Chinese Journal of Applied Chemistry ›› 2022, Vol. 39 ›› Issue (02): 205-222.DOI: 10.19894/j.issn.1000-0518.210059
• Review • Next Articles
Research Progress on the Degradation Mechanism and Cycle Stability Improvement of Lithium-Rich Cathode Materials
Ying ZHAO1, Yi-Jia SHAO1, Luo-Qian LI1, Jian-Wei REN2( ), Shi-Jun LIAO1(
), Shi-Jun LIAO1( )
)
- 1.The Key Laboratory of Fuel Cells Technology of Guangdong Province, School of Chemistry and Chemical Engineering, South China University of Technology, Guangzhou 510641, China
2.Department of Mechanical Engineering Science, University of Johannesburg, Johannesburg ZA-2092, South Africa
-
Received:2021-02-02Accepted:2021-06-09Published:2022-02-10Online:2022-02-09 -
Contact:Jian-Wei REN,Shi-Jun LIAO -
Supported by:National Key Research and Development Program of China(2017YFB0102900);National Natural Science Foundation of China(51971094);Guangdong Provincial Department of Science and Technology(2015A030312007)
CLC Number:
Cite this article
Ying ZHAO, Yi-Jia SHAO, Luo-Qian LI, Jian-Wei REN, Shi-Jun LIAO. Research Progress on the Degradation Mechanism and Cycle Stability Improvement of Lithium-Rich Cathode Materials[J]. Chinese Journal of Applied Chemistry, 2022, 39(02): 205-222.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: http://yyhx.ciac.jl.cn/EN/10.19894/j.issn.1000-0518.210059

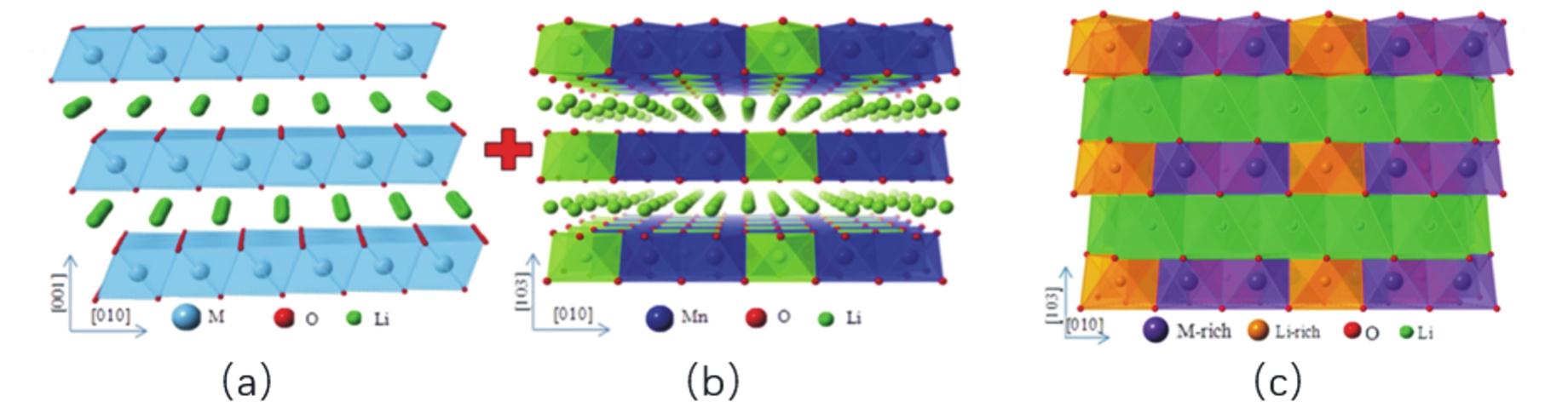
Fig.4 (a)Honeycomb-type cation arrangement in the TM layer of Li1.2Ni0.13Co0.13Mn0.54O2;(b)Oxygen in the honeycomb structure coordinated by Mn4+and Li+;(c)O-/O2-Energy and state density diagram[47]

Fig.5 Figures of the fitting results of PDF spectra at different states of OCV,pristine,charged to 4.4 V,and charged to 4.8 V(left);Figures of the local structure around the center Mn atom. Purple:Mn,red:O(right)[49]
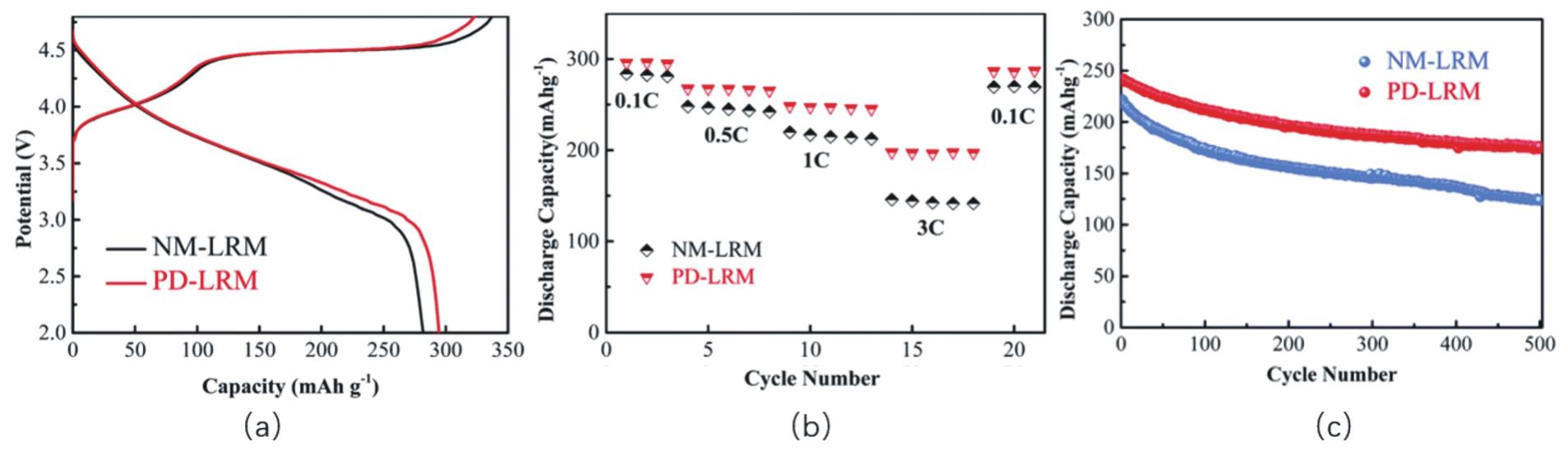
Fig.9 (a)First charge-discharge characteristics of NM-LRM and PD-LRM at 0.1 C;(b)Rate performance of NM-LRM and PD-LRM;(c)Cycle performance of NM-LRM and PD-LRM at 1 C[79]
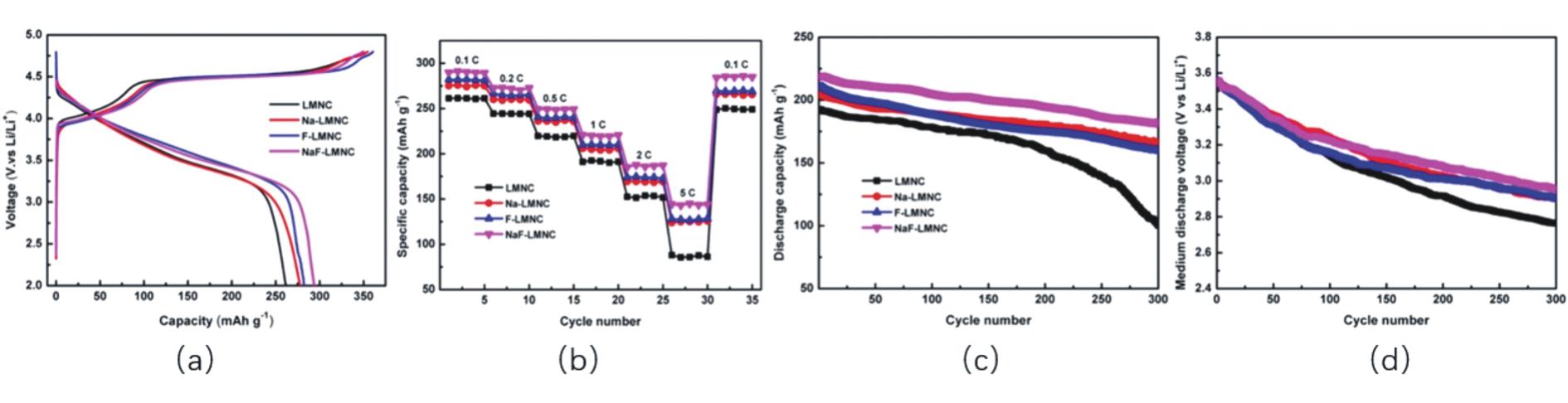
Fig.10 (a)First charge-discharge characteristics of LMNC,Na-LMNC,F-LMNC,and NaF-LMNC at 0.1 C;(b)Rate performance of LMNC,Na-LMNC,F-LMNC,and NaF-LMNC;(c)Cycle performance of LMNC,Na-LMNC,F-LMNC,and NaF-LMNC at 1 C;(d)Voltage fading of LMNC,Na-LMNC,F-LMNC,and NaF-LMNC[86]
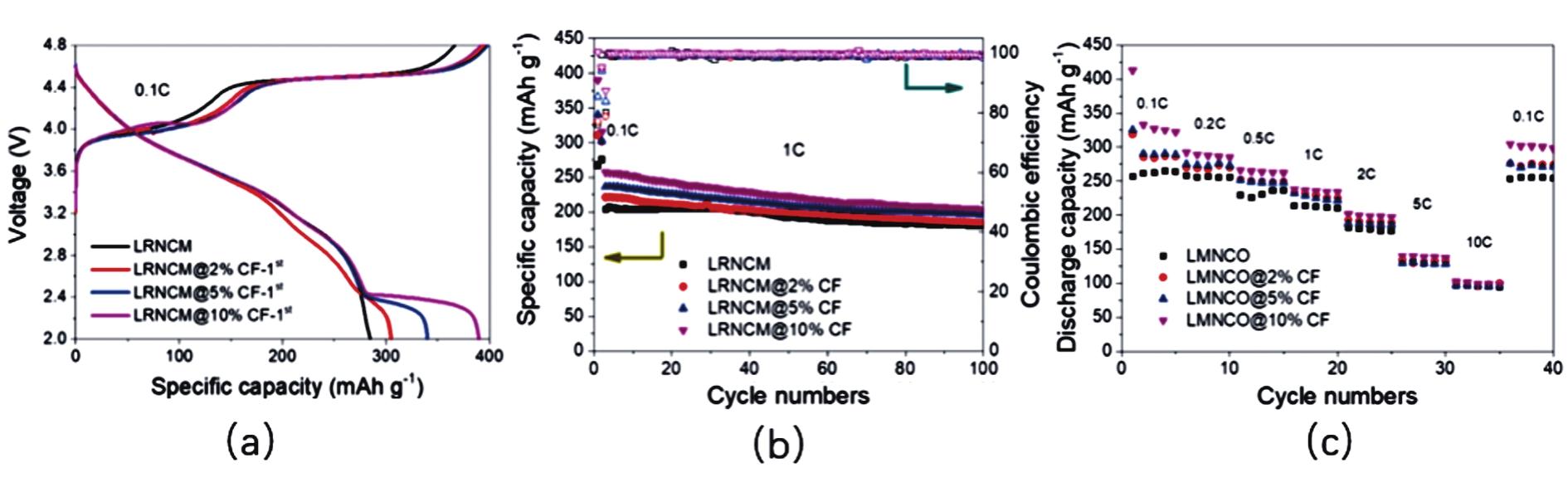
Fig.12 (a)First charge-discharge characteristics of materials before and after modification at 0.1 C;(b)Cycling performance of materials before and after modification at 1 C;(c)Rate performance of materials before and after modification at 0.1 C[104]
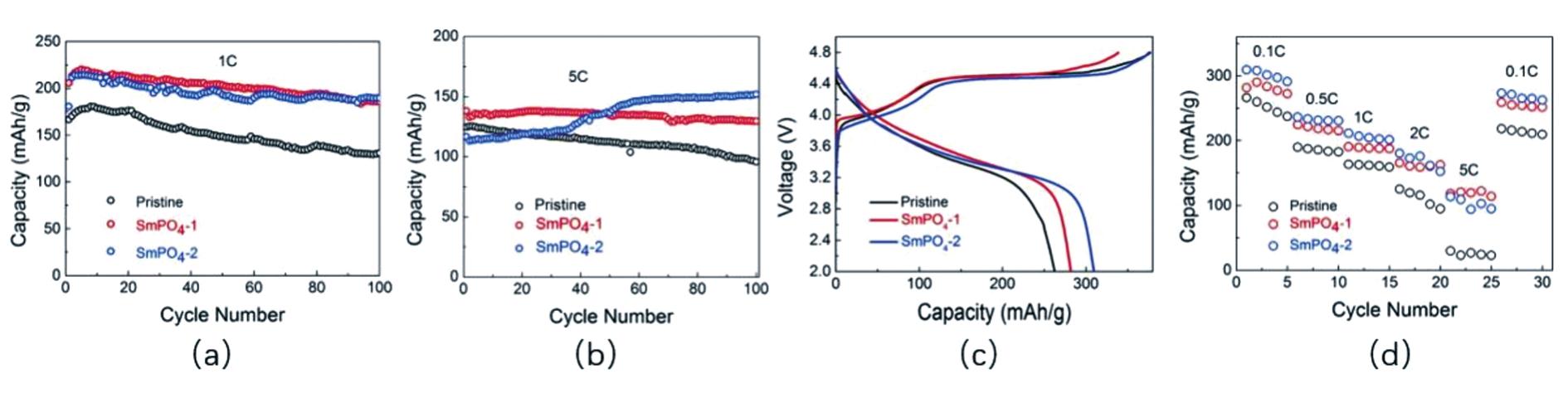
Fig.14 (a)First charge-discharge performance of materials before and after modification at 0.1 C;(b)rate performance of materials before and after modification;cycling performance of materials before and after modification at(c)1 C,(d)5 C[120]
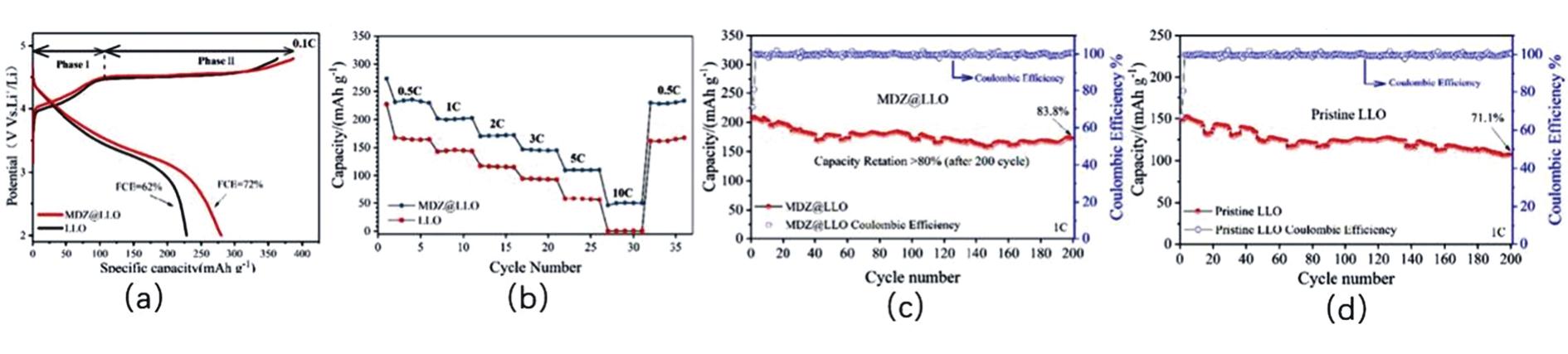
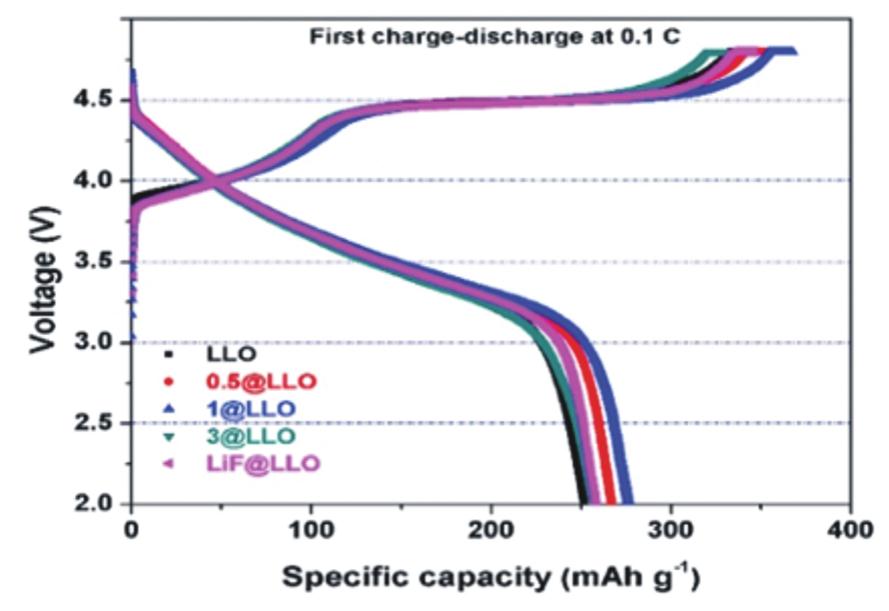
Fig.15 (a)first charge-discharge performance of LLO@MDZ and LLO at 0.1 C;(b)Rate performance of LLO@MDZ and LLO;(c)Cycling performance and coulombic efficiency of LLO@MDZ at 1 C;(d)Cycling performance and coulombic efficiency of LLO at 1 C[131]
| [1] | CROGUENNEC L, PALACIN M R. Recent achievements on inorganic electrode materials for lithium-ion batteries[J]. J Am Chem Soc, 2015, 137(9):3140-3156. |
| [2] | ETACHERI V, MAROM R, ELAZARI R, et al. Challenges in the development of advanced Li-ion batteries: a review[J]. Energy Environ Sci, 2011, 4(9):3243-3262. |
| [3] | NITTA N, WU F, LEE J T, et al. Li-ion battery materials: present and future[J]. Mater Today, 2015, 18(5):252-264. |
| [4] | DUNN B, KAMATH H, TARASCON J M. Electrical energy storage for the grid: a battery of choices[J]. Science, 2011, 334(6058):928. |
| [5] | ARMSTRONG A R, LYNESS C, PANCHMATIA P M, et al. The lithium intercalation process in the low-voltage lithium battery anode Li1+x V1-x O2 [J]. Nat Mater, 2011, 10:223. |
| [6] | HU Y, GAO Y, FAN L, et al. Electrochemical study of poly (2, 6-anthraquinonyl sulfide) as cathode for alkali-metal-ion batteries[J]. Adv Energy Mater, 2020, 10(48):2002780. |
| [7] | SU C, HAN B, MA J, et al. A novel anthraquinone-containing poly (triphenylamine) derivative: preparation and electrochemical performance as cathode for lithium-ion batteries[J]. Chem Electro Chem, 2020, 7(19):4101-4107. |
| [8] | KOBAYASHI H, TSUKASAKI T, OGASAWARA Y, et al. Cation-disorder-assisted reversible topotactic phase transition between antifluorite and rocksalt toward high-capacity lithium-ion batteries[J]. ACS Appl Mater Interfaces, 2020, 12(39):43605-43613. |
| [9] | CHANG Q, LUO Z, FU L, et al. A new cathode material of NiF2for thermal batteries with high specific power[J]. Electrochim Acta, 2020, 361:137051. |
| [10] | LUO L W, ZHANG C, XIONG P, et al. A redox-active conjugated microporous polymer cathode for high-performance lithium/potassium-organic batteries[J]. Sci China Chem, 2020, 64(1):72-81. |
| [11] | YU Z, WANG X, WAN P, et al. Li1.233Mo0.467Fe0.3O2as an advanced cathode material for high-performance lithium-ion battery[J]. Mater Lett, 2019, 249:45-48. |
| [12] | SHAO Y, HUANG B, LU Z, et al. High-performance 3D pinecone-like LiNi1/3Co1/3Mn1/3O2cathode for lithium-ion batteries[J]. Energy Technol, 2019, 7(4):1800769. |
| [13] | KALLURI S, YOON M, JO M, et al. Surface engineering trategies of layered LiCoO2cathode material to realize high-energy and high-voltage Li-ion cells[J]. Adv Energy Mater, 2017, 7(1):1601507. |
| [14] | WEI Y, ZHENG J, CUI S, et al. Kinetics tuning of Li-ion diffusion in layered Li (Ni x Mn y Coz) O2 [J]. J Am Chem Soc, 2015, 137(26):8364-8367. |
| [15] | LU Z H, MACNEIL D D, DAHN J R. Layered cathode materials Li Ni x Li(1/3-2x/3)Mn(2/3-x/3)O2for lithium-ion batteries[J]. Electrochem Solid State Lett, 2001, 4(11):A191-A194. |
| [16] | LU Z H, BEAULIEU L Y, DONABERGER R A, et al. Synthesis, structure, and electrochemical behavior of Li Ni x Li1/3-2x/3Mn2/3-x/3O2 [J]. J Electrochem Soc, 2002, 149(6):A778-A791. |
| [17] | LU Z H, DAHN J R. Understanding the anomalous capacity of Li/Li Ni x Li(1/3-2x/3)Mn(2/3-x/3)O2cells using in situ X-ray diffraction and electrochemical studies[J]. J Electrochem Soc, 2002, 149(7):A815-A822. |
| [18] | SHUNMUGASUNDARAM R, SENTHIL ARUMUGAM R, DAHN J R. High capacity Li-rich positive electrode materials with reduced first-cycle irreversible capacity loss[J]. Chem Mater, 2015, 27(3):757-767. |
| [19] | JOHNSON C S, KIM J S, LEFIEF C, et al. The significance of the Li2MnO3component in‘composite’xLi2MnO3·(1-x) LiMn0.5Ni0.5O2electrodes[J]. Electrochem Commun, 2004, 6(10):1085-1091. |
| [20] | KIM J S, JOHNSON C S, VAUGHEY J T, et al. Electrochemical and structural properties of xLi2M′O3·(1-x) LiMn0.5Ni0.5O2electrodes for lithium batteries (M′=Ti, Mn, Zr;0≤x-0.3)[J]. Chem Mater, 2004, 16(10):1996-2006. |
| [21] | ZHANG M, CHEN M, SHAO Y, et al. Enhanced performance of LiNi0.03Mo0.01Mn1.96O4cathode materials coated with biomass-derived carbon layer[J]. Ionics, 2018, 25(3):917-925. |
| [22] | 邵奕嘉, 黄斌, 刘全兵, 等. 三元镍钴锰正极材料的制备及改性[J]. 化学进展, 2018, 30(4):410-419. |
| SHAO Y J, HUANG B, LIU Q B, et al. Preparation and Modification of Ni-Co-Mn ternary cathode materials[J]. Chem Ind Eng Prog, 2018, 30(4):410-419. | |
| [23] | 廖世军, 卲奕嘉, 叶跃坤, 等. 一种溶剂热法制备三元正极材料及其制备方法[P]. 广东:CN107959022A, 2018-04-24. |
| LIAO S J, SHAO Y J, YE Y, K, et al. A solvothermal method for preparing ternary cathode material and its preparation method[P]. Guangdong:CN107959022A, 2018-04-24. | |
| [24] | 廖世军, 张和, 张梦诗, 等. 一种碳包覆的富锂多元锂离子电池正极材料及其制备方法[P]. 广东:CN107369817A, 2017-11-21. |
| LIAO S J, ZHAO H, ZHANG M S, et al. A Carbon-coated lithium-rich multi-element lithium-ion battery cathode material and its preparation method[P]. Guangdong:CN107369817A, 2017-11-21. | |
| [25] | CHEN M, REN W, SHAO Y, et al. Lithium-rich layered nickel-manganese oxides as high-performance cathode materials: the effects of composition and PEG on performance[J]. Ionics, 2016, 22(11):2067-2073. |
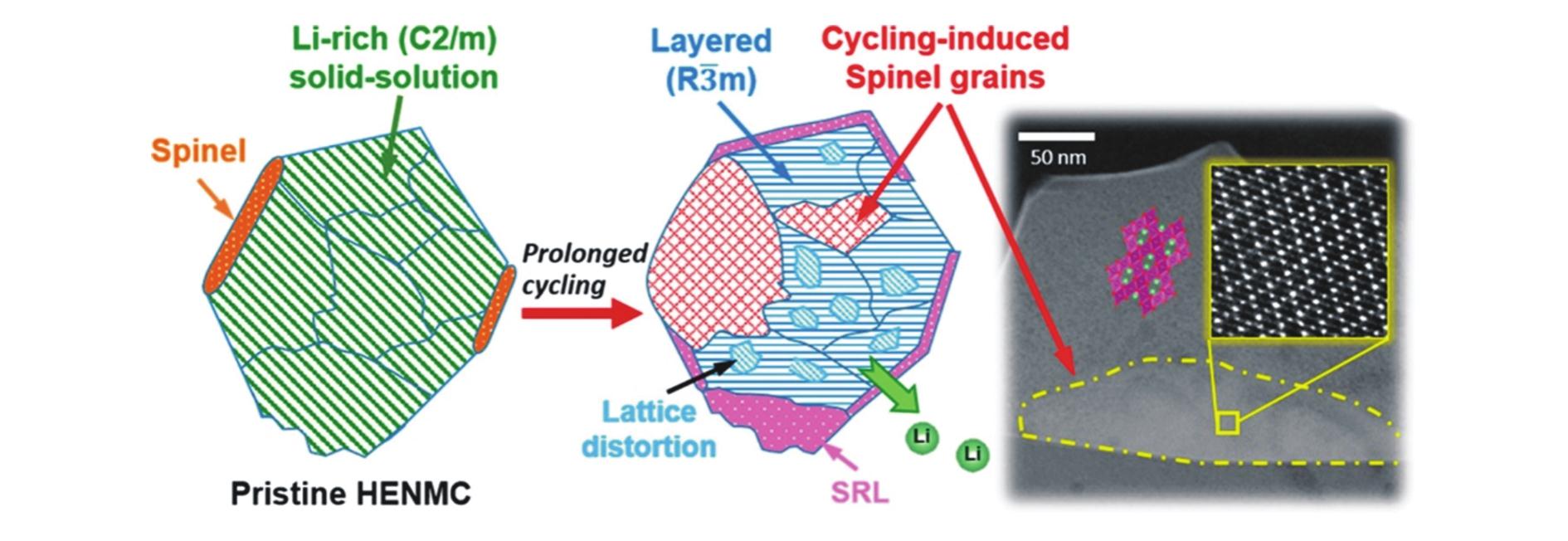
| [26] | 鲁志远, 刘燕妮, 廖世军. 锂离子电池富锂锰基层状正极材料的稳定性[J]. 化学进展, 2020, 32(10):1504-1514. |
| LU Z Y, LIU Y N, LIAO S J. Enhancing the stability of lithium-rich manganese-based layered cathode materials for Li-ion batteries application[J]. Chem Ind Eng Prog, 2020, 32(10):1504-1514. | |
| [27] | 张和, 张梦诗, 廖世军. 富锂三元层状正极材料的研究进展[J]. 应用化学, 2018, 35(11):1277-1288. |
| ZHANG H, ZHANG M S, LIAO S J. Research progress of lithium-rich ternary layered cathode materials[J]. Chinese J Appl Chem, 2018, 35(11):1277-1288. | |
| [28] | 廖世军, 鲁志远, 赵莹. 一种提升富锂三元材料循环稳定性及容量的方法及其材料[P]. 广东省:CN110518210A, 2019-11-29. |
| LIAO S J, LU Z Y, ZHAO Y. A method and material for improving the cycling stability and capacity of lithium-rich ternary materials[P]. Guangdong Province:CN110518210A, 2019-11-29. | |
| [29] | 廖世军, 鲁志远, 赵莹. 一种富锂三元正极材料及其绿色制备方法[P]. 广东省:CN109768272A, 2019-05-17. |
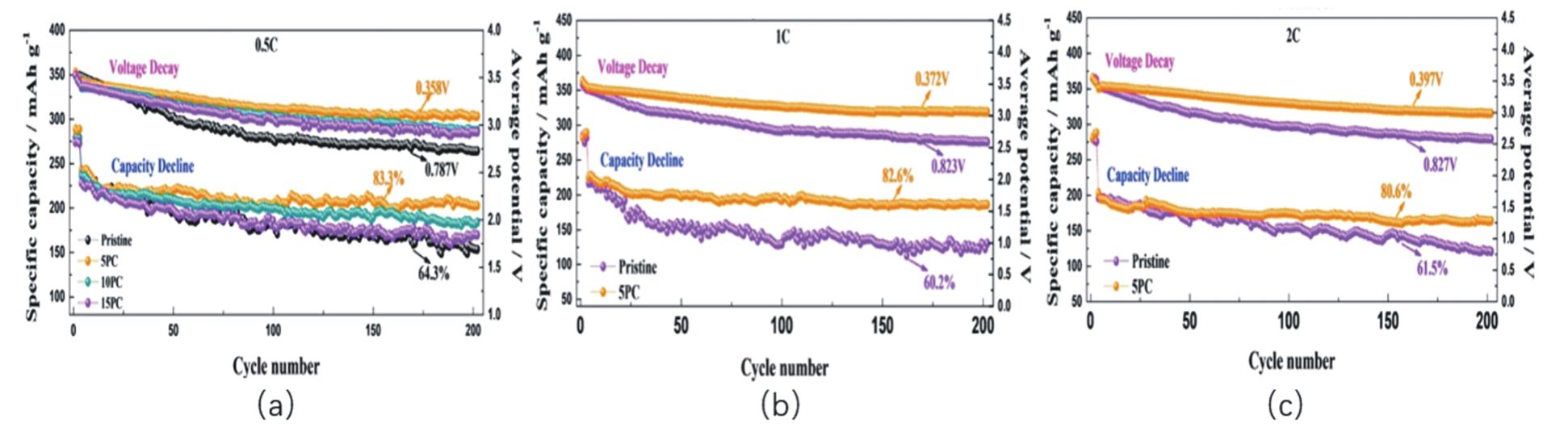
| LIAO S J, LU Z Y, ZHAO Y. A lithium-rich ternary cathode material and its green preparation method[P]. Guangdong Province:CN109768272A, 2019-05-17. | |
| [30] | ZHENG J, MYEONG S, CHO W, et al. Li-and Mn-rich cathode materials: challenges to commercialization[J]. Adv Energy Mater, 2016, 7(6):1601284. |
| [31] | LEE W, MUHAMMAD S, SERGEY C, et al. Advances in the cathode materials for lithium rechargeable batteries[J]. Angew Chem Int Ed Engl, 2020, 59(7):2578-2605. |
| [32] | YU H, ZHOU H. High-energy cathode materials (Li2MnO3-LiMO2) for lithium-ion batteries[J]. J Phys Chem Lett, 2013, 4(8):1268-1280. |
| [33] | BOULINEAU A, SIMONIN L, COLIN J F, et al. Evolutions of Li1.2Mn0.61Ni0.18Mg0.01O2during the initial charge/discharge cycle studied by advanced electron microscopy[J]. Chem Mater, 2012, 24(18):3558-3566. |
| [34] | YUH, ISHIKAWAR, SOYG, et al. Direct atomic-resolution observation of two phases in the Li1.2Mn0.567Ni0.166Co0.067O2cathode material for lithium-ion batteries[J]. Angew Chem Int Ed Engl, 2013, 52(23):5969-5973. |
| [35] | OHZUKU T, NAGAYAMA M, TSUJI K, et al. High-capacity lithium insertion materials of lithium nickel manganese oxides for advanced lithium-ion batteries: toward rechargeable capacity more than 300 mA·h·g-1 [J]. J Mater Chem, 2011, 21(27):10179-10188. |
| [36] | AMMUNDSEN B, PAULSEN J, DAVIDSON I, et al. Local structure and first cycle redox mechanism of layered Li1.2Cr0.4Mn0.4O2cathode material[J]. J Electrochem Soc, 2002, 149(4):A431-A436. |
| [37] | MOHANTY D, KALNAUS S, MEISNER R A, et al. Structural transformation of a lithium-rich Li1.2Co0.1Mn0.55Ni0.15O2 cathode during high voltage cycling resolved by in situ X-ray diffraction[J]. J Power Sources, 2013, 229:239-248. |
| [38] | WU Y, MANTHIRAM A. Effect of Al3+and F-doping on the irreversible oxygen loss from layered Li Li0.17Mn0.58Ni0.25O2 cathodes[J]. Electrochem Solid State Lett, 2007, 10(6):A151-A154. |
| [39] | WU F, LI W, CHEN L, et al. Renovating the electrode-electrolyte interphase for layered lithium- &manganese-rich oxides[J]. Energy Storage Mater, 2020, 28:383-392. |
| [40] | ZHAO S, YAN K, ZHANG J, et al. Reaction mechanisms of layered lithium-rich cathode materials for high-energy lithium-ion batteries[J]. Angew Chem Int Ed Engl, 2021, 60(5):2208-2220. |
| [41] | KONISHI H, HIRANO T, TAKAMATSU D, et al. Electrochemical reaction mechanism of two components in xLi2MnO3-(1-x)LiNi0.5Mn0.5O2and effect of x on the electrochemical performance in lithium-ion battery[J]. J Electroanal Chem, 2020, 873:114402. |
| [42] | ARMSTRONG A R, HOLZAPFEL M, NOVAK P, et al. Demonstrating oxygen loss and associated structural reorganization in the lithium battery cathode LiNi0.2Li0.2Mn0.6O2 [J]. J Am Chem Soc, 2006, 128(26):8694-8698. |
| [43] | TANG Z K, XUE Y F, TEOBALDI G, et al. The oxygen vacancy in Li-ion battery cathode materials[J]. Nanoscale Horiz, 2020, 5(11):1453-1466. |
| [44] | CUI S L, ZHANG X, WU X W, et al. Understanding the structure-performance relationship of lithium-rich cathode materials from an oxygen-vacancy perspective[J]. ACS Appl Mater Interfaces, 2020, 12(42):47655-47666. |
| [45] | DING X, LI Y X, WANG S, et al. Towards improved structural stability and electrochemical properties of a Li-rich material by a strategy of double gradient surface modification[J]. Nano Energy, 2019, 61:411-419. |
| [46] | MUHAMMAD S, KIM H, KIM Y, et al. Evidence of reversible oxygen participation in anomalously high capacity Li-and Mn-rich cathodes for Li-ion batteries[J]. Nano Energy, 2016, 21:172-184. |
| [47] | LUO K, ROBERTS M R, HAO R, et al. Charge-compensation in 3D-transition-metal-oxide intercalation cathodes through the generation of localized electron holes on oxygen[J]. Nat Chem, 2016, 8(7):684-691. |
| [48] | NAKAYAMA K, ISHIKAWA R, KOBAYASHI S, et al. Dislocation and oxygen-release driven delithiation in Li2MnO3 [J]. Nat Commun, 2020, 11(1):4452. |
| [49] | WANG L, DAI A, XU W, et al. Structural distortion induced by manganese activation in a lithium-rich layered cathode[J]. J Am Chem Soc, 2020, 142(35):14966-14973. |
| [50] | HONG J, LIM H D, LEE M, et al. Critical role of oxygen evolved from layered Li-excess metal oxides in lithium rechargeable batteries[J]. Chem Mater, 2012, 24(14):2692-2697. |
| [51] | YU X Y, YU L, LOU X W. Metal sulfide hollow nanostructures for electrochemical energy storage[J]. Adv Energy Mater, 2016, 6(3):1501333. |
| [52] | SINGER A, ZHANG M, HY S, et al. Nucleation of dislocations and their dynamics in layered oxide cathode materials during battery charging[J]. Nat Energy, 2018, 3(8):641-647. |
| [53] | LIU H, HARRIS K J, JIANG M, et al. Unraveling the rapid performance decay of layered high-energy cathodes: from nanoscale degradation to drastic bulk evolution[J]. ACS Nano, 2018, 12(3):2708-2718. |
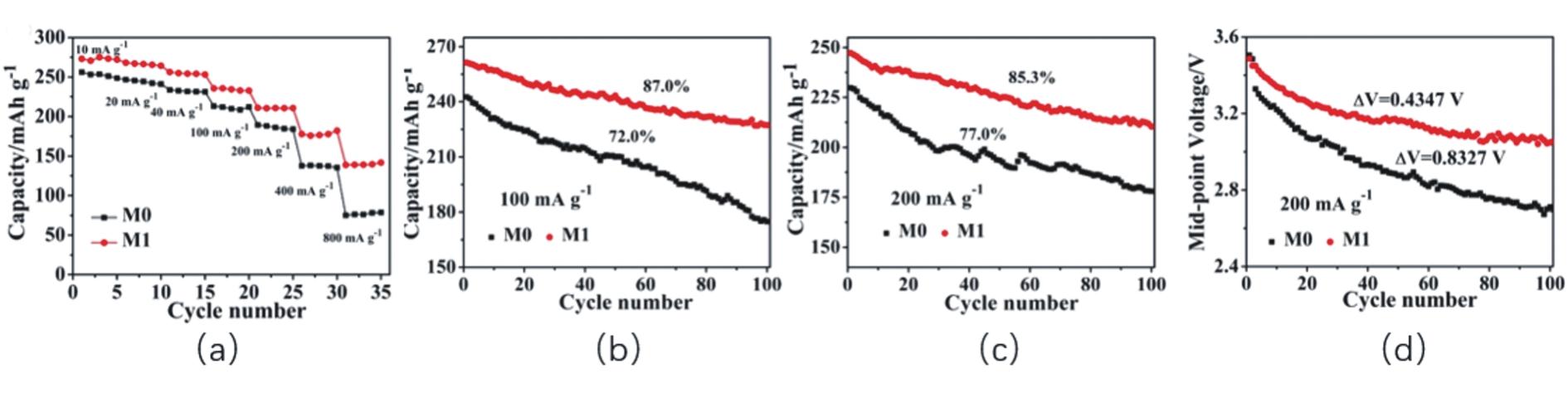
| [54] | DING X, LI Y X, DENG M M, et al. Cesium doping to improve the electrochemical performance of layered Li1.2Ni0.13Co0.13Mn0.54O2cathode material[J]. J Alloys Compd, 2019, 791:100-108. |
| [55] | ZHANG K, SHENG H, WU X, et al. Improving electrochemical properties by Sodium doping for lithium-rich layered oxides[J]. ACS Appl Energ Mater, 2020, 3(9):8953-8959. |
| [56] | CHEN B, ZHAO B, ZHOU J, et al. Enhanced electrochemical performance of Li1.2Ni0.2Mn0.6O2cathode materials through facile layered/spinel phase tuning[J]. J Solid State Electrochem, 2018, 22(8):2587-2596. |
| [57] | LIU Y, LIU D, WU H H, et al. Improved cycling stability of Na-doped cathode materials Li1.2Ni0.2Mn0.6O2 via a facile synthesis[J]. ACS Sustainable Chem Eng, 2018, 6(10):13045-13055. |
| [58] | XUE Z, QI X, LI L, et al. Sodium doping to enhance electrochemical performance of overlithiated oxide cathode materials for Li-ion batteries via Li/Na ion-exchange method[J]. ACS Appl Mater Interfaces, 2018, 10(32):27141-27149. |
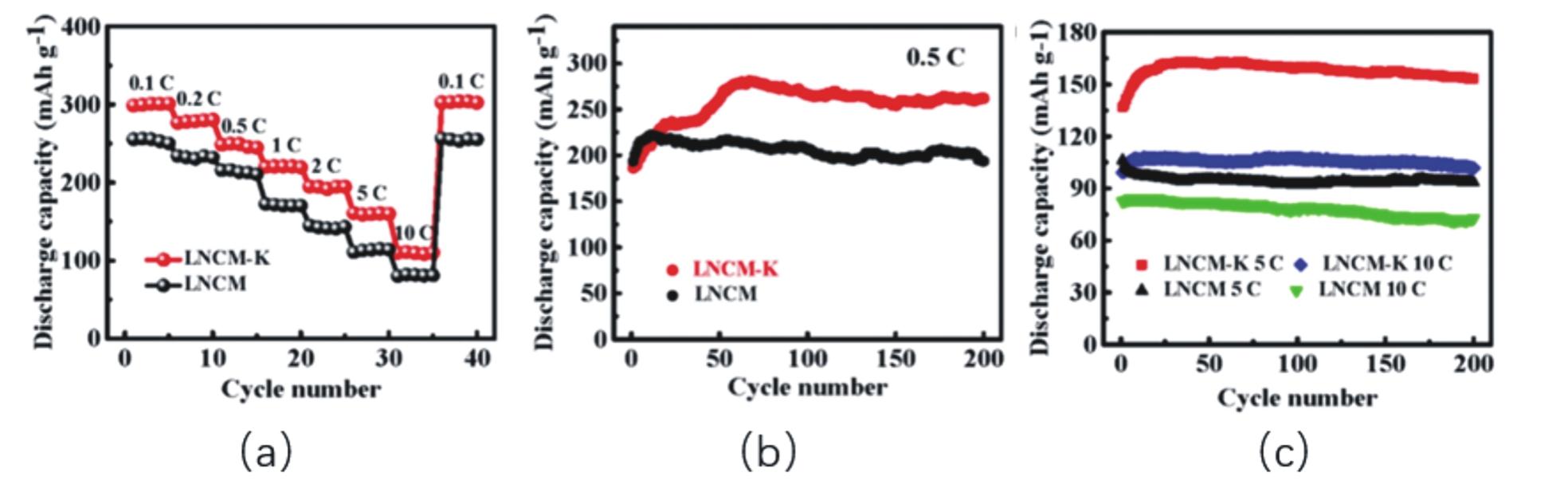
| [59] | LIU Z, ZHANG Z, LIU Y, et al. Facile and scalable fabrication of K+-doped Li1.2Ni0.2Co0.08Mn0.52O2cathode with ultra high capacity and enhanced cycling stability for lithium-ion batteries[J]. Solid State Ion, 2019, 332:47-54. |
| [60] | YANG M, HU B, GENG F, et al. Mitigating voltage decay in high-capacity Li1.2Ni0.2Mn0.6O2cathode material by surface K+doping[J]. Electrochim Acta, 2018, 291:278-286. |
| [61] | JIN Y, XU Y, REN F, et al. Mg-doped Li1.133Ni0.2Co0.2Mn0.467O2in Li site as high-performance cathode material for Li-ion batteries[J]. Solid State Ion, 2019, 336:87-94. |
| [62] | MENG F, GUO H, WANG Z, et al. Magnesium-doped Li[Li0.2Mn0.54Ni0.13Co0.13]O2cathode with high rate capability and improved cyclic stability[J]. Ionics, 2018, 25(5):1967-1977. |
| [63] | LOU M, FAN S S, YU H T, et al. Mg-doped Li1.2Mn0.54Ni0.13Co0.13O2nano flakes with improved electrochemical performance for lithium-ion battery application[J]. J Alloys Compd, 2018, 739:607-615. |
| [64] | XU G, XUE Q, LI J, et al. Understanding the enhanced electrochemical performance of Samarium substituted Li[Li0.2Mn0.54-x Sm x Co0.13Ni0.13]O2cathode material for lithium ion batteries[J]. Solid State Ion, 2016, 293:7-12. |
| [65] | ETEFAGH R, ROZATI S M, ARABI H. Al-doped Li1.21[Mn0.54Ni0.125Co0.125]O2cathode material with enhanced electrochemical properties for lithium-ion battery[J]. Appl Phys A, 2020, 126(10):814. |
| [66] | SETENIB, RAPULENYANEN, NGILAJC, et al. Structural and electrochemical behavior of Li1.2Mn0.54Ni0.13Co0.13-x Al x O2(x=0.05) positive electrode material for lithium-ion battery[J]. Mater Today: Proc, 2018, 5(4):10479-10487. |
| [67] | LIU S, LIU Z, SHEN X, et al. Surface doping to enhance structural integrity and performance of Li-rich layered oxide[J]. Adv Energy Mater, 2018, 8(31):1802105. |
| [68] | LI H, JIAN Z, YANG P, et al. Niobium doping of Li1.2Mn0.54Ni0.13Co0.13O2cathode materials with enhanced sructural stability and electrochemical performance[J]. Ceram Int, 2020, 46(15):23773-23779. |
| [69] | BAO L, YANG Z, CHEN L, et al. The effects of trace Yb doping on the electrochemical performance of Li-rich layered oxides[J]. ChemSusChem, 2019, 12(10):2294-2301. |
| [70] | CHEN J, ZOU G, DENG W, et al. Pseudo-bonding and electric-field harmony for Li-rich Mn-based oxide cathode[J]. Adv Funct Mater, 2020, 30(46):2004302. |
| [71] | LIU Y, LI J, YANG Z, et al. Suppressing the voltage decay of lithium-rich cathode for Li-ion batteries via Pt nanoparticles surface modification[J]. Ceram Int, 2020, 46(17):26564-26571. |
| [72] | SORBONI Y G, ARABI H, KOMPANY A. Effect of Cu doping on the structural and electrochemical properties of lithium-rich Li1.25Mn0.50Ni0.125Co0.125O2nanopowders as a cathode material[J]. Ceram Int, 2019, 45(2):2139-2145. |
| [73] | WANG Y, GU H T, SONG J H, et al. Suppressing Mn reduction of Li-rich Mn-based cathodes by F-doping for advanced lithium-ion batteries[J]. J Phys Chem C, 2018, 122(49):27836-27842. |
| [74] | TENG R, YU H T, GUO C F, et al. Effect of F dopant on the structural stability, redox mechanism, and electrochemical performance of Li2MoO3cathode materials[J]. Adv Sustainable Syst, 2020, 4(12):2000104. |
| [75] | LIUT, ZHAOSX, GOULL, et al. Electrochemical performance of Li-rich cathode material, 0.3Li2MnO3-0.7LiMn1/3Ni1/3Co1/3O2microspheres with F-doping[J]. Rare Met, 2018, 38(3):189-198. |
| [76] | RICHARDS W D, DACEK S T, Kitchaev D A, et al. Fluorination of lithium-excess transition metal oxide cathode materials[J]. Adv Energy Mater, 2018, 8(5):1701533. |
| [77] | LUN Z, OUYANG B, KITCHAEV D A, et al. Improved cycling performance of Li-excess cation-disordered cathode materials upon fluorine e substitution[J]. Adv Energy Mater, 2019, 9(2):1802959. |
| [78] | KAPYLOU A, SONG J H, MISSIUL A, et al. Improved thermal stability of lithium-rich layered oxides by fluorine doping[J]. Chemphyschem, 2018, 19(1):116-122. |
| [79] | WANG M J, YU F D, SUN G, et al. Co-regulating the surface and bulk structure of Li-rich layered oxides by a phosphor doping strategy for high-energy Li-ion batteries[J]. J Mater Chem A, 2019, 7(14):8302-8314. |
| [80] | LIU D, FAN X, LI Z, et al. A cation/anion co-soped Li1.12Na0.08Ni0.2Mn0.6O1.95F0.05cathode for lithium-ion batteries[J]. Nano Energy, 2019, 58:786-796. |
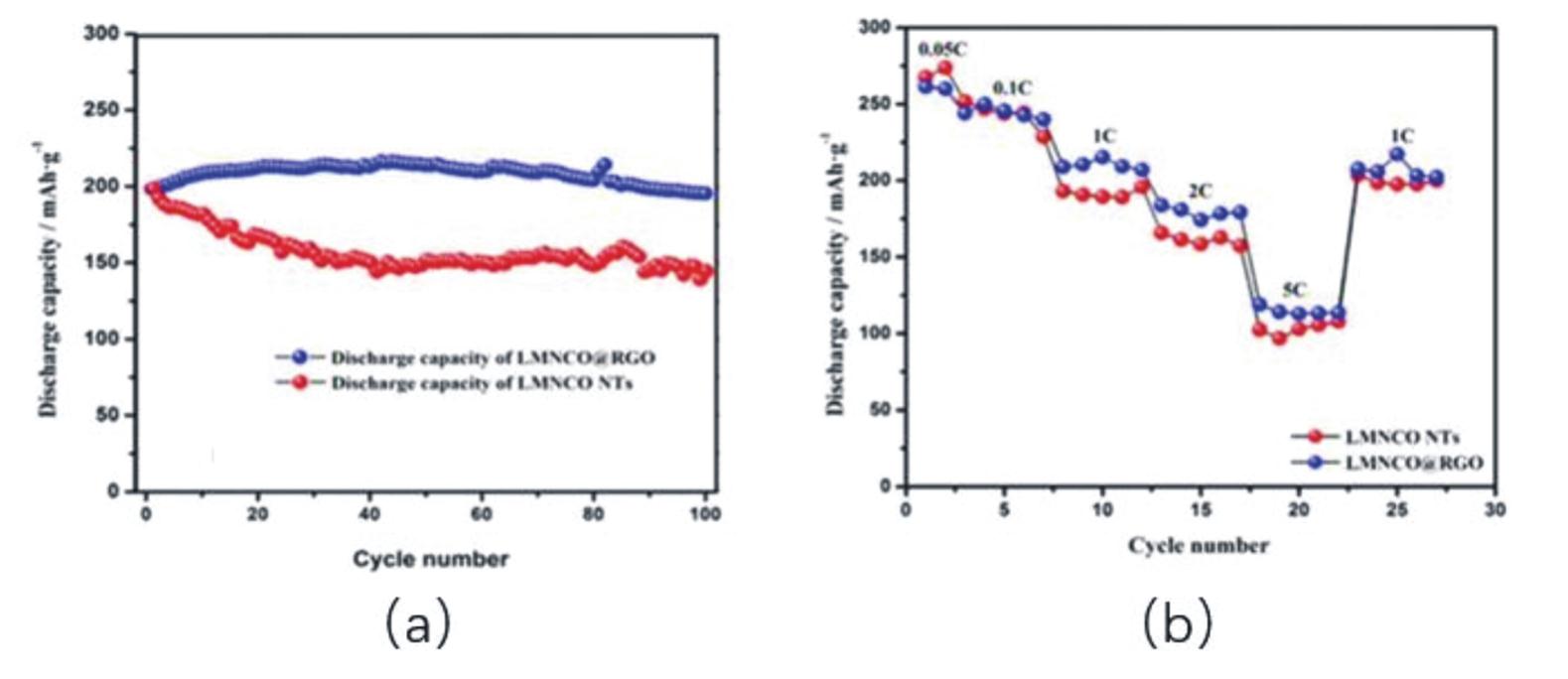
| [81] | PENG Z, MU K, CAO Y, et al. Enhanced electrochemical performance of layered Li-rich cathode materials for lithium-ion batteries via aluminum and boron dual-doping[J]. Ceram Int, 2019, 45(4):4184-4192. |
| [82] | SUN Y, WU Q, ZHAO L. A new doping element to improve the electrochemical performance of Li1.2Mn0.54Ni0.13Co0.13O2 materials for Li-ion batteries[J]. Ceram Int, 2019, 45(1):1339-1347. |
| [83] | VANAPHUTI P, BAI J, MA L, et al. Unraveling Na and F coupling effects in stabilizing Li, Mn-rich layered oxide cathodes via local ordering modification[J]. Energy Storage Mater, 2020, 31:459-469. |
| [84] | LI M, WANG H, ZHAO L, et al. Improving the electrochemical performance of lithium-rich oxide layer material with Mg and La co-doping[J]. J Alloys Compd, 2019, 782:451-460. |
| [85] | HU K, LV G, ZHANG J, et al. Na2S Treatment and coherent interface modification of the Li-rich cathode to address capacity and voltage decay[J]. ACS Appl Mater Interfaces, 2020, 12(38):42660-42668. |
| [86] | ZHANG P, ZHAI X, HUANG H, et al. Synergistic Na+and F-co-doping modification strategy to improve the electrochemical performance of Li-rich Li1.20Mn0.54Ni0.13Co0.13O2cathode[J]. Ceram Int, 2020, 46(15):24723-24736. |
| [87] | LIM SN, SEOJY, JUNGDS, et al. The crystal structure and electrochemical performance of Li1.167Mn0.548Ni0.18Co0.105O2composite cathodes doped and co-doped with Mg and F[J]. J Electroanal Chem, 2015, 740:88-94. |
| [88] | ZENG Z, GAO D, YANG G, et al. Ultrathin interfacial modification of lithium-rich layered oxide electrode/sulfide solid electrolyte via atomic layer deposition for high electrochemical performance batteries[J]. Nanotechnology, 2020, 31(45):454001. |
| [89] | MENG F, GUO H, WANG Z, et al. Modification of Li[Li0.2Mn0.54Ni0.13Co0.13]O2cathode with α-MoO3 via a simple wet chemical coating process[J]. Appl Surf Sci, 2019, 479:1277-1286. |
| [90] | ZHANG C, FENG Y, WEI B, et al. Heteroepitaxial oxygen-buffering interface enables a highly stable cobalt-free Li-rich layered oxide cathode[J]. Nano Energy, 2020, 75:104995. |
| [91] | HUANG Z, CAI M, SONG Z, et al. Surface modification of hierarchical Li1.2Mn0.56Ni0.16Co0.08O2with melting impregnation method for lithium-ion batteries[J]. J Alloys Compd, 2020, 840:155678. |
| [92] | JAMES ABRAHAM J, NISAR U, MONAWWAR H, et al. Improved electrochemical performance of SiO2-coated lithium-rich layered oxides-Li1.2Ni0.13Mn0.54Co0.13O2 [J]. J Mater Sci-Mater El, 2020, 31(21):19475-19486. |
| [93] | WANX, CHEW, ZHANGD, et al. Improved electrochemical behavior of Li-rich cathode Li1.4Mn0.61Ni0.18Co0.18Al0.03O2.4 via Y2O3surface coating[J]. Mater Charact, 2020, 169:110602. |
| [94] | LI Y, HUANG H, YU J, et al. Improved high rate capability of Li[Li0.2Mn0.534Co0.133Ni0.133]O2cathode material by surface modification with Co3O4 [J]. J Alloys Compd, 2019, 783:349-356. |
| [95] | WANG C C, LIN J W, YU Y H, et al. Nanolaminated ZnO-TiO2coated lithium-rich layered oxide cathodes by atomic layer deposition for enhanced electrochemical performances[J]. J Alloys Compd, 2020, 842:155845. |
| [96] | RAN X, TAO J, CHEN Z, et al. Surface heterostructure induced by TiO2modification in Li-rich cathode materials for enhanced electrochemical performances[J]. Electrochim Acta, 2020, 353:135959. |
| [97] | VIVEKANANTHA M, PARTHEEBAN T, KESAVAN T, et al. Alleviating the initial coulombic efficiency loss and enhancing the electrochemical performance of Li1.2Mn0.54Ni0.13Co0.13O2using β-MnO2 [J]. Appl Surf Sci, 2019, 489:336-345. |
| [98] | WEN X, LIANG K, TIAN L, et al. Al2O3coating on Li1.256Ni0.198Co0.082Mn0.689O2.25with spinel-structure interface layer for superior performance lithium-ion batteries[J]. Electrochim Acta, 2018, 260:549-556. |
| [99] | ZUO Y, HUANG B, JIAO C, et al. Enhanced electrochemical properties of Li[Li0.2Mn0.54Ni0.13Co0.13]O2with ZrF4 surface modification as cathode for Li-ion batteries[J]. J Mater Sci-Mater Electron, 2017, 29(1):524-534. |
| [100] | LI C D, YAO Z L, XU J, et al. Surface-modified Li[Li0.2Mn0.54Ni0.13Co0.13]O2nanoparticles with LaF3as cathode for Li-ion battery[J]. Ionics, 2016, 23(3):549-558. |
| [101] | ZHU W, CHONG S, SUN J, et al. The enhanced electrochemical performance of Li1.2Ni0.2Mn0.6O2through coating MnF2 nano protective layer[J]. Energy Technol, 2019, 7(10):1900443. |
| [102] | LIU B, ZHANG Z, WAN J, et al. Improved electrochemical properties of YF3-coated Li1.2Mn0.54Ni0.13Co0.13O2as cathode for Li-ion batteries[J]. Ionics, 2017, 23(6):1365-1374. |
| [103] | ZHAO S, SUN B, YAN K, et al. Aegis of lithium-rich cathode material via heterostructured LiAlF4coating for high-performance lithium-ion batteries[J]. ACS Appl Mater Interfaces, 2018, 10(39):33260-33268. |
| [104] | JIANG B, LUO B, LI J, et al. Electrochemical effect of graphite fluoride modification on Li-rich cathode material in lithium-ion batteries[J]. Ceram Int, 2019, 45(1):160-167. |
| [105] | WU F, XUE Q, LI L, et al. The positive role of (NH4)3AlF6coating on Li[Li0.2Ni0.2Mn0.6]O2oxide as the cathode material for lithium-ion batteries[J]. RSC Adv, 2017, 7(2):1191-1199. |
| [106] | LEE H, LIM S B, KIM J Y, et al. Characterization and control of irreversible reaction in Li-rich cathode during the initial charge process[J]. ACS Appl Mater Interfaces, 2018, 10(13):10804-10818. |
| [107] | YU Z. Electrochemical performances of carbon-coated Li[Li0.29Mn0.57Co0.14]O2cathode caterials for lithium-ion batteries[J]. Int J Electrochem Sci, 2019:4216-4225. |
| [108] | LEE S J, KIM S J, SON J T. Evaluations of discharge capacity and cycle stability for a graphene-added Li1.9Ni0.35Mn0.65O2cathode fabricated by using carbonate co-precipitation[J]. J Korean Phys Soc, 2020, 77(11):1035-1039. |
| [109] | PANG S, ZHU M, XU K, et al. The glucose-based treatment: a green and cost-efficient lithium-rich layered oxide modification strategy[J]. Ceram Int, 2019, 45(15):19268-19274. |
| [110] | LI X, ZHENG L, ZANG Z, et al. Multiply depolarized composite cathode of Li1.2Mn0.54Ni0.13Co0.13O2embedded in a combinatory conductive network for lithium-ion battery with superior overall performances[J]. J Alloys Compd, 2018, 744:41-50. |
| [111] | PARK K, KIM J, PARK J H, et al. Synchronous phase transition and carbon coating on the surface of Li-rich layered oxide cathode materials for rechargeable Li-ion batteries[J]. J Power Sources, 2018, 408:105-110. |
| [112] | LIU D, WANG F, WANG G, et al. Well-wrapped Li-rich layered cathodes by reduced graphene oxide towards high-performance Li-ion batteries[J]. Molecules, 2019, 24(9):1680. |
| [113] | CHEN J J, LI Z D, XIANG H F, et al. Bifunctional effects of carbon coating on high-capacity Li1.2Ni0.13Co0.13Mn0.54O2 cathode for lithium-ion batteries[J]. J Solid State Electrochem, 2014, 19(4):1027-1035. |
| [114] | MA D, LI Y, WU M, et al. Enhanced cycling stability of Li-rich nanotube cathodes by 3D graphene gierarchical architectures for Li-ion batteries[J]. Acta Mater, 2016, 112:11-19. |
| [115] | DING F, LI J, DENG F, et al. Surface heterostructure induced by PrPO4modification in Li1.2[Mn0.54Ni0.13Co0.13]O2 cathode material for high-performance lithium-ion batteries with mitigating voltage decay[J]. ACS Appl Mater Interfaces, 2017, 9(33):27936-27945. |
| [116] | ZHOU L, YIN Z, TIAN H, et al. Spinel-embedded and Li3PO4modified Li[Li0.2Mn0.54Ni0.13Co0.13]O2cathode materials for high-performance Li-ion battries[J]. Appl Surf Sci, 2018, 456:763-770. |
| [117] | XIAO B, WANG B, LIU J, et al. Highly stable Li1.2Mn0.54Co0.13Ni0.13O2enabled by novel atomic layer deposited AlPO4 coating[J]. Nano Energy, 2017, 34:120-130. |
| [118] | LIU Y, FAN X, HUANG X, et al. Electrochemical performance of Li1.2Ni0.2Mn0.6O2coated with a facilely synthesized Li1.3Al0.3Ti1.7(PO4)3 [J]. J Power Sources, 2018, 403:27-37. |
| [119] | CHEN D, ZHENG F, LI L, et al. Effect of Li3PO4coating of layered lithium-rich oxide on electrochemical performance[J]. J Power Sources, 2017, 341:147-155. |
| [120] | HE L, XU J, HAN T, et al. SmPO4-coated Li1.2Mn0.54Ni0.13Co0.13O2as a cathode material with enhanced cycling stability for lithium-ion batteries[J]. Ceram Int, 2017, 43(6):5267-5273. |
| [121] | SONG J, WANG Y, FENG Z, et al. Investigation on the electrochemical properties and stabilized surface/interface of nano-AlPO4-coated Li1.15Ni0.17Co0.11Mn0.57O2as the cathode for lithium-ion batteries[J]. ACS Appl Mater Interfaces, 2018, 10(32):27326-27332. |
| [122] | ZHANG X, HAO J, WU L, et al. Enhanced electrochemical performance of perovskite LaNiO3coating on Li1.2Mn0.54Ni0.13Co0.13O2as cathode materials for Li-ion batteries[J]. Electrochim Acta, 2018, 283:1203-1212. |
| [123] | WU F, LI Q, BAO L, et al. Role of LaNiO3in suppressing voltage decay of layered lithium-rich cathode materials[J]. Electrochim Acta, 2018, 260:986-993. |
| [124] | XU Z, CI L, YUAN Y, et al. Potassium prussian blue-coated Li-rich cathode with enhanced lithium-ion storage property[J]. Nano Energy, 2020, 75:104942. |
| [125] | LUO S, GUO H, FENG S, et al. Clearing surficial charge-transport obstacles to boost the performance of lithium-rich layered oxides[J]. Chem Eng J, 2020, 399:125142. |
| [126] | WANG E, ZHAO Y, XIAO D, et al. Composite nanostructure construction on the grain surface of Li-rich layered oxides[J]. Adv Mater, 2020:e1906070. |
| [127] | WANG D, ZHANG X, XIAO R, et al. Electrochemical performance of Li-rich Li[Li0.2Mn0.56Ni0.17Co0.07]O2cathode stabilized by metastable Li2SiO3surface modification for advanced Li-ion batteries[J]. Electrochim Acta, 2018, 265:244-253. |
| [128] | ZHANG M, LI Z, YU L, et al. Enhanced long-term cyclability in Li-rich layered oxides by electrochemically constructing a Li x TM3-x O4-type spinel shell[J]. Nano Energy, 2020, 77:105188. |
| [129] | SHEVTSOV A, HAN H, MOROZOV A, et al. Protective spinel coating for Li1.17Ni0.17Mn0.50Co0.17O2cathode for Li-ion batteries through sngle-source precursor approach[J]. Nanomaterials, 2020, 10(9):1870. |
| [130] | TANG W, CHEN Z, HUANG H, et al. PVP-bridged γ-LiAlO2nanolayer on Li1.2Ni0.182Co0.08Mn0.538O2cathode materials for improving the rate capability and cycling stability[J]. Chem Eng Sci, 2021, 229:116126. |
| [131] | XIEY, CHENS, LINZ, et al. Enhanced electrochemical performance of Li-rich layered oxide, Li1.2Mn0.54Co0.13Ni0.13O2, by surface modification derived from a MOF-assisted treatment[J]. Electrochem Commun, 2019, 99:65-70. |
| [1] | Fang-Zheng HU, Xing GAO, Lei LIU, Tian-Heng YUAN, Ning CAO, Kai LI, Ya-Tao WANG, Jian-Hua LI, Hui-Qin LIAN, Xiao-Dong WANG, Xiu-Guo CUI. Advances in Black Phosphorus Anode Advantages and Optimization in Li-ion Battery Anodes [J]. Chinese Journal of Applied Chemistry, 2023, 40(4): 571-582. |
| [2] | Lin-Hu SONG, Shi-You LI, Jie WANG, Jing-Jing ZHANG, Ning-Shuang ZHANG, Dong-Ni ZHAO, Fei XU. Research Progress of Additives for Acid and Water Removal in Electrolyte of Lithium Ion Battery [J]. Chinese Journal of Applied Chemistry, 2022, 39(5): 697-706. |
| [3] | Yu-Le WANG, Ke-Li YANG, Yan-Fang GAO. Preparation and Electrochemical Properties of Molybdenum Carbide Modified Silica [J]. Chinese Journal of Applied Chemistry, 2022, 39(11): 1716-1725. |
| [4] | HU Chen,JIN Yi,ZHU Shaoqing,XU Ye,SHUI Jianglan. Methods for Improving Low-Temperature Performance of Lithium Iron Phosphate Based Li-Ion Battery [J]. Chinese Journal of Applied Chemistry, 2020, 37(4): 380-386. |
| [5] | LIU Lixin, DONG Jianhong, ZHANG Guanghui, ZHU Luyi, WANG Xinqiang, XU Dong, CHOW Yuktak. Preparation and Properties of Polyvinylidene Fluoride@Diatomite Fiber Membranes by Eletrospinning as Separator of Lithium-Ion Batteries [J]. Chinese Journal of Applied Chemistry, 2020, 37(12): 1441-1446. |
| [6] | ZUO Zicheng,LI Yuliang. Applications of Graphdiyne in Li+/Na+ Battery Anodes [J]. Chinese Journal of Applied Chemistry, 2018, 35(9): 1057-1066. |
| [7] | LIU Jianben1*, MO Rubao2, WU Xianming1. Preparation and Electrochemical Performances of Li(Ni1/3Co1/3Mn1/3)0.95Al0.05O2 Cathode Material for Lithium-ion Batteries [J]. Chinese Journal of Applied Chemistry, 2014, 31(04): 462-468. |
| [8] | ZHANG Kai, BAI Hongmei, CHENG Fangyi, CHEN Jun*. Preparation of Sn Films by Vacuum Evaporation and Their Electrochemical Properties as Lithium-storage Materials [J]. Chinese Journal of Applied Chemistry, 2011, 28(08): 918-923. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||