应用化学 ›› 2022, Vol. 39 ›› Issue (7): 1026-1038.DOI: 10.19894/j.issn.1000-0518.210313
熔盐电化学还原二氧化碳制备碳材料研究进展
乔志强, 纪德强, 王鹏, 赫英明, 李志达, 纪德彬, 吴红军( )
)
- 东北石油大学化学化工学院,大庆 163318
-
收稿日期:2021-06-25接受日期:2021-10-19出版日期:2022-07-01发布日期:2022-07-11 -
通讯作者:吴红军 -
基金资助:黑龙江省杰出青年科学基金(JC2017002)
Progress in Preparation of Carbon Materials by Electrochemical Reduction of Carbon Dioxide in Molten Salt
Zhi-Qiang QIAO, De-Qiang JI, Peng WANG, Ying-Ming HE, Zhi-Da LI, De-Bin JI, Hong-Jun WU( )
)
- College of Chemistry & Chemical Engineering,Northeast Petroleum University,Daqing 163318,China
-
Received:2021-06-25Accepted:2021-10-19Published:2022-07-01Online:2022-07-11 -
Contact:Hong-Jun WU -
About author:hjw@nepu.edu.cn
-
Supported by:the Excellent Youth Foundation of Heilongjiang Scientific Committee(JC2017002)
摘要:
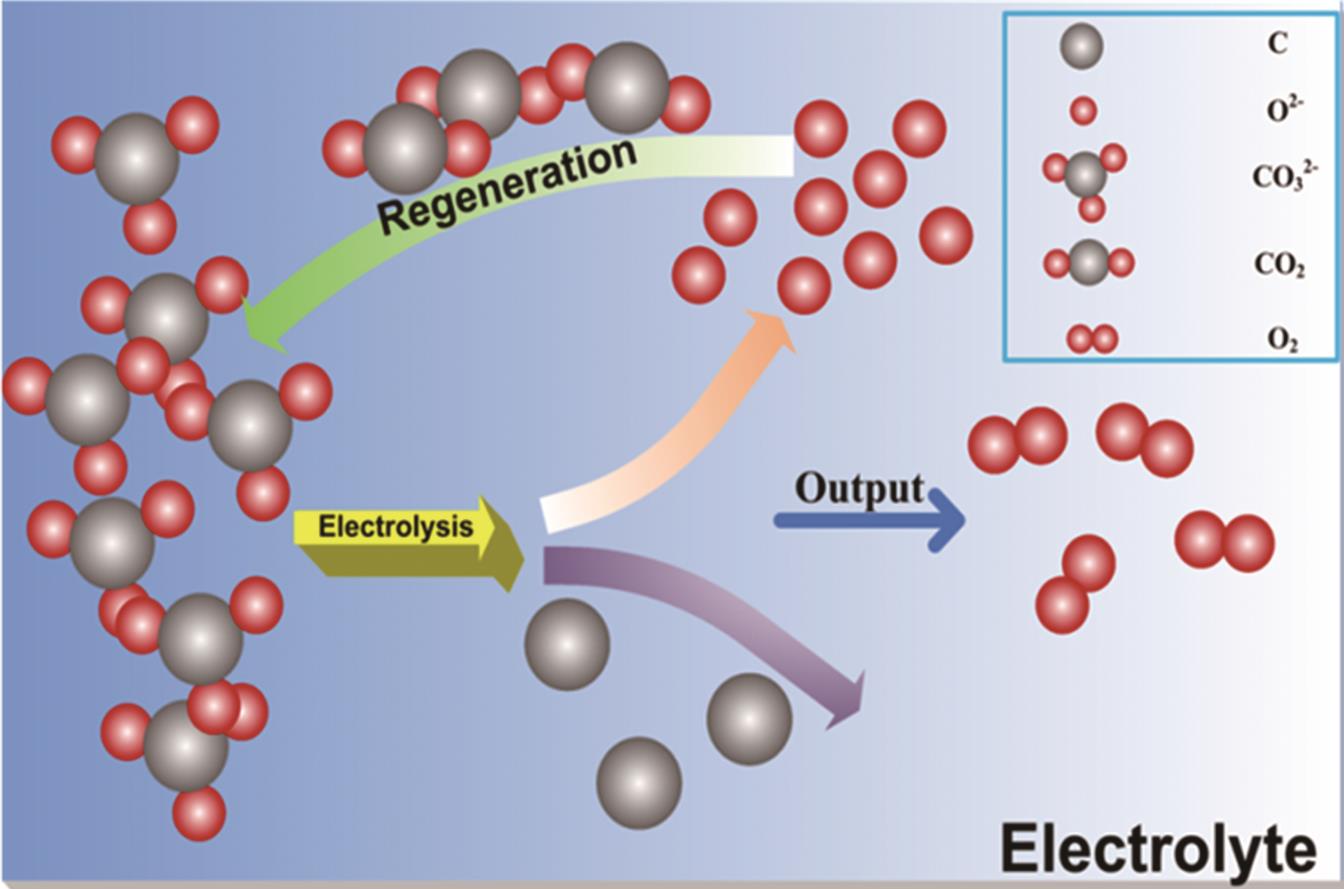
二氧化碳浓度持续升高导致的温室效应已在全球范围内引发极端天气、冰川融化等一系列生态环境问题。为降低二氧化碳含量,改善气候变暖带来的恶劣影响,研发高效、绿色、安全的二氧化碳处理技术,促进碳资源循环可持续发展刻不容缓。熔盐离子液体作为一种良好的电化学转化介质,为二氧化碳还原提供了一条极具应用前景的技术路线。综述了国内外近几年高温熔盐中二氧化碳的捕获与电化学还原的研究,简述了熔盐电沉积碳的电化学机理和热力学机制,对不同形貌高附加值碳材料:无定形碳、碳球和碳纳米管的制备进行了总结,最后对未来发展方向做出展望。
中图分类号:
引用本文
乔志强, 纪德强, 王鹏, 赫英明, 李志达, 纪德彬, 吴红军. 熔盐电化学还原二氧化碳制备碳材料研究进展[J]. 应用化学, 2022, 39(7): 1026-1038.
Zhi-Qiang QIAO, De-Qiang JI, Peng WANG, Ying-Ming HE, Zhi-Da LI, De-Bin JI, Hong-Jun WU. Progress in Preparation of Carbon Materials by Electrochemical Reduction of Carbon Dioxide in Molten Salt[J]. Chinese Journal of Applied Chemistry, 2022, 39(7): 1026-1038.
熔盐 Molten salt | CO2平衡分压(Pa, a(M y O)=0.001 mol/L) Equilibrium partial pressure of CO2(Pa, a(M y O)=0.001 mol/L) |
|---|---|
| Li2CO3 | 1.039 |
| Na2CO3 | 2.462 × 10-8 |
| K2CO3 | 2.054 × 10-1 |
| CaCO3 | 2.309 × 103 |
| BaCO3 | 1.793 × 10-3 |
表1 450 ℃不同熔融碳酸盐中氧化物对CO2的吸收能力[29]
Table 1 Carbon dioxide absorption capacity of oxides in different molten carbonate at 450 ℃[29]
熔盐 Molten salt | CO2平衡分压(Pa, a(M y O)=0.001 mol/L) Equilibrium partial pressure of CO2(Pa, a(M y O)=0.001 mol/L) |
|---|---|
| Li2CO3 | 1.039 |
| Na2CO3 | 2.462 × 10-8 |
| K2CO3 | 2.054 × 10-1 |
| CaCO3 | 2.309 × 103 |
| BaCO3 | 1.793 × 10-3 |
温度 Temperature/℃ | 金属 Metal | 还原为碱金属的 吉布斯自由能 | 还原为碱金属的电解电势 | 还原为单质碳的 吉布斯自由能 | 还原为单质碳的电解电势 |
|---|---|---|---|---|---|
| Li | 581.3 | -3.01 | 661.4 | -1.71 | |
| 540 | Na | 500.3 | -2.59 | 978.1 | -2.53 |
| K | 511.0 | -2.65 | 1181.8 | -3.06 | |
| Li | 562.5 | -2.91 | 638.4 | -1.65 | |
| 620 | Na | 480.5 | -2.49 | 952.1 | -2.47 |
| K | 489.7 | -2.54 | 1150.1 | -2.98 | |
| Li | 543.6 | -2.82 | 615.3 | -1.59 | |
| 700 | Na | 460.7 | -2.39 | 926.2 | -2.40 |
| K | 468.5 | -2.43 | 1118.4 | -2.90 | |
| Li | 531.8 | -2.76 | 599.6 | -1.55 | |
| 750 | Na | 451.8 | -2.34 | 917.1 | -2.38 |
| K | 459.3 | -2.38 | 1110.7 | -2.88 |
表2 碱金属碳酸盐电化学还原反应的Gibbs自由能及相应的标准还原电势[59-60]
Table 2 Gibbs free energy and corresponding standard reduction potential of the electrochemical reduction reaction of alkali metal carbonate[59-60]
温度 Temperature/℃ | 金属 Metal | 还原为碱金属的 吉布斯自由能 | 还原为碱金属的电解电势 | 还原为单质碳的 吉布斯自由能 | 还原为单质碳的电解电势 |
|---|---|---|---|---|---|
| Li | 581.3 | -3.01 | 661.4 | -1.71 | |
| 540 | Na | 500.3 | -2.59 | 978.1 | -2.53 |
| K | 511.0 | -2.65 | 1181.8 | -3.06 | |
| Li | 562.5 | -2.91 | 638.4 | -1.65 | |
| 620 | Na | 480.5 | -2.49 | 952.1 | -2.47 |
| K | 489.7 | -2.54 | 1150.1 | -2.98 | |
| Li | 543.6 | -2.82 | 615.3 | -1.59 | |
| 700 | Na | 460.7 | -2.39 | 926.2 | -2.40 |
| K | 468.5 | -2.43 | 1118.4 | -2.90 | |
| Li | 531.8 | -2.76 | 599.6 | -1.55 | |
| 750 | Na | 451.8 | -2.34 | 917.1 | -2.38 |
| K | 459.3 | -2.38 | 1110.7 | -2.88 |
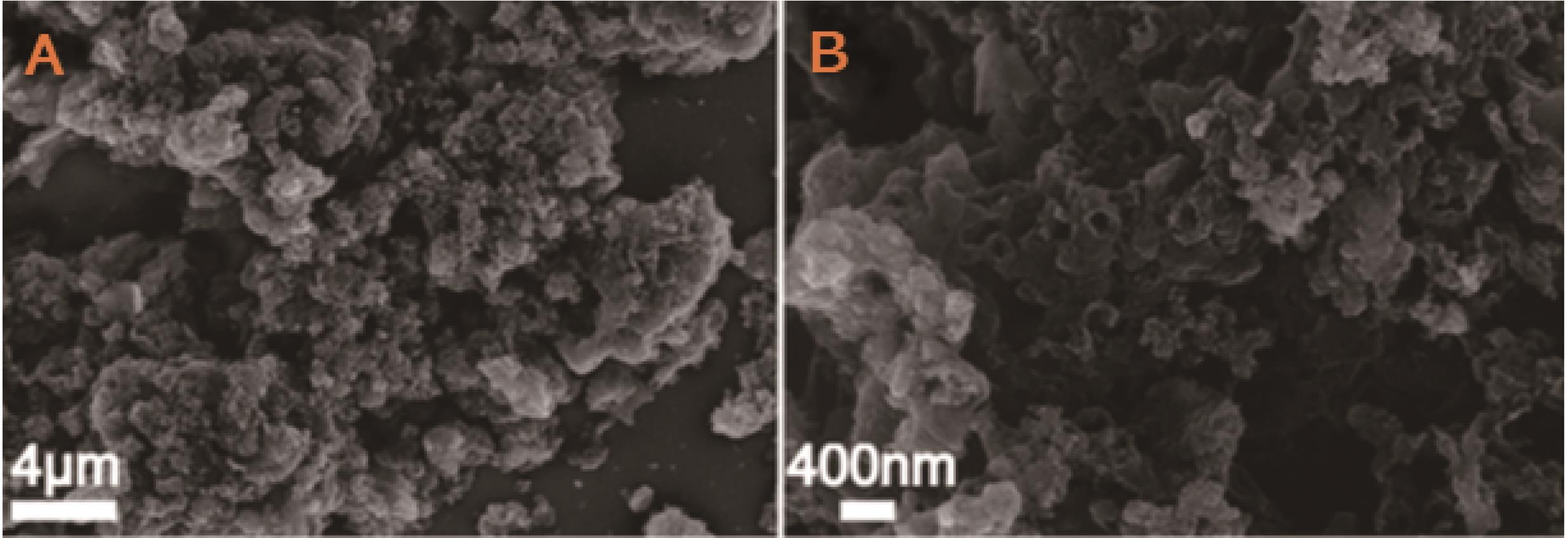
图2 Li2-Na2-K2CO3(质量分数33.3%、33.3%和33.3%)混合体系制备无定形碳不同倍率扫描电子显微镜图[65]
Fig.2 SEM images of amorphous carbon prepared by salt mixture system Li2-Na2-K2CO3(mass fraction 33.3%, 33.3%, 33.3%) at different magnification[65]
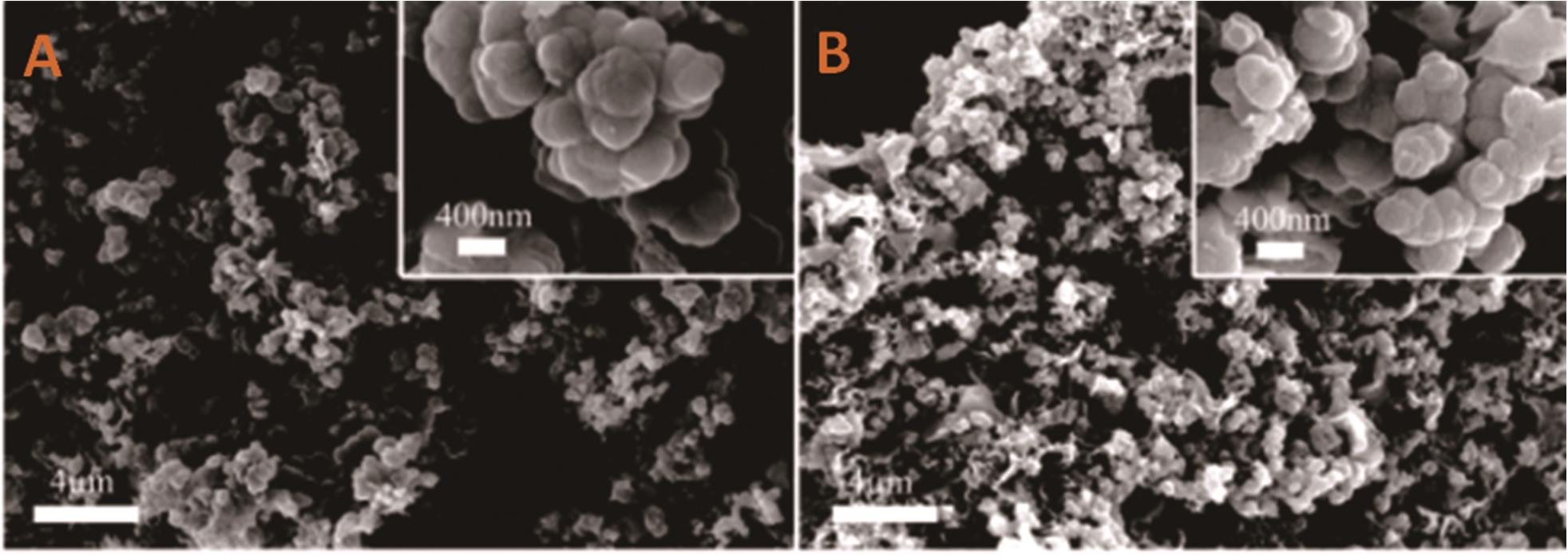
图3 混合体系制备碳球扫描电子显微镜图(A)Li2-Ca-Na2CO3(质量分数66.7%、20%和13.3%)及(B)Li2-Ca-K2CO3(质量分数66.7%、20%和13.3%)[67]
Fig.3 SEM images of carbon spheres prepared by the salt mixture system (A) Li2-Ca-Na2CO3(mass fraction 66.7%, 20%, 13.3%) and (B) Li2-Ca-K2CO3(mass fraction 66.7%, 20%, 13.3%)[67]
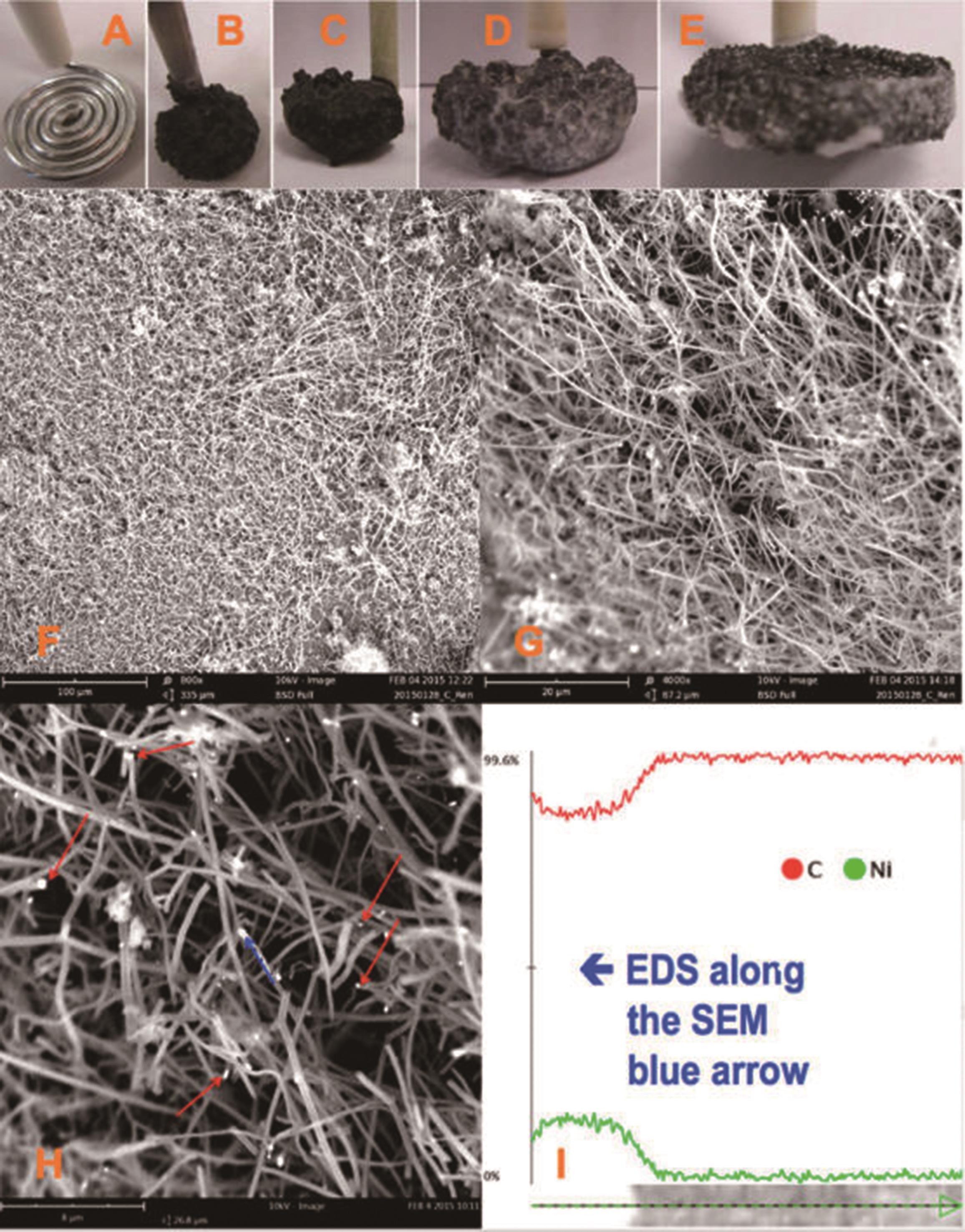
图4 (A)反应前阴极的形态; (B-E)反应后产物析出在阴极后的形态; (F-H)碳酸锂体系制备碳纳米纤维不同倍率下扫描电子显微镜图; (H)红色箭头所指位置为Ni的成核位点,蓝色箭头表示一段从成核位点生长的碳纳米纤维; (I)该段碳纳米纤维X射线能谱分析[81]
Fig.4 (A) The shape of the cathode before the reaction; (B-E) The shape of product precipitated in the cathode after reaction; (F-H) The scanning electron microscope image of carbon nanofibers prepared by Li2CO3 system at different magnification; (H) The red arrow indicates the nucleation site of Ni, and the blue arrow represents a segment of carbon nanofiber growing from the nucleation site; (I) EOS spectrum of carbon snarofiber shownon blue arrow.[81]
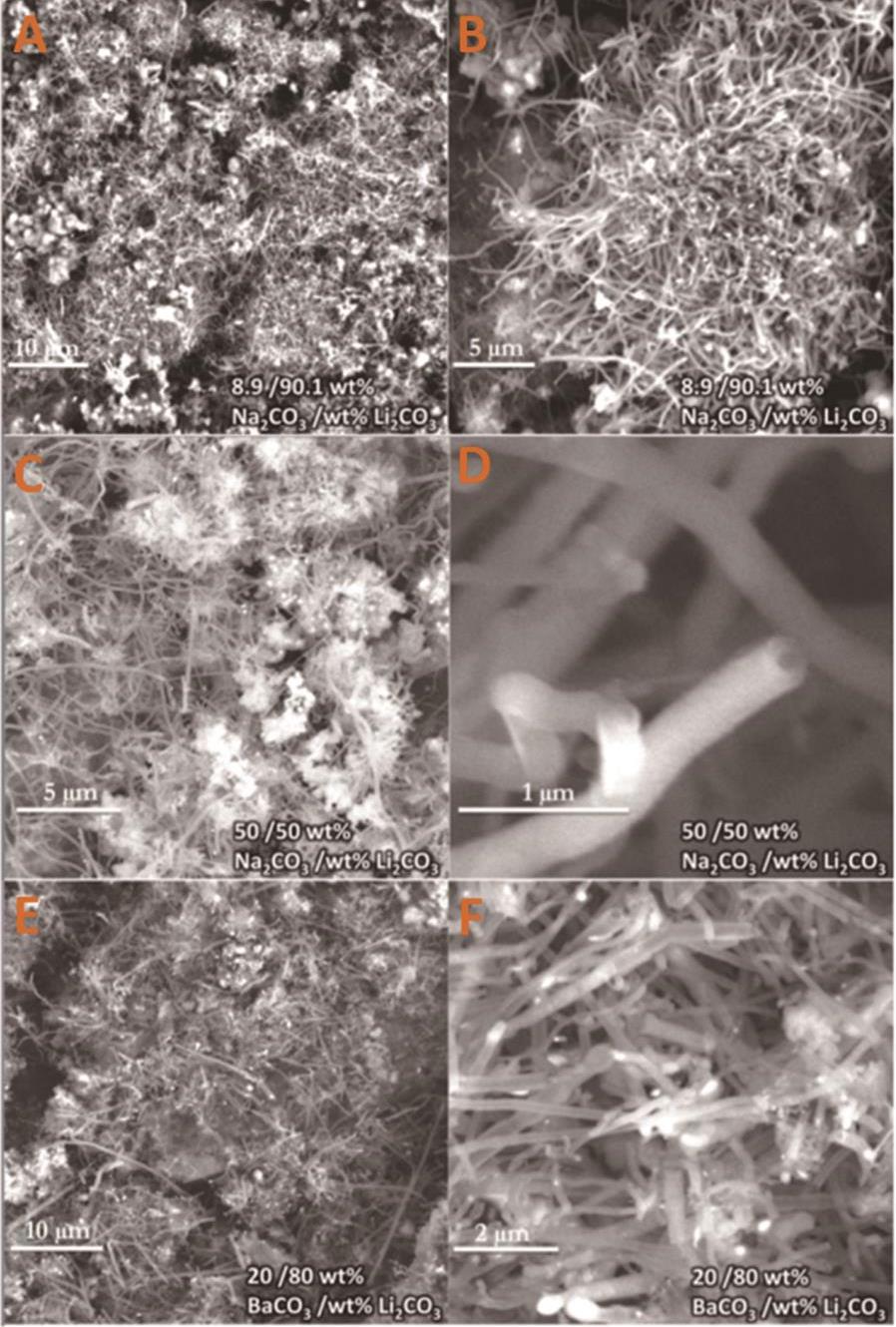
图5 混盐体系制备碳纳米管低倍率(A,C,E)和高倍率(B,D,F)扫描电子显微镜图,(A,B)Na2CO3-Li2CO3(质量分数8.9%和91.1%)、(C,D)Na2CO3-Li2CO3(质量分数50%和50%)和(E,F)BaCO3-Li2CO3(质量分数20%和80%)[44]
Fig.5 Low(A,C,E) and high(B,D,F) magnification SEM images of carbon sphere prepared by salt mixture system, (A,B)Na2CO3-Li2CO3(mass fraction 8.9%,91.1%),(C,D)Na2CO3-Li2CO3(mass fraction 50%,50%) and (E,F)BaCO3-Li2CO3(mass fraction 20%,80%)[44]
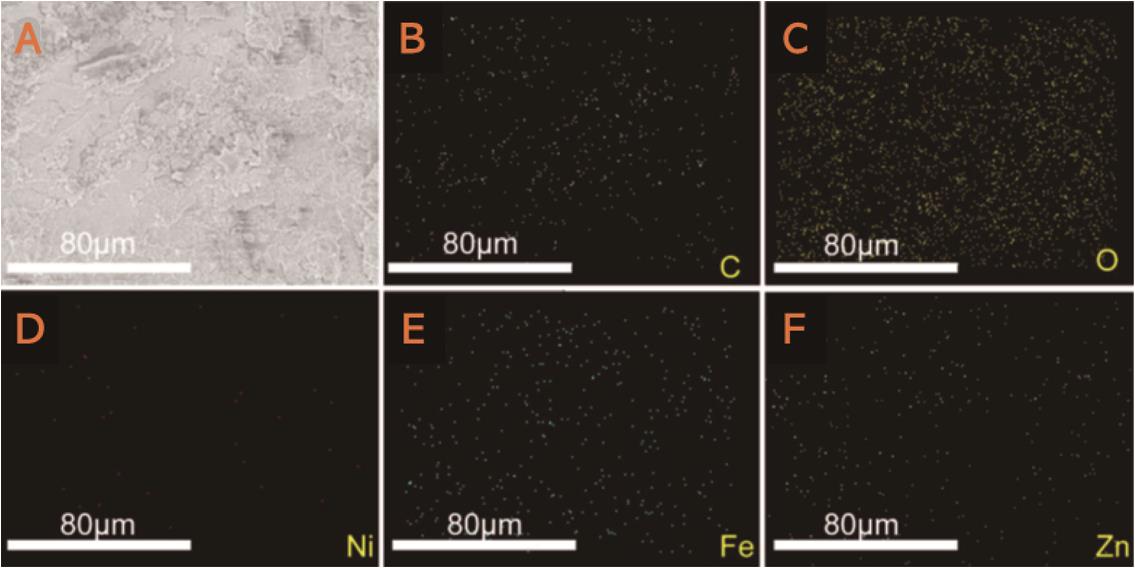
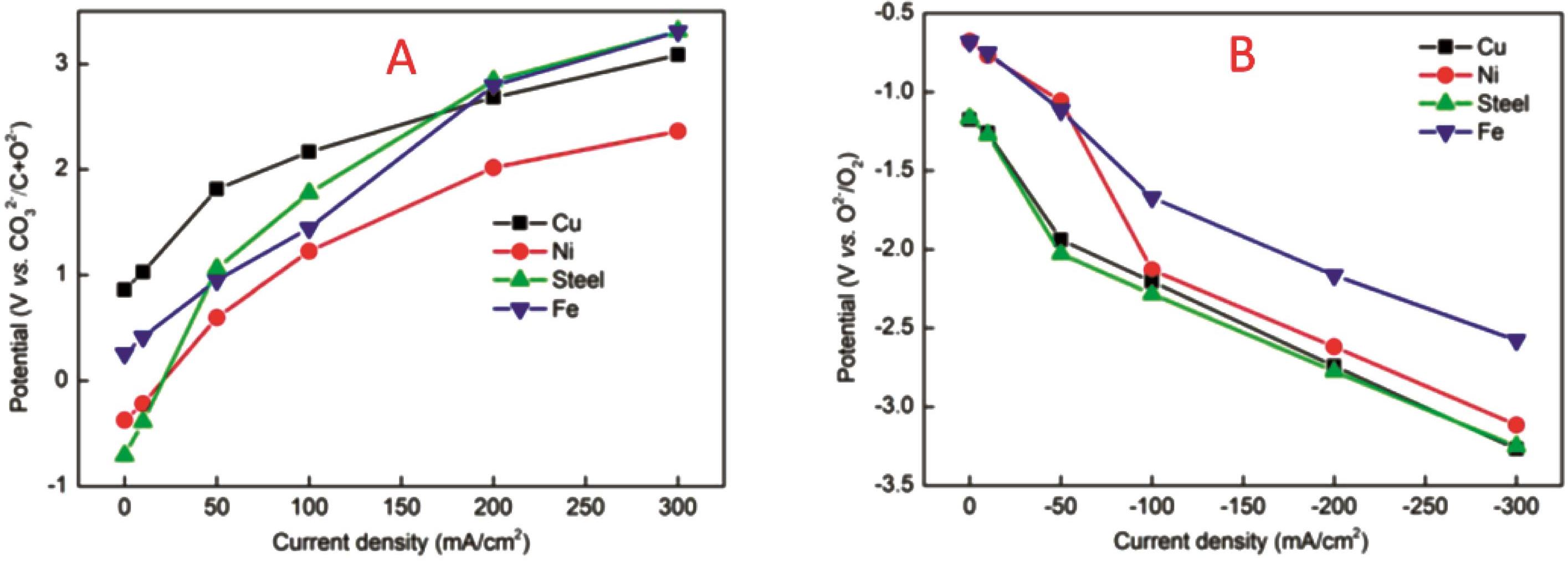
图7 (A)小电流电解后Fe阴极的扫描电子显微镜图;(B-F)分别为Fe阴极上C、O、Ni、Fe和Zn元素EDS能谱分析分布图[49]
Fig.7 (A) SEM image of Fe cathode after small current electrolysis, (B-F) EDS spetrum distributions of C, O, Ni, Fe and Zn at the Fe cathode[49]
| 1 | Climate change: atmospheric carbon dioxide[Z/OL]. [2020-8-14]. https://www.climate.gov/news-features/understanding-climate/climate-change-atmospheric-carbon-dioxide. |
| 2 | PETER S C. Reduction of CO2 to chemicals and fuels: a solution to global warming and energy crisis[J]. ACS Energy Lett, 2018, 3(7): 1557-1561. |
| 3 | DONG F, WANG Y, SU B, et al. The process of peak CO2 emissions in developed economies: a perspective of industrialization and urbanization[J]. Resour Conserv Recy, 2019, 141: 61-75. |
| 4 | CASERINI S, BARRETO B, LANFREDI C, et al. Affordable CO2 negative emission through hydrogen from biomass, ocean liming, and CO2 storage[J]. Mitig Adapt Strat Gl, 2019, 24(7): 1321-1248. |
| 5 | MARDANI A, STREIMIKIENE D, CAVALLARO F, et al. Carbon dioxide (CO2) emissions and economic growth: a systematic review of two decades of research from 1995 to 2017[J]. Sci Total Environ, 2019, 649: 31-49. |
| 6 | 郭红霞, 崔继方, 刘利. 氧空位增强光催化还原CO2性能方面的研究进展[J]. 应用化学, 2020, 37(3): 256-263. |
| GUO H X, CUI J F, LIU L. Research progress in photocatalytic reduction of CO2 enhanced by oxygen vacancy[J]. Chinese J Appl Chem, 2020, 37(3): 256-263. | |
| 7 | DING Q, KHATTAK S I, AHMAD M. Towards sustainable production and consumption: assessing the impact of energy productivity and eco-innovation on consumption-based carbon dioxide emissions (CCO2) in G-7 nations[J]. Sustain Prod Consump, 2021, 27: 254-268. |
| 8 | 巩金龙. CO2化学转化研究进展概述[J]. 化工学报, 2017, 68(4): 1282-1285. |
| GONG J L. A brief overview on recent progress on chemical conversion of CO2[J]. CIESC J, 2017, 68(4): 1282-1285. | |
| 9 | Climate change: Global temperature[Z/OL]. [2020-3-15]. https://www.climate.gov/news-features/understanding-climate/climate-change-global-temperature. |
| 10 | 毛涛. 碳达峰与碳中和背景下工业低碳发展制度研究[J/OL]. 广西社会科学: 1-9[2021-09-10]. http://kns.cnki.net/-kcms/detail/45.1185.C.20210910.1404.006.html. |
| MAO T. Research on industrial low-carbon development system under the background of carbon peak and carbon neutralization[J/OL]. Guangxi Soc Sci: 1-9[2021-09-10]. http://kns.cnki.net/-kcms/detail/45.1185.C. 20210910. 1404.006.html. | |
| 11 | ALAFNAN S, FALOLA Y, MANSOUR O A, et al. Enhanced recovery from organic-rich shales through carbon dioxide injection: molecular-level investigation[J]. Energ Fuel, 2020. 34(12): 16089-16098. |
| 12 | HUANG C H, TAN C S. A review: CO2 utilization[J]. Aerosol Air Qual Res, 2014, 14(2): 480-499. |
| 13 | 陈倩倩, 顾宇, 唐志永, 等. 以二氧化碳规模化利用技术为核心的碳减排方案[J]. 中国科学院院刊, 2019, 34(4): 478-487. |
| CHEN Q Q, GU Y, TANG Z Y, et al. Carbon dioxide sizable utilization technology based carbon reduction solutions[J]. Bull Chinese Acad Sci, 2019, 34(4): 478-487. | |
| 14 | FUKUMOTO M, SUGIUCHI K, NAKAJIMA K. Formation of porous Ni surface by electrodeposition and dissolution in molten salt[J]. Int J Hydrogen Energ, 2020, 45(53): 28252-28259. |
| 15 | CHEN G Z, FRAY D J, FARTHING T W. Direct electrochemical reduction of titanium dioxide to titanium in molten calcium chloride[J]. Nature, 2000, 407(6802): 361-364. |
| 16 | LIANG Y, LI Y K, XUE L Y, et al. Extraction of rare earth elements from fluoride molten salt electrolytic slag by mineral phase reconstruction[J]. J Clean Prod, 2018, 177: 567-572. |
| 17 | JIAO H, SONG W L, CHEN H S, et al. Sustainable recycling of titanium scraps and purity titanium production via molten salt electrolysis[J]. J Clean Prod, 2020, 261: 121314. |
| 18 | 张明杰. 熔盐电化学原理与应用[M]. 北京: 化学工业出版社, 2006: 1-40. |
| ZHANG M J. Principle and application of molten salt electrochemistry[M]. Beijing: Chemical Industry Press, 2006: 1-40. | |
| 19 | KAPLAN V, WACHTEL E, GARTSMAN K, et al. Conversion of CO2 to CO by electrolysis of molten lithium carbonate[J]. J Electrochem Soc, 2010, 157(4): B552-B556. |
| 20 | LIU Y, YUAN D D, JI D Q, et al. Syngas production: diverse H2/CO range by regulating carbonates electrolyte composition from CO2/H2O via co-electrolysis in eutectic molten salts[J]. RSC Adv, 2017, 7(83): 52414-52422. |
| 21 | 刘军, 孟桂祥, 姚胜, 等. 超低浓度气氛中CO2吸附动力学影响因素分析[J]. 化工进展, 2017, 36(8): 3092-3099. |
| LIU J, MENG G X, YAO S, et al. Analysis on influence factors of CO2 adsorption kinetics under ultra-low CO2 atmosphere[J]. Chem Ind Eng Prog, 2017, 36(8): 3092-3099. | |
| 22 | CHUNG W, ROH K, LEE J H. Design and evaluation of CO2 capture plants for the steelmaking industry by means of amine scrubbing and membrane separation[J]. Int J Greenh Gas Con, 2018, 74: 259-270. |
| 23 | GHASEM N. Advances in carbon capture[M]. RAHIMPOUR M R. Britain: Woodhead Publishing, 2020: 479-501. |
| 24 | 徐志明, 王颖聪, 郜时旺, 等. 碳酸钾溶液捕集CO2的吸收热研究[J]. 中国电机工程学报, 2015, 35(9): 2254-2260. |
| XU Z M, WANG Y C, GAO S W, et al. Heat of CO2 absorption in aqueous potassium carbonate solution[J]. Proc Chin Soc Electrical Eng, 2015, 35(9): 2254-2260. | |
| 25 | 赵文波, 李广振, 许胜超, 等. 相变吸收酸性气体的发展现状[J]. 化工进展, 2021, 40(1): 401-414. |
| ZHAO W B, LI G Z, XU S C, et al. Recent developments of acid gas absorption by phase-change[J]. Chem Ind Eng Prog, 2021, 40(1): 401-414. | |
| 26 | LIAN Y H, DENG S, LI S J, et al. Numerical analysis on CO2 capture process of temperature swing adsorption (TSA): optimization of reactor geometry[J]. Int J Greenh Gas Con, 2019, 85: 187-198. |
| 27 | GREEN D A, TURK B S, PORTZER Z W, et al. Capture of carbon dioxide from flue gas using solid regenerable sorbents[J]. Int J Environ Technol, 2004, 4(1): 53-67. |
| 28 | 赵传文, 陈晓平, 赵长遂. 钾基CO2吸收剂的碳酸化反应特性[J]. 化工学报, 2008, 59(9): 2328-2333. |
| ZHAO C W, CHEN X P, ZHAO C S. Carbonation reaction characteristics of dry potassium-based sorbent for CO2 capture[J]. CIESC J, 2008, 59(9): 2328-2333. | |
| 29 | 邓博文, 尹华意, 汪的华. CO2高效资源化利用的高温熔盐电化学技术研究[J]. 电化学, 2020, 26(5): 628-638. |
| DENG B W, YIN H Y, WANG D H. Highly efficient CO2 utilization via molten salt CO2 capture and electrochemical transformation technology[J]. J Electrochem, 2020, 26(5): 628-638. | |
| 30 | 徐敏杰, 朱明辉, 陈天元, 等. CO2高值化利用: CO2加氢制甲醇催化剂研究进展[J]. 化工进展, 2021, 40(2): 565-576. |
| XU M J, ZHU M H, CHEN T Y, et al. High value utilization of CO2: research progress of catalyst for hydrogenation of CO2 to methanol[J]. Chem Ind Eng Prog, 2021, 40(2): 565-576. | |
| 31 | MATSUURA F, WAKAMATSU T, NATSUI S, et al. CO gas production by molten salt electrolysis from CO2 gas[J]. ISIJ Int, 2015, 55(2): 404-408. |
| 32 | WU H J, LIU Y, JI D Q, et al. Renewable and high efficient syngas production from carbon dioxide and water through solar energy assisted electrolysis in eutectic molten salts[J]. J Power Sources, 2017, 362: 92-104. |
| 33 | YIN H Y, MAO X H, TANG D Y, et al. Capture and electrochemical conversion of CO2 to value-added carbon and oxygen by molten salt electrolysis[J]. Energ Environ Sci, 2013, 6(5): 1538-1545. |
| 34 | REN J W, JOHNSON M, SINGHAL R, et al. Transformation of the greenhouse gas CO2 by molten electrolysis into a wide controlled selection of carbon nanotubes[J]. J CO2 Util, 2017, 18: 335-344. |
| 35 | DOUGLAS A, CARTER R E, LI M, et al. Toward small-diameter carbon nanotubes synthesized from captured carbon dioxide: critical role of catalyst coarsening[J]. ACS Appl Mater Int, 2018, 10(22): 19010-19018. |
| 36 | WANG X R, LIU X Y, LICHT G, et al. Calcium metaborate induced thin walled carbon nanotube syntheses from CO2 by molten carbonate electrolysis[J]. Sci Rep-UK, 2020, 10(1): 1-7. |
| 37 | LICHT S, WANG B H, GHOSH S, et al. A new solar carbon capture process: solar thermal electrochemical photo (STEP) carbon capture[J]. J Phys Chem Lett, 2010, 1(15): 2363-2368. |
| 38 | LICHT S, WANG B H, WU H J. STEP—a solar chemical process to end anthropogenic global warming. II: experimental results[J]. J Phys Chem C, 2011, 115(23): 11803-11821. |
| 39 | 吴红军, 董维, 王宝辉, 等. 太阳能光-热-电化学耦合法理论及其化学利用新技术进展[J]. 化工进展, 2014, 33(7): 1718-1724. |
| WU H J, DONG W, WANG B H, et al. STEP theory and its new technology in chemical utilization[J]. Chem Ind Eng Prog, 2014, 33(7): 1718-1724. | |
| 40 | GROULT H, KAPLAN B, KOMABA S, et al. Lithium insertion into carbonaceous anode materials prepared by electrolysis of molten Li-K-Na carbonates[J]. J Electrochem Soc, 2002, 150(2): G67-G75. |
| 41 | GROULT H, KAPLAN B, LANTELME F, et al. Preparation of carbon nanoparticles from electrolysis of molten carbonates and use as anode materials in lithium-ion batteries[J]. Solid State Ionics, 2006, 177(9/10): 869-875. |
| 42 | DOUGLAS A, CARTER R, MURALIDHARAN N, et al. Iron catalyzed growth of crystalline multi-walled carbon nanotubes from ambient carbon dioxide mediated by molten carbonates[J]. Carbon, 2017, 116: 572-578. |
| 43 | DOUGLAS A, CARTER R E, LI M Y, et al. Toward small diameter carbon nanotubes synthesized from captured carbon dioxide: critical role of catalyst coarsening[J]. ACS Appl Mater Inter, 2018, 10(22): 19010-19018. |
| 44 | WU H J, LI Z D, JI D Q, et al. One-pot synthesis of nanostructured carbon materials from carbon dioxide via electrolysis in molten carbonate salts[J]. Carbon, 2016, 106: 208-217. |
| 45 | WU H J, LI Z D, JI D Q, et al. Effect of molten carbonate composition on the generation of carbon material[J]. RSC Adv, 2017, 7(14): 8467-8473. |
| 46 | LI Z D, YUAN D D, WU H J, et al. A novel route to synthesize carbon spheres and carbon nanotubes from carbon dioxide in a molten carbonate electrolyzer[J]. Inorg Chem Front, 2018, 5(1): 208-216. |
| 47 | LI, Z D, WANG G Z, ZHANG W Y, et al. Carbon nanotubes synthesis from CO2 based on the molten salts electrochemistry: effect of alkaline earth carbonate additives on the diameter of the carbon nanotubes[J]. J Electrochem Soc, 2019, 166(10): D415-D420. |
| 48 | YU Y Y, LI Z D, ZHANG W Y, et al. Facile synthesis of capacitive carbon materials via electrolyzing carbonates eutectic with varying metallic anodes[J]. J Electrochem Soc, 2018, 165(13): D612-D619. |
| 49 | LI Z D, ZHANG W Y, JI D Q, et al. Electrochemical conversion of CO2 into valuable carbon nanotubes: the insights into metallic electrodes screening[J]. J Electrochem Soc, 2020, 167: 042501. |
| 50 | MASSOT L, CHAMELOT P, BOUYER F, et al. Electrodeposition of carbon films from molten alkaline fluoride media[J]. Electrochim Acta, 2003, 47(12): 1949-1957. |
| 51 | NOVOSELOVA I A, OLIINYK N F, VOLKOV S V, et al. Electrolytic synthesis of carbon nanotubes from carbon dioxide in molten salts and their characterization[J]. Phys E, 2008, 40(7): 2231-2237. |
| 52 | DENG B W, CHEN Z G, GAO M X, et al. Molten salt CO2 capture and electro-transformation (MSCC-ET) into capacitive carbon at medium temperature: effect of the electrolyte composition[J]. Faraday Discuss, 2016, 190: 241-258. |
| 53 | 王宝辉, 洪美花, 吴红军, 等. 熔盐电解还原二氧化碳制碳技术[J]. 化工进展, 2013, 32(9): 2120-2125. |
| WANG B H, HONG M H, WU H J, et al. Study on carbon generation from carbon dioxide by molten salt electrolysis[J]. Chem Ind Eng Prog, 2013, 32(9): 2120-2125. | |
| 54 | INGRAM M D, BARON B, JANZ G J. The electrolytic deposition of carbon from fused carbonates[J]. Electrochim Acta, 1966, 11(11): 1629-1639. |
| 55 | DELIMARSKII Y K, GORODYSKII A V, GRISHCHENKO V F. Cathode liberation of carbon from molten carbonates[J]. Dokl Akad Nauk SSSR, 1964, 156(3): 650-651. |
| 56 | KAWAMURA H, ITO Y. Electrodeposition of cohesive carbon films on aluminum in a LiCl-KCl-K2CO3 melt[J]. J Appl Electrochem, 2000, 30(5): 571-574. |
| 57 | NOVOSELOVA I A, OLIINYK N F, VOLKOV S V, et al. Electrolytic synthesis of carbon nanotubes from carbon dioxide in molten salts and their characterization[J]. Phys E, 2008, 40(7): 2231-2237. |
| 58 | STERN K H, ROLSION D R. Theme and variations on tantalum-carbonate reactions in molten fluorides[J]. J Electrochem Soc, 1989, 136(12): 3760-3767. |
| 59 | 吕旺燕, 刘世念, 苏伟, 等. 熔盐电沉积碳材料的研究进展[J]. 材料导报, 2012, 26(2): 248-251. |
| LV W Y, LIU S N, SU W, et al. Research progresses in the electro-deposition of carbonaceous materials in molten salts[J]. Mater Rep, 2012, 26(2): 248-251. | |
| 60 | IJIJE H V, LAWRENCE R C, CHEN G Z. Carbon electrodeposition in molten salts: electrode reactions and applications[J]. Rsc Adv, 2014, 4(67): 35808-35817. |
| 61 | KAPLAN B, GROULT H, BARHOUN A, et al. Synthesis and structural characterization of carbon powder by electrolytic reduction of molten Li2CO3-Na2CO3-K2CO3[J]. J Electrochem Soc, 2002, 149(5): D72-D78. |
| 62 | IJIJE H V, SUN C G, CHEN G Z. Indirect electrochemical reduction of carbon dioxide to carbon nanopowders in molten alkali carbonates: process variables and product properties[J]. Carbon, 2014, 73: 163-174. |
| 63 | 尹华意. 基于高温熔盐化学的减碳和固碳技术研究[D]. 武汉: 武汉大学, 2012. |
| YIN H Y. Research on carbon reduction and fixation technology based on high temperature molten salt chemistry[D]. Wuhan: Wuhan University, 2012. | |
| 64 | YIN H Y, MAO X H, TANG D Y, et al. Capture and electrochemical conversion of CO2 to value-added carbon and oxygen by molten salt electrolysis[J]. Energ Environ Sci, 2013, 6(5): 1538-1545. |
| 65 | YU Y Y, LI Z D, ZHANG W Y, et al. Effect of BaCO3 addition on the CO2-derived carbon deposition in molten carbonates electrolyzer[J]. New J Chem, 2018, 42(2): 1208-1215. |
| 66 | VAN K L, GROULT H, LANTELME F, et al. Electrochemical formation of carbon nano-powders with various porosities in molten alkali carbonates[J]. Electrochim Acta, 2009, 54(19): 4566-4573. |
| 67 | 李志达, 李金莲, 吴红军. 熔盐体系组成对二氧化碳电化学合成新型碳材料形貌影响[J]. 化工进展, 2019, 38(9): 4174-4182. |
| LI Z D, LI J L, WU H J. Impact of molten salts conformation on the morphology of the electrochemically synthesized carbon materials[J]. Chem Ind Eng Prog, 2019, 38(9): 4174-4182. | |
| 68 | JIANG M P, LI Z D, YU Y Y, et al. Efficient conversion of greenhouse gas of CO2 into carbon products with desirable structures via molten carbonates electrolysis[J]. J Electrochem Soc, 2017, 164(14): D1022-D1027. |
| 69 | DENG B W, MAO X H, XIAO W, et al. Microbubble effect-assisted electrolytic synthesis of hollow carbon spheres from CO2[J]. J Mater Chem A, 2017, 5(25): 12822-12827. |
| 70 | NIWASE K, HOMAE T, NAKAMURA K G, et al. Generation of giant carbon hollow spheres from C60 fullerene by shock-compression[J]. Chem Phys Lett, 2002, 362(1): 47-50. |
| 71 | BORGOHAIN R, YANG J, SELEGUE J P, et al. Controlled synthesis, efficient purification, and electrochemical characterization of arc-discharge carbon nano-onions[J]. Carbon, 2014, 66: 272-284. |
| 72 | SU F, ZHAO X S, WANG Y, et al. Hollow carbon spheres with a controllable shell structure[J]. J Mater Chem, 2006, 16(45): 4413-4419. |
| 73 | HU F P, WANG Z U, LI Y L, et al. Improved performance of Pd electrocatalyst supported on ultrahigh surface area hollow carbon spheres for direct alcohol fuel cells[J]. J Power Sources, 2008, 177(1): 61-66.. |
| 74 | FU J W, XU Q, CHEN J F, et al. Controlled fabrication of uniform hollow core porous shell carbon spheres by the pyrolysis of core/shell polystyrene/cross-linked polyphosphazene composites[J]. Chem Commun, 2010, 46(35): 6563-6565. |
| 75 | HSU W K, HARE J P, TERRONES M, et al. Condensed-phase nanotubes[J]. Nature, 1995, 377(6551): 687-687. |
| 76 | HSU W K, TERRONES M, HARE J P, et al. Electrolytic formation of carbon nanostructures[J]. Chem Phys Lett, 1996, 262(1): 161-166. |
| 77 | CHEN G Z, FAN X D, LUGET A, et al. Electrolytic conversion of graphite to carbon nanotubes in fused salts[J]. J Electroanal Chem, 1998, 446(1): 1-6. |
| 78 | CHEN G Z, KINLOCH I, SHAFFER M S P, et al. Electrochemical investigation of the formation of carbon nanotubes in molten salts[J]. High Temp Mater P-US, 1998, 2(4): 459-469. |
| 79 | SCHWANDT C, DIMITROV A T, FRAY D J. The preparation of nano-structured carbon materials by electrolysis of molten lithium chloride at graphite electrodes[J]. J Electroanal Chem, 2010, 647(2): 150-158. |
| 80 | DIMITROV A T. Study of molten Li2CO3 electrolysis as a method for production of carbon nanotubes[J]. Maced J Chem Chem En, 2009, 28(1): 111-118. |
| 81 | REN J W, L F F, LAU J, et al. One-pot synthesis of carbon nanofibers from CO2[J]. Nano Lett, 2015, 15(9): 6142-6148. |
| 82 | REN J W, LICHT S. Tracking airborne CO2 mitigation and low cost transformation into valuable carbon nanotubes[J]. Sci Rep-UK, 2016, 6(1): 1-11. |
| 83 | LICHT S, DOUGLAS A, REN J W, et al. Carbon nanotubes produced from ambient carbon dioxide for environmentally sustainable lithium-ion and sodium-ion battery anodes[J]. ACS Comb Sci, 2016, 2(3): 162-168. |
| [1] | 周嘉成, 王冬冬, 高云宝, 金晶, 姜伟. 基于聚二氧化碳树脂压敏胶的流变及粘结性能[J]. 应用化学, 2023, 40(6): 871-878. |
| [2] | 朱凤, 彭小连, 张文彬. 质子给受体对电催化反应影响的研究进展[J]. 应用化学, 2023, 40(5): 666-680. |
| [3] | 王小梅, 郭江飞, 王立志, 黄健涵. 卟啉基超交联聚合物的合成及其对二氧化碳和汞(Ⅱ)离子的吸附[J]. 应用化学, 2022, 39(7): 1083-1089. |
| [4] | 商林杰, 刘江, 兰亚乾. 共价有机框架材料用于光/电催化CO2还原的研究进展[J]. 应用化学, 2022, 39(4): 559-584. |
| [5] | 喻奥, 马国铭, 朱龙涛, 彭平, 李芳芳. 电化学还原二氧化碳合成碳材料电催化还原氧气合成过氧化氢[J]. 应用化学, 2022, 39(4): 657-665. |
| [6] | 叶祥志, 邓云水, 刘源, 周咏柳, 贺建雄, 熊春荣. 玻璃球负载非晶态有机钛聚合物提高光催化还原CO2的转换频率[J]. 应用化学, 2022, 39(10): 1554-1563. |
| [7] | 陆新宇, 马彬泽, 罗皓, 齐欢, 李强, 伍广朋. 二氧化碳基聚碳酸环己撑酯电子束光刻胶显影工艺优化[J]. 应用化学, 2021, 38(9): 0-0. |
| [8] | 陆新宇, 马彬泽, 罗皓, 齐欢, 李强, 伍广朋. 二氧化碳基聚碳酸环己撑酯电子束光刻胶显影工艺优化[J]. 应用化学, 2021, 38(9): 1189-1198. |
| [9] | 李宫, 金龙一, 姚鹏飞, 刘聪, 徐维林. 介孔炭负载铂纳米粒子的高效氧还原催化剂的可控设计[J]. 应用化学, 2021, 38(12): 1639-1646. |
| [10] | 张守村, 皮茂. 单体对聚乙烯醇乳化行为的影响及大孔材料的制备[J]. 应用化学, 2021, 38(1): 77-83. |
| [11] | 徐小龙, 王绥军, 金翼, 汪浩. 介孔碳材料抑制锂电池负极枝晶生长[J]. 应用化学, 2020, 37(6): 703-708. |
| [12] | 刘军辉, 宋亚坤, 宋春山, 郭新闻. 金属-有机骨架衍生催化剂在二氧化碳加氢和费托合成反应中的应用[J]. 应用化学, 2020, 37(10): 1099-1111. |
| [13] | 郭洪辰, 秦玉升, 王献红, 王佛松. 铝卟啉配合物催化二氧化碳与环氧丙烷共聚反应[J]. 应用化学, 2019, 36(10): 1118-1127. |
| [14] | 冷爽, 王韬, 杨敏, 赵彦芝, 陆伟, 王若明, 孙国英. 基于聚多巴胺的氮掺杂碳材料的制备及其电化学性能[J]. 应用化学, 2018, 35(4): 477-483. |
| [15] | 丁凡舒,聂小娃,刘民,宋春山,郭新闻. Fe基催化剂上二氧化碳加氢制C2+烃的研究进展[J]. 应用化学, 2016, 33(2): 123-132. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||