应用化学 ›› 2023, Vol. 40 ›› Issue (2): 155-168.DOI: 10.19894/j.issn.1000-0518.220259
• 综合评述 • 下一篇
金属-有机框架MIL-88A(Fe)及其复合材料在水处理中的研究进展
- 华北理工大学化学工程学院,唐山 063210
-
收稿日期:2022-08-01接受日期:2022-11-06出版日期:2023-02-01发布日期:2023-02-27 -
通讯作者:葛明 -
基金资助:河北省自然科学基金(B2019209373)
Research Progress of Metal-organic Framework MIL-88A(Fe) and Its Composites in Water Treatment
Hua-Yu WANG, Chao ZHANG, Ke-Ming CHEN, Ming GE( )
)
- School of Chemical Engineering,North China University of Science and Technology,Tangshan 063210,China
-
Received:2022-08-01Accepted:2022-11-06Published:2023-02-01Online:2023-02-27 -
Contact:Ming GE -
About author:geminggena@163.com
-
Supported by:the Natural Science Foundation of Hebei Province(B2019209373)
摘要:
具有多重功能的金属-有机框架MIL-88A(Fe)作为一种新兴的材料在水处理领域具有一定的应用潜力。利用MIL-88A(Fe)独特的理化特性(如多孔结构、不饱和金属位点、优良的可见光吸收能力),将其和其它功能材料(如碳材料、无机半导体材料)异质复合,可以提升MIL-88A(Fe)的吸附及催化性能。详细综述了MIL-88A(Fe)及其复合材料作为吸附剂和催化剂在水处理中的应用,总结它们吸附去除污染物(尤其是重金属离子)的机制、介绍了它们作为光催化技术、类芬顿技术、过二硫酸盐高级氧化技术和催化臭氧技术的催化剂来降解水体中有机污染物的反应机理。指出基于MIL-88A(Fe)的功能材料处理水体污染存在适用pH范围窄和难回收利用等问题。未来研究需优化MIL-88A(Fe)的制备条件来提高产率和保证MIL-88A(Fe)的规整形貌、小尺寸和高结晶度,通过表面包裹技术改善MIL-88A(Fe)的稳定性以及赋予MIL-88A(Fe)磁性来提升回收利用性能。另外,需要根据目标有机污染物的结构和水质条件,合理调控基于MIL-88A(Fe)的高级氧化过程中自由基途径和非自由基途径对目标物的降解贡献,以期达到最佳去污效果。
中图分类号:
引用本文
王华宇, 张超, 陈柯铭, 葛明. 金属-有机框架MIL-88A(Fe)及其复合材料在水处理中的研究进展[J]. 应用化学, 2023, 40(2): 155-168.
Hua-Yu WANG, Chao ZHANG, Ke-Ming CHEN, Ming GE. Research Progress of Metal-organic Framework MIL-88A(Fe) and Its Composites in Water Treatment[J]. Chinese Journal of Applied Chemistry, 2023, 40(2): 155-168.
| Method | Synthesis process | Advantage | Disadvantage |
|---|---|---|---|
| Ultrasoundmethod[ | Through high-frequency vibration, the bubbles and bursts were rapidly generated in the mixed solution of FeCl3·6H2O and fumaric acid, and the temperature of the local solution was increased to synthesize MIL-88A(Fe)[ | The operation is simple and the time-consuming is relatively short[ | The product morphology is irregular and the yield is low[ |
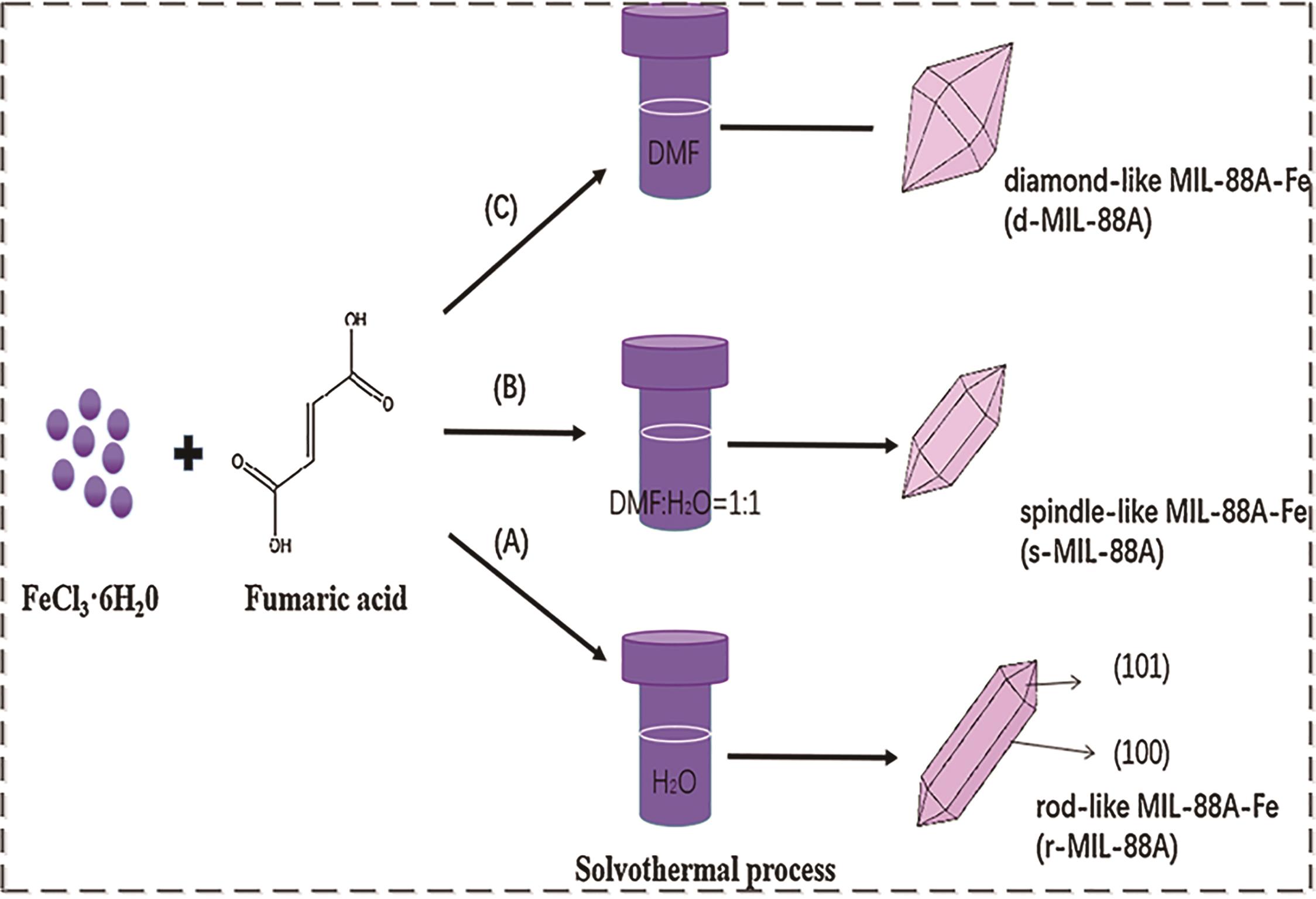
| Hydrothermal/solvothermal method[ | FeCl3·6H2O and fumaric acid were completely dissolved in water/DMF according to a certain proportion, and the mixed solution was put into an autoclave and heated at 65~105 ℃ for 12 h or longer[ | The operation is simple, the crystal morphology of MIL-88(Fe) is good, and the yield is high[ | High energy consumption and time-consuming |
| Microwave method[ | The energy is transferred to the reaction solution by microwave radiation, and the temperature of the reaction solution was increased after absorbing the energy, thereby generating MIL-88A(Fe) | The operation is simple and the time-consuming is short, and it is one of the convenient and quick methods for the preparation of MIL-88A(Fe) in large quantities[ | The crystallization rate is too fast to obtain a product with a regular morphology |
| Room temperature stirring method[ | The reaction solution was continuously stirred for a certain period of time at room temperature. During this process, FeCl3·6H2O and fumaric acid reacted slowly to form MIL-88A(Fe)[ | Simple operation and low energy consumption[ | It takes a long time, the product is difficult to separate out, and the yield is low |
| Mechanicalgrindingmethod[ | The reaction occured by the input of mechanical energy to the reactant, generally referring to the solid reaction. FeCl3·6HO and pure water were added into the mortar, grind to completely dissolve the metal salt, then adding fumaric acid and continue grinding to obtain MIL-88A(Fe) | No organic solvent is used, the yield is high and the product particle size is small[ | The product is less crystalline and may contain amorphous impurities |
表1 合成MIL-88A(Fe)的方法
Table 1 Method for synthesis of MIL-88A(Fe)
| Method | Synthesis process | Advantage | Disadvantage |
|---|---|---|---|
| Ultrasoundmethod[ | Through high-frequency vibration, the bubbles and bursts were rapidly generated in the mixed solution of FeCl3·6H2O and fumaric acid, and the temperature of the local solution was increased to synthesize MIL-88A(Fe)[ | The operation is simple and the time-consuming is relatively short[ | The product morphology is irregular and the yield is low[ |
| Hydrothermal/solvothermal method[ | FeCl3·6H2O and fumaric acid were completely dissolved in water/DMF according to a certain proportion, and the mixed solution was put into an autoclave and heated at 65~105 ℃ for 12 h or longer[ | The operation is simple, the crystal morphology of MIL-88(Fe) is good, and the yield is high[ | High energy consumption and time-consuming |
| Microwave method[ | The energy is transferred to the reaction solution by microwave radiation, and the temperature of the reaction solution was increased after absorbing the energy, thereby generating MIL-88A(Fe) | The operation is simple and the time-consuming is short, and it is one of the convenient and quick methods for the preparation of MIL-88A(Fe) in large quantities[ | The crystallization rate is too fast to obtain a product with a regular morphology |
| Room temperature stirring method[ | The reaction solution was continuously stirred for a certain period of time at room temperature. During this process, FeCl3·6H2O and fumaric acid reacted slowly to form MIL-88A(Fe)[ | Simple operation and low energy consumption[ | It takes a long time, the product is difficult to separate out, and the yield is low |
| Mechanicalgrindingmethod[ | The reaction occured by the input of mechanical energy to the reactant, generally referring to the solid reaction. FeCl3·6HO and pure water were added into the mortar, grind to completely dissolve the metal salt, then adding fumaric acid and continue grinding to obtain MIL-88A(Fe) | No organic solvent is used, the yield is high and the product particle size is small[ | The product is less crystalline and may contain amorphous impurities |
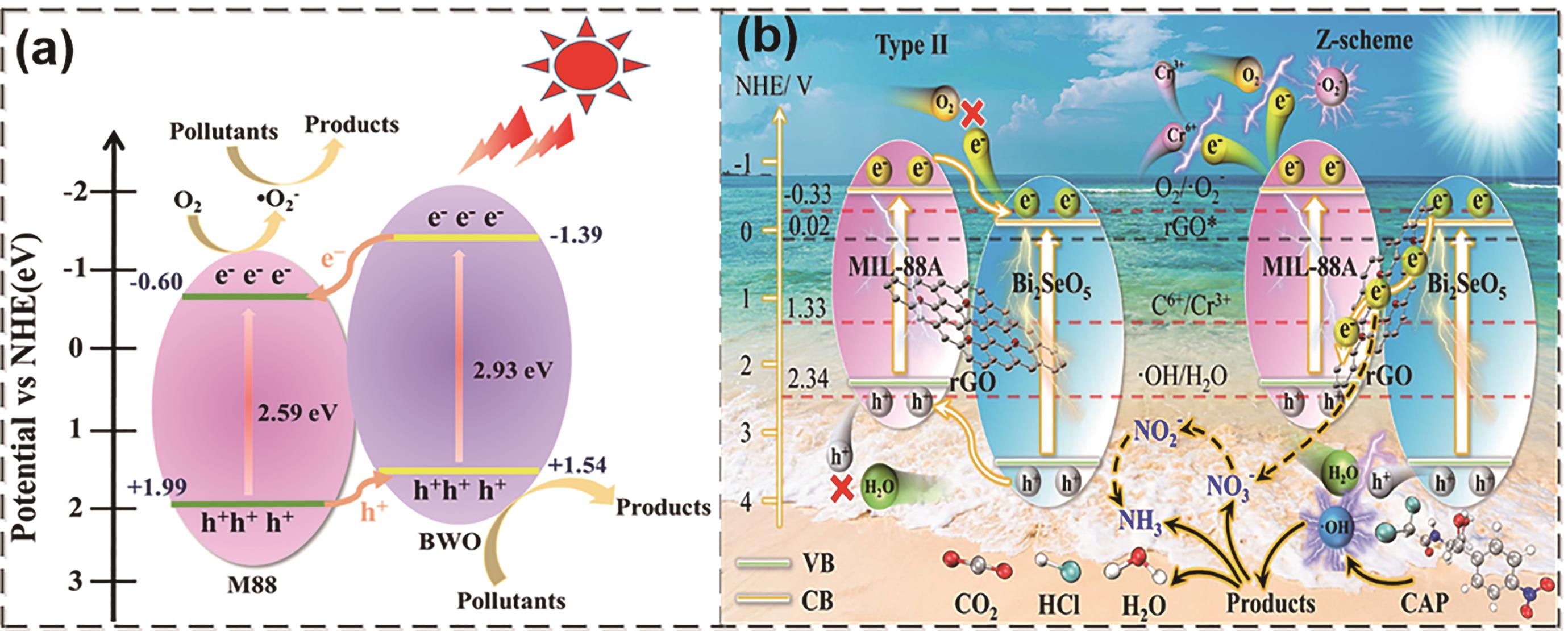
图1 (a) MIL-88A(Fe)/Bi2WO6 Ⅱ型异质结[16];(b) Z型Bi2SeO5/rGO/MIL-88A(Fe)光催化体系[17]
Fig.1 (a) Type II heterojunction of MIL-88A(Fe)/Bi2WO6[16]; (b) Z-type photocatalytic system of Bi2SeO5/rGO/MIL-88A(Fe)[17]
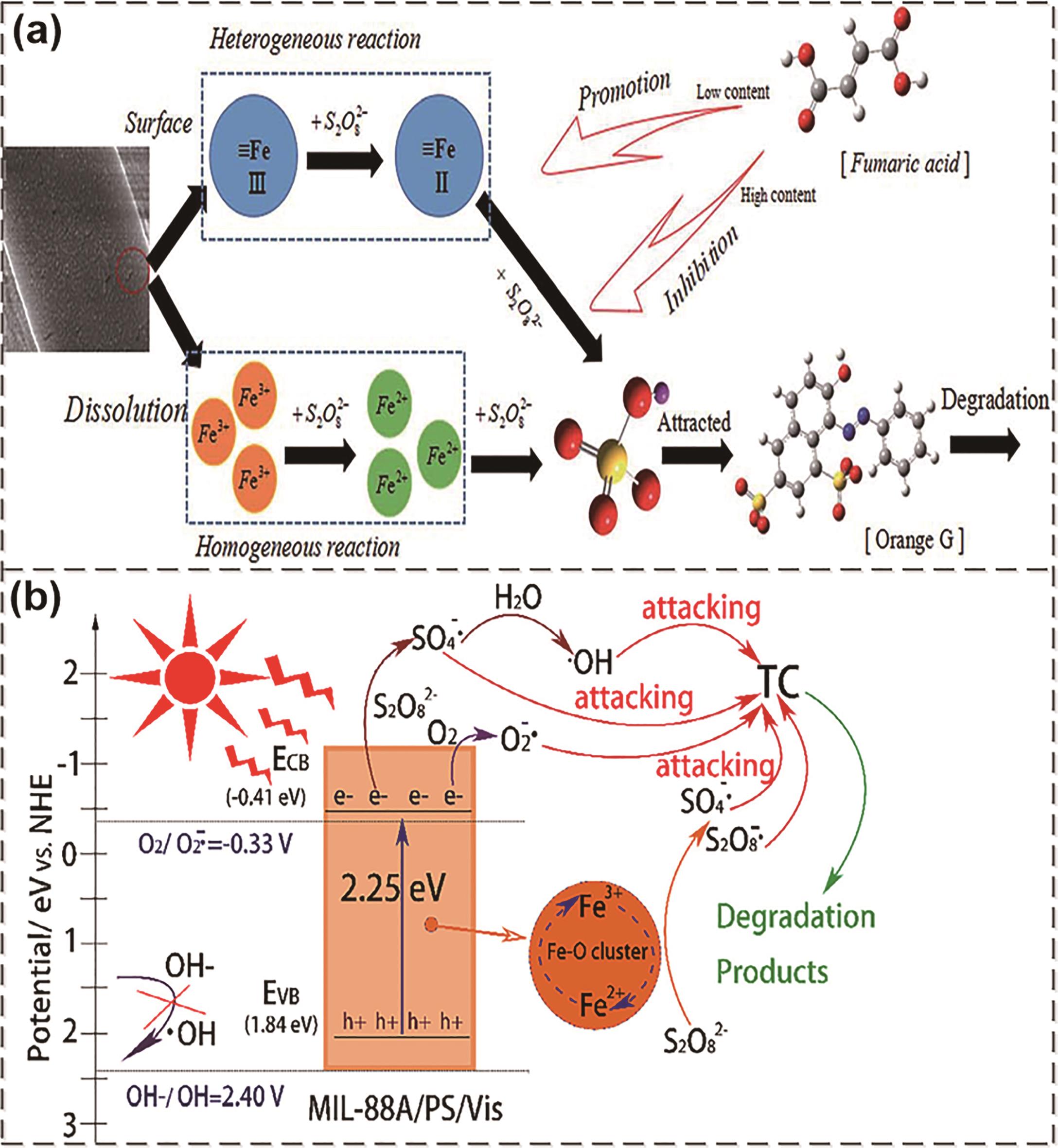
图3 (a) MIL-88A(Fe)活化PDS降解OG的机制[63];(b) 可见光协助MIL-88A(Fe)活化PDS去除水中TC的反应机理[65]
Fig.3 (a) The mechanism of MIL-88A(Fe)?-activated PDS to degrade OG[63]; (b) The reaction mechanism of visible light-assisted MIL-88A(Fe)-activated PDS to remove TC in water[65]
| Technology category | Advantage | Disadvantage |
|---|---|---|
| Adsorption | MIL-88A(Fe) has a larger specific surface area and pore size, and has an abnormal expansion of 85% in water, which expands its internal channels and pores, and bonds easily with heavy metal ions[ | MIL-88A(Fe) is not easy to desorb after adsorbing heavy metals, and the recycling rate is low |
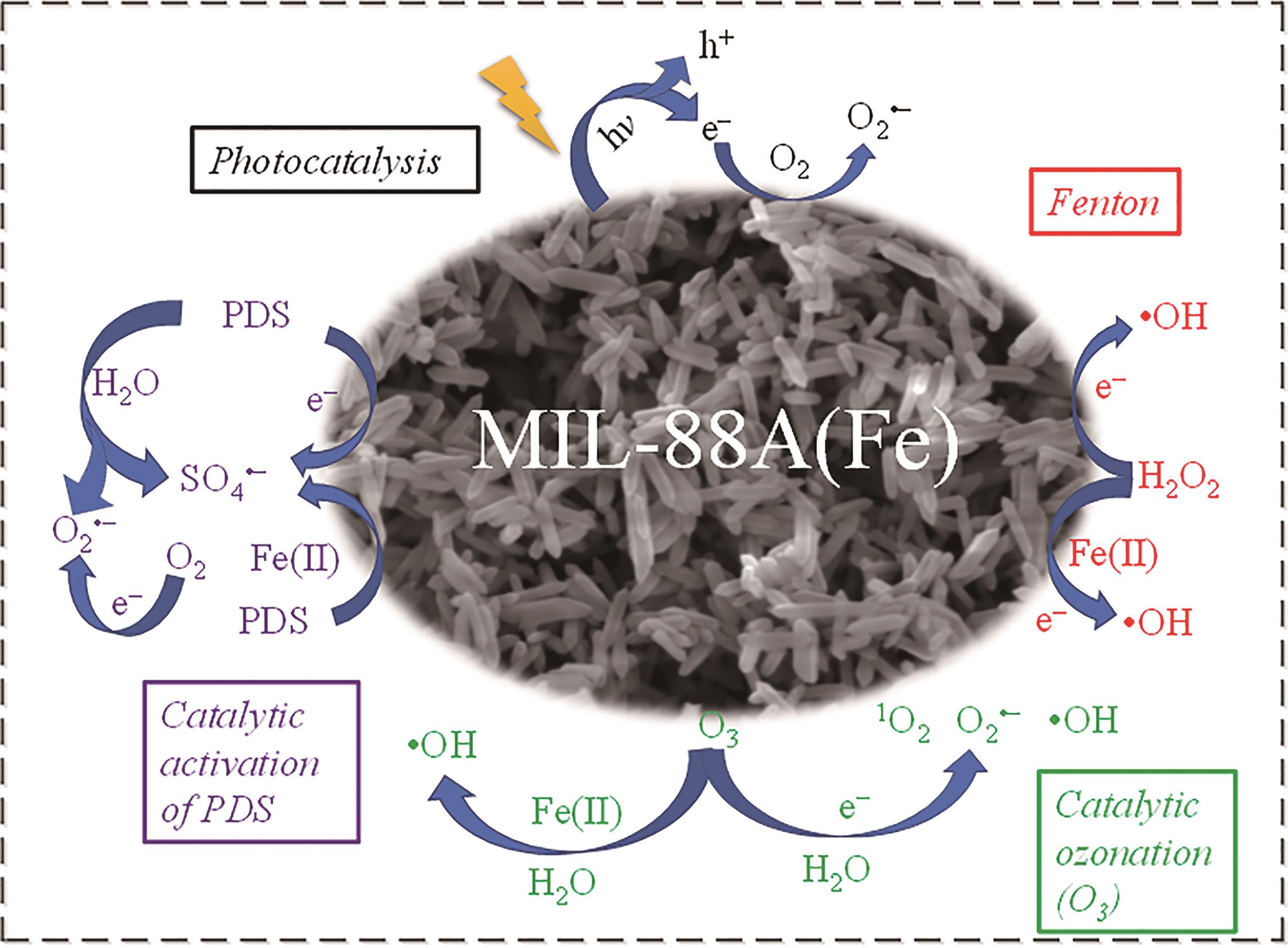
| Photocatalytic | MIL-88A(Fe) has a wide range of visible light response and can utilize solar energy; the regular morphology of MIL-88A(Fe) is easy to construct a high activity composite catalyst with other photocatalysts[ | High energy consumption for continuous light irradiation |
| Fenton-like | The exposed crystal face of MIL-88A(Fe) is easy to control, and the catalytic activation efficiency of H2O2 is efficient[ | The ?OH generated by catalytic activation of H2O2 can destroy the structure of MIL-88A(Fe), and at the same time Fe ions can beleached, which leads to a decrease in stability |
| PDS advanced oxidation | MIL-88A(Fe) has many reactive sites to catalyze activation of PDS[ | During the reaction, the rate of Fe(Ⅲ) reduction to Fe(Ⅱ) is slow, and a large amount of SO |
| Catalytic O3 oxidation | MIL-88A(Fe) has many Lewis acid sites, which can efficiently catalyze O3 to produce a variety of reactive species[ | O3 is not easy to transport and needs to be prepared on site, which increases the cost of equipment |
表2 基于MIL-88A(Fe)的水处理技术的优缺点对比
Table 2 Comparison of advantages and disadvantages of water treatment technology based on MIL-88A(Fe)
| Technology category | Advantage | Disadvantage |
|---|---|---|
| Adsorption | MIL-88A(Fe) has a larger specific surface area and pore size, and has an abnormal expansion of 85% in water, which expands its internal channels and pores, and bonds easily with heavy metal ions[ | MIL-88A(Fe) is not easy to desorb after adsorbing heavy metals, and the recycling rate is low |
| Photocatalytic | MIL-88A(Fe) has a wide range of visible light response and can utilize solar energy; the regular morphology of MIL-88A(Fe) is easy to construct a high activity composite catalyst with other photocatalysts[ | High energy consumption for continuous light irradiation |
| Fenton-like | The exposed crystal face of MIL-88A(Fe) is easy to control, and the catalytic activation efficiency of H2O2 is efficient[ | The ?OH generated by catalytic activation of H2O2 can destroy the structure of MIL-88A(Fe), and at the same time Fe ions can beleached, which leads to a decrease in stability |
| PDS advanced oxidation | MIL-88A(Fe) has many reactive sites to catalyze activation of PDS[ | During the reaction, the rate of Fe(Ⅲ) reduction to Fe(Ⅱ) is slow, and a large amount of SO |
| Catalytic O3 oxidation | MIL-88A(Fe) has many Lewis acid sites, which can efficiently catalyze O3 to produce a variety of reactive species[ | O3 is not easy to transport and needs to be prepared on site, which increases the cost of equipment |
| 1 | MOROZAN A, JAOUEN F. Metal organic frameworks for electrochemical applications[J]. Energy Environ Sci, 2012, 5(11): 9269-9290. |
| 2 | CHANG L M, LI Q H, WEIDLER P, et al. Surface-oriented assembly of cyclodextrin metal-organic framework film for enhanced peptide-enantiomers sensing[J]. CCS Chem, 2022, https://doi.org/10.31635/ccschem.022.202101708. |
| 3 | YU D, WU M, HU Q, et al. Iron-based metal-organic frameworks as novel platforms for catalytic ozonation of organic pollutant: efficiency and mechanism[J]. J Hazard Mater, 2019, 367: 456-464. |
| 4 | LIU Z, FANG A, HE J, et al. Association of habitually low intake of dietary calcium with blood pressure and hypertension in a population with predominantly plant-based diets[J]. Nutrients, 2018, 10(5): 603. |
| 5 | AMARO-GAHETE J, KLEE R, ESQUIVEL D, et al. Fast ultrasound-assisted synthesis of highly crystalline MIL-88A particles and their application as ethylene adsorbents[J]. Ultrason Sonochem, 2019, 50: 59-66. |
| 6 | LIAO X, WANG F, WANG F, et al. Synthesis of (100) surface oriented MIL-88A-Fe with rod-like structure and its enhanced fenton-like performance for phenol removal[J]. Appl Catal B: Environ, 2019, 259: 118064. |
| 7 | CHALATI T, HORCAJADA P, GREF R, et al. Optimisation of the synthesis of MOF nanoparticles made of flexible porous iron fumarate MIL-88A[J]. J Mater Chem, 2011, 21: 2220-2227. |
| 8 | FU H, SONG X X, WU L, et al. Room-temperature preparation of MIL-88A as a heterogeneous photo-fenton catalyst for degradation of rhodamine B and bisphenol a under visible light[J]. Mater Res Bull, 2020, 125: 110806. |
| 9 | YI X H, JI H D, WANG C C, et al. Photocatalysis-activated SR-AOP over PDINH/MIL-88A(Fe) composites for boosted chloroquine phosphate degradation: performance, mechanism, pathway and DFT calculations[J]. Appl Catal B: Environ, 2021, 293: 120229. |
| 10 | YANG J C, YIN X B. CoFe2O4@MIL-100(Fe) hybrid magnetic nanoparticles exhibit fast and selective adsorption of arsenic with high adsorption capacity[J]. Sci Rep, 2017, 7: 40955. |
| 11 | ZHENG S Y, KONG Z, MENG L J, et al. MIL-88A grown in-situ on graphitic carbon nitride (g-C3N4) as a novel sorbent: synthesis, characterization, and high-performance of tetracycline removal and mechanism[J]. Adv Powder Technol, 2020, 31(10): 4344-4353. |
| 12 | XUE B, DU L, JIN J, et al. In situ growth of MIL-88A into polyacrylate and its application in highly efficient photocatalytic degradation of organic pollutants in water[J]. Appl Surf Sci, 2021, 564: 150404. |
| 13 | REN G L, ZHAO K, ZHAO L, et al. A fenton-like method using ZnO doped MIL-88A for degradation of methylene blue dyes[J]. RSC Adv, 2020, 10(66): 39973-39980. |
| 14 | LIN K Y A, CHANG H A, HSU C J, et al. Iron-based metal organic framework, MIL-88A, as a heterogeneous persulfate catalyst for decolorization of rhodamine B in water[J]. RSC Adv, 2015, 5(41): 32520-32530. |
| 15 | YU D, LI L, WU M, et al. Enhanced photocatalytic ozonation of organic pollutants using an iron-based metal-organic framework[J]. Appl Catal, B, 2019, 251: 66-75. |
| 16 | LI Q W, LI L M, LONG X Y, et al. Novel visible-light-driven CQDs/Bi2WO6 hybrid materials with enhanced photocatalytic activity toward organic pollutants degradation and mechanism insight[J]. Opt Mater, 2021, 118: 111260. |
| 17 | LIU C, WANG P, QIAO Y, et al. Self-assembled Bi2SeO5/rGO/MIL-88A Z-scheme heterojunction boosting carrier separation for simultaneous removal of Cr(Ⅵ) and chloramphenicol[J]. Chem Eng J, 2022, 431: 133289. |
| 18 | LIU N, HUANG W Y, ZHANG X D, et al. Ultrathin graphene oxide encapsulated in uniform MIL-88A(Fe) for enhanced visible light-driven photodegradation of RhB[J]. Appl Catal B: Environ, 2018, 221: 119-128. |
| 19 | JUNG B K, JUN J W, HASAN Z, et al. Adsorptive removal of p-arsanilic acid from water using mesoporous zeolitic imidazolate framework-8[J]. Chem Eng J, 2015, 267: 9-15. |
| 20 | WU H, MA M D, GAI W Z, et al. Arsenic removal from water by metal-organic framework MIL-88A microrods[J]. Environ Sci Pollut Res, 2018, 25(27): 27196-27202. |
| 21 | PANG D, WANG C C, WANG P, et al. Superior removal of inorganic and organic arsenic pollutants from water with MIL-88A(Fe) decorated on cotton fibers[J]. Chemosphere, 2020, 254: 126829. |
| 22 | WANG C, LUAN J, WU C, et al. Metal-organic frameworks for aquatic arsenic removal[J]. Water Res, 2019, 158: 370-382. |
| 23 | LI J, WANG X X, ZHAO G X, et al. Metal-organic framework-based materials: superior adsorbents for the capture of toxic and radioactive metal ions[J]. Chem Soc Rev, 2018, 47(7): 2322-2356. |
| 24 | WEN J, FANG Y, ZENG G M, et al. Progress and prospect of adsorptive removal of heavy metal ions from aqueous solution using metal-organic frameworks: a review of studies from the last decade[J]. Chemosphere, 2018, 201: 627-643. |
| 25 | RAMSAHYE N A, TRUNG T K, SCOTT L, et al. Impact of the flexible character of MIL-88 iron(Ⅲ) dicarboxylates on the adsorption of n-alkanes [J]. Chem Mater, 2013, 25(3): 479-488. |
| 26 | SONG X X, FU H, WANG P, et al. The selectively fluorescent sensing detection and adsorptive removal of Pb2+ with a stable [delta-Mo8O26]-based hybrid[J]. J Colloid Interface Sci, 2018, 532: 598-604. |
| 27 | LI J, WANG H, YUAN X Z, et al. Metal-organic framework membranes for wastewater treatment and water regeneration[J]. Coor Chem Rev, 2020, 404: 213116. |
| 28 | LUO F, CHEN J L, DANG L L, et al. High-performance Hg2+ removal from ultra-low-concentration aqueous solution using both acylamide- and hydroxyl-functionalized metal-organic framework[J]. J Mater Chem A, 2015, 3(18): 9616-9620. |
| 29 | ZHENG S Y, HUANG M H, SUN S M, et al. Synergistic effect of MIL-88A/g-C3N4 and MoS2 to construct a self-cleaning multifunctional electrospun membrane[J]. Chem Eng J, 2021, 421: 129621. |
| 30 | LI M H, LIU Y B, SHEN C S, et al. One-step Sb(Ⅲ) decontamination using a bifunctional photoelectrochemical filter[J]. J Hazard Mater, 2020, 389: 121840. |
| 31 | MADHUMITA M, SUJIT S. Advanced oxidation process: a sustainable technology for treating refractory organic compounds present in industrial wastewater[J]. Environ Sci Pollut Res, 2022,https:∥doi.org/10.1007/s11356-022-19435-0. |
| 32 | WANG C C, YI X H, WANG P, et al. Powerful combination of MOFs and C3N4 for enhanced photocatalytic performance[J]. Appl Catal B: Environ, 2019, 247: 24-48. |
| 33 | LIU N, HUANG W, ZHANG X, et al. Ultrathin graphene oxide encapsulated in uniform MIL-88A(Fe) for enhanced visible light-driven photodegradation of RhB[J]. Appl Catal B: Environ, 2018, 221: 119-128. |
| 34 | HUANG W, JING C, ZHANG X, et al. Integration of plasmonic effect into spindle-shaped MIL-88A(Fe): steering charge flow for enhanced visible-light photocatalytic degradation of ibuprofen[J]. Chem Eng J, 2018, 349: 603-612. |
| 35 | DI J, XIA J, GE Y, et al. Novel visible-light-driven CQDs/Bi2WO6 hybrid materials with enhanced photocatalytic activity toward organic pollutants degradation and mechanism insight[J]. Appl Catal B: Environ, 2015, 168-169: 51-61. |
| 36 | SARAVANAKUMAR K, PARK C M. Rational design of a novel LaFeO3/g-C3N4/BiFeO3 double Z-scheme structure: photocatalytic performance for antibiotic degradation and mechanistic insight[J]. Chem Eng J, 2021, 423: 130076. |
| 37 | ZHUANG S, XU X, FENG B, et al. Photogenerated carriers transfer in dye-graphene-SnO2 composites for highly efficient visible-light photocatalysis[J]. ACS Appl Mater Interfaces, 2014, 6(1): 613-621. |
| 38 | CHEN H, LIU Y, CAI T, et al. Boosting photocatalytic performance in mixed-valence MIL-53(Fe) by changing FeII/FeIII ratio[J]. ACS Appl Mater Interfaces, 2019, 11(32): 28791-28800. |
| 39 | YANG Z, XIA X, SHAO L, et al. Efficient photocatalytic degradation of tetracycline under visible light by Z-scheme Ag3PO4/mixed-valence MIL-88A(Fe) heterojunctions: mechanism insight, degradation pathways and DFT calculation[J]. Chem Eng J, 2021, 410: 128454. |
| 40 | YUAN R, QIU J, YUE C, et al. Self-assembled hierarchical and bifunctional MIL-88A(Fe)@ZnIn2S4 heterostructure as a reusable sunlight-driven photocatalyst for highly efficient water purification[J]. Chem Eng J, 2020, 401: 126020. |
| 41 | KHASEVANI S G, GHOLAMI M R. Evaluation of the reaction mechanism for photocatalytic degradation of organic pollutants with MIL-88A/BiOI structure under visible light irradiation[J]. Res Chem Intermed, 2019, 45(3): 1341-1356. |
| 42 | SHAO Z, ZHANG D, LI H, et al. Fabrication of MIL-88A/g-C3N4 direct Z-scheme heterojunction with enhanced visible-light photocatalytic activity[J]. Sep Purif Technol, 2019, 220: 16-24. |
| 43 | TAN C E, SU E C, WEY M Y. Development of physicochemically stable Z-scheme MIL-88A/g-C3N4 heterojunction photocatalyst with excellent charge transfer for improving acid red 1 dye decomposition efficiency[J]. Appl Surf Sci, 2022, 590: 152954. |
| 44 | CHEN Q, LI J, CHENG L, et al. Construction of CdLa2S4/MIL-88A(Fe) heterojunctions for enhanced photocatalytic H2-evolution activity via a direct Z-scheme electron transfer[J]. Chem Eng J, 2020, 379: 122389. |
| 45 | TANG J, YU X, ZHOU R, et al. Plasmonic coated spindle-shaped MIL-88A(Fe) ternary composites heterojunction for photocatalytic degradation of tetracycline: mechanism studies and theoretical calculation[J]. Appl Surf Sci, 2022, 603: 154429. |
| 46 | GU J B, LI Q W, LONG X Y, et al. Fabrication of magnetic dual Z-scheme heterojunction materials for efficient photocatalytic performance: the study of ternary novel MIL-88A(Fe)/BiOBr/SrFe12O19 nanocomposite[J]. Sep Purif Technol, 2022, 289: 120778. |
| 47 | GAO C, CHEN S, QUAN X, et al. Enhanced Fenton-like catalysis by iron-based metal organic frameworks for degradation of organic pollutants[J]. J Catal, 2017, 356: 125-132. |
| 48 | TAO S, YANG J, HOU H, et al. Enhanced sludge dewatering via homogeneous and heterogeneous Fenton reactions initiated by Fe-rich biochar derived from sludge[J]. Chem Eng J, 2019, 372: 966-977. |
| 49 | SUDARSANAM P, ZHONG R Y, VAN DEN BOSCH S, et al. Functionalised heterogeneous catalysts for sustainable biomass valorisation[J]. Chem Soc Rev, 2018, 47(22): 8349-8402. |
| 50 | YAO Y, CHEN H, LIAN C, et al. Fe, Co, Ni nanocrystals encapsulated in nitrogen-doped carbon nanotubes as Fenton-like catalysts for organic pollutant removal[J]. J Hazard Mater, 2016, 314: 129-139. |
| 51 | WANG J F, LIU Y, SHAO P, et al. Efficient ofloxacin degradation via photo-Fenton process over eco-friendly MIL-88A(Fe): performance, degradation pathways, intermediate library establishment and toxicity evaluation[J]. Environ Res J, 2022, 210: 112937. |
| 52 | XU W T, MA L, KE F, et al. Metal-organic frameworks MIL-88A hexagonal microrods as a new photocatalyst for efficient decolorization of methylene blue dye[J]. Dalton Trans, 2014, 43(9): 3792-3798. |
| 53 | LIU J, LI X, LIU B, et al. Shape-controlled synthesis of metal-organic frameworks with adjustable Fenton-like catalytic activity[J]. ACS Appl Mater Interfaces, 2018, 10(44): 38051-38056. |
| 54 | ZHANG R, DU B, LI Q, et al. α-Fe2O3 nanoclusters confined into UiO-66 for efficient visible-light photodegradation performance[J]. Appl Surf Sci, 2019, 466: 956-963. |
| 55 | CHEN D D, YI X H, LING L, et al. Photocatalytic Cr(Ⅵ) sequestration and photo-Fenton bisphenol A decomposition over white light responsive PANI/MIL-88A(Fe)[J]. Appl Organomet Chem, 2020, 34(9): e5795. |
| 56 | KUZHAROV A, GRITSAI M,BUTOVA V, et al. One-step electrochemical synthesis of γ-Fe2O3@MIL-88a magnetic composite for heterogeneous Fenton-like catalysis[J]. Ceram Int, 2022, https://doi.org/10.1016/j.ceramint.2022.08.076. |
| 57 | SHI K X, QIU F G, WANG P, et al. Magnetic MgFe2O4/MIL-88A catalyst for photo-Fenton sulfamethoxazole decomposition under visible light[J]. Sep Purif Technol, 2022, 301: 121965. |
| 58 | 黄智辉, 纪志永, 陈希, 等. 过硫酸盐高级氧化降解水体中有机污染物研究进展[J]. 化工进展, 2019, 38(5): 2461-2470. |
| HUANG Z H, JI Z Y, CHEN X, et al. Degradation of organic pollutants in water by persulfate advanced oxidation[J]. Chem Ind Eng Prog, 2019, 38(5): 2461-2470. | |
| 59 | WANG J L, WANG S Z. Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants[J]. Chem Eng J, 2018, 334: 1502-1517. |
| 60 | SUBRAMANIAN G, PARAKH P, PRAKASH H, et al. Photodegradation of methyl orange and photoinactivation of bacteria by visible light activation of persulphate using a tris(2,2'-bipyridyl) ruthenium(Ⅱ) complex[J]. Photochem Photobiol Sci, 2013, 12(3): 456-466. |
| 61 | ANTONIOU M G, DE LA CRUZ A A, DIONYSIOU D D, et al. Degradation of microcystin-LR using sulfate radicals generated through photolysis, thermolysis and e-transfer mechanisms[J]. Appl Catal B: Environ, 2010, 96(3): 290-298. |
| 62 | BOUGIE S, DUBE J S. Oxidation of dichlorobenzene isomers with the help of thermally activated sodium persulfate[J]. J Environ Eng Sci, 2007, 6(4): 397-407. |
| 63 | WANG J M, WAN J Q, MA Y W, et al. Metal-organic frameworks MIL-88A with suitable synthesis conditions and optimal dosage for effective catalytic degradation of orange G through persulfate activation[J]. RSC Adv, 2016, 6(113): 112502-112511. |
| 64 | YUAN S, FENG L, WANG K C, et al. Stable metal-organic frameworks: design, synthesis, and applications[J]. Adv Mater, 2018, 30(37): 1704303. |
| 65 | ZHANG Y, ZHOU J B, CHEN X, et al. Coupling of heterogeneous advanced oxidation processes and photocatalysis in efficient degradation of tetracycline hydrochloride by Fe-based MOFs: synergistic effect and degradation pathway[J]. Chem Eng J, 2019, 369: 745-757. |
| 66 | WANG J S, YI X H, XU X, et al. Eliminating tetracycline antibiotics matrix via photoactivated sulfate radical-based advanced oxidation process over the immobilized MIL-88A: batch and continuous experiments[J]. Chem Eng J, 2022, 431: 133213. |
| 67 | CHEN T M, XU C C, ZOU C, et al. Self-assembly of PDINH/TiO2/Bi2WO6 nanocomposites for improved photocatalytic activity based on a rapid electron transfer channel[J]. Appl Surf Sci, 2022, 584: 152667. |
| 68 | 王九妹, 关泽宇, 万金泉, 等. MIL-88A@MIP催化活化过硫酸盐靶向降解邻苯二甲酸二丁酯[J]. 环境科学, 2017, 38(12): 5124-5131. |
| WANG J M, GUAN Z Y, WAN J Q, et al. MIL-88A@MIP activated persulfate for targeted degradation of dibutyl phthalate[J]. Environ Sci, 2017, 38(12): 5124-5131. | |
| 69 | ZHANG X W, WANG F, WANG C C, et al. Photocatalysis activation of peroxodisulfate over the supported Fe3O4 catalyst derived from MIL-88A(Fe) for efficient tetracycline hydrochloride degradation[J]. Chem Eng J, 2021, 426: 131927. |
| 70 | YANG T T, PENG J M, ZHENG Y, et al. Enhanced photocatalytic ozonation degradation of organic pollutants by ZnO modified TiO2 nanocomposites[J]. Appl Catal B: Environ, 2018, 221: 223-234. |
| 71 | LECLERC H, VIONT A, LAVALLEY J C, et al. Infrared study of the influence of reducible iron(Ⅲ) metal sites on the adsorption of CO, CO2, propane, propene and propyne in the mesoporous metal-organic framework MIL-100[J]. Phys Chem Chem Phys, 2011, 13(24): 11748-11756. |
| 72 | TANG J, WANG J. Metal organic framework with coordinatively unsaturated sites as efficient Fenton-like catalyst for enhanced degradation of sulfamethazine[J]. Environ Sci Technol, 2018, 52(9): 5367-5377. |
| 73 | BING J, HU C, NIE Y, et al. Mechanism of catalytic ozonation in Fe2O3/Al2O3@SBA-15 aqueous suspension for destruction of ibuprofen[J]. Environ Sci Technol, 2015, 49(3): 1690-1697. |
| [1] | 元宁, 马洁, 张晋玲, 张建胜. 蒸气辅助合成PCN-6(M)双金属有机框架材料及其CH4和CO2吸附性能[J]. 应用化学, 2023, 40(6): 896-903. |
| [2] | 雷学博, 刘慧景, 丁赫宇, 申国栋, 孙润军. 用于降解印染废水中有机污染物的光催化剂的研究进展[J]. 应用化学, 2023, 40(5): 681-696. |
| [3] | 兰晓琳, 郑红星, 张依帆, 赵振, 肖和业, 王志江, 邓鹏飏. 常压高温固相反应制备SiC陶瓷粉体的研究进展[J]. 应用化学, 2023, 40(4): 476-485. |
| [4] | 许祥民, 邓杰, 杜雨琪, 沈红亮, 安泽坤, 孙才英. 螺环磷酰咪唑阻燃棉织物的热解挥发物分析及热解机理推测[J]. 应用化学, 2023, 40(3): 380-388. |
| [5] | 张琴, 刘文彬, 樊利娇, 谢宇铭, 黄国林. 功能化介孔二氧化硅的制备及其吸附分离水中铀的研究进展[J]. 应用化学, 2023, 40(2): 169-187. |
| [6] | 王兵, 唐敏, 王颖, 刘志光. 微氧化烧结制备掺杂Y2O3的SiC陶瓷及含镉模拟废水处理[J]. 应用化学, 2022, 39(8): 1312-1318. |
| [7] | 邵姗, 张剑, 邓凯强, 杨杰, 杨绍明. 镍钴双金属-卟啉有机框架复合纳米材料构建的无酶传感器检测多巴胺[J]. 应用化学, 2022, 39(7): 1098-1107. |
| [8] | 宋林虎, 李世友, 王洁, 张晶晶, 张宁霜, 赵冬妮, 徐菲. 锂离子电池电解液除酸除水添加剂的研究进展[J]. 应用化学, 2022, 39(5): 697-706. |
| [9] | 王雪, 王意波, 王显, 祝建兵, 葛君杰, 刘长鹏, 邢巍. 酸性电解水过程中氧析出反应的机理及铱基催化剂的研究进展[J]. 应用化学, 2022, 39(4): 616-628. |
| [10] | 杜慧, 姚晨阳, 彭皓, 姜波, 李顺祥, 姚俊烈, 郑方, 杨方, 吴爱国. 过渡金属掺杂磁性纳米粒子在生物医学领域中的研究进展[J]. 应用化学, 2022, 39(3): 391-406. |
| [11] | 黄小梅, 邓祥, 邢浪漫, 陈伟, 孙莉, 朱晓玉. Cu(Ⅱ)Co(Ⅱ)双金属碳纳米片用于无酶葡萄糖传感器[J]. 应用化学, 2022, 39(12): 1891-1902. |
| [12] | 周玉凤, 周川巍, 胡桐泽, 段展鹏, 王颢潼, 石淑云. Fe/V-Sb2O3复合材料的构筑及光催化降解医药废水[J]. 应用化学, 2022, 39(10): 1572-1578. |
| [13] | 赵莹, 邵奕嘉, 李罗钱, 任建伟, 廖世军. 富锂正极材料的衰减机理及循环稳定性提升的研究进展[J]. 应用化学, 2022, 39(02): 205-222. |
| [14] | 赵星鹏, 王娅乔, 高生旺, 朱建超王国英, 夏训峰, 王洪良, 王书平. BiOBr/CeO2复合材料的制备及光催化降解磺胺异恶唑[J]. 应用化学, 2021, 38(4): 422-430. |
| [15] | 魏雪莹, 吴玮, 乃永宁, 姜梦圆, 田诗伟, 毛国梁. 膦胺铬和双膦铬催化乙烯选择性齐聚研究进展[J]. 应用化学, 2021, 38(2): 136-156. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||