应用化学 ›› 2024, Vol. 41 ›› Issue (7): 1010-1023.DOI: 10.19894/j.issn.1000-0518.240031
窄带隙 β-CuFeO2铁电光催化剂性质及表面析氧反应特性
- 1.中国科学院长春应用化学研究所,稀土资源利用国家重点实验室,长春 130022
2.中国科学技术大学,合肥 230026
-
收稿日期:2024-01-30接受日期:2024-05-07出版日期:2024-07-01发布日期:2024-08-03 -
通讯作者:刘孝娟 -
基金资助:国家自然科学基金(U2130114);吉林省自然科学基金项目(YDZJ202201ZYS378)
The Properties of Narrow Bandgap β-CuFeO2 Ferroelectric Photocatalysts and Surface Oxygen Evolution Reaction Characteristics
Dong-Hao LYU1,2, Lan-Lan XU1, Xiao-Juan LIU1,2( )
)
- 1.State Key Laboratory of Rare Earth Resources Utilization,Changchun Institute of Applied Chemistry,Chinese Academy of Sciences,Changchun 130022,China
2.University of Science and Technology of China,Hefei 230026,China
-
Received:2024-01-30Accepted:2024-05-07Published:2024-07-01Online:2024-08-03 -
Contact:Xiao-Juan LIU -
About author:lxjuan@ciac.ac.cn
-
Supported by:the National Natural Science Foundation of China(U2130114);the Natural Science Foundation of Jilin Province(YDZJ202201ZYS378)
摘要:
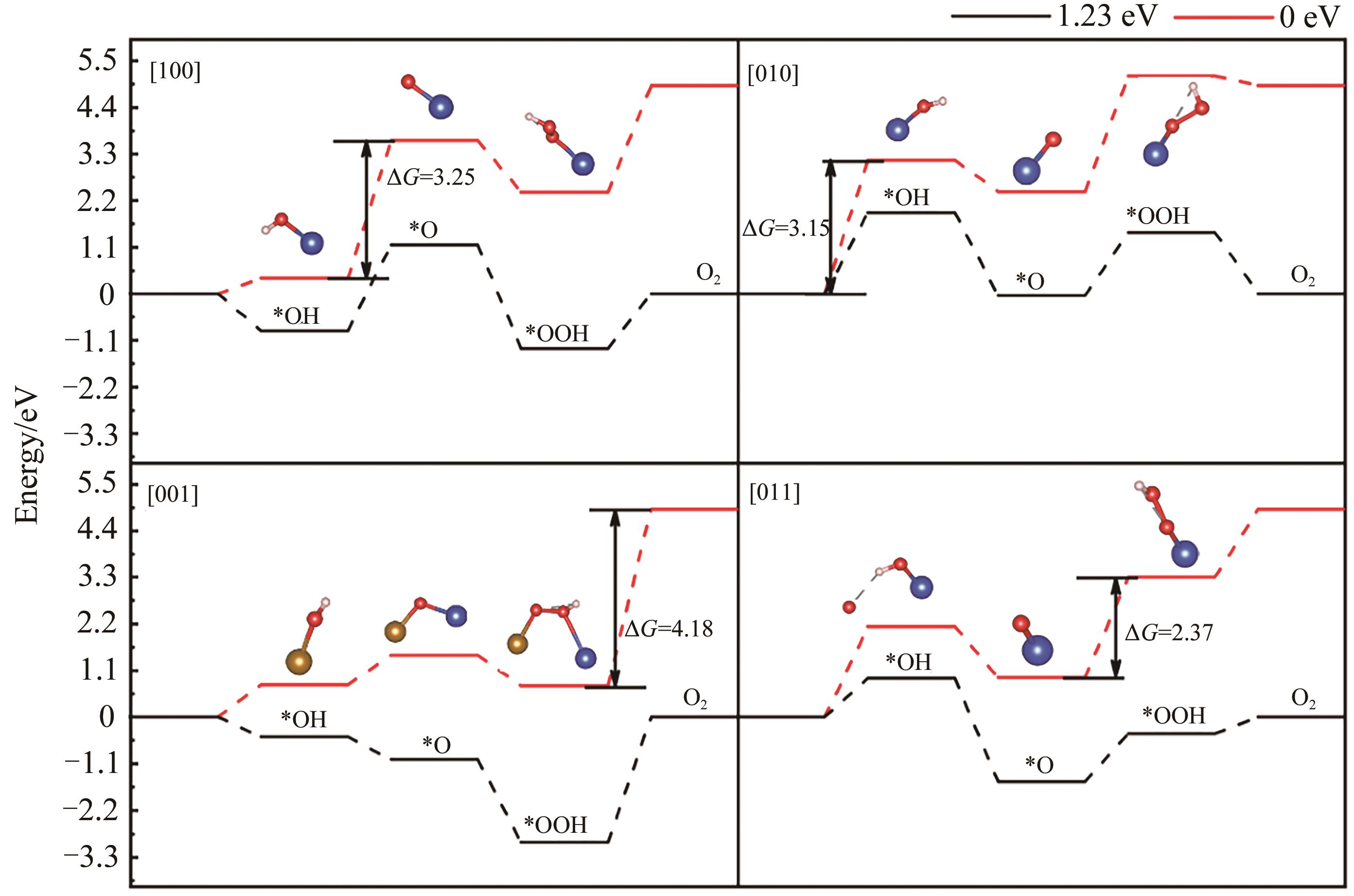
铜铁矿(delafossite)型CuFeO2具有窄带隙、良好稳定性被广泛用于光催化领域的研究,而其中心对称的层状结构使得光生载流子容易复合,限制了其光催化效果。 β-CuFeO2是一种具有相稳定,窄带隙,强极化的本征铁电半导体,利用其内禀的铁电极化性质,构建光生电子空穴生成位点交替排列的[011]表面,在铁电内建电场作用下促进电荷分离,从而提升光催化性能。 基于第一性原理计算,本文首先确定β-CuFeO2具有热力学稳定性,磁基态为C型反铁磁,且带隙为1.37 eV的直接带隙半导体,理论铁电极化为83.466 μC/cm2,是良好的光催化剂载体。 进一步地,以表面析氧反应(OER)为模型,通过构建非极化表面[100]、[010]和极化表面[001]、[011]研究铁电极化对OER的影响。 结果表明,β-CuFeO2的表面价带顶氧化还原电势大部分大于水氧化电势(1.23 eV),且极化表面更易形成。 此外,在极化方向上,完全暴露Cu-O原子,Cu,Fe原子层交替排列的[011]表面最易吸附水分子且具有最优OER催化活性。 对[011]表面OER决速步骤进行电子结构分析,发现在*O中间体上具有2个电子口袋,反应生成*OOH后消耗一个电子口袋,即空轨道上得到电子。 这是[011]极化方向表面OER决速步骤的内在机理。 本工作构建了β-CuFeO2铁电半导体并通过理论模拟计算了其基本性质,构建了不同方向表面研究铁电极化对光催化OER活性的影响,将为铁电光催化剂设计提供理论依据。
中图分类号:
引用本文
吕东昊, 徐兰兰, 刘孝娟. 窄带隙 β-CuFeO2铁电光催化剂性质及表面析氧反应特性[J]. 应用化学, 2024, 41(7): 1010-1023.
Dong-Hao LYU, Lan-Lan XU, Xiao-Juan LIU. The Properties of Narrow Bandgap β-CuFeO2 Ferroelectric Photocatalysts and Surface Oxygen Evolution Reaction Characteristics[J]. Chinese Journal of Applied Chemistry, 2024, 41(7): 1010-1023.
使用本文
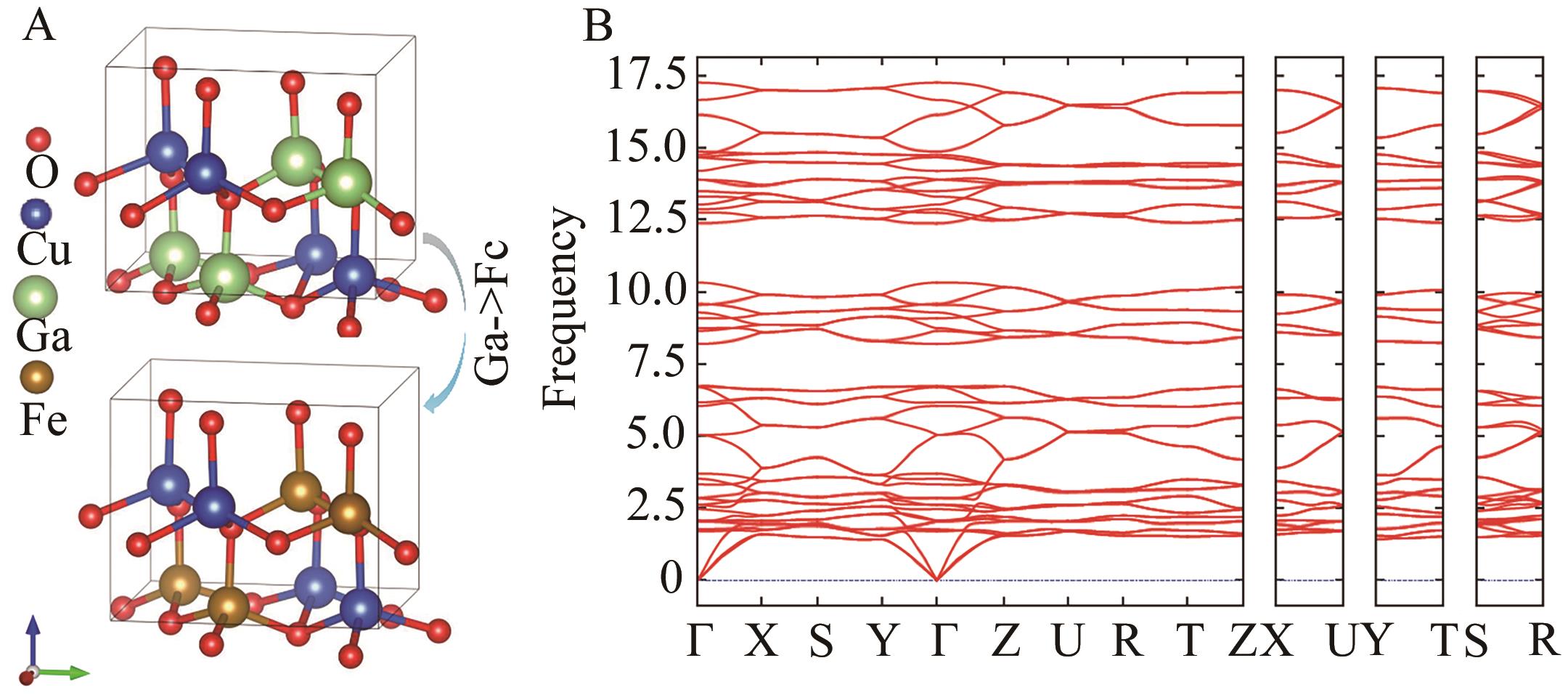
图1 (A) β-CuFeO2体相结构构建示意图及(B) β-CuFeO2体相声子谱计算
Fig.1 (A) Schematic structure construction of β-CuFeO2 bulk phase and (B) β-CuFeO2 bulk phase phonon spectrum calculation
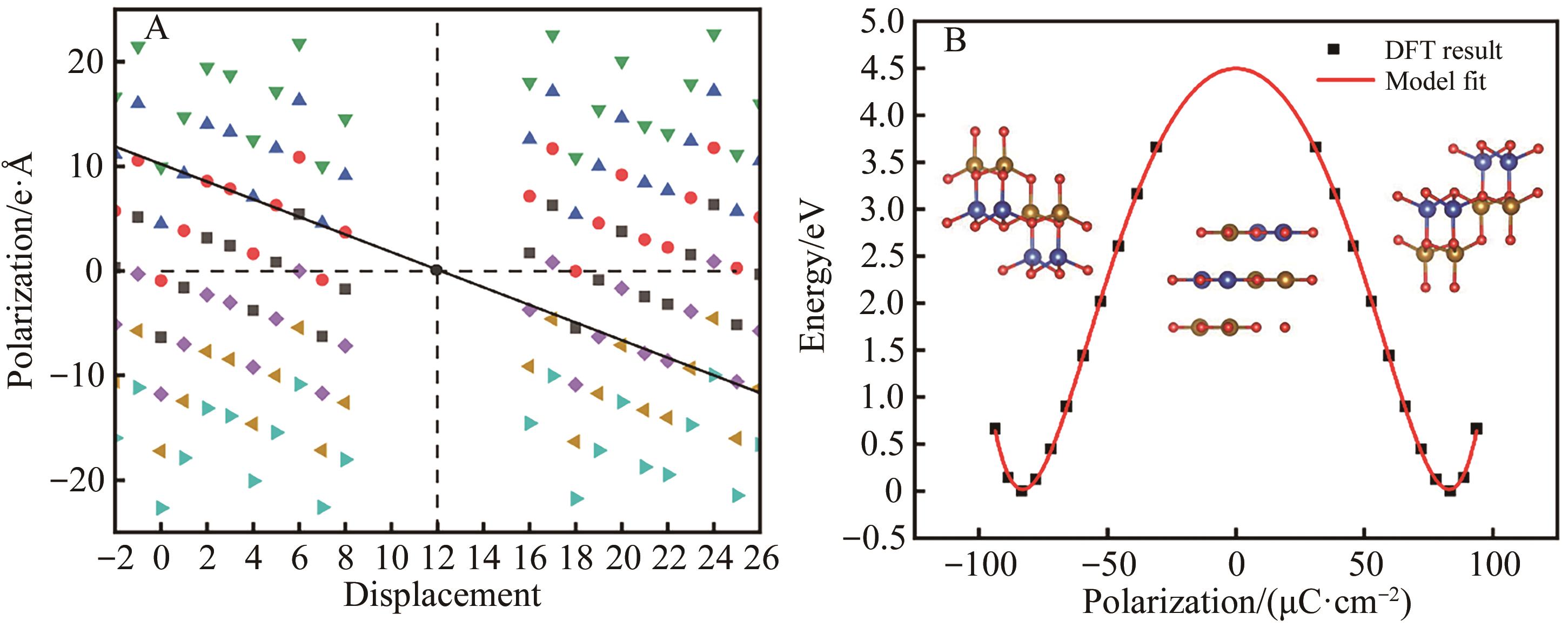
图2 (A) β-CuFeO2体相偏移与极化强度的关系及(B) β-CuFeO2极化与能量的关系
Fig.2 (A) The relationship between phase migration and polarization intensity of β-CuFeO2 and (B) the relationship between polarization and energy of β-CuFeO2
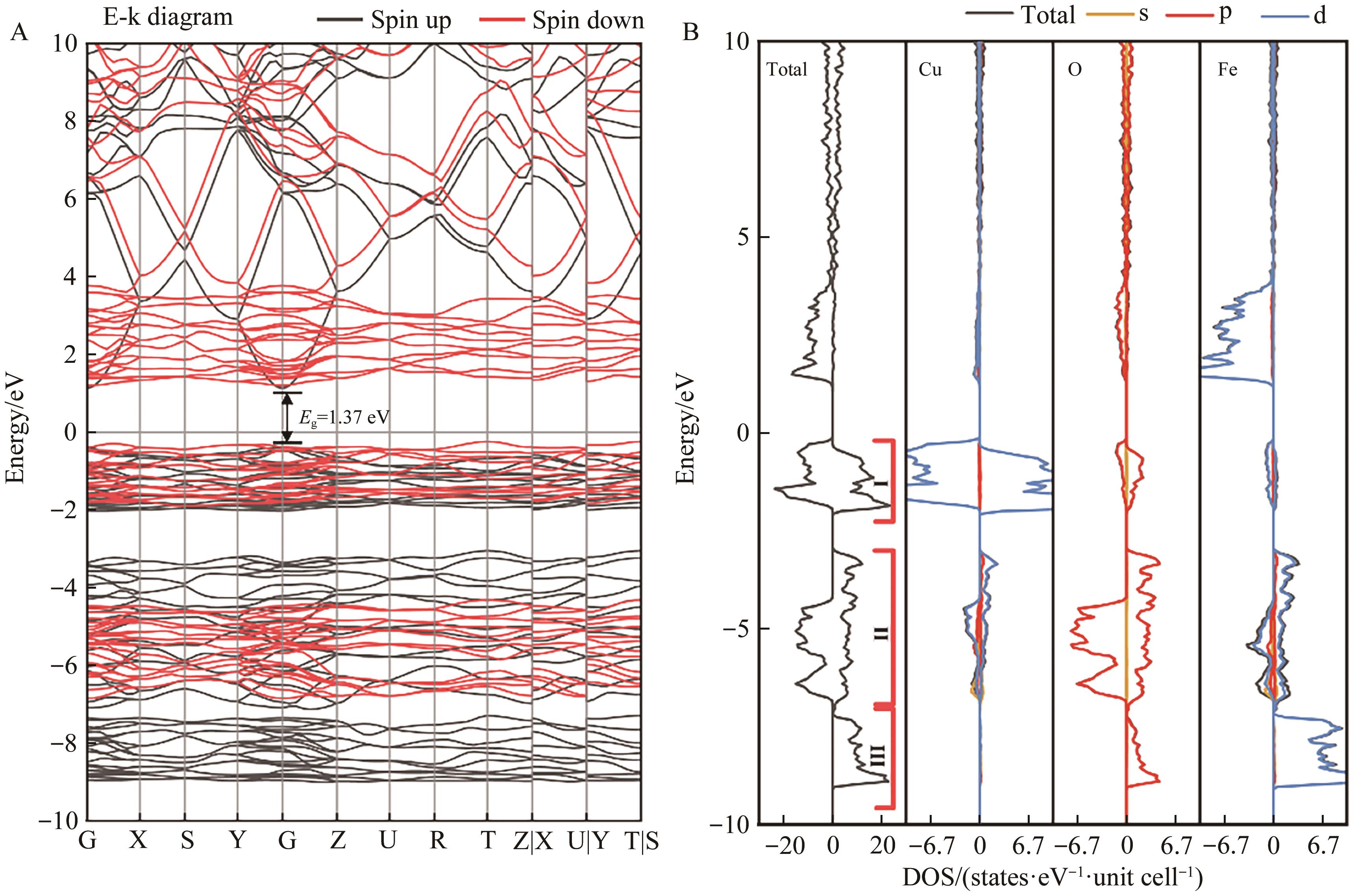
图3 (A)体相β-CuFeO2能带结构及(B) β-CuFeO2总态密度以及各个元素分波态密度
Fig.3 (A) Energy band structure of the bulk phase β-CuFeO2 and (B) total density of states of β-CuFeO2 and the partial wave state densityof each element
| Method | Gap/eV | VBM/eV | CBM/eV | |
|---|---|---|---|---|
| Hse06 | 1.37 | -0.242 | 1.125 | |
| GGA+U | UFe=5 eV, UCu=8 eV | 0.76 | -0.235 | 0.521 |
| UFe=6 eV, UCu=8 eV | 0.89 | -0.247 | 0.639 | |
| UFe=7 eV, UCu=8 eV | 0.92 | -0.247 | 0.668 | |
| UFe=8 eV, UCu=8 eV | 0.91 | -0.238 | 0.671 | |
| UFe=9 eV, UCu=8 eV | 0.90 | -0.223 | 0.675 | |
| UFe=7 eV, UCu=6 eV | 0.76 | -0.238 | 0.517 | |
| UFe=7 eV, UCu=7 eV | 0.83 | -0.221 | 0.614 | |
| UFe=7 eV, UCu=9 eV | 1.00 | -0.226 | 0.773 | |
表1 β-CuFeO2不同U值下带隙以及带边位置
Table 1 Band gap and band edge position under different U values of β-CuFeO2
| Method | Gap/eV | VBM/eV | CBM/eV | |
|---|---|---|---|---|
| Hse06 | 1.37 | -0.242 | 1.125 | |
| GGA+U | UFe=5 eV, UCu=8 eV | 0.76 | -0.235 | 0.521 |
| UFe=6 eV, UCu=8 eV | 0.89 | -0.247 | 0.639 | |
| UFe=7 eV, UCu=8 eV | 0.92 | -0.247 | 0.668 | |
| UFe=8 eV, UCu=8 eV | 0.91 | -0.238 | 0.671 | |
| UFe=9 eV, UCu=8 eV | 0.90 | -0.223 | 0.675 | |
| UFe=7 eV, UCu=6 eV | 0.76 | -0.238 | 0.517 | |
| UFe=7 eV, UCu=7 eV | 0.83 | -0.221 | 0.614 | |
| UFe=7 eV, UCu=9 eV | 1.00 | -0.226 | 0.773 | |
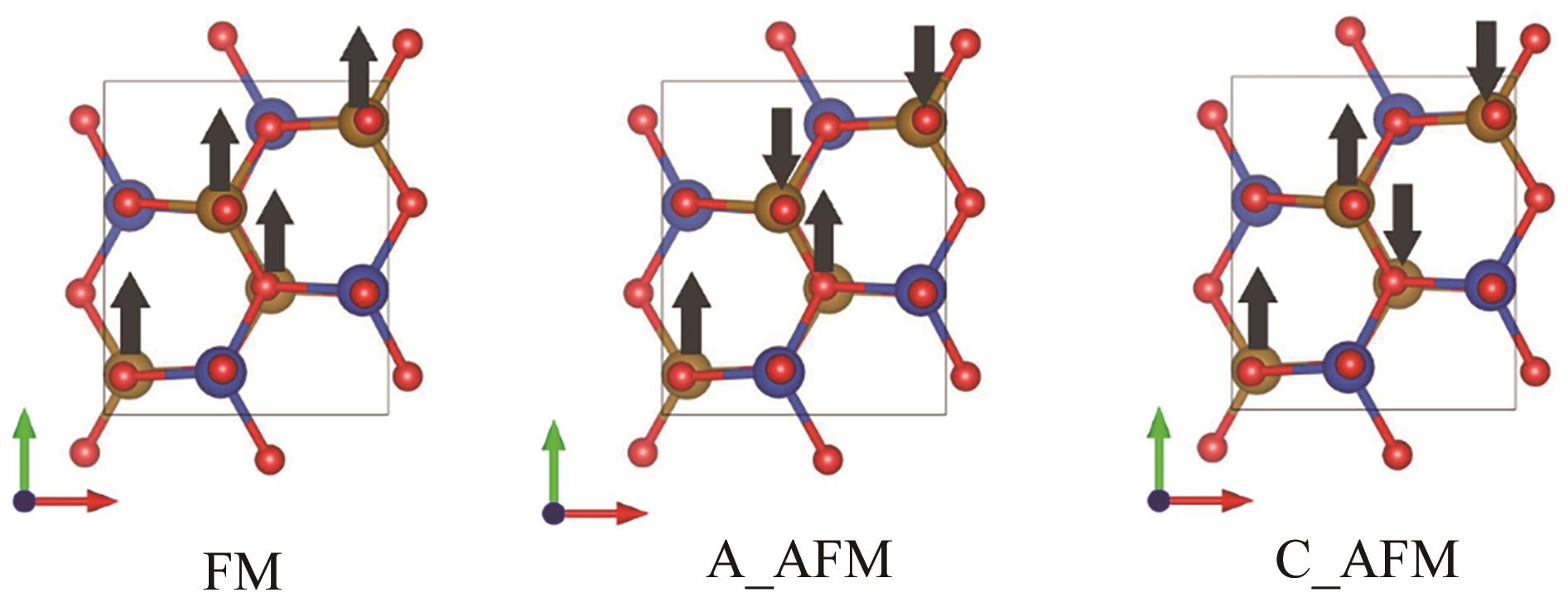
| Magnetic structure | Lattice parameters/? | Energy/eV | Mean magnetic moment (Fe)/μB |
|---|---|---|---|
| FM | a=5.376, b=6.245, c=5.384 | -107.446 | 3.74 |
| A_AFM | a=5.375, b=6.189, c=5.394 | -108.426 | 3.54 |
| C_AFM | a=5.353, b=6.164, c=5.380 | -109.375 | 3.40 |
表2 β-CuFeO2不同磁构型下结构参数、能量以及磁矩
Table 2 Structural parameters, energies, and magnetic moments in different magnetic configurations
| Magnetic structure | Lattice parameters/? | Energy/eV | Mean magnetic moment (Fe)/μB |
|---|---|---|---|
| FM | a=5.376, b=6.245, c=5.384 | -107.446 | 3.74 |
| A_AFM | a=5.375, b=6.189, c=5.394 | -108.426 | 3.54 |
| C_AFM | a=5.353, b=6.164, c=5.380 | -109.375 | 3.40 |
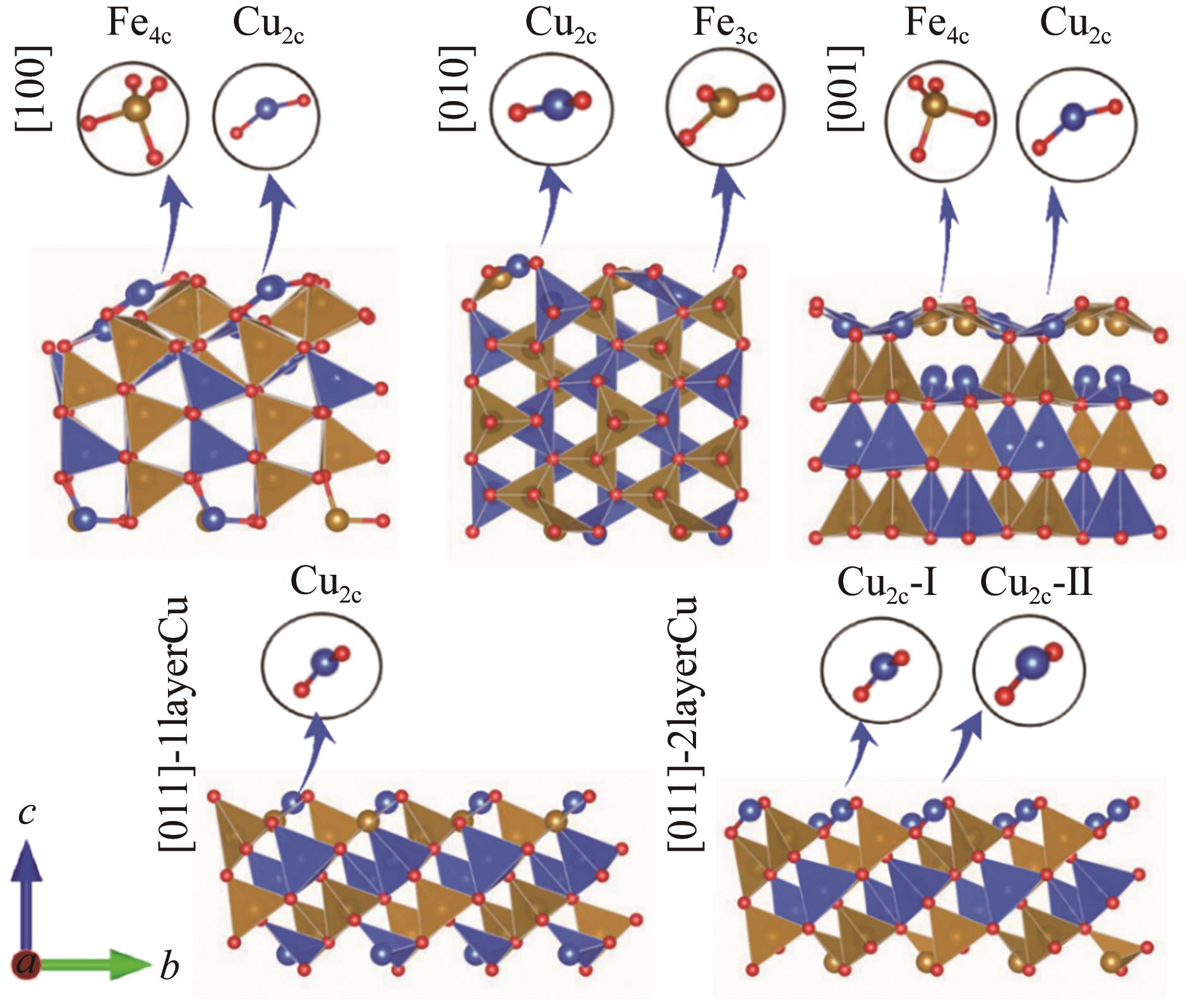
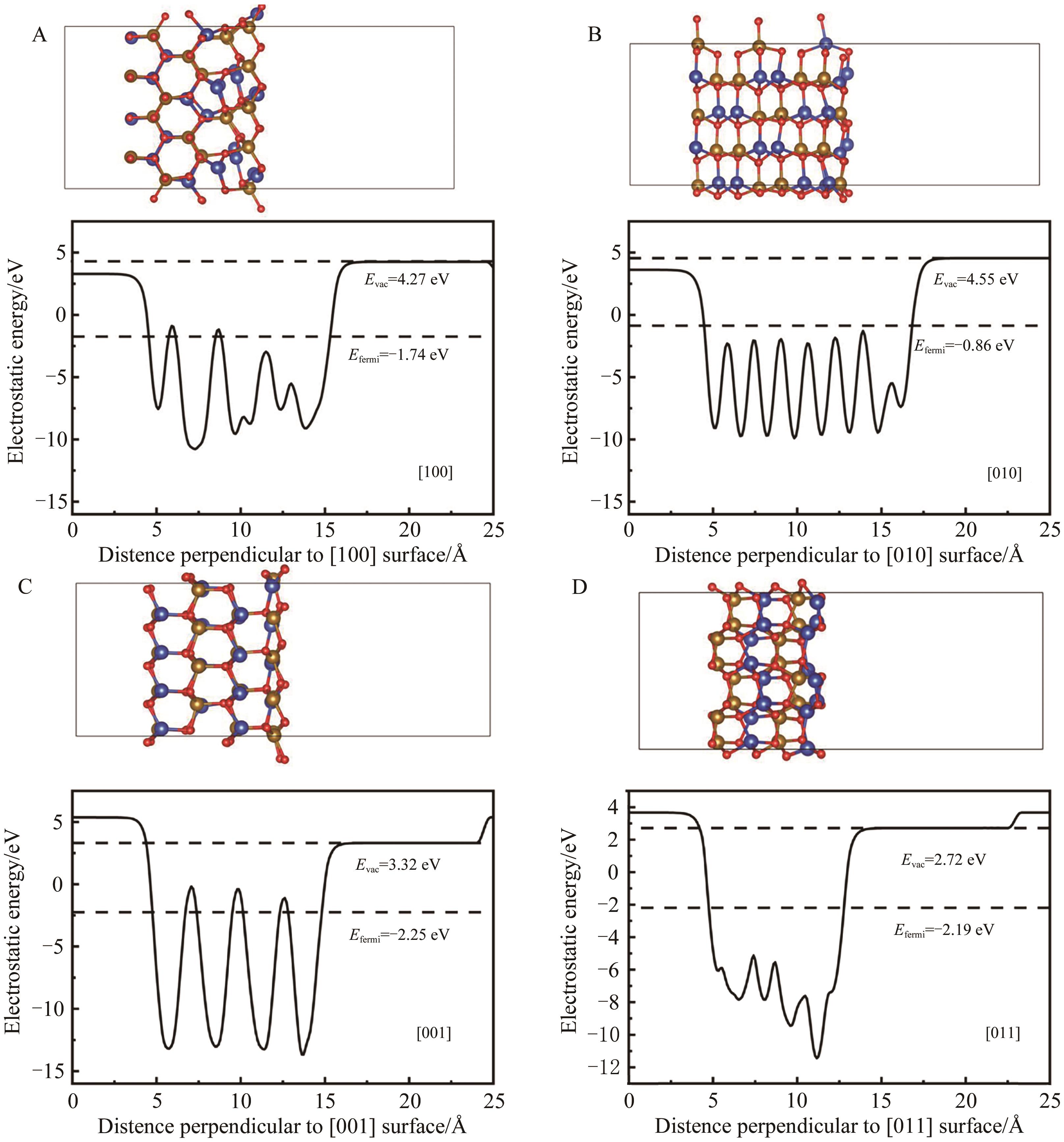
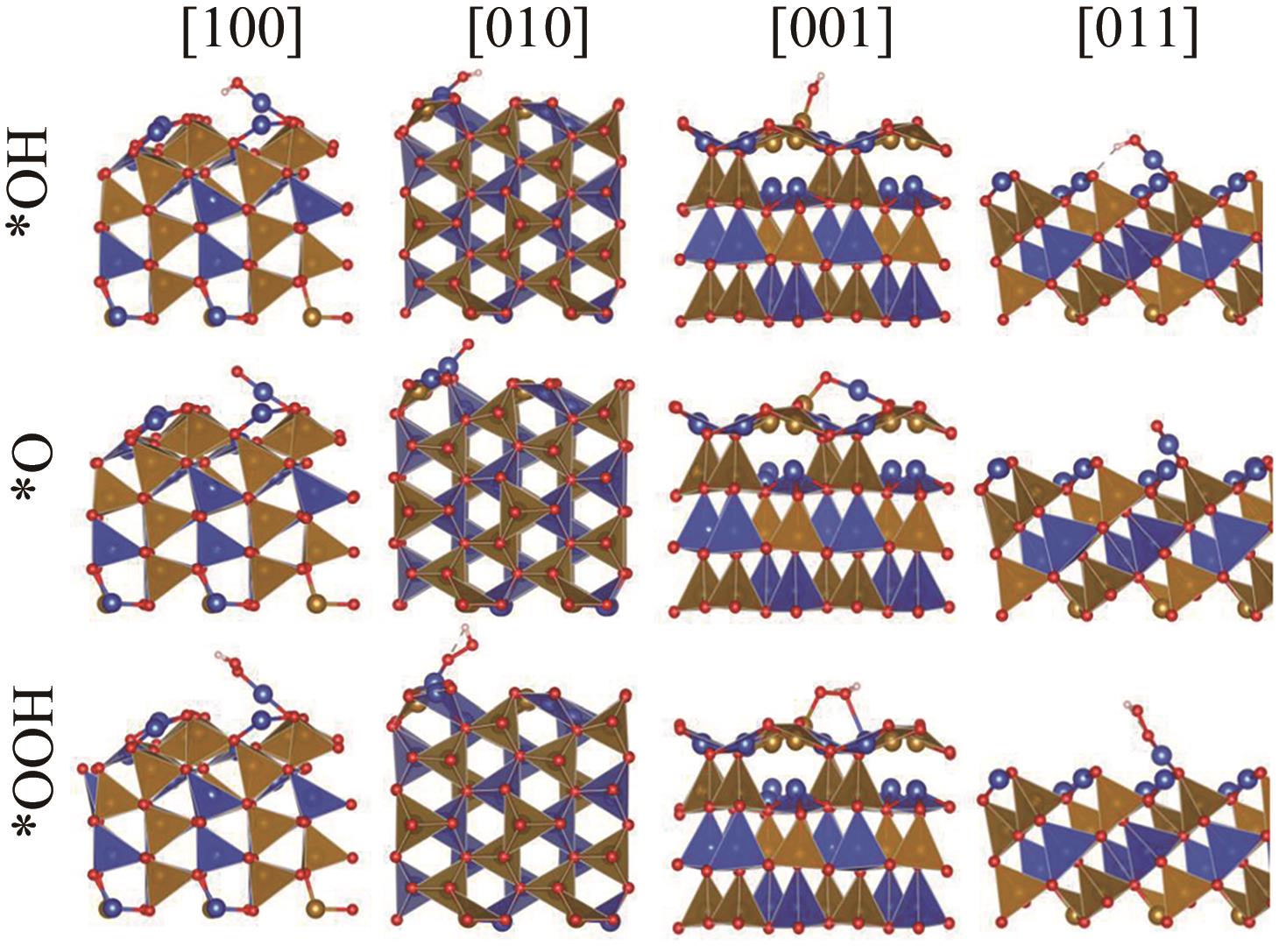
图5 基于DFT优化的[100]、[010]、[001]和[011]取向的β-CuFeO2表面在a方向示意图
Fig.5 The schematic diagrams of the a-direction of the β-CuFeO2 surfaces of [100], [010], [001] and [011] based on DFT optimized slab structures.
| Surfaces | Coordination (unrelax) | ||||
|---|---|---|---|---|---|
| Cu | Fe | ||||
| [100] | 5.19 | -1.24 | 3.95 | 2 | 2 |
| [010] | 4.89 | -1.52 | 3.37 | 3 | 3 |
| [001] | 4.92 | -2.71 | 2.20 | 3 | 3 |
| [011]-2layerCu | 3.84 | -0.94 | 2.90 | 3,2 | 4 |
| [011]-1layerCu | 3.84 | -0.86 | 2.97 | 2 | 3 |
表3 β-CuFeO2不同取向表面的表面能大小和未弛豫表面Cu、Fe原子配位数
Table 3 Surface energy of differently oriented surfaces of β-CuFeO2 and the Cu, Fe atomic coordination number of the surface without structural optimization
| Surfaces | Coordination (unrelax) | ||||
|---|---|---|---|---|---|
| Cu | Fe | ||||
| [100] | 5.19 | -1.24 | 3.95 | 2 | 2 |
| [010] | 4.89 | -1.52 | 3.37 | 3 | 3 |
| [001] | 4.92 | -2.71 | 2.20 | 3 | 3 |
| [011]-2layerCu | 3.84 | -0.94 | 2.90 | 3,2 | 4 |
| [011]-1layerCu | 3.84 | -0.86 | 2.97 | 2 | 3 |
| Surface | Coordination | Surface | Coordination | ||
|---|---|---|---|---|---|
| [100] | 4Fe2c->4c | 0.722 | [010] | 4Fe3c->3c | -0.032 |
| 4Cu2c->2c | 0.112 | 2Cu3c->2c | -0.381 | ||
| 4O2c->2c | -0.126 8 | 2Cu3c->3c | 0.215 | ||
| 4O2c->3c | -0.192 | 8O3c->3c | -0.314 | ||
| [001] | 8Fe3c->3c | 0.005 | 8Cu2c->2c | 0.469 | |
| 8Cu3c->3c | 0.316 | [011]-2layerCu | 8O4c->4c | -0.221 | |
| 8O4c->4c | -0.264 | 8O3c->3c | -0.550 | ||
| 8O4c->3c | -1.219 | 8Cu2c->2c | 0.555 | ||
| [011]-1layerCu | 8O4c->4c | -1.300 | |||
| 8O3c->3c | -0.517 |
表4 β-CuFeO2不同取向表面的表层原子弛豫位移
Table 4 Relaxation displacements of surface atoms on differently oriented surfaces of β-CuFeO2
| Surface | Coordination | Surface | Coordination | ||
|---|---|---|---|---|---|
| [100] | 4Fe2c->4c | 0.722 | [010] | 4Fe3c->3c | -0.032 |
| 4Cu2c->2c | 0.112 | 2Cu3c->2c | -0.381 | ||
| 4O2c->2c | -0.126 8 | 2Cu3c->3c | 0.215 | ||
| 4O2c->3c | -0.192 | 8O3c->3c | -0.314 | ||
| [001] | 8Fe3c->3c | 0.005 | 8Cu2c->2c | 0.469 | |
| 8Cu3c->3c | 0.316 | [011]-2layerCu | 8O4c->4c | -0.221 | |
| 8O4c->4c | -0.264 | 8O3c->3c | -0.550 | ||
| 8O4c->3c | -1.219 | 8Cu2c->2c | 0.555 | ||
| [011]-1layerCu | 8O4c->4c | -1.300 | |||
| 8O3c->3c | -0.517 |
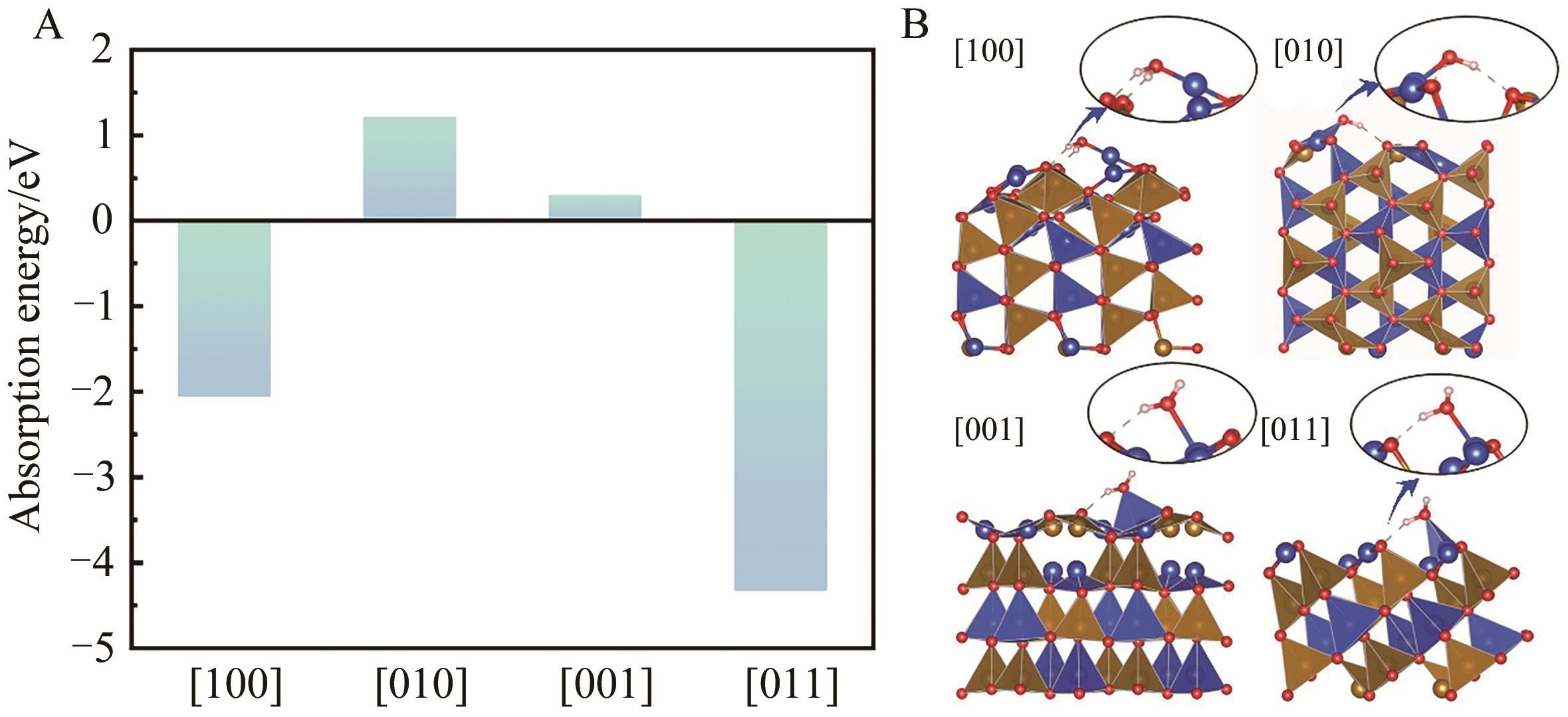
图8 (A) [100]、[010]、[001]和[011]取向表面水吸附能; (B)表面水分子吸附示意图
Fig.8 (A) The water adsorption energy on the [100], [010], [001] and [011] oriented surfaces; (B) Diagram of adsorption of surface water
| Surface | lCu—O/? | lH—O/? | θ/(°) | |||
|---|---|---|---|---|---|---|
| [100] | 1.891 | 0.974 | 1.755 | 1.004 | 1.016 | 99.589 |
| [010] | 2.063 | -0.039 | 1.834 | 0.997 | 0.992 | 104.666 |
| [001] | 2.340 | 0.182 | 1.906 | 0.973 | 0.988 | 107.153 |
| [011] | 1.902 | 1.079 | 1.504 | 0.973 | 1.047 | 109.004 |
表5 β-CuFeO2不同取向表面的表层原子弛豫位移
Table 5 Relaxation displacements of surface atoms on differently oriented surfaces of β-CuFeO2
| Surface | lCu—O/? | lH—O/? | θ/(°) | |||
|---|---|---|---|---|---|---|
| [100] | 1.891 | 0.974 | 1.755 | 1.004 | 1.016 | 99.589 |
| [010] | 2.063 | -0.039 | 1.834 | 0.997 | 0.992 | 104.666 |
| [001] | 2.340 | 0.182 | 1.906 | 0.973 | 0.988 | 107.153 |
| [011] | 1.902 | 1.079 | 1.504 | 0.973 | 1.047 | 109.004 |
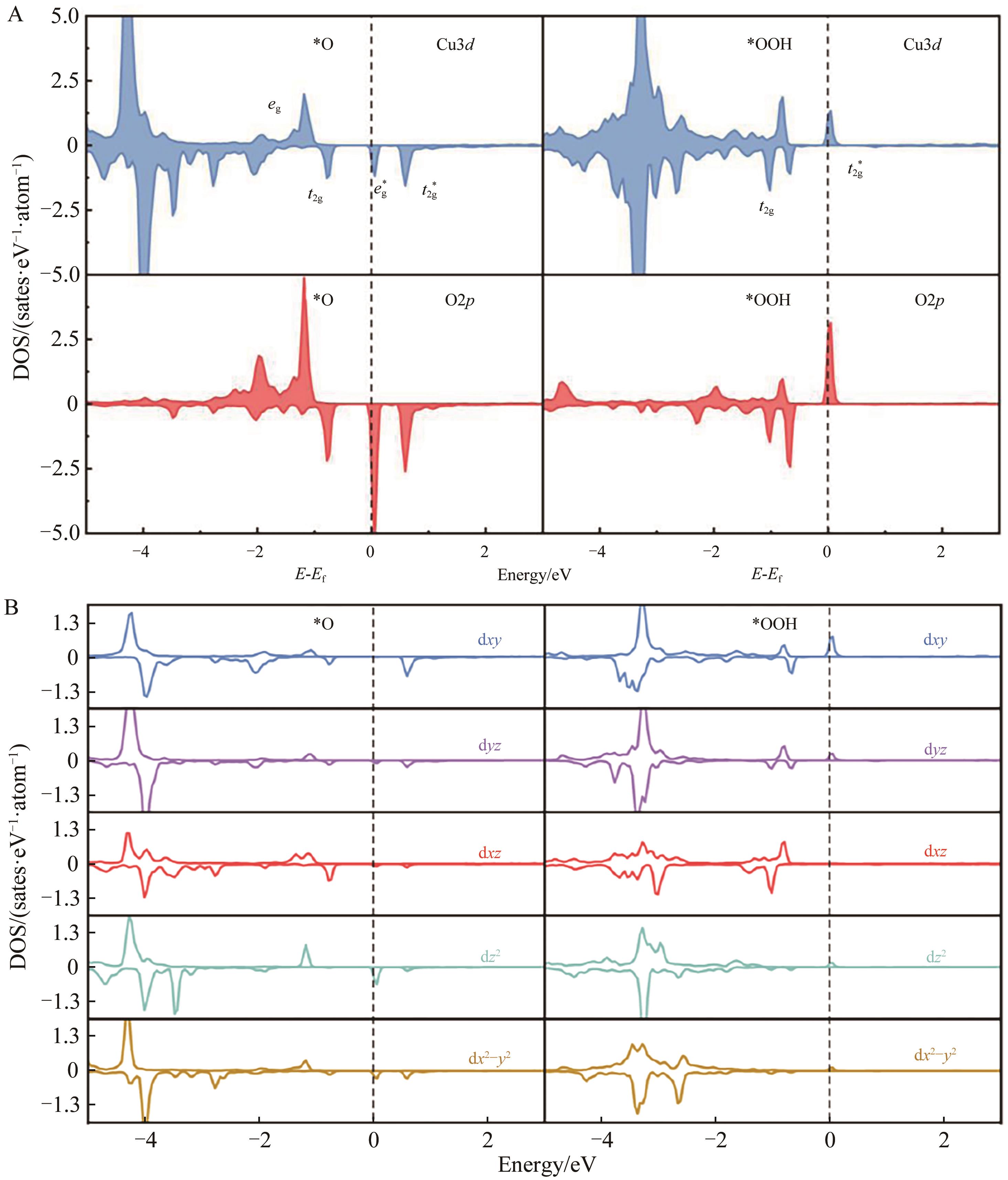
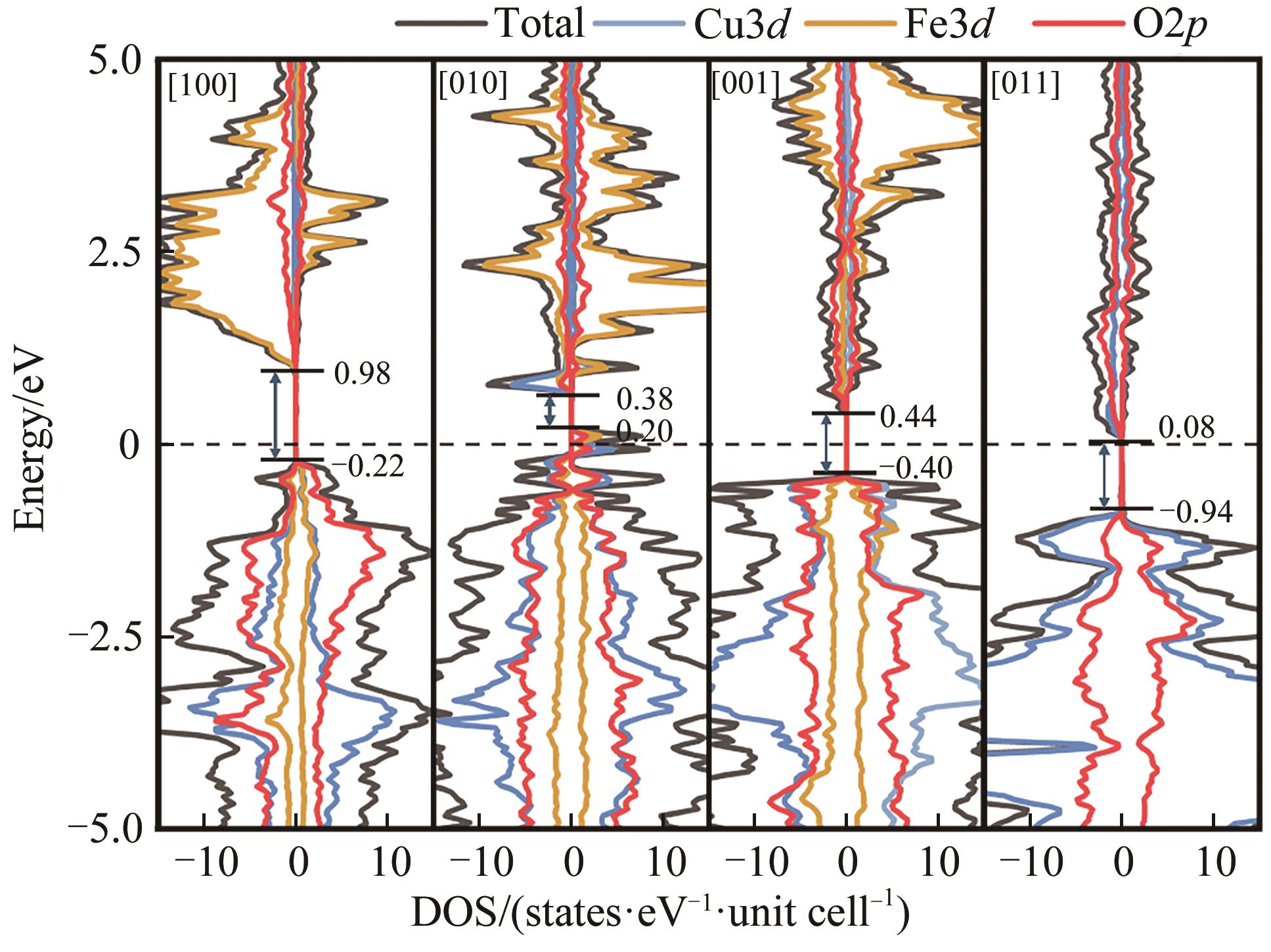
图11 (A) [011]表面OER决速步骤态密度图及(B)反应位点Cu3d轨道分波态密度
Fig.11 (A) Density of states of the rate-determining step for OER and (B) partial wave state density for the reaction site of Cu3d
| 1 | YAN S C, LI Z S, ZOU Z G. Photodegradation performance of g-C3N4 fabricated by directly heating melamine[J]. Langmuir, 2009, 25(17): 10397-10401. |
| 2 | HOUAS A, LACHHEB H, KSIBI M, et al. Photocatalytic degradation pathway of methylene blue in water[J]. Appl Catal B: Environ, 2001, 31(2): 145-157. |
| 3 | TURCHI C S, OLLIS D F. Photocatalytic degradation of organic-water contaminants-mechanisms involving hydroxyl radical attack[J]. J Catal, 1990, 122(1): 178-192. |
| 4 | ZOU Z G, YE J H, SAYAMA K, et al. Direct splitting of water under visible light irradiation with an oxide semiconductor photocatalyst[J]. Nature, 2001, 414(6864): 625-627. |
| 5 | XIANG Q, YU J, JARONIEC M. Synergetic effect of MoS2 and graphene as cocatalysts for enhanced photocatalytic H2 production activity of TiO2 nanoparticles[J]. J Am Chem Soc, 2012, 134(15): 6575-6578. |
| 6 | ZONG X, YAN H, WU G, et al. Enhancement of photocatalytic H2 evolution on CdS by loading MOS2 as cocatalyst under visible light irradiation[J]. J Am Chem Soc, 2008, 130(23): 7176-7177 |
| 7 | YAN S C, LI Z S, ZOU Z G. Photodegradation of rhodamine B and methyl orange over boron-doped g-C3N4 under visible light irradiation[J]. Langmuir, 2010, 26(6): 3894-3901. |
| 8 | PENG C, XU Z, LUO G, et al. Highly exposed single interlayered Cu edges enable high rate CO2 to CH4 electrosynthesis[J]. Adv Energy Mater, 2022, 12(15): 2200195.. |
| 9 | HAGFELDT A, GRAETZEL M. Light-induced redox reactions in nanocrystalline systems[J]. Chem Rev, 1995, 95(1): 49-68. |
| 10 | FANG Y, HOU Y, FU X, et al. Semiconducting polymers for oxygen evolution reaction under light illumination[J]. Chem Rev, 2022, 122(3): 4204-4256. |
| 11 | TEE S Y, WIN K Y, TEO W S, et al. Recent progress in energy-driven water splitting[J]. Adv Sci, 2017, 4(5): 1600337. |
| 12 | TAKANABE K. Photocatalytic water splitting: quantitative approaches toward photocatalyst by design[J]. ACS Catal, 2017, 7(11): 8006-8022. |
| 13 | WANG Q, DOMEN K. Particulate photocatalysts for light-driven water splitting: mechanisms, challenges, and design strategies[J]. Chem Rev, 2020, 120(2): 919-985. |
| 14 | LIN B, CHATURVEDI A, DI J, et al. Ferroelectric-field accelerated charge transfer in 2D CuInP2S6 heterostructure for enhanced photocatalytic H2 evolution[J]. Nano Energy, 2020, 76: 104972 . |
| 15 | HUANG H, TU S, DU X, et al. Ferroelectric spontaneous polarization steering charge carriers migration for promoting photocatalysis and molecular oxygen activation[J]. J Colloid Interface Sci, 2018, 509: 113-122. |
| 16 | HUANG X, LEI R, YUAN J, et al. Insight into the piezo-photo coupling effect of PbTiO3/CdS composites for piezo-photocatalytic hydrogen production[J]. Appl Catal B: Environ, 2021, 282: 119586. |
| 17 | CUI Y, BRISCOE J, DUNN S. Effect of ferroelectricity on solar-light-driven photocatalytic activity of BaTiO3-influence on the carrier separation and stern layer formation[J]. Chem Mat, 2013, 25(21): 4215-4223. |
| 18 | WAN G, YIN L, CHEN X, et al. Photocatalytic overall water splitting over PbTiO3 modulated by oxygen vacancy and ferroelectric polarization[J]. J Am Chem Soc, 2022, 144(44): 20342-20350. |
| 19 | WANG R, XU L, LIU Q, et al. Search for simple β-AIMIIIO2-type intrinsic ferroelectric semiconductors with simultaneous robust built-in electric field and full-spectrum absorption for superior photocatalysts[J]. J Mater Chem A, 2023, 11(10): 5233-5244. |
| 20 | YAO M C, WU X J, XU L L, et al. β-CuGaO2: a ferroelectric semiconductor with narrow band gap as degradation catalyst for wastewater environmental remediation[J]. Rare Met, 2021, 41(3): 972-981. |
| 21 | OUYANG S, LI Z, OUYANG Z, et al. Correlation of crystal structures, electronic structures, and photocatalytic properties in a series of Ag-based oxides: AgAlO2, AgCrO2, and Ag2CrO4[J]. J Phys Chem C, 2008, 112(8): 3134-3141. |
| 22 | WANG R, AN H, ZHANG H, et al. High active radicals induced from peroxymonosulfate by mixed crystal types of CuFeO2 as catalysts in the water[J]. Appl Surf Sci, 2019, 484: 1118-1127. |
| 23 | LIU Q L, ZHAO Z Y, ZHAO R D, et al. Fundamental properties of delafossite CuFeO2 as photocatalyst for solar energy conversion[J]. J Alloy Compd, 2020, 819: 153032. |
| 24 | JIANG T, XU C, ZHANG Y, et al. Wet chemical epitaxial growth of a cactus-like CuFeO2/ZnO heterojunction for improved photocatalysis[J]. Dalton Trans, 2020, 49(28): 9574-9578. |
| 25 | TU L W, CHANG K S. Hydrothermal fabrication and photocatalytic study of delafossite (CuFeO2) thin films on fluorine-doped tin oxide substrates[J]. Mater Chem Phys, 2021, 267: 124620. |
| 26 | WANG S, LIAO W, SU H, et al. Review on the application of semiconductor heterostructures in photocatalytic hydrogen evolution: state of the art and outlook[J]. Energy Fuels, 2023, 37(3): 1633-1656. |
| 27 | KRESSE G, FURTHMüLLER J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set[J]. Phys Rev B, 1996, 54(16): 11169-11186. |
| 28 | KRESSE G, FURTHMÜLLER J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set[J]. Comput Mater Sci, 1996, 6(1): 15-50. |
| 29 | PERDEW J P, BURKE K, ERNZERHOF M. Generalized gradient approximation made simple[J]. Phys Rev Lett, 1996, 77(18): 3865-3868. |
| 30 | AMRUTE A P, ŁODZIANA Z, MONDELLI C, et al. Solid-state chemistry of cuprous delafossites: synthesis and stability aspects[J]. Chem Mater, 2013, 25(21): 4423-4435. |
| 31 | TOGO A. First-principles phonon calculations with Phonopy and Phono3py[J]. J Phys Soc Jpn, 2022, 92(1): 012001. |
| 32 | KRUKAU A V, VYDROV O A, IZMAYLOV A F, et al. Influence of the exchange screening parameter on the performance of screened hybrid functionals[J]. J Chem Phys, 2006, 125(22): 224106. |
| 33 | WANG V, XU N, LIU J C, et al. VASPKIT: a user-friendly interface facilitating high-throughput computing and analysis using VASP code[J]. Comput Phys Commun, 2019, 267: 108033. |
| 34 | NøRSKOV J K, ROSSMEISL J, LOGADOTTIR A, et al. Origin of the overpotential for oxygen reduction at a fuel-cell cathode[J]. J Phys Chem B, 2004, 108(46): 17886-17892. |
| 35 | VALDÉS Á, QU Z W, KROES G J, et al. Oxidation and photo-oxidation of water on TiO2 surface[J]. J Phys Chem C, 2008, 112(26): 9872-9879. |
| 36 | OMATA T, NAGATANI H, SUZUKI I, et al. Wurtzite CuGaO2: a new direct and narrow band gap oxide semiconductor applicable as a solar cell absorber[J]. J Am Chem Soc, 2014, 136(9): 3378-3381. |
| 37 | HENKELMAN G, UBERUAGA B P, JÓNSSON H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths[J]. J Chem Phys, 2000, 113(22): 9901-9904. |
| 38 | MA X Y, LYU H Y, HAO K R, et al. Large family of two-dimensional ferroelectric metals discovered via machine learning[J]. Sci Bull, 2021, 66(3): 233-242. |
| 39 | MAOUHOUBI A, OUZAROUAL L, TOUAL Y, et al. Structural, electronic and magnetic properties of the CuFeO2 multiferroic compound[J]. Arab J Chem, 2024, 17(1): 105437. |
| 40 | YOON S H, KANG U, PARK H, et al. Computational density functional theory study on the selective conversion of CO2 to formate on homogeneously and heterogeneously mixed CuFeO2 and CuO surfaces[J]. Catal Today, 2019, 335: 345-353. |
| 41 | RIBEIRO R A P, ANDRÉS J, LONGO E, et al. Magnetism and multiferroic properties at MnTiO3 surfaces: a DFT study[J]. Appl Surf Sci, 2018, 452: 463-472. |
| 42 | LI Q, RELLAN-PINEIRO M, ALMORA-BARRIOS N, et al. Shape control in concave metal nanoparticles by etching[J]. Nanoscale, 2017, 9(35): 13089-13094. |
| 43 | JU L, SHANG J, TANG X, et al. Tunable photocatalytic water splitting by the ferroelectric switch in a 2D AgBiP2Se6 monolayer[J]. J Am Chem Soc, 2020, 142(3): 1492-1500. |
| 44 | BERGERHOFF G, HUNDT R, SIEVERS R, et al. The inorganic crystal structure data base[J]. J Chem Inf Comput Sci, 1983, 23(2): 66-69. |
| [1] | 李英维, 韩吉, 关卜源. 二维介孔材料的合成方法、设计与应用研究进展[J]. 应用化学, 2024, 41(6): 767-782. |
| [2] | 万冰洁, 刘小雪, 齐林光, 贾长超, 刘健. TiO2基光催化CO2还原研究进展[J]. 应用化学, 2024, 41(5): 637-658. |
| [3] | 王静, 李喜兰, 郭秀芬. 五氧化二铌光催化剂的制备及其光催化性能[J]. 应用化学, 2024, 41(3): 415-421. |
| [4] | 钱音男, 石钏, 张卫, 罗兆艳. 酸性环境下析氧反应Ir、Ru贵金属电催化剂的研究进展[J]. 应用化学, 2023, 40(8): 1126-1139. |
| [5] | 雷学博, 刘慧景, 丁赫宇, 申国栋, 孙润军. 用于降解印染废水中有机污染物的光催化剂的研究进展[J]. 应用化学, 2023, 40(5): 681-696. |
| [6] | 杜志鹏, 周洋, 赵三根. 银簇化合物Ag3B6O10I的合成、晶体结构和双折射性质[J]. 应用化学, 2023, 40(2): 229-235. |
| [7] | 蔡国庆, 董静茹, 莫君明. N‑苄基亚砜亚胺的绿色合成及其抑菌活性[J]. 应用化学, 2023, 40(12): 1693-1699. |
| [8] | 尉枫, 邢海东, 修子媛, 邢德峰, 韩晓军. 卤氧化铋基光催化剂的构建及其应用在能源转化领域的研究进展[J]. 应用化学, 2023, 40(11): 1518-1530. |
| [9] | 刘盛杰, 叶永杰, 刘银怡, 林淑满, 谢浩源, 刘文婷, 许伟钦. 基于泡沫铜的多孔碳均匀负载Cu3P纳米颗粒的制备及其光催化降解染料性能[J]. 应用化学, 2022, 39(7): 1090-1097. |
| [10] | 张晓丽, 彭玉美, 王庆伟, 秦利霞, 刘肖霞, 康诗钊, 李向清. 纳米Ag/TiO2纳米管阵列基底构建及表面增强拉曼散射光谱检测与降解盐酸四环素[J]. 应用化学, 2022, 39(7): 1147-1156. |
| [11] | 徐一鑫, 王爽, 全静, 高婉婷, 宋天群, 杨梅. 二硫化钼量子点/还原氧化石墨烯复合材料的制备及其光催化降解有机染料、四环素和Cr(VI)[J]. 应用化学, 2022, 39(5): 769-778. |
| [12] | 曹维锦, 白璐, 武兰兰, 李敬德, 宋术岩. 多壳层中空镍钴双金属磷化物纳米球用于高效电催化析氧[J]. 应用化学, 2022, 39(4): 666-672. |
| [13] | 商林杰, 刘江, 兰亚乾. 共价有机框架材料用于光/电催化CO2还原的研究进展[J]. 应用化学, 2022, 39(4): 559-584. |
| [14] | 王佳赫, 刘大勇, 刘伟, 王林, 董彪. 纳米TiO2光催化抗菌应用的研究进展[J]. 应用化学, 2022, 39(4): 629-646. |
| [15] | 逯慧, 李江, 王丽华, 诸颖, 陈静. 原子级精确的币金属纳米团簇在光催化应用方面的研究进展[J]. 应用化学, 2022, 39(11): 1652-1664. |
| 阅读次数 | ||||||||||||||||||||||||||||||||||||||||||||||||||
|
全文 167
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||
|
摘要 224
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||