
应用化学 ›› 2022, Vol. 39 ›› Issue (4): 513-527.DOI: 10.19894/j.issn.1000-0518.210331
单原子催化剂在锂硫电池中的研究进展
- 吉林大学物理学院,新型电池物理与技术教育部重点实验室,长春 130012
-
收稿日期:2021-07-08接受日期:2021-10-19出版日期:2022-04-01发布日期:2022-04-19 -
通讯作者:张冬,杜菲 -
作者简介:E-mail: dufei@jlu.edu.cn
E-mail: dongzhang@jlu.edu.cn;
-
基金资助:国家自然科学基金(21771086);吉林省教育厅“十三五”科学研究规划项目(JJKH20211034KJ)
Recent Progress of Single⁃Atom Catalytic Materials for Lithium⁃Sulfur Batteries
WANG-Xin, ZHANG-Dong( ), DU-Fei(
), DU-Fei( )
)
- Key Laboratory of Physics and Technology for Advanced Batteries,Ministry of Education,College of Physics,Jilin University,Changchun 130012,China
-
Received:2021-07-08Accepted:2021-10-19Published:2022-04-01Online:2022-04-19 -
Contact:ZHANG-Dong, DU-Fei -
Supported by:the National Natural Science Foundation of China(21771086);Jilin Provincial Department of Education “13th Five-Year” Scientific Research Project(JJKH20211034KJ)
摘要:
锂硫电池因其较高的理论比容量和能量密度而成为最有前途的下一代储能系统之一。然而,硫和放电产物硫化锂的低导电率、可溶性多硫化锂(LiPSs)的穿梭以及缓慢的反应动力学致使锂硫电池的循环寿命短、倍率性能低。近年来,研究表明具有强催化活性的单原子(SAs)是理想的LiPSs锚定中心和催化位点。用SAs修饰正极和隔膜有助于吸附多硫化物并催化其转化,修饰负极则可显著提高锂的剥离/沉积效率,抑制锂枝晶的生长。本文综述了SAs在锂硫电池中的研究进展,包括材料合成、表征方法以及应用方向。最后,对SAs应用在电池中所面临的挑战和未来发展方向进行总结。
中图分类号:
引用本文
王欣, 张冬, 杜菲. 单原子催化剂在锂硫电池中的研究进展[J]. 应用化学, 2022, 39(4): 513-527.
WANG-Xin, ZHANG-Dong, DU-Fei. Recent Progress of Single⁃Atom Catalytic Materials for Lithium⁃Sulfur Batteries[J]. Chinese Journal of Applied Chemistry, 2022, 39(4): 513-527.
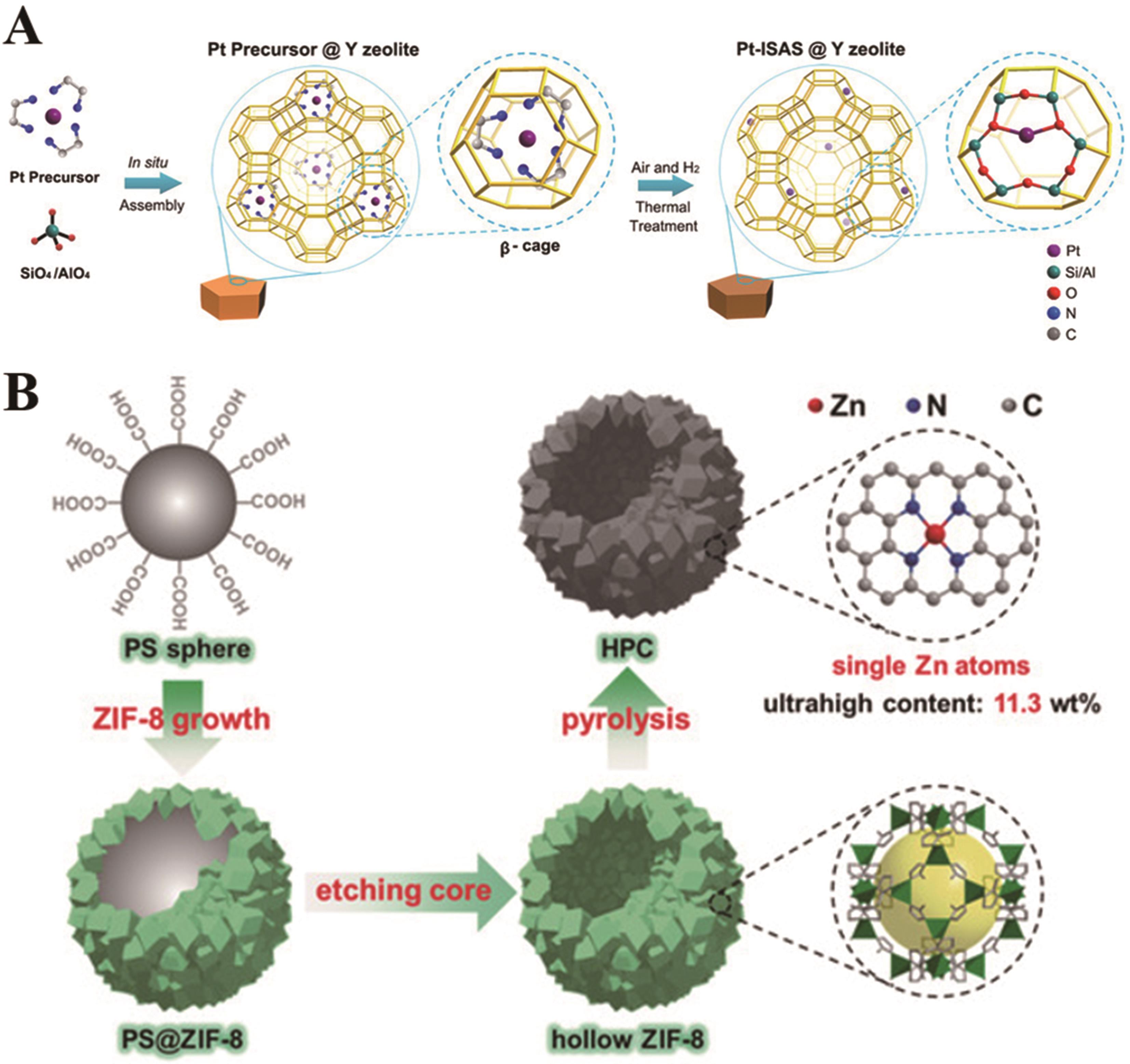
图1 (A)热处理后在β?分子筛中原位分离和限制铂前驱体的示意图[41];(B)HPC的制造过程示意图[42]
Fig.1 (A) Schematic illustration of the in situ separation and confinement of a platinum precursor in a β-cage followed by thermal treatment[41]; (B) Schematic illustration of the fabrication process of HPC[42]
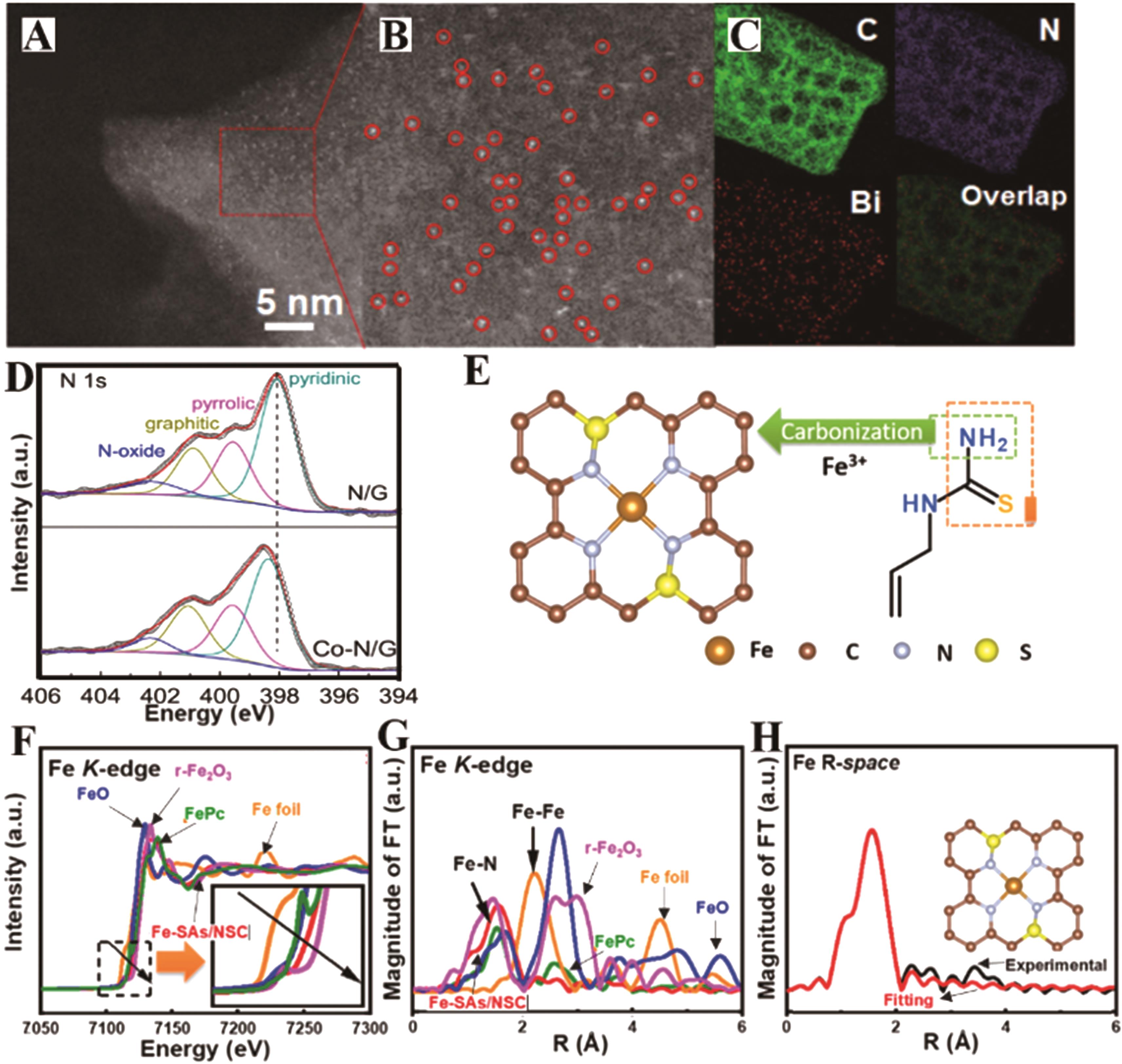
图2 BiSAs/NC的(A-B)HAADF-STEM图像和(C)BiSAs/NC的能量色散X射线能谱(EDS)元素映射图[43];(D)Co N/G和N/G的高分辨率XPS N 1s谱[44];(E)Fe-SAs/NSC的结构示意图[45]。 Fe-SAs/NSC和参考材料在Fe K边的(F)归一化XANES谱和(G)k3加权傅里叶变EXAFS谱[45];(H) Fe-SAs/NSC的EXAFS实验数据与拟合曲线[45]
Fig.2 (A, B) Magnified HAADF-STEM images and (C) energy dispersive X-ray spectroscopy (EDS) elemental mapping results of BiSAs/NC, with C (green), N (blue), and Bi (red)[43]; (D) high-resolution XPS N 1s spectra of Co-N/G and N/G [44]; (E) Schematic illustration of the formation of Fe-SAs/NSC[45]; the normalized XANES spectra and (F) the k3-weighted Fourier transform of EXAFS spectra at Fe K-edge of Fe-SAs/NSC and the reference materials[45]; (G) EXAFS curves between the experimental data and the fit of Fe-SAs/NSC[45]

图3 (A) (1)多硫化物溶液被(2)PNC和(3)Fe-PNC吸附后颜色变化的照片[60];(B)硫在N-C和Fe-N4-C的还原能量分布[61]。Li2S在(C)Fe-N4-C和(D)N-C上分解势垒,绿色、黄色、银色和棕色的球分别代表Li、S、N和Fe原子[61];(E)在0.5 mol/L Li2S6电解液中,Fe-N/MHCS对称电池的CV曲线[61];(F)Li2S/SACo复合材料的合成示意图[62]
Fig.3 (A) photograph showing the variation in color of (1) the polysulfide solution after adsorption by (2) PNC and (3) Fe-PNC [60]; (B) Energy profiles for the sulfur reduction on N-C and Fe-N4-C substrates (the inset in shows the optimized adsorption configurations)[61]; Energy profiles for the dissociation of the Li2S cluster on (C) N-C and (D) Fe-N4-C, The green, yellow, silver and brown balls represent Li, S, N and Fe atoms, respectively[61]; (E) CV curves of Fe-N/MHCS symmetric cells in 0.5 mol/L Li2S6 electrolyte at a scan rate of 1 mV/s[61]; (F) Schematic synthesis illustration of the converted-Li2S nanocomposite with SACo catalyst[62]
材料 Material | 硫负载量 Sulphur load/ (mg·cm-2) | 倍率性能 Rate capability/ (mA?h·g-1) | 电流/循环圈数/容量保持率 Current/number of cycles/ capacity retention rate | 参考文献 Ref. |
|---|---|---|---|---|
| Co-N4@2D/3D carbon | 1 | 1171 (0.2 C)/695(5 C) | 1 C/500/73.5% | [ |
| Co?PCNF | 1.7 | 1373.5 (0.2C)/914.3 (2C) | 0.5 C/100/95.5% | [ |
| Mn/C?(N, O) | 1.1 | ~1100 (0.2 C)/~500 (4 C) | 1 C/1000/50% | [ |
| FeNSC | 1 | 1193 (0.05 C)/550.2 (4 C) | 1 C/1000/53% | [ |
| Fe?N/MHCS | 2 | 1110 (0.2 C)/949 (2 C) | 1 C/1000/81.3% | [ |
| ZnS and Co-N-C DEB sites | 9 | 0.6 C/6.5 mA·h/cm2 | 0.6 C/100/86.7% | [ |
| CoSA?N?C@S | ||||
| FeSA?CN | 2.4 | 1123 (0.05 C)/605 (4 C) | [ | |
| S?SAV@NG | 2 | 1230 (0.2 C)/645 (3 C) | 0.5 C/400/70.64% | [ |
表1 单原子材料修饰锂硫电池正极的电化学性能总结
Table 1 The electrochemical performance of Li?S batteries using SAs as sulfur hosts
材料 Material | 硫负载量 Sulphur load/ (mg·cm-2) | 倍率性能 Rate capability/ (mA?h·g-1) | 电流/循环圈数/容量保持率 Current/number of cycles/ capacity retention rate | 参考文献 Ref. |
|---|---|---|---|---|
| Co-N4@2D/3D carbon | 1 | 1171 (0.2 C)/695(5 C) | 1 C/500/73.5% | [ |
| Co?PCNF | 1.7 | 1373.5 (0.2C)/914.3 (2C) | 0.5 C/100/95.5% | [ |
| Mn/C?(N, O) | 1.1 | ~1100 (0.2 C)/~500 (4 C) | 1 C/1000/50% | [ |
| FeNSC | 1 | 1193 (0.05 C)/550.2 (4 C) | 1 C/1000/53% | [ |
| Fe?N/MHCS | 2 | 1110 (0.2 C)/949 (2 C) | 1 C/1000/81.3% | [ |
| ZnS and Co-N-C DEB sites | 9 | 0.6 C/6.5 mA·h/cm2 | 0.6 C/100/86.7% | [ |
| CoSA?N?C@S | ||||
| FeSA?CN | 2.4 | 1123 (0.05 C)/605 (4 C) | [ | |
| S?SAV@NG | 2 | 1230 (0.2 C)/645 (3 C) | 0.5 C/400/70.64% | [ |
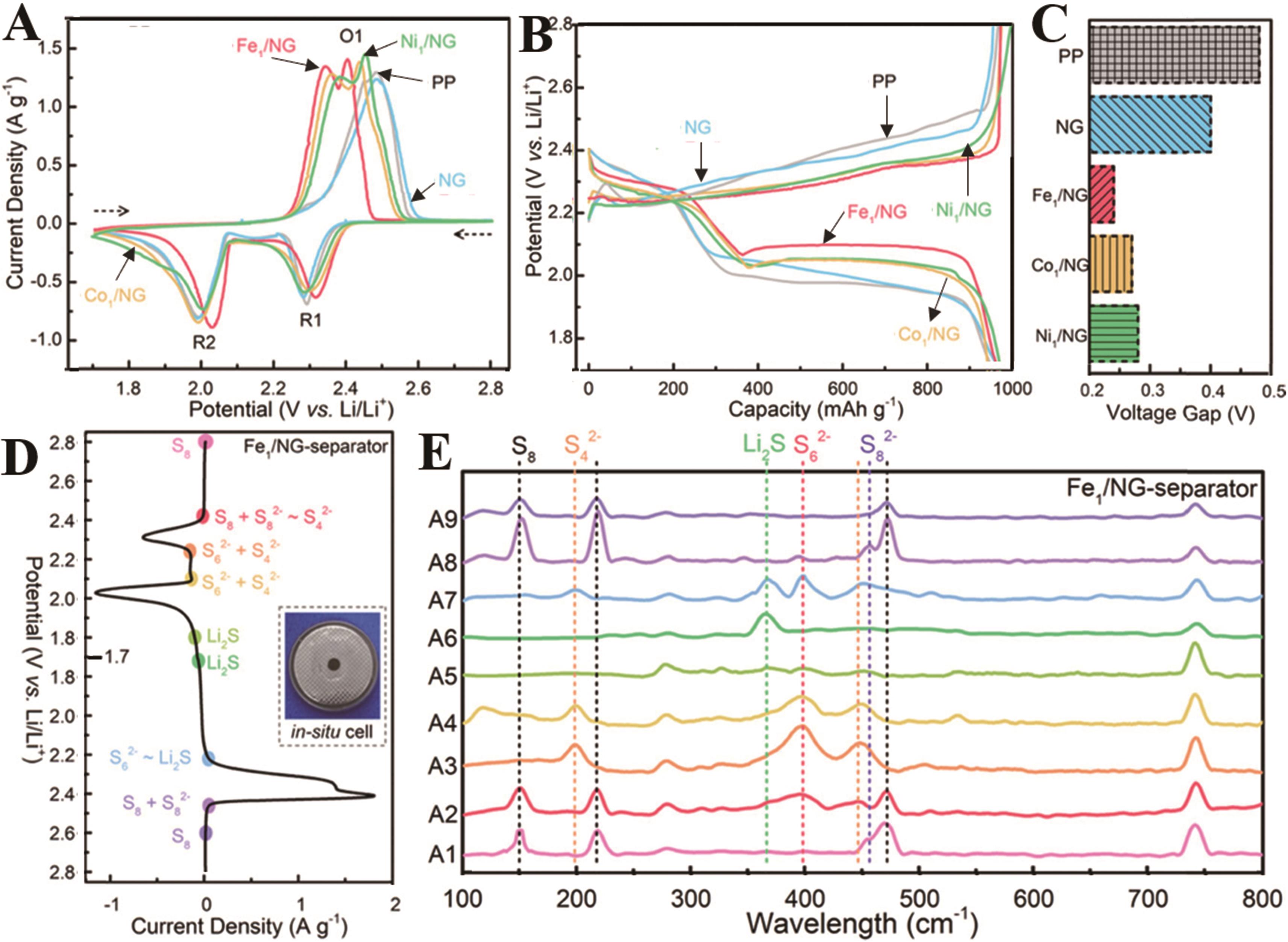
图4 装配有PP隔膜、NG和M1/NG改性隔膜电池的(A)CV曲线、(B)充放电曲线和(C)电池容量为60 mA·h/g时的电势差[71];(D) 使用Fe1/NG改性隔膜的电池的CV曲线,插图:测量原位拉曼电池的照片[71];(E)不同电压下使用Fe1/NG修饰隔膜的电池的原位拉曼光谱图[71]
Fig.4 (A) CV profiles of the Li-S batteries with unmodified PP, the NG, or M1/NG-modified separators at 0.1 mV/s[71]; (B) Charge-discharge curves of the Li-S batteries at 0.5 C[71]; (C) Voltage gaps of the Li-S batteries with various separators at 600 mA·h/g[71]; (D) CV profiles of the Li-S cell with the Fe1/NG-modified separator, inset: digital photo of the in situ Raman cell; in situ Raman spectra of the Li-S cell with the Fe1/NG-modified separator at different voltages as indicated in (E)[71]
材料 Material | 硫负载量 Sulphur load/(mg·cm-2) | 倍率性能 Rate capability/(mA·h·g-1) | 电流/循环圈数/容量保持率 Current/number of cycles/capacity retention rate | 参考文献 Ref. |
|---|---|---|---|---|
| 1.1 | 1375 (0.2 C)/678 (10 C) | 2 C/1000/55% | [ | |
| NC@SA?Co | 1 | 1160 (0.1 C)/582 (5 C) | 2 C/700/59.4% | [ |
| 1.2 | [ | |||
| [ |
表2 单原子材料在锂硫电池隔膜中的性能
Table 2 The electrochemical performance of Li?S batteries using SAs to modify the separator
材料 Material | 硫负载量 Sulphur load/(mg·cm-2) | 倍率性能 Rate capability/(mA·h·g-1) | 电流/循环圈数/容量保持率 Current/number of cycles/capacity retention rate | 参考文献 Ref. |
|---|---|---|---|---|
| 1.1 | 1375 (0.2 C)/678 (10 C) | 2 C/1000/55% | [ | |
| NC@SA?Co | 1 | 1160 (0.1 C)/582 (5 C) | 2 C/700/59.4% | [ |
| 1.2 | [ | |||
| [ |
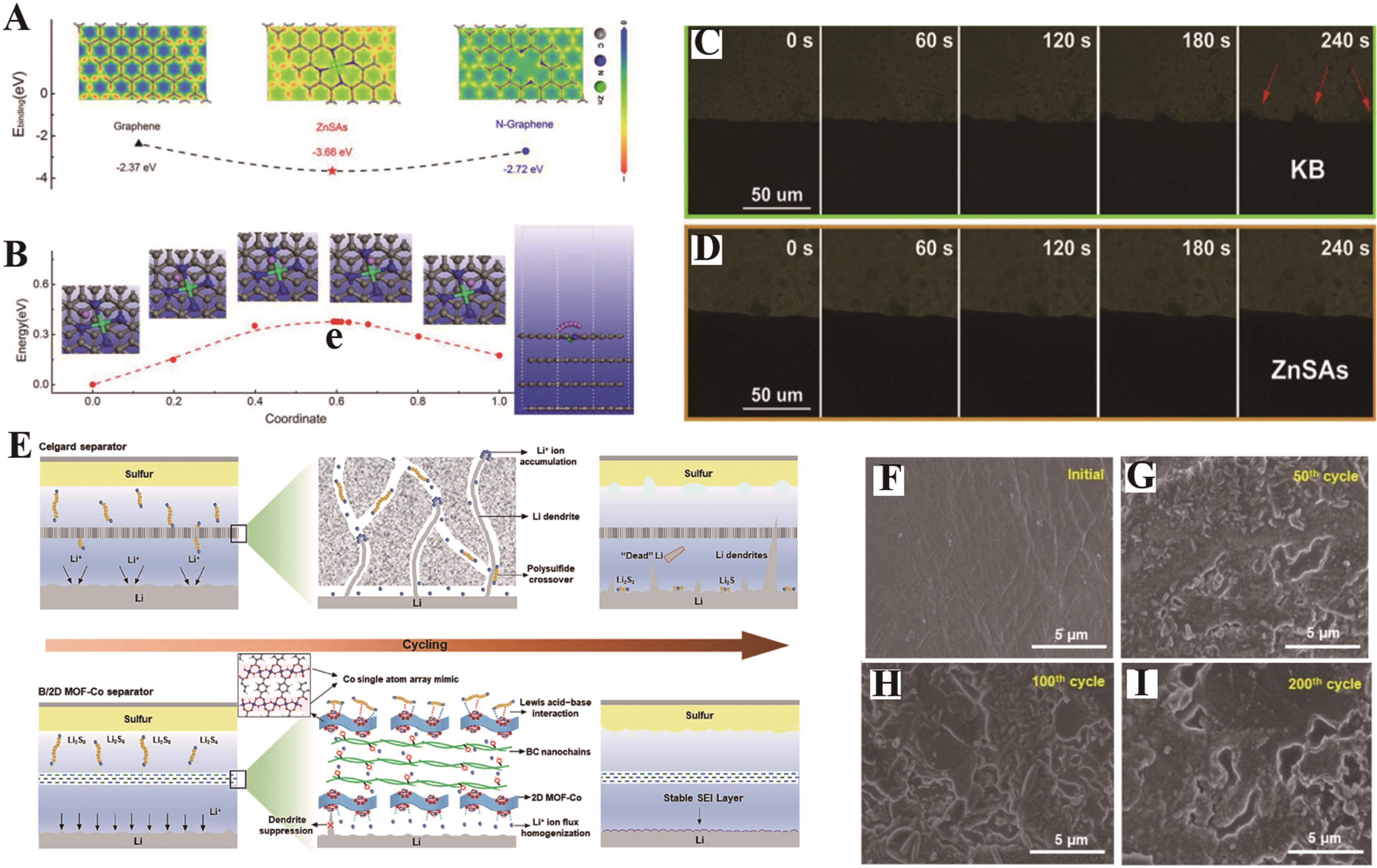
图5 (A)石墨烯、ZnSAs和N-石墨烯的表面结合能[78];(B)ZnSAs上Li+的迁移途径和势垒[78];Li+电沉积在(C)KB和(D)ZnSAs上的原位观测[78];(E)装配有Celgard和B/2D MOF-Co隔膜的锂硫电池示意图[79];(F - I) B/2D MOF-Co修饰隔膜后锂负极的初始和循环后SEM图像[79]
Fig.5 (A) Electron density difference and surface binding energy of graphene, ZnSAs, and N-graphene[78]; (B) Li migration pathways and barriers on ZnSAs[78]; (C - D) In situ observation of Li electrodepositing on KB and ZnSAs[78]; (E) Schematic illustration for the Li-S batteries with celgard and B/2D MOF-Co separators[79]; (F - I) SEM images of the initial and cycled Li anodes treated with B/2D MOF-Co[79]
材料 Materials | 电流密度/电压/循环寿命 Current density (1 mA·cm-2)/ voltage (mV)/cycle life (h) | 锂硫电池的硫负载量 Sulphur Load of lithium?sulfur battery/ (mg·cm-2) | 锂硫电池的电流密度/循环圈数/容量 Current of lithium?sulfur battery /Number of cycles/Capacity(mA?h/g) | 参考文献 Ref. |
|---|---|---|---|---|
| Co?O?G SA | 1/18/780 | 1 | 0.5 C/1000/915 | [ |
| SACo | 0.5/15/1600 | 5.4 | 1 mA/cm2/60/542 | [ |
| Zn?HNC | 3/16/1200 | 1.5 | 1 C/300/1149 | [ |
表3 单原子材料在锂硫电池负极中的性能
Table 3 The electrochemical performance of Li?S batteries using SAs to modify the anode
材料 Materials | 电流密度/电压/循环寿命 Current density (1 mA·cm-2)/ voltage (mV)/cycle life (h) | 锂硫电池的硫负载量 Sulphur Load of lithium?sulfur battery/ (mg·cm-2) | 锂硫电池的电流密度/循环圈数/容量 Current of lithium?sulfur battery /Number of cycles/Capacity(mA?h/g) | 参考文献 Ref. |
|---|---|---|---|---|
| Co?O?G SA | 1/18/780 | 1 | 0.5 C/1000/915 | [ |
| SACo | 0.5/15/1600 | 5.4 | 1 mA/cm2/60/542 | [ |
| Zn?HNC | 3/16/1200 | 1.5 | 1 C/300/1149 | [ |
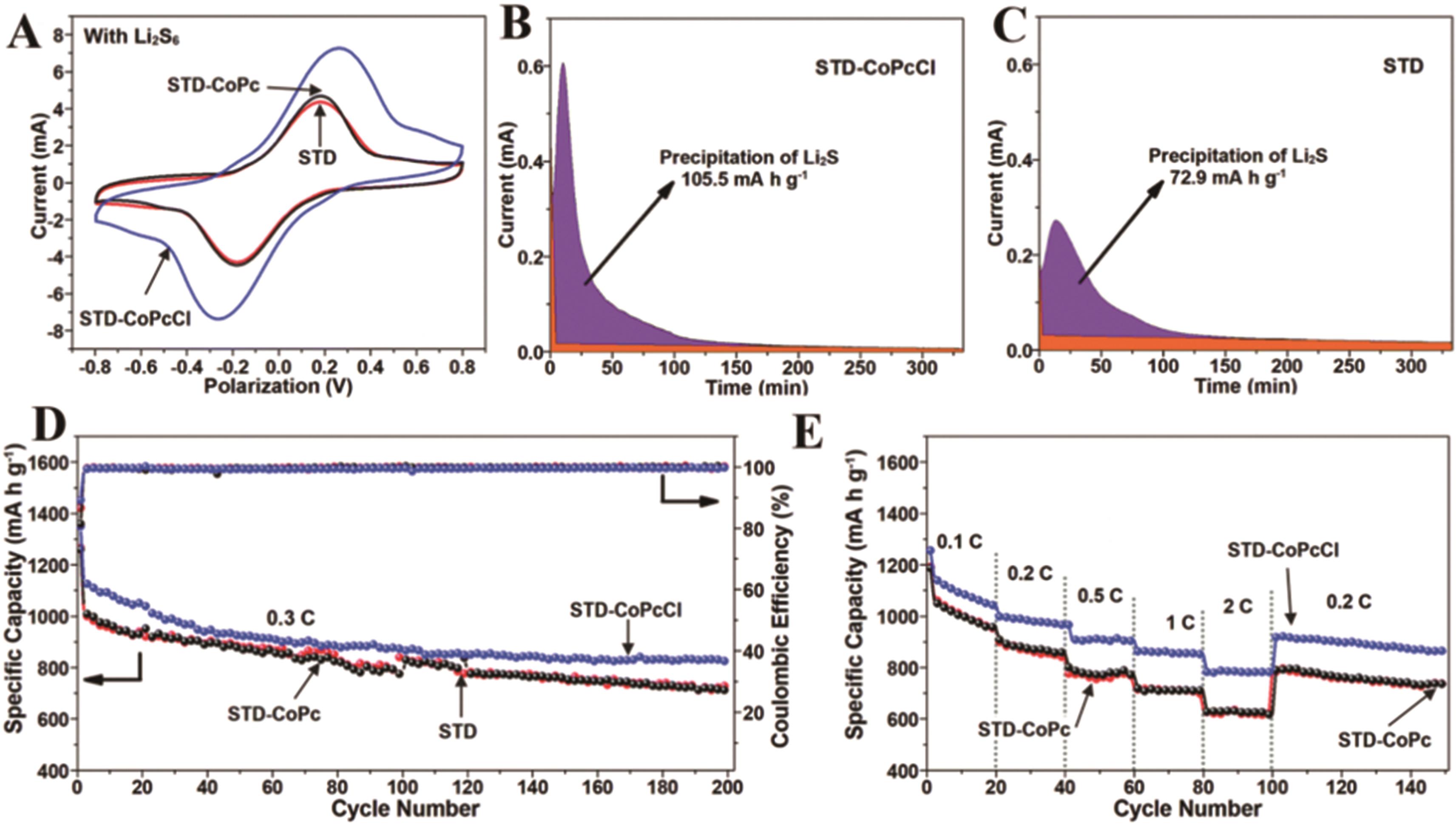
图6 (A)Li2S6对称电池在50 mV/s扫描速率下的CV曲线;使用(B) STD-CoPcCl和(C) STD电解液在CNT衬底上放电至2.07 V时Li2S6溶液的恒电势放电曲线;(D) Li-S电池的长期循环和 (E) 倍率性能[83]
Fig.6 CV curves of the symmetric cells with (A) Li2S6 at a scan rate of 50 mV/s; Potentiostatic discharge profiles of Li2S6 solution at 2.07 V on the CNT substrate using STD-CoPcCl (B) and STD (C) electrolytes; (D) Long-term cycling and (E) rate performance of Li-S batteries[83]
| 1 | NI S, LIU J, CHAO D, et al. Vanadate-based materials for Li-ion batteries: the search for anodes for practical applications[J]. Adv Energy Mater, 2019, 9(14): 1803324. |
| 2 | LI P, HWANG J, SUN Y. Nano/microstructured silicon-graphite composite anode for high-energy-density Li-ion battery[J]. ACS Nano, 2019, 13(2): 2624-2633. |
| 3 | LARCHER D, TARASCON J. Towards greener and more sustainable batteries for electrical energy storage[J]. Nat Chem, 2015, 7(1): 19-29. |
| 4 | HUANG L, LI J, LIU B, et al. Electrode design for lithium-sulfur batteries: problems and solutions[J]. Adv Funct Mater, 2020, 30(22): 1910375. |
| 5 | BETZ J, BIEKER G, MEISTER P, et al. Theoretical versus practical energy: a plea for more transparency in the energy calculation of different rechargeable battery systems[J]. Adv Energy Mater, 2018, 9(6): 1803170. |
| 6 | LIU X, HUANG J, ZHANG Q, et al. Nanostructured metal oxides and sulfides for lithium-sulfur batteries[J]. Adv Mater, 2017, 29(20): 1601759. |
| 7 | JANA M, XU R, CHENG X. Rational design of two-dimensional nanomaterials for lithium-sulfur batteries[J]. Energy Environ Sci, 2020, 13(4): 1049-1075. |
| 8 | HOU L, YUAN H, ZHAO C, et al. Improved interfacial electronic contacts powering high sulfur utilization in all-solid-state lithium-sulfur batteries[J]. Energy Storage Mater, 2020, 25: 436-442. |
| 9 | ZHAO Q, ZHENG J, ARCHER L. Interphases in lithium-sulfur batteries: toward deployable devices with competitive energy density and stability[J]. ACS Energy Lett, 2018, 3(9): 2104-2113. |
| 10 | HUANG L, LI J, LIU B, et al. Electrode design for lithium-sulfur batteries: problems and solutions[J]. Adv Funct Mater, 2020, 30(22): 1910375. |
| 11 | WANG X, TAN Y, SHEN G, et al. Recent progress in fluorinated electrolytes for improving the performance of Li-S batteries[J]. J Energy Chem, 2020, 41: 149-170. |
| 12 | SEH Z, SUN Y, ZHANG Q, et al. Designing high-energy lithium-sulfur batteries[J]. Chem Soc Rev, 2016, 45(20): 5605-5634. |
| 13 | LI S, FAN Z. Encapsulation methods of sulfur particles for lithium-sulfur batteries: a review[J]. Energy Storage Mater, 2021, 34: 107-127. |
| 14 | CHEON S, CHOI S, HAN J, et al. Capacity fading mechanisms on cycling a high-capacity secondary sulfur cathode[J]. J Electrochem Soc, 2004, 151(12): A2067-A2073. |
| 15 | LIU Y, LIU S, LI G, et al. Strategy of enhancing the volumetric energy density for lithium-sulfur batteries[J]. Adv Mater, 2021, 33(8): 2003955. |
| 16 | HE X, REN J, WANG L, et al. Expansion and shrinkage of the sulfur composite electrode in rechargeable lithium batteries[J]. J Power Sources, 2009, 190(1): 154-156. |
| 17 | ZHAO Q, HAO Z, TANG J, et al. Cation-selective separators for addressing the lithium-sulfur battery challenges[J]. ChemSusChem, 2021, 14(3): 792-807. |
| 18 | WANG M, SONG Y, WEI N, et al. Universal interface and defect engineering dual-strategy for graphene-oxide heterostructures toward promoted Li-S chemistry[J]. Chem Eng J, 2021, 418: 129407. |
| 19 | QI F, SUN Z, FAN X, et al. Tunable interaction between metal-organic frameworks and electroactive components in lithium-sulfur batteries: status and perspectives[J]. Adv Energy Mater, 2021, 11(20): 2100387. |
| 20 | JANA M, XU R, CHENG X, et al. Rational design of two-dimensional nanomaterials for lithium-sulfur batteries[J]. Energy Environ Sci, 2020, 13(4): 1049-1075. |
| 21 | YANG X, YU Y, LIN X, et al. Multi-functional nanowall arrays with unrestricted Li+ transport channels and an integrated conductive network for high-areal-capacity Li-S batteries[J]. J Mater Chem A, 2018, 6(45): 22958-22965. |
| 22 | LIAO K, CHEN S, WEI H, et al. Micropores of pure nanographite spheres for long cycle life and high-rate lithium-sulfur batteries[J]. J Mater Chem A, 2018, 6(45): 23062-23070. |
| 23 | QIU X, HUA Q, DAI Z, et al. High sulfur loading application with the assistance of an extremely light-weight multifunctional layer on the separator for lithium-sulfur batteries[J]. Ionics, 2019, 26(3): 1139-1147. |
| 24 | WANG Y, HE J, ZHANG Z, et al. Graphdiyne-modified polyimide separator: a polysulfide-immobilizing net hinders the shuttling of polysulfides in lithium-sulfur battery[J]. ACS Appl Mater Interfaces, 2019, 11(39): 35738-35745. |
| 25 | SONG Y, ZHOU H, LONG X, et al. Dual-heterostructures decorated interweaved carbon nanofibers sulfur host for high performance lithium-sulfur batteries[J]. Chem Eng J, 2021, 418: 129388. |
| 26 | HU Y, CHEN W, LEI T, et al. Strategies toward high-loading lithium-sulfur battery[J]. Adv Energy Mater, 2020, 10(17): 2000082. |
| 27 | LI N, XU Z, WANG P, et al. High-rate lithium-sulfur batteries enabled via vanadium nitride nanoparticle/3D porous graphene through regulating the polysulfides transformation[J]. Chem Eng J, 2020, 398: 125432. |
| 28 | HE J, MANTHIRAM A. Long-life, high-rate lithium-sulfur cells with a carbon-free VN host as an efficient polysulfide adsorbent and lithium dendrite inhibitor[J]. Adv Energy Mater, 2019, 10(3): 1903241. |
| 29 | YU Y, YAN M, DONG W, et al. Optimizing inner voids in yolk-shell TiO2 nanostructure for high-performance and ultralong-life lithium-sulfur batteries[J]. Chem Eng J, 2021, 417: 129241. |
| 30 | QI B, ZHAO X, WANG S, et al. Mesoporous TiN microspheres as an efficient polysulfide barrier for lithium-sulfur batteries[J]. J Mater Chem A, 2018, 6(29): 14359-14366. |
| 31 | LIU Y, MA S, LIU L, et al. Nitrogen doping improves the immobilization and catalytic effects of Co9S8 in Li-S batteries[J]. Adv Funct Mater, 2020, 30(32): 2002462. |
| 32 | WANG X, ZHAO X, MA C, et al. Electrospun carbon nanofibers with MnS sulfiphilic sites as efficient polysulfide barriers for high-performance wide-temperature-range Li-S batteries[J]. J Mater Chem A, 2020, 8(3): 1212-1220. |
| 33 | QIAO B, WANG A, YANG X, et al. Single-atom catalysis of CO oxidation using Pt1/FeOx[J]. Nat Chem, 2011, 3(8): 634-641. |
| 34 | WANG J, DING B, LU X, et al. Single atom-based nanoarchitectured electrodes for high-performance lithium-sulfur batteries[J]. Adv Mater Interfaces, 2021, 8(8): 2002159. |
| 35 | PENG Y, LU B, CHEN S. Carbon-supported single atom catalysts for electrochemical energy conversion and storage[J]. Adv Mater, 2018, 30(48): 1801995. |
| 36 | JIAO L, ZHANG R, WAN G, et al. Nanocasting SiO2 into metal-organic frameworks imparts dual protection to high-loading Fe single-atom electrocatalysts[J]. Nat Commun, 2020, 11(1): 2831. |
| 37 | SUN S, ZHANG G, GAUQUELIN N, et al. Single-atom catalysis using Pt/graphene achieved through atomic layer deposition[J]. Sci Rep, 2013, 3(1): 1775. |
| 38 | CHENG N, SUN X. Single atom catalyst by atomic layer deposition technique[J]. Chinese J Catal, 2017, 38(9): 1508-1514. |
| 39 | HE X, DENG Y, ZHANG Y, et al. Mechanochemical kilogram-scale synthesis of noble metal single-atom catalysts[J]. Cell Rep Phys Sci, 2020, 1(1): 100004. |
| 40 | FEI H, DONG J, WAN C, et al. Microwave-assisted rapid synthesis of graphene-supported single atomic metals[J]. Adv Mater, 2018, 30(35): 1802146. |
| 41 | LIU Y, LI Z, YU Q, et al. A general strategy for fabricating isolated single metal atomic site catalysts in Y zeolite[J]. J Am Chem Soc, 2019, 141(23): 9305-9311. |
| 42 | YANG Q, YANG C, LIN C, et al. Metal-organic-framework-derived hollow N-doped porous carbon with ultrahigh concentrations of single Zn atoms for efficient carbon dioxide conversion[J]. Angew Chem Int Ed, 2019, 58(11): 3511-3515. |
| 43 | ZHANG E, WANG T, YU K, et al. Bismuth single atoms resulting from transformation of metal-organic frameworks and their use as electrocatalysts for CO2 reduction[J]. J Am Chem Soc, 2019, 141(42): 16569-16573. |
| 44 | DU Z, CHEN X, HU W, et al. Cobalt in nitrogen-doped graphene as single-atom catalyst for high-sulfur content lithium-sulfur batteries[J]. J Am Chem Soc, 2019, 141(9): 3977-3985. |
| 45 | ZHANG J, ZHAO Y, CHEN C, et al. Tuning the coordination environment in single-atom catalysts to achieve highly efficient oxygen reduction reactions[J]. J Am Chem Soc, 2019, 141(51): 20118-20126. |
| 46 | ZHAO L, ZHANG Y, HUANG L, et al. Cascade anchoring strategy for general mass production of high-loading single-atomic metal-nitrogen catalysts[J]. Nat Commun, 2019, 10(1): 1278. |
| 47 | HAN J, MENG X, LU L, et al. Single-atom Fe-Nx-C as an efficient electrocatalyst for zinc-air batteries[J]. Adv Funct Mater, 2019, 29(41): 1808872. |
| 48 | LI M, LU J, AMINE K. Nanotechnology for sulfur cathodes[J]. ACS Nano, 2021, 15(5): 8087-8094. |
| 49 | WANG C, YI Y, LI H, et al. Rapid gas-assisted exfoliation promises V2O5 nanosheets for high performance lithium-sulfur batteries[J]. Nano Energy, 2020, 67: 104253. |
| 50 | GAO X, ZHU X, GU L, et al. Efficient polysulfides anchoring for Li-S batteries: ombined physical adsorption and chemical conversion in V2O5 hollow spheres wrapped in nitrogen-doped graphene network[J]. Chem Eng J, 2019, 378: 122189. |
| 51 | CHENG S, WANG J, DUAN S, et al. Anionic oxygen vacancies in Nb2O5- x/carbon hybrid host endow rapid catalytic behaviors for high-performance high areal loading lithium sulfur pouch cell[J]. Chem Eng J, 2021, 417: 128172. |
| 52 | ZHONG Y, HAN T, CHENG M, et al. Self-reduction preparation of porous multi-walled ZnCo2O4 spheres as sulfur host for lithium-sulfur battery cathodes with long cycling life and stable rate-performance[J]. J Electroanal Chem, 2021, 880: 114860. |
| 53 | CHENG Z, CHEN Y, YANG Y. et al. Metallic MoS2 nanoflowers decorated graphene nanosheet catalytically boosts the volumetric capacity and cycle life of lithium-sulfur batteries[J]. Adv Energy Mater, 2021, 11(12): 2003718. |
| 54 | LI B, SU Q, YU L, et al. Tuning the band structure of MoS2 via Co9S8@MoS2 core-shell structure to boost catalytic activity for lithium-sulfur batteries[J]. ACS Nano, 2020, 14(12): 17285-17294. |
| 55 | ZHU X, ZHAO W, SONG Y, et al. In situ assembly of 2D conductive vanadium disulfide with graphene as a high-sulfur-loading host for lithium-sulfur batteries[J]. Adv Energy Mater, 2018, 8(20): 1800201. |
| 56 | XIAO K, WANG J, CHEN Z, et al. Improving polysulfides adsorption and redox kinetics by the Co4N nanoparticle/ N-doped carbon composites for lithium-sulfur batteries[J]. Small, 2019, 15(25): 1901454. |
| 57 | DENG D, XUE F, JIA Y, et al. Co4N nanosheet assembled mesoporous sphere as a matrix for ultrahigh sulfur content lithium-sulfur batteries[J]. ACS Nano 2017, 11(6): 6031-6039. |
| 58 | CI H, CAI J, MA H, et al. Defective VSe2-graphene heterostructures enabling in situ electrocatalyst evolution for lithium-sulfur batteries[J]. ACS Nano, 2020, 14(9): 11929-11938. |
| 59 | HE J, MANTHIRAM A. 3D CoSe@C aerogel as a host for dendrite-free lithium-metal anode and efficient sulfur cathode in Li-S full cells[J]. Adv Energy Mater, 2020, 10(41): 2002654. |
| 60 | LIU Z, ZHOU L, GE Q, et al. Atomic iron catalysis of polysulfide conversion in lithium-sulfur batteries[J]. ACS Appl Mater Interfaces, 2018, 10(23): 19311-19317. |
| 61 | SHAO Q, XU L, GUO D, et al. Atomic level design of single iron atom embedded mesoporous hollow carbon spheres as multi-effect nanoreactors for advanced lithium-sulfur batteries[J]. J Mater Chem A, 2020, 8(45): 23772-23783. |
| 62 | WANG J, JIA L, DUAN S, et al. Single atomic cobalt catalyst significantly accelerates lithium ion diffusion in high mass loading Li2S cathode[J]. Energy Storage Mater, 2020, 28: 375-382. |
| 63 | WANG R, WU R, DING C, et al. Porous carbon architecture assembled by cross-linked carbon leaves with implanted atomic cobalt for high-performance Li-S batteries[J]. Nano-Micro Lett, 2021, 13(1): 151. |
| 64 | HUANG T, SUN Y, WU J, et al. A dual-functional fibrous skeleton implanted with single-atomic Co-Nx dispersions for longevous Li-S full batteries[J]. ACS Nano, 2021, 15(9): 14105-14115. |
| 65 | LIU Y, WEI Z, ZHONG B, et al. O-, N-Coordinated single Mn atoms accelerating polysulfides transformation in lithium-sulfur batteries[J]. Energy Stor Mater, 2021, 35: 12-18. |
| 66 | HAO H, TIAN B, SU C, et al. Single-Atom iron and doped sulfur improve the catalysis of polysulfide conversion for obtaining high-performance lithium-sulfur batteries[J]. ACS Appl Mater Interfaces, 2021, 13(6): 7171-7177. |
| 67 | ZHAO C, XU G, YU Z, et al. A high-energy and long-cycling lithium-sulfur pouch cell via a macroporous catalytic cathode with double-end binding sites[J]. Nat Nanotechnol, 2021, 16(2): 166-173. |
| 68 | LI Y, WU J, ZHANG B, et al. Fast conversion and controlled deposition of lithium (poly)sulfides in lithium-sulfur batteries using high-loading cobalt single atoms[J]. Energy Storge Mater, 2020, 30: 250-259. |
| 69 | WANG C, SONG H, YU C, et al. Iron single-atom catalyst anchored on nitrogen-rich MOF-derived carbon nanocage to accelerate polysulfide redox conversion for lithium sulfur batteries[J]. J Mater Chem A, 2020, 8(6): 3421-3430. |
| 70 | ZHOU G, WANG S, WANG T, et al. Theoretical calculation guided design of single-atom catalysts toward fast kinetic and long-life Li-S batteries[J]. Nano Lett, 2020, 20(2): 1252-1261. |
| 71 | ZHANG K, CHEN Z, NING R, et al. Single-atom coated separator for robust lithium-sulfur batteries[J]. ACS Appl Mater Interfaces, 2019, 11(28): 25147-25154. |
| 72 | SU Y, MANTHIRAM A. A new approach to improve cycle performance of rechargeable lithium-sulfur batteries by inserting a free-standing MWCNT interlayer[J]. Chem Commun, 2012, 48(70): 8817-8819. |
| 73 | LI Y, ZHOU P, LI H, et al. A freestanding flexible single-atom cobalt-based multifunctional interlayer toward reversible and durable lithium-sulfur batteries[J]. Small Methods, 2020, 4(3): 1900701. |
| 74 | WANG R, WU R, DING C, et al. Atomic tungsten on graphene with unique coordination enabling kinetically boosted lithium-sulfur batteries[J]. Angew Chem Int Ed, 2021, 60(28): 15563-15571. |
| 75 | ZHANG L, LIU D, WAN F, et al. Single nickel atoms on nitrogen-doped graphene enabling enhanced kinetics of lithium-sulfur batteries[J]. Adv Mater, 2019, 31(40): 1903955. |
| 76 | XIE J, LI B, PENG H, et al. Implanting atomic cobalt within mesoporous carbon toward highly stable lithium-sulfur batteries[J]. Adv Mater, 2019, 31(43): 1903813. |
| 77 | LIU H, CHEN X, CHENG X, et al. Uniform lithium nucleation guided by atomically dispersed lithiophilic CoNx sites for safe lithium metal batteries[J]. Small Methods, 2018, 3(9): 1800354. |
| 78 | XU K, ZHU M, WU X, et al. Dendrite-tamed deposition kinetics using single-atom Zn sites for Li metal anode[J]. Energy Storage Mater, 2019, 23: 587-593. |
| 79 | LI Y, LIN S, WANG D, et al. Single atom array mimic on ultrathin MOF nanosheets boosts the safety and life of lithium-sulfur batteries[J]. Adv Mater, 2020, 32(8): 1906722. |
| 80 | SHI H, LI Y, LU P, et al. Single-atom cobalt coordinated to oxygen sites on graphene for stable lithium metal anodes[J]. Acta Phys-Chim Sin, 2021, 37(11): 20080. |
| 81 | WANG J, ZHANG J, CHENG S, et al. Long-Life dendrite-free lithium metal electrode achieved by constructing a single metal atom anchored in a diffusion modulator layer[J]. Nano Lett, 2021, 21(7): 3245-3253. |
| 82 | SHI H, REN X, LU J, et al. Dual-Functional atomic zinc decorated hollow carbon nanoreactors for kinetically accelerated polysulfides conversion and dendrite free lithium sulfur batteries[J]. Adv Energy Mater, 2020, 10(39): 2002271. |
| 83 | SHI Z, WANG L, XU H. et al. A soluble single atom catalyst promotes lithium polysulfide conversion in lithium sulfur batteries[J]. Chem Commun, 2019, 55(80): 12056-12059. |
| 84 | ZHANG D, WANG S, HU R, et al. Catalytic conversion of polysulfides on single atom zinc implanted MXene toward high-rate lithium-sulfur batteries[J]. Adv Funct Mater, 2020, 30(30): 2002471. |
| 85 | LAI W, WANG H, ZHENG L, et al. General synthesis of single-atom catalysts for hydrogen evolution reactions and room-temperature Na-S batteries[J]. Angew Chem Int Ed, 2020, 59(49): 22171-22178. |
| [1] | 王路飞, 甄蒙蒙, 沈伯雄. 贫电解液下电催化剂对调控锂硫电池性能的研究进展[J]. 应用化学, 2023, 40(2): 188-209. |
| [2] | 曹蓉, 夏杰桢, 廖漫华, 赵路超, 赵晨, 吴琪. 单原子催化剂在电化学合成氨中的理论研究进展[J]. 应用化学, 2023, 40(1): 9-23. |
| [3] | 王显, 杨小龙, 马荣鹏, 刘长鹏, 葛君杰, 邢巍. 单原子分散的Ir-N-C燃料电池阳极抗中毒催化剂[J]. 应用化学, 2022, 39(8): 1202-1208. |
| [4] | 张超. 单原子催化剂电催化还原二氧化碳研究进展[J]. 应用化学, 2022, 39(6): 871-887. |
| [5] | 高峰, 徐子迪, 管纯倩, 王研, 臧云浩, 顾建峰, 曲江英. 硫/石墨烯气凝胶的绿色可控制备及锂硫电池性能[J]. 应用化学, 2021, 38(4): 439-446. |
| [6] | 王春丽,孙连山,钟鸣,王立民,程勇. 过渡金属及其化合物应用于锂硫电池的研究进展[J]. 应用化学, 2020, 37(4): 387-404. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||