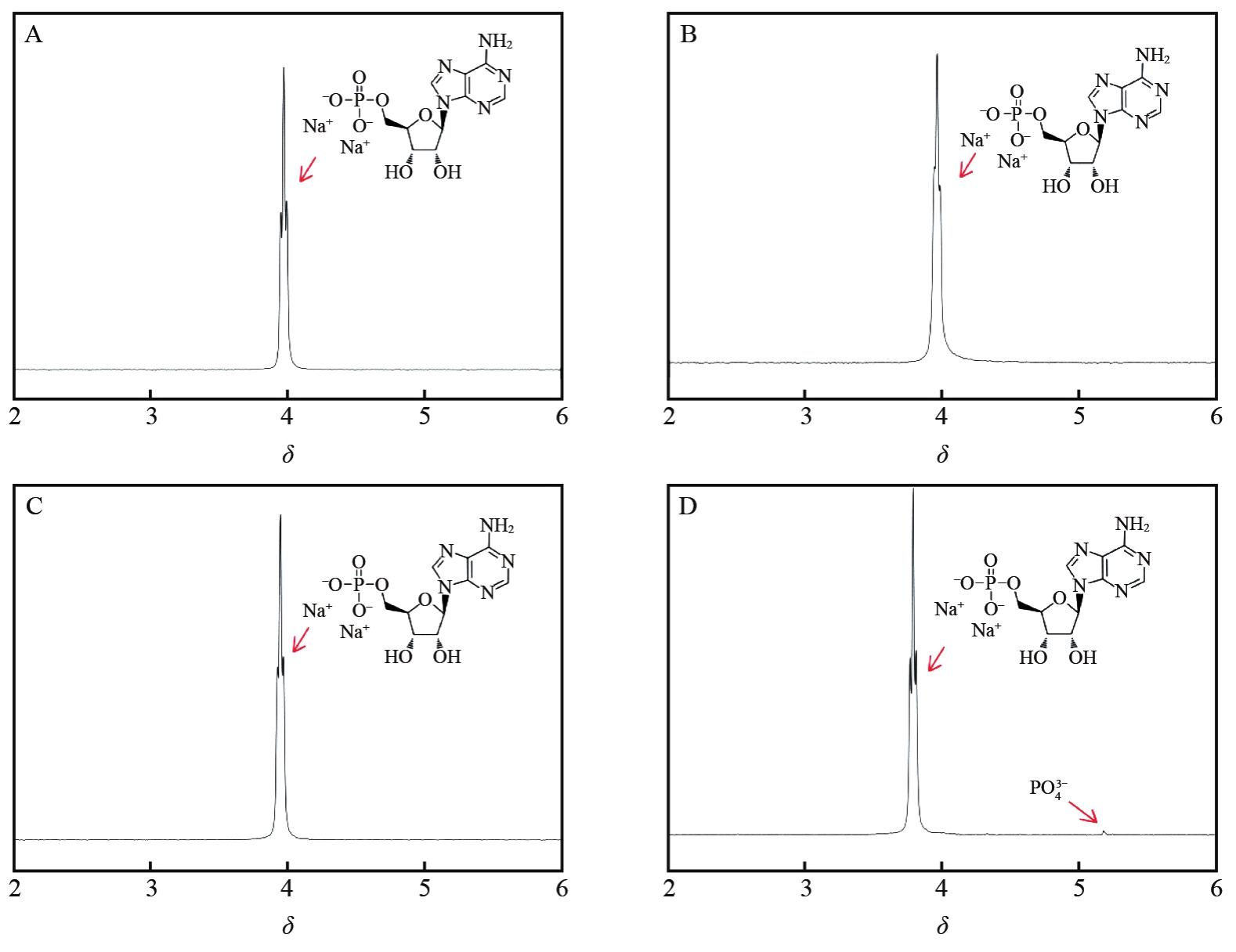
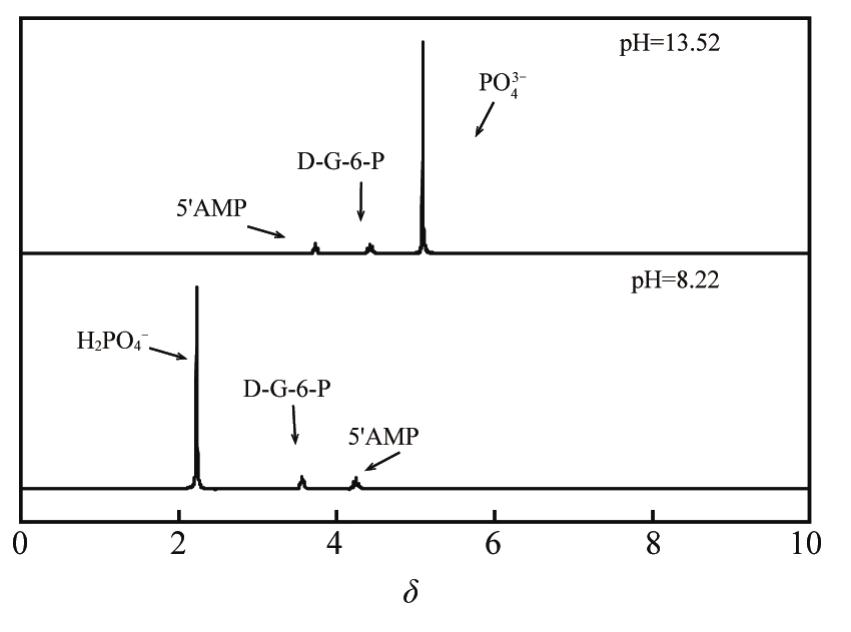
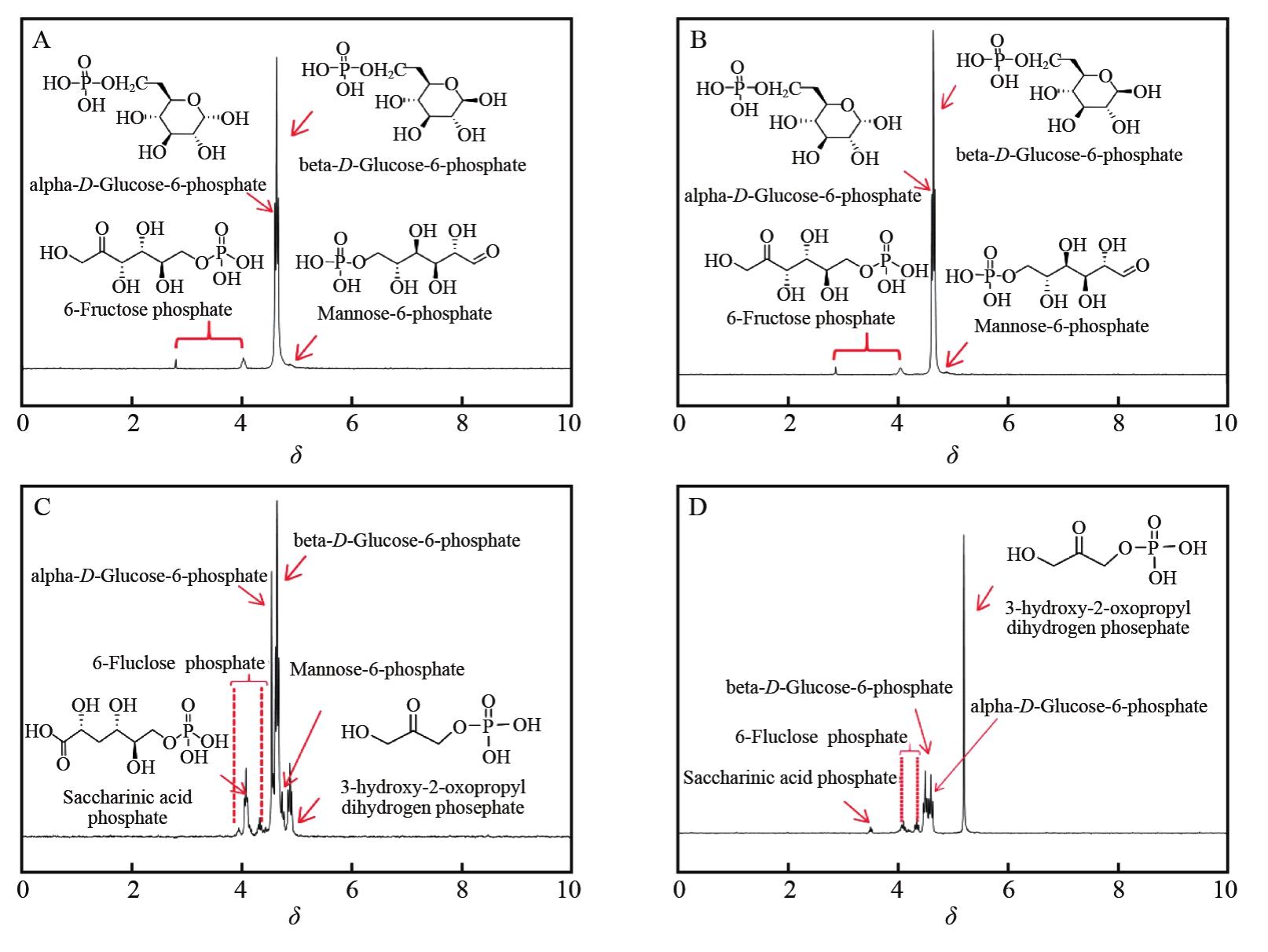
| [1] |
LIU J, HU Y F, YANG J J, et al. Investigation of soil legacy phosphorus transformation in long-term agricultural fields using sequential fractionation, P K-edge XANES and solution P NMR spectroscopy[J]. Environ Sci Technol, 2014, 49(1):168-176.
|
| [2] |
刘瑾, 杨建军, 梁新强, 等, 同步辐射X射线吸收近边结构光谱技术在磷素固相形态研究中的应用[J]. 应用生态学报, 2011, 22(10):2757-2764.
|
|
LIU J, YANG J J, LIANG X Q, et al, Applications of synchrotron-based X-ray absorption near-edge structure spectroscopy in identifying solid state phosphorus speciation: a review[J]. Chinese J Appl Ecol, 2011, 22(10):2757-2764.
|
| [3] |
LIU J, YANG J J, CADE M B J, et al. Complementary phosphorus speciation in agricultural soils by sequential fractionation, solution31P NMR, and P K-edge XANES spectroscopy[J]. J Environ Qual, 2013, 42(6):1763-1770.
|
| [4] |
LIU J, YANG J J, CADE M B J, et al. Molecular speciation and transformation of soil legacy phosphorus with and without long-term phosphorus fertilization: insights from bulk and microprobe spectroscopy[J]. Sci Rep, 2017, 7(1):15354.
|
| [5] |
LIU J, SUI P, CADE M B J, et al. Molecular-level understanding of phosphorus transformation with long-term phosphorus addition and depletion in an alkaline soil[J]. Geoderma, 2019, 353:116-124.
|
| [6] |
LIU J, HAN C Q, ZHAO Y H, et al. The chemical nature of soil phosphorus in response to long-term fertilization practices: Implications for sustainable phosphorus management[J]. J Clean Prod, 2020:123093.
|
| [7] |
CADE M B J. Characterizing phosphorus in environmental and agricultural samples by31P nuclear magnetic resonance spectroscopy[J]. Talanta, 2005, 66(2):359-371.
|
| [8] |
叶汝汉. 含磷化合物核磁共振谱图的研究与应用[D]. 广东工业大学, 2011.
|
|
YE R H. Study and application of NMR spectra of phosphorus containing compounds[D]. Guangdong University of Technology, 2011.
|
| [9] |
LIU J, CADE M B J, YANG J J, et al. Long-term land use affects phosphorus speciation and the composition of phosphorus cycling genes in agricultural soils[J]. Front Microbiol, 2018, 9:1643-1657.
|
| [10] |
LIU J, YANG J J, LIANG X Q. Molecular speciation of phosphorus present in readily dispersible colloids from agricultural soils[J]. Soil Sci Soc Am J, 2014, 78(1):47-53.
|
| [11] |
CROUSE D A, SIERZPUTOWSKAL G H, MIKKELSEN R L. Optimization of sample pH and temperature for phosphorus-31 nuclear magnetic resonance spectroscopy of poultry manure extracts[J]. Commun Soil Sci Plant Anal, 2000, 31(12):229-240.
|
| [12] |
CADE M B J, LIU C W. Solution phosphorus-31 nuclear magnetic resonance spectroscopy of soils from 2005 to 2013: a review of sample preparation and experimental parameters[J]. Soil Sci Soc Am J, 2014, 78(1):19-37.
|
| [13] |
IMOTO T, SHIBATA S, AKASAKA K, et al. Conformation of adenosine 5′monophosphate in aqueous solution as studied by NMR-DESERT method[J]. Biopolymers, 1977, 16(12):2705-2721.
|
| [14] |
PO H N, SENOZAN N M. The henderson-hasselbalch equation: its history and limitations[J]. J Chem Educ, 2001, 80(11):1499-1503.
|
| [15] |
ROBITAILLE P M L, ROBITAILLE P A, BROWM G G. An analysis of the pH-dependent chemical-shift behavior of phosphorus-containing metabolites[J]. J Magn Reson, 1991, 92(1):73-84.
|
| [16] |
BATTERSBY M K, RADDA G K. The stereospecificity of the glucose-6-phosphate binding site of glycogen phosphorylase B[J]. FEBS Lett, 1976, 72(2):319-322.
|
| [17] |
BAILEY J. Studies on mutarotases. V. serum mutarotase levels in renal disease[J]. Clin Biochem, 1970, 3:11-21.
|
| [18] |
SIMONOV A N, PESTUNOVA O P, MATVIENKO L G. 13C NMR studies of isomerization of D-glucose in an aqueous solution of Ca (OH)2 [J]. Russ Chem Bull, 2005, 54(8):1967-1972.
|
| [19] |
LINDHORST T K. Essentials of carbohydrate chemistry and biochemistry[M]. Wiley-VCH, 2003:3-31.
|
| [20] |
MOON R B, RICHARDS J H. Determination of intracellular pH by31P magnetic resonance[J]. J Biol Chem, 1973, 248(20):7276-7278.
|
| [21] |
刘景瑶, 朱晋昌, 蒋丽金. 用NMR研究D-葡萄糖在碱性溶液内的行为[J]. 化学学报 1983(4):73-77.
|
|
LIU J Y, ZHU J C, JIANG L J. A study of the behavior of D-glucose in alkaline solution using NMR technique[J]. Acta Chim Sin, 1983(4):73-77.
|

 )
)