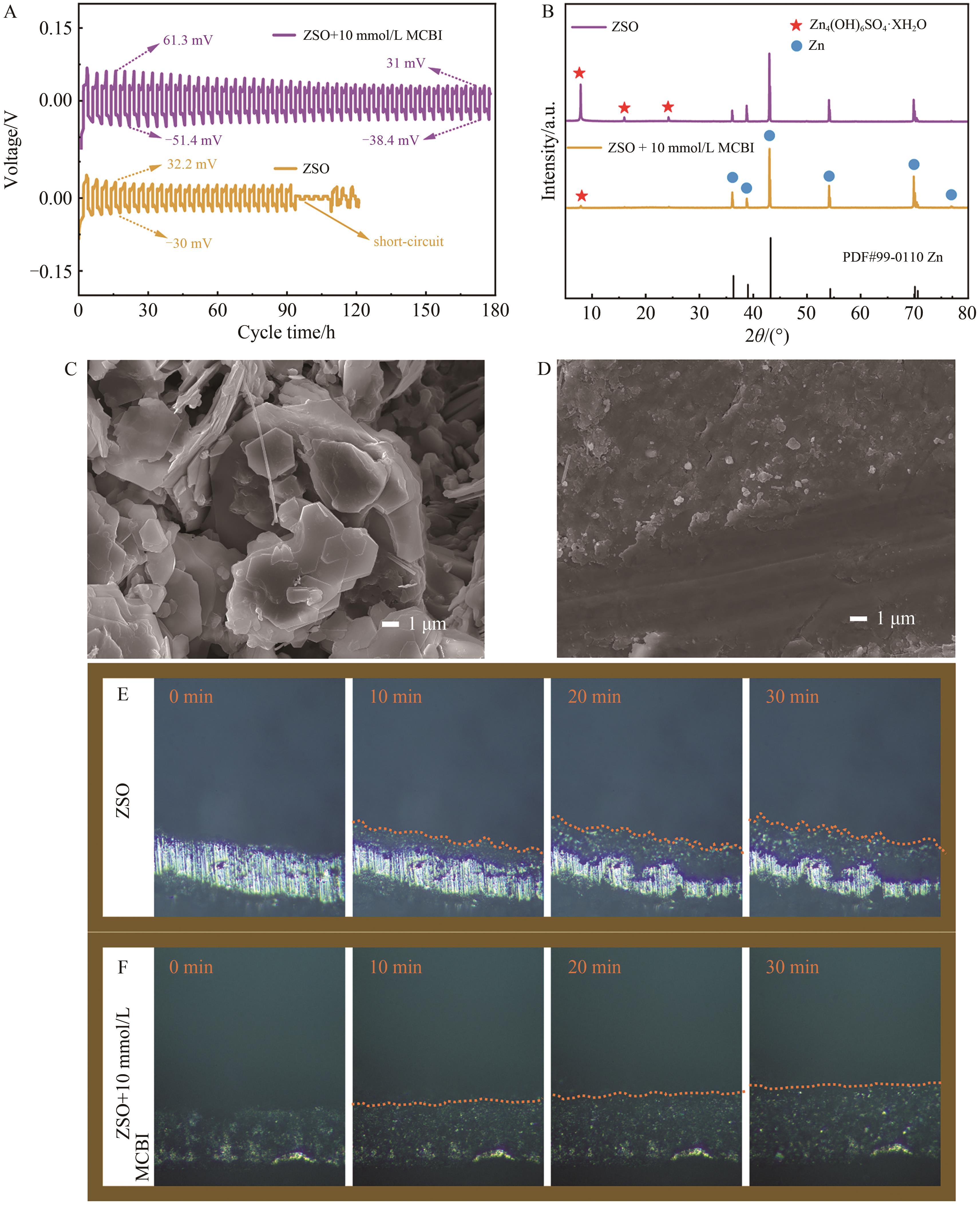
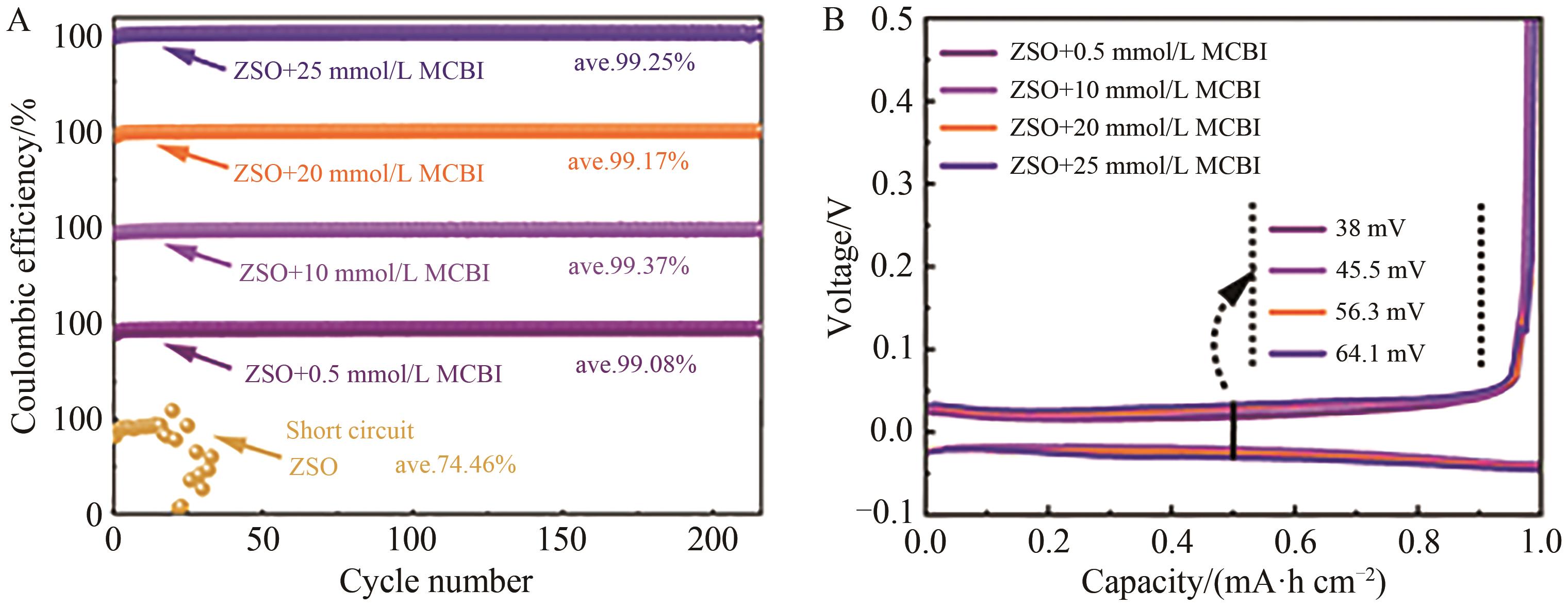
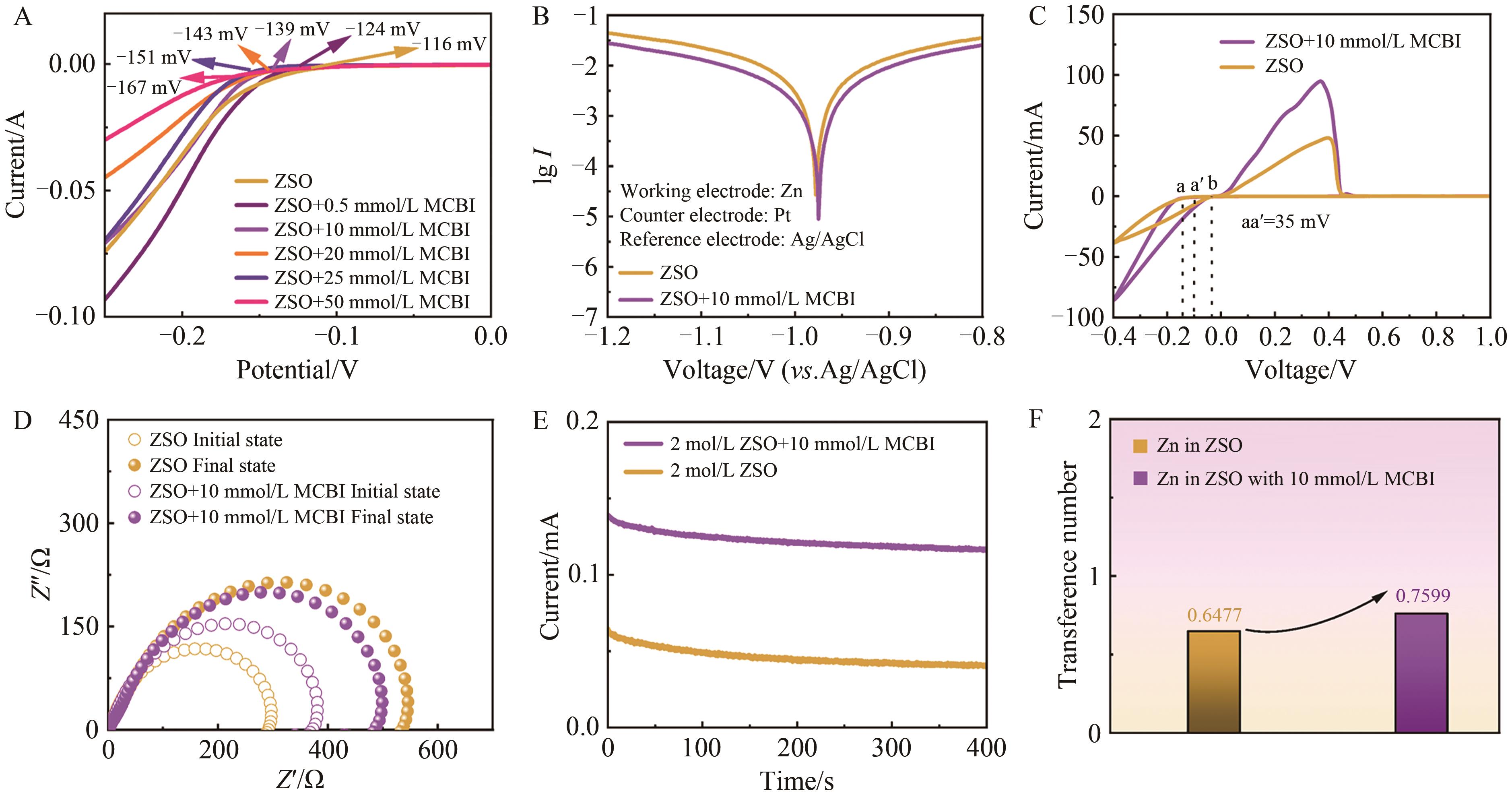
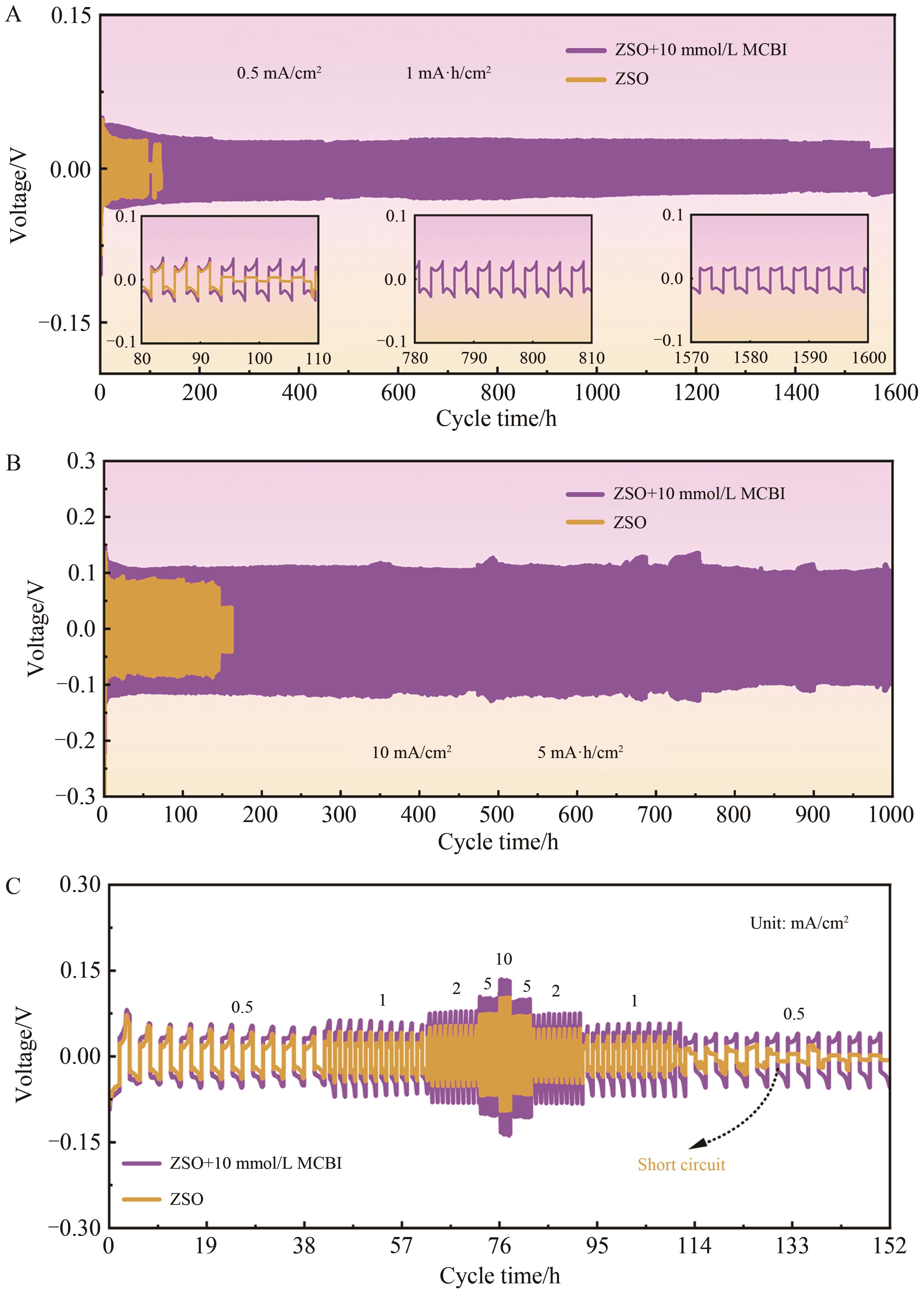
Chinese Journal of Applied Chemistry ›› 2024, Vol. 41 ›› Issue (7): 998-1009.DOI: 10.19894/j.issn.1000-0518.240029
• Full Papers • Previous Articles Next Articles
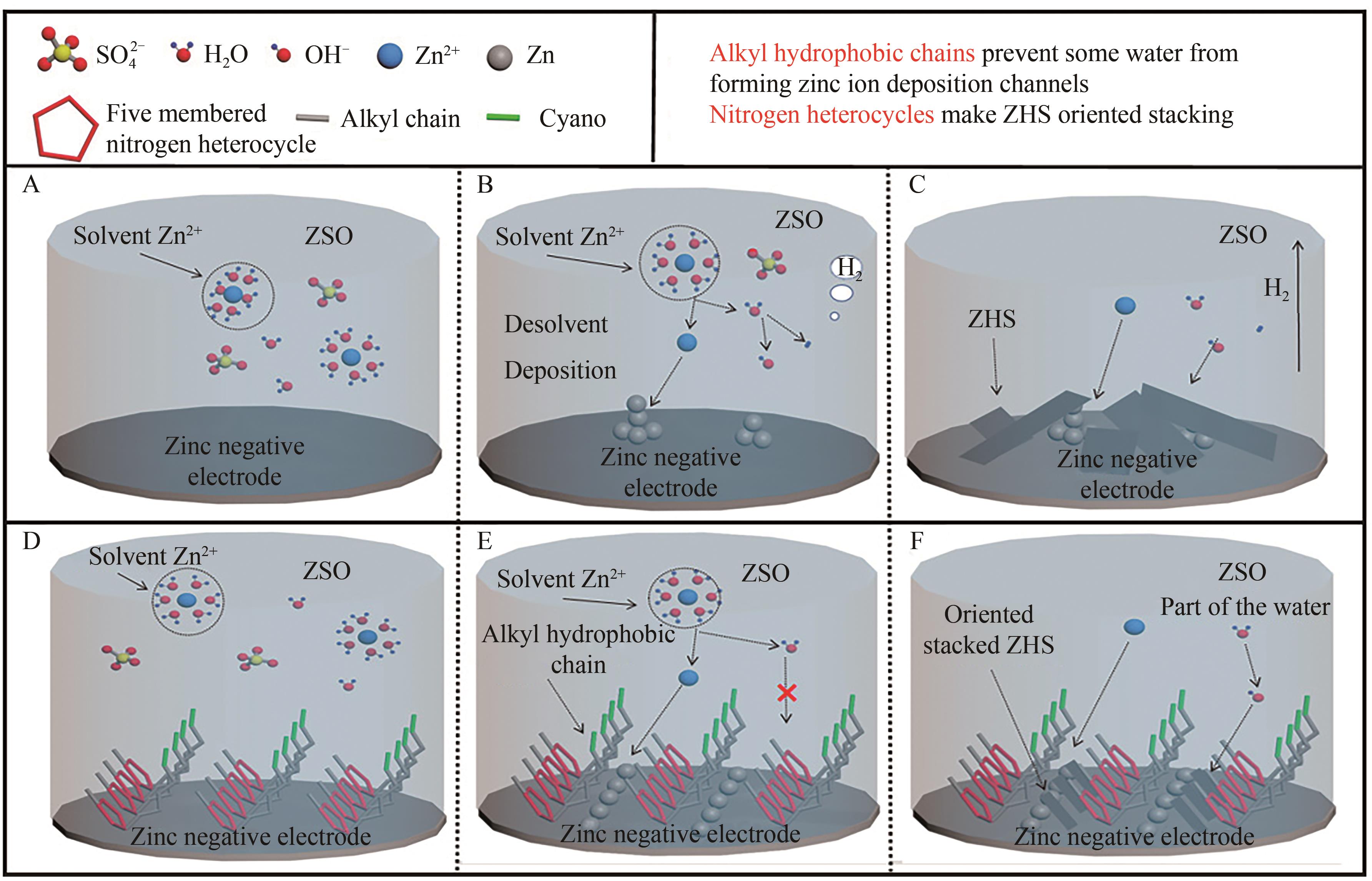
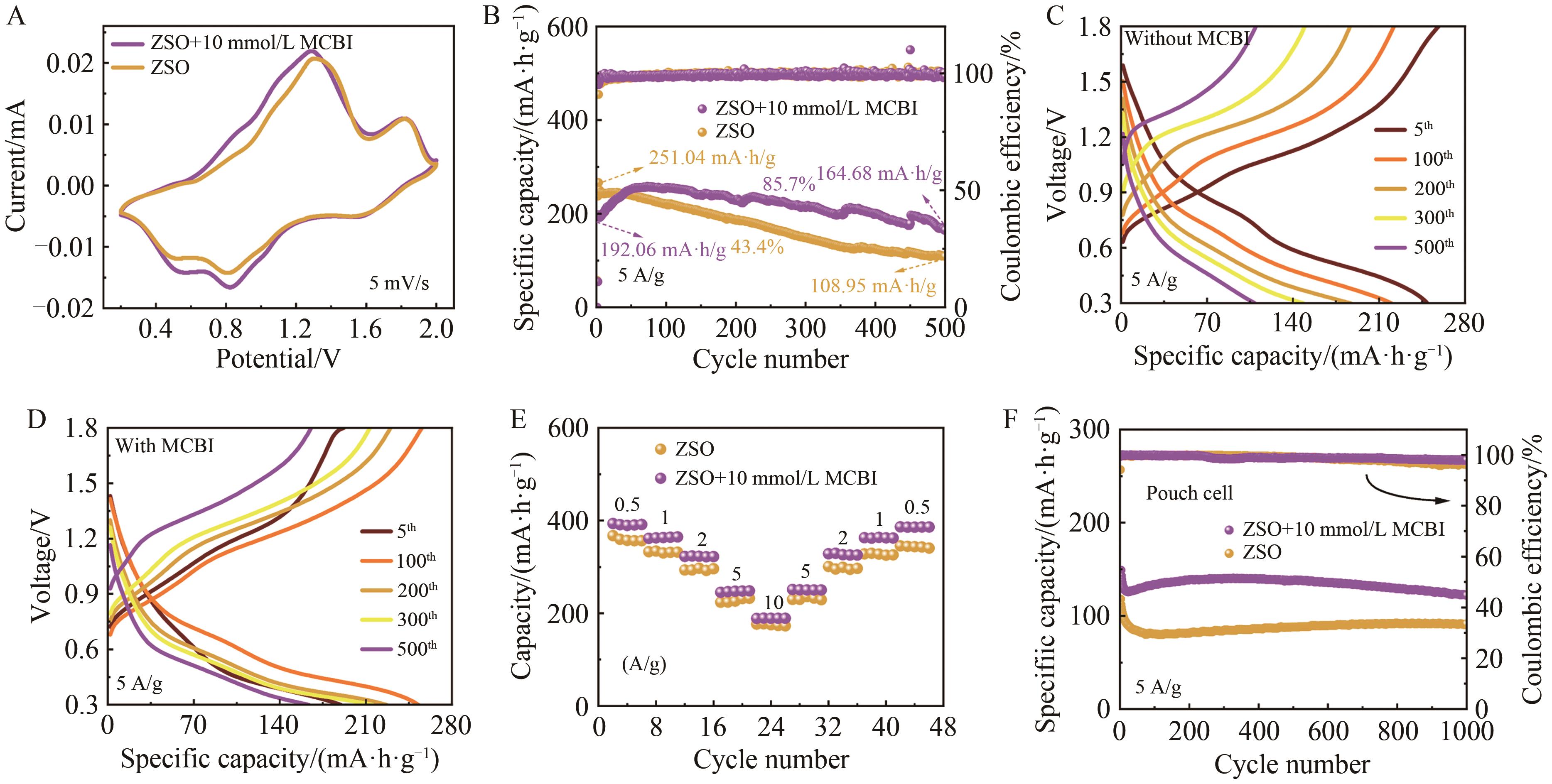
Long-Term Aqueous Zinc-Ion Batteries without Dendrites Protected by Nitrogen Heterocyclic Imidazole Ionic Liquid
Lei XU1,2, Long-Yang WANG1,2, Li TAO1,2( ), Hao-Nan ZHANG1,2, Xin-Wang JIA1,2, Hou-Zhao WAN1,2, Jun ZHANG1,2, Hao WANG1,2(
), Hao-Nan ZHANG1,2, Xin-Wang JIA1,2, Hou-Zhao WAN1,2, Jun ZHANG1,2, Hao WANG1,2( )
)
- 1.School of Microelectronics,Hubei University,Wuhan 430062,China
2.Hubei Yangtze Memory Laboratories,Wuhan 430205,China
-
Received:2024-01-29Accepted:2024-05-15Published:2024-07-01Online:2024-08-03 -
Contact:Li TAO,Hao WANG -
About author:wangh@hubu.edu.cn
litao@hubu.edu.cn
-
Supported by:the Natural Science Foundation of Hubei Province(2022CFB402);the National Natural Science Foundation of China(52272198)
CLC Number:
Cite this article
Lei XU, Long-Yang WANG, Li TAO, Hao-Nan ZHANG, Xin-Wang JIA, Hou-Zhao WAN, Jun ZHANG, Hao WANG. Long-Term Aqueous Zinc-Ion Batteries without Dendrites Protected by Nitrogen Heterocyclic Imidazole Ionic Liquid[J]. Chinese Journal of Applied Chemistry, 2024, 41(7): 998-1009.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: http://yyhx.ciac.jl.cn/EN/10.19894/j.issn.1000-0518.240029
| 1 | CHEN M Z, ZHANG Y Y, XING G C, et al. Electrochemical energy storage devices working in extreme conditions[J]. Energ Environ Sci, 2021,14(6): 3323-3351. |
| 2 | 衡永丽, 谷振一, 郭晋芝, 等. 水系锌离子电池用钒基正极材料的研究进展[J]. 物理化学学报, 2021, 37(3): 2005013. |
| HENG Y L, GU Z Y, GUO J Z, et al. Research progresses on vanadium-based cathode mate‐rials for aqueous zinc-ion batteries[J]. Acta Phys Chim Sin,2021, 37(3): 2005013. | |
| 3 | LU X, WANG Y, XU X, et al. Polymer-based solid-state electrolytes for high-energy-density lithium-ion batteries-review[J]. Adv Energy Mater, 2023, 13(38): 2301746. |
| 4 | WANG H, LI X, ZENG Q, et al. A novel hyperbranched polyurethane solid electrolyte for room temperature ultra-long cycling lithium-ion batteries[J]. Energy Storage Mater, 2024, 66: 103188. |
| 5 | DONG N, ZHANG F L, PAN H L. Towards the practical application of Zn metal anodes for mild aqueous rechargeable Zn batteries[J]. Chem Sci, 2022, 13(28): 8243-8252. |
| 6 | ZHOU J H, WU F, MEI Y, et al. Establishing thermal infusion method for stable zinc metal anodes in aqueous zinc-ion batteries[J]. Adv Mater, 2022, 34(21): 2200782. |
| 7 | LI C, JIN S, ARCHER L A, et al. Toward practical aqueous zinc-ion batteries for electrochemical energy storage[J]. Joule,2022, 6(8): 1733-1738. |
| 8 | WANG J D, ZHANG B, CAI Z, et al. Stable interphase chemistry of textured Zn anode for rechargeable aqueous batteries[J]. Sci Bull, 2022, 67(7): 716-724. |
| 9 | ZHAO J, YING Y P, WANG G L, et al. Covalent organic framework film protected zinc anode for highly stable rechargeable aqueous zinc-ion batteries[J]. Energy Storage Mater, 2022, 48: 82-89. |
| 10 | WANG S N, LI T Y, YIN Y B, et al. High-energy-density aqueous zinc-based hybrid supercapacitor-battery with uniform zinc deposition achieved by multifunctional decoupled additive[J]. Nano Energy, 2022, 96: 107120. |
| 11 | ZHANG X Q, CHEN J, CAO H, et al. Efficient suppression of dendrites and side reactions by strong electrostatic shielding effect via the additive of Rb2SO4 for anodes in aqueous zinc-ion batteries[J]. Small, 2023, 19(52): 2303906. |
| 12 | CHEN R W, ZHANG W, HUANG Q B, et al. Trace amounts of triple-functional additives enable reversible aqueous zinc-ion batteries from a comprehensive perspective[J]. Nano-Micro Lett, 2023, 15(1): 81. |
| 13 | TAO L, GUAN K L, YANG R, et al. Dual-protected zinc anodes for long-life aqueous zinc ion battery with bifunctional interface constructed by zwitterionic surfactants[J]. Energy Storage Mater, 2023, 63: 102981. |
| 14 | SHI M, WANG R, HE J, et al. Multiple redox-active cyano-substituted organic compound integrated with MXene for high-performance flexible aqueous K-ion battery[J]. Chem Eng J, 2022, 450: 138238. |
| 15 | CHEN J, ZHOU W, QUAN Y, et al. Ionic liquid additive enabling anti-freezing aqueous electrolyte and dendrite-free Zn metal electrode with organic/inorganic hybrid solid electrolyte interphase layer[J]. Energy Storage Mater, 2022, 53: 629-637. |
| 16 | YAN Q, HU Z, LIU Z, et al. Synergistic interaction between amphiphilic ion additive groups for stable long-life zinc ion batteries[J]. Energy Storage Mater, 2024, 67: 103299. |
| 17 | 刘欢, 马宇, 曹斌, 等. MXenes 在水系锌离子电池中的应用研究进展[J]. 物理化学学报, 2023, 39(5): 2210027. |
| LIU H, MA Y, CAO B, et al. Recent progress of MXenes in aqueous zinc-ion batteries[J]. Acta Phys Chim Sin, 2023, 39(5): 2210027. | |
| 18 | LIU Z X, WANG R, MA Q W, et al. A dual-functional organic electrolyte additive with regulating suitable overpotential for building highly reversible aqueous zinc ion batteries[J]. Adv Funct Mater, 2023: 2214538. |
| 19 | ZHENG H, HUANG Y, XIAO J, et al. Multi-protection of zinc anode via employing a natural additive in aqueous zinc ion batteries[J]. Chem Eng J, 2023, 468: 143834. |
| 20 | JI H J, HAN Z Q, LIN Y H, et al. Stabilizing zinc anode for high-performance aqueous zinc ion batteries via employing a novel inositol additive[J]. J Alloy Compd, 2022, 914: 165231. |
| 21 | YANG J Z, YIN B S, SUN Y, et al. Zinc anode for mild aqueous zinc-ion batteries: challenges, strategies, and perspectives[J]. Nano-Micro Lett, 2022, 14: 1-47. |
| 22 | GUO S, QIN L P, ZHANG T S, et al. Fundamentals and perspectives of electrolyte additives for aqueous zinc-ion batteries[J]. Energy Storage Mater, 2021, 34: 545-562. |
| 23 | CAO H, HUANG X M, LIU Y, et al. An efficient electrolyte additive of tetramethylammonium sulfate hydrate for dendritic-free zinc anode for aqueous zinc-ion batteries[J]. J Colloid Interface Sci, 2022, 627: 367-374. |
| 24 | GENG Y F, PAN L, PENG Z Y, et al. Electrolyte additive engineering for aqueous Zn ion batteries[J]. Energy Storage Mater, 2022, 51: 733-755. |
| 25 | THIEU N A, LI W, CHEN X J, et al. Synergistically stabilizing zinc anodes by molybdenum dioxide coating and Tween 80 electrolyte additive for high-performance aqueous zinc-ion batteries[J]. ACS Appl Mater Interfaces, 2023, 15: 55570-55586. |
| 26 | LIU Z X, WANG R, GAO Y C, et al. Low-cost multi-function electrolyte additive enabling highly stable interfacial chemical environment for highly reversible aqueous zinc ion batteries[J]. Adv Funct Mater, 2023, 33: 2308463. |
| 27 | ZHOU W J, CHEN M F, TIAN Q H, et al. Stabilizing zinc deposition with sodium lignosulfonate as an electrolyte additive to improve the life span of aqueous zinc-ion batteries[J]. J Colloid Interface Sci, 2021, 601: 486-494. |
| 28 | DONG H Y, YAN S X, LI T F, et al. Chelating dicarboxylic acid as a multi-functional electrolyte additive for advanced Zn anode in aqueous Zn-ion batteries[J]. J Power Sources, 2023, 585: 233593. |
| 29 | YIN J Y, LIU H L, LI P, et al. Integrated electrolyte regulation strategy: trace trifunctional tranexamic acid additive for highly reversible Zn metal anode and stable aqueous zinc ion battery[J]. Energy Storage Mater, 2023, 59: 102800. |
| 30 | HONG L, WU X M, WANG L Y, et al. Highly reversible zinc anode enabled by a cation-exchange coating with Zn-ion selective channels[J]. ACS Nano, 2022, 16(4): 6906-6915. |
| 31 | GUAN Q L, LI J H, LI L J, et al. In situ construction of organic anion-enriched interface achieves ultra-long life aqueous zinc-ion battery[J]. Chem Eng J, 2023, 476: 146534. |
| 32 | YAO R, QIAN L, SUI Y M, et al. A versatile cation additive enabled highly reversible zinc metal anode[J]. Adv Energy Mater, 2022, 12(2): 2102780. |
| 33 | CAO P H, ZHOU X Y, WEI A R, et al. Fast-charging and ultrahigh-capacity zinc metal anode for high-performance aqueous zinc-ion batteries[J]. Adv Funct Mater, 2021, 31(20): 2100398. |
| 34 | TIAN Z, ZOU Y, LIU G, et al. Electrolyte solvation structure design for sodium ion batteries[J]. Adv Sci, 2022, 9(22): 2201207. |
| 35 | CHENG H, SUN Q, LI L, et al. Emerging era of electrolyte solvation structure and interfacial model in batteries[J]. ACS Energy Lett, 2022, 7(1): 490-513. |
| 36 | LI L, CHENG H, ZHANG J, et al. Quantitative chemistry in electrolyte solvation design for aqueous batteries[J]. ACS Energy Lett, 2023, 8(2): 1076-1095. |
| 37 | XIE C L, LI Y H, WANG Q, et al. Issues and solutions toward zinc anode in aqueous zinc-ion batteries: a mini review[J]. Carbon Energy, 2020, 2(4): 540-560. |
| 38 | HAN C, LI W J, LIU H K, et al. Principals and strategies for constructing a highly reversible zinc metal anode in aqueous batteries[J]. Nano Energy, 2020, 74: 104880. |
| 39 | YANG S, CHEN A, TANG Z J, et al. Regulating the electrochemical reduction kinetics by the steric hindrance effect for a robust Zn metal anode[J]. Energ Environ Sci, 2024, 17(3): 1095-1106. |
| 40 | SU K L M, ZHANG X Y, ZHANG X Q, et al. Polar small molecular electrolyte additive for stabilizing Zn anode[J]. Chem Eng J, 2023, 474: 145730. |
| 41 | WU C, SUN C, REN K, et al. 2-Methyl imidazole electrolyte additive enabling ultra-stable Zn anode[J]. Chem Eng J, 2023, 452: 139465. |
| 42 | ZHAO Y, HONG H, ZHONG L, et al. Zn-rejuvenated and SEI-regulated additive in zinc metal battery via the iodine post-functionalized zeolitic imidazolate framework-90[J]. Adv Energy Mater, 2023, 13(28): 2300627. |
| 43 | ZHANG Q, MA Y, LU Y, et al. Designing anion-type water-free Zn2+ solvation structure for robust Zn metal anode[J]. Angew Chem, 2021, 133(43): 23545-23552. |
| 44 | CHEN Y M, GONG F C, DENG W J, et al. Dual-function electrolyte additive enabling simultaneous electrode interface and coordination environment regulation for zinc-ion batteries[J]. Energy Storage Mater, 2023, 58: 20-29. |
| 45 | QUAN Y H, YANG M, CHEN M F, et al. Electrolyte additive of sorbitol rendering aqueous zinc-ion batteries with dendrite-free behavior and good anti-freezing ability[J]. Chem Eng J, 2023, 458: 141392. |
| [1] | Run ZHANG, Hong-Mei ZHAN, Chen-Yang ZHAO, Yan-Xiang CHENG, Chuan-Jiang QIN. Pure Blue Perovskite Light‑Emitting Diodes Modified with Fluorine‑Containing Phosphine Oxide Derivatives [J]. Chinese Journal of Applied Chemistry, 2024, 41(6): 830-838. |
| [2] | Yi ZHANG, Yu-Tong CHEN, Jing-Yu SHI, Ke-Ke HUANG. Research Progress of Optimizing Conductivity of Garnet-Type Solid Electrolyte Li7La3Zr2O12 [J]. Chinese Journal of Applied Chemistry, 2024, 41(4): 484-495. |
| [3] | Yu CHENG, Ling-Jun HE, Chu-Yuan LIN, Hui LIN, Fu-Yu XIAO, Wen-Bin LAI, Qing-Rong QIAN, Xiao-Xia HUANG, Qing-Hua CHEN, Ling-Xing ZENG. Progress Research on Electrolyte Modification Strategy to Improve the Performance of Aqueous Zinc-Ion Batteries Within the Wide Temperature Range [J]. Chinese Journal of Applied Chemistry, 2024, 41(3): 349-364. |
| [4] | Yuan-Jie LI, Bing-Bing FAN, Jun-Li ZHANG, Yan-Mei ZHOU. Research Progress in the Application of Amino Acid Ionic Liquids to Energy Storage and Biomass Resource Utilization [J]. Chinese Journal of Applied Chemistry, 2024, 41(3): 391-404. |
| [5] | Hui-Hui LI, Kai-Sheng YAO, Ya-Nan ZHAO, Li-Na FAN, Yu-Lin TIAN, Wei-Wei LU. Ionic Liquid-Modulated Synthesis of Pt-Pd Bimetallic Nanomaterials and Their Catalytic Performance for Ammonia Borane Hydrolysis to Generate Hydrogen [J]. Chinese Journal of Applied Chemistry, 2023, 40(4): 597-609. |
| [6] | Qi-Hang CHEN, Fei-Jian XU, Feng WANG, Shuang-Chan FU, Ying-Hao YU. Research Progress of Deep Eutectic Solvent and Its Application Prospects as Antistatic Agents [J]. Chinese Journal of Applied Chemistry, 2023, 40(3): 341-359. |
| [7] | Lu-Fei WANG, Meng-Meng ZHEN, Bo-Xiong SHEN. Research Progress of Controlling Lithium-Sulfur Batteries by Electrocatalysts under Lean Electrolyte Conditions [J]. Chinese Journal of Applied Chemistry, 2023, 40(2): 188-209. |
| [8] | Xue-Jian SHI, Wan-Qiang LIU, Chun-Li WANG, Yong CHENG, Li-Min WANG. Research Progress of Sb-based Anode Materials for Potassium Ion Batteries [J]. Chinese Journal of Applied Chemistry, 2023, 40(2): 210-228. |
| [9] | Cheng-Yuan LIU, Jiang-Yu YU, Feng-Cui LI, Zhi-Wei LIU. Research Progress of Raman Spectroscopy Technique in Energy Storage Mechanism of Rechargeable Aluminum-Ion Batteries [J]. Chinese Journal of Applied Chemistry, 2023, 40(10): 1347-1358. |
| [10] | Xin GU, Wen-Qing WANG, Jun-He HOU, Lu GAO, Ming-Hua HUANG, Ge SU. Advances of Inorganic‑inorganic Composite Electrochromic Films [J]. Chinese Journal of Applied Chemistry, 2022, 39(9): 1345-1359. |
| [11] | Lin-Hu SONG, Shi-You LI, Jie WANG, Jing-Jing ZHANG, Ning-Shuang ZHANG, Dong-Ni ZHAO, Fei XU. Research Progress of Additives for Acid and Water Removal in Electrolyte of Lithium Ion Battery [J]. Chinese Journal of Applied Chemistry, 2022, 39(5): 697-706. |
| [12] | Jing ZHOU, Yu-Xuan CHEN, Jun-Ming MA, Xiao-Fei ZHU, De-Feng ZHOU. Effect of MgO and Fe2O3 Incorporation on the Microstructure and Electrochemical Performance of GDCSi System [J]. Chinese Journal of Applied Chemistry, 2022, 39(5): 809-818. |
| [13] | Qi ZHANG, Qian ZHANG, Xiao-Meng SHI, Ya-Qi KONG, Ke-Xin GAO, Ya-Ping DU. Research Progress of Rare Earth Bromides Based Solid Electrolytes for All⁃Solid⁃State Batteries [J]. Chinese Journal of Applied Chemistry, 2022, 39(4): 585-598. |
| [14] | Shuang WU, De-Yang ZHAO, Sheng-Han WU, Li-Gang WEI, Na LIU, Qing-Da AN. Insights into the Dissolution of Lignin Model Phenolic Monomer in 1‑Butyl‑3‑methylimidazolium Methanesulfonate Aqueous Solutions Using Two‑Dimensional Correlation Infrared Spectroscopy [J]. Chinese Journal of Applied Chemistry, 2022, 39(10): 1600-1609. |
| [15] | Ou-Wen HE, Chang-Fu SUN, Hong-Bing YU. Optimization on the Production of Furfural from Corncob in Cheap Ionic Liquid System [J]. Chinese Journal of Applied Chemistry, 2022, 39(02): 272-282. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||