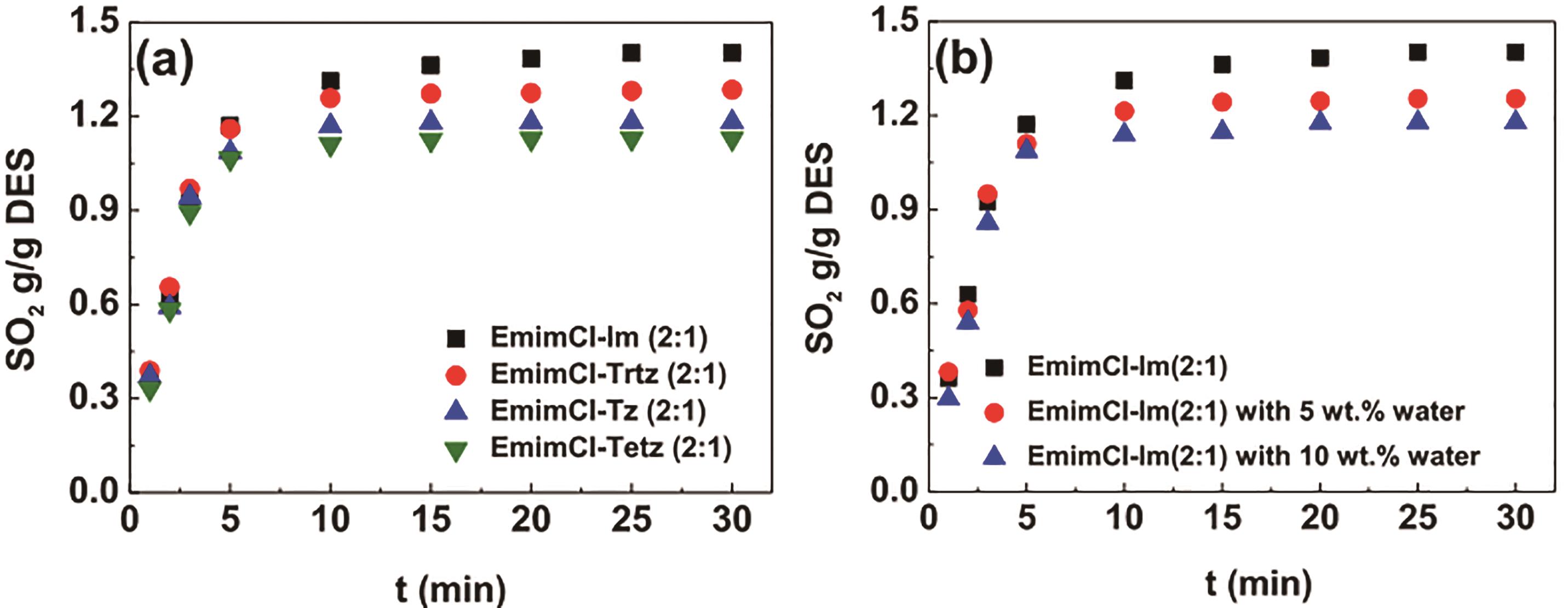
Fig.5 Comparison of SO2 absorption by four DESs as a function of time at 20 ℃ and 1×105 Pa (a). Effect of water on SO2 absorption capacity in EmimCl-Im at 20 ℃ and 1×105 Pa (b)[24]
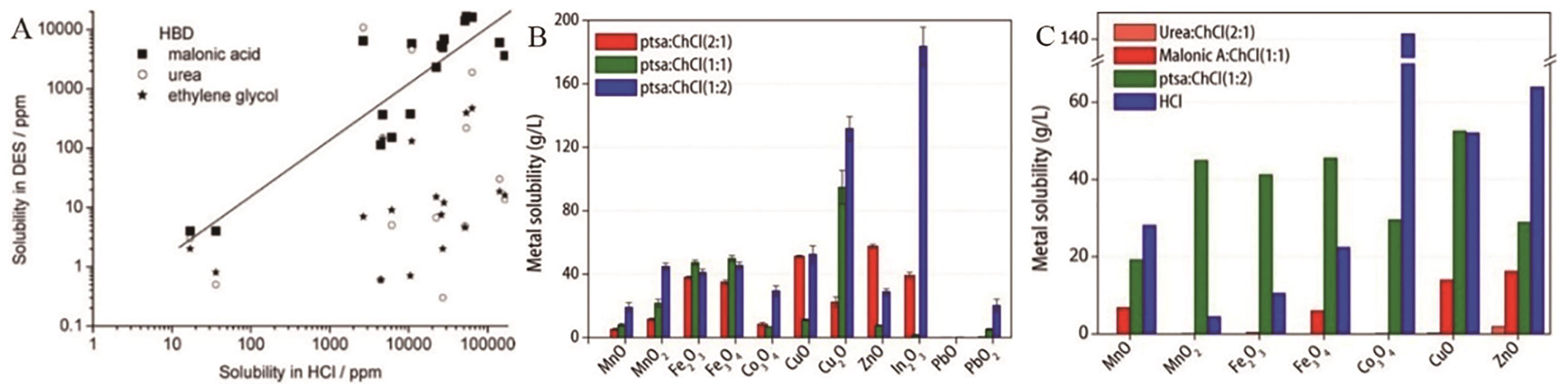
Fig.6 (A) Correlation between the solubility of a variety of metal oxides in three deep eutectic solvents and the solubility in 3.14 mol/L HCl(aq) after equilibration for 2 days at 50 ℃[28]; (B) Metal content after solubilization of metal oxides in the deep eutectic solvent ptsa∶ChCl at different HBD∶HBA molar ratios at 50 ℃ and atmospheric pressure; (C) Solubility of metal oxides in different DESs: ptsa∶ChCl(1∶1), malonic acid∶ChCl(1∶1), urea∶ChCl(2∶1), ethylene glycol∶ChCl(2∶1), and HCl (3.14 mol/L) [30]
| Solvent | iCALB | CALB | CALA | PCL | No enzyme |
|---|---|---|---|---|---|
| ChCl∶Acet | 23 a | 96 | 0.5 | 0.0 | 0.0 |
| ChCl∶EG | 11(99) b | 32(93) b | 3.0 | 0.2 | 0.0 |
| ChCl∶Gly | 96 | 96 | 70 | 22 | 0.0 |
| ChCl∶MA | 30 | 58 | 0.7 | 0.0 | 0.7 |
| ChCl∶Urea | 93 | 99 | 1.6 | 0.8 | 0.0 |
| EAC∶Acet | 63 | 92 | 2.7 | 0.0 | 0.0 |
| EAC∶EG | 23(54) b | 33(79) b | 20 | 0.0 | 0.0 |
| EAC∶Gly | 93 | 91 | 2.1 | 0.5 | 0.0 |
| Toluene | 92 | 92 | 76 | 5.0 | 0.0 |
Table 2 Percentage conversion of ethyl valerate to butyl valerate at 60 ℃[33]
| Solvent | iCALB | CALB | CALA | PCL | No enzyme |
|---|---|---|---|---|---|
| ChCl∶Acet | 23 a | 96 | 0.5 | 0.0 | 0.0 |
| ChCl∶EG | 11(99) b | 32(93) b | 3.0 | 0.2 | 0.0 |
| ChCl∶Gly | 96 | 96 | 70 | 22 | 0.0 |
| ChCl∶MA | 30 | 58 | 0.7 | 0.0 | 0.7 |
| ChCl∶Urea | 93 | 99 | 1.6 | 0.8 | 0.0 |
| EAC∶Acet | 63 | 92 | 2.7 | 0.0 | 0.0 |
| EAC∶EG | 23(54) b | 33(79) b | 20 | 0.0 | 0.0 |
| EAC∶Gly | 93 | 91 | 2.1 | 0.5 | 0.0 |
| Toluene | 92 | 92 | 76 | 5.0 | 0.0 |

Fig.7 (A) Synthesis of Z-vinyl Selenocyanates; (B) Yield comparison of one-pot synthesis of Z-vinylselenocyanate using DESs as catalyst and catalyzed by standard reaction conditions[35]
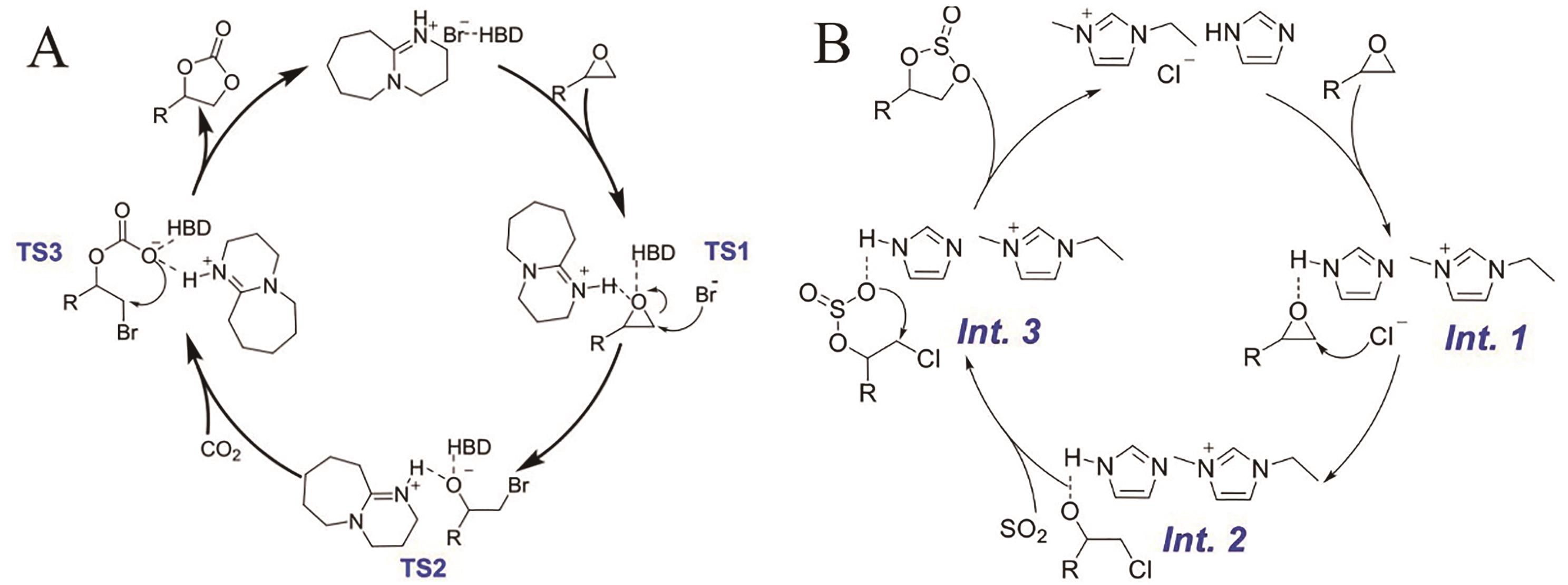
Fig.9 (A) Proposed mechanism of carbon dioxide epoxy fixation catalyzed by protic ionic liquid-based deep eutectic solvents[39]; (B) Proposed mechanism of bisazole-based DESs for oxidative fixation of sulfur dioxide[24]

Fig.10 [PG-CaCl2-0.5]-gel applied in stretchable and flexible electroluminescent devices at room and cold environment: (a) Diagram of the as-prepared electroluminescent device; (b) Luminance of the prepared electroluminescent device as a function of temperature, showing that luminance becomes brighter as the temperature increases; (c) Photos of the prepared electroluminescent device in darkroom at 20 ℃ and -20 ℃. Images of the prepared electroluminescent device in darkroom at 20 ℃ and -20 ℃ with (d) stretching and (e) bending[42]

Fig.11 (a) Two free-standing SSHTPs are cut into two halves, and they can be rejoined into a new one after healing for 24 h (i). It can bear (ii) or lift (iii) a 500 g load without any fracture, which is larger than 500× its own mass; (b) Optical microscopy images of healed SSHTPs with different healing times at 80 ℃[53]
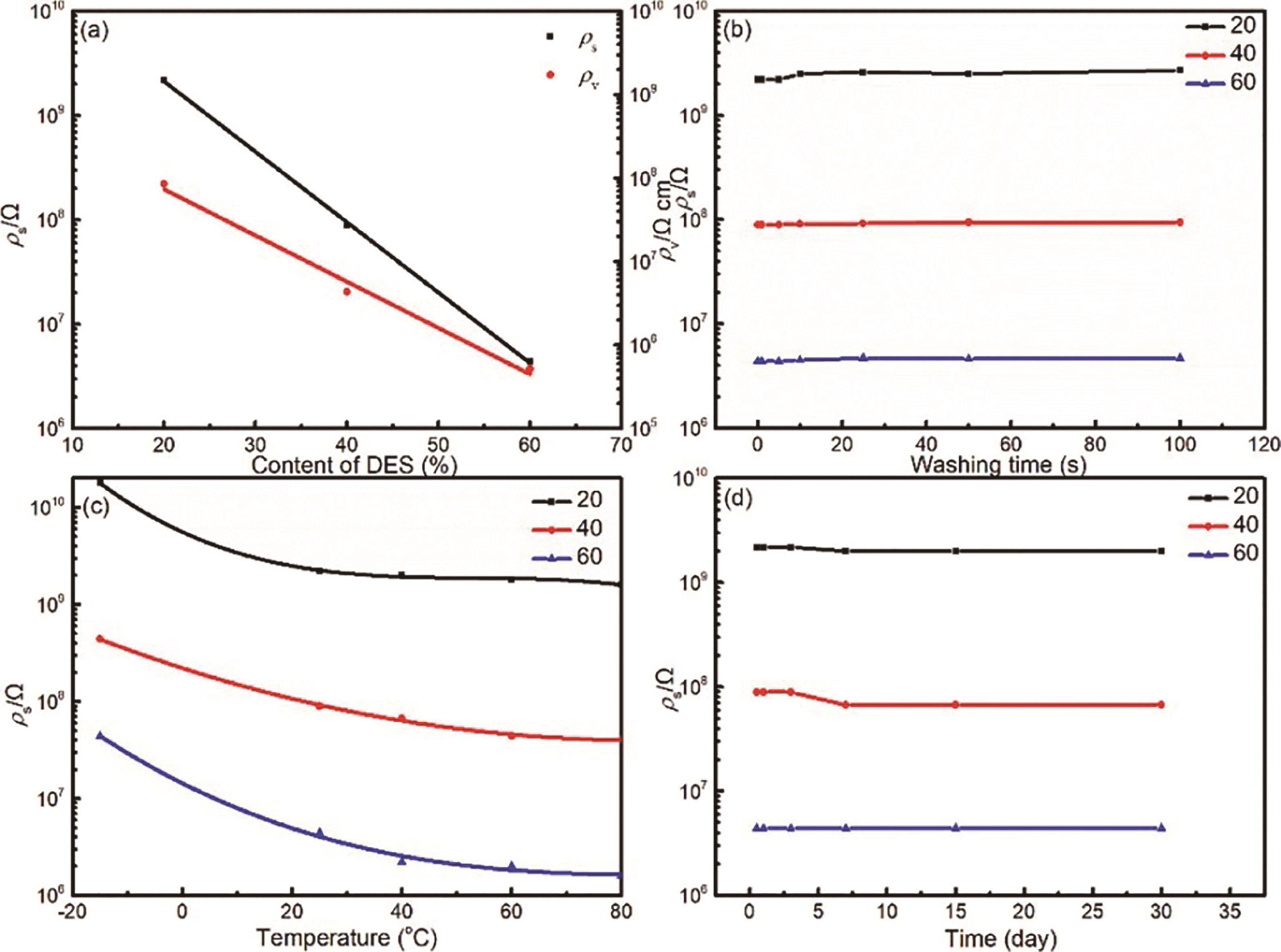
Fig.12 Antistatic effect of the AA/ChCl content on printed parts: ρs and ρv (a), ρsvs temperature (b), ρsvs storage time (c) and ρs after ethanol washing (d)[57]
| 1 | GE X, GU C, WANG, X, et al. Deep eutectic solvents (DESs)-derived advanced functional materials for energy and environmental applications: challenges, opportunities, and future vision[J]. J Mater Chem A, 2017, 5(18): 8209-8229. |
| 2 | PLECHKOVA N V, SEDDON, K R. Applications of ionic liquids in the chemical industry[J]. Chem Soc Rev, 2008, 37(1): 123-150. |
| 3 | ZHANG Q, DE OLIVEIRA VIGIER K, ROYER S, et al. Deep eutectic solvents: syntheses, properties and applications[J]. Chem Soc Rev, 2012, 41(21): 7108-7146. |
| 4 | ABBOTT A P, CAPPER, G, DAVIES D L, et al. Novel solvent properties of choline chloride/urea mixtures[J]. Chem Commun, 2003(1): 70-71. |
| 5 | WELTON T. Ionic liquids: a brief history[J]. Biophys Rev, 2018, 10(3): 691-706. |
| 6 | ABBOTT A P, BOOTHBY D, CAPPER G, et al. Deep eutectic solvents formed between choline chloride and carboxylic acids: versatile alternatives to ionic liquids[J]. J Am Chem Soc, 2004, 126(29): 9142-9147. |
| 7 | HIMEUR F, STEIN I, WRAGG D S, et al. The ionothermal synthesis of metal organic frameworks, Ln(C9O6H3)((CH3NH)2CO)2, using deep eutectic solvents[J]. Solid State Sci, 2010, 12(4): 418-421. |
| 8 | ABBOTT A P, CAPPER, G, MCKENZIE K J, et al. Electrodeposition of zinc-tin alloys from deep eutectic solvents based on choline chloride[J]. J Electroanal Chem, 2007, 599(2): 288-294. |
| 9 | JHONG H R, WONG D S H, WAN C C, et al. A novel deep eutectic solvent-based ionic liquid used as electrolyte for dye-sensitized solar cells[J]. Electrochem Commun, 2009, 11(1): 209-211. |
| 10 | ALVAREZ-VASCO C, MA R S, QUINTERO M, et al. Unique low-molecular-weight lignin with high purity extracted from wood by deep eutectic solvents (DES): a source of lignin for valorization[J]. Green Chem, 2016, 18(19): 5133-5141. |
| 11 | SMITH E L, ABBOTT A P, RYDER K S. Deep eutectic solvents (DESs) and their applications[J]. Chem Rev, 2014, 114(21): 11060-11082. |
| 12 | DI PIETRO M E, MELE A. Deep eutectics and analogues as electrolytes in batteries[J]. J Mol Liq, 2021: 338. |
| 13 | TANG X, ZUO M, LI Z, et al. Green processing of lignocellulosic biomass and its derivatives in deep eutectic solvents[J]. ChemSusChem, 2017, 10(13): 2696-2706. |
| 14 | LIU C, LI M C, CHEN W, et al. Production of lignin-containing cellulose nanofibers using deep eutectic solvents for UV-absorbing polymer reinforcement[J]. Carbohydr Polym, 2020, 246: 116548. |
| 15 | KHANDELWAL S, TAILOR, Y K, KUMAR, M. Deep eutectic solvents (DESs) as eco-friendly and sustainable solvent/catalyst systems in organic transformations[J]. J Mol Liq, 2016, 215: 345-386. |
| 16 | ZHAO J, ZHANG J, YANG W, et al. “Water-in-deep eutectic solvent” electrolytes enable zinc metal anodes for rechargeable aqueous batteries[J]. Nano Energy, 2019, 57: 625-634. |
| 17 | PROTSENKO V S, BOBROVA L S, GOLUBTSOV D E, et al. Electrolytic deposition of hard chromium coatings from electrolyte based on deep eutectic solvent[J]. Russ J Appl Chem, 2018, 91(7): 1106-1111. |
| 18 | LIAO H G, JIANG Y X, ZHOU Z Y, et al. Shape-controlled synthesis of gold nanoparticles in deep eutectic solvents for studies of structure-functionality relationships in electrocatalysis[J]. Angew Chem Int Ed Engl, 2008, 47(47): 9100-9103. |
| 19 | HARTLEY J M, IP C M, FORREST G C, et al. EXAFS study into the speciation of metal salts dissolved in ionic liquids and deep eutectic solvents[J]. Inorg Chem, 2014, 53(12): 6280-6288. |
| 20 | KALHOR P, GHANDI, K. Deep eutectic solvents for pretreatment, extraction, and catalysis of biomass and food waste[J]. Molecules, 2019, 24(22): 4012. |
| 21 | ZUBEIR L F, VAN OSCH D, ROCHA M A A, et al. Carbon dioxide solubilities in decanoic acid-based hydrophobic deep eutectic solvents[J]. J Chem Eng Data, 2018, 63(4): 913-919. |
| 22 | ALIZADEH V, ESSER L, KIRCHNER B. How is CO2 absorbed into a deep eutectic solvent?[J]. J Chem Phys, 2021, 154(9): 094503. |
| 23 | ALHADID A, SAFAROV J, MOKRUSHINA L, et al. Carbon dioxide solubility in nonionic deep eutectic solvents containing phenolic alcohols[J]. Front Chem, 2022, 10: 864663. |
| 24 | LONG G, YANG C, YANG X, et al. Bisazole-based deep eutectic solvents for efficient SO2 absorption and conversion without any additives[J]. ACS Sustain Chem Eng, 2020, 8(7): 2608-2613. |
| 25 | DENG D, ZHANG C, DENG X, et al. Efficient absorption of low partial pressure SO2 by 1-ethyl-3-methylimidazolium chloride plus N-formylmorpholine deep eutectic solvents[J]. Energy Fuels, 2019, 34(1): 665-671. |
| 26 | XING H, LIAO C, YANG Q, et al. Ambient lithium-SO2 batteries with ionic liquids as electrolytes[J]. Angew Chem Int Ed Engl, 2014, 53(8): 2099-2103. |
| 27 | YANG D, ZHANG S, JIANG D E. Efficient absorption of SO2 by deep eutectic solvents formed by biobased aprotic organic compound succinonitrile and 1-ethyl-3-methylimidazolium chloride[J]. ACS Sustain Chem Eng, 2019, 7(10): 9086-9091. |
| 28 | ABBOTT A P, CAPPER G, DAVIES D L, et al. Solubility of metal oxides in deep eutectic solvents based on choline chloride[J]. J Chem Eng Data, 2006, 51(4): 1280-1282. |
| 29 | CHEN W, JIANG J, LAN X, et al. A strategy for the dissolution and separation of rare earth oxides by novel Bronsted acidic deep eutectic solvents[J]. Green Chem, 2019, 21(17): 4748-4756. |
| 30 | RODRIGUEZ N R, MACHIELS L, BINNEMANS K. p-Toluenesulfonic acid-based deep-eutectic solvents for solubilizing metal oxides[J]. ACS Sustain Chem Eng, 2019, 7(4): 3940-3948. |
| 31 | MORRISON H G, SUN C C, NEERVANNAN S. Characterization of thermal behavior of deep eutectic solvents and their potential as drug solubilization vehicles[J]. Int J Pharm, 2009, 378(1/2): 136-139. |
| 32 | ZHANG H, LANG J, LAN P, et al. Study on the dissolution mechanism of cellulose by ChCl-based deep eutectic solvents[J]. Material, 2020, 13(2): 278. |
| 33 | GORKE J T, SRIENC F, KAZLAUSKAS R J. Hydrolase-catalyzed biotransformations in deep eutectic solvents[J]. Chem Commun, 2008, (10): 1235-1237. |
| 34 | SINGH B, LOBO H, SHANKARLING G. Selective N-alkylation of aromatic primary amines catalyzed by bio-catalyst or deep eutectic solvent[J]. Catal Lett, 2011, 141(1): 178-182. |
| 35 | WU C, XIAO H J, WANG S W, et al. Natural deep eutectic solvent-catalyzed selenocyanation of activated alkynes via an intermolecular h-bonding activation process[J]. ACS Sustain Chem Eng, 2019, 7(2): 2169-2175. |
| 36 | MIRJALILI B B F, JALILI BAHABADI N, BAMONIRI A. Triethanolamine-sodium acetate as a novel deep eutectic solvent for promotion of tetrahydrodipyrazolopyridines synthesis under microwave irradiation[J]. J Iran Chem Soc, 2021, 18(8): 2181-2187. |
| 37 | WU M L, BAI Y S, CHEN X J, et al. Deep eutectic solvents used as catalysts for synthesis of 1,10-phenanthroline by improved Skraup reaction[J]. Res Chem Intermed, 2021, 47(9): 3551-3567. |
| 38 | OBST M, SRIVASTAVA A, BASKARAN S, et al. Preparation of propargyl amines in a ZnCl2-dimethylurea deep-eutectic solvent[J]. Synlett, 2018, 29(2): 185-188. |
| 39 | YANG X, ZOU Q, ZHAO T, et al. Deep eutectic solvents as efficient catalysts for fixation of CO2 to cyclic carbonates at ambient temperature and pressure through synergetic catalysis[J]. ACS Sustain Chem Eng, 2021, 9(31): 10437-10443. |
| 40 | ABBOTT A P, EL TTAIB K, FRISCH G, et al. The electrodeposition of silver composites using deep eutectic solvents[J]. Phys Chem Chem Phys, 2012, 14(7): 2443-2449. |
| 41 | CRUZ H, JORDAO N, BRANCO L C. Deep eutectic solvents (DESs) as low-cost and green electrolytes for electrochromic devices[J]. Green Chem, 2017, 19(7): 1653-1658. |
| 42 | LIU C, QI J, HE B, et al. Ionic conductive gels based on deep eutectic solvents[J]. Int J Smart Nano Mat, 2021, 12(3): 337-350. |
| 43 | JAUMAUX P, LIU Q, ZHOU D, et al. Deep-eutectic-solvent-based self-healing polymer electrolyte for safe and long-life lithium-metal batteries[J]. Angew Chem Int Ed, 2020, 59(23): 9134-9142. |
| 44 | BOLDRINI C L, MANFREDI N, PERNA F M, et al. Dye-sensitized solar cells that use an aqueous choline chloride-based deep eutectic solvent as effective electrolyte solution[J]. Energy Technol, 2017, 5(2): 345-353. |
| 45 | HONG S, YUAN Y, LIU C Z, et al. A stretchable and compressible ion gel based on a deep eutectic solvent applied as a strain sensor and electrolyte for supercapacitors[J]. J Mater Chem C, 2020, 8(2): 550-560. |
| 46 | BOISSET A, JACQUEMIN J, ANOUTI M. Physical properties of a new deep eutectic solvent based on lithium bis (trifluoromethyl)sulfonyl imide and N-methylacetamide as superionic suitable electrolyte for lithium ion batteries and electric double layer capacitors[J]. Electrochim Acta, 2013, 102: 120-126. |
| 47 | QIN H, OWYEUNG R E, SONKUSALE S R, et al. Highly stretchable and nonvolatile gelatin-supported deep eutectic solvent gel electrolyte-based ionic skins for strain and pressure sensing[J]. J Mater Chem C, 2019, 7(3): 601-608. |
| 48 | HUANG M K, ANURATHA K S, XIAO Y, et al. Co-solvent modified methylsulfonylmethane-based hybrid deep eutectic solvent electrolytes for high-voltage symmetric supercapacitors[J]. Electrochim Acta, 2022: 424. |
| 49 | GAO Z S, XIE S L, ZHANG B, et al. Ultrathin Mg-Al layered double hydroxide prepared by ionothermal synthesis in a deep eutectic solvent for highly effective boron removal[J]. Chem Eng J, 2017, 319: 108-118. |
| 50 | FENG Y C, YAN G H, WANG T, et al. Synthesis of MCM-41-supported metal catalysts in deep eutectic solvent for the conversion of carbohydrates into 5-hydroxymethylfurfural[J]. ChemSusChem, 2019, 12(5): 978-982. |
| 51 | SEYEDI N, KHABAZZADEH H, SAEEDNIA S. ZnCl2/urea as a deep eutectic solvent for the preparation of bis(indolyl)methanes under ultrasonic conditions[J]. Synth React Inorg M, 2015, 45(10): 1501-1505. |
| 52 | ROKADE S M, BHATE P M. Ferrier reaction in a deep eutectic solvent[J]. Carbohydr Res, 2015, 415: 28-30. |
| 53 | LI R A, ZHANG K L, CHEN G X, et al. Stiff, self-healable, transparent polymers with synergetic hydrogen bonding interactions[J]. Chem Mater, 2021, 33(13): 5189-5196. |
| 54 | LU C, WANG C, WANG J, et al. Integration of hydrogen bonding interaction and Schiff-base chemistry toward self-healing, anti-freezing, and conductive elastomer[J]. Chem Eng J, 2021: 425. |
| 55 | KOSIŃSKI S, RYKOWSKA I, GONSIOR M, et al. Ionic liquids as antistatic additives for polymer composites-a review[J]. Polym Test, 2022, 112: 107649. |
| 56 | GOUDARZI M, MAHYARI M, FATHOLLAHI M, et al. Deep eutectic solvents as sustainable antistatic coating agent for cyclotetramethylenetetranitramine to reduce charge-accumulations[J]. J Electrostat, 2020: 108: 103519. |
| 57 | SU J, LI S, CHEN Y, et al. 3D photoprintable antistatic materials with polymerizable deep eutectic solvents[J]. Ind Eng Chem Res, 2021, 60(49): 17797-17803. |
| [1] | Hui-Hui LI, Kai-Sheng YAO, Ya-Nan ZHAO, Li-Na FAN, Yu-Lin TIAN, Wei-Wei LU. Ionic Liquid-Modulated Synthesis of Pt-Pd Bimetallic Nanomaterials and Their Catalytic Performance for Ammonia Borane Hydrolysis to Generate Hydrogen [J]. Chinese Journal of Applied Chemistry, 2023, 40(4): 597-609. |
| [2] | NIU Zhan-Ning, TANG Hao-Qing, ZHENG Chao, TIAN Tian, ZHENG Li-Yun. Study on [RESA]Br@-COOH@Fe3O4 with Density Functional Theory [J]. Chinese Journal of Applied Chemistry, 2021, 38(7): 825-835. |
| [3] | ZHOU Chao, SHENG Cheng-Ju, WEN Lin-Lin. Preparation of Imidazolium Salt-based Poly(ionic liquids) Antibacterial Agent and Its Application in Hydrogel Dressing [J]. Chinese Journal of Applied Chemistry, 2021, 38(1): 51-59. |
| [4] | LIU Ning, WANG Danfeng, WU Suyun, LIU Shuilin, FU Lin, LIU Yuejin. Catalytic Synthesis of Bisphenol F over Short-Channeled Mesoporous Molecular Sieve Zr-Ce-SBA-15 Supported Acidic Ionic Liquids [J]. Chinese Journal of Applied Chemistry, 2020, 37(9): 1038-1047. |
| [5] | HU Jiale, XUE Dongfeng. Research Progress on the Characteristics of Rare Earth Ions and Rare Earth Functional Materials [J]. Chinese Journal of Applied Chemistry, 2020, 37(3): 245-255. |
| [6] | GAO Bo, YANG Hongwei, TIAN Shaopeng, ZHAO Yuzhen, TIAN Tian. Solubility Properties of 1-Alkyl-4-amino-1,2,4-triazolium Energetic Ionic Liquids [J]. Chinese Journal of Applied Chemistry, 2019, 36(9): 1044-1052. |
| [7] | QU Haonan, BAO Chong, MA Wenjing, MA Yiming, ZHAO Peng, WANG Xinhai, ZHOU Yanmei. Hydrolysis of Cellulose by Solid Carbon Sulfonic Acid Supported 1-Butyl-3-methylimidazolium Chloride [J]. Chinese Journal of Applied Chemistry, 2019, 36(1): 58-64. |
| [8] | XIE Fei,WEI Zhixian. Advances on Tetrazole-1-acetic Acid-Based Metal Coordination Polymers [J]. Chinese Journal of Applied Chemistry, 2017, 34(10): 1099-1109. |
| [9] | WANG Wei, CHEN Ji, LIU Hongzhao, YANG Hualing, CAO Yaohua, GAO Zhaoguo, ZHANG Bo. Research Progress of Task-specific Ionic Liquids Used in Metal Ions Extraction [J]. Chinese Journal of Applied Chemistry, 2015, 32(7): 733-742. |
| [10] | GUO Rui, SAI Mingze, ZHANG Min, ZHONG Shiliang, MA Wentao, CHEN Lei, DING Derun. Preparation and Performance of Chitosan Grafted Polyvinyl Pyrrolidone Film with Iodine [J]. Chinese Journal of Applied Chemistry, 2015, 32(5): 498-503. |
| [11] | JIN Mingyue,FU Luxiang,YOU Jichun,Li Yongjin. Preparation and Properties of Ionic Liquid and Ethylene-Vinyl Acetate Copolymer Composites [J]. Chinese Journal of Applied Chemistry, 2015, 32(10): 1146-1152. |
| [12] | YU Guiqin, LIU Jianjun, LIANG Yongmin. Tribological Investigation of Guanidinium Ionic Liquids as Lubricants for Si3N4/steel Contacts [J]. Chinese Journal of Applied Chemistry, 2015, 32(1): 99-103. |
| [13] | LI Ruirui,ZHOU Yantong,XIA Liangshu ,XIAO Yiqun,LI Guang. Research Progress of Electrochemical and Extractible Characteristics of Actinide Elements in the Molten Salt System [J]. Chinese Journal of Applied Chemistry, 2015, 32(1): 1-9. |
| [14] | ZHAO Haili1, YAO Kaisheng2*, ZHANG Jun2, LU, Weiwei2, HUANG Qingling2. Liquid-liquid Interfacial Synthesis of Nanomaterials [J]. Chinese Journal of Applied Chemistry, 2014, 31(09): 1010-1018. |
| [15] | WANG Fuyu, LIU Yanli, WANG Chong, ZHAO Zhenbo*. Dehydration of Fructose in Presence of Acidic Ionic Liquids to Prepare 5-Hydroxymethylfurfural [J]. Chinese Journal of Applied Chemistry, 2014, 31(04): 424-430. |
| Viewed | ||||||||||||||||||||||||||||||||||||||||||||||||||
|
Full text 1644
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||
|
Abstract 615
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||