
应用化学 ›› 2022, Vol. 39 ›› Issue (6): 871-887.DOI: 10.19894/j.issn.1000-0518.210244
单原子催化剂电催化还原二氧化碳研究进展
- 航天材料及工艺研究所,北京 100076
-
收稿日期:2021-05-18接受日期:2021-10-13出版日期:2022-06-01发布日期:2022-06-27 -
通讯作者:张超 -
基金资助:国家自然科学基金(51472015)
Research Prospect of Single Atom Catalysts Towards Electrocatalytic Reduction of Carbon Dioxide
- Aerospace Research Institute of Materials & Processing Technology,Beijing 100076,China
-
Received:2021-05-18Accepted:2021-10-13Published:2022-06-01Online:2022-06-27 -
Contact:Chao ZHANG -
About author:zhchao@buaa.edu.cn
-
Supported by:the National Natural Science Foundation of China(51472015)
摘要:
电催化CO2还原能够在常温常压下利用电能将CO2转化为含碳清洁能源,具有很好的应用前景。 但其应用仍受缓慢的阴极催化剂限制。众所周知,催化剂的尺寸对其活性有很大的影响,将金属催化剂减少到纳米颗粒级别,能够显著提升其暴露的活性位点数和本征活性,从而提升其催化性能。在这一思路下,如果将催化剂的尺寸降低到单原子分散级别,催化剂的活性能够得到明显提升。近几年,由于单原子分散催化剂具有特殊的微观几何结构和电子态,已经成为电催化还原CO2领域的研究热点。在本综述中,对单原子分散催化剂在电催化还原CO2方面的研究进行了总结和回顾,并对未来单原子分散催化剂在电催化还原CO2领域的难点问题和进一步研究方向进行了分析和讨论。
中图分类号:
引用本文
张超. 单原子催化剂电催化还原二氧化碳研究进展[J]. 应用化学, 2022, 39(6): 871-887.
Chao ZHANG. Research Prospect of Single Atom Catalysts Towards Electrocatalytic Reduction of Carbon Dioxide[J]. Chinese Journal of Applied Chemistry, 2022, 39(6): 871-887.

图1 不同SACs催化CO2还原反应生成多种有机产物的构效关系
Fig.1 The structure-activity relationship of various organic products produced by CO2 reduction reaction catalyzed by different SACs
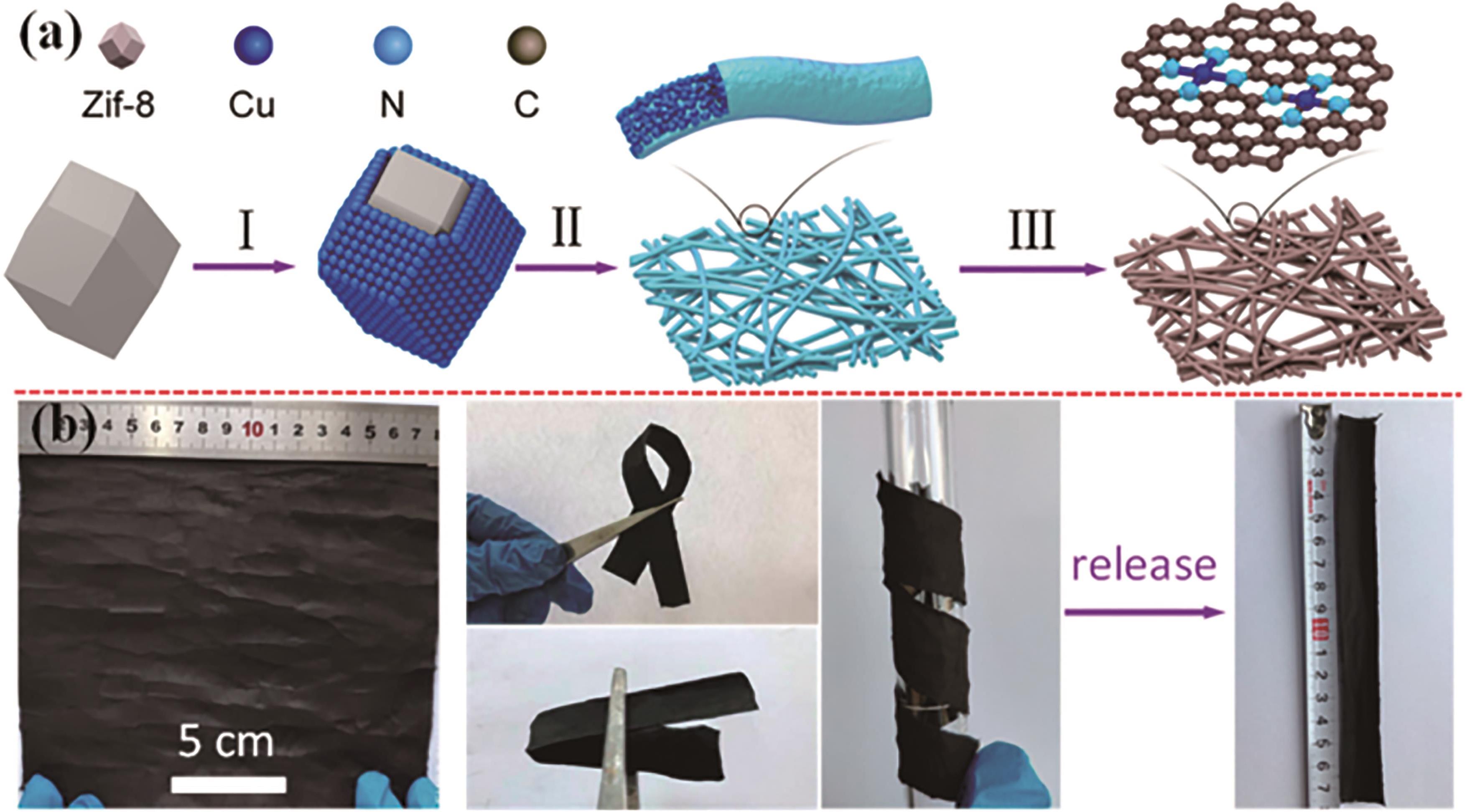
图2 CuSAs/THCF的工艺: (a)CuSAs/THCF的工艺流程,分为含钴ZIF前驱体制备,电纺丝和高温碳化3个流程; (b)CuSAs/THCF的照片及其柔韧性展示[17]
Fig.2 Preparation process of CuSA/THCF: (a) Preparation of CuSA/THCF sample contains synthesis of ZIF precursors, electrospun and high-temperature treatment steps; (b) Digital photos of CuSA-THCF show the flexible of the sample[17]
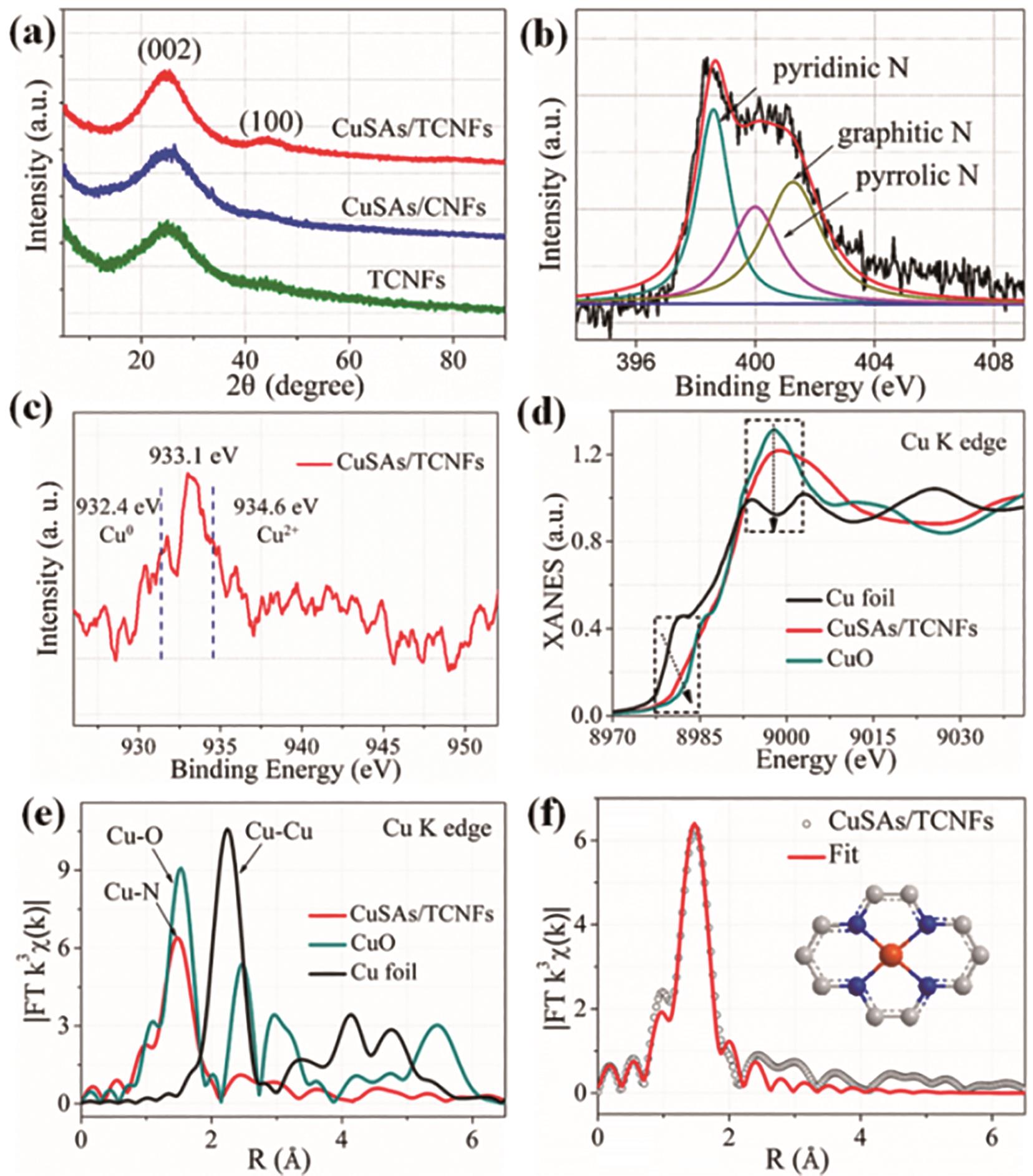
图3 Cu单原子的表征: (a) X射线衍射(XRD)花样表征; (b) CuSAs/THCF的高分辨N1s图谱; (c)CuSAs/THCF的高分辨Cu2p图谱, 证实Cu原子的不饱和价态; (d) CuSAs/THCF、Cu箔和CuO的X射线吸收近边结构图谱(XANES); (e) CuSAs/THCF、Cu箔和CuO的扩展X射线吸收精细结构图谱(EXAFS); (f) CuSAs/THCF扩展X射线吸收精细结构图谱的拟合结果, 证实Cu原子处于Cu-N4结构配位[17]
Fig.3 Structural characterization of Cu single atoms: (a) XRD patterns; (b) High-resolution N1s spectral of CuSA/THCF; (c) High-resolution Cu2p spectral of CuSA/THCF show the unsaturated states of Cu atoms; (d) XANES spectral of CuSA/THCF, Cu foil and CuO; (e) EXAFS spectral of CuSA/THCF, Cu foil and CuO (f) EXAFS fitting results of CuSA/THCF confirm the existence of Cu-N4 coordinations [17]
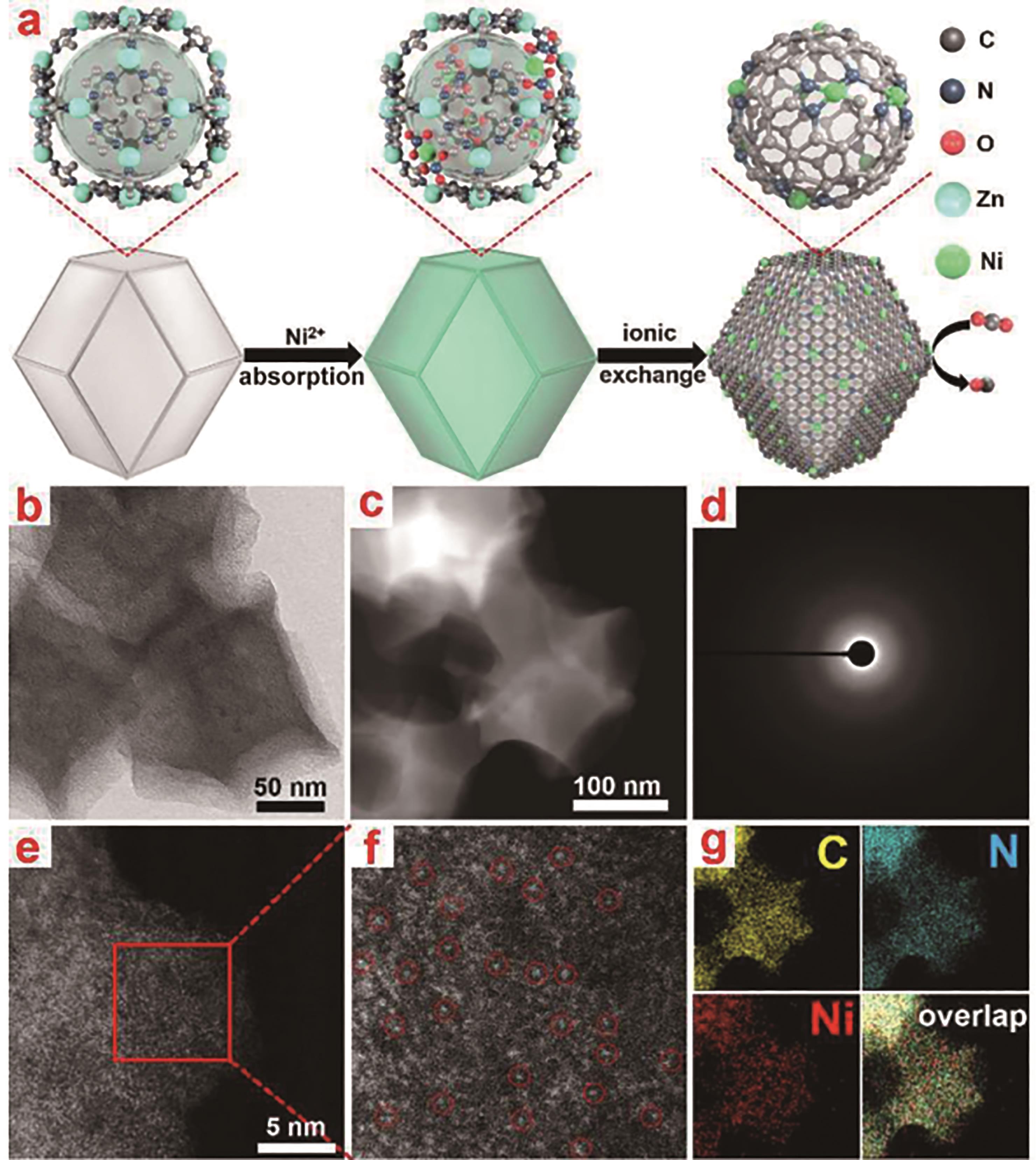
图4 Ni SAs/N-C的表征: (a)Ni SAs/N-C的工艺流程; (b)Ni SAs/N-C的透射电子显微镜(TEM)图像; (c)Ni SAs/N-C的高角环形暗场像-扫描透射电子显微镜(HAADF-STEM)图像; (d)Ni SAs/N-C的选区电子衍射(SAED)花样; (e和f)Ni SAs/N-C不同倍数的HAADF-STEM图像,Ni原子用红圈进行标注; (g)Ni SAs/N-C的EDS能谱图和相应的Ni、N和C的元素面分布图[22]
Fig.4 Characterization of NiSA/N-C: (a) Preparation process of NiSA/N-C; (b) TEM image of NiSA/N-C; (c) HAADF-STEM image of NiSA/N-C; (d) SAED patterns of NiSA/N-C; (e and f) HAADF-STEM images of NiSA/N-C with different magnifications with single atom Ni marked by red circles; (g) EDS results and the corresponding elemental mapping of Ni, N and C species[22]
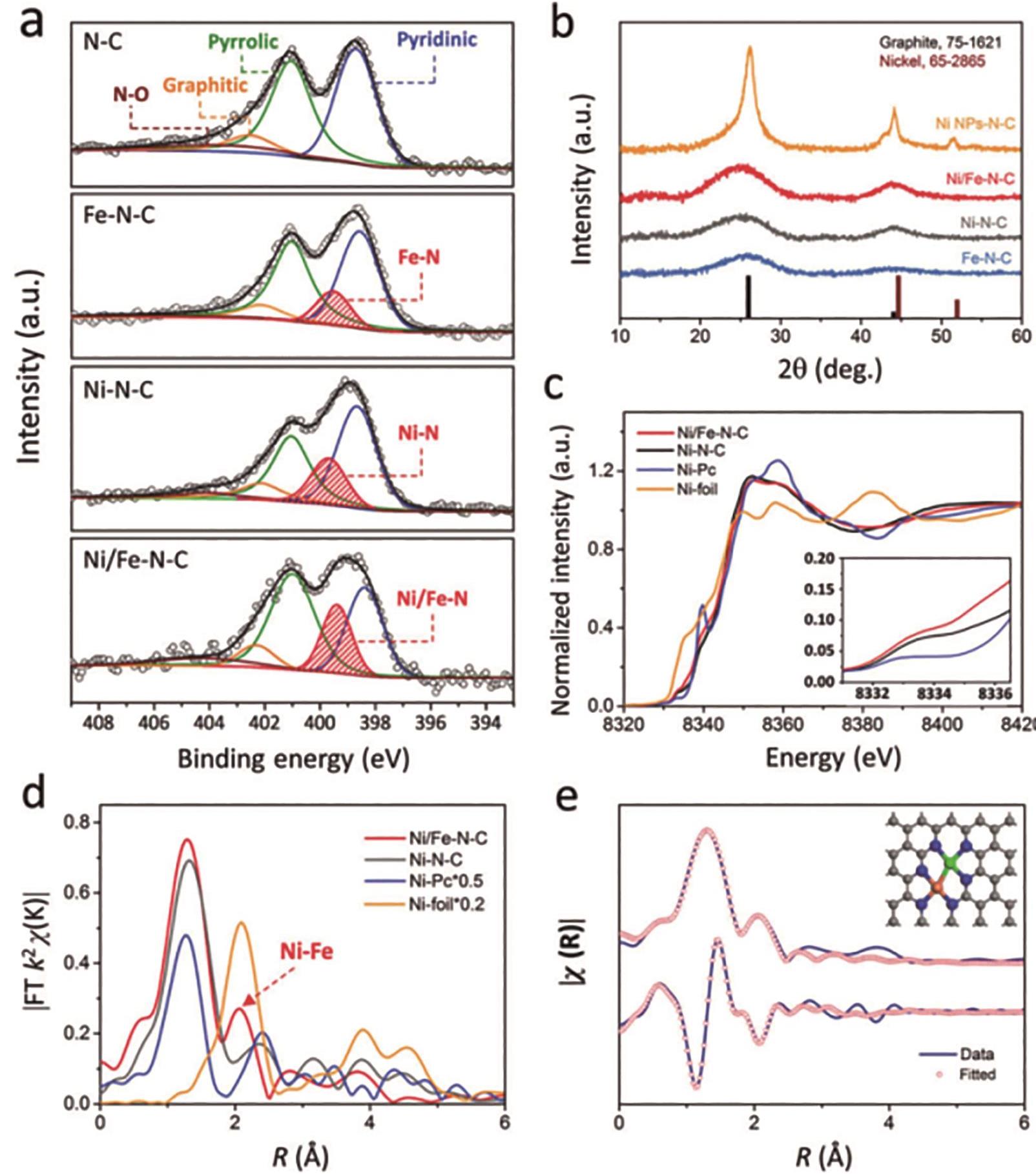
图5 Ni/Fe-N-C的表征: (a和b)Ni/Fe-N-C、Fe-N-C、Ni-N-C和N-C样品的(a)高分辨X射线光电子能谱(XPS)N1s图谱和(b)XRD衍射花样; (c)Ni/Fe-N-C、Ni-N-C、Fe-N-C和Ni箔的K-边XANES图谱; (d)Ni/Fe-N-C、Ni-N-C、Fe-N-C和Ni箔的EXAFS图谱; (e)Ni/Fe-N-C样品中Ni-N和Ni-Fe的模拟EXAFS图谱[22](1 ? = 0.1 nm,下同)
Fig.5 Characterization of Ni/Fe-N-C: (a and b) High-resolution XPS N1s spectral (a) and XRD patterns (b) of Ni/Fe-N-C, Fe-N-C, Ni-N-C and N-C samples; (c) K-edge XANES spectral of Ni/Fe-N-C, Fe-N-C, Ni-N-C and N-C samples; (d) EXAFS spectral of Ni/Fe-N-C, Fe-N-C, Ni-N-C and N-C samples; (e) EXAFS fitting results of Ni/Fe-N-C sample show the presence of Ni-N and Ni-Fe coordination[22]
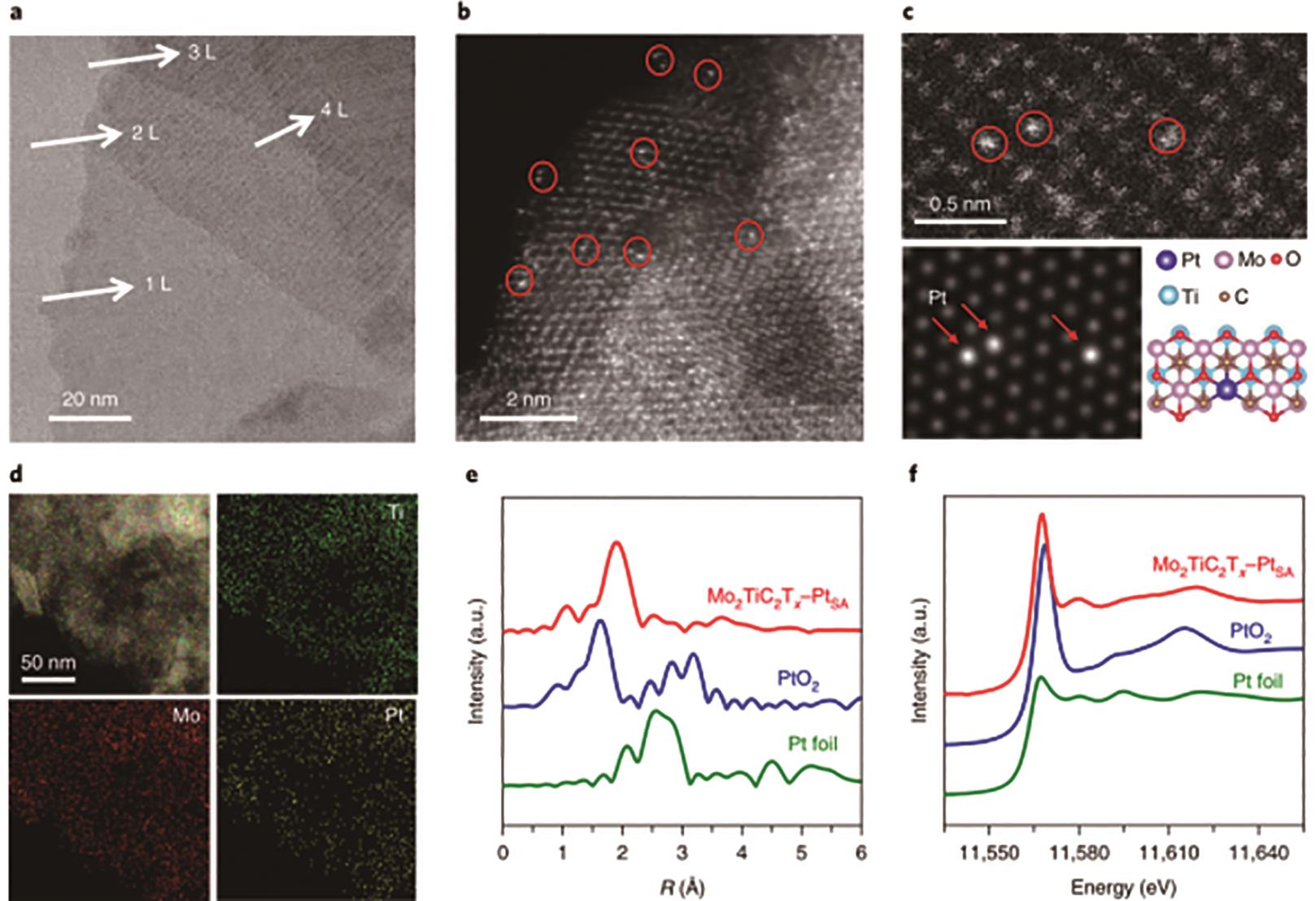
图6 Mo2TiC2T x -PtSA样品的表征: (a)Mo2TiC2T x -PtSA样品的TEM图像; (b)Mo2TiC2T x -PtSA样品的HAADF-STEM图像; (c)Mo2TiC2T x -PtSA样品的高倍HAADF-STEM图像以及其相应的微观结构拟合图像, 可以观测到独立、明亮的斑点,证明Pt的单原子性; (d)Mo2TiC2T x -PtSA样品的STEM-EDS图像; (e)Mo2TiC2T x -PtSA、Pt箔和PtO2的EXAFS图谱; (f)Mo2TiC2T x -PtSA,Pt箔和PtO2归一化的XANES图谱[25]
Fig.6 Characterization of Mo2TiC2T x -PtSA sample: (a) TEM image of Mo2TiC2T x -PtSA sample; (b) HAADF-STEM image of Mo2TiC2T x -PtSA sample; (c) HAADF-STEM images and their corresponding nanostructure fitting result confirm the existence of single atom Pt; (d) STEM-EDS images and corresponding elemental mapping results; (e) EXAFS spectra of Mo2TiC2T x -PtSA, Pt foil and PtO2 samples; (f) EXAFS spectra of Mo2TiC2T x -PtSA, Pt foil and PtO2 samples[25]
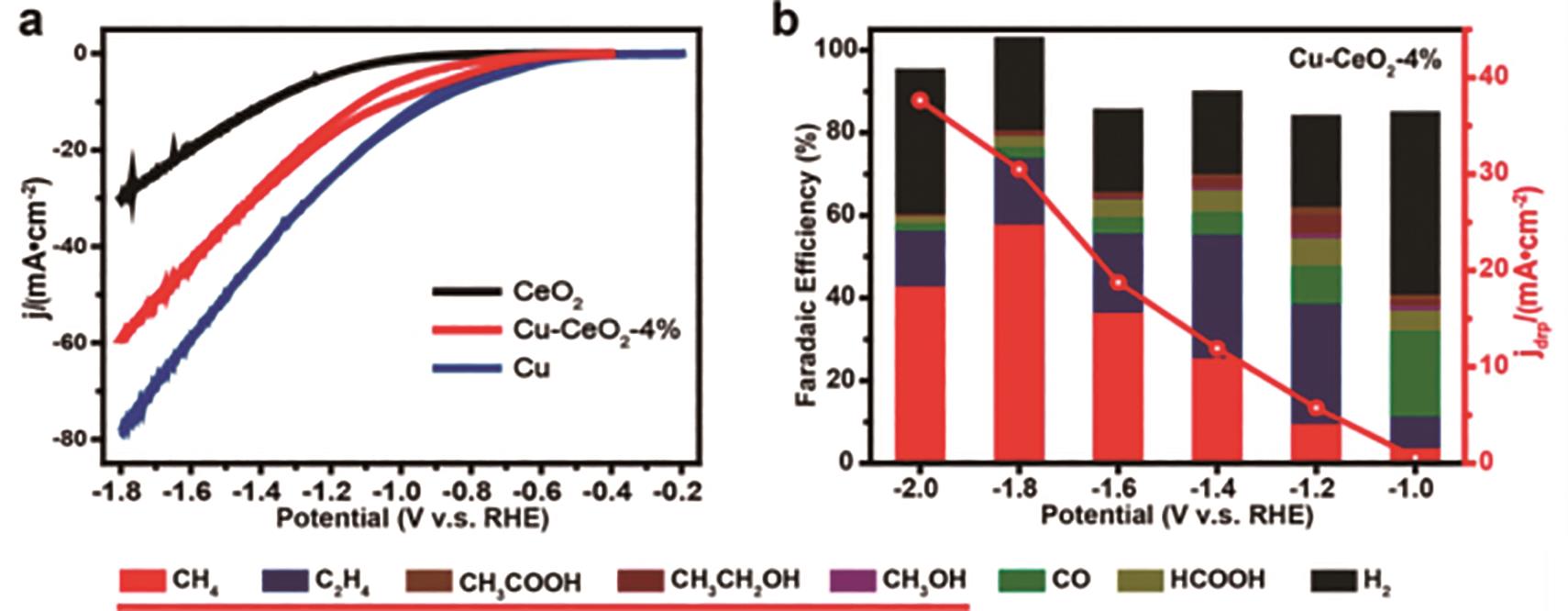
图7 Cu-CeO2-4%样品的CO2还原性能: (a)Cu-CeO2、CeO2和Cu样品的循环伏安曲线; (b)Cu-CeO2-4%样品在不同电位下对不同CO2还原产物的法拉第效率结果[26]
Fig.7 Electrocatalytic CO2 reduction properties of Cu-CeO2-4%: (a) CV curves of Cu-CeO2, CeO2 and Cu samples; (b) Faradaic efficiencies of various CO2 reduction products catalysis by Cu-CeO2-4% under different potentials[26]

图8 原位快速加热制备SACs的方法: (a)快速加热制备SACs的工艺流程图; (b)快速加热过程中的温度-时间曲线,插图为快速加热下材料表面的光学照片; (c)连续10次的快速加热-冷却过程中的时间-温度曲线[27]
Fig.8 In situ high-temperature-shockwave synthesis of HT-SAs: (a) The schematic diagram shows the HT-SA synthesis and dispersion process; (b) Temperature evolution during the shockwave synthesis and a detailed heating/cooling pattern; (c) A ten-pulse shock heating pattern demonstrates the uniform temperature in each cycle with a high-temperature on state and a low temperature off state[27]
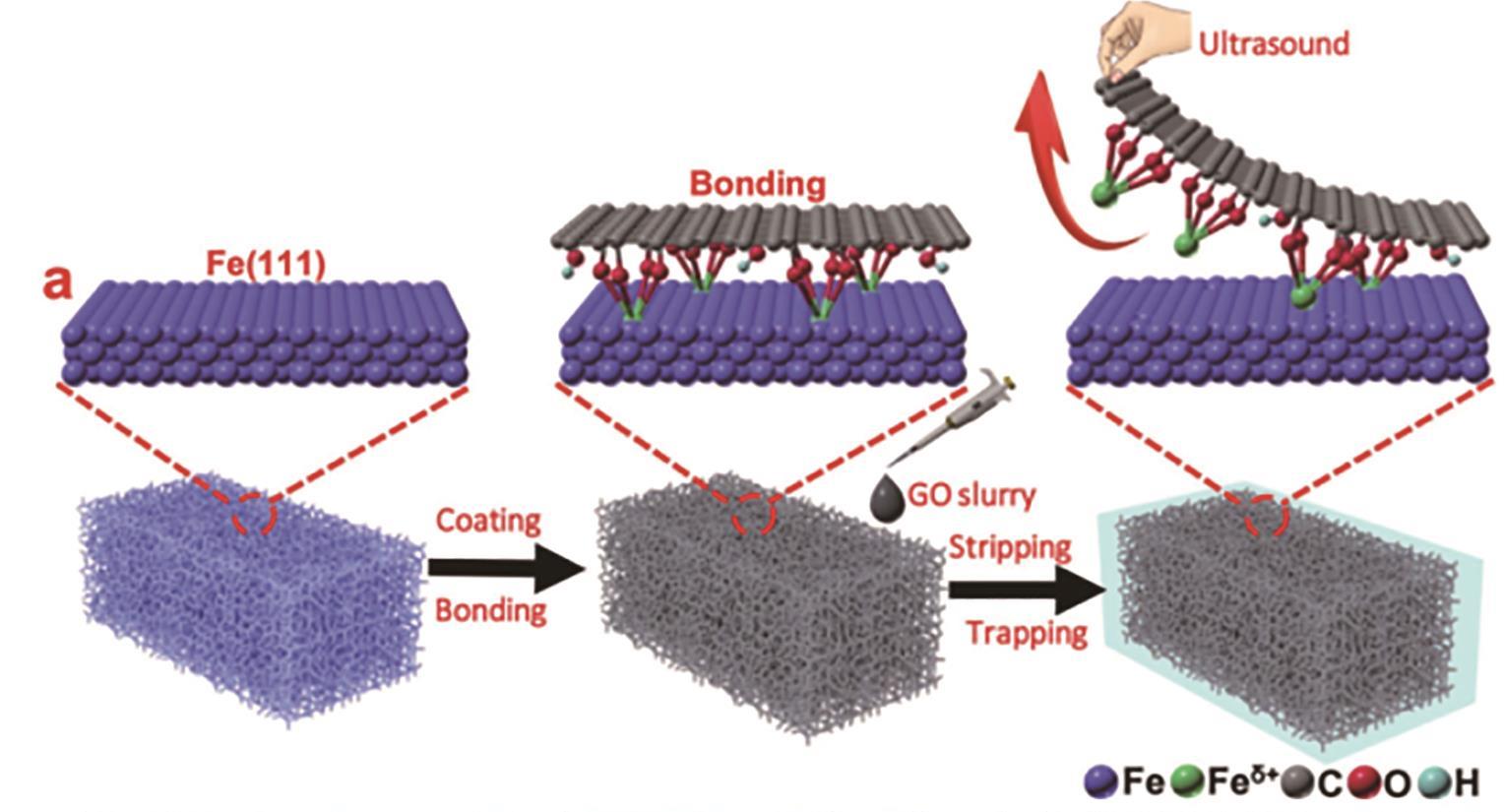
图9 Fe SAs/GO的制备工艺主要包含两步: 1)将GO吸附在泡沫Fe的表面,并与Fe原子形成Fe—O键;2)通过机械力,使GO从泡沫Fe表面剥离,并利用Fe—O强度大于Fe—Fe金属键强度的原理,形成单原子Fe位点[28]
Fig.9 Preparation of Fe SAs/GO involves two steps: 1) Absorbed GO on the surface of Fe foam and formed Fe—O bonds with Fe atoms on the surface; 2) By mechanical force, GO is separated from the foam Fe surface. Using the principle that the strength of Fe—O is greater than the strength of Fe—Fe metal bond, single atomic Fe is formed[28]

图10 单原子分散的Co催化剂的表征: (a) Co-N2、Co-N3和Co-N4配位的单原子分散样品的EXAFS图谱; (b)Co-N2、Co-N3和Co-N4配位的单原子分散样品的XANES图谱[33]
Fig.10 Characterization of single atom Co catalyst: (a) EXAFS spectra of Co-N2, Co-N3 and Co-N4 samples; (b) XANES spectra of Co-N2, Co-N3 and Co-N4 samples[33]
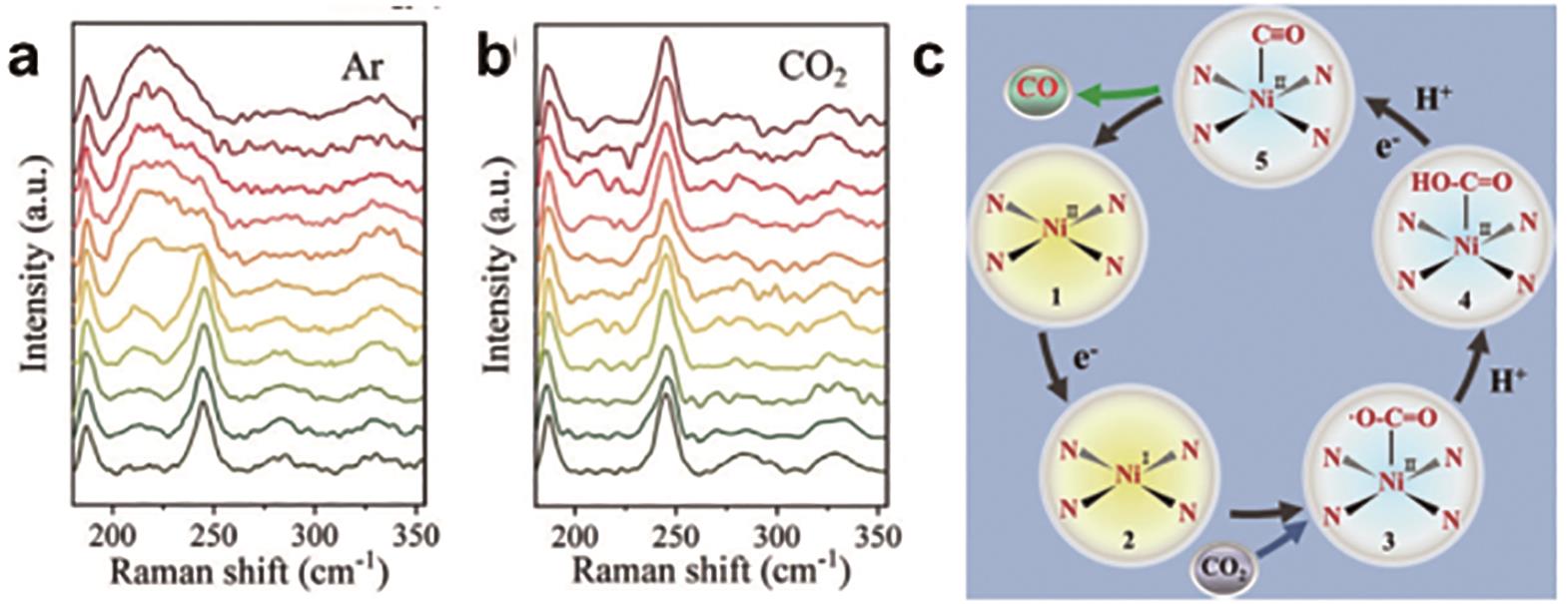
图11 Ni-TAPc催化剂的原位催化机理图示: 金电极表面Ni-TAPc在Ar饱和(a)及CO2饱和(b)的0.5 mol/L KHCO3电解液中,不同电势下的拉曼光谱; (c)Ni-TAPc催化剂的Ni原子动态变化过程[37]
Fig.11 In situ catalytic mechanism illustration of Ni-TAPc catalyst: (a and b) Raman spectra of Ni-TAPc collected on an Au electrode at various potentials (vs.RHE) in 0.5 mol/L KHCO3 aqueous solution at room temperature under an atmosphere of (c) Ar (1.01×105 Pa) and (d) CO2 (1.01×105 Pa); (c) Reaction pathway of CO2 reduction to CO on the Ni SAC[37]
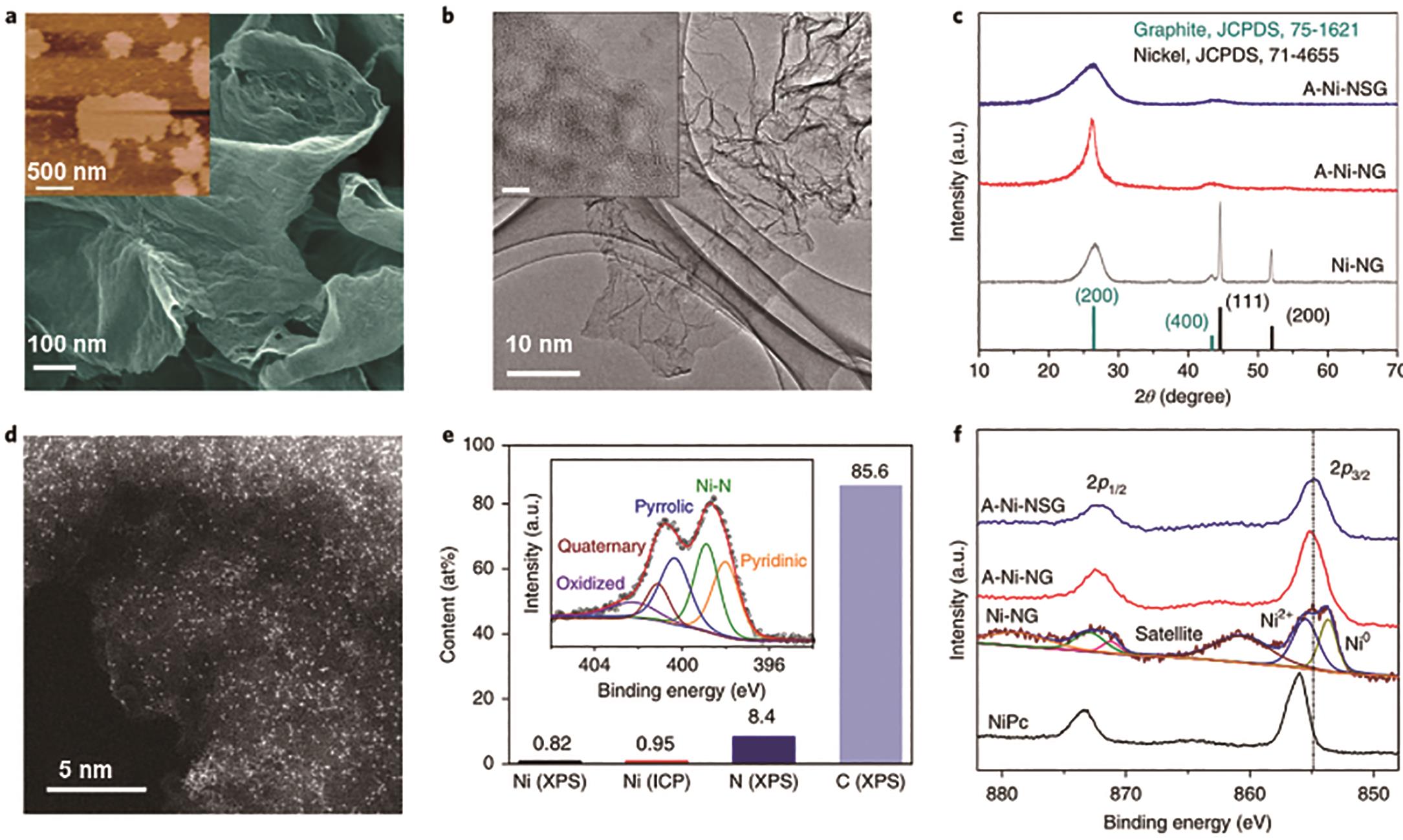
图12 N掺杂石墨烯负载单原子分散Ni的表征: (a)A-Ni-NG的扫描电子显微镜(SEM)图像,插图为AFM图像; (b)A-Ni-NG的TEM图像,插图为高分辨TEM图像; (c)A-Ni-NG、A-Ni-NSG和Ni-NG的XRD衍射花样; (d)A-Ni-NG样品的HAADF-STEM图像; (e)A-Ni-NG样品中的元素含量,插图为高分辨XPS N1s图谱; (f)不同样品的高分辨XPS Ni2p图谱[47]
Fig.12 Characterization of single atom Ni decorated N-doped graphene: (a) SEM image of A-Ni-NG; (b) TEM image of A-Ni-NG; (c) XRD patterns of Ni-NG, A-Ni-NSG and Ni-NG; (d) HAADF-STEM image of A-Ni-NG; (e) Elemental contents of A-Ni-NG, inset: high-resolution XPS N1s spectra; (f) High-resolution Ni2p spectra of different samples[47]
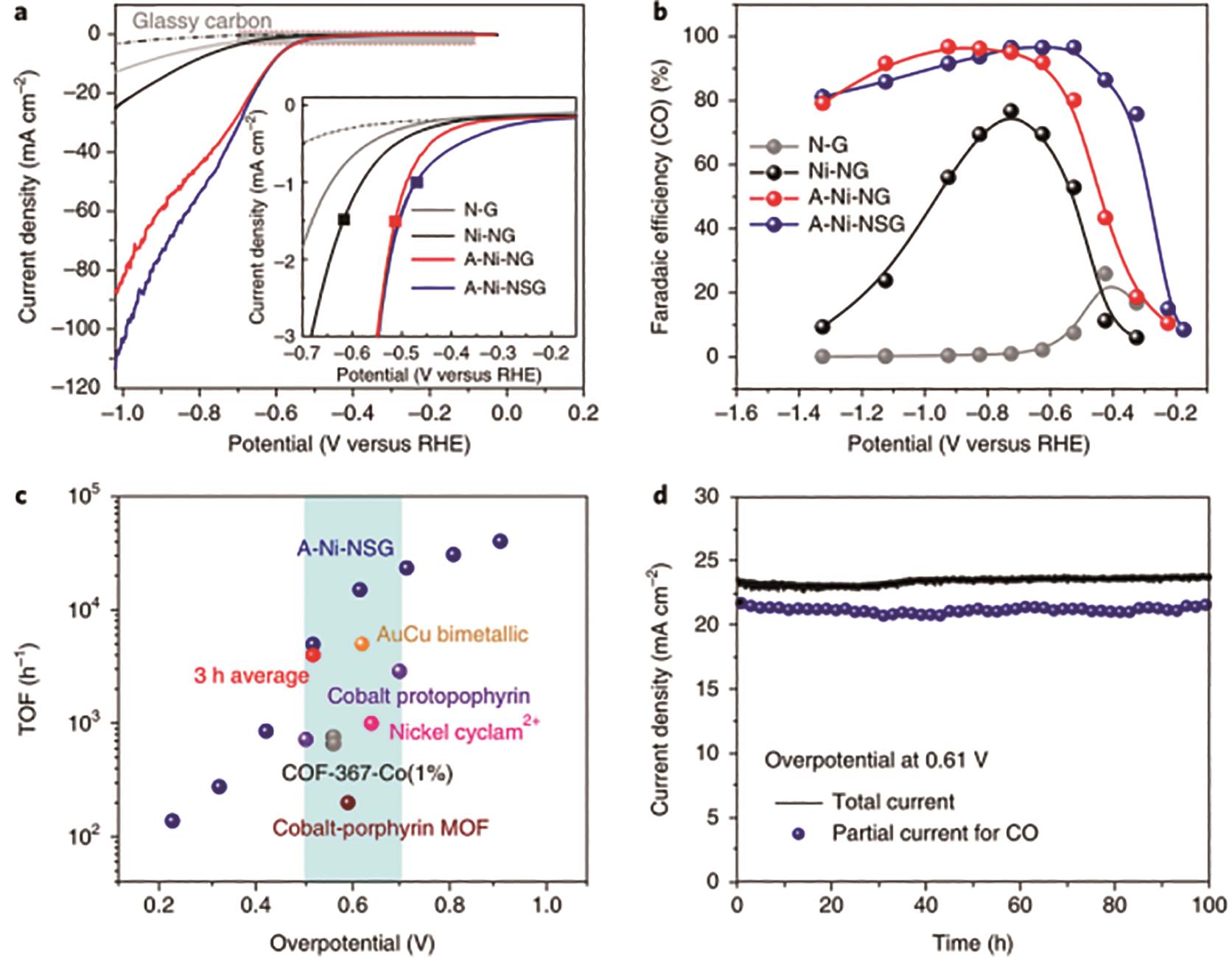
图13 A-Ni-NG电催化CO2还原测试: (a)转速为1600 r/min下不同样品在CO2饱和的0.5 mol/L KHCO3电解液中的LSV曲线; (b)不同样品在不同测试电势下的CO产物法拉第效率结果; (c)A-Ni-NG催化剂和一些其它催化剂的TOF值对比结果; (d)A-Ni-NG催化剂的还原电流密度和CO产物电流密度的100 h稳定性测试结果[47]
Fig.13 Electrocatalytic CO2 reduction results of A-Ni-NG: (a) LSV curves of different samples in 0.5 mol/L KHCO3 electrolyte at the rotation rate of 1600 r/min; (b) CO product Faradaic efficiencies of different samples under different potentials; (c) Comparations of TOF results of A-Ni-NG catalyst with a series of reported catalysts; (d) Total reduction current densities and CO product current densities of A-Ni-NG for 100 h long-term tests[47]
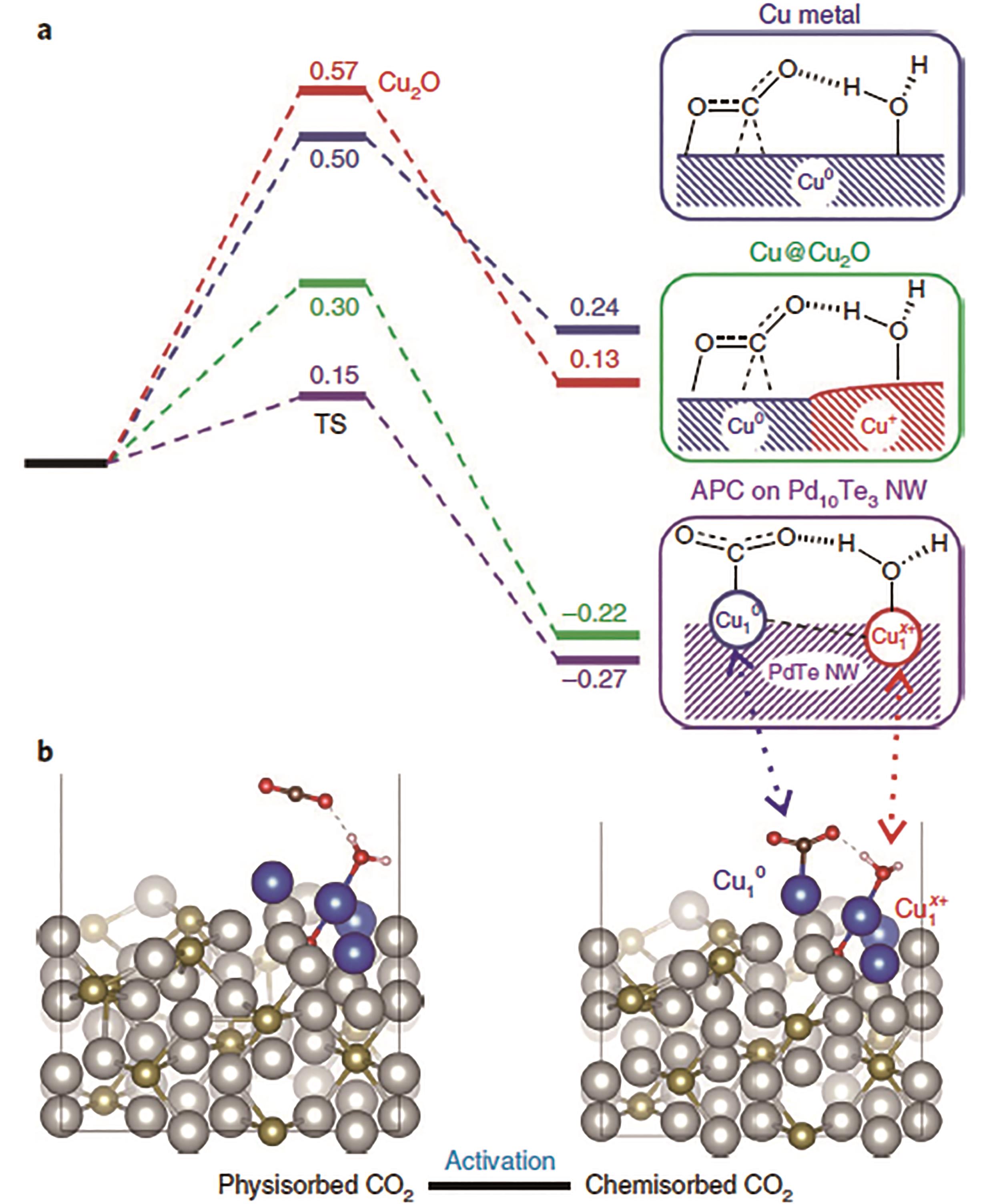
图14 Cu10-Cu1x+原子对催化剂催化机理研究: (a)在-0.78 V(vs.RHE)电势下不同活性位点的CO2活化自由能变化曲线; (b)CO2分子物理和化学吸附在催化剂表面的构型[51]
Fig.14 (a) Free energy profiles (at -0.78 V(vs.RHE)) for CO2 activation on Cu, Cu@Cu2O and APC of Cu10-Cu1x+ on Pd10Te3 nanowires; (b) Configurations of physisorbed CO2 and chemisorbed CO2 on Cu-APC[51]
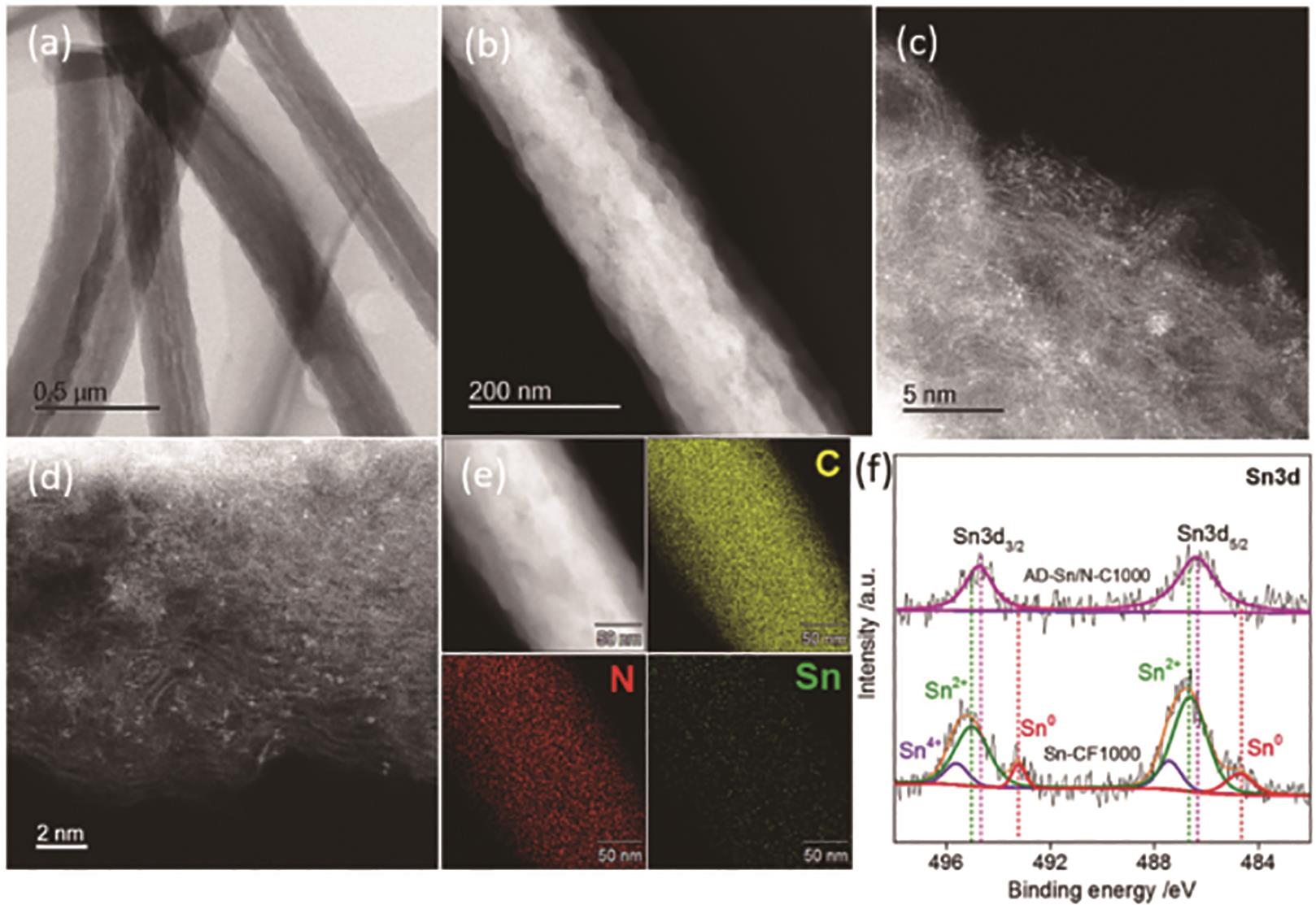
图15 AD-Sn/N-C1000样品的表征: (a)AD-Sn/N-C1000样品的STEM明场像; (b-d)AD-Sn/N-C1000样品不同倍数的HAADF-STEM图像; (e)AD-Sn/N-C1000样品的元素分布图; (f)AD-Sn/N-C1000和Sn-CF1000样品的高分辨XPS Sn3d图谱[53]
Fig.15 Characterization of AD-Sn/N-1000 sample: (a) Bright-field STEM image of AD-Sn/N-1000 sample; (b-d) Dark-field STEM image of AD-Sn/N-1000 sample; (e) Elemental mapping images of AD-Sn/N-1000 sample; (f) High-resolution XPS Sn3d spectral of AD-Sn/N-1000 and Sn-CF1000[53]
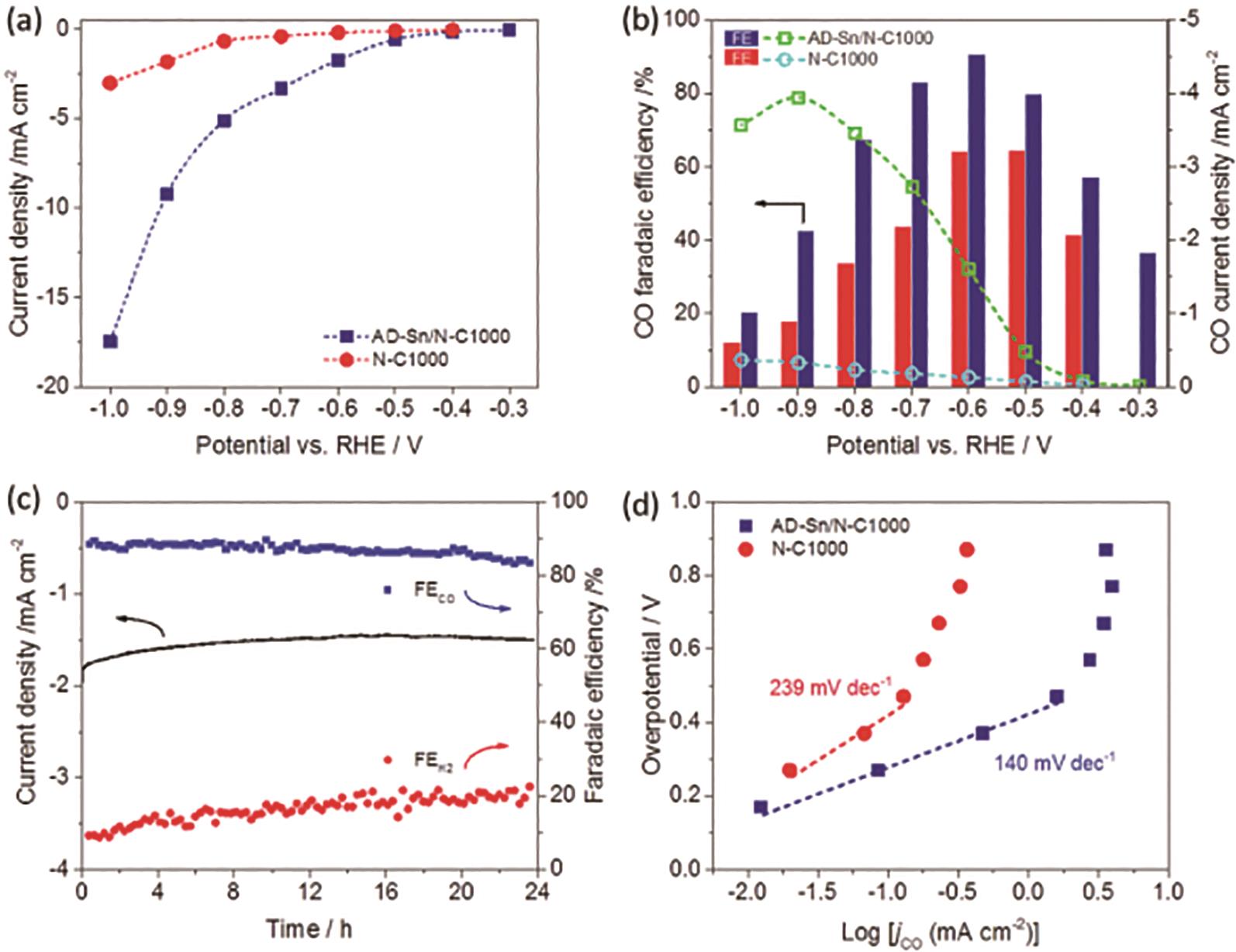
图16 AD-Sn/N-C1000样品的电催化性能: (a)AD-Sn/N-C1000和Sn-CF1000样品在0.1 mol/L KHCO3电解液中,不同电势下的电流密度曲线; (b)AD-Sn/N-C1000和Sn-CF1000样品在不同电势下的法拉第效率测试结果; (c)AD-Sn/N-C1000样品在-0.6 V vs.RHE电势下的稳定性测试结果; (d)AD-Sn/N-C1000和Sn-CF1000样品催化CO产物产生的塔菲尔曲线[53]
Fig.16 Electrocatalytic properties of AD-Sn/N-C1000 sample: (a) Current densities curves of AD-Sn/N-C1000 and Sn-CF1000 samples in 0.1 mol/L KHCO3 electrolyte; (b) Faradaic efficiencies of AD-Sn/N-C1000 and Sn-CF1000 samples at different potentials; (c) Long-term test of AD-Sn/N-C1000 at -0.6 V vs.RHE; (d) Tafel slopes of CO product current densities of AD-Sn/N-C1000 and Sn-CF1000 samples[53]
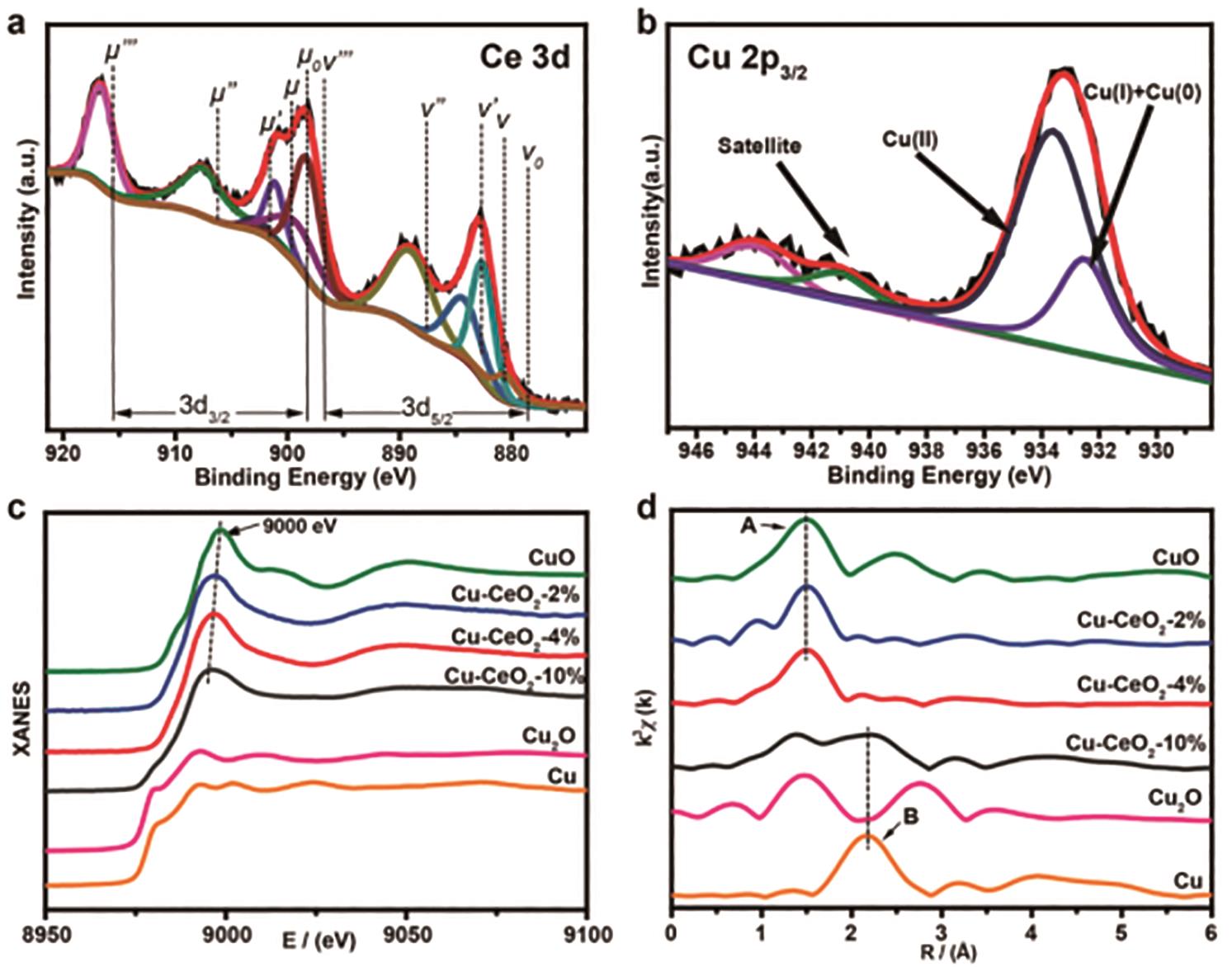
图17 Cu-CeO2样品的XPS和XAFS能谱曲线: (a) Cu-CeO2-4%样品中高分辨XPS Ce3d图谱; (b)Cu-CeO2-4%样品中高分辨XPS Cu2p3/2能谱; (c)含有不同Cu含量的Cu-CeO2、 Cu箔、 CuO和Cu2O样品的XANES图谱; (d)含有不同Cu含量的Cu-CeO2、 Cu箔、 CuO和Cu2O样品的EXAFS图谱[26]
Fig.17 XPS and XAFS spectra of Cu-CeO2 sample: (a) XPS Ce3d spectra of Cu-CeO2-4% sample; (b) XPS Cu2p3/2 spectra of Cu-CeO2-4% sample; (c) XANES spectra of Cu-CeO2, Cu foil, CuO and Cu2O samples; (d) EXAFS spectra of Cu-CeO2, Cu foil, CuO and Cu2O samples[26]
| 1 | DAVIS S J, LEWIS N S, SHANER M, et al. Net-Zero emissions energy systems[J]. Science, 2018, 360(6396): 1419-1427. |
| 2 | SPRINGMANN M, CLARK M, MASON-D'CROZ D, et al. Options for keeping the food system within environmental limits[J]. Nature, 2018, 562(7728): 519-525. |
| 3 | VENTO-TORMO R, EFREMOVA M, BOTTING R A, et al. Single-cell reconstruction of the early maternal-fetal interface in humans[J]. Nature, 2018, 563(7731): 347-353. |
| 4 | GAO D, ZHANG Y, ZHOU Z, et al. Enhancing CO2 electroreduction with the metal-oxide interface[J]. J Am Chem Soc, 2017, 139(16): 5652-5655. |
| 5 | HAO L, KANG L, HUANG H, et al. Surface-halogenation-induced atomic-site activation and local charge separation for superb CO2 photoreduction[J]. Adv Mater, 2019, 31(25): 1900546. |
| 6 | NIU K Y, XU Y, WANG H C, et al. A spongy nickel-organic CO2 reduction photocatalyst for nearly 100% selective CO production[J]. Sci Adv, 2017, 3(7): e1700921. |
| 7 | SZCZESNY J, RUFF A, OLIVEIRA A R, et al. Electroenzymatic CO2 fixation using redox polymer/enzyme-modified gas diffusion electrodes[J]. ACS Energy Lett, 2020, 5(1): 321-327. |
| 8 | LIU M, PANG Y, ZHANG B, et al. Enhanced electrocatalytic CO2 reduction via field-induced reagent concentration[J]. Nature, 2016, 537(7620): 382-386. |
| 9 | CAMERON R, WADE S P, GREGORY V, et al. Synergistic enhancement of electrocatalytic CO2 reduction with gold nanoparticles embedded in functional graphene nanoribbon composite electrodes[J]. J Am Chem Soc, 2017, 139(11): 4052-4061. |
| 10 | HATSUKADE T, KUHL K P, CAVE E R, et al. Insights into the electrocatalytic reduction of CO2 on metallic silver surfaces[J]. Phys Chem Chem Phys, 2014, 16(27): 13814-13819. |
| 11 | SHI M M, BAO D, LI S J, et al. Anchoring PdCu amorphous nanocluster on graphene for electrochemical reduction of N2 to NH3 under ambient conditions in aqueous solution[J]. Adv Energy Mater, 2018, 8(21): 1800124. |
| 12 | SHI M M, BAO D, WULAN B R, et al. Au sub-nanoclusters on TiO2 toward highly efficient and selective electrocatalyst for N2 conversion to NH3 at ambient conditions[J]. Adv Mater, 2017, 29(17): 1606550. |
| 13 | MARCINKOWSKI M D, DARBY M T, LIU J, et al. Pt/Cu single-atom alloys as coke-resistant catalysts for efficient C-H activation[J]. Nat Chem, 2018, 10(3): 325-332. |
| 14 | XU H, WAN J, ZHANG H, et al. A new platinum-like efficient electrocatalyst for hydrogen evolution reaction at all pH: single-crystal metallic interweaved V8C7 networks[J]. Adv Energy Mater, 2018, 8(23): 1800575. |
| 15 | JIANG K, SIAHROSTAMI S, ZHENG T, et al. Isolated Ni single atoms in graphene nanosheets for high-performance CO2 reduction[J]. Energy Environ Sci, 2018, 11(4): 893-903. |
| 16 | YANG F, SONG P, LIU X Z, et al. Highly efficient CO2 electroreduction on ZnN4-based single-atom catalyst[J]. Angew Chem, 2018, 57(38): 12303-12307. |
| 17 | YANG H P, WU Y, LI G D, et al. Scalable production of efficient single-atom copper decorated carbon membranes for CO2 electroreduction to methanol[J]. J Am Chem Soc, 2019, 141(32): 12717-12723. |
| 18 | LI J, CHEN S G, YANG N, et al. Ultrahigh-loading zinc single-atom catalyst for highly efficient oxygen reduction in both acidic and alkaline media[J]. Angew Chem, 2019, 58(21): 7035-7039. |
| 19 | TIAN C C, ZHANG H Y, ZHU X, et al. A new trick for an old support: stabilizing gold single atoms on LaFeO3 perovskite[J]. Appl Catal B, 2020, 261: 118178. |
| 20 | WU W Z, NIU C Y, WEI C, et al. Activation of MoS2 basal planes for hydrogen evolution by zinc[J]. Angew Chem, 2019, 58(7): 2029-2033. |
| 21 | GU J A, ZHU Q, SHI Y Z, et al. Single zinc atoms immobilized on MXene (Ti3C2Clx) layers toward dendrite-free lithium metal anodes[J]. ACS Nano, 2020, 14(1): 891-898. |
| 22 | ZHAO C M, DAI X Y, YAO T, et al. Ionic exchange of metal organic frameworks to access single nickel sites for efficient electroreduction of CO2[J]. J Am Chem Soc, 2017, 139(24): 8078-8081. |
| 23 | REN W B, TAN X, YANG W F, et al. Isolated diatomic Ni-Fe metal-nitrogen sites for synergistic electroreduction of CO2 [J]. Angew Chem, 2019, 58(21): 6972-6976. |
| 24 | ZHANG C, FU Z H, ZHAO Q, et al. Single-atom-Ni-decorated, nitrogen-doped carbon layers for efficient electrocatalytic CO2 reduction reaction[J]. Electrochem Commun, 2020, 116: 106758. |
| 25 | ZHANG J Q, ZHAO Y F, GUO X, et al. Single platinum atoms immobilized on an MXene as an efficient catalyst for the hydrogen evolution reaction[J]. Nat Catal, 2018, 1(12): 985-992. |
| 26 | WANG Y, CHEN Z, HAN P, et al. Single-atomic Cu with multiple oxygen vacancies on ceria for electrocatalytic CO2 reduction to CH4[J]. ACS Catal, 2018, 8(8): 7113-7119. |
| 27 | YAO Y G, HUANG Z N, XIE P F, et al. High temperature shockwave stabilized single atoms[J]. Nat Nanotechnol, 2019, 14(9): 851-857. |
| 28 | QU Y T, WANG L G, LI Z J, et al. Ambient synthesis of single-atom catalysts from bulk metal via trapping of atoms by surface dangling bonds[J]. Adv Mater, 2019, 31(44): 1904496. |
| 29 | RAMALINGAM V, VARADHAN P, FU H C, et al. Heteroatom-mediated interactions between ruthenium single atoms and an MXene support for efficient hydrogen evolution[J]. Adv Mater, 2019: 1903841. |
| 30 | HAN L L, LIU X J, CHEN J P, et al. Atomically dispersed molybdenum catalysts for efficient ambient nitrogen fixation[J]. Angew Chem, 2019, 58(8): 2321-2325. |
| 31 | LI Q H, CHEN W X, XIAO H, et al. Fe isolated single atoms on S, N codoped carbon by copolymer pyrolysis strategy for highly efficient oxygen reduction reaction[J]. Adv Mater, 2018, 30(25): 1800588. |
| 32 | HAN Y H, WANG Y G, CHEN W X, et al. Hollow N-doped carbon spheres with isolated cobalt single atomic sites: superior electrocatalysts for oxygen reduction[J]. J Am Chem Soc, 2017, 139(48): 17269-17272. |
| 33 | WANG X Q, CHEN Z, ZHAO X Y, et al. Regulation of coordination number over single Co sites: triggering the efficient electroreduction of CO2[J]. Angew Chem, 2018, 57(7): 1944-1948. |
| 34 | MISTRY H, VARELA A S, BONIFACIO C S, et al. Highly selective plasma-activated copper catalysts for carbon dioxide reduction to ethylene[J]. Nat Commun, 2016, 7: 12123. |
| 35 | BIRDJA Y Y, PEREZ-GALLENT E, FIGUEIREDO M C, et al. Advances and challenges in understanding the electrocatalytic conversion of carbon dioxide to fuels[J]. Nat Energy, 2019, 4: 732-745. |
| 36 | WANG Y H, WANG Z Y, DINH C T, et al. Catalyst synthesis under CO2 electroreduction favours faceting and promotes renewable fuels electrosynthesis[J]. Nat Catal, 2020, 3: 98-106. |
| 37 | LIU S, YANG H B, HUNG S F, et al. Elucidating the electrocatalytic CO2 reduction reaction over a model single-atom nickel catalyst[J]. Angew Chem, 2020, 59(2): 798-803. |
| 38 | WUTTING A, LIU C, PENG Q, et al. Tracking a common surface-bound intermediate during CO2-to-fuels catalysis[J]. ACS Cent Sci, 2016, 2(8): 522-528. |
| 39 | LUNA P D, QUINTERO-BERMUDEZ R, DINH C T, et al. Catalyst electro-redeposition controls morphology and oxidation state for selective carbon dioxide reduction[J]. Nat Catal, 2018,1: 103-110. |
| 40 | GENG Z G, KONG X D, CHEN W W, et al. Oxygen vacancies in ZnO nanosheets enhance CO2 electrochemical reduction to CO[J]. Angew Chem, 2018, 57(21): 6054-6059. |
| 41 | LI F W, XUE M Q, LI J Z, et al. Unlocking the electrocatalytic activity of antimony for CO2 reduction by two-dimensional engineering of the bulk material[J]. Angew Chem, 2017, 56(46): 14718-14722. |
| 42 | LIU Y M, ZHANG Y J, CHENG K, et al. Selective electrochemical reduction of carbon dioxide to ethanol on a boron- and nitrogen-Co-doped nanodiamond[J]. Angew Chem, 2017, 56(49): 15607-15611. |
| 43 | WON D H, SHIN H, KOH J, et al. Highly efficient, selective, and stable CO2 electroreduction on a hexagonal Zn catalyst[J]. Angew Chem, 2016, 55(32): 9297-9300. |
| 44 | NITOPI S, BERTHEUSSEN E, SCOTT S B, et al. Progress and perspectives of electrochemical CO2 reduction on copper in aqueous electrolyte[J]. Chem Rev, 2019, 119(12): 7610-7672. |
| 45 | MA M, TRZESNIEWSKI B J, XIE J, et al. Selective and efficient reduction of carbon dioxide to carbon monoxide on oxide-derived nanostructured silver electrocatalysts[J]. Angew Chem, 2016, 55(33): 9748-9752. |
| 46 | KORNIENKO N, ZHAO Y B, KLEY C S, et al. Metal-organic frameworks for electrocatalytic reduction of carbon dioxide[J]. J Am Chem Soc, 2015, 137(44): 14129-14135. |
| 47 | YANG H B, HUNG S F, LIU S, et al. Atomically dispersed Ni(I) as the active site for electrochemical CO2 reduction[J]. Nat Energy, 2018, 3(2): 140-147. |
| 48 | ZHANG C H, YANG S Z, WU J J, et al. Electrochemical CO2 reduction with atomic iron-dispersed on nitrogen-doped graphene[J]. Adv Energy Mater, 2018, 8(19): 1703487. |
| 49 | DING C M, FENG C C, MEI Y H, et al. Carbon nitride embedded with transition metals for selective electrocatalytic CO2 reduction[J]. Appl Catal B, 2020, 268: 118391. |
| 50 | ZHANG H C, CHANG X X, CHEN J G, et al. Computational and experimental demonstrations of one-pot tandem catalysis for electrochemical carbon dioxide reduction to methane[J]. Nat Commun, 2019, 10: 3340. |
| 51 | JIAO J Q, LIN R, LIU S J, et al. Copper atom-pair catalyst anchored on alloy nanowires for selective and efficient electrochemical reduction of CO2[J]. Nat Chem, 2019, 11: 222-228. |
| 52 | WANG Q J, CHEN L Y, LIU Z, et al. Phosphate-mediated immobilization of high-performance AuPd nanoparticles for dehydrogenation of formic acid at room temperature[J]. Adv Funct Mater, 2019, 29(39): 1903341. |
| 53 | ZHAO Y, LIANG J J, WANG C Y, et al. Tunable and efficient tin modified nitrogen-doped carbon nanofibers for electrochemical reduction of aqueous carbon dioxide[J]. Adv Energy Mater, 2018, 8(10): 1702524. |
| 54 | ZHAO Q, ZHANG C, HU R M, et al. Selective etching quaternary MAX phase toward single atom copper immobilized MXene (Ti3C2Clx) for efficient CO2 electroreduction to methanol[J]. ACS Nano, 2021, 15(3): 4927-4936. |
| 55 | ZHENG W Z, YANG J, CHEN H Q, et al. Atomically defined undercoordinated active sites for highly efficient CO2 electroreduction[J]. Adv Funct Mater, 2019, 30(4): 1907658. |
| 56 | ZHANG H C, CHANG X X, CHEN J G, et al. Computational and experimental demonstrations of one-pot tandem catalysis for electrochemical carbon dioxide reduction to methane[J]. Nat Commun, 2019, 10: 3340. |
| [1] | 王路飞, 甄蒙蒙, 沈伯雄. 贫电解液下电催化剂对调控锂硫电池性能的研究进展[J]. 应用化学, 2023, 40(2): 188-209. |
| [2] | 曹蓉, 夏杰桢, 廖漫华, 赵路超, 赵晨, 吴琪. 单原子催化剂在电化学合成氨中的理论研究进展[J]. 应用化学, 2023, 40(1): 9-23. |
| [3] | 王显, 杨小龙, 马荣鹏, 刘长鹏, 葛君杰, 邢巍. 单原子分散的Ir-N-C燃料电池阳极抗中毒催化剂[J]. 应用化学, 2022, 39(8): 1202-1208. |
| [4] | 杜卫民, 刘欣, 朱琳, 付佳敏, 郭文山, 杨晓晴, 双培硕. 三元镍基硫属化物纳米棒阵列的简单合成及其高效的电催化析氧性能[J]. 应用化学, 2022, 39(8): 1252-1261. |
| [5] | 王岩, 张树聪, 汪兴坤, 刘志承, 王焕磊, 黄明华. 电解海水析氢反应过渡金属基催化剂的研究进展[J]. 应用化学, 2022, 39(6): 927-940. |
| [6] | 曹维锦, 白璐, 武兰兰, 李敬德, 宋术岩. 多壳层中空镍钴双金属磷化物纳米球用于高效电催化析氧[J]. 应用化学, 2022, 39(4): 666-672. |
| [7] | 陶荟冰, 田震, 谢勇, 孙瑜, 汪莉, 康卓, 张跃. 电解水催化材料动态构效关系的原位拉曼研究进展[J]. 应用化学, 2022, 39(4): 528-539. |
| [8] | 商林杰, 刘江, 兰亚乾. 共价有机框架材料用于光/电催化CO2还原的研究进展[J]. 应用化学, 2022, 39(4): 559-584. |
| [9] | 张艺潆, 李翠艳, 赵杰, 余笑明, 方千荣. 卟啉-硫醚基共价有机框架材料用于氧还原反应电催化剂[J]. 应用化学, 2022, 39(4): 647-656. |
| [10] | 孙立智, 吕浩, 闵晓文, 刘犇. 介孔钯-硼合金纳米颗粒的制备和甲醇氧化电催化性能[J]. 应用化学, 2022, 39(4): 673-684. |
| [11] | 吴小峰, 陈德顺, 马伟, 黄科科. 电喷雾沉积WO3/Fe2TiO5复合光阳极及其光电催化水裂解性能[J]. 应用化学, 2022, 39(4): 694-696. |
| [12] | 毕一飘, 宫雪, 杨发, 阮明波, 宋平, 徐维林. 多价MnOx/C电催化剂用于高效氮还原反应[J]. 应用化学, 2020, 37(9): 1048-1055. |
| [13] | 李琳, 任慧敏, 卫博慧, 李军, 王杰, 李晖, 姚陈忠. 钒-氮共掺杂碳基介孔纳米材料的制备及氮还原电催化性能[J]. 应用化学, 2020, 37(8): 930-938. |
| [14] | 蒙阳, 杨婵, 彭娟. 基于铁、钴、镍金属磷化物纳米催化剂的碱性条件下电解水制氢的研究进展[J]. 应用化学, 2020, 37(7): 733-745. |
| [15] | 陈佳琪, 周焱, 孙敬文, 朱俊武, 汪信, 付永胜. 基于金属有机框架中空材料的研究进展[J]. 应用化学, 2020, 37(11): 1221-1235. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
