
Chinese Journal of Applied Chemistry ›› 2022, Vol. 39 ›› Issue (10): 1488-1500.DOI: 10.19894/j.issn.1000-0518.210573
• Review • Previous Articles Next Articles
Research Progress of Carbon‑Encapsulated Iron‑Based Nanoparticles Electrocatalysts for Zinc‑Air Batteries
Dan WANG, Xian-Biao HOU, Xing-Kun WANG, Zhi-Cheng LIU, Huan-Lei WANG, Ming-Hua HUANG( )
)
- School of Materials Science and Engineering,Ocean University of China,Qingdao 266100,China
-
Received:2021-12-21Accepted:2022-04-20Published:2022-10-01Online:2022-10-05 -
Contact:Ming-Hua HUANG -
About author:huangminghua@ouc.edu.cn
-
Supported by:the National Natural Science Foundation of China(21775142);the Natural Science Foundation of Shandong Province(ZR2020ME038)
CLC Number:
Cite this article
Dan WANG, Xian-Biao HOU, Xing-Kun WANG, Zhi-Cheng LIU, Huan-Lei WANG, Ming-Hua HUANG. Research Progress of Carbon‑Encapsulated Iron‑Based Nanoparticles Electrocatalysts for Zinc‑Air Batteries[J]. Chinese Journal of Applied Chemistry, 2022, 39(10): 1488-1500.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: http://yyhx.ciac.jl.cn/EN/10.19894/j.issn.1000-0518.210573
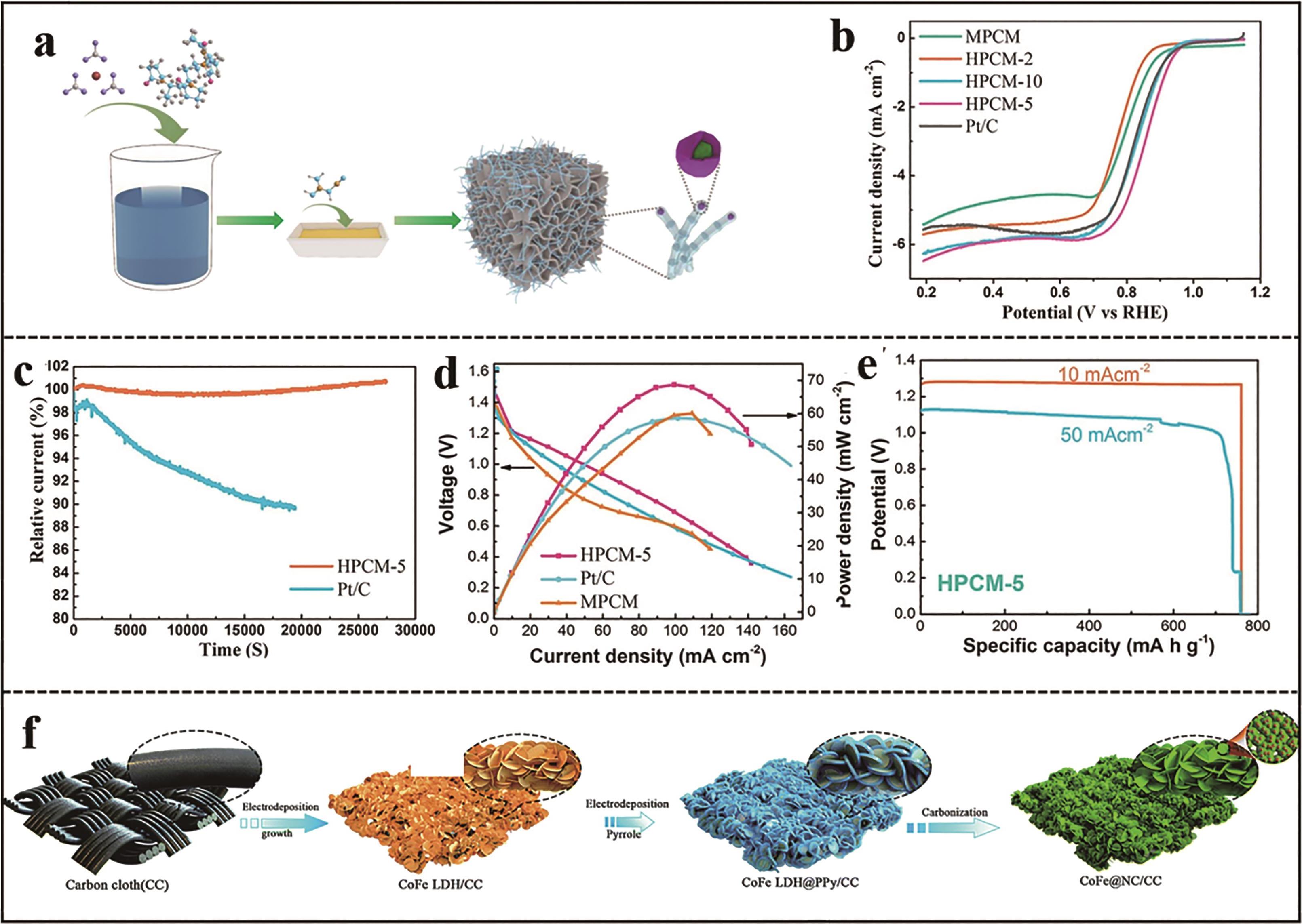
Fig.2 (a) Schematic illustration for the synthesis of HPCM-5[22]; (b) ORR polarization curves of HPCM-5 and Pt/C in O2 saturated 0.1 mol/L KOH electrolyte[22]; (c) Chronoamperometric curves for HPCM-5 and Pt/C at 0.5 V[22]; (d) Discharge polarization curves and corresponding power density of the zinc air batteries assembled as cathodes by HPCM-5 and Pt/C[22]; (e) Long-term discharge curves of zinc air battery based on HPCM-5 at a current density of 10 and 50 mA/cm2[22]; (f) Schematic illustration for the synthesis of CoFe@NC/CC[23]
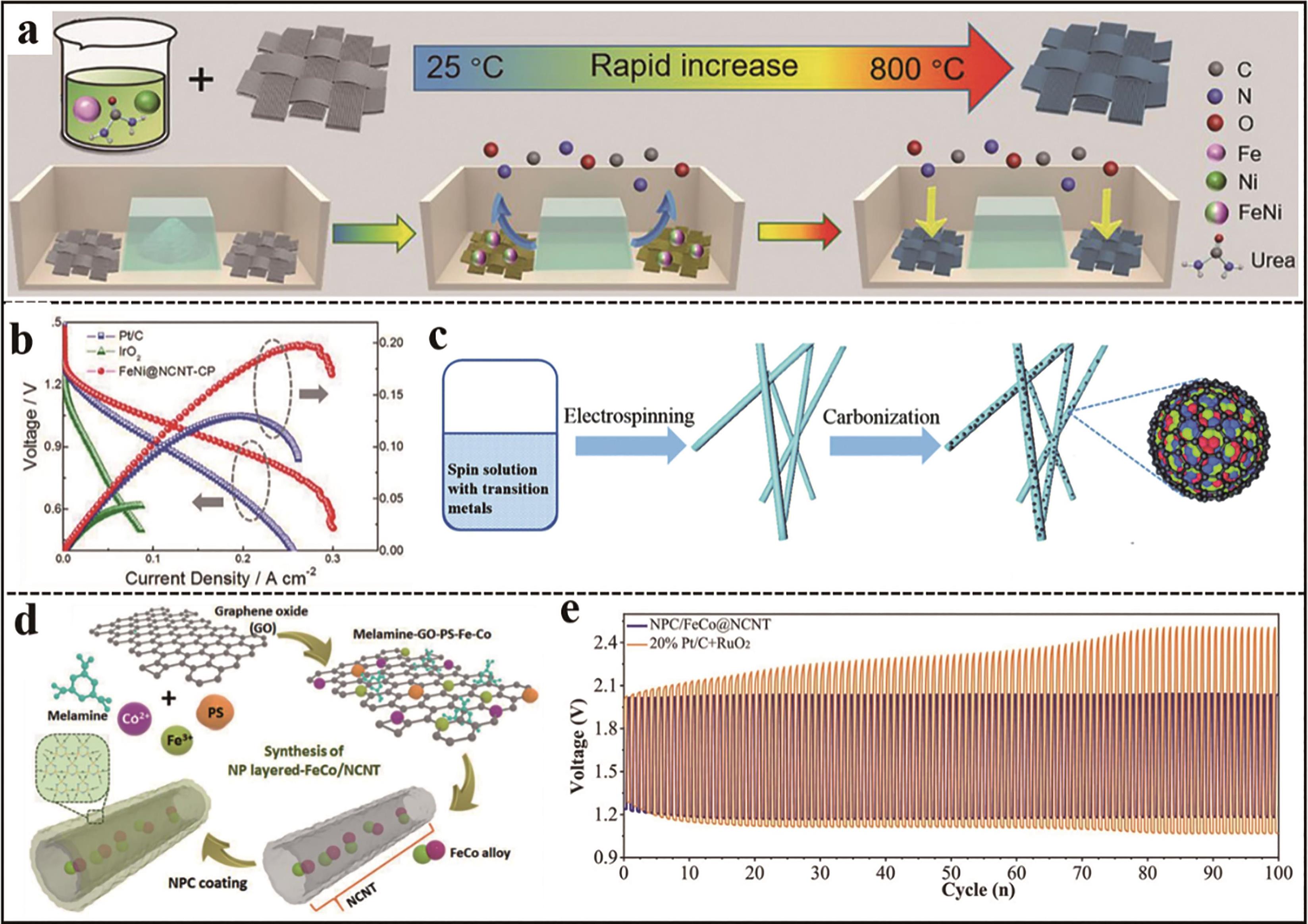
Fig.3 (a) Schematic illustration for preparation of FeNi@NCNT-CP[24]; (b) Discharge polarization and power density curves of zinc air battery based on FeNi@NCNT-CP, Pt/C and IrO2, respectively[24]; (c) Schematic illustration for preparation of NiCoFe@N-CNFs[25]; (d) Schematic illustration for preparation of NPC/FeCo@NCNT[26]; (e) Galvanostatic charge-discharge cycling curves of zinc air battery assembled by NPC/FeCo@NCNT and Pt/C+RuO2 at 10 mA/cm2, respectively[26]
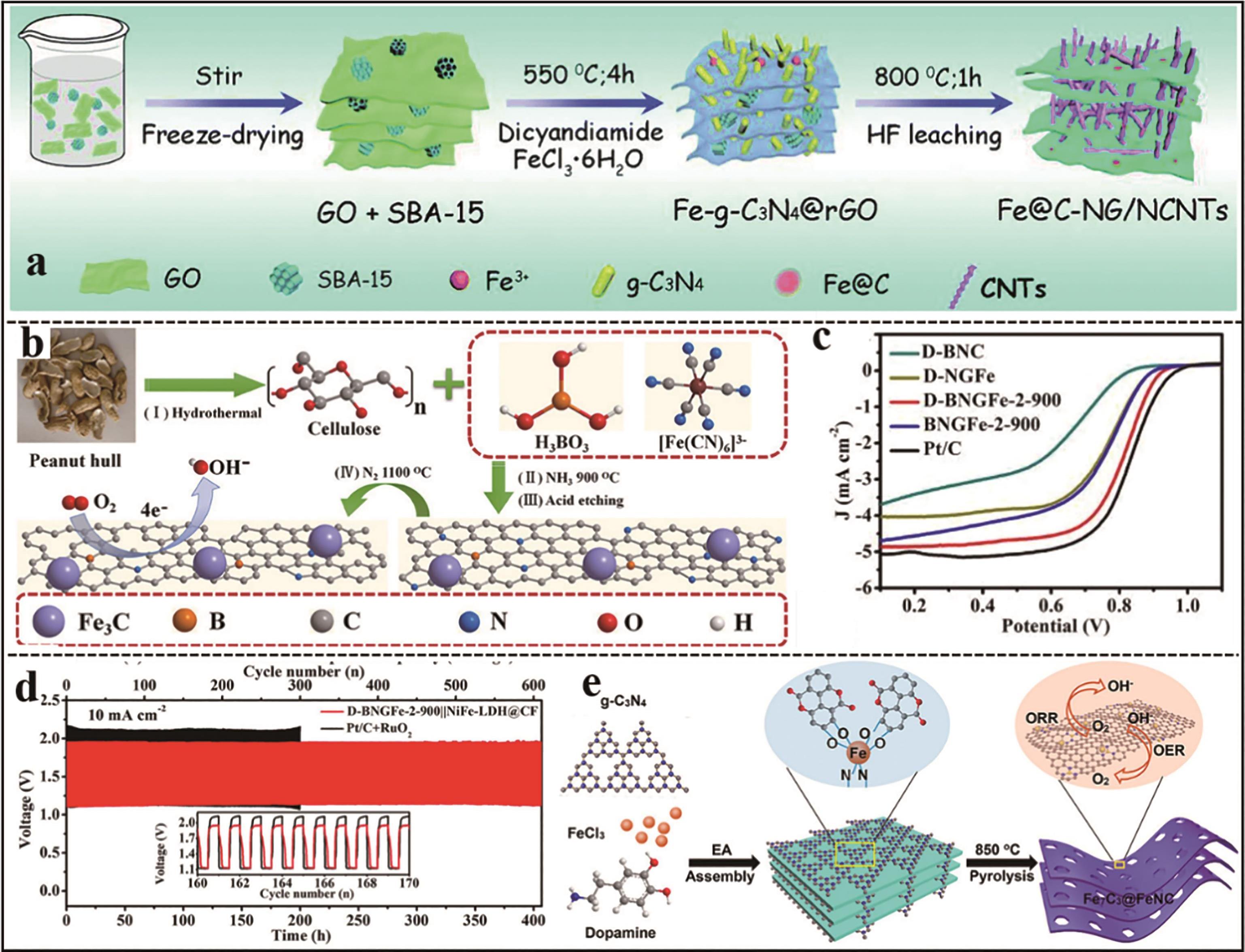
Fig.4 (a) Schematic illustration of the synthesis procedure for Fe@C-NG/NCNTs[27]; (b) Schematic illustration of the synthesis procedure for D-BNGFe-2-900[28]; (c) ORR polarization curves of D-BNGFe-2-900 and Pt/C[28]; (d) Galvanostatic charge-discharge cycling curves of zinc air batteries based on D-BNGFe-2-900∥NiFe-LDH@CF and Pt/C+RuO2 (40 min for one cycle) at 10 mA/cm2, respectively[28]; (e) Schematic illustration of the synthesis procedure for Fe7C3@FeNC[31]
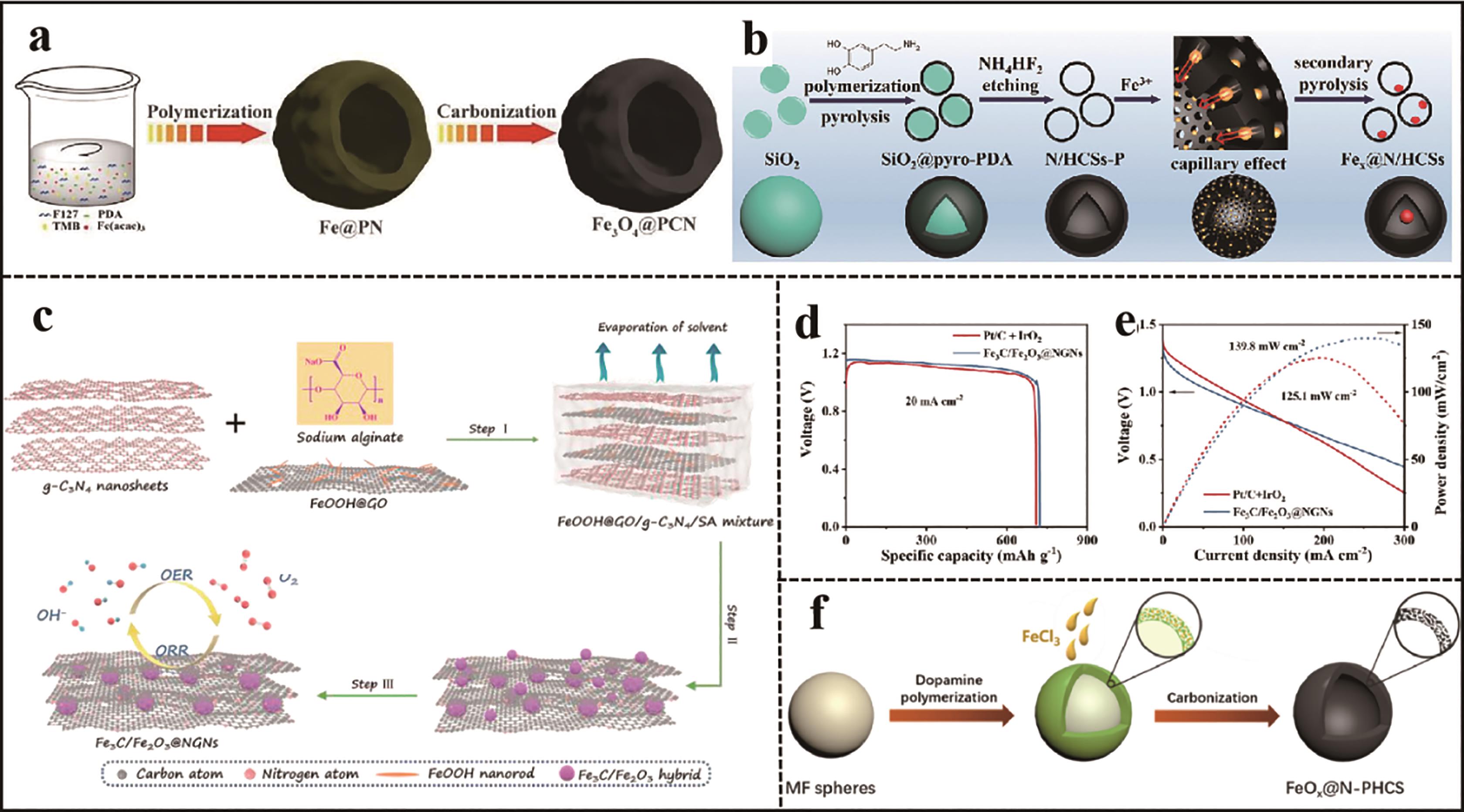
Fig.5 (a) Schematic illustration of the fabricated process of Fe3O4@PCN[34]; (b) Schematic illustration of the fabricated process of Fe x @N/HCSs[36];(c) Schematic illustration of the fabricated process of Fe3C/Fe2O3@NGNs[37]; The zinc air batteries performances with Fe3C/Fe2O3@NGNs and Pt/C+IrO2; (d) Specific capacity at current density of 20 mA/cm2[37]; (e) Discharge polarization and power density curves[37]; (f) Schematic illustration of the fabricated process of FeO x @N-PHCS[38]

Fig.6 (a) Schematic illustration for the synthesis of Fe2P/FeP-PNC[42]; (b) ORR polarization curves of Fe2P/FeP-PNC in 0.1 mol/L HClO4 electrolyte[42]; (c) Discharging polarization and corresponding power density curves with cathode catalysts of Fe2P/FeP-PNC[42]; (d) Schematic illustration for the synthesis of C-ZIF/LFP[43]; (e) Schematic illustration for the synthesis of FECNFS-NP[41]; (f) Polarization curves and corresponding power density curves of the zinc air battery with FECNFS-NP as the catalyst[41]; (g) Long-time discharge curves of the zinc air battery with FECNFS-NP as the catalyst[41]
分类 Classification | 催化剂 Catalyst | 电解液 Electrolyte | 开路电压 Open circuit voltage/V | 最大功率密度 Peak power density/ (mW·cm-2) | 循环次数 Cycle number | 参考 文献 Ref. |
|---|---|---|---|---|---|---|
铁及合金纳米颗粒 Iron and ferroalloys nanoparticles | Fe@C?NG/NCNTs | 6 mol/L KOH+0.2 mol/L Zn(Ac)2 | 1.37 | 101.2 | 297 | [ |
| 1.5FeNi@NCNT | 6 mol/L KOH+0.2 mol/L ZnCl2 | 1.44 | 114 | 100 | [ | |
| NiCoFe@N?CNFs | 6 mol/L KOH+0.2 mol/L Zn(Ac)2 | 1.32 | 147 | 120 | [ | |
| Fe1.2Co@NC/NCNT | 6 mol/L KOH+0.2 mol/L Zn(Ac)2 | 1.43 | 194 | 300 | [ | |
| FeCo?N/C | 6 mol/L KOH | - | 89.9 | 140 | [ | |
| FeNi@NCNT?CP | 6 mol/L KOH+0.2 mol/L Zn(Ac)2 | 1.551 | 200 | 250 | [ | |
| NPC/FeCo@NCNT | 6 mol/L KOH+0.2 mol/L Zn(Ac)2 | 1.429 | 151.3 | 100 | [ | |
| FeCo@NC?g | 6 mol/L KOH+0.2 mol/L ZnCl2 | 1.456 | 190.2 | 120 | [ | |
| CoFe@NC?SE | 6 mol/L KOH+0.2 mol/L Zn(Ac)2 | 1.58 | 102 | 288 | [ | |
碳化物纳米颗粒 Carbide nanoparticles | Fe/Fe3C@NCNT?750 | 6 mol/L KOH+0.2 mol/L Zn(Ac)2 | - | 198 | 500 | [ |
| D?BNGFe?2?900 | 6 mol/L KOH+0.2 mol/L Zn(Ac)2 | - | 142 | 610 | [ | |
| Fe?2?WNPC?NCNTs | 6 mol/L KOH+0.2 mol/L Zn(Ac)2 | 1.43 | 101.3 | 200 | [ | |
| Fe7C3@FeNC | 6 mol/L KOH+0.2 mol/L Zn(Ac)2 | 1.41 | 66.8 | 120 | [ | |
| Fe/Fe3C@Fe?Nx?C | 6 mol/L KOH | - | 147 | 200 | [ | |
氧化物纳米颗粒 Oxide nanoparticles | 4Fe3O4@PCN?800 | 6 mol/L KOH | 1.423 | 156.8 | - | [ |
| FeOx@N?PHCS | 6 mol/L KOH+0.2 mol/L Zn(Ac)2 | 1.41 | 93.6 | 25 | [ | |
| Fe20@N/HCSs | 6 mol/L KOH+0.2 mol/L Zn(Ac)2 | 1.57 | 140.8 | - | [ | |
| CoCx/(Co0.55Fe1.945)2P@C | 6 mol/L KOH+0.2 mol/L Zn(Ac)2 | 1.50 | 131 | 60 | [ | |
| Fe?CNSs?N | 6 mol/L KOH+0.2 mol/L Zn(Ac)2 | 1.27 | 106.8 | - | [ | |
| C?FePPDA?900 | 6 mol/L KOH | - | 106 | - | [ | |
| Fe3C/Fe2O3@NGNs | 6 mol/L KOH | 1.46 | 139.8 | - | [ | |
磷化物纳米颗粒 Phosphide nanoparticles | FeCo?P?2 | 6 mol/L KOH+0.2 mol/L Zn(Ac)2 | 1.415 | 205 | - | [ |
| C?ZIF/LFP | 6 mol/L KOH+0.2 mol/L Zn(Ac)2 | 1.5 | 140 | - | [ | |
| FeCNFs?NP | 6 mol/L KOH+0.2 mol/L Zn(Ac)2 | 1.4 | 97 | - | [ |
Table 1 Performance comparison of carbon?encapsulated iron?based catalysts applied in zinc air batteries
分类 Classification | 催化剂 Catalyst | 电解液 Electrolyte | 开路电压 Open circuit voltage/V | 最大功率密度 Peak power density/ (mW·cm-2) | 循环次数 Cycle number | 参考 文献 Ref. |
|---|---|---|---|---|---|---|
铁及合金纳米颗粒 Iron and ferroalloys nanoparticles | Fe@C?NG/NCNTs | 6 mol/L KOH+0.2 mol/L Zn(Ac)2 | 1.37 | 101.2 | 297 | [ |
| 1.5FeNi@NCNT | 6 mol/L KOH+0.2 mol/L ZnCl2 | 1.44 | 114 | 100 | [ | |
| NiCoFe@N?CNFs | 6 mol/L KOH+0.2 mol/L Zn(Ac)2 | 1.32 | 147 | 120 | [ | |
| Fe1.2Co@NC/NCNT | 6 mol/L KOH+0.2 mol/L Zn(Ac)2 | 1.43 | 194 | 300 | [ | |
| FeCo?N/C | 6 mol/L KOH | - | 89.9 | 140 | [ | |
| FeNi@NCNT?CP | 6 mol/L KOH+0.2 mol/L Zn(Ac)2 | 1.551 | 200 | 250 | [ | |
| NPC/FeCo@NCNT | 6 mol/L KOH+0.2 mol/L Zn(Ac)2 | 1.429 | 151.3 | 100 | [ | |
| FeCo@NC?g | 6 mol/L KOH+0.2 mol/L ZnCl2 | 1.456 | 190.2 | 120 | [ | |
| CoFe@NC?SE | 6 mol/L KOH+0.2 mol/L Zn(Ac)2 | 1.58 | 102 | 288 | [ | |
碳化物纳米颗粒 Carbide nanoparticles | Fe/Fe3C@NCNT?750 | 6 mol/L KOH+0.2 mol/L Zn(Ac)2 | - | 198 | 500 | [ |
| D?BNGFe?2?900 | 6 mol/L KOH+0.2 mol/L Zn(Ac)2 | - | 142 | 610 | [ | |
| Fe?2?WNPC?NCNTs | 6 mol/L KOH+0.2 mol/L Zn(Ac)2 | 1.43 | 101.3 | 200 | [ | |
| Fe7C3@FeNC | 6 mol/L KOH+0.2 mol/L Zn(Ac)2 | 1.41 | 66.8 | 120 | [ | |
| Fe/Fe3C@Fe?Nx?C | 6 mol/L KOH | - | 147 | 200 | [ | |
氧化物纳米颗粒 Oxide nanoparticles | 4Fe3O4@PCN?800 | 6 mol/L KOH | 1.423 | 156.8 | - | [ |
| FeOx@N?PHCS | 6 mol/L KOH+0.2 mol/L Zn(Ac)2 | 1.41 | 93.6 | 25 | [ | |
| Fe20@N/HCSs | 6 mol/L KOH+0.2 mol/L Zn(Ac)2 | 1.57 | 140.8 | - | [ | |
| CoCx/(Co0.55Fe1.945)2P@C | 6 mol/L KOH+0.2 mol/L Zn(Ac)2 | 1.50 | 131 | 60 | [ | |
| Fe?CNSs?N | 6 mol/L KOH+0.2 mol/L Zn(Ac)2 | 1.27 | 106.8 | - | [ | |
| C?FePPDA?900 | 6 mol/L KOH | - | 106 | - | [ | |
| Fe3C/Fe2O3@NGNs | 6 mol/L KOH | 1.46 | 139.8 | - | [ | |
磷化物纳米颗粒 Phosphide nanoparticles | FeCo?P?2 | 6 mol/L KOH+0.2 mol/L Zn(Ac)2 | 1.415 | 205 | - | [ |
| C?ZIF/LFP | 6 mol/L KOH+0.2 mol/L Zn(Ac)2 | 1.5 | 140 | - | [ | |
| FeCNFs?NP | 6 mol/L KOH+0.2 mol/L Zn(Ac)2 | 1.4 | 97 | - | [ |
| 1 | WANG H F, TANG C, ZHANG Q, et al. A review of precious-metal-free bifunctional oxygen electrocatalysts: rational design and applications in Zn-air batteries[J]. Adv Funct Mater, 2018, 28(46): 1803329. |
| 2 | XU R, WANG X K, ZHANG C H, et al. Engineering solid-liquid-gas interfaces of single-atom cobalt catalyst for enhancing the robust stability of neutral Zn-air batteries under high current density[J]. Chem Eng J, 2022, 433: 133685. |
| 3 | WANG H F, XU Q. Materials design for rechargeable metal-air batteries[J]. Matter-US, 2019, 1(3): 565-595. |
| 4 | ZHOU T P, ZHANG N, WU C Z, et al. Surface/interface nanoengineering for rechargeable Zn-air batteries[J]. Energy Environ Sci, 2020, 13(4): 1132-1153. |
| 5 | 李宫, 金龙一, 姚鹏飞, 等. 介孔炭负载铂纳米粒子的高效氧还原催化剂的可控设计[J]. 应用化学, 2021, 38(12): 1639-1646. |
| LI G, JIN L Y, YAO P F, et al. Controllability design of high performance oxygen reduction catalysts supported by platinum nanoparticles loaded on mesoporous carbon[J]. Chinese J Appl Chem, 2021, 38(12): 1639-1646. | |
| 6 | 高国锋, 李丹丹, 郝根彦, 等. 铁基阳极析氧催化剂的研究进展与展望[J]. 应用化学, 2016, 33(5): 505-511. |
| GAO G F, LI D D, HAO G Y, et al. Progress and prospect of iron based anodic oxygen evolving catalysts[J]. Chinese J Appl Chem, 2016, 33(5): 505-511. | |
| 7 | ZHAO C X, LIU J N, WANG J, et al. A ΔE=0.63 V bifunctional oxygen electrocatalyst enables high-rate and long‐cycling zinc-air batteries[J]. Adv Mater, 2021, 33(15): 2008606. |
| 8 | SUN W, WANG F, ZHANG B, et al. A rechargeable zinc-air battery based on zinc peroxide chemistry[J]. Science, 2021, 371(6524): 46-51. |
| 9 | YI J, LIANG P P, LIU X Y, et al. Challenges, mitigation strategies and perspectives in development of zinc-electrode materials and fabrication for rechargeable zinc air batteries[J]. Energy Environ Sci, 2018, 11(11): 3075-3095. |
| 10 | WANG X K, ZHAN G M, WANG Y R, et al. Engineering core-shell Co9S8/Co nanoparticles on reduced graphene oxide: efficient bifunctional mott-schottky electrocatalysts in neutral rechargeable Zn-air batteries[J]. J Energy Chem, 2022, 68: 113-123. |
| 11 | HUANG Y P, LIU K, KAN S T, et al. Highly dispersed Fe-Nx active sites on graphitic-N dominated porous carbon for synergetic catalysis of oxygen reduction reaction[J]. Carbon, 2021, 171: 1-9. |
| 12 | WANG X K, GAI H Y, CHEN Z K, et al. The marriage of crystalline/amorphous Co/Co3O4 heterostructures with N-doped hollow carbon spheres: efficient and durable catalysts for oxygen reduction[J]. Mater Today Energy, 2020, 18: 100497. |
| 13 | REN S S, DUAN X D, LIANG S, et al. Bifunctional electrocatalysts for Zn-air batteries: recent developments and future perspectives[J]. J Mater Chem A, 2020, 8(13): 6144-6182. |
| 14 | WANG D, PAN X, YANG P, et al. Transition metal and nitrogen co-doped carbon-based electrocatalysts for the oxygen reduction reaction: from active site insights to the rational design of precursors and structures[J]. ChemSusChem, 2021, 14(1): 33-55. |
| 15 | DENG J, DENG D H, BAO X H, et al. Robust catalysis on 2D materials encapsulating metals: concept, application, and perspective[J]. Adv Mater, 2017, 29(43): 1606967. |
| 16 | MENG F L, LIU K H, ZHANG Y, et al. Recent advances toward the rational design of efficient bifunctional air electrodes for rechargeable Zn-air batteries[J]. Small, 2018, 14(32): 1703843. |
| 17 | DU D, ZHAO S, ZHU Z, et al. Photo-excited oxygen reduction and oxygen evolution reactions enable a high-performance Zn-air battery[J]. Angew Chem Int Ed, 2020, 59(41): 18140-18144. |
| 18 | ZHANG H, LIU J, TIAN Z, et al. A general strategy toward transition metal carbide/carbon core/shell nanospheres and their application for supercapacitor electrode[J]. Carbon, 2016, 100: 590-599. |
| 19 | DENG D H, YU L, CHEN X Q, et al. Iron encapsulated within pod-like carbon nanotubes for oxygen reduction reaction[J]. Angew Chem Int Ed, 2013, 52(1): 371-375. |
| 20 | WANG J, WU H H, GAO D F, et al. High-density iron nanoparticles encapsulated within nitrogen-doped carbon nanoshell as efficient oxygen electrocatalyst for zinc-air battery[J]. Nano Energy, 2015, 13: 387-396. |
| 21 | DENG J, REN P J, DENG D H, et al. Enhanced electron penetration through an ultrathin graphene layer for highly efficient catalysis of the hydrogen evolution reaction[J]. Angew Chem Int Ed, 2015, 54(7): 2100-2104. |
| 22 | SUN Z H, WANG Y K M, ZHANG L B, et al. Simultaneously realizing rapid electron transfer and mass transport in jellyfish‐like mott-schottky nanoreactors for oxygen reduction reaction[J]. Adv Funct Mater, 2020, 30(15): 1910482. |
| 23 | LIU Q, LIU X, XIE Y, et al. N-Doped carbon coating enhances the bifunctional oxygen reaction activity of CoFe nanoparticles for a highly stable Zn-air battery[J]. J Mater Chem A, 2020, 8(40): 21189-21198. |
| 24 | ZHENG X J, CAO X C, ZENG K, et al. A self-jet vapor-phase growth of 3D FeNi@NCNT clusters as efficient oxygen electrocatalysts for zinc air batteries[J]. Small, 2021, 17(4): 2006183. |
| 25 | CAO F, YANG X, SHEN C, et al. Electrospinning synthesis of transition metal alloy nanoparticles encapsulated in nitrogen-doped carbon layers as an advanced bifunctional oxygen electrode[J]. J Mater Chem A, 2020, 8(15): 7245-7252. |
| 26 | HAO X Q, JIANG Z Q, ZHANG B A, et al. N-doped carbon nanotubes derived from graphene oxide with embedment of FeCo nanoparticles as bifunctional air electrode for rechargeable liquid and flexible all-solid-state zinc air batteries[J]. Adv Sci, 2021, 8(10): 2004572. |
| 27 | WANG Q C, LEI Y P, CHEN Z Y, et al. Fe/Fe3C@C nanoparticles encapsulated in N-doped graphene-CNTs framework as an efficient bifunctional oxygen electrocatalyst for robust rechargeable Zn-air batteries[J]. J Mater Chem A, 2018, 6(2): 516-526. |
| 28 | ZHANG G, LIU X, WANG L, et al. B, N-doped defective carbon entangled Fe3C nanoparticles as the superior oxygen reduction electrocatalyst for Zn-air batteries[J]. ACS Sustain Chem Eng, 2019, 7(23): 19104-19112. |
| 29 | LIU Z, ZHU Y F, XIAO K K, et al. Fe/Fe3C embedded in N-doped worm-like porous carbon for highrate catalysis in rechargeable zinc-air batteries[J]. ACS Appl Mater Inter, 2021, 13(21): 24710-24722. |
| 30 | DI J, GUO J X, WANG N N, et al. Multicomponent doped sugar-coated haws stick-like nanofibers as efficient oxygen reduction reaction catalysts for the Zn-air battery[J]. ACS Sustain Chem Eng, 2019, 7(8): 24710-24722. |
| 31 | NIU Y L, TENG X, WANG J Y, et al. Space-confined strategy to Fe7C3 nanoparticles wrapped in porous Fe-/N-doped carbon nanosheets for efficient oxygen electrocatalysis[J]. ACS Sustain Chem Eng, 2019, 7(15): 13576-13583. |
| 32 | LIU Z, ZHU Y F, XIAO K K, et al. Fe/Fe3C embedded in N-doped worm-like porous carbon for high-rate catalysis in rechargeable zinc-air batteries[J]. ACS Appl Mater Inter 2021, 13(21): 24710-24722. |
| 33 | YANG J, H J T, WENG M Y, et al. Fe-cluster pushing electrons to N-doped graphitic layers with Fe3C(Fe) hybrid nanostructure to enhance O2 reduction catalysis of Zn-air batteries[J]. ACS Appl Mater Inter, 2017, 9(5): 4587-4596. |
| 34 | ZHANG H M, ZHAO Y, ZHANG Y J, et al. Fe3O4 encapsulated in porous carbon nanobowls as efficient oxygen reduction reaction catalyst for Zn-air batteries[J]. Chem Eng J, 2019, 375: 122058. |
| 35 | DENG Y J, TIAN X L, SHEN G H, et al. Coupling hollow Fe3O4 nanoparticles with oxygen vacancy on mesoporous carbon as a high-efficiency ORR electrocatalyst for Zn-air battery [J]. J Colloid Interf Sci, 2020, 567: 410-418. |
| 36 | WANG B, YE Y Z, XU L, et al. Space-confined yolk-shell construction of Fe3O4 nanoparticles inside N-doped hollow mesoporous carbon spheres as bifunctional electrocatalysts for long-term rechargeable zinc air batteries[J]. Adv Funct Mater, 2020, 30(51): 2005834. |
| 37 | TIAN Y H, XU L, QIAN J C, et al. Fe3C/Fe2O3 heterostructure embedded in N-doped graphene as a bifunctional catalyst for quasi-solid-state zinc air batteries[J]. Carbon, 2019, 146: 763-771. |
| 38 | HAO R, REN J T, LV X W, et al. N-doped porous carbon hollow microspheres encapsulated with iron-based nanocomposites as advanced bifunctional catalysts for rechargeable Zn-air battery[J]. J Energy Chem, 2020, 49: 14-21. |
| 39 | PU Z H, LIU T T, AMIINU I S, et al. Transition-metal phosphides: activity origin, energy-related electrocatalysis applications, and synthetic strategies[J]. Adv Funct Mater, 2020, 30(45): 2004009. |
| 40 | CHEN K, WEN Z H, CAI P W, et al. One-pot scalable route to tri-functional electrocatalysts FeCoPx nanoparticles for integrated electrochemical devices[J]. Appl Catal B: Environ, 2021, 295: 120275. |
| 41 | WANG M, ZHANG C T, MENG T, et al. Iron oxide and phosphide encapsulated within N, P-doped microporous carbon nanofibers as advanced tri-functional electrocatalyst toward oxygen reduction/evolution and hydrogen evolution reactions and zinc air batteries[J]. J Power Sources, 2019, 413: 367-375. |
| 42 | XUE D X, YU F J, YING Q, et al. Phosphate-assisted dispersion of iron phosphide in carbon nanosheets towards efficient and durable ORR catalysts in acidic and alkaline media[J]. ChemCatChem, 2021, 13(20): 4431-4441. |
| 43 | JIN H H, ZHOU H, JI P X, et al. ZIF-8/LiFePO4 derived Fe-N-P Co-doped carbon nanotube encapsulated Fe2P nanoparticles for efficient oxygen reduction and Zn-air batteries[J]. Nano Res, 2020, 13(3): 818-823. |
| 44 | WU M C, GUO B K, NIE A, et al. Tailored architectures of FeNi alloy embedded in N-doped carbon as bifunctional oxygen electrocatalyst for rechargeable zinc-air battery[J]. J Colloid Interf Sci, 2020, 561: 585-592. |
| 45 | LI S S, CHEN W H, PAN H Z, et al. FeCo alloy nanoparticles coated by an ultrathin N-doped carbon layer and encapsulated in carbon nanotubes as a highly efficient bifunctional air electrode for rechargeable Zn-air batteries[J]. ACS Sustain Chem Eng, 2019, 7(9): 8530-8541. |
| 46 | GAO X W, YANG J H, SONG K Y, et al. Robust FeCo nanoparticles embedded in a N-doped porous carbon framework for high oxygen conversion catalytic activity in alkaline and acidic media[J]. J Mater Chem A, 2018, 6(46): 23445-23456. |
| 47 | HUANG L B, ZHAO L, ZHANG Y, et al. Engineering carbon-shells of M@NC bifunctional oxygen electrocatalyst towards stable aqueous rechargeable Zn-air batteries[J]. Chem Eng J, 2021, 418: 129409. |
| 48 | SAMANTA A, RETNA RAJ C R. Bifunctional nitrogen-doped hybrid catalyst based on onion-like carbon and graphitic carbon encapsulated transition metal alloy nanostructure for rechargeable zinc-air battery[J]. J Power Sources, 2020, 455: 227975. |
| 49 | JIA J C, YANG H J, WANG G X, et al. Fe/Fe3C nanoparticles embedded in nitrogen-doped carbon nanotubes as multifunctional electrocatalysts for oxygen catalysis and CO2 reduction[J]. ChemElectroChem, 2018, 5(3): 471-477. |
| 50 | LIU Z, ZHU Y F, XIAO K K, et al. Fe/Fe3C embedded in N-doped worm-like porous carbon for high-rate catalysis in rechargeable zinc air batteries[J]. ACS Appl Mater Inter, 2021, 13(21): 24710-24722. |
| 51 | ZONG L B, CHEN X, LIU S L, et al. Ultrafine Fe/Fe3C decorated on Fe-N-C as bifunctional oxygen electrocatalysts for efficient Zn-air batteries[J]. J Energy Chem, 2021, 56: 72-79. |
| 52 | WU Y L, XIAO Z X, JIN Z C, et al. The cobalt carbide/bimetallic CoFe phosphide dispersed on carbon nanospheres as advanced bifunctional electrocatalysts for the ORR, OER, and rechargeable Zn-air batteries[J]. J Colloid Interf Sci, 2021, 590: 321-329. |
| 53 | WANG Y L, GAN R H, LIU H, et al. Fe3O4/Fe2O3/Fe nanoparticles anchored on N-doped hierarchically porous carbon nanospheres as a high-efficiency ORR electrocatalyst for rechargeable Zn-air batteries[J]. J Mater Chem A, 2021, 9(5): 2764-2774. |
| [1] | Wei-Min DU, Xin LIU, Lin ZHU, Jia-Min FU, Wen-Shan GUO, Xiao-Qing YANG, Pei-Shuo SHUANG. Facile Synthesis and High⁃Efficiency Electrocatalytic Oxygen Evolution Performance of Ternary Nickel⁃Based Chalcogenide Nanorod Arrays [J]. Chinese Journal of Applied Chemistry, 2022, 39(8): 1252-1261. |
| [2] | Wei-Jin CAO, Lu BAI, Lan-Lan WU, Jing-De LI, Shu-Yan SONG. Multi⁃Shell Hollow Nickel⁃Cobalt Bimetallic Phosphide Nanospheres for Highly Efficient Oxygen Evolution Reaction [J]. Chinese Journal of Applied Chemistry, 2022, 39(4): 666-672. |
| [3] | Xue WANG, Yi-Bo WANG, Xian WANG, Jian-Bing ZHU, Jun-Jie GE, Chang-Peng LIU, Wei XING. Research Progress of Mechanism of Acidic Oxygen Evolution Reaction and Development of Ir⁃based Catalysts [J]. Chinese Journal of Applied Chemistry, 2022, 39(4): 616-628. |
| [4] | He LI, Gong LI, Xue GONG, Ming-Bo RUAN, Ce HAN, Ping SONG, Wei-Lin XU. Research on Performance Decay Mechanism of Pt/C Catalyst in Long‑Term ORR Test [J]. Chinese Journal of Applied Chemistry, 2022, 39(10): 1564-1571. |
| [5] | LI Gong, JIN Long-Yi, YAO Peng-Fei, LIU Cong, XU Wei-Lin. Controllability Design of High Performance Oxygen Reduction Catalysts Supported by Platinum Nanoparticles Loaded on Mesoporous Carbon [J]. Chinese Journal of Applied Chemistry, 2021, 38(12): 1639-1646. |
| [6] | WEI Zhenye, MENG Junling, WANG Haocong, ZHANG Wenwen, LIU Xiaojuan, MENG Jian. Improving the Electrocatalytic Activity of La2NiO4+δ Cathode by Surface Modification with Conformal Heterojunction [J]. Chinese Journal of Applied Chemistry, 2020, 37(8): 939-951. |
| [7] | CHEN Si,SUN Lizhen,SHU Xinxin,ZHANG Jintao. Graphene-based Catalysts for Efficient Electrocatalytic Applications [J]. Chinese Journal of Applied Chemistry, 2018, 35(3): 272-285. |
| [8] | SONG Hongwei, HUANG Hui, TAN Ning, CHENG Buming, GUO Zhongcheng. Electrochemical Behavior of Al/Pb-0.2%Ag Anode in Fluoride Ion and Sulfuric Acid [J]. Chinese Journal of Applied Chemistry, 2016, 33(12): 1455-1461. |
| [9] | KULISONG Hayierbiek, ZENG Han*. Direct Electrochemical Behavior and Sensing Performance of Nitrogen-Doped Meso-Porous Carbon and Chitosan Composite Immobilized with Laccase Modified Electrode [J]. Chinese Journal of Applied Chemistry, 2013, 30(10): 1194-1201. |
| [10] | ZHAO Shuxian, ZENG Han*. Kinetic Analysis of Oxygen Reduction Reaction Catalyzed by Multi-copper Oxidase in Presence of Electron Relay [J]. Chinese Journal of Applied Chemistry, 2013, 30(09): 1073-1081. |
| [11] | WANG Lipin, WANG Senlin*, DUAN Qianhua. Ni/NiFe2O4 Composite Electrode Prepared by Electro-deposition and Its Electro-catalytic Performance Towards Oxygen Evolution Reaction [J]. Chinese Journal of Applied Chemistry, 2013, 30(06): 690-697. |
| [12] | ZENG Han*, ZHAO Shuxian, GONG Lanxin, SU Zhi. Catalysis of Poly Benzimidazole and Laccase Composite Modified Electrode for Oxygen Reduction Reaction Without Electron Transfer Mediators [J]. Chinese Journal of Applied Chemistry, 2013, 30(04): 436-443. |
| [13] | ZHANG Yongchun1, CHEN Buming1, GUO Zhongcheng1,2*, LIU Jianhua1. Corrosion Resistance of Electrodeposited Al/Pb-Ag Anode During Zinc Electrowinning [J]. Chinese Journal of Applied Chemistry, 2013, 30(04): 458-463. |
| [14] | ZENG Han* , ZHAO Shu-Xian, GONG Lan-Xin, XU Guo-Qiang. Immobilization of Laccase from Trametes Versicolor on the Matrix of N,N-ethylene bis(acrylamide) Cross-linked Poly(methyl acrylic acid) and the Electrochemical Behavior of its Modified Glassy Carbon Electrode [J]. Chinese Journal of Applied Chemistry, 2010, 27(09): 1076-1082. |
| [15] | ZHAO Wei-Li, ZHOU De-Bi*, SUN Xin-Yang, TAN Long-Hui. One-step Preparation Process of Carbon Supported Co-phthalocyanine and Its Catalytic Performance for Electroreduction of Oxygen in Base Medium [J]. Chinese Journal of Applied Chemistry, 2010, 27(02): 183-190. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
