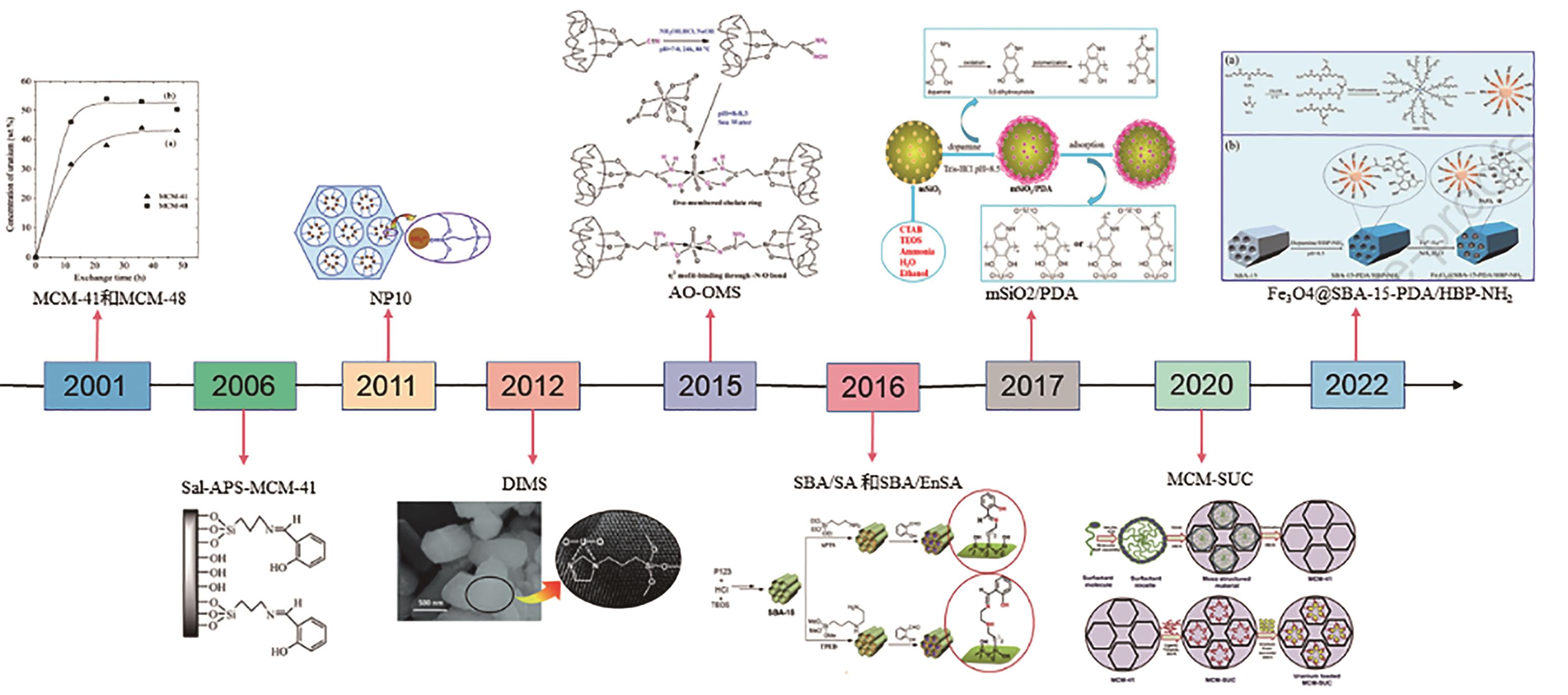
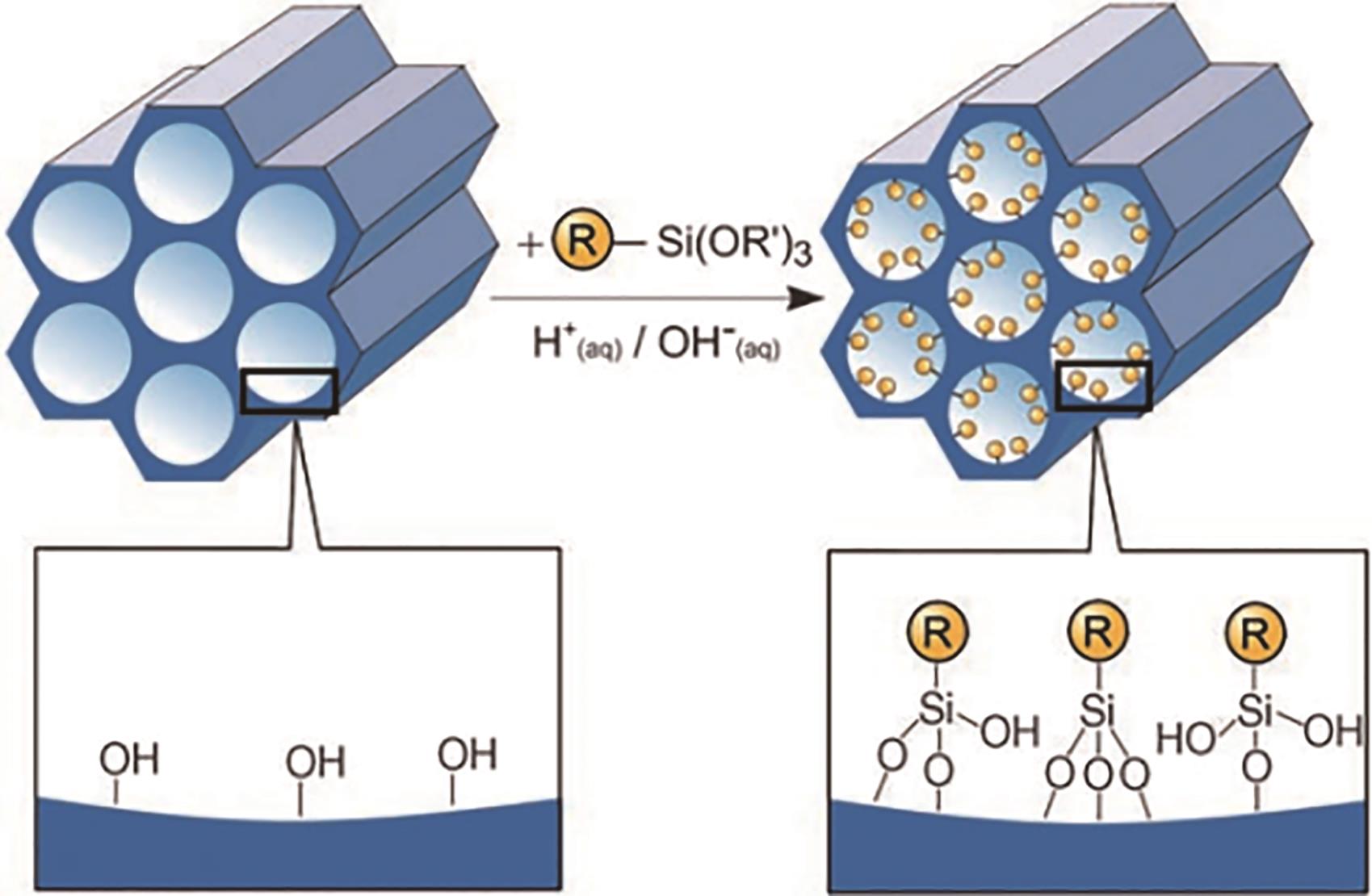
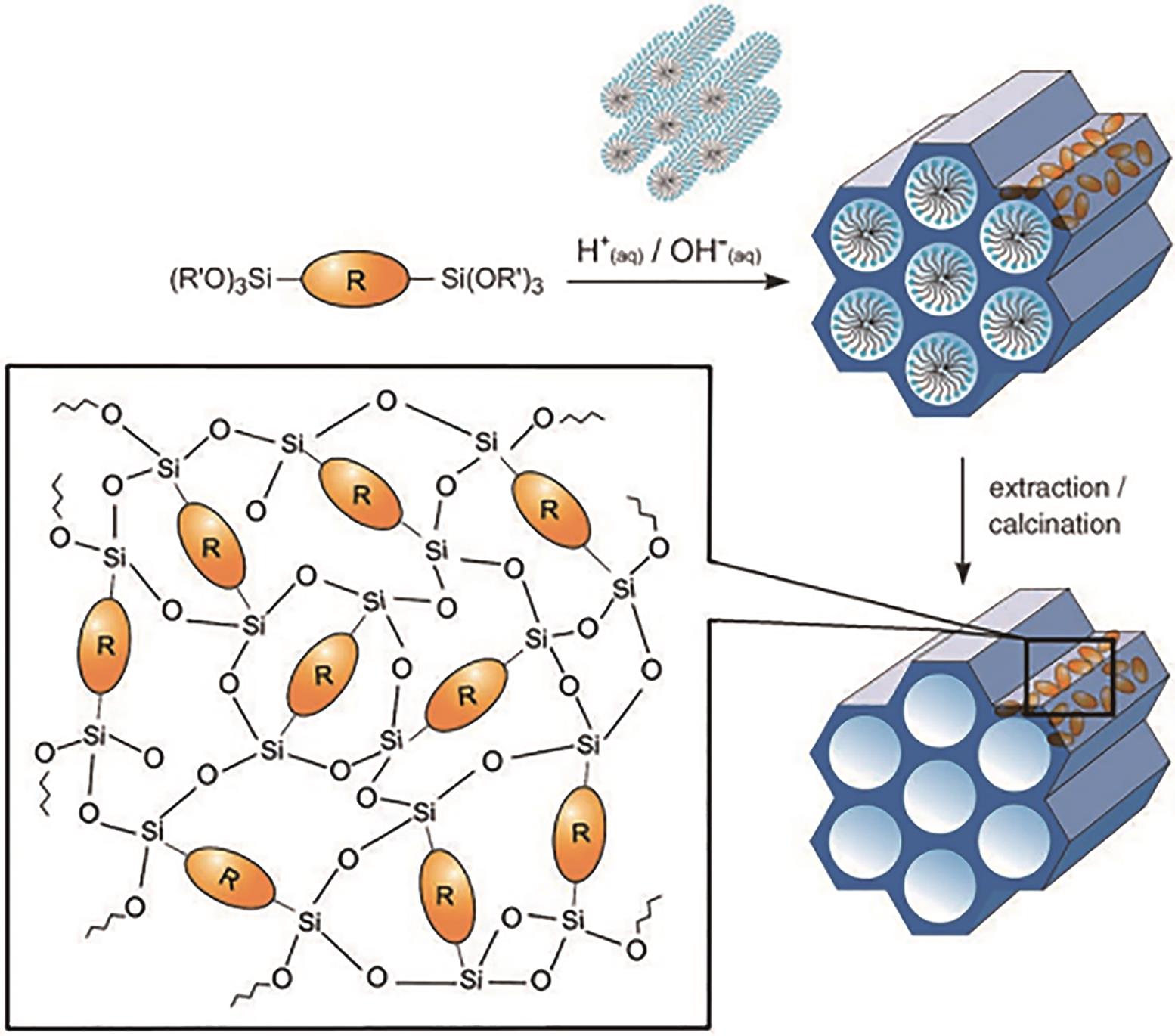
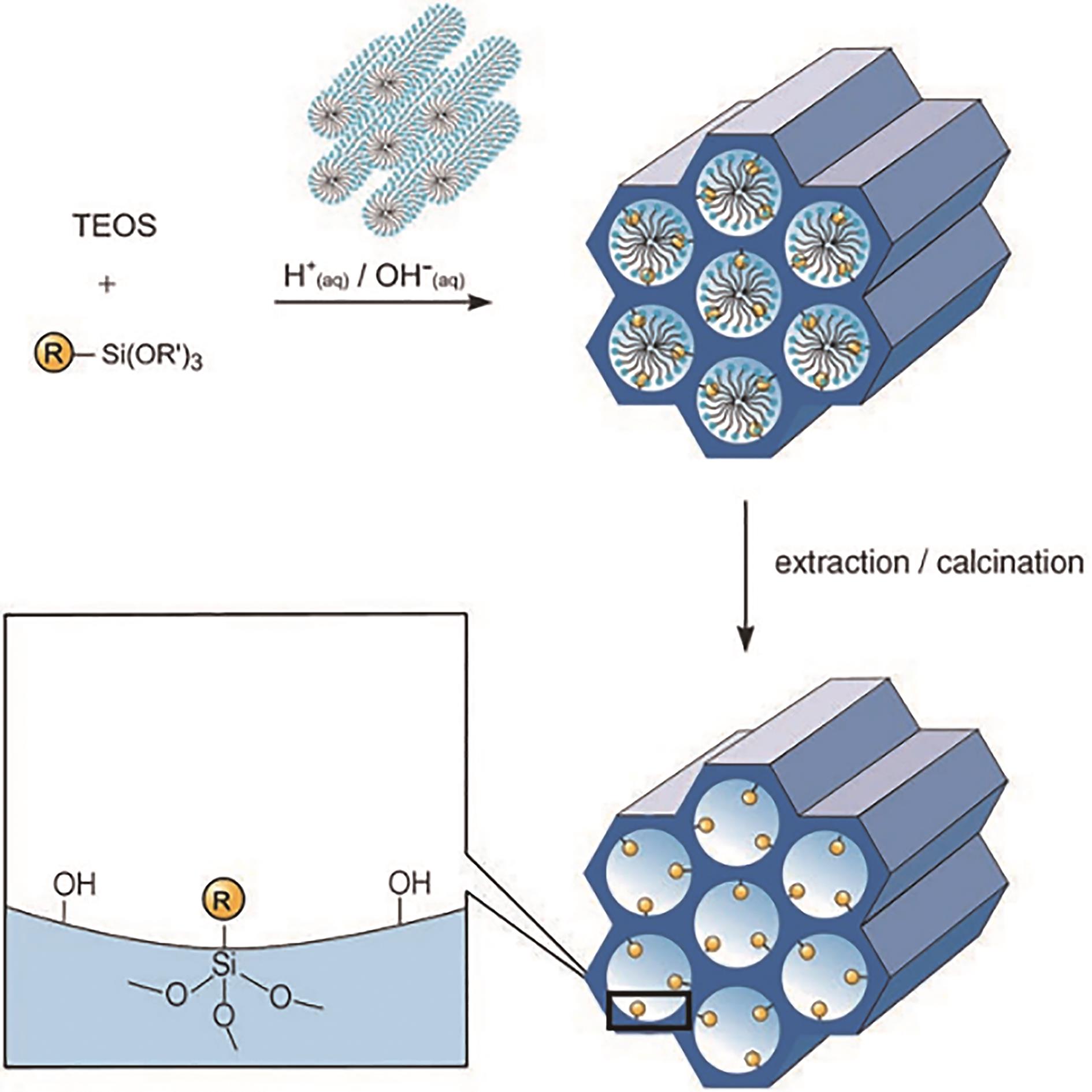
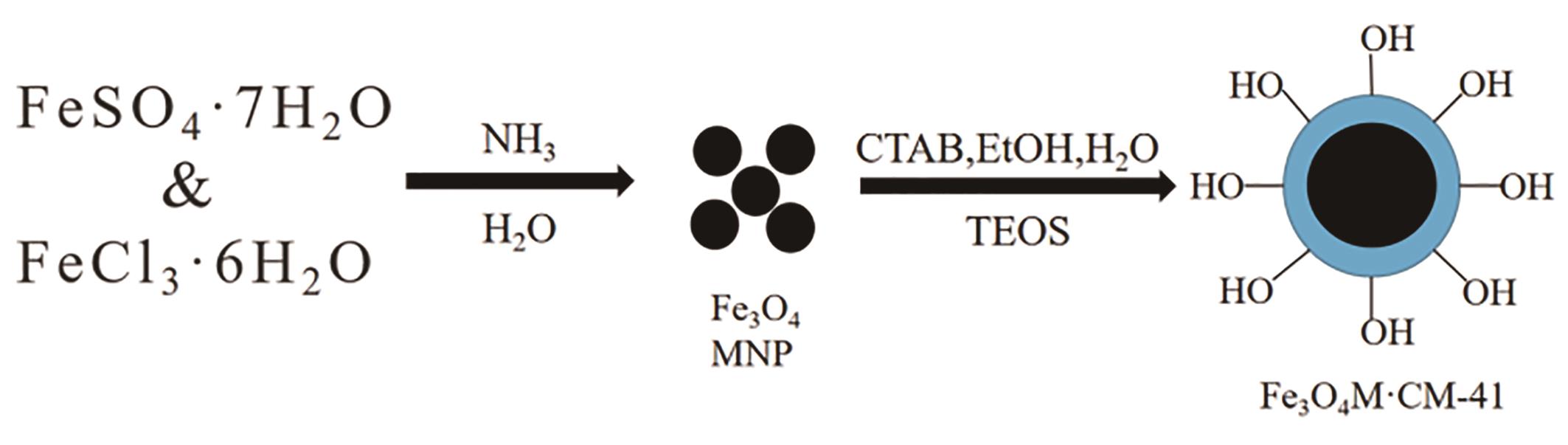
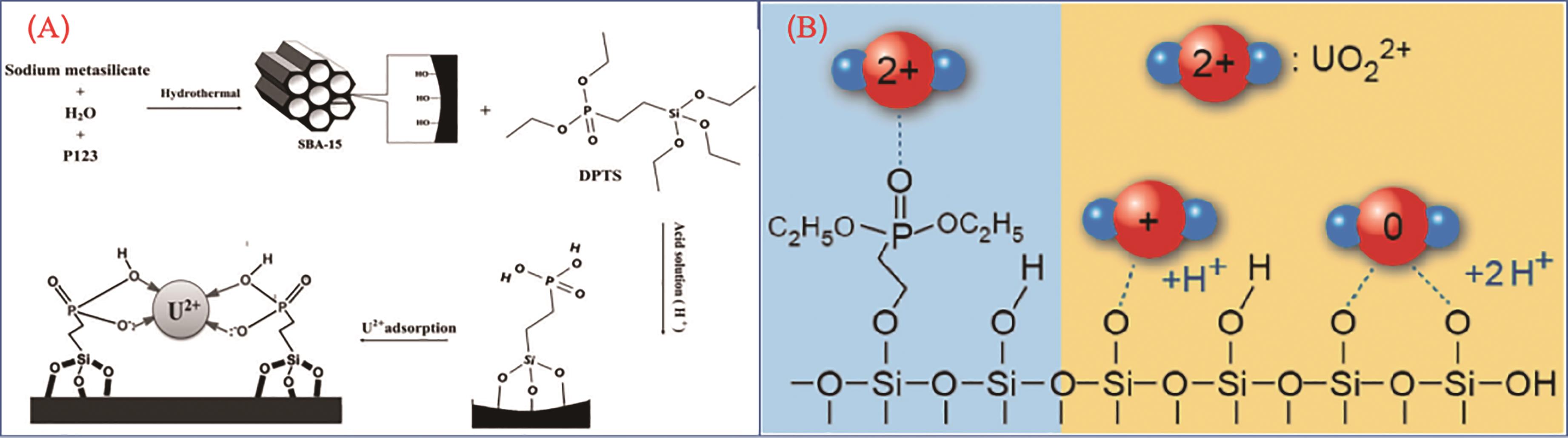
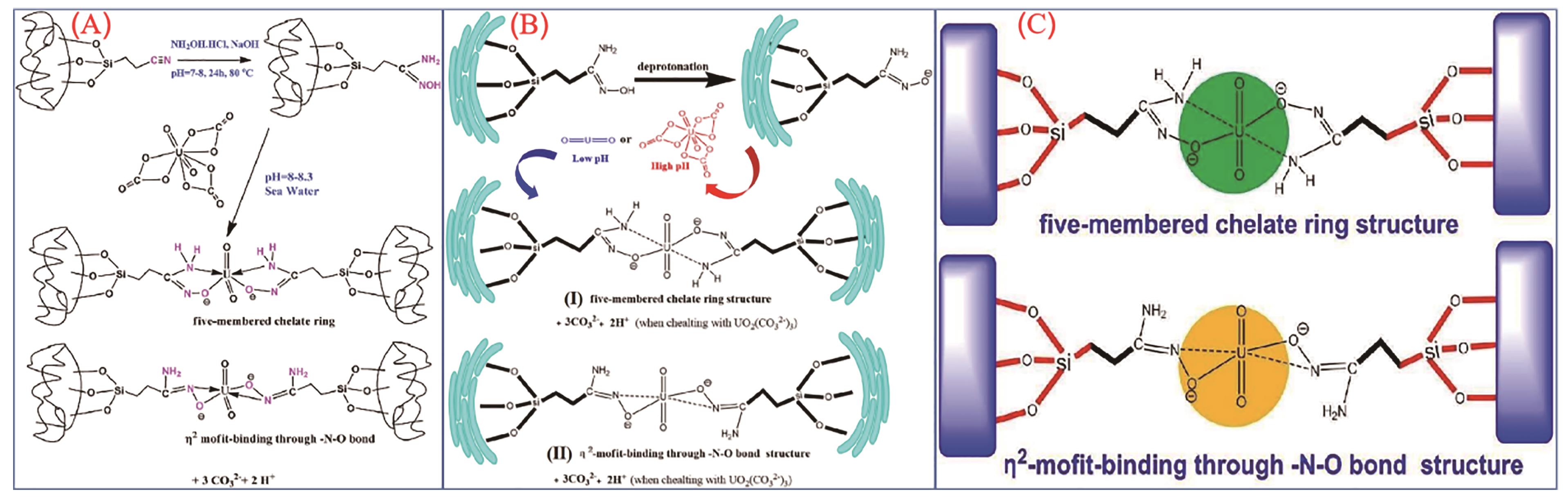
应用化学 ›› 2023, Vol. 40 ›› Issue (2): 169-187.DOI: 10.19894/j.issn.1000-0518.220236
功能化介孔二氧化硅的制备及其吸附分离水中铀的研究进展
- 东华理工大学化学生物与材料科学学院,南昌 330013
-
收稿日期:2022-07-08接受日期:2022-10-28出版日期:2023-02-01发布日期:2023-02-27 -
通讯作者:黄国林 -
基金资助:国家自然科学基金(21866005)
Research Progress in the Preparation of Functionalized Mesoporous Silica and Its Application in Adsorption and Separation of Uranium from Water
Qin ZHANG, Wen-Bin LIU, Li-Jiao FAN, Yu-Ming XIE, Guo-Lin HUANG( )
)
- School of Chemistry,Biology and Material Science,East China University of Technology,Nanchang 330013,China
-
Received:2022-07-08Accepted:2022-10-28Published:2023-02-01Online:2023-02-27 -
Contact:Guo-Lin HUANG -
About author:guolinhuang@sina.com
-
Supported by:the National Natural Science Foundation of China(21866005)
摘要:
铀是一种高效、清洁的核能燃料,但在核工业中不可避免地会产生含铀废水。如果不及时处理,泄漏到环境中,将对动植物和人类的健康构成威胁。因此,从能源回收和环境保护的角度来说,研究水溶液中U(Ⅵ)的分离工艺迫在眉睫。吸附技术因其可行性、效率高和操作简单等优点备受关注。功能化介孔二氧化硅材料具有比表面积大、孔容量大和吸附能力强等优点,是一种理想的吸附剂,在铀的吸附分离领域有着广泛的应用。本文在功能化介孔二氧化硅制备方法的基础上,结合X射线光电子能谱、傅里叶变换红外光谱、X射线吸收精细结构谱、X射线能谱分析和拉曼光谱等分析方法,对国内外目前水溶液中U(Ⅵ)吸附的表征及吸附机理进行了综述。虽然功能化介孔硅吸附铀已经取得了令人鼓舞和潜在的发展,但新型多功能吸附剂的设计和批量生产在实际环境的应用方面仍具有挑战性。
中图分类号:
引用本文
张琴, 刘文彬, 樊利娇, 谢宇铭, 黄国林. 功能化介孔二氧化硅的制备及其吸附分离水中铀的研究进展[J]. 应用化学, 2023, 40(2): 169-187.
Qin ZHANG, Wen-Bin LIU, Li-Jiao FAN, Yu-Ming XIE, Guo-Lin HUANG. Research Progress in the Preparation of Functionalized Mesoporous Silica and Its Application in Adsorption and Separation of Uranium from Water[J]. Chinese Journal of Applied Chemistry, 2023, 40(2): 169-187.
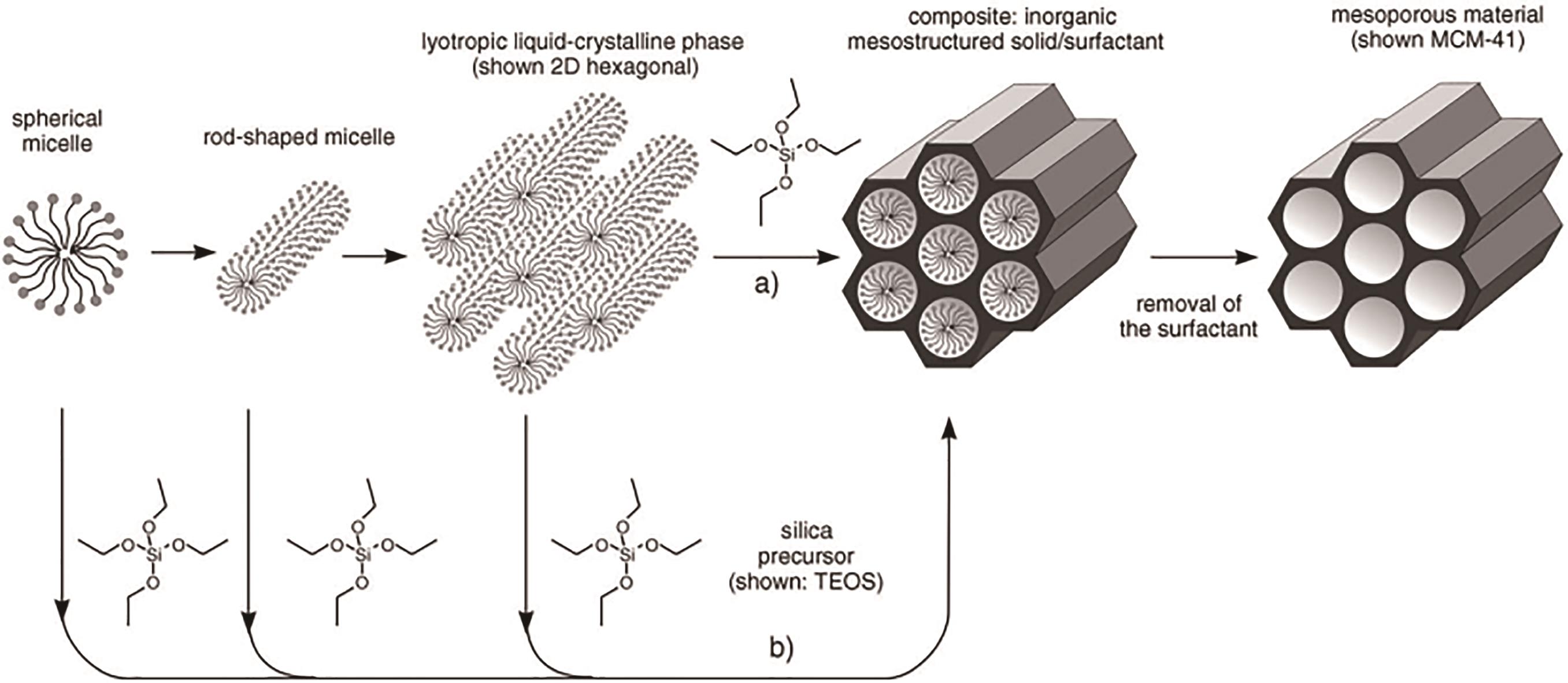
图2 结构导向剂形成介孔材料[38]
Fig.2 Formation of mesoporous materials by structure-directing agents[38]Note: (a) True liquid-crystal template mechanism; (b) Cooperative liquid-crystal template mechanism

图3 (a-d, f)分别为杆状、球状、纤维状、立方体状、血小板状介孔硅的扫描电子显微镜图(SEM)[41-44],(e)为纤维球介孔硅的透射电子显微镜图(TEM)[45]
Fig.3 (a-d, f)Scanning electron micrographs(SEM) of rod-shaped, spherical, fibrous, cuboidal, and platelet-shaped mesoporous silicon, respectively[41-44], and (e) transmission electron micrographs(TEM) of fibrous spherical mesoporous silicon[45]
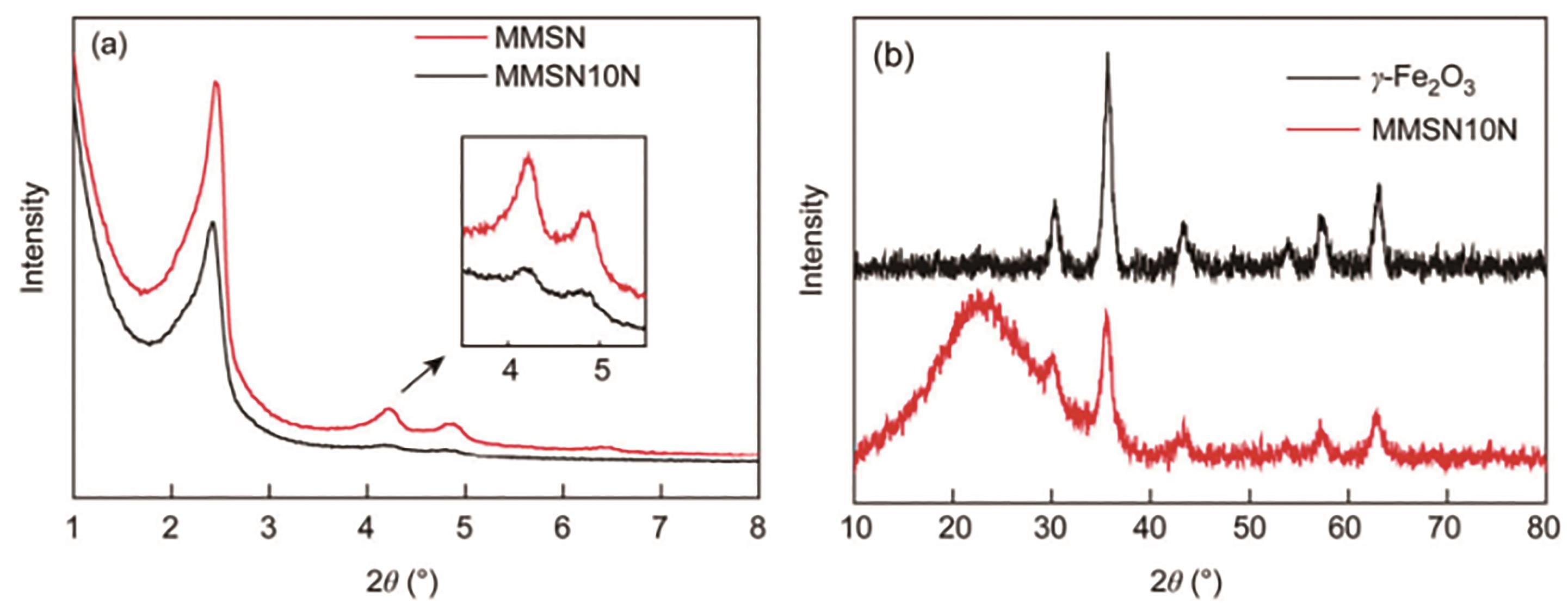
图7 MMSN和MMSN10N的PXRD谱[54](a)Low-angle PXRD patterns of MMSN and MMSN10N; (b)Wide-angle PXRD patterns of γ-Fe2O3 and MMSN10N
Fig.7 PXRD patterns of MMSN and MMSN10N[54]
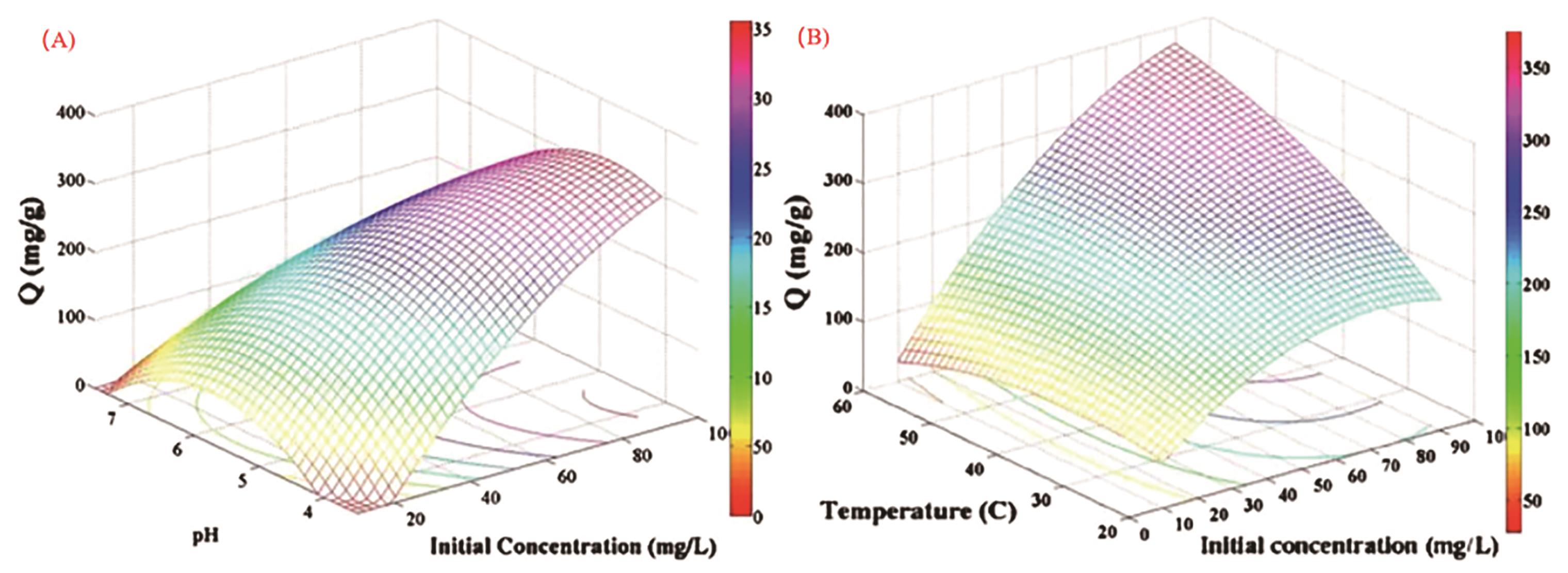
图9 初始浓度和pH值、初始浓度和温度对NH2-MCM-41从水溶液中吸附UO22+ 影响的响应面图[67]
Fig.9 Response surface diagram of the effect of initial concentration and pH, initial concentration and temperature on the adsorption of UO22+ from aqueous solution by NH2-MCM-41[67]
| Adsorbents | Pore diameter/nm | BET surface area/(m2·g-1) | Equilibrium time /min | Qmax/ (mg·g-1) | Isotherm model | Ref. |
|---|---|---|---|---|---|---|
| NP10 | 2.7 | 920 | 30 | 303 | Langmuir model | [ |
| DIMs | 6.7 | — | 10 | 268 | Langmuir model | [ |
| MCM-SUC | 2.3 | 539 | 10 | 807 | Langmuir model | [ |
| MMSN10N | 2.88- | 815 | 360 | 160 | Langmuir model | [ |
| MMS-AO | 2.2 | 287.1 | 120 | 277.3 | Langmuir model | [ |
| Fe-MCM-SUC | 3-13 | 233 | — | 430 | Langmuir model | [ |
| NH2-MCM-41 | 1.9 | 577 | 173 | 435 | Langmuir model | [ |
| MCM-TEPA | 3.86 | 1052 | 30 | 454 | Langmuir model | [ |
| SBA-15-N2C1 | 6.1 | 267 | 30 | 573 | Langmuir model | [ |
| PFG-MSs | 4.6 | 1.5 | 60 | 207.6 | Freundlich model | [ |
| SMS-Ph | 7.73 | 4.28 | 60 | 820.7 | Freundlich model | [ |
| Fe3O4@SiO2-AO | — | — | 120 | 104.96 | Langmuir model | [ |
| AO-MCM-41 | — | — | 40 | 442.3 | Temkin and Freundlich | [ |
| Ami-MSN | 0.619 | 676 | 150 | 200.41 | Langmuir model | [ |
| MCM-41-AO | — | — | 90 | 384.59 | Langmuir model | [ |
| MCC/MS-AO | 2.85 | 358.45 | 90 | 315.46 | Langmuir model | [ |
| Al/MS-AO | 0.75 | 308.89 | 180 | 328.68 | Langmuir model | [ |
表1 部分功能化介孔硅对铀的吸附性能及主要参数研究
Table 1 The adsorption properties and main parameters of some functionalized mesoporous silicon for uranium
| Adsorbents | Pore diameter/nm | BET surface area/(m2·g-1) | Equilibrium time /min | Qmax/ (mg·g-1) | Isotherm model | Ref. |
|---|---|---|---|---|---|---|
| NP10 | 2.7 | 920 | 30 | 303 | Langmuir model | [ |
| DIMs | 6.7 | — | 10 | 268 | Langmuir model | [ |
| MCM-SUC | 2.3 | 539 | 10 | 807 | Langmuir model | [ |
| MMSN10N | 2.88- | 815 | 360 | 160 | Langmuir model | [ |
| MMS-AO | 2.2 | 287.1 | 120 | 277.3 | Langmuir model | [ |
| Fe-MCM-SUC | 3-13 | 233 | — | 430 | Langmuir model | [ |
| NH2-MCM-41 | 1.9 | 577 | 173 | 435 | Langmuir model | [ |
| MCM-TEPA | 3.86 | 1052 | 30 | 454 | Langmuir model | [ |
| SBA-15-N2C1 | 6.1 | 267 | 30 | 573 | Langmuir model | [ |
| PFG-MSs | 4.6 | 1.5 | 60 | 207.6 | Freundlich model | [ |
| SMS-Ph | 7.73 | 4.28 | 60 | 820.7 | Freundlich model | [ |
| Fe3O4@SiO2-AO | — | — | 120 | 104.96 | Langmuir model | [ |
| AO-MCM-41 | — | — | 40 | 442.3 | Temkin and Freundlich | [ |
| Ami-MSN | 0.619 | 676 | 150 | 200.41 | Langmuir model | [ |
| MCM-41-AO | — | — | 90 | 384.59 | Langmuir model | [ |
| MCC/MS-AO | 2.85 | 358.45 | 90 | 315.46 | Langmuir model | [ |
| Al/MS-AO | 0.75 | 308.89 | 180 | 328.68 | Langmuir model | [ |
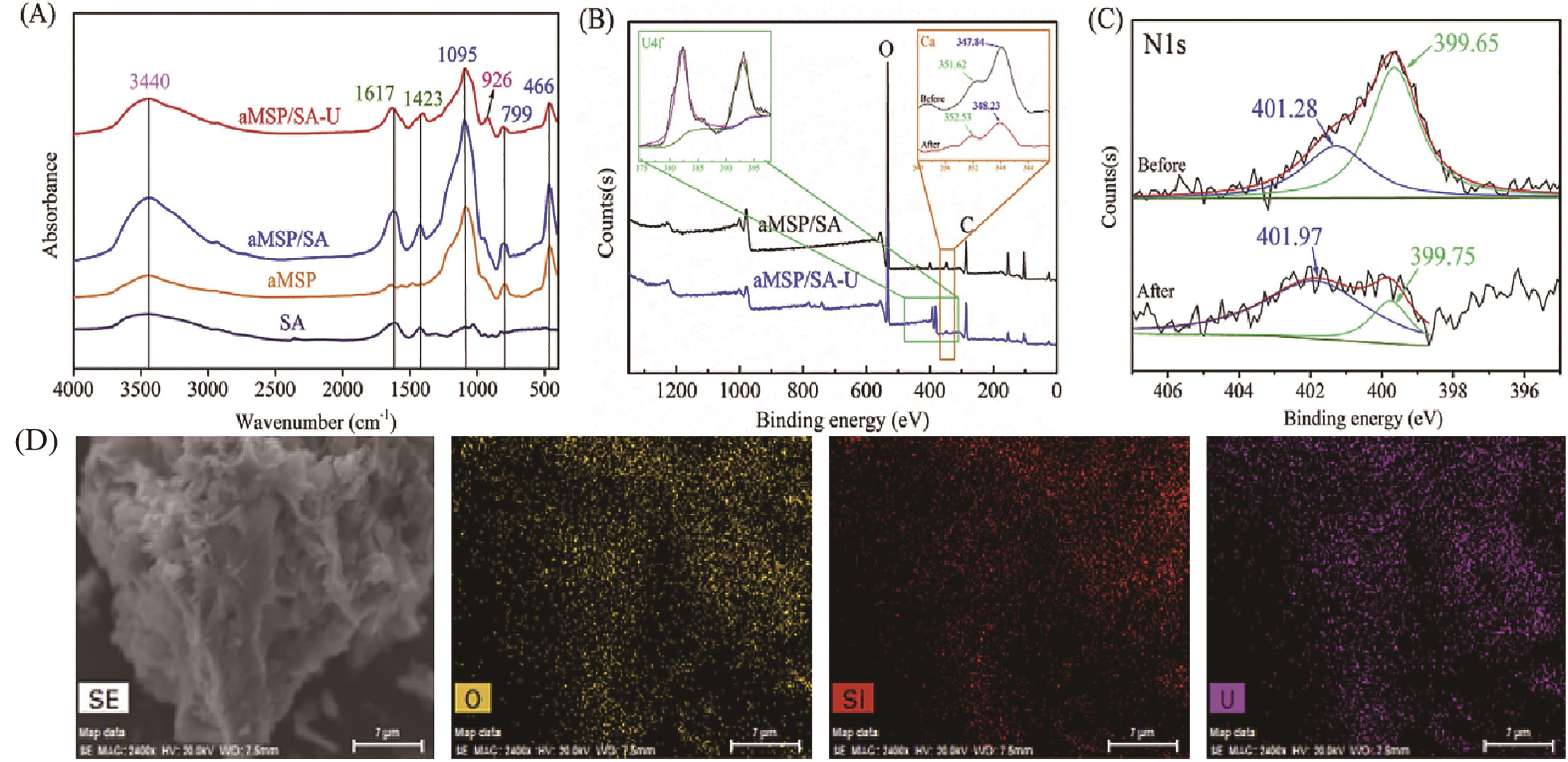
图 10 (A) SA、aMSP和aMSP/SA吸附前后的FT-IR图谱;(B) 吸附前后aMSP/SA的XPS光谱;(C) 吸附前后aMSP/SA的曲线拟合N1s的XPS光谱;(D)吸附后aMSP/SA的背散射电子图像[87]
Fig.10 (A) FT-IR spectra of SA, aMSP, and aMSP/SA before and after adsorption; (B) XPS spectra for aMSP/SA before and after adsorption; (C) XPS spectra of curve fitted N1s of aMSP/SA before and after adsorption; (D) backscattered electron image of aMSP/SA after adsorption[87]
| Atom.c/% | O | Si | Cl | Ca | U |
|---|---|---|---|---|---|
| aMSP/SA | 70.0 | 14.9 | 1.9 | 11.5 | Not detected |
| aMSP/SA-U | 73.3 | 17.6 | 6.1 | Not detected | 3.0 |
表2 样品表面积元素组成(EDS分析)(只列出大于质量分数0.1%的元素)
Table 2 Sample surface area element composition (EDS analysis) (only elements greater than 0.1% are listed)
| Atom.c/% | O | Si | Cl | Ca | U |
|---|---|---|---|---|---|
| aMSP/SA | 70.0 | 14.9 | 1.9 | 11.5 | Not detected |
| aMSP/SA-U | 73.3 | 17.6 | 6.1 | Not detected | 3.0 |
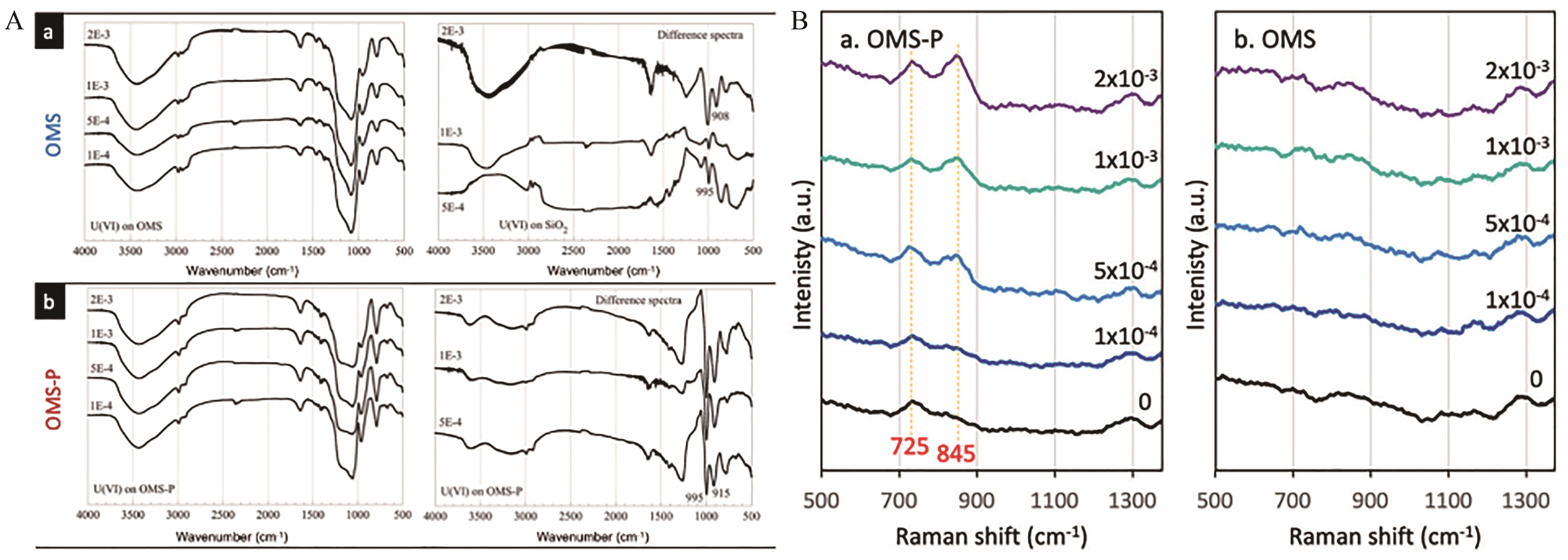
图12 (A)OMS(a)和OMS-P(b)与不同初始/总浓度的U(Ⅵ)溶液接触得到的FT-IR光谱和差分光谱;(B)OMS-P和OMS在不同初始/总U(Ⅵ)浓度下吸附U(Ⅵ)后的拉曼光谱[92]
Fig.12 (A)FT-IR spectra and difference spectra obtained for OMS(a) and OMS-P(b) in contact with U(Ⅵ) solutions of various uranium initial/total concentrations;(B)Raman spectra of OMS-P and OMS after U(?Ⅵ) adsorption at different initial/total U(Ⅵ) concentration[92]
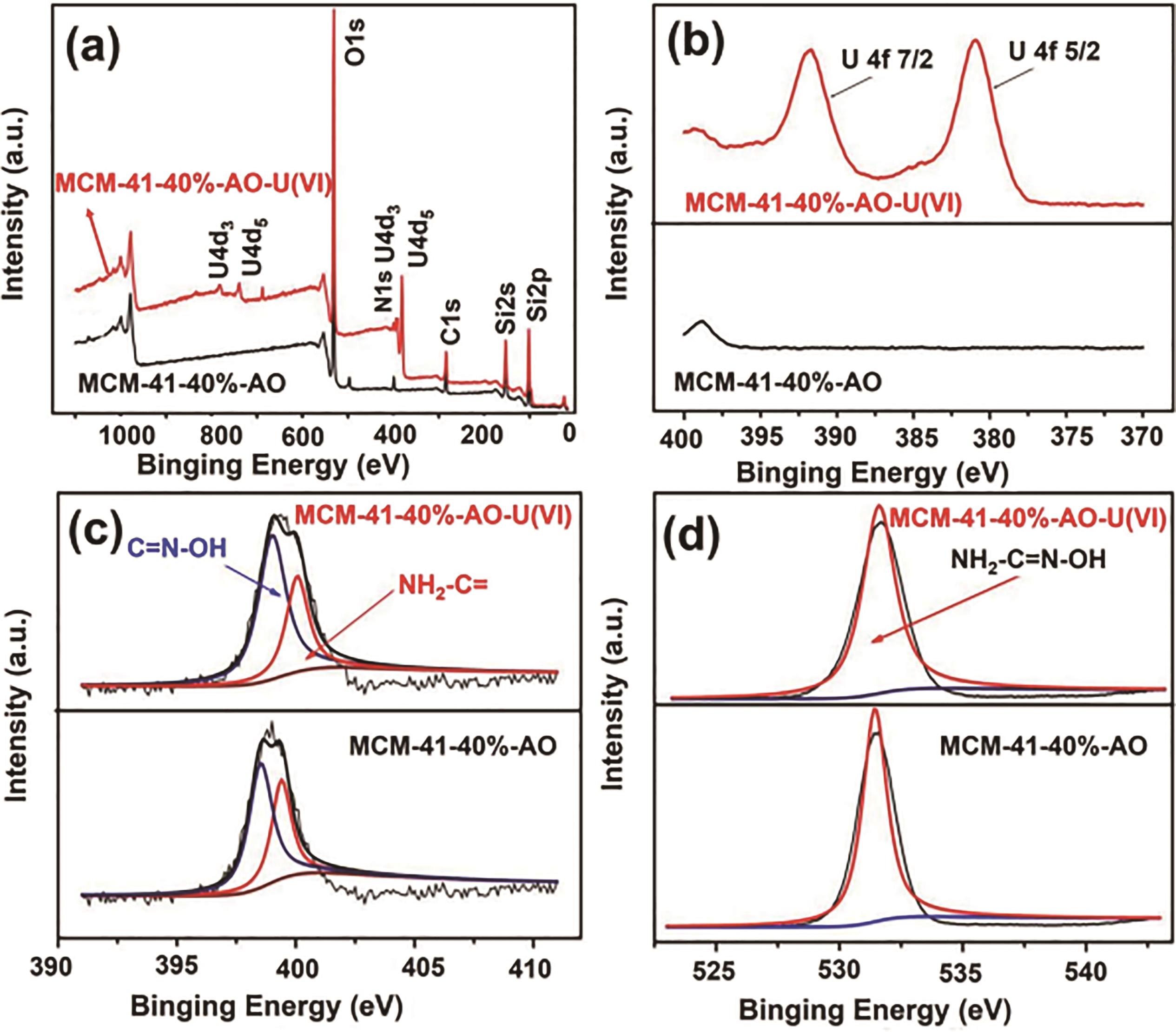
图14 (a)MCM-41-AO-40% 和 MCM-41-AO-40%-U的XPS 谱图; (b)U4f的高分辨率光谱; (c)和(d)MCM-41-AO-40% 和MCM-41-AO-40%-U的N1s和O1s核心级光谱[78]
Fig. 14 (a)XPS spectra of MCM-41-AO-40% and MCM-41-AO-40%-U; (b)High resolution spectra of U4f; (c)N1s and(d)O1s core-level spectra of MCM-41-AO-40% and MCM-41-AO-40%-U[78]
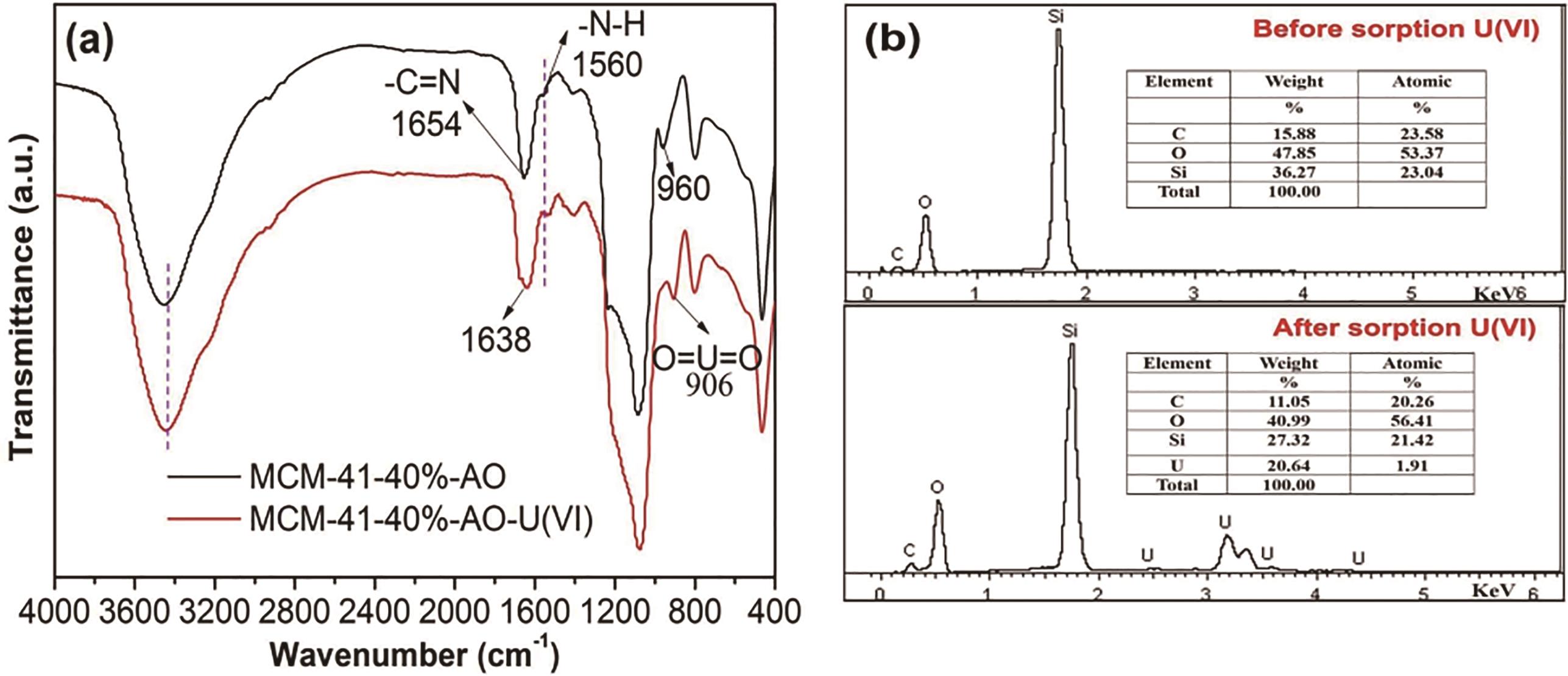
图15 MCM-41-40%-AO吸附U(Ⅵ)前后的 FT-IR 光谱(a)和EDS(b)[78]
Fig.15 FT-IR spectra(a) and EDS patterns(b) of MCM-41-40%-AO before and after adsorption of U(Ⅵ)[78]
| 1 | CHAUDHARY M, SINGH L, REKHA P, et al. Adsorption of uranium from aqueous solution as well as seawater conditions by nitrogen-enriched nanoporous polytriazine[J]. Chem Eng J, 2019, 378: 122236. |
| 2 | LADEIRA A C Q, MORAIS C A. Uranium recovery from industrial effluent by ion exchange-column experiments[J]. Miner Eng, 2005, 18(13): 1337-1340. |
| 3 | AL-HOBAIB A S, AL-SUHYBANI A A. Removal of uranyl ions from aqueous solutions using barium titanate[J]. J Radioanal Nucl Chem, 2014, 299(1): 559-567. |
| 4 | WANG J, YIN M, LIU J, et al. Geochemical and U-Th isotopic insights on uranium enrichment in reservoir sediments[J]. J Hazard Mater, 2021, 414: 125466. |
| 5 | BELTRAMI D, CHAGNES A, HADDAD M, et al. Recovery of uranium(Ⅵ) from concentrated phosphoric acid by mixtures of new bis(1,3-dialkyloxypropan-2-yl)phosphoric acids and tri-n-octylphosphine oxide[J]. Hydrometallurgy, 2013, 140: 28-33. |
| 6 | DINIS M D, FIÚZA A. Mitigation of uranium mining impacts-a review on groundwater remediation technologies[J]. Geosciences, 2021, 11(6): 250. |
| 7 | HUANG G, PENG W, YANG S. Synthesis of magnetic chitosan/graphene oxide nanocomposites and its application for U(Ⅵ) adsorption from aqueous solution[J]. J Radioanal Nucl Chem, 2018, 317(1): 337-344. |
| 8 | PENG W, HUANG G, YANG S, et al. Performance of biopolymer/graphene oxide gels for the effective adsorption of U(Ⅵ) from aqueous solution[J]. J Radioanal Nucl Chem, 2019, 322(2): 861-868. |
| 9 | 刘小冬,陈沈. 二氧化钍纳米球在碳酸铀酰铵溶液中对铀的吸附性能[J]. 应用化学, 2017, 34(10): 1177-1185. |
| LIU X D, CHEN S. Adsorption performance of thorium dioxide nanospheres towards uranium in the aqueous solution of ammonium uranyl tricarbonate[J].Chin J Appl Chem, 2017, 34(10): 1177-1185. | |
| 10 | HE H, ZONG M, DONG F, et al. Simultaneous removal and recovery of uranium from aqueous solution using TiO2 photoelectrochemical reduction method[J]. J Radioanal Nucl Chem, 2017, 313(1): 59-67. |
| 11 | MOON H S, MCGUINNESS L, KUKKADAPU R K, et al. Microbial reduction of uranium under iron- and sulfate-reducing conditions: effect of amended goethite on microbial community composition and dynamics[J]. Water Res, 2010, 44(14): 4015-4028. |
| 12 | DA'NA E. Adsorption of heavy metals on functionalized-mesoporous silica: a review[J]. Microporous Mesoporous Mater, 2017, 247: 145-157. |
| 13 | KRESGE C T, LEONOWICZ M E, ROTH W J, et al. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism[J]. Nature, 1992, 359(6397): 710-712. |
| 14 | BECK J S, VARTULI J C, ROTH W J, et al. A new family of mesoporous molecular sieves prepared with liquid crystal templates[J]. J Am Chem Soc, 1992, 114(27): 10834-10843. |
| 15 | LYU Y, ASOH T A, UYAMA H. Hierarchical silica monolith prepared using cellulose monolith as template[J]. Polym Degrad Stab, 2020, 177: 109164. |
| 16 | WU S H, MOU C Y, LIN H P. Synthesis of mesoporous silica nanoparticles[J]. Chem Soc Rev, 2013, 42(9): 3862-3875. |
| 17 | ZHANG Z, LIU H, WU L, et al. Preparation of amino-Fe(Ⅲ) functionalized mesoporous silica for synergistic adsorption of tetracycline and copper[J]. Chemosphere, 2015, 138: 625-632. |
| 18 | ZHAO D, HUO Q, FENG J, et al. Nonionic triblock and star diblock copolymer and oligomeric surfactant syntheses of highly ordered, hydrothermally stable, mesoporous silica structures[J]. J Am Chem Soc, 1998, 120(24): 6024-6036. |
| 19 | INAGAKI S, FUKUSHIMA Y, KURODA K. Synthesis of highly ordered mesoporous materials from a layered polysilicate[J]. J Chem Soc, Chem Commun, 1993(8): 680-682. |
| 20 | BAGSHAW STEPHEN A, PROUZET E, PINNAVAIA THOMAS J. Templating of mesoporous molecular sieves by nonionic polyethylene oxide surfactants[J]. Science, 1995, 269(5228): 1242-1244. |
| 21 | KIM S S, ZHANG W, PINNAVAIA T J. Ultrastable mesostructured silica vesicles[J]. Science, 1998, 282(5392): 1302-1305. |
| 22 | KLEITZ F, HEI CHOI S, RYOO R. Cubic Ia3d large mesoporous silica: synthesis and replication to platinum nanowires, carbon nanorods and carbon nanotubes[J]. Chem Commun, 2003(17): 2136-2137. |
| 23 | FAN J, YU C, GAO F, et al. Cubic mesoporous silica with large controllable entrance sizes and advanced adsorption properties[J]. Angew Chem Int Ed, 2003, 42(27): 3146-3150. |
| 24 | VIDYA K, DAPURKAR S E, SELVAM P, et al. The entrapment of U O 2 2 + in mesoporous MCM-41 and MCM-48 molecular sieves[J]. Microporous Mesoporous Mater, 2001, 50(2): 173-179. |
| 25 | JAMALI M R, ASSADI Y, SHEMIRANI F, et al. Synthesis of salicylaldehyde-modified mesoporous silica and its application as a new sorbent for separation, preconcentration and determination of uranium by inductively coupled plasma atomic emission spectrometry[J]. Anal Chim Acta, 2006, 579(1): 68-73. |
| 26 | YUAN L Y, LIU Y L, SHI W Q, et al. High performance of phosphonate-functionalized mesoporous silica for U(Ⅵ)sorption from aqueous solution[J]. Dalton Trans, 2011, 40(28): 7446-7453. |
| 27 | YUAN L Y, LIU Y L, SHI W Q, et al. A novel mesoporous material for uranium extraction, dihydroimidazole functionalized SBA-15[J]. J Mater Chem, 2012, 22(33): 17019-17026. |
| 28 | GUNATHILAKE C, GÓRKA J, DAI S, et al. Amidoxime-modified mesoporous silica for uranium adsorption under seawater conditions[J]. J Mater Chem A, 2015, 3(21): 11650-11659. |
| 29 | DOLATYARI L, YAFTIAN M R, ROSTAMNIA S. Removal of uranium(Ⅵ) ions from aqueous solutions using Schiff base functionalized SBA-15 mesoporous silica materials[J]. J Environ Manage, 2016, 169: 8-17. |
| 30 | BAI L, DUAN S, JIANG W, et al. High performance polydopamine-functionalized mesoporous silica nanospheres for U(Ⅵ) removal[J]. Appl Surf Sci, 2017, 426: 1121-1132. |
| 31 | AMESH P, SUNEESH A S, VENKATESAN K A, et al. High capacity amidic succinic acid functionalized mesoporous silica for the adsorption of uranium[J]. Colloid Surface A, 2020, 602: 125053. |
| 32 | LIU F, WANG A, XIANG M, et al. Effective adsorption and immobilization of Cr(Ⅵ) and U(Ⅵ) from aqueous solution by magnetic amine-functionalized SBA-15[J]. Sep Purif Technol, 2022, 282: 120042. |
| 33 | 张春. 介孔SiO2及其氧化物复合材料的制备与表征[D]. 合肥: 安徽大学, 2010. |
| ZHANG C. Preparation and characterization of mesoporous SiO2 and oxide/SiO, nanocomposites[D]. Hefei: Anhui University, 2010. | |
| 34 | 段昌龙, 谢昆沛, 陈权, 等. 多级介孔MCM-48的水热法制备及其对三唑酮的负载[J]. 仲恺农业工程学院学报, 2019, 32(4): 19-25. |
| DUAN C L, XIE K P, CHEN Q, et al. Hydrothermal synthesis of multistage mesoporous MCM-48 and its loading on triadimefon[J]. J Zhongkai Inst Agric Eng, 2019, 32(4): 19-25. | |
| 35 | COSTA J A S, VEDOVELLO P, PARANHOS C M. Use of ionic liquid as template for hydrothermal synthesis of the MCM-41 mesoporous material[J]. Silicon, 2020, 12(2): 289-294. |
| 36 | 姚汝亮, 张炜, 周达. 溶胶-凝胶法原位生成SiO2改性硅基耐烧蚀材料[J]. 高分子学报, 2009(3): 264-267. |
| YAO R L, ZHANG W, ZHOU D. In-situ silica preparation in sillicon-based ablation-resistant material by sol-gel method[J]. Acta Polym Sin, 2009(3): 264-267. | |
| 37 | 秦君. 介孔二氧化硅纳米材料的合成及其在室温磷光分析中的应用[D]. 太原: 山西师范大学, 2017. |
| QIN J. Synthesis of mesoporous silica nanomaterials and the application in room temperature phosphorescence analysis[D].Taiyuan: Shanxi Normal University, 2017. | |
| 38 | HOFFMANN F, CORNELIUS M, MORELL J, et al. Silica-based mesoporous organic-inorganic hybrid materials[J]. Angew Chem Int Ed, 2006, 45(20): 3216-3251. |
| 39 | HUO Q, MARGOLESE D I, CIESLA U, et al. Generalized synthesis of periodic surfactant/inorganic composite materials[J]. Nature, 1994, 368(6469): 317-321. |
| 40 | MENG C, ZHIKUN W, QIANG L, et al. Preparation of amino-functionalized Fe3O4@mSiO2 core-shell magnetic nanoparticles and their application for aqueous Fe3+ removal[J]. J Hazard Mater, 2018, 341: 198-206. |
| 41 | NEAMANI S, MORADI L, SUN M. Synthesis of magnetic hollow mesoporous N-doped silica rods as a basic catalyst for the preparation of some spirooxindole-1,4-dihydropyridine derivatives[J]. Appl Surf Sci, 2020, 504: 144466. |
| 42 | MELÉNDEZ-ORTIZ H I, GARCÍA-CERDA L A, OLIVARES-MALDONADO Y, et al. Preparation of spherical MCM-41 molecular sieve at room temperature: influence of the synthesis conditions in the structural properties[J]. Ceram Int, 2012, 38(8): 6353-6358. |
| 43 | AGHAYAN H, KHANCHI A R, YOUSEFI T, et al. Tungsten substituted molybdophosphoric acid loaded on various types of mesoporous silica SBA-15 for application of thorium ion adsorption[J]. J Nucl Mater, 2017, 496: 207-214. |
| 44 | XIAO J, JING Y, YAO Y, et al. Synthesis of amidoxime-decorated 3D cubic mesoporous silica via self-assembly co-condensation as a superior uranium(Ⅵ) adsorbent[J]. J Mol Liq, 2019, 277: 843-855. |
| 45 | POLSHETTIWAR V, CHA D, ZHANG X, et al. High-surface-area silica nanospheres(KCC-1) with a fibrous morphology[J]. Angew Chem Int Ed, 2010, 49(50): 9652-9656. |
| 46 | WANG X, JI G, ZHU G, et al. Surface hydroxylation of SBA-15 via alkaline for efficient amidoxime-functionalization and enhanced uranium adsorption[J]. Sep Purif Technol, 2019, 209: 623-635. |
| 47 | DAI Y, JIN J, ZHOU L, et al. Preparation of hollow SiO2 microspheres functionalized with amidoxime groups for highly efficient adsorption of U(Ⅵ) from aqueous solution[J]. J Radioanal Nucl Chem, 2017, 311(3): 2029-2037. |
| 48 | LU D, XU S, QIU W, et al. Adsorption and desorption behaviors of antibiotic ciprofloxacin on functionalized spherical MCM-41 for water treatment[J]. J Clean Prod, 2020, 264: 121644. |
| 49 | KISHOR R, GHOSHAL A K. APTES grafted ordered mesoporous silica KIT-6 for CO2 adsorption[J]. Chem Eng J,2015, 262: 882-890. |
| 50 | FAROOGHI A, SAYADI M H, REZAEI M R, et al. An efficient removal of lead from aqueous solutions using FeNi3@SiO2 magnetic nanocomposite[J]. Surf Interfaces, 2018, 10: 58-64. |
| 51 | DASHTIAN K, ZARE-DORABEI R. Synthesis and characterization of functionalized mesoprous SBA-15 decorated with Fe3O4 nanoparticles for removal of Ce(Ⅲ) ions from aqueous solution: ICP-OES detection and central composite design optimization[J]. J Colloid Interface Sci, 2017, 494: 114-123. |
| 52 | ZHANG X, ZHANG Y, ZHANG X, et al. Nitrogen rich core-shell magnetic mesoporous silica as an effective adsorbent for removal of silver nanoparticles from water[J]. J Hazard Mater, 2017, 337: 1-9. |
| 53 | FAN R, MIN H, HONG X, et al. Plant tannin immobilized Fe3O4@SiO2 microspheres: a novel and green magnetic bio-sorbent with superior adsorption capacities for gold and palladium[J]. J Hazard Mater, 2019, 364: 780-790. |
| 54 | GUO W, NIE C, WANG L, et al. Easily prepared and stable functionalized magnetic ordered mesoporous silica for efficient uranium extraction[J]. Sci China Chem, 2016, 59(5): 629-636. |
| 55 | LI D, EGODAWATTE S, KAPLAN D I, et al. Sequestration of U(Ⅵ) from acidic, alkaline, and high ionic-strength aqueous media by functionalized magnetic mesoporous silica nanoparticles: capacity and binding mechanisms[J]. Environ Sci Technol, 2017, 51(24): 14330-14341. |
| 56 | ZHAO Y, LI J, ZHANG S, et al. Amidoxime-functionalized magnetic mesoporous silica for selective sorption of U(Ⅵ)[J]. RSC Adv, 2014, 4(62): 32710-32717. |
| 57 | FAN F L, QIN Z, BAI J, et al. Rapid removal of uranium from aqueous solutions using magnetic Fe3O4@SiO2 composite particles[J]. J Environ Radioact, 2012, 106: 40-46. |
| 58 | AMESH P A, VENKATESAN K, SUNEESH A S, et al. Succinic acid functionalized magnetic mesoporous silica for the magnetic assisted separation of uranium from aqueous solution[J]. J Radioanal Nucl Chem, 2022, 331(6): 2719-2733. |
| 59 | ULU A, NOMA S A A, KOYTEPE S, et al. Chloro-modified magnetic Fe3O4@MCM-41 core-shell nanoparticles for L-asparaginase immobilization with improved catalytic activity, reusability, and storage stability[J]. Appl Biochem Biotechnol, 2019, 187(3): 938-956. |
| 60 | WANG S, WANG K, DAI C, et al. Adsorption of Pb2+ on amino-functionalized core-shell magnetic mesoporous SBA-15 silica composite[J]. Chem Eng J, 2015, 262: 897-903. |
| 61 | FU Y, SUN Y, CHEN Z, et al. Functionalized magnetic mesoporous silica/poly(m-aminothiophenol)nanocomposite for Hg(Ⅱ) rapid uptake and high catalytic activity of spent Hg(Ⅱ) adsorbent[J]. Sci Total Environ, 2019, 691: 664-674. |
| 62 | VOJOUDI H, BADIEI A, BAHAR S, et al. A new nano-sorbent for fast and efficient removal of heavy metals from aqueous solutions based on modification of magnetic mesoporous silica nanospheres[J]. J Magn Magn Mater, 2017, 441: 193-203. |
| 63 | ZHANG Y, YUE Q, ZAGHO M M, et al. Core-shell magnetic mesoporous silica microspheres with large mesopores for enzyme immobilization in biocatalysis[J]. ACS Appl Mater Interfaces, 2019, 11(10): 10356-10363. |
| 64 | FU X, CHEN X, WANG J, et al. Fabrication of carboxylic functionalized superparamagnetic mesoporous silica microspheres and their application for removal basic dye pollutants from water[J]. Microporous Mesoporous Mater, 2011, 139(1): 8-15. |
| 65 | WANG F, TANG Y, ZHANG B, et al. Preparation of novel magnetic hollow mesoporous silica microspheres and their efficient adsorption[J]. J Colloid Interface Sci, 2012, 386(1): 129-134. |
| 66 | LOBATO N C C, FERREIRA A D M, WEIDLER P G, et al. Microstructure and chemical stability analysis of magnetic core coated with SILICA and functionalized with silane OTS[J]. Appl Surf Sci, 2020, 505: 144565. |
| 67 | SERT Ş, ERAL M. Uranium adsorption studies on aminopropyl modified mesoporous sorbent(NH2-MCM-41)using statistical design method[J]. J Nucl Mater, 2010, 406(3): 285-292. |
| 68 | 张宗波, 袁亚莉, 周智慧, 等. 多氨基含氮配体改性有序介孔材料的制备及对铀(Ⅵ)的吸附性能研究[J]. 应用化工, 2016, 45(4): 603-607. |
| ZHANG Z B, YUAN Y L, ZHOU Z H, et al. Preparation and adsorption of uranyl of multi-amino nitrogen ligands functionalized mesoporous silica materials[J]. Appl Chem Ind, 2016, 45(4): 603-607. | |
| 69 | HUYNH J, PALACIO R, SAFIZADEH F, et al. Adsorption of uranium over NH2-functionalized ordered silica in aqueous solutions[J]. ACS Appl Mater Interfaces, 2017, 9(18): 15672-15684. |
| 70 | HUYNH J, PALACIO R, ALLAVENA A, et al. Selective adsorption of U(Ⅵ) from real mine water using an NH2-functionalized silica packed column[J]. Chem Eng J, 2021, 405: 126912. |
| 71 | SARAFRAZ H, MINUCHEHR A, ALAHYARIZADEH G, et al. Synthesis of enhanced phosphonic functional groups mesoporous silica for uranium selective adsorption from aqueous solutions[J]. Sci Rep, 2017, 7(1): 11675. |
| 72 | SARAFRAZ H, ALAHYARIZADEH G, MINUCHEHR A, et al. Economic and efficient phosphonic functional groups mesoporous silica for uranium selective adsorption from aqueous solutions[J]. Sci Rep, 2019, 9(1): 9686. |
| 73 | WANG Q, WANG Z, DING K, et al. Novel amidoxime-functionalized SBA-15-incorporated polymer membrane-type adsorbent for uranium extraction from wastewater[J]. J Water Process Eng, 2021, 43: 102316. |
| 74 | XIE Y, WANG J, WANG M, et al. Fabrication of fibrous amidoxime-functionalized mesoporous silica microsphere and its selectively adsorption property for Pb2+ in aqueous solution[J]. J Hazard Mater, 2015, 297: 66-73. |
| 75 | ZHAO Y, LI J, ZHAO L, et al. Synthesis of amidoxime-functionalized Fe3O4@SiO2 core-shell magnetic microspheres for highly efficient sorption of U(Ⅵ)[J]. Chem Eng J, 2014, 235: 275-283. |
| 76 | BAYRAMOGLU G, ARICA M Y. MCM-41 silica particles grafted with polyacrylonitrile: modification in to amidoxime and carboxyl groups for enhanced uranium removal from aqueous medium[J]. Microporous Mesoporous Mater, 2016, 226: 117-124 |
| 77 | WANG B, ZHOU Y, LI L, et al. Preparation of amidoxime-functionalized mesoporous silica nanospheres(ami-MSN) from coal fly ash for the removal of U(Ⅵ)[J]. Sci Total Environ, 2018, 626: 219-227. |
| 78 | XIAO J, JING Y, WANG X, et al. Preconcentration of uranium(Ⅵ) from aqueous solution by amidoxime-functionalized microspheres silica material: kinetics, isotherm and mechanism study[J]. ChemistrySelect, 2018, 3(43): 12346-12356. |
| 79 | YIN X, BAI J, TIAN W, et al. Uranium sorption from saline lake brine by amidoximated silica[J]. J Radioanal Nucl Chem, 2017, 313(1): 113-121. |
| 80 | ZHAO Y, WANG X, LI J, et al. Amidoxime functionalization of mesoporous silica and its high removal of U(Ⅵ)[J]. Polym Chem, 2015, 6(30): 5376-5384. |
| 81 | ALLEN D, BASTON G, BRADLEY A E, et al. An investigation of the radiochemical stability of ionic liquids[J].Green Chem, 2002, 4(2): 152-158. |
| 82 | LIU W, HUANG Y, HUANG G, et al. Eco-friendly and low-cost amidoxime-functionalized microcrystalline cellulose/mesoporous silica composite for the selective adsorption of U(Ⅵ) from aqueous solution[J]. J Radioanal Nucl Chem, 2022, 331(5): 2055-2068. |
| 83 | LIU W, HUANG Y, HUANG G, et al. Facile strategy to separate uranium(Ⅵ) using glued amidoxime-functionalized composite beads synthesized from aqueous solution[J]. Sep Purif Technol, 2022, 293: 121132. |
| 84 | DENG S, BAI R. Removal of trivalent and hexavalent chromium with aminated polyacrylonitrile fibers: performance and mechanisms[J]. Water Res, 2004, 38(9): 2424-2432. |
| 85 | ZHANG X, BAI R. Adsorption behavior of humic acid onto polypyrrole-coated nylon 6,6 granules[J]. J Mater Chem, 2002, 12(9): 2733-2739. |
| 86 | SCOTT T B, RIBA TORT O, ALLEN G C. Aqueous uptake of uranium onto pyrite surfaces; reactivity of fresh versus weathered material[J].Geochim Cosmochim Acta, 2007, 71(21): 5044-5053. |
| 87 | JIANG X, WANG H, WANG Q, et al. Immobilizing amino-functionalized mesoporous silica into sodium alginate for efficiently removing low concentrations of uranium[J]. J Clean Prod, 2020, 247: 119162. |
| 88 | GIANNAKOUDAKIS D A, ANASTOPOULOS I, BARCZAK M, et al. Enhanced uranium removal from acidic wastewater by phosphonate-functionalized ordered mesoporous silica: surface chemistry matters the most[J]. J Hazard Mater, 2021, 413: 125279. |
| 89 | QUILÈS F, BURNEAU A. Infrared and Raman spectra of uranyl(Ⅵ) oxo-hydroxo complexes in acid aqueous solutions: a chemometric study[J]. Vib Spectrosc, 2000, 23(2): 231-241. |
| 90 | NGUYEN TRUNG C, BEGUN G M, PALMER D A. Aqueous uranium complexes. 2. Raman spectroscopic study of the complex formation of the dioxouranium(Ⅵ) ion with a variety of inorganic and organic ligands[J]. Inorg Chem, 1992, 31(25): 5280-5287. |
| 91 | NGUYEN-TRUNG C, PALMER D A, BEGUN G M, et al. Aqueous uranyl complexes 1. Raman spectroscopic study of the hydrolysis of uranyl(Ⅵ) in solutions of trifluoromethanesulfonic acid and/or tetramethylammonium hydroxide at 25 ℃ and 0.1 MPa[J]. J Solution Chem, 2000, 29(2): 101-129. |
| 92 | ZHANG A, ASAKURA T, UCHIYAMA G. The adsorption mechanism of uranium(Ⅵ) from seawater on a macroporous fibrous polymeric adsorbent containing amidoxime chelating functional group[J]. React Funct Polym, 2003, 57(1): 67-76. |
| [1] | 元宁, 马洁, 张晋玲, 张建胜. 蒸气辅助合成PCN-6(M)双金属有机框架材料及其CH4和CO2吸附性能[J]. 应用化学, 2023, 40(6): 896-903. |
| [2] | 张元华, 李晟冉, 于喜飞. 氟功能化胆碱磷酸脂质体用于胰岛素的口服给药[J]. 应用化学, 2023, 40(5): 730-742. |
| [3] | 郝晨丽, 丁庆伟, 贾世昌, 毛泱博, 王松柏, 马骏. 硫辛酸修饰钛酸纳米管吸附亚甲基蓝的性能[J]. 应用化学, 2023, 40(5): 749-757. |
| [4] | 兰晓琳, 郑红星, 张依帆, 赵振, 肖和业, 王志江, 邓鹏飏. 常压高温固相反应制备SiC陶瓷粉体的研究进展[J]. 应用化学, 2023, 40(4): 476-485. |
| [5] | 赵金丽, 于宗仁, 苏伯民. 墓葬壁画中蛋清胶结材料的热裂解-气质联用分析[J]. 应用化学, 2023, 40(4): 562-570. |
| [6] | 许祥民, 邓杰, 杜雨琪, 沈红亮, 安泽坤, 孙才英. 螺环磷酰咪唑阻燃棉织物的热解挥发物分析及热解机理推测[J]. 应用化学, 2023, 40(3): 380-388. |
| [7] | 熊波, 黎泰华, 周武平, 刘长宇, 徐晓龙. 一步热聚合法制备Cu2O/CuO-g-C3N4吸附剂及其对甲基橙吸附的性能[J]. 应用化学, 2023, 40(3): 420-429. |
| [8] | 王华宇, 张超, 陈柯铭, 葛明. 金属-有机框架MIL-88A(Fe)及其复合材料在水处理中的研究进展[J]. 应用化学, 2023, 40(2): 155-168. |
| [9] | 赵跃华, 王大鹏. 氨基化氧化石墨烯和脂肪酸在水-油界面的共吸附动力学[J]. 应用化学, 2022, 39(8): 1274-1284. |
| [10] | 王文栋, 李在均. 钌-石墨烯量子点人工酶合成及用于胡萝卜中辛硫磷的光度检测[J]. 应用化学, 2022, 39(8): 1285-1293. |
| [11] | 曹从军, 马含笑, 侯成敏, 丁小健, 管飙. 乙基纤维素磁性复合材料对溶液中铜离子的吸附性能[J]. 应用化学, 2022, 39(6): 969-979. |
| [12] | 薛松松, 解正峰, 何佳伟, 张天怡, 夏保平, 李雨芹. 高选择性快速识别汞(Ⅱ)离子的磺酰腙型探针的合成及在吸附中的应用[J]. 应用化学, 2022, 39(5): 760-768. |
| [13] | 宋林虎, 李世友, 王洁, 张晶晶, 张宁霜, 赵冬妮, 徐菲. 锂离子电池电解液除酸除水添加剂的研究进展[J]. 应用化学, 2022, 39(5): 697-706. |
| [14] | 王雪, 王意波, 王显, 祝建兵, 葛君杰, 刘长鹏, 邢巍. 酸性电解水过程中氧析出反应的机理及铱基催化剂的研究进展[J]. 应用化学, 2022, 39(4): 616-628. |
| [15] | 杜慧, 姚晨阳, 彭皓, 姜波, 李顺祥, 姚俊烈, 郑方, 杨方, 吴爱国. 过渡金属掺杂磁性纳米粒子在生物医学领域中的研究进展[J]. 应用化学, 2022, 39(3): 391-406. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||