应用化学 ›› 2024, Vol. 41 ›› Issue (5): 637-658.DOI: 10.19894/j.issn.1000-0518.230369
• 综合评述 • 上一篇
TiO2基光催化CO2还原研究进展
万冰洁1, 刘小雪1, 齐林光1, 贾长超1( ), 刘健1,2(
), 刘健1,2( )
)
- 1.青岛科技大学材料科学与工程学院,青岛 266042
2.中国科学院青岛生物能源与过程研究所,太阳能光电转化与利用重点实验室,青岛新能源山东省实验室,青岛 266101
-
收稿日期:2023-11-24接受日期:2024-03-15出版日期:2024-05-01发布日期:2024-06-03 -
通讯作者:贾长超,刘健
Research Progress of TiO2-Based Photocatalytic CO2 Reduction
Bing-Jie WAN1, Xiao-Xue LIU1, Lin-Guang QI1, Chang-Chao JIA1( ), Jian LIU1,2(
), Jian LIU1,2( )
)
- 1.College of Materials Science and Engineering,Qingdao University of Science and Technology,Qingdao 266042,China
2.Qingdao Institute of Biomass Energy and Bioprocess Technology,Chinese Academy of Sciences,Key Laboratory of Photoelectric Conversion and Utilization of Solar Energy,Qingdao New Energy Shandong Laboratory,Qingdao 266101,China
-
Received:2023-11-24Accepted:2024-03-15Published:2024-05-01Online:2024-06-03 -
Contact:Chang-Chao JIA,Jian LIU -
About author:jiachangchao@qust.edu.cn
liujian@qibebt.ac.cn
-
Supported by:the Qingdao Natural Science Foundation(23-2-1-250-zyyd-jch);China Postdoctoral Science Foundation(2022M711743);the National Natural Science Foundation of China(22175104);the Natural Science Foundation of Shandong Province(ZR2019ZD47);the Education Department of Shandong Province(2019KJC006)
摘要:
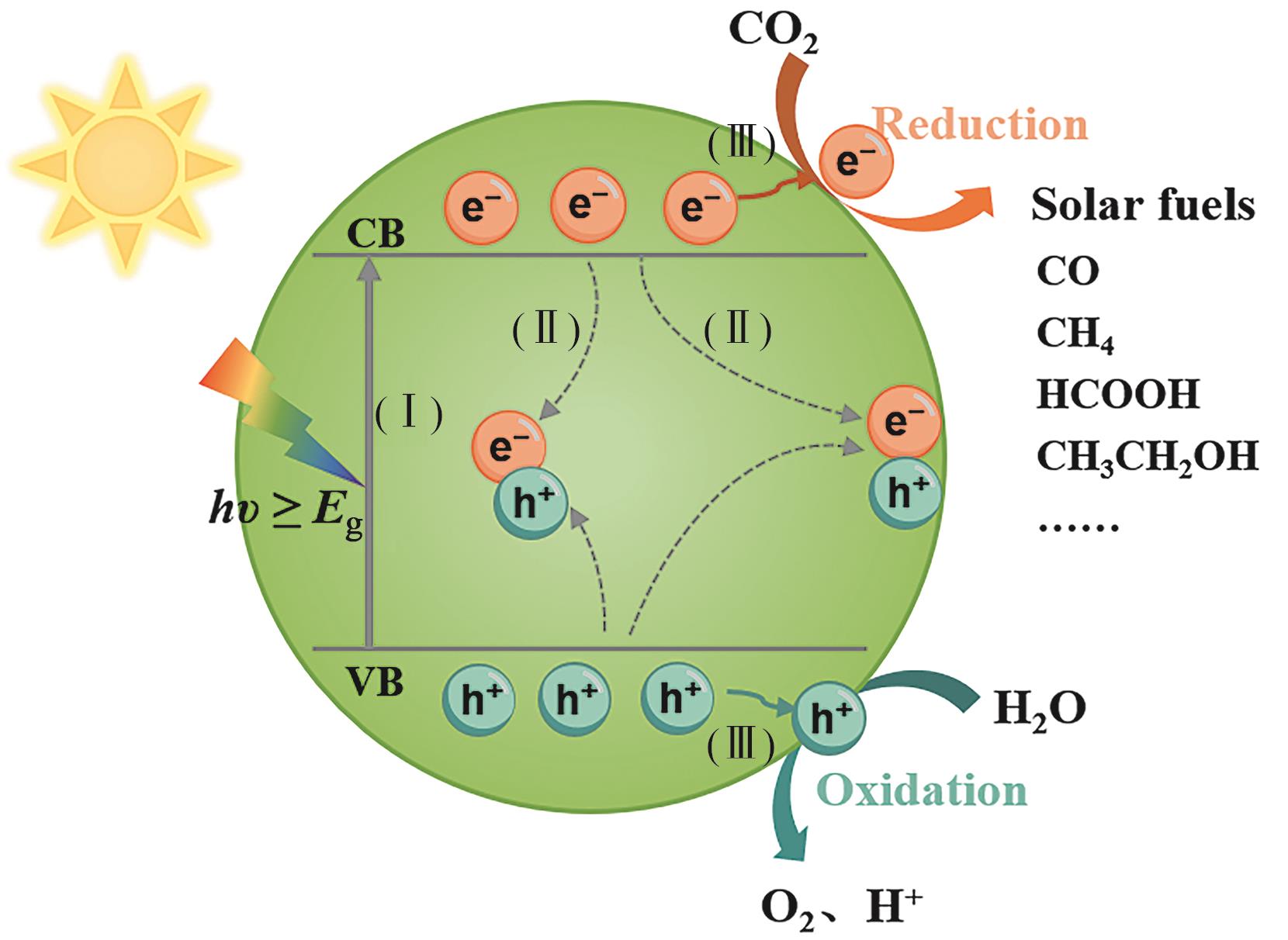
自二氧化钛(TiO2)材料被发现具有光催化性能以来,至今已有50余年的发展历程。因其优异的催化活性以及化学稳定性在光催化领域备受青睐。本文总结了近年来TiO2基材料在光催化CO2还原(CO2RR)方面的发展,有助于全面认识和把握该领域的研究进展和发展趋势,为双碳目标的顺利实现提供有力支撑。本文主要从光催化CO2还原机理、CO2还原反应体系和TiO2基催化剂在光催化CO2还原方面的研究进展进行论述; 详细阐述了对TiO2基光催化剂活性位点的调控,重点探讨了钛氧簇的设计、路易斯酸碱对的构筑、异质结的建立以及浸润性调节对TiO2基光催化剂性能的提高; 最后,对该领域面临的挑战和发展机遇进行了分析和展望。
中图分类号:
引用本文
万冰洁, 刘小雪, 齐林光, 贾长超, 刘健. TiO2基光催化CO2还原研究进展[J]. 应用化学, 2024, 41(5): 637-658.
Bing-Jie WAN, Xiao-Xue LIU, Lin-Guang QI, Chang-Chao JIA, Jian LIU. Research Progress of TiO2-Based Photocatalytic CO2 Reduction[J]. Chinese Journal of Applied Chemistry, 2024, 41(5): 637-658.
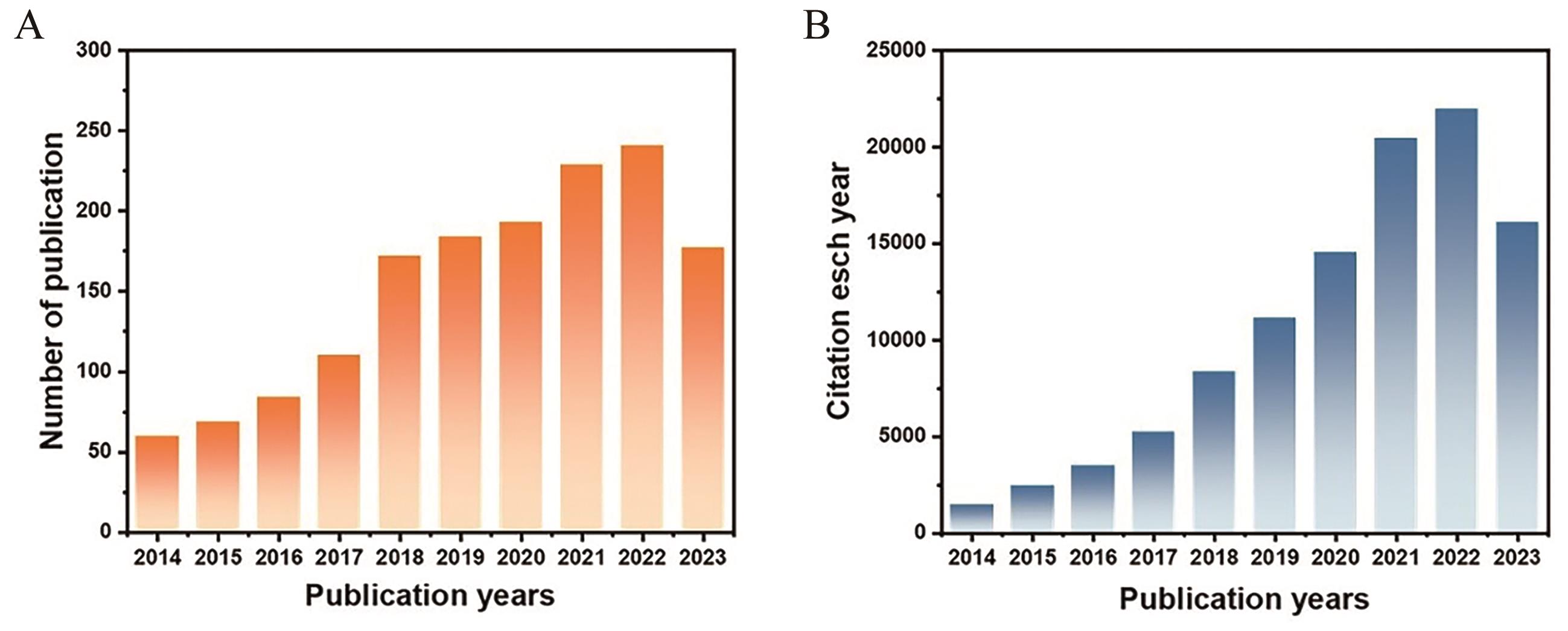
图1 近10年来关于TiO2基光催化剂在CO2还原领域(A)出版以及(B)引用情况
Fig.1 Publications (A) and citations (B) of TiO2-based photocatalysts in the field of CO2 reduction during last 10 years (The data are achieved on the search results from the Web of Science 2023.11)
| Product | Reaction | E/V(vs.NHE) |
|---|---|---|
| Hydrogen | 2H2O + 2e- | -0.41 |
| Methane | CO2 + 8H+ + 8e - | -0.24 |
| Carbon monoxide | CO2 + 2H + + 2e - | -0.51 |
| Methanol | CO2 + 6H + + 6e - | -0.38 |
| Formic acid | CO2 + 2H + + 2e - | -0.61 |
| Methanal | CO2 + 4H+ + 4e - | -0.48 |
| Ethylene | 2CO2 + 12H+ + 12e - | -0.34 |
| Ethane | 2CO2 + 14H + + 14e - | -0.27 |
| Ethanol | 2CO2 + 12H+ + 12e- | -0.33 |
| Oxalate | 2CO2 + 2H + + 2e - | -0.87 |
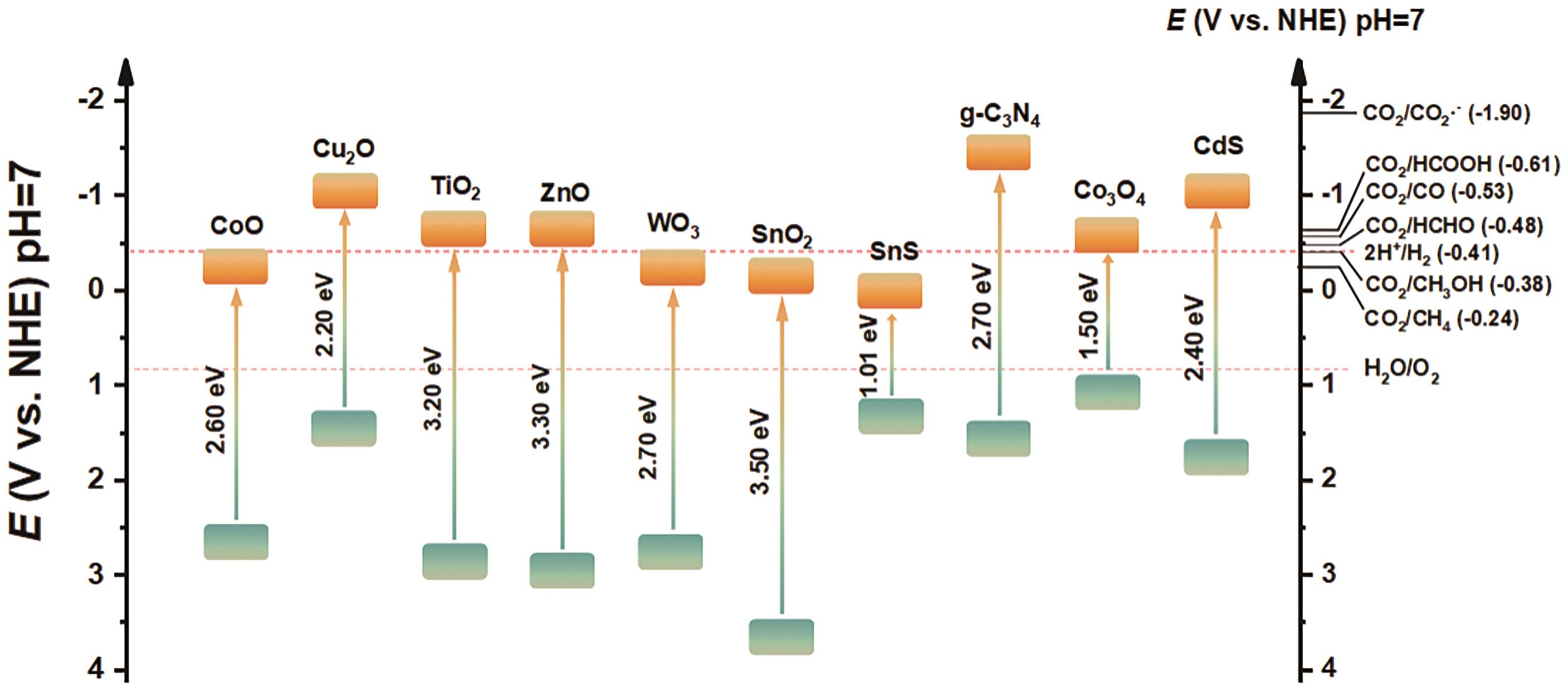
表 1 CO2转化形成不同产物所需的反应势能
Table 1 Reaction potential energy required for CO2 conversion to form different products
| Product | Reaction | E/V(vs.NHE) |
|---|---|---|
| Hydrogen | 2H2O + 2e- | -0.41 |
| Methane | CO2 + 8H+ + 8e - | -0.24 |
| Carbon monoxide | CO2 + 2H + + 2e - | -0.51 |
| Methanol | CO2 + 6H + + 6e - | -0.38 |
| Formic acid | CO2 + 2H + + 2e - | -0.61 |
| Methanal | CO2 + 4H+ + 4e - | -0.48 |
| Ethylene | 2CO2 + 12H+ + 12e - | -0.34 |
| Ethane | 2CO2 + 14H + + 14e - | -0.27 |
| Ethanol | 2CO2 + 12H+ + 12e- | -0.33 |
| Oxalate | 2CO2 + 2H + + 2e - | -0.87 |
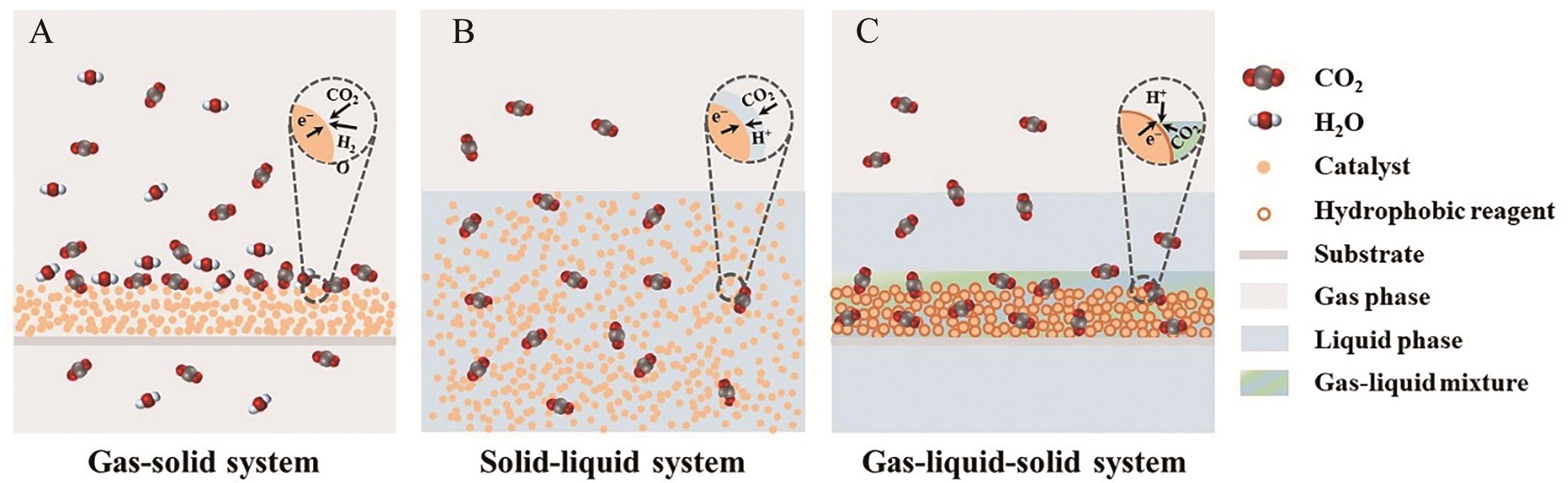
图4 (A)气-固反应体系、(B)固-液反应体系和(C)气-液-固反应体系光催化CO2还原体系示意图
Fig.4 Schematic diagram of photocatalytic CO2 reduction system in (A) gas-solid system, (B) solid-liquid system and (C) gas-liquid-solid system
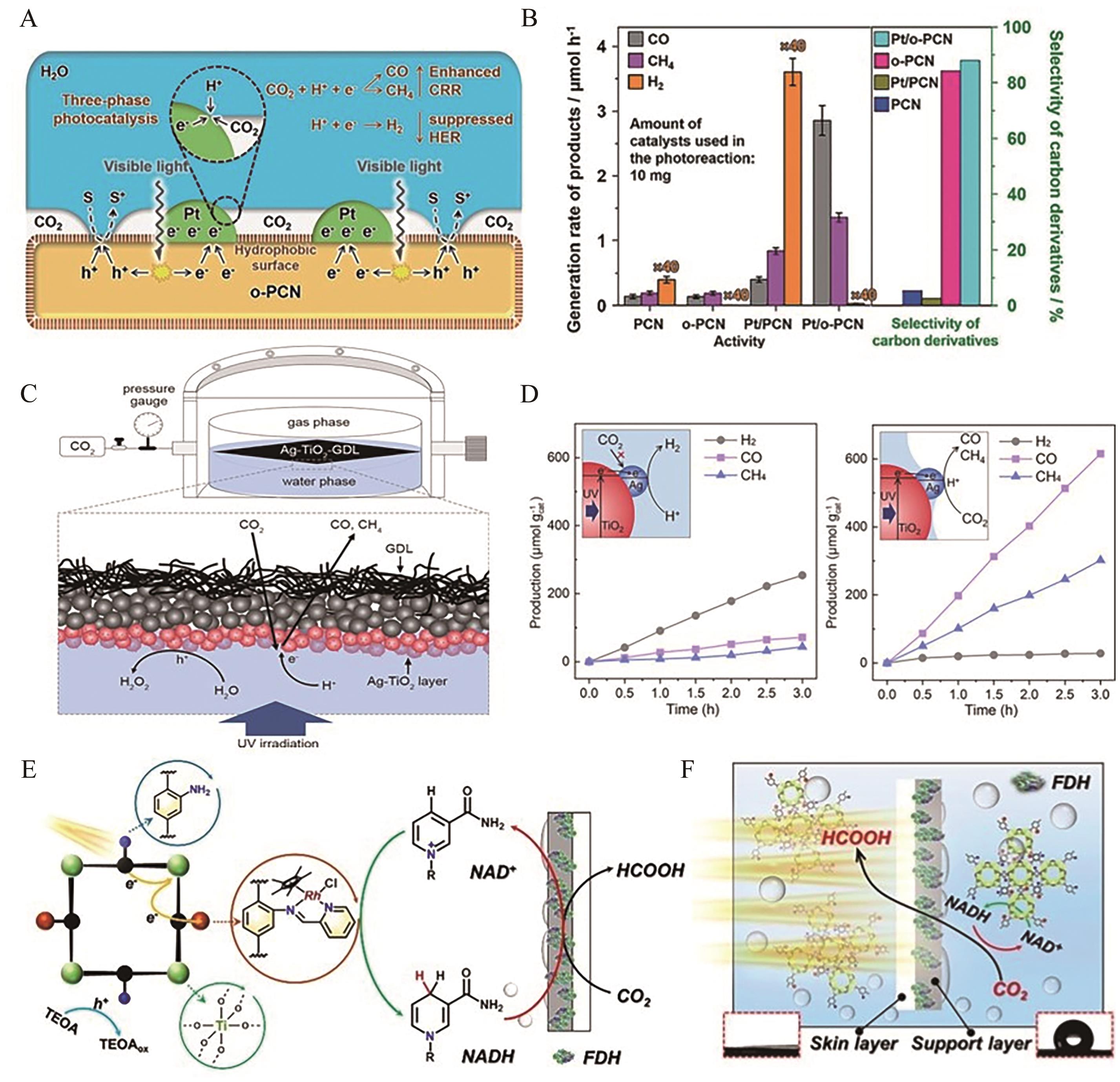
图5 (A) Pt/o-PCN三相光催化体系示意图; (B) Pt/o-PCN光催化活性和碳基产物的选择性[46]; (C)气-液-固界面负载的Ag-TiO2三相光催化体系示意图; (D)Ag-TiO2-GDL体系还原CO2产物[47]; (E)具有亲水/疏水层膜上光-酶集成催化系统示意图; (F)光酶催化CO2还原示意图[52]
Fig.5 (A) Schematic diagram of Pt/o-PCN three-phase photocatalytic system; (B) Photocatalytic activity and selectivity of Pt/o-PCN to carbon products[46]; (C) Schematic diagram of Ag-TiO2 three-phase photocatalytic system supported by gas-liquid-solid interface; (D) CO2 reduction products at Ag-TiO2-GDL[47]; (E) Schematic diagram of an integrated photoenzyme catalytic system with a hydrophilic and hydrophobic layer on the membrane; (F) Schematic diagram of CO2 reduction catalyzed by photoenzyme[52]
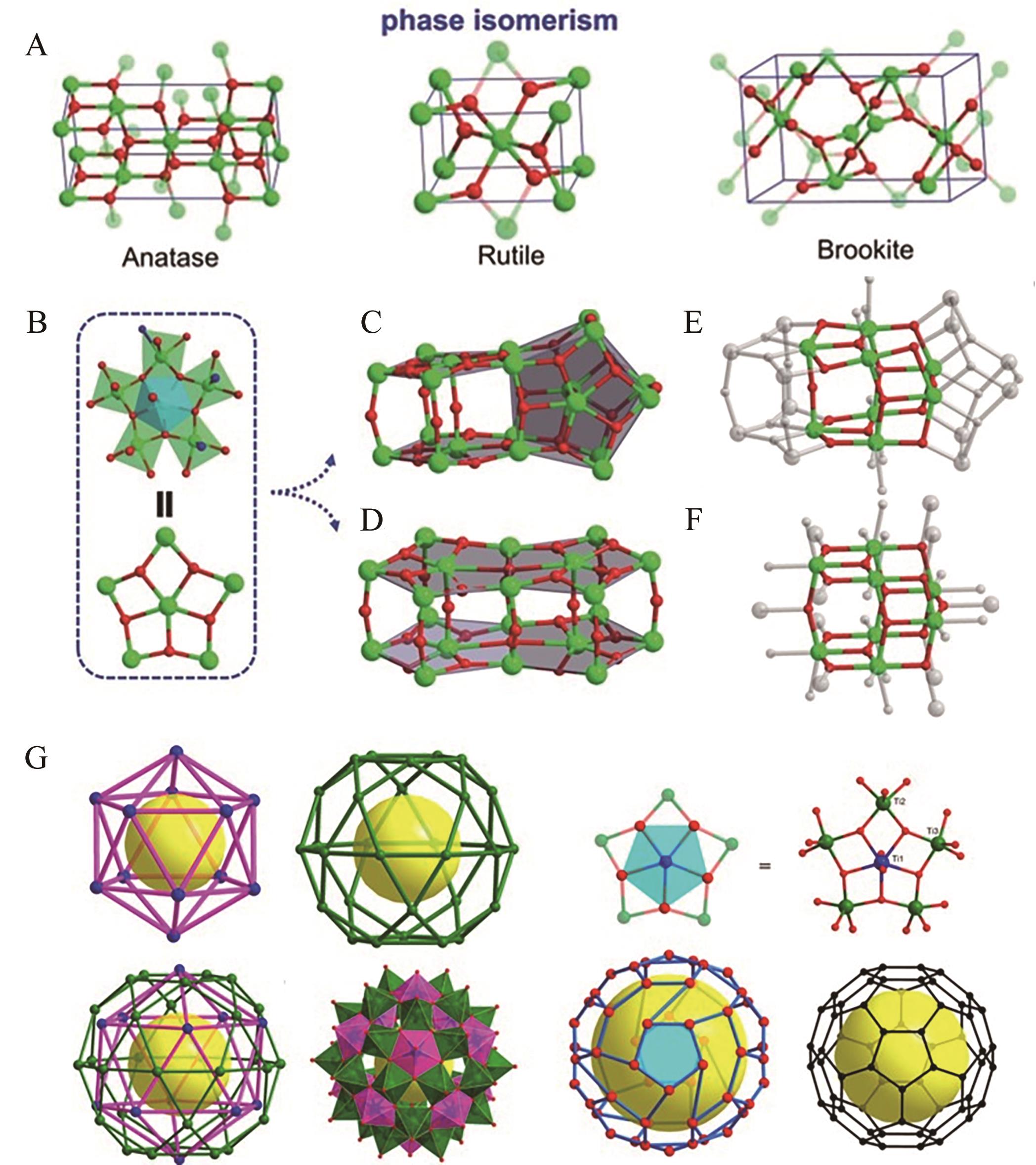
图6 (A) TiO2分子模型; (B){Ti(Ti5)}五边形结构单元示意图; Ti—O团簇结构(C)PTC-49V和(D)PTC-49H;{Ti8O14}分子结构模型(E)PTC-49H和(F)锐钛矿TiO2[58]; (G)Ti42分子结构示意图[59]
Fig.6 (A) Molecular configuration of TiO2; (B) illustration of the pentagonal {Ti(Ti5)} building units; the Ti—O core structures of (C) PTC-49V and (D) PTC-49H; illustration of the highlighted anatase-type {Ti8O14} moiety in (E) PTC-49H and (F) anatase TiO2[58]; (G) Molecular structure diagram of Ti42[59]
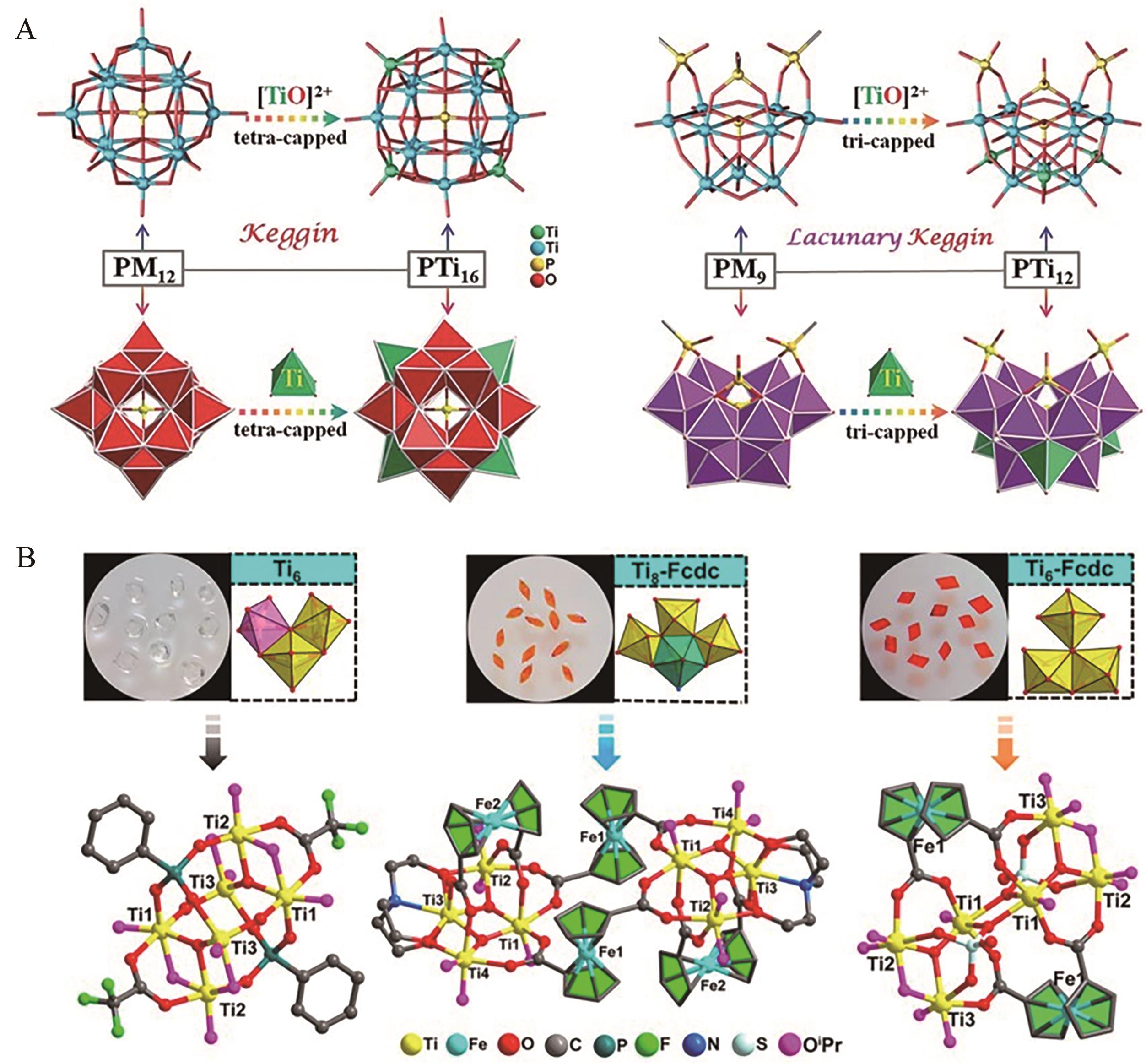
图7 (A)PTi16和PTi12的杂原子Keggin结构示意图[60]及(B)Ti6、Ti8-Fcdc和Ti6-Fcdc的晶体照片及晶体结构[62]
Fig.7 (A) Structural and polyhedral relationships between PTi16, PTi12 and original heteroatom Keggin structure[60] and (B) the morphology and molecular structure of Ti6, Ti8-Fcdc and Ti6-Fcdc[62]
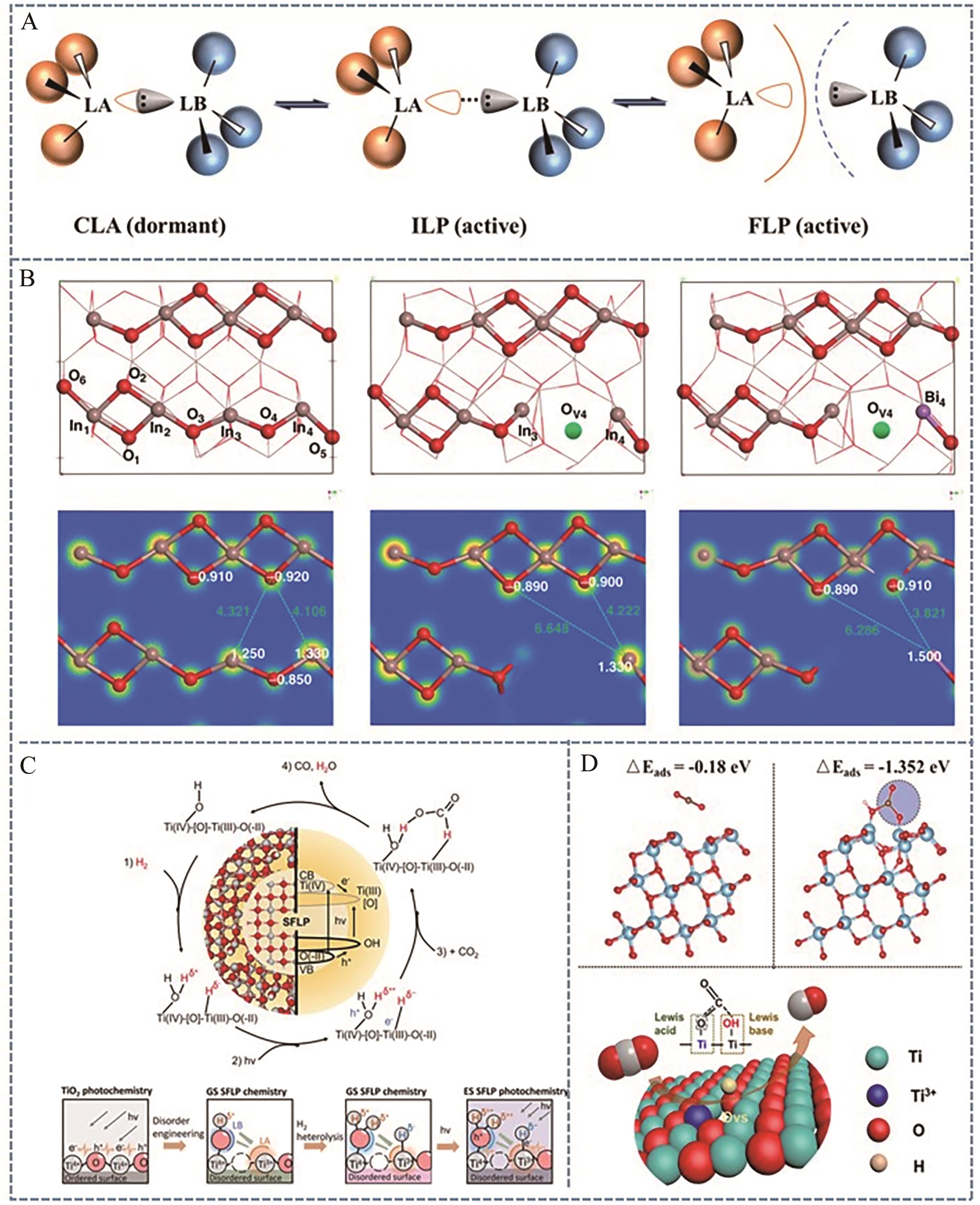
图8 (A)路易斯酸碱对示意图; (B) Bi3+取代In位点Bi x In2-x O3的FLPs反应性示意图[70]; (C) c-TiO2@a-TiO2-x (OH)y异质结构表面路易斯酸碱对[71]及(D)TiO2-x 表面受阻路易斯酸碱对[72]
Fig.8 (A) Schematic illustration of the classical Lewis acid-base pair; (B) Reactivity of FLPs in single-site Bi3+ substituted Bi x In2–x O3[70]; (C) c-TiO2@a-TiO2-x (OH)y heterogeneous surface FLPs[71] and (D) the surface of TiO2-x is hindered by Lewis acid-base pairs[72]
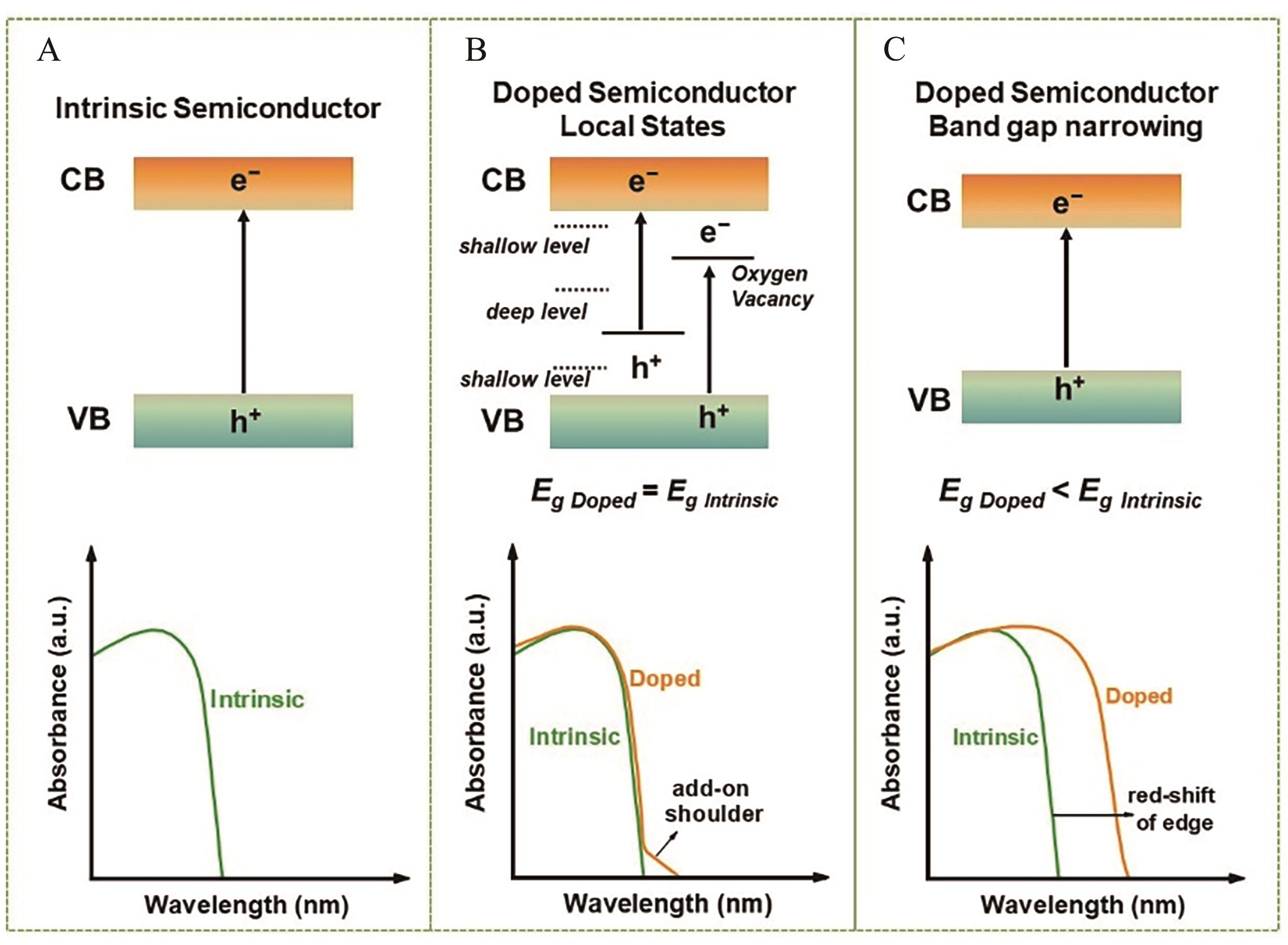
图9 半导体电子能带结构及光吸收图谱示意图[88]The band structure and optical absorption curves of (A) an intrinsic semiconductor, (B) doping-induced intraband energy states, (C) doping-induced band gap narrowing
Fig.9 Schematic for engineering the electronic band structure of semiconductors[88]
| Photocatalyst | Light source for photocatalytic reaction | Product | Yield/(μmol·g-1·h-1) | Selectivity/% | Year∣Ref. | |
|---|---|---|---|---|---|---|
| Cu/3DOM-TiO2 | Gas-solid | 300 W Xe lamp with a UV filter (320~780 nm) | CH4 | 43.15 | 83.3 | 2022∣[ |
| Cu/3DOM-TiO2 | Solid-liquid | 300 W Xe lamp with a UV filter (320~780 nm) | C2H4 | 6.99 | 58.4 | 2022∣[ |
| Cu/TiO2 | Gas-solid | 300 W Xe lamp UV enhanced | CO | 15.27 | 95.9 | 2022∣[ |
| Cu NCs/TiO2 | Gas-solid | 300 W Xe lamp | CO | 40.3 | 93 | 2022∣[ |
| H-TiO2@Cu | Gas-solid | 300 W Xe lamp | CO | 23.5 | 84.5 | 2021∣[ |
| Cu-O/Ti0.91O2-SL | Solid-liquid | 300 W Xe lamp | CO | 61.0 | 84.4 | 2023∣[ |
| Cu-Ti-OVs/Ti0.91O2-SL | Solid-liquid | 300 W Xe lamp | C2H4 C3H8 | 7.6 13.8 | 17.8 32.4 | 2023∣[ |
| Au/TiO2 | Gas-solid | 300 W Xe lamp with an AM 1.5G filter | CO | 608 | 99 | 2022∣[ |
| Au-TiO2-C3N4 | Gas-solid | 300 W Xe lamp, 420 nm cut-off | CH4 | 140 | -- | 2018∣[ |
| Au-NG-TiO2 | Gas-solid | 300 W Xe lamp with an AM 1.5G filter | CH4 | 83.72 | -- | 2022∣[ |
| AuCu-TiO2-x NSs | Gas-solid | 300 W Xe lamp | CH4 | 22.47 | 90.55 | 2021∣[ |
| Cu0.8Au0.2/TiO2 | Gas-solid | 300 W Xe lamp with an AM 1.5G filter | CH4 C2H4 | 3578.9 369.8 | 77.1 11.9 | 2021∣[ |
| Ag/TiO2 | Solid-liquid | 300 W Xe lamp | CO | 0.58 | 100 | 2021∣[ |
| Ag-TiO2-GDL | Gas-liquid-solid | 300 W Xe lamp with a UV filter (<400 nm) | CO CH4 | 205.1 100.6 | 65.4 32.0 | 2022∣[ |
| Ag-TiO2 | Solid-liquid | 300 W Xe lamp with a UV filter (<400 nm) | CO CH4 | 23.9 14.5 | 19.4 11.8 | 2022∣[ |
| 1% Pt/TiO2 nanocrystals | Gas-solid | 300 W Xe lamp with a UV filter (320~780 nm) | CH4 | 4.6 | -- | 2017∣[ |
| Pt-TiO2-x | Gas-solid | 300 W Xe lamp | CO CH4 | 54.2 66.4 | -- | 2019∣[ |
0.4% Pt/Ti3+-TiO2 | Gas-solid | 300 W Xe lamp with an AM 1.5G filter | CH4 | 80 | -- | 2017∣117] |
| Pd0.2Ag0.04/TiO2 | Gas-solid | 300 W Xe lamp with a UV filter (<400 nm) | CO C2H5OH CH4 | 17 13 4 | -- | 2017∣[ |
| Pd-HPP-TiO2 | Gas-solid | 300 W Xe lamp with a UV filter (325~780 nm) | CO CH4 | 34 48 | -- 59 | 2022∣[ |
| Pd-HCPs-TiO2 | Gas-solid | 300 W Xe lamp | CH4 | 237.4 | 99.9 | 2021∣[ |
表2 TiO2负载不同金属光催化CO2还原性能
Table 2 Photocatalytic CO2 reduction performance of TiO2 loaded with different metals
| Photocatalyst | Light source for photocatalytic reaction | Product | Yield/(μmol·g-1·h-1) | Selectivity/% | Year∣Ref. | |
|---|---|---|---|---|---|---|
| Cu/3DOM-TiO2 | Gas-solid | 300 W Xe lamp with a UV filter (320~780 nm) | CH4 | 43.15 | 83.3 | 2022∣[ |
| Cu/3DOM-TiO2 | Solid-liquid | 300 W Xe lamp with a UV filter (320~780 nm) | C2H4 | 6.99 | 58.4 | 2022∣[ |
| Cu/TiO2 | Gas-solid | 300 W Xe lamp UV enhanced | CO | 15.27 | 95.9 | 2022∣[ |
| Cu NCs/TiO2 | Gas-solid | 300 W Xe lamp | CO | 40.3 | 93 | 2022∣[ |
| H-TiO2@Cu | Gas-solid | 300 W Xe lamp | CO | 23.5 | 84.5 | 2021∣[ |
| Cu-O/Ti0.91O2-SL | Solid-liquid | 300 W Xe lamp | CO | 61.0 | 84.4 | 2023∣[ |
| Cu-Ti-OVs/Ti0.91O2-SL | Solid-liquid | 300 W Xe lamp | C2H4 C3H8 | 7.6 13.8 | 17.8 32.4 | 2023∣[ |
| Au/TiO2 | Gas-solid | 300 W Xe lamp with an AM 1.5G filter | CO | 608 | 99 | 2022∣[ |
| Au-TiO2-C3N4 | Gas-solid | 300 W Xe lamp, 420 nm cut-off | CH4 | 140 | -- | 2018∣[ |
| Au-NG-TiO2 | Gas-solid | 300 W Xe lamp with an AM 1.5G filter | CH4 | 83.72 | -- | 2022∣[ |
| AuCu-TiO2-x NSs | Gas-solid | 300 W Xe lamp | CH4 | 22.47 | 90.55 | 2021∣[ |
| Cu0.8Au0.2/TiO2 | Gas-solid | 300 W Xe lamp with an AM 1.5G filter | CH4 C2H4 | 3578.9 369.8 | 77.1 11.9 | 2021∣[ |
| Ag/TiO2 | Solid-liquid | 300 W Xe lamp | CO | 0.58 | 100 | 2021∣[ |
| Ag-TiO2-GDL | Gas-liquid-solid | 300 W Xe lamp with a UV filter (<400 nm) | CO CH4 | 205.1 100.6 | 65.4 32.0 | 2022∣[ |
| Ag-TiO2 | Solid-liquid | 300 W Xe lamp with a UV filter (<400 nm) | CO CH4 | 23.9 14.5 | 19.4 11.8 | 2022∣[ |
| 1% Pt/TiO2 nanocrystals | Gas-solid | 300 W Xe lamp with a UV filter (320~780 nm) | CH4 | 4.6 | -- | 2017∣[ |
| Pt-TiO2-x | Gas-solid | 300 W Xe lamp | CO CH4 | 54.2 66.4 | -- | 2019∣[ |
0.4% Pt/Ti3+-TiO2 | Gas-solid | 300 W Xe lamp with an AM 1.5G filter | CH4 | 80 | -- | 2017∣117] |
| Pd0.2Ag0.04/TiO2 | Gas-solid | 300 W Xe lamp with a UV filter (<400 nm) | CO C2H5OH CH4 | 17 13 4 | -- | 2017∣[ |
| Pd-HPP-TiO2 | Gas-solid | 300 W Xe lamp with a UV filter (325~780 nm) | CO CH4 | 34 48 | -- 59 | 2022∣[ |
| Pd-HCPs-TiO2 | Gas-solid | 300 W Xe lamp | CH4 | 237.4 | 99.9 | 2021∣[ |
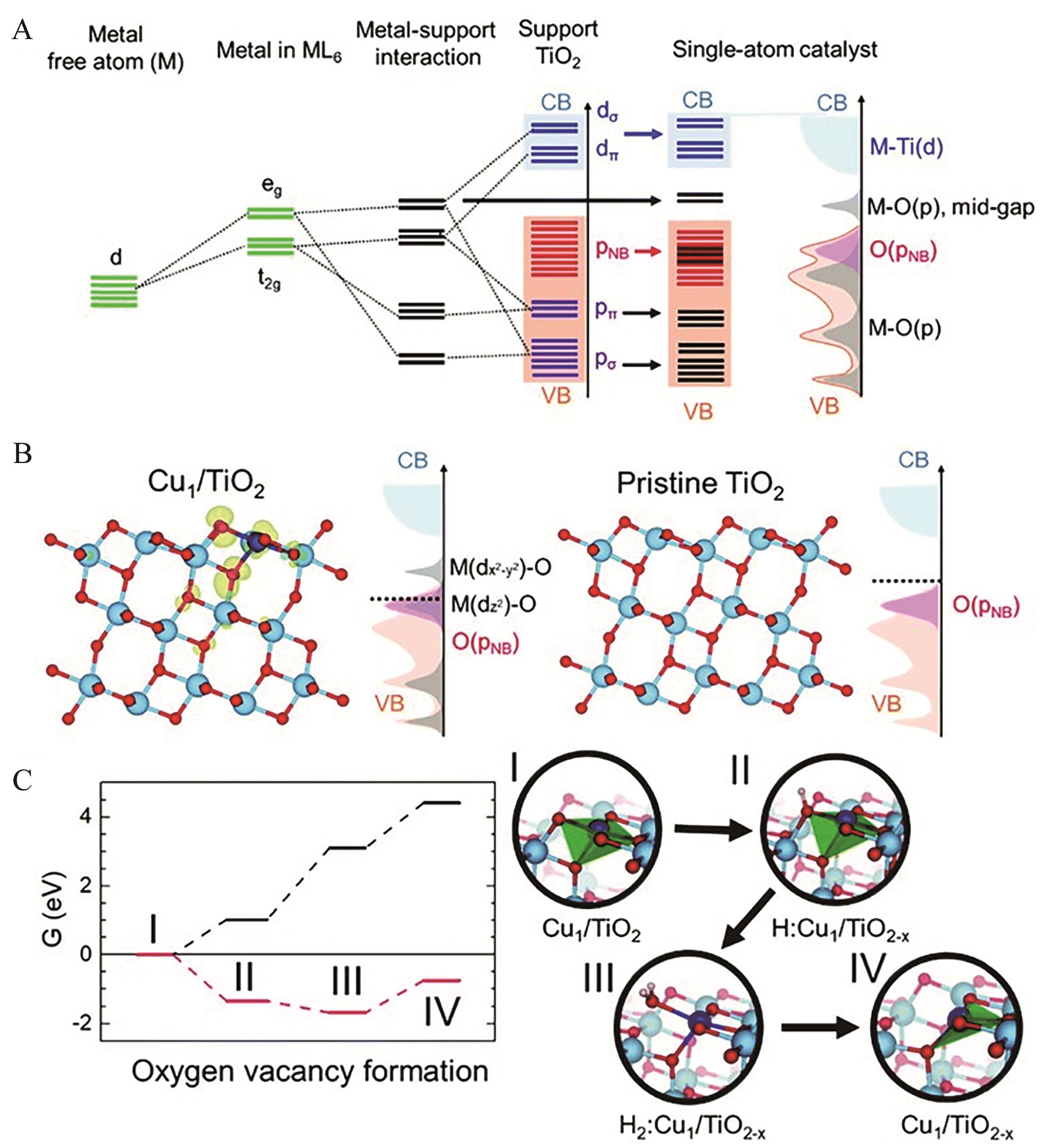
图10 金属原子与基底材料的电子交互作用: (A)单原子催化剂金属原子d轨道分裂的排列示意图; (B) Cu1/TiO2和原始TiO2的结构和态密度示意图; (C)氧空位DFT计算[124]
Fig.10 The electronic interactions at a single Cu atom and the surrounding TiO2: (A) Schematic alignments of the d orbital splitting of a metal atom in a coordinate complex; (B) Structure and schematic illustration of the density of states of Cu1/TiO2 and pristine TiO2; (C) DFT energetics of oxygen vacancy formation[124]
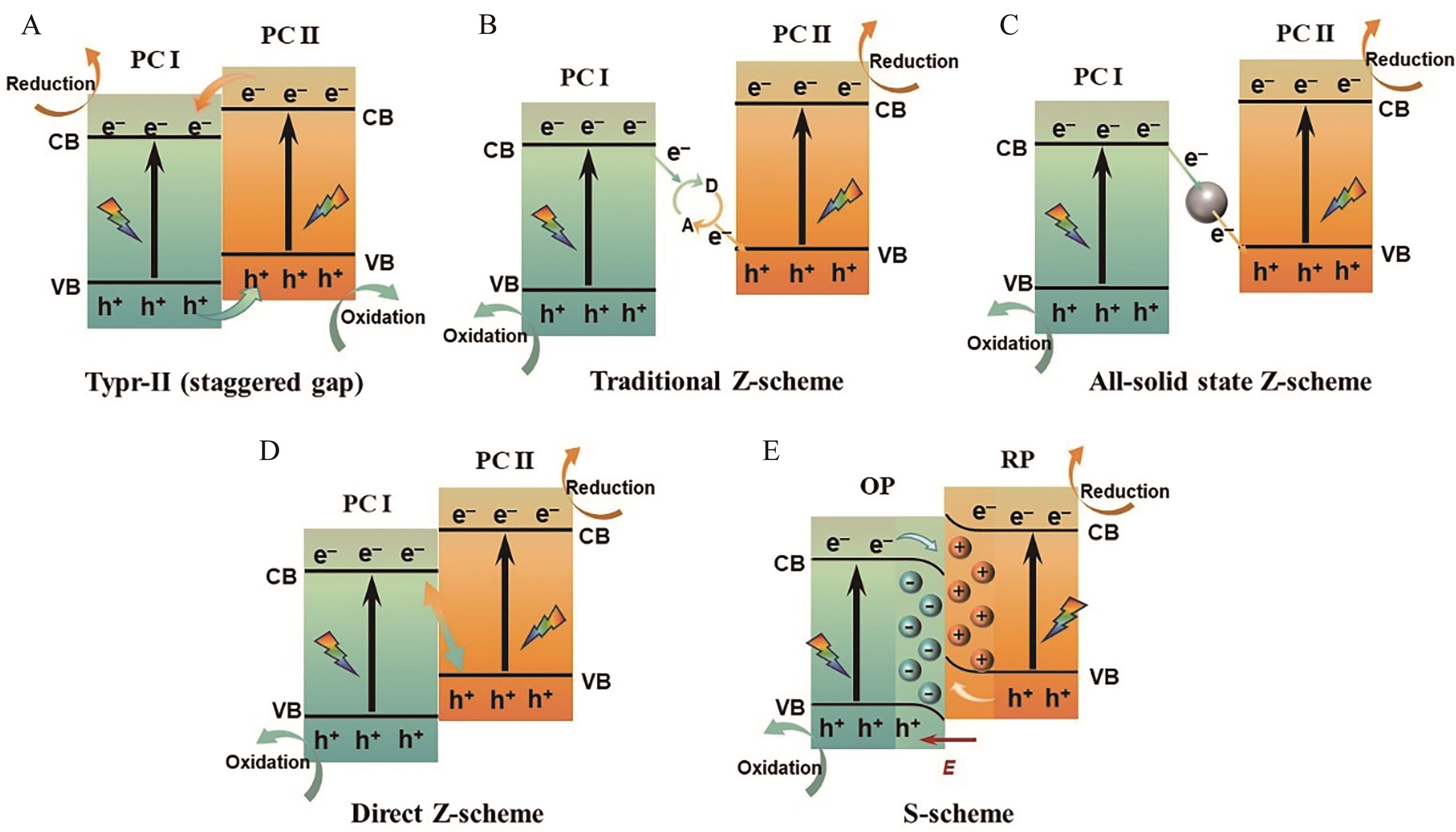
图11 不同类型异质结光响应情况下的电子-空穴对分离示意图: (A)Ⅱ型异质结; (B)传统Z型异质结; (C)全固态Z型异质结; (D)直接Z型异质结; (E)S型异质结
Fig.11 Schematic illustration of electron-hole pair separation under different types of heterojunction: (A) Typr-Ⅱ? scheme; (B) Traditional Z-scheme; (C) All-solid state Z-scheme; (D) Direct Z-scheme; (E) S-scheme photoresponse
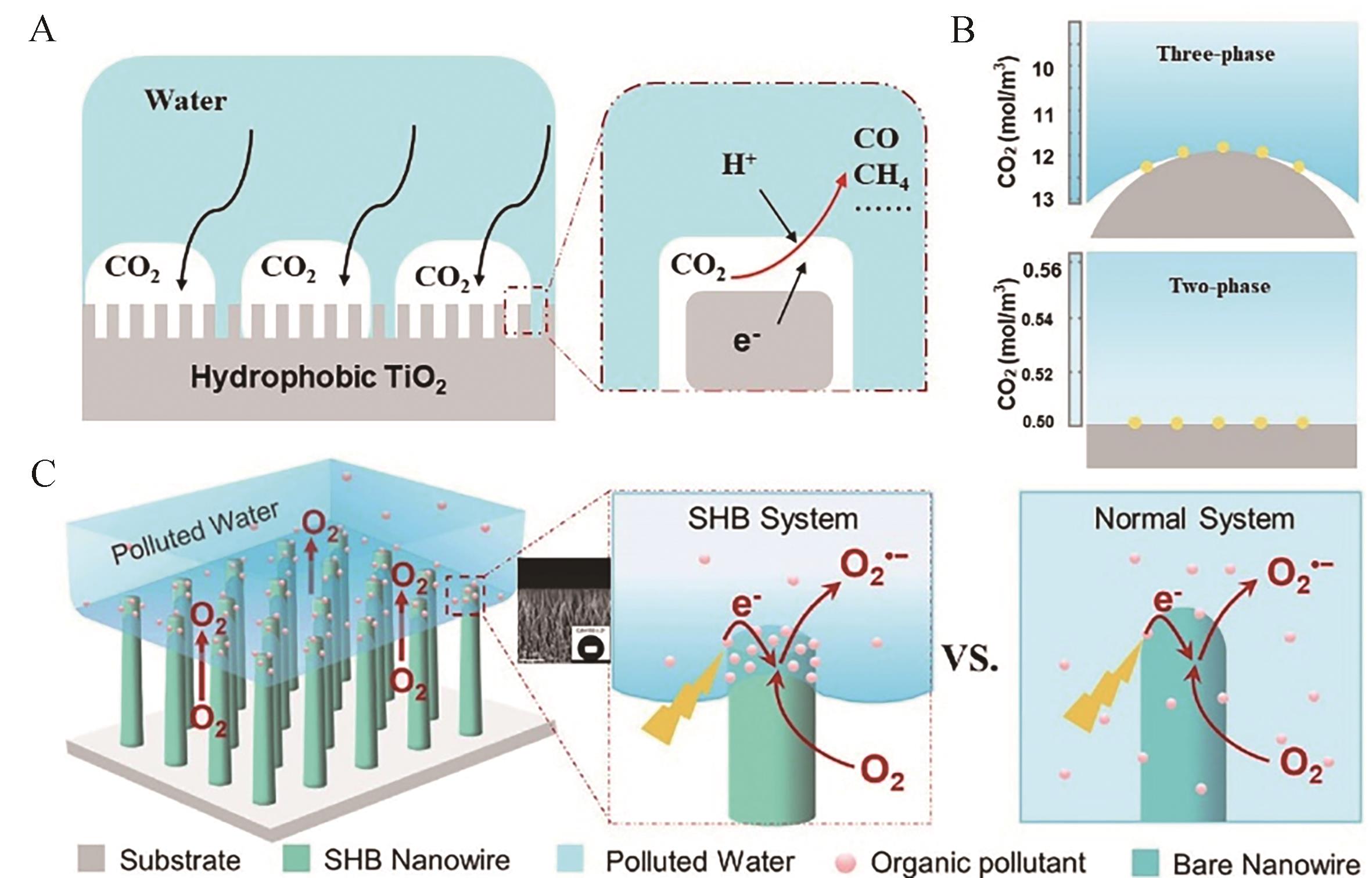
图12 (A)超疏水TiO2纳米线体系示意图及反应界面微环境示意图; (B)疏水型TiO2光催化CO2还原反应体系示意图及(C)三相和两相体系中CO2溶解度示意图[149]
Fig.12 (A) Schematic diagram of superhydrophobic TiO2 nanowire system and reaction interface microenvironment; (B) Schematic diagram of hydrophobic TiO2 photocatalytic CO2 reduction reaction system and (C) Schematic diagram of CO2 solubility in three-phase and two-phase systems[149]
| 1 | HUSSAIN I, JALIL A, HASSAN N, et al. Recent advances in catalytic systems for CO2 conversion to substitute natural gas (SNG): perspective and challenges[J]. J Energy Chem, 2021, 62: 377-407. |
| 2 | ZHAO Y F, WATERHOUSE G I N, CHEN G B, et al. Two-dimensional-related catalytic materials for solar-driven conversion of COx into valuable chemical feedstocks[J]. Chem Soc Rev, 2019, 48(7): 1972-2010. |
| 3 | ZHANG H N, LI Y F, WANG J Z, et al. An unprecedent hydride transfer pathway for selective photocatalytic reduction of CO2 to formic acid on TiO2[J]. Appl Catal B: Environ, 2021, 284: 119692. |
| 4 | SUN Z Y, MA T, TAO H C, et al. Fundamentals and challenges of electrochemical CO2 reduction using two-dimensional materials[J]. Chem, 2017, 3(4): 560-587. |
| 5 | WEI J, YAO R W, HAN Y, et al. Towards the development of the emerging process of CO2 heterogenous hydrogenation into high-value unsaturated heavy hydrocarbons[J]. Chem Soc Rev, 2021, 50(19): 10764-10805. |
| 6 | LI M J, SUN Z X, HU Y H. Catalysts for CO2 reforming of CH4: a review[J]. J Mater Chem A, 2021, 9(21): 12495-12520. |
| 7 | WAGNER A, SAHM C D, REISNER E. Towards molecular understanding of local chemical environment effects in electro-and photocatalytic CO2 reduction[J]. Nat Catal, 2020, 3(10): 775-786. |
| 8 | WANG J, GUO R T, BI Z X, et al. A review on TiO2- x-based materials for photocatalytic CO2 reduction[J]. Nanoscale, 2022, 14(32): 11512-11528. |
| 9 | FUJISHIMA A, HONDA K. Electrochemical photolysis of water at a semiconductor electrode[J]. Nature, 1972, 238(5358): 37-38. |
| 10 | INOUE T, FUJISHIMA A, KONISHI S, et al. Photoelectrocatalytic reduction of carbon dioxide in aqueous suspensions of semiconductor powders[J]. Nature, 1979, 277(5698): 637-638. |
| 11 | CAO S W, LOW J X, YU J G, et al. Polymeric photocatalysts based on graphitic carbon nitride[J]. Adv Mater, 2015, 27(13): 2150-2176. |
| 12 | WANG S L, XU M, PENG T Y, et al. Porous hypercrosslinked polymer-TiO2-graphene composite photocatalysts for visible-light-driven CO2 conversion[J]. Nat Commun, 2019, 10(1): 676. |
| 13 | JIANG Z, XU X H, MA Y H, et al. Filling metal-organic framework mesopores with TiO2 for CO2 photoreduction[J]. Nature, 2020, 586(7830): 549-554. |
| 14 | NGUYEN N T, ALTOMARE M, YOO J, et al. Efficient photocatalytic H2 evolution: controlled dewetting-dealloying to fabricate site-selective high-activity nanoporous Au particles on highly ordered TiO2 nanotube arrays[J]. Adv Mater, 2015, 27(20): 3208-3215. |
| 15 | NGUYEN T P, NGUYEN D L T, NGUYEN V H, et al. Recent advances in TiO2-based photocatalysts for reduction of CO2 to fuels[J]. Nanomaterials, 2020, 10(2): 337. |
| 16 | LI D, ZHAO Y, MIAO Y, et al. Accelerating electron-transfer dynamics by TiO2-immobilized reversible single-atom copper for enhanced artificial photosynthesis of urea[J]. Adv Mater, 2022, 34(51): 2207793. |
| 17 | FANG S, RAHAMAN M, BHARTI J, et al. Photocatalytic CO2 reduction[J]. Nat Rev Methods Primers, 2023, 3(1): 61. |
| 18 | YANG S, ZHANG W, PAN G, et al. Photocatalytic Co-reduction of N2 and CO2 with CeO2 catalyst for urea synthesis[J]. Angew Chem Int Ed, 2023, 62(43): e202312076. |
| 19 | HORVáTH E, ROSSI L, MERCIER C, et al. Photocatalytic nanowires-based air filter: towards reusable protective masks[J]. Adv Funct Mater, 2020, 30(40): 2004615. |
| 20 | BERNADET S, TAVERNIER E, TA D M, et al. Bulk photodriven CO2 conversion through TiO2@Si(HIPE) monolithic macrocellular foams[J]. Adv Funct Mater, 2019, 29(9): 1807767. |
| 21 | ZHANG P, SUI X Y, WANG Y, et al. Surface Ru—H bipyridine complexes-grafted TiO2 nanohybrids for efficient photocatalytic CO2 methanation[J]. J Am Chem Soc, 2023, 145(10): 5769-5777. |
| 22 | PARK J H, KIM J H. Selective CO2 photoconversion on low-coordinate TiO2[J]. Chem, 2020, 6(10): 2435-2436. |
| 23 | BAI S, YIN W J, WANG L L, et al. Surface and interface design in cocatalysts for photocatalytic water splitting and CO2 reduction[J]. RSC Adv, 2016, 6(62): 57446-57463. |
| 24 | CHANG X X, WANG T, GONG J L. CO2 photo-reduction: insights into CO2 activation and reaction on surfaces of photocatalysts[J]. Energy Environ Sci, 2016, 9(7): 2177-2196. |
| 25 | CHAUHAN D K, SHARMA N, KAILASAM K. A critical review on emerging photocatalysts for syngas generation via CO2 reduction under aqueous media: a sustainable paradigm[J]. Mater Adv, 2022, 3(13): 5274-5298. |
| 26 | JIA C C, CHEN H S, YANG P. Construction of hollow waxberry-like rutile-/anatase-TiO2/SnO2 towards enhanced photocatalysis[J]. J Ind Eng Chem, 2018, 58: 278-289. |
| 27 | HERRMANN J M. Photocatalysis fundamentals revisited to avoid several misconceptions[J]. Appl Catal B: Environ, 2010, 99(3/4): 461-468. |
| 28 | WENG B, QI M Y, HAN C, et al. Photocorrosion inhibition of semiconductor-based photocatalysts: basic principle, current development, and future perspective[J]. ACS Catal, 2019, 9(5): 4642-4687. |
| 29 | MEISSNER D, MEMMING R, KASTENING B. Photoelectrochemistry of cadmium sulfide. 1. reanalysis of photocorrosion and flat-band potential[J]. J Phys Chem, 2002, 92(12): 3476-3483. |
| 30 | BAHNEMANN D W, KORMANN C, HOFFMANN M R. Preparation and characterization of quantum size zinc oxide: a detailed spectroscopic study[J]. J Phys Chem, 1987, 91(14): 3789-3798. |
| 31 | SONG C S. Global challenges and strategies for control, conversion and utilization of CO2 for sustainable development involving energy, catalysis, adsorption and chemical processing[J]. Catal Today, 2006, 115(1/2/3/4): 2-32. |
| 32 | BENSON E E, KUBIAK C P, SATHRUM A J, et al. Electrocatalytic and homogeneous approaches to conversion of CO2 to liquid fuels[J]. Chem Soc Rev, 2009, 38(1): 89-99. |
| 33 | XIE S J, ZHANG Q H, LIU G D, et al. Photocatalytic and photoelectrocatalytic reduction of CO2 using heterogeneous catalysts with controlled nanostructures[J]. Chem Commun, 2016, 52(1): 35-59. |
| 34 | JIANG S Y, ZHAO K, AL-MAMUN M, et al. Design of three-dimensional hierarchical TiO2/SrTiO3 heterostructures towards selective CO2 photoreduction[J]. Inorg Chem Front, 2019, 6(7): 1667-1674. |
| 35 | CHEN C, WANG T, YAN K, et al. Photocatalytic CO2 reduction on Cu single atoms incorporated in ordered macroporous TiO2 toward tunable products[J]. Inorg Chem Front, 2022, 9(18): 4753-4767. |
| 36 | LI N, LIU J, LIU J J, et al. Adenine components in biomimetic metal-organic frameworks for efficient CO2 photoconversion[J]. Angew Chem Int Ed, 2019, 58(16): 5226-5231. |
| 37 | JIA G G, SUN M Z, WANG Y, et al. Asymmetric coupled dual-atom sites for selective photoreduction of carbon dioxide to acetic acid[J]. Adv Funct Mater, 2022, 32(41): 2206817. |
| 38 | LAM E, REISNER E. A TiO2-Co(terpyridine)2 photocatalyst for the selective oxidation of cellulose to formate coupled to the reduction of CO2 to syngas[J]. Angew Chem Int Ed, 2021, 60(43): 23306-23312. |
| 39 | YOSHINO S, IWASE A, YAMAGUCHI Y, et al. Photocatalytic CO2 reduction using water as an electron donor under visible light irradiation by Z-scheme and photoelectrochemical systems over (CuGa)0.5ZnS2 in the presence of basic additives[J]. J Am Chem Soc, 2022, 144(5): 2323-2332. |
| 40 | KUEHNEL M F, ORCHARD K L, DALLE K E, et al. Selective photocatalytic CO2 reduction in water through anchoring of a molecular Ni catalyst on CdS nanocrystals[J]. J Am Chem Soc, 2017, 139(21): 7217-7223. |
| 41 | XIN Z K, HUANG M Y, WANG Y, et al. Reductive carbon-carbon coupling on metal sites regulates photocatalytic CO2 reduction in water using ZnSe quantum dots[J]. Angew Chem Int Ed, 2022, 61(31): e202207222. |
| 42 | JIA C C, WAN B J, LIU W G, et al. Microenvironment modulation of ultrathin bronze-phase TiO2 nanosheets for highly selective photocatalytic CO2 reduction in water[J]. Adv Funct Mater, 2024, 34(9): 2311663. |
| 43 | LI J, CHEN G X, ZHU Y Y, et al. Efficient electrocatalytic CO2 reduction on a three-phase interface[J]. Nat Catal, 2018, 1(8): 592-600. |
| 44 | WANG P, ZHAO J Q, SHI R, et al. Efficient photocatalytic aerobic oxidation of bisphenol a via gas-liquid-solid triphase interfaces[J]. Mater Today Energy, 2022, 23: 100908. |
| 45 | IIZUKA K, WATO T, MISEKI Y, et al. Photocatalytic reduction of carbon dioxide over ag cocatalyst-loaded ALa4Ti4O15 (A = Ca, Sr, and Ba) using water as a reducing reagent[J]. J Am Chem Soc, 2011, 133(51): 20863-20868. |
| 46 | LI A, CAO Q, ZHOU G Y, et al. Three-phase photocatalysis for the enhanced selectivity and activity of CO2 reduction on a hydrophobic surface[J]. Angew Chem Int Ed, 2019, 58(41): 14549-14555. |
| 47 | HUANG H N, SHI R, LI Z H, et al. Triphase photocatalytic CO2 reduction over silver-decorated titanium oxide at a gas-water boundary[J]. Angew Chem Int Ed, 2022, 61(17): e202200802. |
| 48 | LIU J, ANTONIETTI M. Bio-inspired NADH regeneration by carbon nitride photocatalysis using diatom templates[J]. Energy Environ Sci, 2013, 6(5): 1486-1493. |
| 49 | JU D X, LIN G, XIAO H, et al. Investigation of water-stable perovskite DMASnIxBr3- x for photoenzyme catalysis in aqueous solution [J]. Sol RRL, 2020, 4(12): 2000559. |
| 50 | WANG Y C, LIU H, PAN Q Y, et al. Construction of thiazolo[5,4-d]thiazole-based two-dimensional network for efficient photocatalytic CO2 reduction[J]. ACS Appl Mater Interfaces, 2020, 12(41): 46483-46489. |
| 51 | ZHANG Y Y, LIU J. Bioinspired photocatalytic NADH regeneration by covalently metalated carbon nitride for enhanced CO2 reduction[J]. Chem-Eur J, 2022, 28(55): e202201430. |
| 52 | LIN G, ZHANG Y Y, HUA Y T, et al. Bioinspired metalation of the metal-organic framework MIL-125-NH2 for photocatalytic NADH regeneration and gas-liquid-solid three‐phase enzymatic CO2 reduction[J]. Angew Chem Int Ed, 2022, 61(31): e202206283. |
| 53 | JIAO X C, LI X D, JIN X Y, et al. Partially oxidized SnS2 atomic layers achieving efficient visible-light-driven CO2 reduction[J]. J Am Chem Soc, 2017, 139(49): 18044-18051. |
| 54 | JIA C C, ZHANG X, YANG P. Construction of anatase/rutile TiO2 hollow boxes for highly efficient photocatalytic performance[J]. Appl Surf Sci, 2018, 430: 457-465. |
| 55 | SCHRAUZER G N, GUTH T D. Photolysis of water and photoreduction of nitrogen on titanium dioxide[J]. J Am Chem Soc, 2002, 99(22): 7189-7193. |
| 56 | ZHANG G Y, LI W Y, LIU C Y, et al. Titanium-oxide host clusters with exchangeable guests[J]. J Am Chem Soc, 2017, 140(1): 66-69. |
| 57 | TAN Y X, WANG F, ZHANG J. Design and synthesis of multifunctional metal-organic zeolites[J]. Chem Soc Rev, 2018, 47(6): 2130-2144. |
| 58 | FAN X, WANG J H, WU K F, et al. Isomerism in titanium‐oxo clusters: molecular anatase model with atomic structure and improved photocatalytic activity[J]. Angew Chem Int Ed, 2018, 58(5): 1320-1323. |
| 59 | GAO M Y, WANG F, GU Z G, et al. Fullerene-like polyoxotitanium cage with high solution stability[J]. J Am Chem Soc, 2016, 138(8): 2556-2559. |
| 60 | LI N, LIU J, LIU J J, et al. Self-assembly of a phosphate-centered polyoxo-titanium cluster: discovery of the heteroatom keggin family[J]. Angew Chem Int Ed, 2019, 58(48): 17260-17264. |
| 61 | GAO M Y, BAI H, CUI X F, et al. Precisely tailoring heterometallic polyoxotitanium clusters for the efficient and selective photocatalytic oxidation of hydrocarbons[J]. Angew Chem Int Ed, 2022, 61(52): e202215540. |
| 62 | LIU J J, LI N, SUN J W, et al. Ferrocene-functionalized polyoxo-titanium cluster for CO2 photoreduction[J]. ACS Catal, 2021, 11(8): 4510-4519. |
| 63 | JIANG Z, XU X H, MA Y H, et al. Filling metal-organic framework mesopores with TiO2 for CO2 photoreduction[J]. Nature, 2020, 586(7830): 549-554. |
| 64 | LEWIS G N. Valence and the structure of atoms and molecules[M]. American Chemical Monograph Series, The Chemical Catalog Co., Inc., New York 1923: 172. |
| 65 | WELCH G C, JUAN R R S, MASUDA J D, et al. Reversible, metal-free hydrogen activation[J]. Science, 2006, 314(5802): 1124-1126. |
| 66 | MCCAHILL J S J, WELCH G C, STEPHAN D W. Reactivity of “frustrated lewis pairs”: three-component reactions of phosphines, a borane, and olefins[J]. Angew Chem Int Ed, 2007, 46(26): 4968-4971. |
| 67 | STEPHAN D W. The broadening reach of frustrated Lewis pair chemistry[J]. Science, 2016, 354(6317): aaf7229. |
| 68 | WANG Q L, MIAO Z R, ZHANG Y F, et al. Photocatalytic reduction of CO2 with H2O mediated by Ce-tailored bismuth oxybromide surface frustrated lewis pairs[J]. ACS Catal, 2022, 12(7): 4016-4025. |
| 69 | GHUMAN K K, WOOD T E, HOCH L B, et al. Illuminating CO2 reduction on frustrated Lewis pair surfaces: investigating the role of surface hydroxides and oxygen vacancies on nanocrystalline In2O3- x(OH)y[J]. Phys Chem Chem Phys, 2015, 17(22): 14623-14635. |
| 70 | YAN T J, LI N, WANG L L, et al. Bismuth atom tailoring of indium oxide surface frustrated Lewis pairs boosts heterogeneous CO2 photocatalytic hydrogenation[J]. Nat Commun, 2020, 11(1): 6095. |
| 71 | LI Z, MAO C L, PEI Q J, et al. Engineered disorder in CO2 photocatalysis[J]. Nat Commun, 2022, 13(1): 7205. |
| 72 | JIA C C, KAN X N, ZHANG X, et al. Construction of frustrated lewis pairs on TiO2- x derived from perovskite for enhanced photocatalytic CO2 reduction[J]. Chem Eng J, 2022, 427: 131554. |
| 73 | ZHANG S, HUANG Z Q, MA Y, et al. Solid frustrated-Lewis-pair catalysts constructed by regulations on surface defects of porous nanorods of CeO2[J]. Nat Commun, 2017, 8(1): 15266. |
| 74 | ZHANG S, XIA Z M, ZOU Y, et al. Interfacial frustrated lewis pairs of CeO2 activate CO2 for selective tandem transformation of olefins and CO2 into cyclic carbonates[J]. J Am Chem Soc, 2019, 141(29): 11353-11357. |
| 75 | CHEN X B, LIU L, YU P Y, et al. Increasing solar absorption for photocatalysis with black hydrogenated titanium dioxide nanocrystals[J]. Science, 2011, 331(6018): 746-750. |
| 76 | YU H J, LI J Y, ZHANG Y H, et al. Three-in-one oxygen vacancies: whole visible-spectrum absorption, efficient charge separation, and surface site activation for robust CO2 photoreduction[J]. Angew Chem Int Ed, 2019, 58(12): 3880-3884. |
| 77 | SHI R, ZHAO Y X, WATERHOUSE G I N, et al. Defect engineering in photocatalytic nitrogen fixation[J]. ACS Catal, 2019, 9(11): 9739-9750. |
| 78 | BAI S, ZHANG N, GAO C, et al. Defect engineering in photocatalytic materials[J]. Nano Energy, 2018, 53: 296-336. |
| 79 | RAWOOL S A, YADAV K K, POLSHETTIWAR V. Defective TiO2 for photocatalytic CO2 conversion to fuels and chemicals[J]. Chem Sci, 2021, 12(12): 4267-4299. |
| 80 | ZHANG Y Z, XIA B Q, RAN J R, et al. Atomic-level reactive sites for semiconductor-based photocatalytic CO2 reduction[J]. Adv Energy Mater, 2020, 10(9): 1903879. |
| 81 | XIONG L B, LI J L, YANG B, et al. Ti3+ in the surface of titanium dioxide: generation, properties and photocatalytic application[J]. J Nanomater, 2012, 2012: 1-13. |
| 82 | CHEN X B, LIU L, HUANG F Q. Black titanium dioxide TiO2 nanomaterials[J]. Chem Soc Rev, 2015, 44(7): 1861-1885. |
| 83 | LIN G X, JU Q J, LIU L J, et al. Caged-cation-induced lattice distortion in bronze TiO2 for cohering nanoparticulate hydrogen evolution electrocatalysts[J]. ACS Nano, 2022, 16(6): 9920-9928. |
| 84 | KANG Q, CAO J Y, ZHANG Y J, et al. Reduced TiO2 nanotube arrays for photoelectrochemical water splitting[J]. J Mater Chem A, 2013, 1(18): 5766-5774. |
| 85 | ZHANG W, XUE J B, SHEN Q Q, et al. Black single-crystal TiO2 nanosheet array films with oxygen vacancy on {001} facets for boosting photocatalytic CO2 reduction[J]. J Alloys Compd, 2021, 870: 159400. |
| 86 | JIA G R, WANG Y, CUI X Q, et al. Wet-chemistry hydrogen doped TiO2 with switchable defects control for photocatalytic hydrogen evolution[J]. Matter, 2022, 5(1): 206-218. |
| 87 | LI Y H, REN Z T, GU M L, et al. Synergistic effect of interstitial C doping and oxygen vacancies on the photoreactivity of TiO2 nanofibers towards CO2 reduction[J]. Appl Catal B: Environ, 2022, 317: 121773. |
| 88 | GAO M M, ZHU L L, PEH C K, et al. Solar absorber material and system designs for photothermal water vaporization towards clean water and energy production[J]. Energy Environ Sci, 2019, 12(3): 841-864. |
| 89 | LI X, YU J G, LOW J X, et al. Engineering heterogeneous semiconductors for solar water splitting[J]. J Mater Chem A, 2015, 3(6): 2485-2534. |
| 90 | LI J T, WU N Q. Semiconductor-based photocatalysts and photoelectrochemical cells for solar fuel generation: a review[J]. Catal Sci Technol, 2015, 5(3): 1360-1384. |
| 91 | GUPTA S M, TRIPATHI M. A review of TiO2 nanoparticles[J]. Chin Sci Bull, 2011, 56(16): 1639-1657. |
| 92 | MEDHI R, MARQUEZ M D, LEE T R. Visible-light-active doped metal oxide nanoparticles: review of their synthesis, properties, and applications[J]. ACS Appl Nano Mater, 2020, 3(7): 6156-6185. |
| 93 | CHEN J R, QIU F X, XU W Z, et al. Recent progress in enhancing photocatalytic efficiency of TiO2-based materials[J]. Appl Catal A, 2015, 495: 131-140. |
| 94 | ASAHI R, MORIKAWA T, OHWAKI T, et al. Visible-light photocatalysis in nitrogen-doped titanium oxides[J]. Science, 2001, 293(5528): 269-271. |
| 95 | LEE D K, CHOI J I, LEE G H, et al. Energy states of a core-shell metal oxide photocatalyst enabling visible light absorption and utilization in solar-to-fuel conversion of carbon dioxide[J]. Adv Energy Mater, 2016, 6(14): 1600583. |
| 96 | NI B X, ZHANG G R, WANG H M, et al. Correlating oxidation state and surface ligand motifs with the selectivity of CO2 photoreduction to C2 products[J]. Angew Chem Int Ed, 2022, 62(6): e202215574. |
| 97 | WEN J Q, LI X, LIU W, et al. Photocatalysis fundamentals and surface modification of TiO2 nanomaterials[J]. Chin J Catal, 2015, 36(12): 2049-2070. |
| 98 | PRAKASH J, SUN S H, SWART H C, et al. Noble metals-TiO2 nanocomposites: from fundamental mechanisms to photocatalysis, surface enhanced Raman scattering and antibacterial applications[J]. Appl Mater Today, 2018, 11: 82-135. |
| 99 | ZHU S, CHEN X F, LI Z C, et al. Cooperation between inside and outside of TiO2: lattice Cu+ accelerates carrier migration to the surface of metal copper for photocatalytic CO2 reduction[J]. Appl Catal B: Environ, 2020, 264: 118515. |
| 100 | XIN X Y, XU T, WANG L, et al. Ti3+-self doped brookite TiO2 single-crystalline nanosheets with high solar absorption and excellent photocatalytic CO2 reduction[J]. Sci Rep, 2016, 6(1): 23684. |
| 101 | XU M, WU H, TANG Y W, et al. One-step in-situ synthesis of porous Fe3+-doped TiO2 octahedra toward visible-light photocatalytic conversion of CO2 into solar fuel[J]. Microporous Mesoporous Mater, 2020, 309: 110539. |
| 102 | CHEN L, SONG X L, REN J T, et al. Precisely modifying Co2P/black TiO2 S-scheme heterojunction by in situ formed P and C dopants for enhanced photocatalytic H2 production[J]. Appl Catal B: Environ, 2022, 315: 121546. |
| 103 | WANG J C, QIAO X, SHI W N, et al. Enhanced photothermal selective conversion of CO2 to CH4 in water vapor over rod-like Cu and N Co-doped TiO2[J]. Chin J Struct Chem, 2022, 41(12): 2212033-2212042. |
| 104 | WANG H, QI H F, SUN X, et al. High quantum efficiency of hydrogen production from methanol aqueous solution with PtCu-TiO2 photocatalysts[J]. Nat Mater, 2023, 22(5): 619-626. |
| 105 | ZHU K N, ZHU Q, JIANG M P, et al. Modulating Ti t2g orbital occupancy in a Cu/TiO2 composite for selective photocatalytic CO2 reduction to CO[J]. Angew Chem Int Ed, 2022, 61(34): e202207600. |
| 106 | BAO X L, ZHANG M H, WANG Z Y, et al. Molten-salt assisted synthesis of Cu clusters modified TiO2 with oxygen vacancies for efficient photocatalytic reduction of CO2 to CO[J]. Chem Eng J, 2022, 445: 136718. |
| 107 | LIU M, ZHENG L R, BAO X L, et al. Substrate-dependent ALD of Cux on TiO2 and its performance in photocatalytic CO2 reduction[J]. Chem Eng J, 2021, 405: 126654. |
| 108 | SHEN Y, REN C J, ZHENG L R, et al. Room-temperature photosynthesis of propane from CO2 with Cu single atoms on vacancy-rich TiO2[J]. Nat Commun, 2023, 14(1): 1117. |
| 109 | HUANG H W, ZHAO J W, WENG B, et al. Site-sensitive selective CO2 photoreduction to CO over gold nanoparticles[J]. Angew Chem Int Ed, 2022, 61(28): e202204563. |
| 110 | RAZIQ F, SUN L Q, WANG Y Y, et al. Synthesis of large surface-area g-C3N4 comodified with MnOx and Au-TiO2 as efficient visible-light photocatalysts for fuel production[J]. Adv Energy Mater, 2018, 8(3): 1701580. |
| 111 | KAMAL K M, NARAYAN R, CHANDRAN N, et al. Synergistic enhancement of photocatalytic CO2 reduction by plasmonic Au nanoparticles on TiO2 decorated N-graphene heterostructure catalyst for high selectivity methane production[J]. Appl Catal B: Environ, 2022, 307: 121181. |
| 112 | JIANG D L, ZHOU Y M, ZHANG Q X, et al. Synergistic integration of AuCu Co-catalyst with oxygen vacancies on TiO2 for efficient photocatalytic conversion of CO2 to CH4[J]. ACS Appl Mater Interfaces, 2021, 13(39): 46772-46782. |
| 113 | YU Y Y, DONG X A, CHEN P, et al. Synergistic effect of Cu single atoms and Au-Cu alloy nanoparticles on TiO2 for efficient CO2 photoreduction[J]. ACS Nano, 2021, 15(9): 14453-14464. |
| 114 | LI G H, SUN Y Y, ZHANG Q M, et al. Ag quantum dots modified hierarchically porous and defective TiO2 nanoparticles for improved photocatalytic CO2 reduction[J]. Chem Eng J, 2021, 410: 128397. |
| 115 | XIONG Z, LEI Z, CHEN X X, et al. CO2 photocatalytic reduction over Pt deposited TiO2 nanocrystals with coexposed {101} and {001} facets: effect of deposition method and Pt precursors[J]. Catal Commun, 2017, 96: 1-5. |
| 116 | LIU Y A, MIAO C L, YANG P F, et al. Synergetic promotional effect of oxygen vacancy-rich ultrathin TiO2 and photochemical induced highly dispersed Pt for photoreduction of CO2 with H2O[J]. Appl Catal B: Environ, 2019, 244: 919-930. |
| 117 | SORCAR S, HWANG Y, GRIMES C A, et al. Highly enhanced and stable activity of defect-induced titania nanoparticles for solar light-driven CO2 reduction into CH4[J]. Mater Today, 2017, 20(9): 507-515. |
| 118 | ZHU Y Z, XU Z X, LANG Q Q, et al. Grain boundary engineered metal nanowire cocatalysts for enhanced photocatalytic reduction of carbon dioxide[J]. Appl Catal B: Environ, 2017, 206: 282-292. |
| 119 | MA Y J, YI X X, WANG S L, et al. Selective photocatalytic CO2 reduction in aerobic environment by microporous Pd-porphyrin-based polymers coated hollow TiO2[J]. Nat Commun, 2022, 13(1): 1400. |
| 120 | ZHAN Z, WANG H, HUANG Q, et al. Grafting hypercrosslinked polymers on TiO2 surface for anchoring ultrafine Pd nanoparticles: dramatically enhanced efficiency and selectivity toward photocatalytic reduction of CO2 to CH4[J]. Small, 2021, 18(1): 2105083. |
| 121 | ROSS M B, DE LUNA P, LI Y, et al. Designing materials for electrochemical carbon dioxide recycling[J]. Nat Catal, 2019, 2(8): 648-658. |
| 122 | KUAI L, CHEN Z, LIU S J, et al. Titania supported synergistic palladium single atoms and nanoparticles for room temperature ketone and aldehydes hydrogenation[J]. Nat Commun, 2020, 11(1): 48. |
| 123 | AO X, ZHANG W, LI Z S, et al. Markedly enhanced oxygen reduction activity of single-atom Fe catalysts via integration with Fe nanoclusters[J]. ACS Nano, 2019, 13(10): 11853-11862. |
| 124 | LEE B H, GONG E, KIM M, et al. Electronic interaction between transition metal single-atoms and anatase TiO2 boosts CO2 photoreduction with H2O[J]. Energy Environ Sci, 2022, 15(2): 601-609. |
| 125 | QI K Z, CHENG B, YU J G, et al. A review on TiO2-based Z-scheme photocatalysts[J]. Chin J Catal, 2017, 38(12): 1936-1955. |
| 126 | LOW J X, JIANG C J, CHENG B, et al. A review of direct Z-scheme photocatalysts[J]. Small Methods, 2017, 1(5): 1700080. |
| 127 | ZHANG M, LU M, LANG Z L, et al. Semiconductor/covalent-organic-framework Z-scheme heterojunctions for artificial photosynthesis[J]. Angew Chem Int Ed, 2020, 59(16): 6500-6506. |
| 128 | BARD A J. Photoelectrochemistry and heterogeneous photo-catalysis at semiconductors[J]. J Photochem, 1979, 10(1): 59-75. |
| 129 | MARSCHALL R. Semiconductor composites: strategies for enhancing charge carrier separation to improve photocatalytic activity[J]. Adv Funct Mater, 2014, 24(17): 2421-2440. |
| 130 | JIA C C, ZHANG X, MATRAS-POSTOLEK K, et al. Z-scheme reduced graphene oxide/TiO2-Bronze/W18O49 ternary heterostructure towards efficient full solar-spectrum photocatalysis[J]. Carbon, 2018, 139: 415-426. |
| 131 | LOW J X, DAI B Z, TONG T, et al. In situ irradiated X-ray photoelectron spectroscopy investigation on a direct Z-scheme TiO2/CdS composite film photocatalyst[J]. Adv Mater, 2019, 31(6): e1802981. |
| 132 | BIAN J, ZHANG Z Q, FENG J N, et al. Energy platform for directed charge transfer in the cascade Z-scheme heterojunction: CO2 photoreduction without a cocatalyst[J]. Angew Chem Int Ed, 2021, 60(38): 20906-20914. |
| 133 | LI Q, YANG S C, LIU R H, et al. Synergetic effect of the interface electric field and the plasmon electromagnetic field in Au-Ag alloy mediated Z-type heterostructure for photocatalytic hydrogen production and CO2 reduction[J]. Appl Catal B: Environ, 2023, 331: 122700. |
| 134 | ZHANG L Y, ZHANG J J, YU H G, et al. Emerging S-scheme photocatalyst[J]. Adv Mater, 2022, 34(11): e2107668. |
| 135 | WANG L X, ZHU B C, ZHANG J J, et al. S-scheme heterojunction photocatalysts for CO2 reduction[J]. Matter, 2022, 5(12): 4187-4211. |
| 136 | FU J W, XU Q L, LOW J X, et al. Ultrathin 2D/2D WO3/g-C3N4 step-scheme H2-production photocatalyst[J]. Appl Catal B: Environ, 2019, 243: 556-565. |
| 137 | FU J, Wei, YU J G, JIANG C J, et al. g-C3N4-based heterostructured photocatalysts[J]. Adv Energy Mater, 2018, 8(3): 1701503. |
| 138 | LI J M, WU C C, LI J, et al. 1D/2D TiO2/ZnIn2S4 S-scheme heterojunction photocatalyst for efficient hydrogen evolution[J]. Chin J Catal, 2022, 43(2): 339-349. |
| 139 | WANG L B, CHENG B, ZHANG L Y, et al. In situ irradiated XPS investigation on S‐scheme TiO2@ZnIn2S4 photocatalyst for efficient photocatalytic CO2 reduction [J]. Small, 2021, 17(41): e2103447. |
| 140 | WANG Y L, HE W J, XIONG J, et al. MIL-68(In)-derived In2O3@TiO2 S-scheme heterojunction with hierarchical hollow structure for selective photoconversion of CO2 to hydrocarbon fuels[J]. Fuel, 2023, 331: 125719. |
| 141 | XU F Y, MENG K, CHENG B, et al. Unique S-scheme heterojunctions in self-assembled TiO2/CsPbBr3 hybrids for CO2 photoreduction [J]. Nat Commun, 2020, 11(1): 4613. |
| 142 | LI J, QIN Q H, SHAH A, et al. Oil droplet self-transportation on oleophobic surfaces[J]. Sci Adv, 2016, 2(6): e1600148. |
| 143 | LI H Z, LI A, ZHAO Z P, et al. Heterogeneous wettability surfaces: principle, construction, and applications[J]. Small Struct, 2020, 1(2): 2000028. |
| 144 | YOUNG T. III. An essay on the cohesion of fluids[J]. Philos Trans R Soc London, 1997, 95(95): 65-87. |
| 145 | LIU M J, WANG S T, JIANG L. Nature-inspired superwettability systems[J]. Nat Rev Mater, 2017, 2(7): 17036. |
| 146 | SU B, TIAN Y, JIANG L. Bioinspired interfaces with superwettability: from materials to chemistry[J]. J Am Chem Soc, 2016, 138(6): 1727-1748. |
| 147 | YU Y Y, CUI W, SONG L, et al. Design of organic-free superhydrophobic TiO2 with ultraviolet stability or ultraviolet-induced switchable wettability[J]. ACS Appl Mater Interfaces, 2022, 14(7): 9864-9872. |
| 148 | WAKERLEY D, LAMAISON S, OZANAM F, et al. Bio-inspired hydrophobicity promotes CO2 reduction on a Cu surface[J]. Nat Mater, 2019, 18(11): 1222-1227. |
| 149 | ZHOU H, SHENG X, XIAO J, et al. Increasing the efficiency of photocatalytic reactions via surface microenvironment engineering[J]. J Am Chem Soc, 2020, 142(6): 2738-2743. |
| 150 | DONG C Y, XING M Y, ZHANG J L. Economic hydrophobicity triggering of CO2 photoreduction for selective CH4 generation on noble-metal-free TiO2-SiO2[J]. J Phys Chem Lett, 2016, 7(15): 2962-2966. |
| 151 | KANG S, KHAN H, LEE C, et al. Investigation of hydrophobic MoSe2 grown at edge sites on TiO2 nanofibers for photocatalytic CO2 reduction[J]. Chem Eng J, 2021, 420: 130496. |
| 152 | ZHAO D W, DANG C Z, XUAN Y M, et al. Hydrophobic Ni@N-doped TiO2 nanosheet arrays-carbon paper photocatalyst for CO2 photoreduction at tri-phase interfaces[J]. Adv Sustainable Syst, 2023, 7(4): 2200450. |
| [1] | 王静, 李喜兰, 郭秀芬. 五氧化二铌光催化剂的制备及其光催化性能[J]. 应用化学, 2024, 41(3): 415-421. |
| [2] | 张涛, 张贺, 杜雅欣, 展思辉. Co掺杂Mn2O3复合材料的构筑及活化过氧单硫酸盐降解医药废水[J]. 应用化学, 2024, 41(2): 268-278. |
| [3] | 李钰炫, 赵昱昊, 代雨泽, 姜敏, 张瑛, 周光远. 聚2,5-呋喃二甲酸乙二醇酯/纳米二氧化钛/硅藻土复合材料的制备和表征[J]. 应用化学, 2023, 40(9): 1277-1287. |
| [4] | 雷学博, 刘慧景, 丁赫宇, 申国栋, 孙润军. 用于降解印染废水中有机污染物的光催化剂的研究进展[J]. 应用化学, 2023, 40(5): 681-696. |
| [5] | 蔡国庆, 董静茹, 莫君明. N‑苄基亚砜亚胺的绿色合成及其抑菌活性[J]. 应用化学, 2023, 40(12): 1693-1699. |
| [6] | 尉枫, 邢海东, 修子媛, 邢德峰, 韩晓军. 卤氧化铋基光催化剂的构建及其应用在能源转化领域的研究进展[J]. 应用化学, 2023, 40(11): 1518-1530. |
| [7] | 刘盛杰, 叶永杰, 刘银怡, 林淑满, 谢浩源, 刘文婷, 许伟钦. 基于泡沫铜的多孔碳均匀负载Cu3P纳米颗粒的制备及其光催化降解染料性能[J]. 应用化学, 2022, 39(7): 1090-1097. |
| [8] | 张晓丽, 彭玉美, 王庆伟, 秦利霞, 刘肖霞, 康诗钊, 李向清. 纳米Ag/TiO2纳米管阵列基底构建及表面增强拉曼散射光谱检测与降解盐酸四环素[J]. 应用化学, 2022, 39(7): 1147-1156. |
| [9] | 张超. 单原子催化剂电催化还原二氧化碳研究进展[J]. 应用化学, 2022, 39(6): 871-887. |
| [10] | 徐一鑫, 王爽, 全静, 高婉婷, 宋天群, 杨梅. 二硫化钼量子点/还原氧化石墨烯复合材料的制备及其光催化降解有机染料、四环素和Cr(VI)[J]. 应用化学, 2022, 39(5): 769-778. |
| [11] | 陶荟冰, 田震, 谢勇, 孙瑜, 汪莉, 康卓, 张跃. 电解水催化材料动态构效关系的原位拉曼研究进展[J]. 应用化学, 2022, 39(4): 528-539. |
| [12] | 商林杰, 刘江, 兰亚乾. 共价有机框架材料用于光/电催化CO2还原的研究进展[J]. 应用化学, 2022, 39(4): 559-584. |
| [13] | 王佳赫, 刘大勇, 刘伟, 王林, 董彪. 纳米TiO2光催化抗菌应用的研究进展[J]. 应用化学, 2022, 39(4): 629-646. |
| [14] | 逯慧, 李江, 王丽华, 诸颖, 陈静. 原子级精确的币金属纳米团簇在光催化应用方面的研究进展[J]. 应用化学, 2022, 39(11): 1652-1664. |
| [15] | 叶祥志, 邓云水, 刘源, 周咏柳, 贺建雄, 熊春荣. 玻璃球负载非晶态有机钛聚合物提高光催化还原CO2的转换频率[J]. 应用化学, 2022, 39(10): 1554-1563. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||