应用化学 ›› 2022, Vol. 39 ›› Issue (4): 528-539.DOI: 10.19894/j.issn.1000-0518.220001
电解水催化材料动态构效关系的原位拉曼研究进展
陶荟冰1, 田震2, 谢勇2, 孙瑜2, 汪莉1( ), 康卓2(
), 康卓2( ), 张跃2
), 张跃2
- 1.北京科技大学能源与环境工程学院,北京 100083
2.北京科技大学材料科学与工程学院,前沿交叉科学与技术研究院,新金属材料国家重点实验室,北京 100083
-
收稿日期:2022-01-04接受日期:2022-02-21出版日期:2022-04-01发布日期:2022-04-19 -
通讯作者:汪莉,康卓 -
作者简介:E-mail: zhuokang@ustb.edu.cn
E-mail: wangli@ces.ustb.edu.cn;
第一联系人:褚小立,中石化石油化工科学研究院教授级高工,20余年从事近红外光谱的研究和应用工作,现任中国仪器仪表学会近红外光谱分会秘书长,主编《近红外光谱分析技术手册》《近红外光谱在线仪器设备手册》以及“现代过程分析技术”系列丛书,撰写传记《新青胜蓝惟所盼——陆婉珍传》,组编《“我与近红外的故事”文集》等著作。
赵一霖,吉林大学化学学士,加拿大麦克马斯特大学理论化学博士生。从事电子结构理论研究,主要方向为密度矩阵重整化群方法开发。主导开发量子化学计算软件MoHa,并参与开发量子化学计算软件Horton。精通量子力学和光谱学,文理兼通,多次在诗歌比赛中获奖。
(下转第558页) -
基金资助:国家重点研发计划资助项目(2018YFA0703503);高等学校学科创新引智计划(B14003);国家自然科学基金资助项目(51991340)
Progress of In situ Raman Study on the Dynamic Structure Performance Correlation of Water Splitting Catalysts
Hui-Bing TAO1, Zhen TIAN2, Yong XIE2, Yu SUN2, Li WANG1( ), Zhuo KANG2(
), Zhuo KANG2( ), Yue ZHANG2
), Yue ZHANG2
- 1.Academy for Advanced Interdisciplinary Science and Technology,University of Science and Technology Beijing,Beijing 100083,China
2.School of Materials Science and Engineering,Academy for Advanced Interdisciplinary Science and Technology,State Key Laboratory for Advanced Metals and Materials,University of Science and Technology Beijing,Beijing 100083,China
-
Received:2022-01-04Accepted:2022-02-21Published:2022-04-01Online:2022-04-19 -
Contact:Li WANG,Zhuo KANG -
Supported by:the National Key Research and Development Program of China(2018YFA0703503);the Overseas Expertise Introduction Projects for Discipline Innovation(B14003);the National Natural Science Foundation of China(51991340)
摘要:
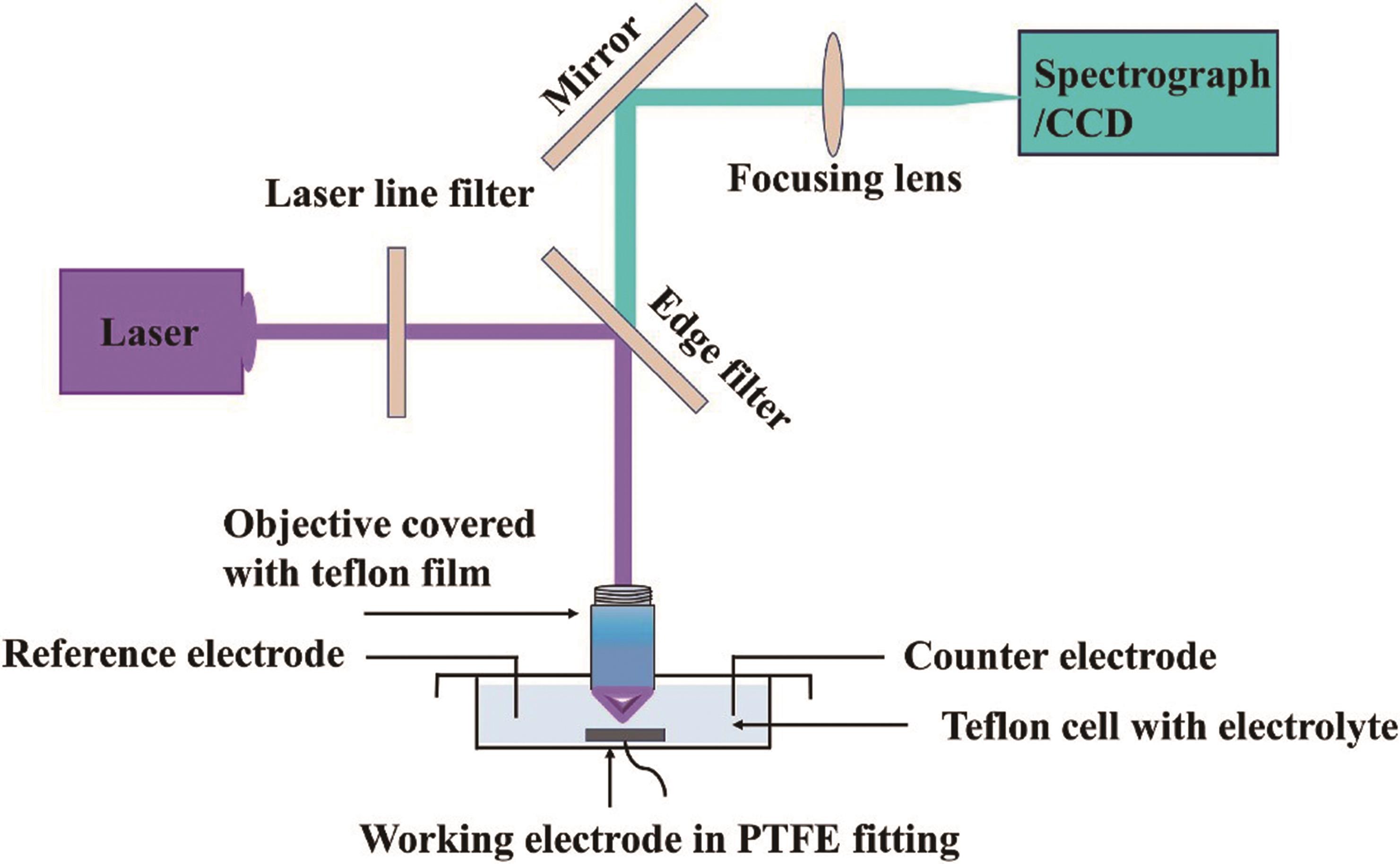
可再生能源电解水产氢对于实现碳中和目标和未来可持续社会的发展具有重要意义。然而,在电解水服役过程中,催化材料往往会发生复杂的结构演变,这对深入理解电解水催化材料反应机制和精准设计高效催化材料造成了挑战。原位电化学拉曼表征技术对催化材料结构动态演变过程的实时监测,是揭示电解水材料动态构效关系,解析催化反应机理的关键。本文介绍了原位电化学拉曼表征技术的基本原理,重点综述了其在催化材料相结构演变、表面活性位点和界面水分子行为中的最新进展,阐述了电解水催化材料在服役过程中结构演变与性能演变之间的变化规律,为实现催化材料全生命周期动态构效关系的精准构建提供了技术基础。最后,分析总结了原位电化学拉曼表征技术在电解水应用过程中存在的问题与挑战,并对先进原位电化学拉曼技术未来的发展进行了展望。
中图分类号:
引用本文
陶荟冰, 田震, 谢勇, 孙瑜, 汪莉, 康卓, 张跃. 电解水催化材料动态构效关系的原位拉曼研究进展[J]. 应用化学, 2022, 39(4): 528-539.
Hui-Bing TAO, Zhen TIAN, Yong XIE, Yu SUN, Li WANG, Zhuo KANG, Yue ZHANG. Progress of In situ Raman Study on the Dynamic Structure Performance Correlation of Water Splitting Catalysts[J]. Chinese Journal of Applied Chemistry, 2022, 39(4): 528-539.
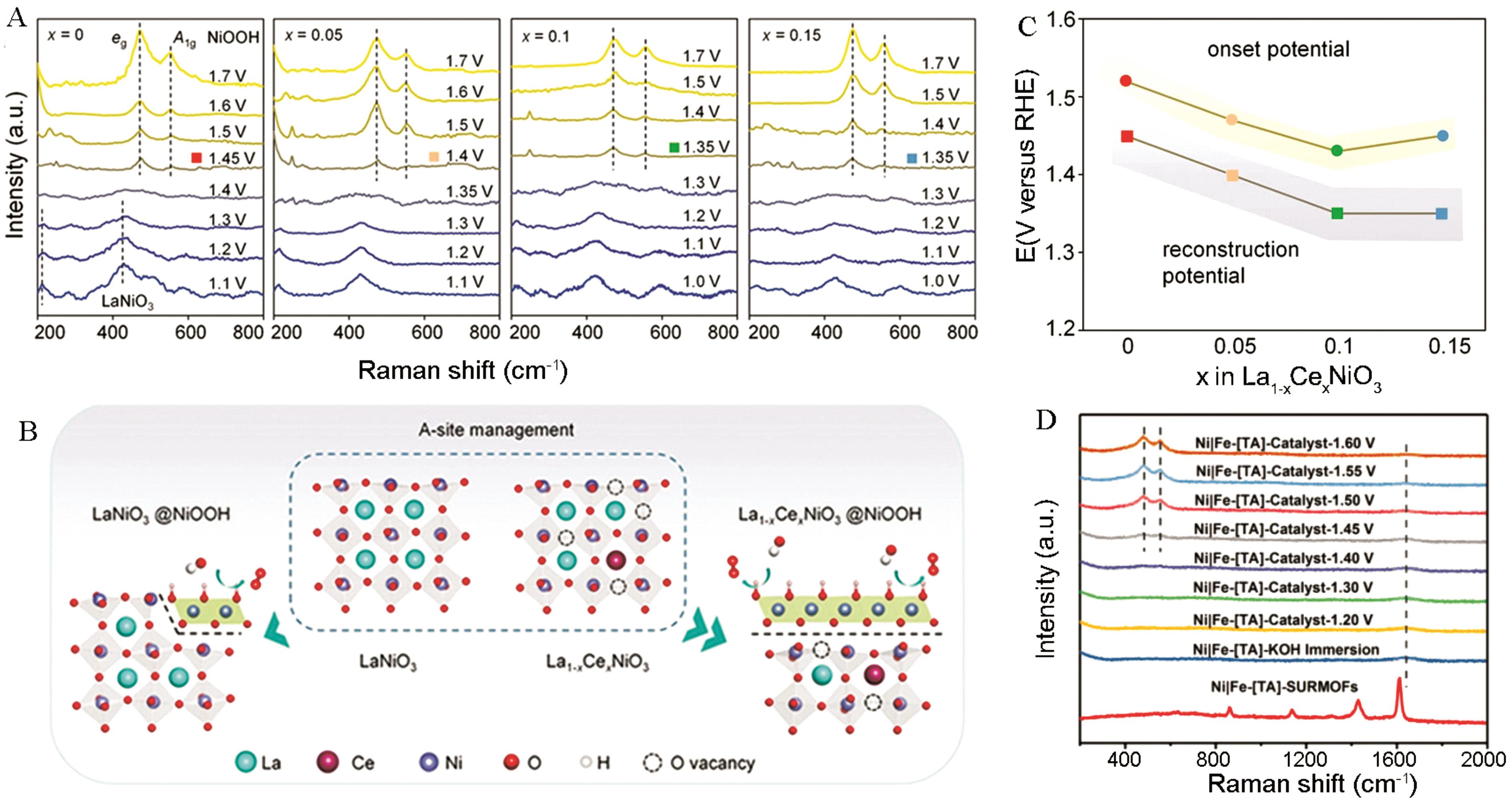
图2 (A) La1-x Ce x NiO3 (x = 0、0.05、0.1和 0.15)的原位拉曼光谱图(1.0~1.7 V vs RHE)。(B) 通过Ce替代策略的A位点掺杂,La1-x Ce x NiO3对NiOOH活性结构的表面重建过程示意图。(C) 表面重构电位与氧演化反应相应起始电位的相关性[33]。(D) 0.1 mol/L KOH电解液中,在200~2000 cm-1的低波数范围收集的原位拉曼光谱图[42]
Fig.2 (A) In situ Raman spectra of La1-x Ce x NiO3 (x=0, 0.05, 0.1 and 0.15) during the OER process (1.0~1.7 V vs RHE). (B) The surface reconstruction process of La1-x Ce x NiO3 to active structure of NiOOH by the A-site management of Ce substitution strategy. The correlation between surface reconstruction potential and the corresponding onset potential for OER. (C) The correlation between surface reconstruction potential and the corresponding onset potential for OER[33]. (D) In situ Raman spectra collected during the OER process in 0.1 mol/L KOH within a low-wavenumber region of 200~2000 cm-1[42]
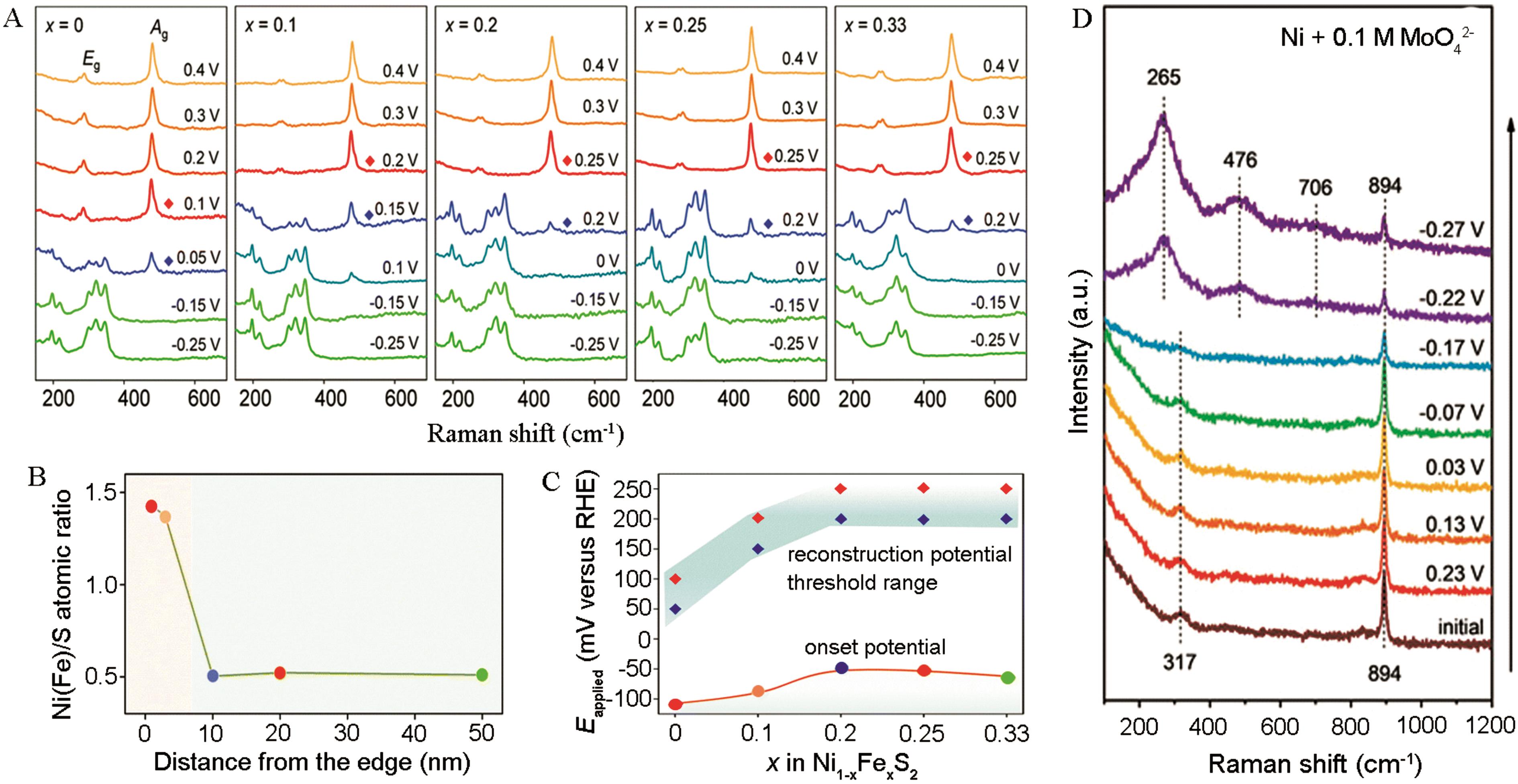
图3 (A) Ni1-x Fe x S2 (x=0、0.1、0.2、0.25和0.33)从0.4~-0.25 V(vs RHE)的原位拉曼光谱图(1 mol/L KOH)。(B) 在从晶体边缘到本体的系列斑点上测定了电解后Ni0.8Fe0.2S2的元素(EDS)组成图,揭示了晶体中Ni(Fe)∶S组成的变化。(C) Ni1-x Fe x S2 (x=0~0.33)样品的表面重构电位阈值范围,以及相应的氢演化起始电位。红色点代表Ni1-x Fe x S2结构中拉曼峰,蓝色点代表Ni3S2拉曼峰。EDS在从晶体边缘到本体的系列斑点上测定了电解后Ni0.8Fe0.2S2的元素组成图,揭示了晶体中Ni(Fe)∶S组成的变化[44]。(D) 在0.1 mol/L MoO42-中金属Ni的原位拉曼光谱图[45]
Fig.3 (A) In situ Raman spectra of the Ni1-x Fe x S2 (x=0, 0.1, 0.2, 0.25 and 0.33) samples at the potentials of 0.4 to -0.25 V versus the reversible hydrogen electrode (vs. RHE) in 1 mol/L KOH. (B) Elemental composition of post-electrolysis Ni0.8Fe0.2S2 determined from EDS at a series of spots along a line from the crystallite edge to the bulk reveals the variation in Ni(Fe)∶S composition across the crystallite. (C) The surface reconstruction potential threshold range for the Ni1-x Fe x S2 (x=0~0.33) samples, and the corresponding onset potentials of hydrogen evolution are plotted to show the dynamic correlation of structure-activity. The red diamond dots represent the final potential of the existence of Raman peaks for the Ni1-x Fe x S2 structures, and the blue diamond dots stand for the potential of emerging Ni3S2 Raman peaks[44]. (D) Potential-dependent in situ Raman spectra of metal Ni with 0.1 mol/L MoO42-[45]
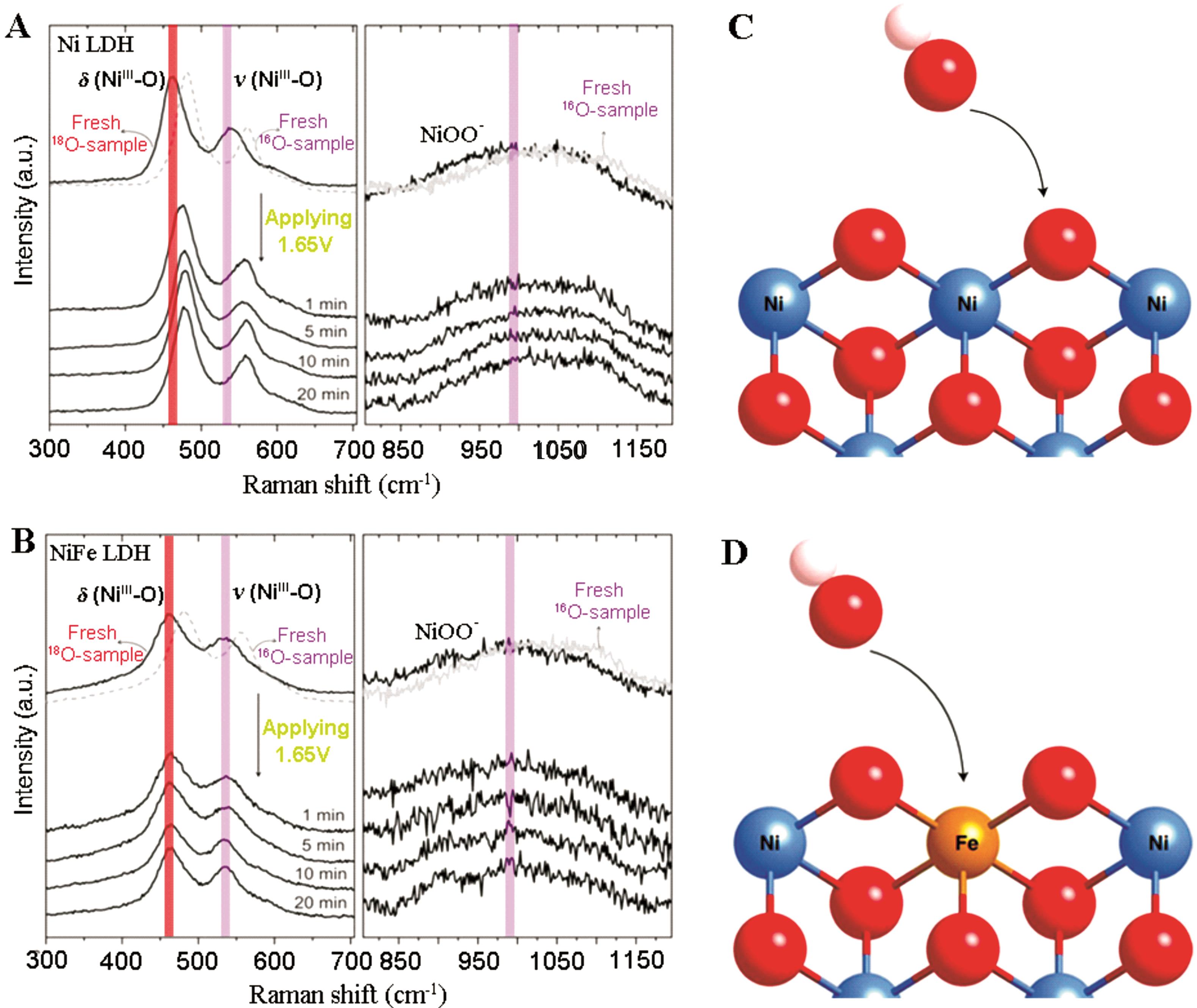
图4 18O标记的 (A) Ni LDHs和 (B) NiFe LDHs的原位拉曼光谱图(vs.RHE)。在0.1 mol/L KOH电解液中,左列和右列分别代表NiOOH和NiOO物种。(C) 晶格氧在Ni和NiCo LDHs中的示意图。(D) 晶格氧不参与NiFe LDH的示意图,M表示Ni或Co[50]
Fig.4 In?situ Raman spectrum of 18O labeled (A) Ni LDHs and (B) NiFe LDHs measured at 1.65 V (vs. RHE) in 0.1 mol/L KOH. The left column and right column represent the NiOOH and NiOO species, respectively. (C) Participation of lattice oxygen in Ni and NiCo LDHs. (D) Nonparticipation of lattice oxygen on NiFe LDH. M=Ni or Co[50]
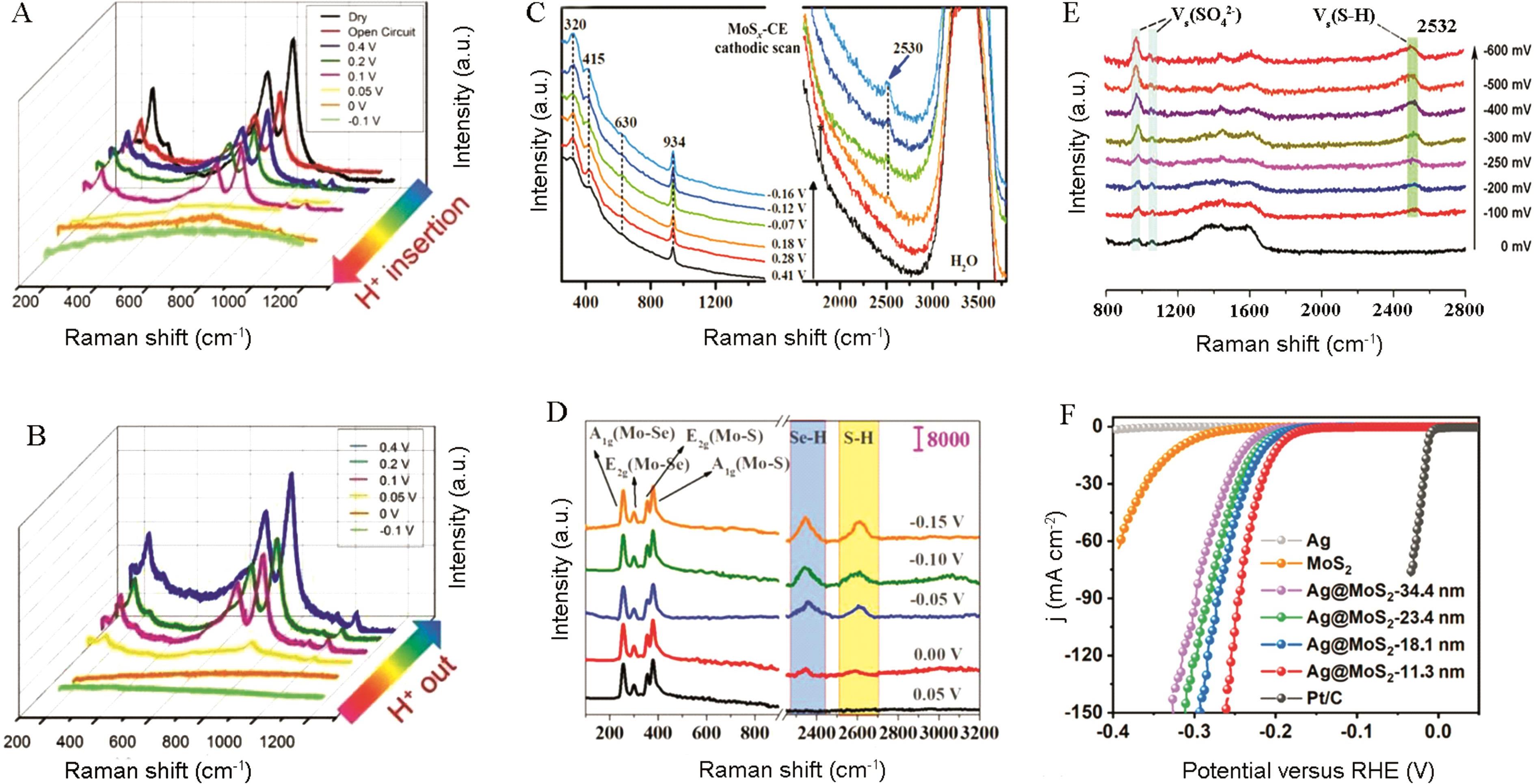
图5 (A)和(B) Pt-WO3在0.4~-0.1 V之间(vs RHE)的原位拉曼光谱图[54]。(C) 在1 mol/L HClO4电解液中,不同阴极电位下阴极沉积的MoS x 的原位拉曼光谱图[59]。(D) 样品MoS0.9Se1.1在0.05、0.00、-0.05、-0.10和-0.15 V电位下的原位拉曼光谱图[60]。(E) 尺寸为(23.4±3.8) nm的Ag@MoS2在0.5 mol/L H2SO4电解液中原位电化学表面增强拉曼光谱图(EC-SERS)。(F)在商业Pt/C、Ag纳米晶体、MoS2纳米片和不同尺寸Ag@MoS2测量的HER的LSV曲线[61]
Fig.5 (A) and (B) in situ Raman spectra of Pt-WO3 from 0.4 to -0.1 V[54]. (C) In situ Raman spectrum of cathodically deposited MoSx at different cathodic potential in 1 mol/L HClO4[59]. (D) In situ Raman spectra of the sample MoS0.9Se1.1 at voltages of 0.05, 0.00, -0.05, -0.10 and -0.15 V[60] respectively. (E) In situ electrochemical surface-enhanced Raman spectroscopy (EC-SERS) of HER on Ag@MoS2 with size of (23.4 ± 3.8) nm in 0.5 mol/L H2SO4 electrolyte. (F) LSV curves for HER measured on the commercial Pt/C, Ag nanocrystals, MoS2 nanosheets, and Ag@MoS2 with different sizes[61]
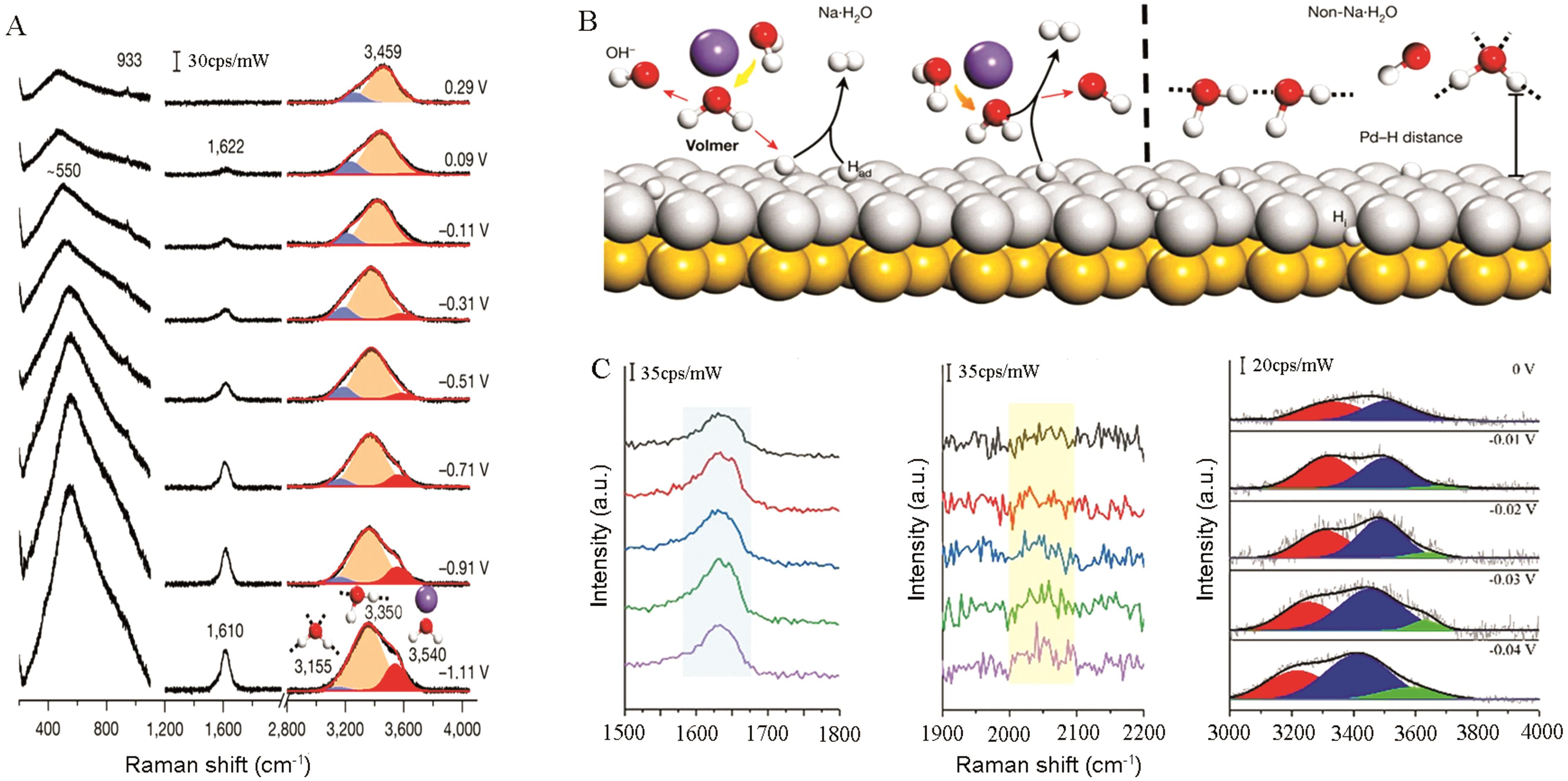
图6 (A)在0.1 mol/L NaClO4溶液(pH=11)中,Pd(111)电极上的界面水的原位拉曼光谱图。3种O—H拉伸模式的高斯拟合分别用蓝色(低波数成分:4-配位氢键水)、橙色(主要成分:2-配位氢键水)和红色(高波数分量:弱氢键相互作用的Na+耦合水)表示。c.p.s.: 每秒钟的计数。(B) Pd(111)表面(Au(111)涂有Pd单层)的界面水离解示意图[21]。(C)在0.1 mol/L NaOH中,Acid-PtNi1.5表面的原位电化学拉曼光谱图[66]
Fig.6 (A) In situ Raman spectra of interfacial water on a Pd (111) electrode in a 0.1 mol/L NaClO4 solution (pH=11). Gaussian fits of three O—H stretching modes are shown in blue, orange and red, respectively. c.p.s., counts per second. (B) Schematic showing interfacial water dissociation on a Pd (111) surface (Au (111) coated with Pd monolayer)[21]. (C) In situ electrochemical Raman spectra at the Acid-PtNi1.5 surface in 0.1 mol/L NaOH[66]
| 1 | DÜRR R N, MALTONI P, TIAN H N, et al. From NiMoO4 to γ-NiOOH: detecting the active catalyst phase by time resolved in situ and operando Raman spectroscopy[J]. ACS Nano, 2021, 15(8): 13504-13515. |
| 2 | 蒙阳, 杨婵, 彭娟. 基于铁、钴、镍金属磷化物纳米催化剂的碱性条件下电解水制氢的研究进展[J]. 应用化学, 2020, 37(7): 733-745. |
| MENG Y, YANG C, PENG J. Progress in iron, cobalt and nickel-based metal phosphide nano-catalysts for hydrogen production under alkaline conditions[J]. Chinese J Appl Chem, 2020, 37(7): 733-745. | |
| 3 | SENTHIL R D, CHUAH X F, LU S Y, et al. In situ grown bimetallic MOF-based composite as highly efficient bifunctional electrocatalyst for overall water splitting with ultrastability at high current densities[J]. Adv Energy Mater, 2018, 8: 1801065. |
| 4 | LIAO X B, LU R H, XIA L X, et al. Density functional theory for electrocatalysis[J]. Energy Environ Mater, 2022, 5(1): 157-185. |
| 5 | WANG Q C, LEI Y P, WANG Y C, et al. Atomic-scale engineering of chemical-vapor-deposition-grown 2D transition metal dichalcogenides for electrocatalysis[J]. Energy Environ Sci, 2020, 13: 1593-1616. |
| 6 | FENG K, WANG Y N, GUO M, et al. In‑situ/operando techniques to identify active sites for thermochemical conversion of CO2 over heterogeneous catalysts[J]. J Energy Chem, 2021, 62: 153-171. |
| 7 | ZHU Y P, WANG J L, CHU H, et al. In situ/operando studies for designing next-generation electrocatalysts[J]. ACS Energy Lett, 2020, 5: 1281-1291. |
| 8 | WANG J, GAO Y, KONG H, et al. Non-precious-metal catalysts for alkaline water electrolysis: operando characterizations, theoretical calculations, and recent advances[J]. Chem Soc Rev, 2020, 49: 9154-9196. |
| 9 | KUAI C G, XU Z R, XI C, et al. Phase segregation reversibility in mixed-metal hydroxide water oxidation catalysts[J]. Nat Catal, 2020, 3: 743-753. |
| 10 | PISHGAR S, GULATI S, STRAIN J M, et al. In situ analytical techniques for the investigation of material stability and interface dynamics in electrocatalytic and photoelectrochemical applications[J]. Small Methods, 2021, 5: 2100322. |
| 11 | WANG X, ZHANG Y W, WU J, et al. Single-atom engineering to ignite 2D transition metal dichalcogenide based catalysis: fundamentals, progress, and beyond[J]. Chem Rev, 2022, 122(1): 1273-1348. |
| 12 | LORIDANT S. Raman spectroscopy as a powerful tool to characterize ceria-based catalysts[J]. Catal Today, 2021, 373: 98-111. |
| 13 | XU Z Z, LIANG Z B, GUO W H, et al. In situ/operando vibrational spectroscopy for the investigation of advanced nanostructured electrocatalysts[J]. Coordin Chem Rev, 2021, 436: 213824. |
| 14 | WEI J, QIN S N, YANG J, et al. Probing single-atom catalysts and catalytic reaction processes by shell-isolated nanoparticle-enhanced Raman spectroscopy[J]. Angew Chem Int Ed, 2021, 60: 9306-9310. |
| 15 | GRAF M, VONBUN-FELDBAUER G B, KOPER M T M. Direct and broadband plasmonic charge transfer to enhance water oxidation on a gold electrode[J]. ACS Nano, 2021, 15(2): 3188-3200. |
| 16 | LI W Q, ZHOU R Y, WANG X T, et al. Identification of the molecular pathways of RuO2 electroreduction by in situ electrochemical surface-enhanced Raman spectroscopy[J]. J Catal, 2021, 400: 367-371. |
| 17 | BAI L, HSU C S, ALEXANDER D T L, et al. Double-atom catalysts as a molecular platform for heterogeneous oxygen evolution electrocatalysis[J]. Nat Energy, 2021, 6: 1054-1066. |
| 18 | LI L G, BU L Z, HUANG B L, et al. Compensating electronic effect enables fast site-to-site electron transfer over ultrathin RuMn nanosheet branches toward highly electroactive and stable water splitting[J]. Adv Mater, 2021: 2105308. |
| 19 | FLEISCHMAN M, HENDRA P J, MCQUILLAN A J. Long-range manifestation of surface-enhanced Raman scattering[J]. Chem Phys Lett, 1974, 26: 163-166. |
| 20 | WANG X, HUANG S C, HU S, et al. Fundamental understanding and applications of plasmon-enhanced Raman spectroscopy[J]. Nat Rev Phys, 2020, 2: 253-271. |
| 21 | WANG Y H, ZHENG S S, YANG W M. In situ Raman spectroscopy reveals the structure and dissociation of interfacial water[J]. Nature, 2021, 600: 81-85. |
| 22 | HARTMAN T, GEITENBEEK R G, WONDERGEM C S, et al. Operando nanoscale sensors in catalysis: all eyes on catalyst particles[J]. ACS Nano, 2020, 14: 3725-3735. |
| 23 | CHENG Y, YUAN P F, XU X H, et al. S-edge-rich MoxSy arrays vertically grown on carbon aerogels as superior bifunctional HER/OER electrocatalysts[J]. Nanoscale, 2019, 11: 20284. |
| 24 | ZHANG H, DUAN S, RADJENOVIC P M, et al. Core-shell nanostructure-enhanced raman spectroscopy for surface catalysis[J]. Acc Chem Res, 2020, 53: 729-739. |
| 25 | ZHANG J, ZHANG Q Y, FENG X L. Support and interface effects in water-splitting electrocatalysts[J]. Adv Mater, 2019, 31: 1808167. |
| 26 | SHI S, QIN D. Bifunctional metal nanocrystals for catalyzing and reporting on chemical reactions[J]. Angew Chem Int Ed, 2020, 59: 3782 -3792. |
| 27 | LAI W, GE L H, LI H M, et al. In situ Raman spectroscopic study towards the growth and excellent HER catalysis of Ni/Ni(OH)2 heterostructure[J]. Int J Hydrogen Energy, 2021, 46: 26861-26872. |
| 28 | HU C J, HU Y F, FAN C H, et al. Surface-enhanced Raman spectroscopic evidence of key intermediate species and role of NiFe dual-catalytic center in water oxidation[J]. Angew Chem Int Ed, 2021, 60: 19774-19778. |
| 29 | NGUYEN T X, LIAO Y C, LIN C C, et al. Advanced high entropy perovskite oxide electrocatalyst for oxygen evolution reaction[J]. Adv Funct Mater, 2021, 31: 2101632. |
| 30 | SARI F N I, ABDILLAH S, TING J M. FeOOH-containing hydrated layered iron vanadate electrocatalyst for superior oxygen evolution reaction and efficient water splitting[J]. Chem Eng J, 2021, 416: 129165. |
| 31 | BOUCLY A, ARITIGLIA L, FABBRI E, et al. Direct evidence of cobalt oxyhydroxide formation on a La0.2Sr0.8CoO3 perovskite water splitting catalyst[J]. J Mater Chem A, 2022, 10: 2434-2444. |
| 32 | OH N K, SEO J, LEE S, et al. Highly efficient and robust noble-metal free bifunctional water electrolysis catalyst achieved via complementary charge transfer[J]. Nat Commun, 2021, 12: 4606. |
| 33 | SUN Y, LI R, CHEN X X, et al. A-site management prompts the dynamic reconstructed active phase of perovskite oxide OER catalysts[J]. Adv Energy Mater, 2021, 11: 2003755. |
| 34 | MENG T, LI Q, YAN M X, et al. Electrochemically induced in‑situ surface self-reconstruction on Ni, Fe, Zn ternary-metal hydroxides towards the oxygen-evolution performance[J]. Chem Eng J, 2021, 410: 128331. |
| 35 | WU J, YU Z J, ZHANG Y Y, et al. Understanding the effect of second metal on CoM (M = Ni, Cu, Zn) metal-organic frameworks for electrocatalytic oxygen evolution reaction[J]. Small, 2021: 2105150. |
| 36 | ZHANG W D, HU Q T, WANG L L, et al. In‑situ generated Ni-MOF/LDH heterostructures with abundant phase interfaces for enhanced oxygen evolution reaction[J]. Appl Catal B: Environ, 2021, 286: 119906. |
| 37 | CHOI Y, CHENA T, KIM D, et al. Transformation of microwave synthesized highly uniform FeMo-MIL-88B nanorod to oxynitride derivate for overall water splitting reaction[J]. Appl Mater Today, 2021, 24: 101093. |
| 38 | ZHAO S L, TAN C H, HE C T, et al. Structural transformation of highly active metal-organic framework electrocatalysts during the oxygen evolution reaction[J]. Nat Energy, 2020, 5: 881-890. |
| 39 | ZHAO S L, WANG Y, DONG J C, et al. Ultrathin metal-organic framework nanosheets for electrocatalytic oxygen evolution[J]. Nat Energy, 2016, 1(12): 1-10. |
| 40 | GUO J, QIN Y T, ZHU Y F, et al. Metal-organic frameworks as catalytic selectivity regulators for organic transformations[J]. Chem Soc Rev, 2021,50: 5366. |
| 41 | ZHENG W, LIU M, LEE L Y S. Electrochemical instability of metal-organic frameworks: in situ spectroelectrochemical investigation of the real active sites[J]. ACS Catal, 2020, 10: 81-92. |
| 42 | HOU S J, LI W J, WATZELE S, et al. Metamorphosis of heterostructured surface-mounted metal-organic frameworks yielding record oxygen evolution mass activities[J]. Adv Mater, 2021, 33: 2103218. |
| 43 | CHENG C C, CHENG P Y, HUANG C L, et al. Gold nanocrystal decorated trimetallic metal organic frameworks as high performance electrocatalysts for oxygen evolution reaction[J]. Appl Catal B: Environ, 2021, 286: 119916. |
| 44 | SUN Y, WU J, ZHANG Z, et al. Phase reconfiguration of multivalent nickel sulfides in hydrogen evolution[J]. Energy Environ Sci, 2022, 15: 633-644. |
| 45 | DU W, SHI Y W, ZHOU W, et al. Unveiling the in situ dissolution and polymerization of Mo in Ni4Mo alloy for promoting the hydrogen evolution reaction[J]. Angew Chem Int Ed, 2021, 60: 7051-7055. |
| 46 | ZE H J, CHEN X, WANG X T, et al. Molecular insight of the critical role of Ni in Pt-based nanocatalysts for improving the oxygen reduction reaction probed using an in situ SERS borrowing strategy[J]. J Am Chem Soc, 2021, 143 (3): 1318-1322. |
| 47 | BALAJI R, MAHESHWARAN S, CHEN S M, et al. High-performance catalytic strips assembled with BiOBr Nano-rose architectures for electrochemical and SERS detection of theophylline[J]. Chem Eng J, 2021, 425: 130616. |
| 48 | LONG C, HAN J Y, GUO J, et al. Operando toolbox for heterogeneous interface in electrocatalysis[J]. Chem Catal, 2021, 19: 509-522. |
| 49 | WANG X, ZHANG Y W, SI H N, et al. Single-atom vacancy defect to trigger high-efficiency hydrogen evolution of MoS2[J]. J Am Chem Soc, 2020, 142: 4298-4308. |
| 50 | LEE S, BANJAC K, LINGENFELDER M, et al. Oxygen isotope labeling experiments reveal different reaction sites for the oxygen evolution reaction on nickel and nickel iron oxides[J]. Angew Chem Int Ed, 2019, 58: 10295-10299. |
| 51 | MA M, KUMAR A, WANG D N, et al. Boosting the bifunctional oxygen electrocatalytic performance of atomically dispersed Fe site via atomic Ni neighboring[J]. Appl Catal B: Environ, 2020, 274: 119091. |
| 52 | PANG B B, LIU X K, LIU T Y, et al. Laser-assisted high-performance PtRu alloy for pH-universal hydrogen evolution[J]. Energy Environ Sci, 2022, 15: 102-108. |
| 53 | LEE W H, KO Y J, KIM J H, et al. High crystallinity design of Ir-based catalysts drives catalytic reversibility for water electrolysis and fuel cells[J]. Nat Commun, 2021, 12: 4271. |
| 54 | XIE C, CHEN W, DU S Q, et al. In‑situ phase transition of WO3 boosting electron and hydrogen transfer for enhancing hydrogen evolution on Pt[J]. Nano Energy 2020, 71: 104653. |
| 55 | WANG X, ZHANG Y W, SI H N, et al. Single-atom vacancy defect to trigger high-efficiency hydrogen evolution of MoS2[J]. J Am Chem Soc, 2020, 142: 4298-4308. |
| 56 | DAHIYA Y, HARIRAM M, KUMAR M, et al. Modified transition metal chalcogenides for high performance supercapacitors: current trends and emerging opportunities[J]. Coordin Chem Rev, 2021, 451: 214265. |
| 57 | HINNEMANN B, MOSES P G, BONDE J, et al. Biomimetic hydrogen evolution: MoS2 nanoparticles as catalyst for hydrogen evolution[J]. J Am Chem Soc, 2005, 127: 5308-5309. |
| 58 | JARAMILLO T F, JORGENSEN K P, BONDE J, et al. Identification of active edge sites for electrochemical H2 evolution from MoS2 nanocatalysts[J]. Science, 2007, 317: 100-102. |
| 59 | DENG Y L, TING L R L, NEO P H L, et al. Operando Raman spectroscopy of amorphous molybdenum sulfide (MoSx) during the electrochemical hydrogen evolution reaction: identification of sulfur atoms as catalytically active sites for H+ reduction[J]. ACS Catal, 2016, 6: 7790-7798. |
| 60 | GUO S H, LI Y H, TANG S W, et al. Monitoring hydrogen evolution reaction intermediates of transition metal dichalcogenides via operando Raman spectroscopy[J]. Adv Funct Mater, 2020, 30: 2003035. |
| 61 | CHEN J Z, LIU G G, ZHU Y Z, et al. Ag@MoS2 core-shell heterostructure as SERS platform to reveal the hydrogen evolution active sites of single-layer MoS2[J]. J Am Chem Soc, 2020, 142: 7161-7167. |
| 62 | SOLOMON G, LANDSTRÖM A, MAZZARO R, et al. NiMoO4@Co3O4 core-shell nanorods: in situ catalyst reconstruction toward high efficiency oxygen evolution reaction[J]. Adv Energy Mater, 2021, 11: 2101324. |
| 63 | FENG J X, WU J Q, TONG Y X, et al. Efficient hydrogen evolution on Cu nanodots-decorated Ni3S2 nanotubes by optimizing atomic hydrogen adsorption and desorption[J]. J Am Chem Soc, 2018, 140: 610-617. |
| 64 | BAI J, MEI J, LIAO T, et al. Molybdenum-promoted surface reconstruction in polymorphic cobalt for initiating rapid oxygen evolution[J]. Adv Energy Mater, 2022, 12(5): 213247. |
| 65 | XIA L, LI G K. Recent progress of microfluidics in surface-enhanced Raman spectroscopic analysis[J]. J Sep Sci, 2021, 44:1752-1768. |
| 66 | SHEN L F, LU B A, LI Y Y, et al. Interfacial structure of water as a new descriptor of the hydrogen evolution reaction[J]. Angew Chem Int Ed, 2020, 59: 22397-22402. |
| 67 | WEN B Y, CHEN Q Q, RADJENOVIC P M, et al. In situ surface-enhanced Raman spectroscopy characterization of electrocatalysis with different nanostructures[J]. Annu Rev Phys Chem, 2021, 72: 331-351. |
| 68 | CHEN H Q, ZOU L,WEI D Y, et al. In situ studies of energy-related electrochemical reactions using Raman and X-ray absorption spectroscopy[J]. Chinese J Catal, 2022, 43: 33-46. |
| 69 | HESS C. New advances in using raman spectroscopy for the characterization of catalysts and catalytic reactions[J]. Chem Soc Rev, 2021, 50: 3519-3546. |
| [1] | 于红丽, 周思仪, 洪琛, 罗稳. 基于黄酮骨架的“关-开”型荧光探针用于检测活细胞内丁酰胆碱酯酶[J]. 应用化学, 2023, 40(4): 500-508. |
| [2] | 宋金萍, 马琦, 梁晓敏, 尚建鹏, 董川. 高荧光量子产率钕、氮双掺杂碳点用于柳氮磺吡啶检测及Hela细胞成像[J]. 应用化学, 2022, 39(11): 1726-1734. |
| [3] | 杨振华, 孙宣森, 张月霞, 曹宇娟, 张琪琦, 郭峤志, 范小鹏, 李忠平, 董川. 氮硫共掺杂碳点的制备及其对牛奶中土霉素的检测[J]. 应用化学, 2022, 39(9): 1382-1390. |
| [4] | 程军杰, 曹智, 杨灿然, 李连顺, 王健, 林庆宇. 便携式远程激光诱导击穿光谱系统及其定量分析性能[J]. 应用化学, 2022, 39(9): 1447-1452. |
| [5] | 何欣, 蒋彩云, 丁涛, 王玉萍. 有序表面增强拉曼散射基底制备的研究发展[J]. 应用化学, 2022, 39(8): 1167-1176. |
| [6] | 张晓丽, 彭玉美, 王庆伟, 秦利霞, 刘肖霞, 康诗钊, 李向清. 纳米Ag/TiO2纳米管阵列基底构建及表面增强拉曼散射光谱检测与降解盐酸四环素[J]. 应用化学, 2022, 39(7): 1147-1156. |
| [7] | 温景惠, 赵冰, 阚伟, 王丽艳, 孙立, 宋天舒, 宋波. 可区别铁离子荧光探针异构体的合成及其水样分析[J]. 应用化学, 2022, 39(5): 787-796. |
| [8] | 程久庚, 苏朝晖. 原子力-红外光谱定量分析聚1-丁烯/聚丙烯共混物的相区组成[J]. 应用化学, 2022, 39(02): 266-271. |
| [9] | 黄译文, 王丽艳, 赵冰, 宋波. 一种水溶性甲氧基萘乙烯半菁合成及对铬(Ⅲ)离子的荧光检测[J]. 应用化学, 2021, 38(11): 1503-1511. |
| [10] | 刘巧玲, 任博荣, 刘睿蓉, 李雨霞, 王桂香, 任紫薇, 董川. N-掺杂的荧光碳点比率识别Ag+的性能[J]. 应用化学, 2021, 38(11): 1512-1520. |
| [11] | 张萌, 陈东圳, 任研伟, 宁攀. 纳米岛状银膜@金纳米针尖表面增强拉曼散射传感界面及多巴胺分子的传感分析[J]. 应用化学, 2021, 38(7): 866-873. |
| [12] | 温广明, 焦婷, 杜孝艳, 李忠平. 硫氮共掺杂碳量子点的制备及应用[J]. 应用化学, 2021, 38(6): 722-730. |
| [13] | 张春晖, 刘西京, 章蓉, 图雅. 蒙药荜茇不同提取物的红外光谱[J]. 应用化学, 2021, 38(3): 271-275. |
| [14] | 郭永艳, 田雁飞, 党铭铭, 杨萍, 龙云飞. 碱性环境下硫脲协调铬黑T稳定的银纳米簇的制备[J]. 应用化学, 2021, 38(2): 195-201. |
| [15] | 闫月荣, 高成庄, 张艳青, 褚文娅, 李双双, 赵伟, 周群, 李晓伟. 钴岛膜上对巯基苯甲酸的表面增强红外吸收光谱[J]. 应用化学, 2015, 32(2): 221-224. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||