应用化学 ›› 2024, Vol. 41 ›› Issue (6): 813-829.DOI: 10.19894/j.issn.1000-0518.230351
连朴饮基准样品高效液相色谱指纹图谱建立及化学成分鉴定
- 长春中医药大学药学院,长春 130117
-
收稿日期:2023-11-07接受日期:2024-02-01出版日期:2024-06-01发布日期:2024-07-09 -
通讯作者:徐可进 -
基金资助:吉林省创新能力建设项目(2021C002);吉林省教育厅科学研究项目(JJKH20241093KJ);重大疫情防治经典名方制剂储备库建设项目(吉中医药发[2021]11号)
Establishment of High Performance Liquid Chromatographic Fingerprints and Identification of Chemical Constituents in Benchmark Samples of Lianpo Decoction
Jie WANG, Shu-Hang WANG, Shao-Yan ZHOU, Xiao-Yun LIANG, Ke-Jin XU( )
)
- Changchun University of Traditional Chinese Medicine,Changchun 130117,China
-
Received:2023-11-07Accepted:2024-02-01Published:2024-06-01Online:2024-07-09 -
Contact:Ke-Jin XU -
About author:xukj@ccucm.edu.cn
-
Supported by:Jilin Province Innovation Capacity Building Project(2021C002);Scientific Research Project of Jilin Provincial Department of Education(JJKH20241093KJ);Construction Project of Reserve Bank of Classical Famous Formulas for Prevention and Control of Major Epidemics ([2021] No.11)
摘要:
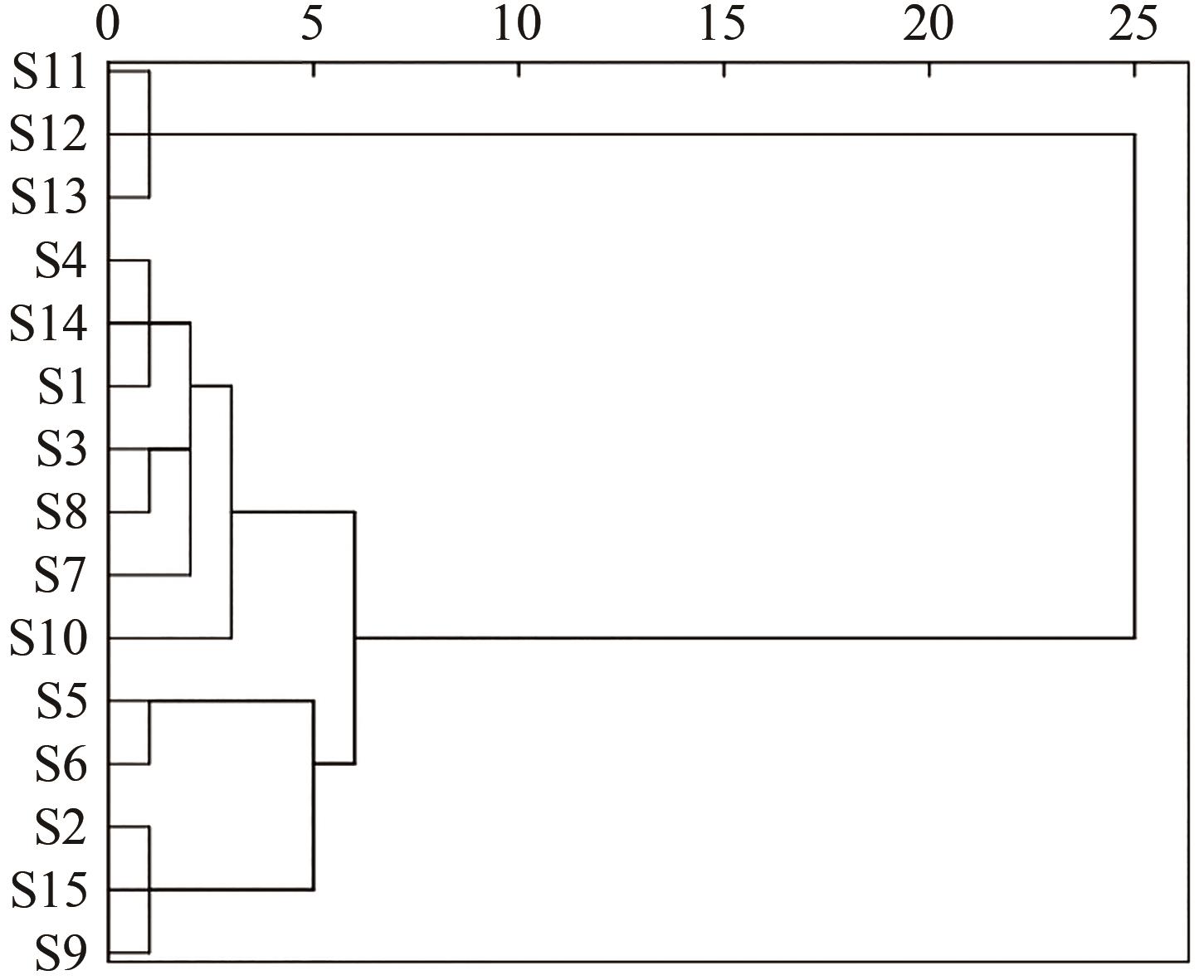
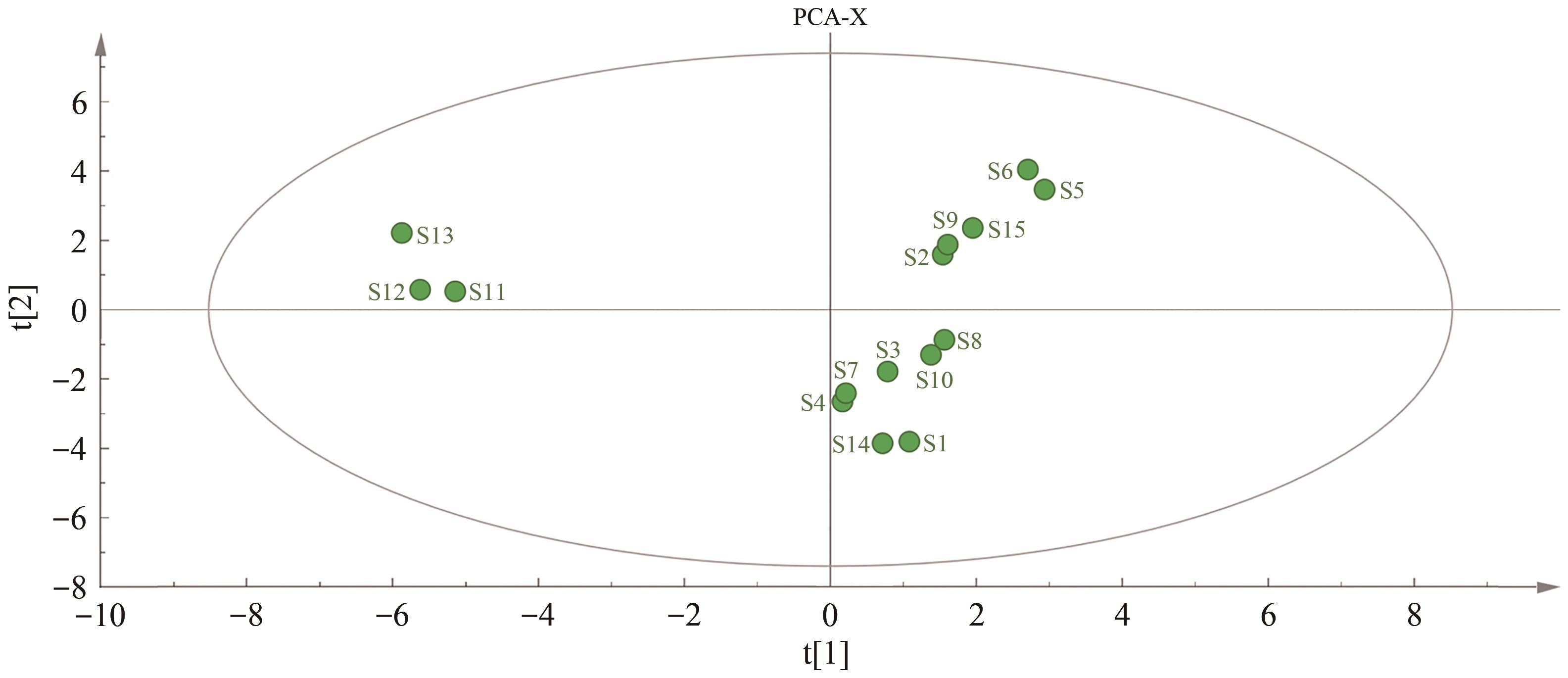
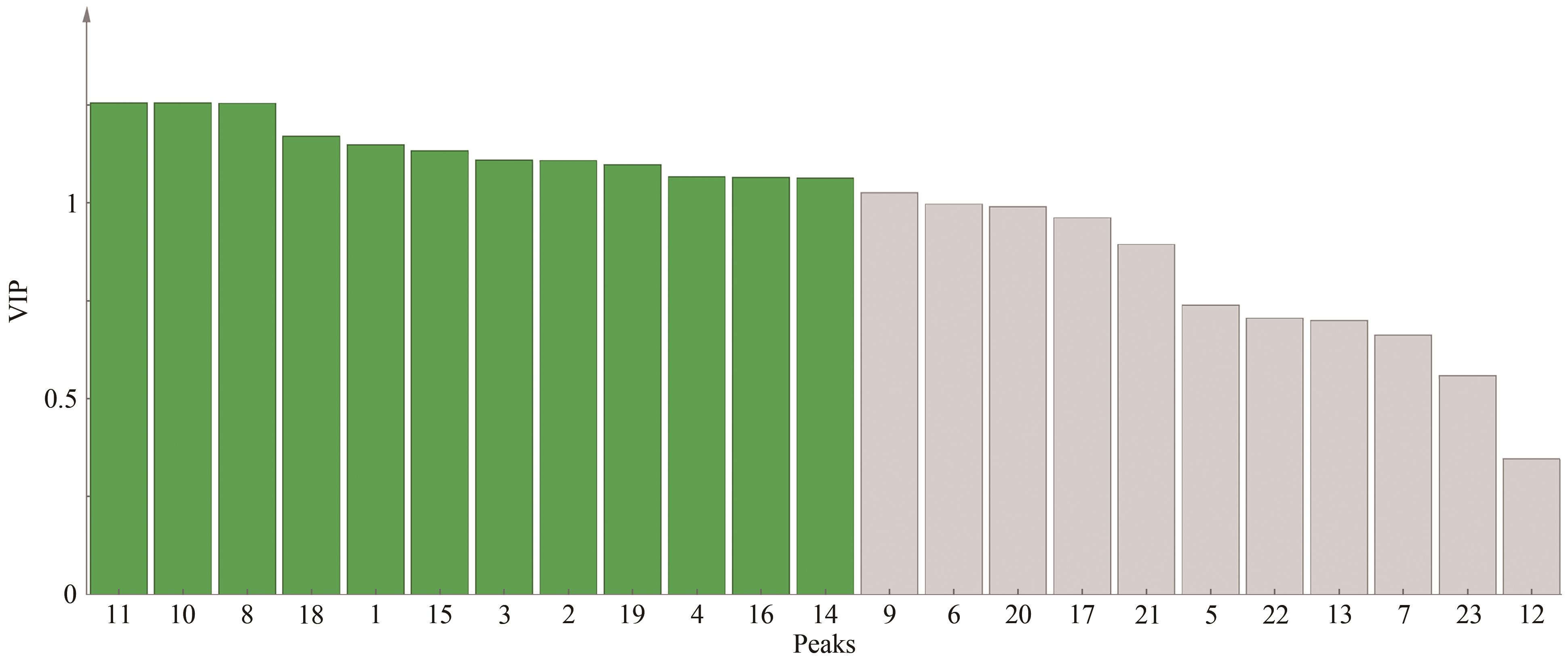
采用高效液相色谱(HPLC)技术建立连朴饮(LPD)基准样品的指纹图谱,并对其中的化学成分进行分析。该实验使用Welch Ultimate?XB-C18(250 mm×4.6 mm,5 μm)色谱柱,以甲醇-0.1%磷酸溶液作为流动相,梯度洗脱,检测波长为254 nm,柱温为30 ℃,体积流量为1.0 mL/min,进样量为10 μL。通过相似度评价、聚类分析(CA)、主成分分析(PCA)和正交偏最小二乘法-判别分析(OPLS-DA)对不同批次连朴饮进行质量评价。运用超高效液相色谱-四极杆-静电场轨道阱高分辨质谱(UPLC-Q-Orbitrap HRMS)技术,识别连朴饮中的化学成分。结果表明,15批连朴饮基准样品与对照指纹图谱的相似度均高于0.9,连朴饮基准样品HPLC指纹图谱方法学验证良好。确定23个共有峰,指认8个成分,分别为3号峰栀子苷、7号峰表小檗碱、8号峰盐酸黄连碱、11号峰盐酸小檗碱、12号峰盐酸巴马汀、19号峰染料木素、21号峰和厚朴酚和22号峰厚朴酚。通过聚类分析和主成分分析2种化学模式识别方法,将15批基准样品划分为3类,结果互相印证。主成分1-4是影响其质量评价的主要因子,OPLS-DA共确定12个差异标志物。在连朴饮中鉴定出了91个化合物,主要包含环烯醚萜类、有机酸类、黄酮苷类、黄酮类和异黄酮类、生物碱类、木脂素类和氨基酸类。建立的连朴饮基准样品HPLC指纹图谱方法简单且稳定性、重复性良好,与质谱检测方法相结合,可为其后续制剂开发和质量控制研究提供参考。
中图分类号:
引用本文
王婕, 王书航, 周绍岩, 梁潇云, 徐可进. 连朴饮基准样品高效液相色谱指纹图谱建立及化学成分鉴定[J]. 应用化学, 2024, 41(6): 813-829.
Jie WANG, Shu-Hang WANG, Shao-Yan ZHOU, Xiao-Yun LIANG, Ke-Jin XU. Establishment of High Performance Liquid Chromatographic Fingerprints and Identification of Chemical Constituents in Benchmark Samples of Lianpo Decoction[J]. Chinese Journal of Applied Chemistry, 2024, 41(6): 813-829.
| No. | Ginger juice Magnoliae Officinalis Cortex | Coptidis Rhizoma processed by ginger juice | Semen Sojae Praeparatum | Fructus Gardeniae Praeparatus | Rhizoma Pinellinae Praeparata | Rhizoma Graminei | Rhizoma Phragmitis |
|---|---|---|---|---|---|---|---|
| S1 | Sichuan (20211004) | Chongqing (20220501) | Anhui (221030) | Jiangxi (210323) | Guizhou (220414) | Sichuan (20210915) | Hebei (211018) |
| S2 | Sichuan (20211117) | Shaanxi (20211209) | Jiangsu (210421) | Sichuan (20210421) | Gansu (210517) | Anhui (210511) | Anhui (220517) |
| S3 | Sichuan (20211018) | Sichuan (20220815) | Anhui (210918) | Chongqing (210913) | Hubei (210912) | Sichuan (20221023) | Hebei (220317) |
| S4 | Anhui (220326) | Shaanxi (20210621) | Hubei (220813) | Henan (20210714) | Gansu (220503) | Sichuan (20221204) | Zhejiang (210823) |
| S5 | Anhui (220215) | Hubei 20220811 | Sichuan (20220518) | Hubei (20220503) | Guizhou (210912) | Anhui (210503) | Anhui (220519) |
| S6 | Anhui (220613) | Sichuan (20220503) | Sichuan (20220913) | Jiangxi (220913) | Gansu (210202) | Anhui (221201) | Hebei (211210) |
| S7 | Hubei (20220712) | Sichuan (20220704) | Zhejiang (221108) | Sichuan (20221013) | Anhui (220802) | Hubei (20220316) | Zhejiang (220701) |
| S8 | Hubei (20220825) | Chongqing (20211217) | Anhui (220402) | Fujian (221117) | Anhui (220920) | Sichuan (20221017) | Hebei (220723) |
| S9 | Sichuan (20220918) | Shaanxi (20220601) | Anhui (220601) | Sichuan (20220617) | Guizhou (210414) | Anhui (220921) | Anhui (220515) |
| S10 | Sichuan (20221011) | Chongqing (20220301) | Henan (20220702) | Anhui (220814) | Sichuan (20220520) | Hubei (20210719) | Zhejiang (220801) |
| S11 | Sichuan (20221119) | Chongqing (20220926) | Anhui (220822) | Sichuan (20221101) | Gansu (220906) | Sichuan (20221024) | Anhui (220823) |
| S12 | Sichuan (20221121) | Hubei (20221113) | Sichuan (20220924) | Sichuan (20221223) | Gansu (220823) | Sichuan (20221113) | Hebei (221016) |
| S13 | Hubei (20221202) | Chongqing (20221008) | Anhui (220822) | Jiangxi (220921) | Anhui (221105) | Anhui (220820) | Zhejiang (221115) |
| S14 | Hubei (20221029) | Hubei (20221117) | Sichuan (20220920) | Jiangxi (220928) | Anhui (221223) | Anhui (221227) | Anhui (220725) |
| S15 | Anhui (220923) | Sichuan (20221024) | Sichuan (20221027) | Jiangxi (221211) | Guizhou (211102) | Sichuan (20221119) | Hebei (220816) |
表1 15批连朴饮组方药材产地及批号
Table 1 15 batches of lianpo decoction group medicinal materials origin and batch number
| No. | Ginger juice Magnoliae Officinalis Cortex | Coptidis Rhizoma processed by ginger juice | Semen Sojae Praeparatum | Fructus Gardeniae Praeparatus | Rhizoma Pinellinae Praeparata | Rhizoma Graminei | Rhizoma Phragmitis |
|---|---|---|---|---|---|---|---|
| S1 | Sichuan (20211004) | Chongqing (20220501) | Anhui (221030) | Jiangxi (210323) | Guizhou (220414) | Sichuan (20210915) | Hebei (211018) |
| S2 | Sichuan (20211117) | Shaanxi (20211209) | Jiangsu (210421) | Sichuan (20210421) | Gansu (210517) | Anhui (210511) | Anhui (220517) |
| S3 | Sichuan (20211018) | Sichuan (20220815) | Anhui (210918) | Chongqing (210913) | Hubei (210912) | Sichuan (20221023) | Hebei (220317) |
| S4 | Anhui (220326) | Shaanxi (20210621) | Hubei (220813) | Henan (20210714) | Gansu (220503) | Sichuan (20221204) | Zhejiang (210823) |
| S5 | Anhui (220215) | Hubei 20220811 | Sichuan (20220518) | Hubei (20220503) | Guizhou (210912) | Anhui (210503) | Anhui (220519) |
| S6 | Anhui (220613) | Sichuan (20220503) | Sichuan (20220913) | Jiangxi (220913) | Gansu (210202) | Anhui (221201) | Hebei (211210) |
| S7 | Hubei (20220712) | Sichuan (20220704) | Zhejiang (221108) | Sichuan (20221013) | Anhui (220802) | Hubei (20220316) | Zhejiang (220701) |
| S8 | Hubei (20220825) | Chongqing (20211217) | Anhui (220402) | Fujian (221117) | Anhui (220920) | Sichuan (20221017) | Hebei (220723) |
| S9 | Sichuan (20220918) | Shaanxi (20220601) | Anhui (220601) | Sichuan (20220617) | Guizhou (210414) | Anhui (220921) | Anhui (220515) |
| S10 | Sichuan (20221011) | Chongqing (20220301) | Henan (20220702) | Anhui (220814) | Sichuan (20220520) | Hubei (20210719) | Zhejiang (220801) |
| S11 | Sichuan (20221119) | Chongqing (20220926) | Anhui (220822) | Sichuan (20221101) | Gansu (220906) | Sichuan (20221024) | Anhui (220823) |
| S12 | Sichuan (20221121) | Hubei (20221113) | Sichuan (20220924) | Sichuan (20221223) | Gansu (220823) | Sichuan (20221113) | Hebei (221016) |
| S13 | Hubei (20221202) | Chongqing (20221008) | Anhui (220822) | Jiangxi (220921) | Anhui (221105) | Anhui (220820) | Zhejiang (221115) |
| S14 | Hubei (20221029) | Hubei (20221117) | Sichuan (20220920) | Jiangxi (220928) | Anhui (221223) | Anhui (221227) | Anhui (220725) |
| S15 | Anhui (220923) | Sichuan (20221024) | Sichuan (20221027) | Jiangxi (221211) | Guizhou (211102) | Sichuan (20221119) | Hebei (220816) |
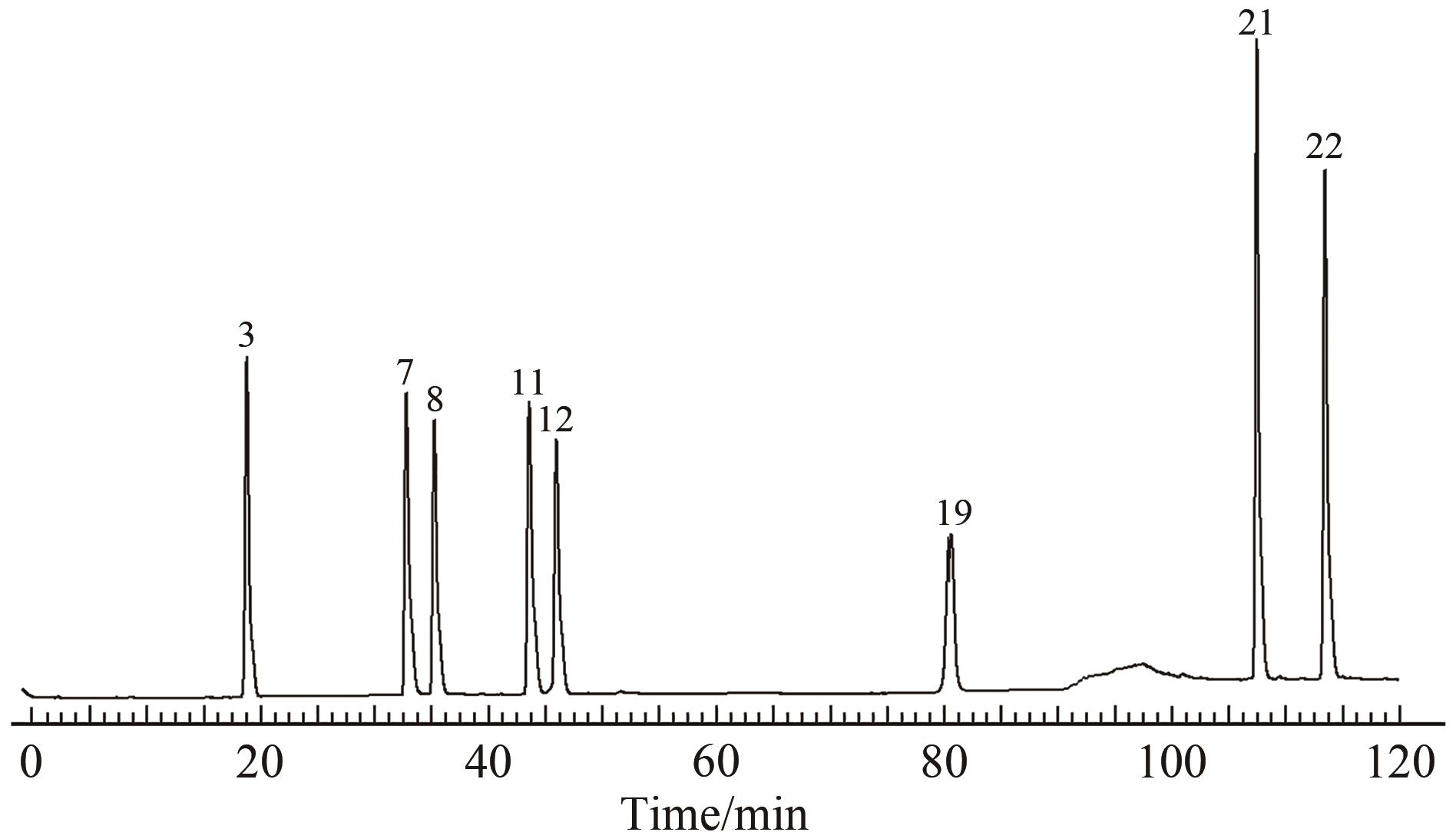
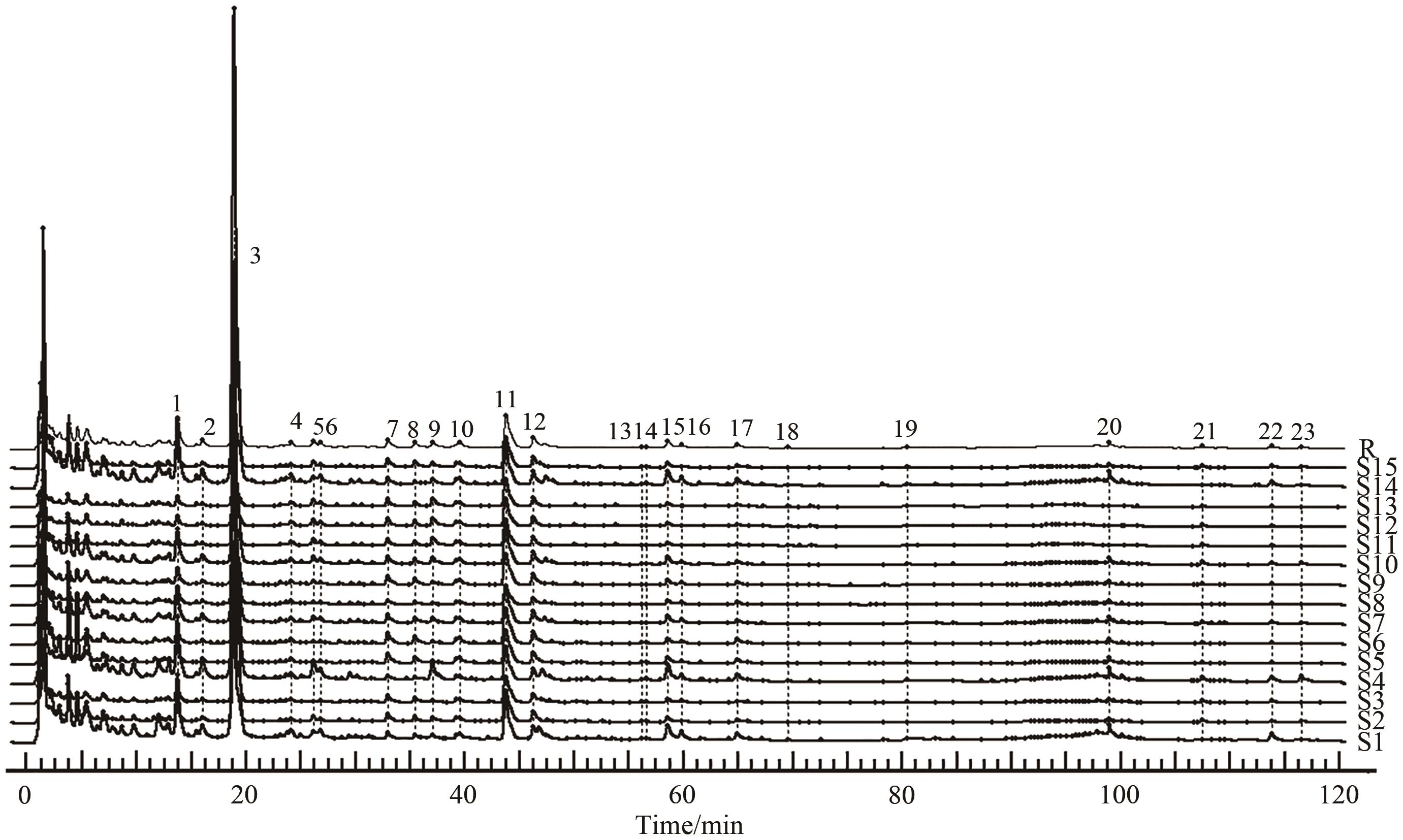
图2 连朴饮混合对照品溶液HPLC图
Fig.2 HPLC diagram of Lianpo Decoction mixed reference solutionNote: 3.geniposide; 7.epiberine; 8.coptis hydrochloride; 11,berberine hydrochloride; 12.palmatine hydrochloride;19.genistein; 21.honokiol; 22.magnolol
| No. | S1 | S2 | S2 | S4 | S5 | S6 | S7 | S8 | S9 | S10 | S11 | S12 | S13 | S14 | S15 | R |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S1 | 1.000 | 0.971 | 0.982 | 0.999 | 0.950 | 0.934 | 0.993 | 0.995 | 0.975 | 0.997 | 0.917 | 0.918 | 0.926 | 0.998 | 0.971 | 0.981 |
| S2 | 0.971 | 1.000 | 0.998 | 0.970 | 0.995 | 0.990 | 0.993 | 0.989 | 0.999 | 0.986 | 0.957 | 0.957 | 0.962 | 0.974 | 0.999 | 0.999 |
| S3 | 0.982 | 0.998 | 1.000 | 0.980 | 0.991 | 0.984 | 0.997 | 0.996 | 0.999 | 0.992 | 0.940 | 0.940 | 0.945 | 0.984 | 0.998 | 0.999 |
| S4 | 0.999 | 0.970 | 0.980 | 1.000 | 0.947 | 0.931 | 0.992 | 0.993 | 0.973 | 0.997 | 0.917 | 0.919 | 0.907 | 0.999 | 0.970 | 0.979 |
| S5 | 0.950 | 0.995 | 0.991 | 0.947 | 1.000 | 0.998 | 0.980 | 0.976 | 0.995 | 0.968 | 0.972 | 0.971 | 0.974 | 0.954 | 0.995 | 0.992 |
| S6 | 0.934 | 0.990 | 0.984 | 0.931 | 0.998 | 1.000 | 0.969 | 0.964 | 0.989 | 0.955 | 0.980 | 0.979 | 0.981 | 0.938 | 0.990 | 0.985 |
| S7 | 0.993 | 0.993 | 0.997 | 0.992 | 0.980 | 0.969 | 1.000 | 0.999 | 0.994 | 0.998 | 0.919 | 0.920 | 0.926 | 0.994 | 0.992 | 0.997 |
| S8 | 0.995 | 0.989 | 0.996 | 0.993 | 0.976 | 0.964 | 0.999 | 1.000 | 0.992 | 0.999 | 0.909 | 0.910 | 0.916 | 0.996 | 0.989 | 0.995 |
| S9 | 0.975 | 0.999 | 0.999 | 0.973 | 0.995 | 0.989 | 0.994 | 0.992 | 1.000 | 0.988 | 0.951 | 0.951 | 0.955 | 0.977 | 0.999 | 0.999 |
| S10 | 0.997 | 0.986 | 0.992 | 0.997 | 0.968 | 0.955 | 0.998 | 0.999 | 0.988 | 1.000 | 0.918 | 0.919 | 0.916 | 0.998 | 0.985 | 0.992 |
| S11 | 0.917 | 0.957 | 0.940 | 0.917 | 0.972 | 0.980 | 0.919 | 0.909 | 0.951 | 0.918 | 1.000 | 0.998 | 0.999 | 0.912 | 0.958 | 0.946 |
| S12 | 0.918 | 0.957 | 0.940 | 0.919 | 0.971 | 0.979 | 0.920 | 0.910 | 0.951 | 0.919 | 0.998 | 1.000 | 0.999 | 0.913 | 0.958 | 0.947 |
| S13 | 0.926 | 0.962 | 0.945 | 0.907 | 0.974 | 0.981 | 0.926 | 0.916 | 0.955 | 0.916 | 0.999 | 0.999 | 1.000 | 0.911 | 0.963 | 0.952 |
| S14 | 0.998 | 0.974 | 0.984 | 0.999 | 0.954 | 0.938 | 0.994 | 0.996 | 0.977 | 0.998 | 0.912 | 0.913 | 0.911 | 1.000 | 0.973 | 0.983 |
| S15 | 0.971 | 0.999 | 0.998 | 0.970 | 0.995 | 0.990 | 0.992 | 0.989 | 0.999 | 0.985 | 0.958 | 0.958 | 0.963 | 0.973 | 1.000 | 0.999 |
| R | 0.981 | 0.999 | 0.999 | 0.979 | 0.992 | 0.985 | 0.997 | 0.995 | 0.999 | 0.992 | 0.946 | 0.947 | 0.952 | 0.983 | 0.999 | 1.000 |
表2 15批连朴饮指纹图谱相似度结果
Table 2 Similarity results of fingerprints of 15 batches of Lianpo Decoction
| No. | S1 | S2 | S2 | S4 | S5 | S6 | S7 | S8 | S9 | S10 | S11 | S12 | S13 | S14 | S15 | R |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S1 | 1.000 | 0.971 | 0.982 | 0.999 | 0.950 | 0.934 | 0.993 | 0.995 | 0.975 | 0.997 | 0.917 | 0.918 | 0.926 | 0.998 | 0.971 | 0.981 |
| S2 | 0.971 | 1.000 | 0.998 | 0.970 | 0.995 | 0.990 | 0.993 | 0.989 | 0.999 | 0.986 | 0.957 | 0.957 | 0.962 | 0.974 | 0.999 | 0.999 |
| S3 | 0.982 | 0.998 | 1.000 | 0.980 | 0.991 | 0.984 | 0.997 | 0.996 | 0.999 | 0.992 | 0.940 | 0.940 | 0.945 | 0.984 | 0.998 | 0.999 |
| S4 | 0.999 | 0.970 | 0.980 | 1.000 | 0.947 | 0.931 | 0.992 | 0.993 | 0.973 | 0.997 | 0.917 | 0.919 | 0.907 | 0.999 | 0.970 | 0.979 |
| S5 | 0.950 | 0.995 | 0.991 | 0.947 | 1.000 | 0.998 | 0.980 | 0.976 | 0.995 | 0.968 | 0.972 | 0.971 | 0.974 | 0.954 | 0.995 | 0.992 |
| S6 | 0.934 | 0.990 | 0.984 | 0.931 | 0.998 | 1.000 | 0.969 | 0.964 | 0.989 | 0.955 | 0.980 | 0.979 | 0.981 | 0.938 | 0.990 | 0.985 |
| S7 | 0.993 | 0.993 | 0.997 | 0.992 | 0.980 | 0.969 | 1.000 | 0.999 | 0.994 | 0.998 | 0.919 | 0.920 | 0.926 | 0.994 | 0.992 | 0.997 |
| S8 | 0.995 | 0.989 | 0.996 | 0.993 | 0.976 | 0.964 | 0.999 | 1.000 | 0.992 | 0.999 | 0.909 | 0.910 | 0.916 | 0.996 | 0.989 | 0.995 |
| S9 | 0.975 | 0.999 | 0.999 | 0.973 | 0.995 | 0.989 | 0.994 | 0.992 | 1.000 | 0.988 | 0.951 | 0.951 | 0.955 | 0.977 | 0.999 | 0.999 |
| S10 | 0.997 | 0.986 | 0.992 | 0.997 | 0.968 | 0.955 | 0.998 | 0.999 | 0.988 | 1.000 | 0.918 | 0.919 | 0.916 | 0.998 | 0.985 | 0.992 |
| S11 | 0.917 | 0.957 | 0.940 | 0.917 | 0.972 | 0.980 | 0.919 | 0.909 | 0.951 | 0.918 | 1.000 | 0.998 | 0.999 | 0.912 | 0.958 | 0.946 |
| S12 | 0.918 | 0.957 | 0.940 | 0.919 | 0.971 | 0.979 | 0.920 | 0.910 | 0.951 | 0.919 | 0.998 | 1.000 | 0.999 | 0.913 | 0.958 | 0.947 |
| S13 | 0.926 | 0.962 | 0.945 | 0.907 | 0.974 | 0.981 | 0.926 | 0.916 | 0.955 | 0.916 | 0.999 | 0.999 | 1.000 | 0.911 | 0.963 | 0.952 |
| S14 | 0.998 | 0.974 | 0.984 | 0.999 | 0.954 | 0.938 | 0.994 | 0.996 | 0.977 | 0.998 | 0.912 | 0.913 | 0.911 | 1.000 | 0.973 | 0.983 |
| S15 | 0.971 | 0.999 | 0.998 | 0.970 | 0.995 | 0.990 | 0.992 | 0.989 | 0.999 | 0.985 | 0.958 | 0.958 | 0.963 | 0.973 | 1.000 | 0.999 |
| R | 0.981 | 0.999 | 0.999 | 0.979 | 0.992 | 0.985 | 0.997 | 0.995 | 0.999 | 0.992 | 0.946 | 0.947 | 0.952 | 0.983 | 0.999 | 1.000 |
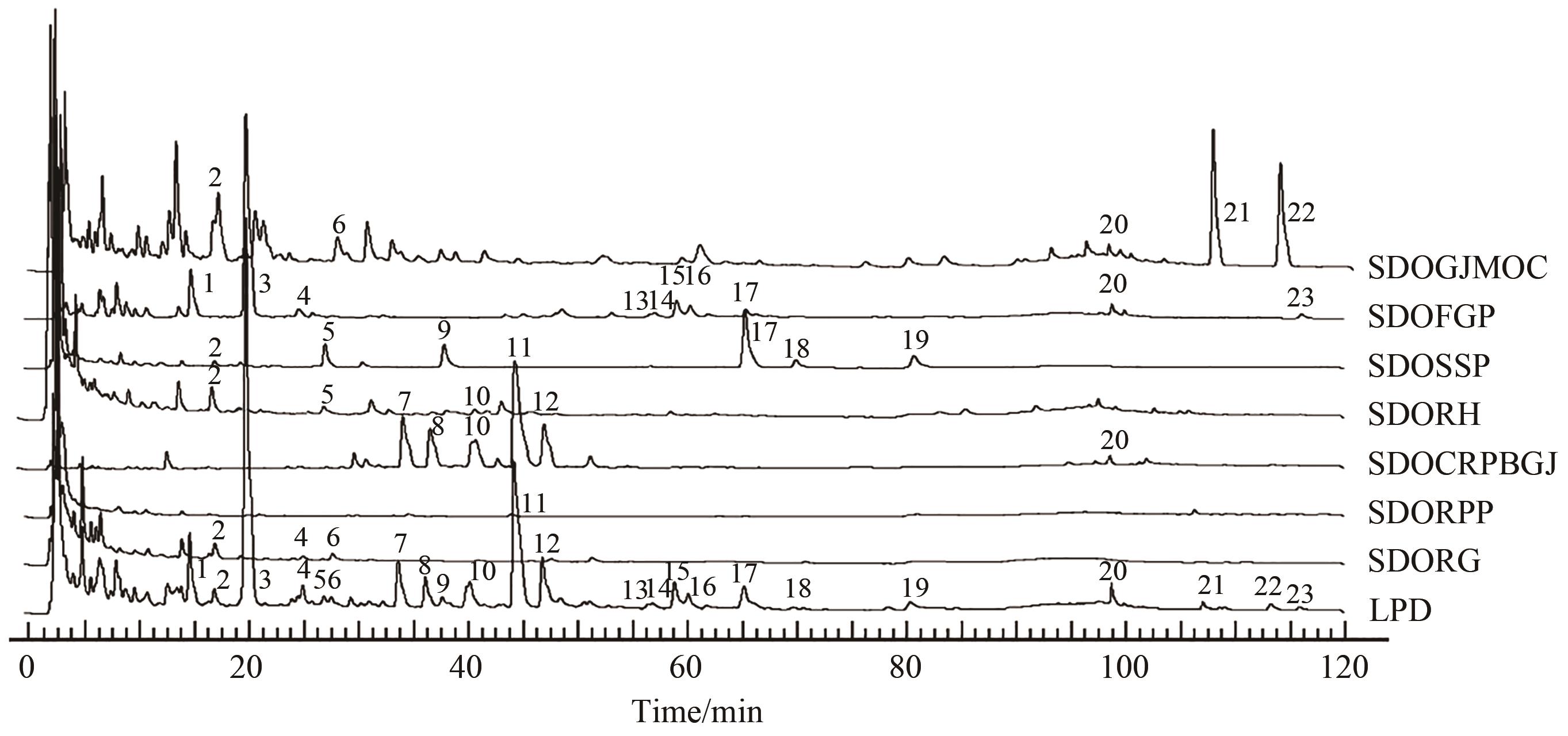
图3 单味药与连朴饮基准样品的HPLC指纹图谱对比
Fig.3 Comparison of HPLC fingerprints of single herb and benchmark samples of Lianpo DecoctionNote: SDOGJMOC, Single Decoction of Ginger juice Magnoliae Officinalis Cortex; SDOFGP, Single Decoction of Fructus Gardeniae Praeparatus; SDOSSP, Single Decoction of Semen Sojae Praeparatum; SDORH, Single Decoction of Rhizoma Phragmitis; SDOCRPBGJ, Single Decoction of Coptidis Rhizoma processed by ginger juice; SDORPP, Single Decoction of Rhizoma Pinellinae Praeparata; SDORG, Single Decoction of Rhizoma Graminei
| Principal component | Eigenvalue | Variance contribution/% | Cumulative variance contribution/% |
|---|---|---|---|
| 1 | 8.57 | 37.26 | 37.26 |
| 2 | 8.10 | 35.20 | 72.45 |
| 3 | 3.66 | 15.91 | 88.36 |
| 4 | 1.12 | 4.88 | 93.24 |
表3 特征值与累积方差贡献率
Table 3 Eigenvalues and cumulative variance contribution ratio
| Principal component | Eigenvalue | Variance contribution/% | Cumulative variance contribution/% |
|---|---|---|---|
| 1 | 8.57 | 37.26 | 37.26 |
| 2 | 8.10 | 35.20 | 72.45 |
| 3 | 3.66 | 15.91 | 88.36 |
| 4 | 1.12 | 4.88 | 93.24 |
| Peak No. | Principal component 1 | Principal component 2 | Principal component 3 | Principal component 4 |
|---|---|---|---|---|
| 1 | -0.612 | 0.672 | 0.345 | 0.06 |
| 2 | -0.48 | 0.513 | 0.663 | 0.206 |
| 3 | -0.633 | 0.65 | 0.33 | 0.006 |
| 4 | 0.859 | -0.306 | -0.302 | -0.054 |
| 5 | 0.465 | -0.721 | 0.34 | 0.358 |
| 6 | 0.221 | -0.811 | 0.283 | 0.243 |
| 7 | 0.847 | 0.408 | 0.265 | -0.192 |
| 8 | 0.812 | 0.411 | 0.359 | -0.174 |
| 9 | 0.583 | -0.696 | 0.364 | 0.145 |
| 10 | 0.839 | 0.4 | 0.261 | -0.21 |
| 11 | 0.83 | 0.382 | 0.331 | -0.201 |
| 12 | 0.866 | 0.38 | 0.214 | -0.145 |
| 13 | 0.585 | 0.602 | -0.294 | 0.755 |
| 14 | 0.197 | 0.945 | -0.088 | 0.226 |
| 15 | -0.056 | 0.913 | 0.047 | 0.303 |
| 16 | -0.172 | 0.867 | -0.43 | -0.082 |
| 17 | 0.565 | 0.74 | -0.26 | 0.229 |
| 18 | 0.741 | 0.518 | -0.229 | 0.259 |
| 19 | 0.847 | 0.048 | -0.374 | 0.122 |
| 20 | -0.535 | 0.724 | -0.321 | -0.07 |
| 21 | 0.464 | 0.022 | 0.766 | -0.084 |
| 22 | -0.351 | 0.411 | 0.516 | -0.423 |
| 23 | -0.278 | 0.252 | 0.808 | 0.392 |
表4 主成分因子载荷矩阵
Table 4 Principal component factor loading matrix
| Peak No. | Principal component 1 | Principal component 2 | Principal component 3 | Principal component 4 |
|---|---|---|---|---|
| 1 | -0.612 | 0.672 | 0.345 | 0.06 |
| 2 | -0.48 | 0.513 | 0.663 | 0.206 |
| 3 | -0.633 | 0.65 | 0.33 | 0.006 |
| 4 | 0.859 | -0.306 | -0.302 | -0.054 |
| 5 | 0.465 | -0.721 | 0.34 | 0.358 |
| 6 | 0.221 | -0.811 | 0.283 | 0.243 |
| 7 | 0.847 | 0.408 | 0.265 | -0.192 |
| 8 | 0.812 | 0.411 | 0.359 | -0.174 |
| 9 | 0.583 | -0.696 | 0.364 | 0.145 |
| 10 | 0.839 | 0.4 | 0.261 | -0.21 |
| 11 | 0.83 | 0.382 | 0.331 | -0.201 |
| 12 | 0.866 | 0.38 | 0.214 | -0.145 |
| 13 | 0.585 | 0.602 | -0.294 | 0.755 |
| 14 | 0.197 | 0.945 | -0.088 | 0.226 |
| 15 | -0.056 | 0.913 | 0.047 | 0.303 |
| 16 | -0.172 | 0.867 | -0.43 | -0.082 |
| 17 | 0.565 | 0.74 | -0.26 | 0.229 |
| 18 | 0.741 | 0.518 | -0.229 | 0.259 |
| 19 | 0.847 | 0.048 | -0.374 | 0.122 |
| 20 | -0.535 | 0.724 | -0.321 | -0.07 |
| 21 | 0.464 | 0.022 | 0.766 | -0.084 |
| 22 | -0.351 | 0.411 | 0.516 | -0.423 |
| 23 | -0.278 | 0.252 | 0.808 | 0.392 |
| No. | F1 | F2 | F3 | F4 | F | Rank |
|---|---|---|---|---|---|---|
| S1 | -4.208 | -0.654 | -1.513 | -0.682 | -2.224 | 14 |
| S2 | -0.022 | 0.004 | 3.447 | -0.313 | 0.566 | 5 |
| S3 | -0.764 | 0.69 | 0.628 | -2.396 | -0.063 | 7 |
| S4 | -3.444 | -0.92 | 0.621 | 2.615 | -1.482 | 13 |
| S5 | 1.822 | 4.451 | -1.166 | -0.228 | 2.196 | 2 |
| S6 | 4.293 | 5.025 | -2.148 | 0.568 | 3.274 | 1 |
| S7 | -2.243 | -0.965 | -0.606 | -0.437 | -1.387 | 12 |
| S8 | -1.568 | 1.017 | -1.774 | 0.182 | -0.538 | 9 |
| S9 | 1.285 | 3.426 | 0.883 | 0.447 | 1.980 | 3 |
| S10 | -2.169 | 0.161 | 1.15 | 0.757 | -0.571 | 11 |
| S11 | 2.83 | -3.984 | -0.834 | -0.543 | -0.541 | 10 |
| S12 | 3.261 | -4.363 | -0.723 | -0.176 | -0.473 | 8 |
| S13 | 4.605 | -3.949 | 0.084 | 0.827 | 0.411 | 6 |
| S14 | -3.787 | -1.023 | -2.309 | -0.254 | -2.309 | 15 |
| S15 | 0.109 | 1.084 | 4.261 | -0.366 | 1.162 | 4 |
表5 主成分得分、综合得分和排名
Table 5 Principal component scores, composite scores and rankings
| No. | F1 | F2 | F3 | F4 | F | Rank |
|---|---|---|---|---|---|---|
| S1 | -4.208 | -0.654 | -1.513 | -0.682 | -2.224 | 14 |
| S2 | -0.022 | 0.004 | 3.447 | -0.313 | 0.566 | 5 |
| S3 | -0.764 | 0.69 | 0.628 | -2.396 | -0.063 | 7 |
| S4 | -3.444 | -0.92 | 0.621 | 2.615 | -1.482 | 13 |
| S5 | 1.822 | 4.451 | -1.166 | -0.228 | 2.196 | 2 |
| S6 | 4.293 | 5.025 | -2.148 | 0.568 | 3.274 | 1 |
| S7 | -2.243 | -0.965 | -0.606 | -0.437 | -1.387 | 12 |
| S8 | -1.568 | 1.017 | -1.774 | 0.182 | -0.538 | 9 |
| S9 | 1.285 | 3.426 | 0.883 | 0.447 | 1.980 | 3 |
| S10 | -2.169 | 0.161 | 1.15 | 0.757 | -0.571 | 11 |
| S11 | 2.83 | -3.984 | -0.834 | -0.543 | -0.541 | 10 |
| S12 | 3.261 | -4.363 | -0.723 | -0.176 | -0.473 | 8 |
| S13 | 4.605 | -3.949 | 0.084 | 0.827 | 0.411 | 6 |
| S14 | -3.787 | -1.023 | -2.309 | -0.254 | -2.309 | 15 |
| S15 | 0.109 | 1.084 | 4.261 | -0.366 | 1.162 | 4 |
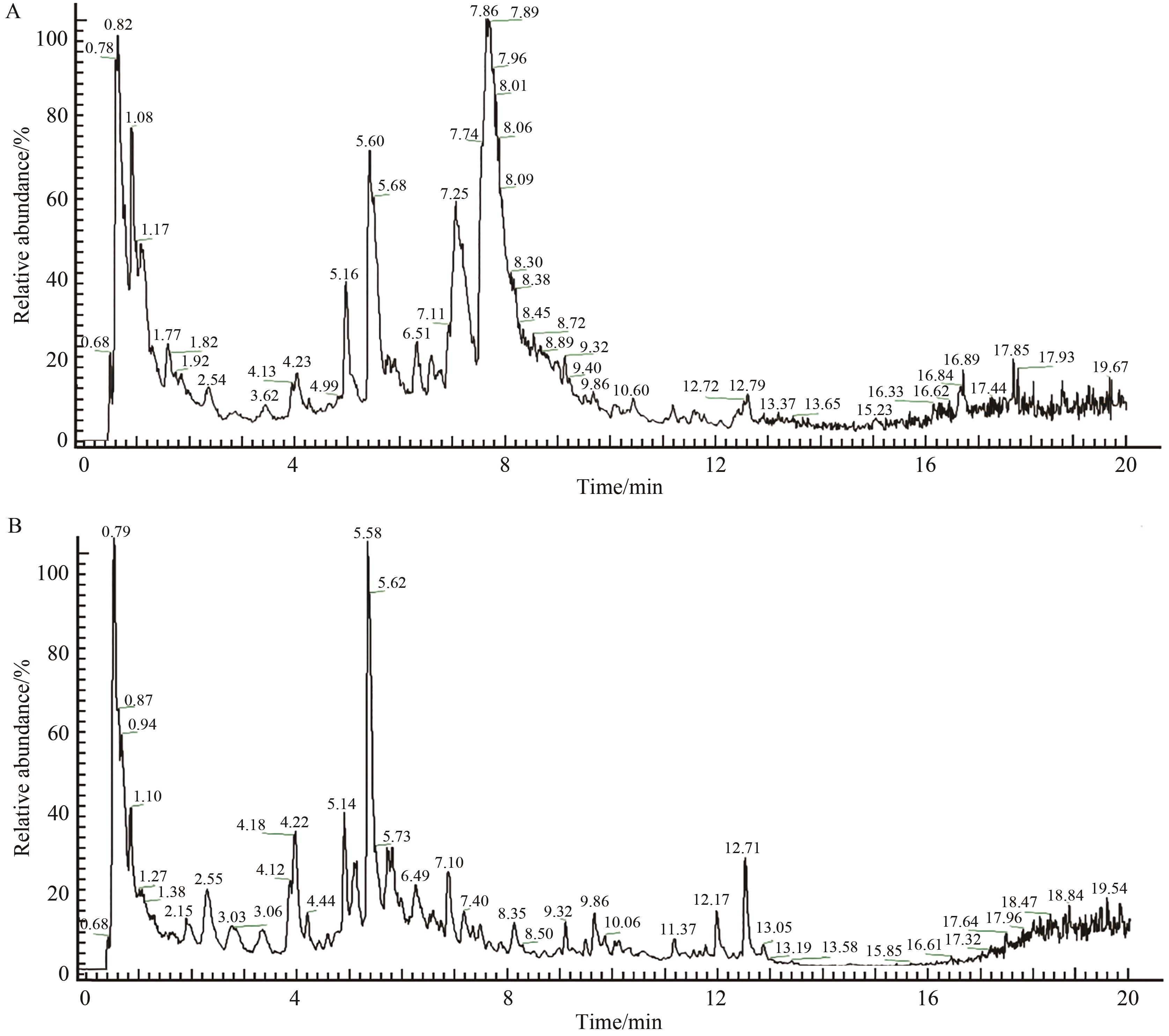
图7 正离子(A)和负离子(B)模式下连朴饮基准样品总离子流图
Fig.7 Total ion current chromatogram pattern of Lianpo Decoction benchmark samples in positive (A) and negative ion (B) modes
| No. | Ionic form | Source | ||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 0.78 | Choline[ | C5H13NO | 103.099 7 | 103.099 8 | 1.01 | P | E |
| 2 | 0.798 | DL-Arginine[ | C6H14N4O2 | 174.111 7 | 174.111 3 | -2.07 | P | E |
| 3 | 0.941 | Gluconic acid[ | C6H12O7 | 196.058 3 | 196.057 4 | -4.75 | P | A |
| 4 | 0.942 | Asparagine | C4H8N2O3 | 132.053 5 | 132.053 3 | -1.73 | P | G |
| 5 | 1.04 | Adenine[ | C5H5N5 | 135.054 5 | 135.054 3 | -1.16 | P | E |
| 6 | 1.088 | Uracil[ | C4H4N2O2 | 112.027 3 | 112.027 3 | 0.27 | P | E |
| 7 | 1.159 | Adenosine[ | C10H13N5O4 | 267.096 8 | 267.096 2 | -2.27 | P | E |
| 8 | 1.162 | 2-Hydroxycinnamic acid[ | C9H8O3 | 164.047 3 | 164.047 1 | -1.61 | P | A |
| 9 | 1.21 | L-Isoleucine[ | C6H13NO2 | 131.094 6 | 131.094 4 | -1.57 | P | E |
| 10 | 1.256 | Guanosine[ | C10H13N5O5 | 283.091 7 | 283.090 9 | -2.83 | P | E |
| 11 | 1.381 | Thymine[ | C5H6N2O2 | 126.042 9 | 126.042 8 | -1.07 | P | E |
| 12 | 1.886 | Geniposidic acid[ | C16H22O10 | 374.121 3 | 374.121 1 | -0.62 | N | D |
| 13 | 2.543 | 2,4,5-Trimethoxybenzoic acid[ | C10H12O5 | 212.068 5 | 212.068 0 | -2.47 | P | F |
| 14 | 3.328 | Coixol | C8H7NO3 | 165.042 6 | 165.042 3 | -1.83 | P | G |
| 15 | 3.53 | Protocatechuic acid | C7H6O4 | 154.026 6 | 154.026 3 | -2.13 | P | E |
| 16 | 3.611 | Maltol[ | C6H6O3 | 126.031 7 | 126.031 6 | -1.03 | P | G |
| 17 | 4.108 | Hypocretin methyl ester[ | C17H24O11 | 404.131 9 | 404.131 7 | -0.53 | N | D |
| 18 | 4.131 | Coumarin[ | C9H6O2 | 146.036 8 | 146.036 4 | -2.49 | P | A |
| 19 | 4.22 | Anethole[ | C10H12O | 148.088 8 | 148.088 6 | -1.7 | P | E |
| 20 | 4.226 | Neochlorogenic acid[ | C16H18O9 | 354.095 1 | 354.094 7 | -1.21 | N | BDF |
| 21 | 4.232 | Jasmin G[ | C16H26O8 | 346.162 8 | 346.161 8 | -2.79 | P | D |
| 22 | 4.273 | 5-Hydroxyindole-3-acetic acid[ | C10H9NO3 | 191.058 2 | 191.057 8 | -2.31 | P | B |
| 23 | 4.606 | Normorphine[ | C16H17NO3 | 271.120 8 | 271.120 2 | -2.5 | P | B |
| 24 | 4.618 | Sinomenine[ | C19H23NO4 | 329.162 7 | 329.161 9 | -2.6 | P | A |
| 25 | 4.805 | Jade leaf auriculosidic acid[ | C21H32O10 | 444.199 6 | 444.199 4 | -0.43 | N | D |
| 26 | 5.002 | Salicylic acid[ | C7H6O3 | 138.031 7 | 138.031 2 | -3.32 | P | BDF |
| 27 | 5.029 | 3-Acetylmorphine[ | C19H21NO4 | 327.147 1 | 327.146 1 | -2.82 | P | B |
| 28 | 5.059 | Ferulaldehyde | C10H10O3 | 178.063 0 | 178.062 9 | -0.7 | P | G |
| 29 | 5.128 | Chlorogenic acid[ | C16H18O9 | 354.095 1 | 354.094 8 | -0.83 | N | BD |
| 30 | 5.142 | Genipin 1-O-β-D-gentiobioside[ | C23H34O15 | 550.189 8 | 550.189 5 | -0.55 | N | D |
| 31 | 5.162 | Ethylmorphine[ | C19H23NO3 | 313.167 8 | 313.166 7 | -3.48 | P | A |
| 32 | 5.295 | Methyl chlorogenate[ | C17H20O9 | 368.110 7 | 368.109 7 | -2.95 | P | BD |
| 33 | 5.301 | Methyl chlorogenate[ | C17H20O9 | 368.110 7 | 368.110 3 | -1.06 | N | BD |
| 34 | 5.573 | 6-Acetylmorphine | C19H21NO4 | 327.147 1 | 327.146 0 | -3.1 | P | B |
| 35 | 5.59 | Geniposide[ | C17H24O10 | 388.137 0 | 388.136 7 | -0.64 | N | D |
| 36 | 5.596 | Genipin[ | C11H14O5 | 226.084 1 | 226.083 5 | -2.99 | N | D |
| 37 | 5.599 | Isoferulic acid[ | C10H10O4 | 194.057 9 | 194.057 3 | -3.12 | P | G |
| 38 | 5.7 | 6-Acetylcodeine[ | C20H23NO4 | 341.162 7 | 341.161 6 | -3.23 | P | A |
| 39 | 5.719 | Saffronic acid[ | C16H26O8 | 346.162 8 | 346.162 6 | -0.48 | N | D |
| 40 | 5.769 | Syringic acid[ | C9H10O5 | 198.052 8 | 198.052 4 | -2.37 | N | A |
| 41 | 5.79 | Jasmin T[ | C21H34O11 | 462.210 1 | 462.208 8 | -2.82 | P | D |
| 42 | 5.962 | 4-Hydroxybenzaldehyde[ | C7H6O2 | 122.036 8 | 122.036 7 | -1.04 | P | G |
| 43 | 5.963 | Caffeic acid[ | C9H8O4 | 180.042 3 | 180.041 8 | -2.34 | N | CDA |
| 44 | 5.976 | Jasmin A[ | C16H26O7 | 330.167 9 | 330.166 9 | -2.99 | P | D |
| 45 | 6.046 | Methyl chlorogenate[ | C17H20O9 | 368.110 7 | 368.110 5 | -0.61 | N | BD |
| 46 | 6.052 | Daidzin[ | C21H20O9 | 416.110 7 | 416.109 5 | -3 | P | C |
| 47 | 6.156 | Glycitin[ | C22H22O10 | 446.121 3 | 446.120 3 | -2.33 | P | C |
| 48 | 6.357 | Erigerin L[ | C27H36O12 | 552.220 7 | 552.220 5 | -0.25 | N | D |
| 49 | 6.409 | Rutin[ | C27H30O16 | 610.153 4 | 610.153 3 | -0.08 | P | D |
| 50 | 6.486 | Lariciresinol 4-O-glucoside[ | C26H34O11 | 522.210 1 | 522.209 9 | -0.44 | N | A |
| 51 | 6.571 | Demethyleneberberine[ | C21H22NO4 | 352.115 8 | 352.114 7 | -3.27 | P | B |
| 52 | 6.604 | Ferulic acid[ | C10H10O4 | 194.057 9 | 194.057 5 | -2.17 | P | G |
| 53 | 6.648 | Kaempferol 7-neohesperidoside[ | C27H30O15 | 594.158 5 | 594.158 5 | -0.01 | N | F |
| 54 | 6.694 | Butyraldehyde | C9H10O4 | 182.057 9 | 182.057 5 | -2.18 | P | G |
| 55 | 6.737 | 6'-o-trans-Mustardoyl gardenia neoside[ | C27H32O14 | 580.179 2 | 580.177 9 | -2.29 | P | D |
| 56 | 6.807 | Coptisine[ | C19H14NO4 | 320.092 3 | 320.092 6 | 1.1 | N | B |
| 57 | 7.102 | 4-Coumaric acid[ | C9H8O3 | 164.047 3 | 164.046 9 | -2.73 | P | A |
| 58 | 7.136 | 6″-o-trans-Mustard acylkinetic acid gentiobioside[ | C34H44O19 | 756.247 7 | 756.245 7 | -2.59 | N | D |
| 59 | 7.218 | 4,5-Dicaffeoylquinic acid[ | C25H24O12 | 516.126 8 | 516.126 4 | -0.71 | N | D |
| 60 | 7.243 | Jatrorrhizine[ | C20H19NO4 | 337.131 4 | 337.130 0 | -4.11 | P | B |
| 61 | 7.266 | 6″-O-Acetylsoyoside[ | C23H22O10 | 458.121 3 | 458.119 8 | -3.34 | P | C |
| 62 | 7.694 | 6'-o-trans-Mustard acyl kynephoside[ | C28H34O14 | 594.194 9 | 594.193 2 | -2.79 | N | D |
| 63 | 7.893 | Berberine[ | C20H17NO4 | 335.115 8 | 335.114 5 | -3.7 | P | B |
| 64 | 8.297 | Feruloyl tyramine[ | C18H19NO4 | 313.131 4 | 313.130 4 | -3.26 | P | F |
| 65 | 8.354 | Daidzein[ | C15H10O4 | 254.057 9 | 254.057 2 | -2.89 | N | C |
| 66 | 8.519 | Disinapoyl Hexoside[ | C28H32O14 | 592.179 2 | 592.179 0 | -0.4 | N | B |
| 67 | 8.56 | 2,4,5-Trimethoxybenzaldehyde[ | C10H12O4 | 196.073 6 | 196.073 1 | -2.46 | P | F |
| 68 | 8.611 | Glycitein[ | C16H12O5 | 284.068 5 | 284.067 6 | -2.92 | N | C |
| 69 | 8.93 | Quercetin[ | C15H10O7 | 302.042 7 | 302.042 3 | -1.03 | N | D |
| 70 | 9.153 | Crocin[ | C44H64O24 | 976.378 8 | 976.378 5 | -0.23 | N | D |
| 71 | 9.314 | Crocetin[ | C20H24O4 | 328.167 5 | 328.166 5 | -2.95 | P | D |
| 72 | 9.679 | Isophorone[ | C9H14O | 138.104 5 | 138.104 2 | -2.11 | P | D |
| 73 | 9.682 | Crocin Ⅱ[ | C38H54O19 | 814.325 9 | 814.325 7 | -0.25 | N | D |
| 74 | 9.694 | Genistein[ | C15H10O5 | 270.052 8 | 270.052 1 | -2.75 | P | C |
| 75 | 10.32 | Crocin 3[ | C32H44O14 | 652.273 1 | 652.273 1 | -0.08 | N | D |
| 76 | 10.675 | Oxyberberine[ | C20H17NO5 | 351.110 7 | 351.109 6 | -3.13 | P | B |
| 77 | 10.849 | (-)-Caryophyllene oxide[ | C15H24O | 220.182 7 | 220.182 2 | -2.42 | P | F |
| 78 | 10.877 | Berberrubine[ | C19H15NO4 | 340.118 5 | 340.117 5 | -2.91 | P | B |
| 79 | 10.983 | Sphingomyelin[ | C18H39NO3 | 317.293 0 | 317.292 1 | -2.91 | P | E |
| 80 | 11.041 | Honokiol[ | C18H18O2 | 266.131 0 | 266.129 0 | -2.92 | P | A |
| 81 | 11.153 | Medicarpin[ | C16H14O4 | 270.089 2 | 270.088 4 | -3.01 | P | B |
| 82 | 11.155 | Prespatane[ | C15H24 | 204.187 8 | 204.187 3 | -2.36 | P | F |
| 83 | 11.586 | Crocetin[ | C20H24O4 | 328.167 5 | 328.167 1 | -1.1 | N | D |
| 84 | 12.185 | Magnolol[ | C18H18O2 | 266.130 7 | 266.129 9 | -2.93 | P | A |
| 85 | 13.431 | β-Asarone[ | C12H16O3 | 208.109 9 | 208.109 4 | -2.43 | P | F |
| 86 | 13.522 | α-Linolenic acid[ | C18H30O2 | 278.224 6 | 278.223 8 | -2.79 | P | E |
| 87 | 14.352 | Linoleoyl ethanolamide[ | C20H37NO2 | 323.282 4 | 323.281 6 | -2.65 | P | E |
| 88 | 16.333 | Hexadecanamide[ | C16H33NO | 255.256 2 | 255.255 3 | -3.46 | P | E |
| 89 | 16.456 | Ursolic acid[ | C30H48O3 | 456.360 4 | 456.359 2 | -2.47 | P | D |
| 90 | 16.989 | Ceratodictyol[ | C19H38O4 | 330.277 0 | 330.276 0 | -3.03 | P | E |
| 91 | 18.422 | Oleic acid[ | C18H34O2 | 282.255 9 | 282.255 6 | -1.11 | N | FE |
表6 连朴饮基准样品成分鉴定结果
Table 6 Compositional identification results of benchmark samples of Lianpo Decoction
| No. | Ionic form | Source | ||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 0.78 | Choline[ | C5H13NO | 103.099 7 | 103.099 8 | 1.01 | P | E |
| 2 | 0.798 | DL-Arginine[ | C6H14N4O2 | 174.111 7 | 174.111 3 | -2.07 | P | E |
| 3 | 0.941 | Gluconic acid[ | C6H12O7 | 196.058 3 | 196.057 4 | -4.75 | P | A |
| 4 | 0.942 | Asparagine | C4H8N2O3 | 132.053 5 | 132.053 3 | -1.73 | P | G |
| 5 | 1.04 | Adenine[ | C5H5N5 | 135.054 5 | 135.054 3 | -1.16 | P | E |
| 6 | 1.088 | Uracil[ | C4H4N2O2 | 112.027 3 | 112.027 3 | 0.27 | P | E |
| 7 | 1.159 | Adenosine[ | C10H13N5O4 | 267.096 8 | 267.096 2 | -2.27 | P | E |
| 8 | 1.162 | 2-Hydroxycinnamic acid[ | C9H8O3 | 164.047 3 | 164.047 1 | -1.61 | P | A |
| 9 | 1.21 | L-Isoleucine[ | C6H13NO2 | 131.094 6 | 131.094 4 | -1.57 | P | E |
| 10 | 1.256 | Guanosine[ | C10H13N5O5 | 283.091 7 | 283.090 9 | -2.83 | P | E |
| 11 | 1.381 | Thymine[ | C5H6N2O2 | 126.042 9 | 126.042 8 | -1.07 | P | E |
| 12 | 1.886 | Geniposidic acid[ | C16H22O10 | 374.121 3 | 374.121 1 | -0.62 | N | D |
| 13 | 2.543 | 2,4,5-Trimethoxybenzoic acid[ | C10H12O5 | 212.068 5 | 212.068 0 | -2.47 | P | F |
| 14 | 3.328 | Coixol | C8H7NO3 | 165.042 6 | 165.042 3 | -1.83 | P | G |
| 15 | 3.53 | Protocatechuic acid | C7H6O4 | 154.026 6 | 154.026 3 | -2.13 | P | E |
| 16 | 3.611 | Maltol[ | C6H6O3 | 126.031 7 | 126.031 6 | -1.03 | P | G |
| 17 | 4.108 | Hypocretin methyl ester[ | C17H24O11 | 404.131 9 | 404.131 7 | -0.53 | N | D |
| 18 | 4.131 | Coumarin[ | C9H6O2 | 146.036 8 | 146.036 4 | -2.49 | P | A |
| 19 | 4.22 | Anethole[ | C10H12O | 148.088 8 | 148.088 6 | -1.7 | P | E |
| 20 | 4.226 | Neochlorogenic acid[ | C16H18O9 | 354.095 1 | 354.094 7 | -1.21 | N | BDF |
| 21 | 4.232 | Jasmin G[ | C16H26O8 | 346.162 8 | 346.161 8 | -2.79 | P | D |
| 22 | 4.273 | 5-Hydroxyindole-3-acetic acid[ | C10H9NO3 | 191.058 2 | 191.057 8 | -2.31 | P | B |
| 23 | 4.606 | Normorphine[ | C16H17NO3 | 271.120 8 | 271.120 2 | -2.5 | P | B |
| 24 | 4.618 | Sinomenine[ | C19H23NO4 | 329.162 7 | 329.161 9 | -2.6 | P | A |
| 25 | 4.805 | Jade leaf auriculosidic acid[ | C21H32O10 | 444.199 6 | 444.199 4 | -0.43 | N | D |
| 26 | 5.002 | Salicylic acid[ | C7H6O3 | 138.031 7 | 138.031 2 | -3.32 | P | BDF |
| 27 | 5.029 | 3-Acetylmorphine[ | C19H21NO4 | 327.147 1 | 327.146 1 | -2.82 | P | B |
| 28 | 5.059 | Ferulaldehyde | C10H10O3 | 178.063 0 | 178.062 9 | -0.7 | P | G |
| 29 | 5.128 | Chlorogenic acid[ | C16H18O9 | 354.095 1 | 354.094 8 | -0.83 | N | BD |
| 30 | 5.142 | Genipin 1-O-β-D-gentiobioside[ | C23H34O15 | 550.189 8 | 550.189 5 | -0.55 | N | D |
| 31 | 5.162 | Ethylmorphine[ | C19H23NO3 | 313.167 8 | 313.166 7 | -3.48 | P | A |
| 32 | 5.295 | Methyl chlorogenate[ | C17H20O9 | 368.110 7 | 368.109 7 | -2.95 | P | BD |
| 33 | 5.301 | Methyl chlorogenate[ | C17H20O9 | 368.110 7 | 368.110 3 | -1.06 | N | BD |
| 34 | 5.573 | 6-Acetylmorphine | C19H21NO4 | 327.147 1 | 327.146 0 | -3.1 | P | B |
| 35 | 5.59 | Geniposide[ | C17H24O10 | 388.137 0 | 388.136 7 | -0.64 | N | D |
| 36 | 5.596 | Genipin[ | C11H14O5 | 226.084 1 | 226.083 5 | -2.99 | N | D |
| 37 | 5.599 | Isoferulic acid[ | C10H10O4 | 194.057 9 | 194.057 3 | -3.12 | P | G |
| 38 | 5.7 | 6-Acetylcodeine[ | C20H23NO4 | 341.162 7 | 341.161 6 | -3.23 | P | A |
| 39 | 5.719 | Saffronic acid[ | C16H26O8 | 346.162 8 | 346.162 6 | -0.48 | N | D |
| 40 | 5.769 | Syringic acid[ | C9H10O5 | 198.052 8 | 198.052 4 | -2.37 | N | A |
| 41 | 5.79 | Jasmin T[ | C21H34O11 | 462.210 1 | 462.208 8 | -2.82 | P | D |
| 42 | 5.962 | 4-Hydroxybenzaldehyde[ | C7H6O2 | 122.036 8 | 122.036 7 | -1.04 | P | G |
| 43 | 5.963 | Caffeic acid[ | C9H8O4 | 180.042 3 | 180.041 8 | -2.34 | N | CDA |
| 44 | 5.976 | Jasmin A[ | C16H26O7 | 330.167 9 | 330.166 9 | -2.99 | P | D |
| 45 | 6.046 | Methyl chlorogenate[ | C17H20O9 | 368.110 7 | 368.110 5 | -0.61 | N | BD |
| 46 | 6.052 | Daidzin[ | C21H20O9 | 416.110 7 | 416.109 5 | -3 | P | C |
| 47 | 6.156 | Glycitin[ | C22H22O10 | 446.121 3 | 446.120 3 | -2.33 | P | C |
| 48 | 6.357 | Erigerin L[ | C27H36O12 | 552.220 7 | 552.220 5 | -0.25 | N | D |
| 49 | 6.409 | Rutin[ | C27H30O16 | 610.153 4 | 610.153 3 | -0.08 | P | D |
| 50 | 6.486 | Lariciresinol 4-O-glucoside[ | C26H34O11 | 522.210 1 | 522.209 9 | -0.44 | N | A |
| 51 | 6.571 | Demethyleneberberine[ | C21H22NO4 | 352.115 8 | 352.114 7 | -3.27 | P | B |
| 52 | 6.604 | Ferulic acid[ | C10H10O4 | 194.057 9 | 194.057 5 | -2.17 | P | G |
| 53 | 6.648 | Kaempferol 7-neohesperidoside[ | C27H30O15 | 594.158 5 | 594.158 5 | -0.01 | N | F |
| 54 | 6.694 | Butyraldehyde | C9H10O4 | 182.057 9 | 182.057 5 | -2.18 | P | G |
| 55 | 6.737 | 6'-o-trans-Mustardoyl gardenia neoside[ | C27H32O14 | 580.179 2 | 580.177 9 | -2.29 | P | D |
| 56 | 6.807 | Coptisine[ | C19H14NO4 | 320.092 3 | 320.092 6 | 1.1 | N | B |
| 57 | 7.102 | 4-Coumaric acid[ | C9H8O3 | 164.047 3 | 164.046 9 | -2.73 | P | A |
| 58 | 7.136 | 6″-o-trans-Mustard acylkinetic acid gentiobioside[ | C34H44O19 | 756.247 7 | 756.245 7 | -2.59 | N | D |
| 59 | 7.218 | 4,5-Dicaffeoylquinic acid[ | C25H24O12 | 516.126 8 | 516.126 4 | -0.71 | N | D |
| 60 | 7.243 | Jatrorrhizine[ | C20H19NO4 | 337.131 4 | 337.130 0 | -4.11 | P | B |
| 61 | 7.266 | 6″-O-Acetylsoyoside[ | C23H22O10 | 458.121 3 | 458.119 8 | -3.34 | P | C |
| 62 | 7.694 | 6'-o-trans-Mustard acyl kynephoside[ | C28H34O14 | 594.194 9 | 594.193 2 | -2.79 | N | D |
| 63 | 7.893 | Berberine[ | C20H17NO4 | 335.115 8 | 335.114 5 | -3.7 | P | B |
| 64 | 8.297 | Feruloyl tyramine[ | C18H19NO4 | 313.131 4 | 313.130 4 | -3.26 | P | F |
| 65 | 8.354 | Daidzein[ | C15H10O4 | 254.057 9 | 254.057 2 | -2.89 | N | C |
| 66 | 8.519 | Disinapoyl Hexoside[ | C28H32O14 | 592.179 2 | 592.179 0 | -0.4 | N | B |
| 67 | 8.56 | 2,4,5-Trimethoxybenzaldehyde[ | C10H12O4 | 196.073 6 | 196.073 1 | -2.46 | P | F |
| 68 | 8.611 | Glycitein[ | C16H12O5 | 284.068 5 | 284.067 6 | -2.92 | N | C |
| 69 | 8.93 | Quercetin[ | C15H10O7 | 302.042 7 | 302.042 3 | -1.03 | N | D |
| 70 | 9.153 | Crocin[ | C44H64O24 | 976.378 8 | 976.378 5 | -0.23 | N | D |
| 71 | 9.314 | Crocetin[ | C20H24O4 | 328.167 5 | 328.166 5 | -2.95 | P | D |
| 72 | 9.679 | Isophorone[ | C9H14O | 138.104 5 | 138.104 2 | -2.11 | P | D |
| 73 | 9.682 | Crocin Ⅱ[ | C38H54O19 | 814.325 9 | 814.325 7 | -0.25 | N | D |
| 74 | 9.694 | Genistein[ | C15H10O5 | 270.052 8 | 270.052 1 | -2.75 | P | C |
| 75 | 10.32 | Crocin 3[ | C32H44O14 | 652.273 1 | 652.273 1 | -0.08 | N | D |
| 76 | 10.675 | Oxyberberine[ | C20H17NO5 | 351.110 7 | 351.109 6 | -3.13 | P | B |
| 77 | 10.849 | (-)-Caryophyllene oxide[ | C15H24O | 220.182 7 | 220.182 2 | -2.42 | P | F |
| 78 | 10.877 | Berberrubine[ | C19H15NO4 | 340.118 5 | 340.117 5 | -2.91 | P | B |
| 79 | 10.983 | Sphingomyelin[ | C18H39NO3 | 317.293 0 | 317.292 1 | -2.91 | P | E |
| 80 | 11.041 | Honokiol[ | C18H18O2 | 266.131 0 | 266.129 0 | -2.92 | P | A |
| 81 | 11.153 | Medicarpin[ | C16H14O4 | 270.089 2 | 270.088 4 | -3.01 | P | B |
| 82 | 11.155 | Prespatane[ | C15H24 | 204.187 8 | 204.187 3 | -2.36 | P | F |
| 83 | 11.586 | Crocetin[ | C20H24O4 | 328.167 5 | 328.167 1 | -1.1 | N | D |
| 84 | 12.185 | Magnolol[ | C18H18O2 | 266.130 7 | 266.129 9 | -2.93 | P | A |
| 85 | 13.431 | β-Asarone[ | C12H16O3 | 208.109 9 | 208.109 4 | -2.43 | P | F |
| 86 | 13.522 | α-Linolenic acid[ | C18H30O2 | 278.224 6 | 278.223 8 | -2.79 | P | E |
| 87 | 14.352 | Linoleoyl ethanolamide[ | C20H37NO2 | 323.282 4 | 323.281 6 | -2.65 | P | E |
| 88 | 16.333 | Hexadecanamide[ | C16H33NO | 255.256 2 | 255.255 3 | -3.46 | P | E |
| 89 | 16.456 | Ursolic acid[ | C30H48O3 | 456.360 4 | 456.359 2 | -2.47 | P | D |
| 90 | 16.989 | Ceratodictyol[ | C19H38O4 | 330.277 0 | 330.276 0 | -3.03 | P | E |
| 91 | 18.422 | Oleic acid[ | C18H34O2 | 282.255 9 | 282.255 6 | -1.11 | N | FE |
| 1 | 王曦宇. 连朴饮加减联合四联疗法治疗幽门螺杆菌相关性胃炎脾胃湿热证的临床研究[J]. 中国医药指南, 2019, 17(1): 153-154. |
| WANG X Y. Clinical study on the treatment of Helicobacter pylori-associated gastritis with damp-heat syndrome of spleen and stomach by combination of Lian Pu Drink and quadruple therapy[J]. Guide Chin Med, 2019, 17(1): 153-154. | |
| 2 | 何学梅. 王氏连朴饮加味治疗脾胃湿热型幽门螺杆菌相关慢性胃炎30例[J]. 浙江中医杂志, 2019, 54(12): 892. |
| HE X M. Treatment of Helicobacter pylori-associated chronic gastritis with damp-heat in spleen and stomach by adding flavor of Wang's Lianpu Drink in 30 cases[J]. Zhejiang J Tradit Chin Med, 2019, 54(12): 892. | |
| 3 | 褚璨灿,师为人,陈云志,等. 连朴饮的临床应用与实验研究进展[J]. 中华中医药学刊, 2018, 36(10): 2478-2480. |
| CHU C C, SHI W R, CHEN Y Z, et al. Progress of clinical application and experimental research on Lianpuo drink[J]. Chin J Tradit Chin Med, 2018, 36(10): 2478-2480. | |
| 4 | 王彩萍, 张科防, 苗慧霞, 等. 幽门螺杆菌相关性胃炎脾胃湿热证连朴饮加减联合四联疗法治疗临床效果分析[J]. 临床医药文献电子杂志, 2019, 6(86): 8-9. |
| WANG C P, ZHANG K F, MIAO H X, et al. Clinical effect analysis of Helicobacter pylori-associated gastritis with damp-heat syndrome of spleen and stomach combined with Lianpu Drink and quadruple therapy[J]. Electronic J Clin Med Literature, 2019, 6(86): 8-9. | |
| 5 | 孟金金. 黄连苦寒成分体内代谢过程对药性的影响研究[J]. 抗感染药学, 2017, 14(2): 256-258. |
| MENG J J. Studies on the effects of in vivo metabolic processes of bitter and cold components of Coptis chinensis on the medicinal properties of the drug[J]. Anti-Infection Pharm, 2017, 14(2): 256-258. | |
| 6 | 杨红兵, 杨晓琴, 陈学昆, 等. 厚朴不同部位的体外抑菌试验研究[J]. 湖北中医药大学学报, 2016, 18(1): 40-43. |
| YANG H B, YANG X Q, CHEN X K, et al. In vitro bacteriostatic test study on different parts of Huperzia serrata[J]. J Hubei Univ Chin Med, 2016, 18(1): 40-43. | |
| 7 | 王志强, 宓伟, 刘现兵, 等. 厚朴体外抑菌作用研究[J]. 时珍国医国药, 2007(11): 2763. |
| WANG Z Q, MI W, LIU X B, et al. In vitro bacteriostatic test study on different parts of Huperzia serrata.[J]. Lishizhen Med Mater Med Res, 2007(11): 2763. | |
| 8 | 伍晓丽, 陈义嘉, 王钰, 等. 黄连研究进展综述[J]. 安徽农学通报, 2023, 29(7): 37-41. |
| WU X L, CHEN Y J, WANG Y, et al. Synthesis of the progress of research on Coptis chinensis[J]. Anhui Agric Sci Bull, 2023, 29(7): 37-41. | |
| 9 | 李家奇, 薛珍珍, 杨滨. 不同产地和树龄厚朴样本姜炙前后化学成分的定性定量分析[J]. 中国中药杂志, 2023, 48(9): 2435-2454. |
| LI J Q, XUE Z Z, YANG B. Qualitative and quantitative analysis of chemical constituents of Houpao samples of different origins and ages before and after ginger-roasting[J]. Chin J Chin Mater Med, 2023, 48(9): 2435-2454. | |
| 10 | 穆成林, 周欣, 卢焘韬, 等. 基于HPLC与化学计量学方法的姜黄连饮片指纹图谱研究[J]. 中药材, 2019, 42(9): 2086-2090. |
| MU C L, ZHOU X, LU T T, et al. Fingerprinting study of Jianghuanglian drinking tablets based on HPLC and chemometric methods[J]. Chin Med Mat, 2019, 42(9): 2086-2090. | |
| 11 | 黄玲. 黄连化学成分及有效成分药理活性的研究进展[J]. 中西医结合心血管病电子杂志, 2020, 8(17): 136-137. |
| HUANG L. Progress in the study of chemical composition and pharmacological activity of active ingredients of Rhizoma Coptidis[J]. Cardiovascular Dis J Integr Tradit Chin West Med, 2020, 8(17): 136-137. | |
| 12 | 张景, 冯亭亭, 张明柱. 淡豆豉UPLC指纹图谱[J]. 中成药, 2017, 39(7): 1531-1533. |
| ZHANG J, FENG T T, ZHANG M Z. UPLC fingerprint of tempeh[J]. Chin Traditi Pat Med, 2017, 39(7): 1531-1533. | |
| 13 | 雷磊, 王玉, 霍志鹏, 等. LCMS-IT-TOF分析栀子炒焦前后化学成分的变化[J]. 中国实验方剂学杂志, 2019, 25(17): 88-97. |
| LEI L, WANG Y, HUO Z P, et al. LCMS-IT-TOF analysis of changes in chemical composition of Gardenia jasminoides before and after frying and scorching[J]. Chin J Exp Tradit Med Formulae, 2019, 25(17): 88-97. | |
| 14 | 马莎莎. 石菖蒲的化学指纹图谱及易混淆品化学成分比较研究[D]. 昆明: 昆明理工大学, 2018. |
| MA S S. Chemical fingerprinting of Acorus calamus and comparative study on the chemical constituents of the confusibles[D]. Kunming: Kunming University of Science and Technology, 2018. | |
| 15 | 王中华, 郭庆梅, 周凤琴. 芦根化学成分、药理作用及开发利用研究进展[J]. 辽宁中医药大学学报, 2014, 16(12): 81-83. |
| WANG Z H, GUO Q M, ZHOU F Q. Advances in chemical composition, pharmacological effects and development and utilization of rutabaga root[J]. J Liaoning UnivTradit Chin Med, 2014, 16(12): 81-83. | |
| 16 | 左军, 牟景光, 胡晓阳. 半夏化学成分及现代药理作用研究进展[J]. 辽宁中医药大学学报, 2019, 21(9): 26-29. |
| ZUO J, MOU J G, HU X Y. Progress in the study of chemical constituents and modern pharmacological effects of Pinellia ternata[J]. J Liaoning Univ Tradit Chin Med, 2019, 21(9): 26-29. | |
| 17 | 汤书婉, 李新亮, 马莉, 等. 基于HPLC指纹图谱和LC-Q-TOF/MS的加味黄芪桂枝五物汤化学成分研究[J]. 中草药, 2023, 54(3): 711-721. |
| TANG S G, LI X L, MA L, et al. Study on the chemical constituents of Flavored Astragalus and Gui Zhi Wu Wu Wu Tang based on HPLC fingerprints and LC-Q-TOF/MS[J]. Chin Tradit Herbal Drugs, 2023, 54(3): 711-721. | |
| 18 | 张维方, 张强, 贾豪, 等. 经典名方一贯煎HPLC指纹图谱及化学模式识别研究[J]. 药物分析杂志, 2022, 42(12): 2169-2178. |
| ZHANG W F, ZHANG Q, JIA H, et al. HPLC fingerprinting and chemical pattern recognition study of the classical famous formula consistently decoction[J]. Chin J Pharm Anal, 2022, 42(12): 2169-2178. | |
| 19 | 薛晓霞, 靳如娜, 王学圆, 等. 经典名方二冬汤基准样品的指标成分含量测定及量值传递规律探索[J]. 中国实验方剂学杂志, 2022, 28(11): 1-7. |
| XUE X X, JIN R N, WANG X Y, et al. Determination of indicator components in benchmark samples of the classic formula Erdong Tang and exploration of the law of quantitative value transmission[J]. Chin J Exp Tradit Med Formulae, 2022, 28(11): 1-7. | |
| 20 | 国家中医药管理局、卫生部关于印发医疗机构中药煎药室管理规范的通知(国中医药发〔2009〕3号)[J]. 2009(6): 29-31. |
| State Administration of Traditional Chinese Medicine, Ministry of Health on the issuance of medical institutions of Chinese medicine decoction room management norms notice (State Administration of Traditional Chinese Medicine issued [2009] No. 3)[J]. 2009(6): 29-31. | |
| 21 | 崔美娜, 钟凌云, 兰泽伦, 等. 基于UPLC-Q-TOF-MS/MS分析多物料多流程炮制对半夏化学成分的影响[J]. 中草药, 2021, 52(24): 7428-7437. |
| CUI M N, ZHONG L Y, LAN Z L, et al. UPLC-Q-TOF-MS/MS-based analysis of the effects of multi-material and multi-process concoctions on the chemical composition of Pinellia ternata[J]. Chin Tradit Herbal Drugs, 2021, 52(24): 7428-7437. | |
| 22 | 聂黎行, 王馨平, 黄烈岩, 等. 板蓝根化学成分信息库的构建[J]. 中国药学杂志, 2022, 57(6): 428-452. |
| NIE L X, WANG X P, HUANG L Y, et al. Construction of an information base of chemical components of Panax quinquefolium[J]. Chin Pharm J, 2022, 57(6): 428-452. | |
| 23 | CHEN Q C, ZHANG W Y, YOUN U, et al. Iridoid glycosides from Gardeniae Fructus for treatment of ankle sprain[J]. Phytochemistry, 2009, 70(6): 779-784. |
| 24 | HAN Y, WEN J, ZHOU T, et al. Chemical fingerprinting of Gardenia jasminoides Ellis by HPLC-DAD-ESI-MS combined with chemometrics methods[J]. Food Chem, 2015, 188: 648-657. |
| 25 | WANG L, LIU S, ZHANG X, et al. A strategy for identification and structural characterization of compounds from Gardenia jasminoides by integrating macroporous resin column chromatography and liquid chromatography-tandem mass spectrometry combined with ion-mobility spectrometry[J]. J Chromatogr, 2016, 1452: 47-57. |
| 26 | 田世民, 谢锦, 尚强, 等. 基于UPLC-Q-Orbitrap HRMS和GC-MS技术的抗病毒颗粒化学成分分析[J]. 中国医院药学杂志, 2023, 43(2): 134-144. |
| TIAN S M, XIE J, SHANG Q, et al. Chemical composition analysis of antiviral particles based on UPLC-Q-Orbitrap HRMS and GC-MS techniques[J]. Chin J Hosp Pharm, 2023, 43(2): 134-144. | |
| 27 | 孙淑玲. 中药芦根的药理作用及临床应用[J]. 中西医结合心血管病电子杂志, 2016, 4(36): 165. |
| SUN S L. Pharmacological actions and clinical applications of the Chinese medicine rehmannia glutinosa[J]. Cardiovascular Dis J Integr Tradit Chin West Med, 2016, 4(36): 165. | |
| 28 | 陈梦倩, 王允吉, 冯芳. 基于UPLC-Q-Exactive Orbitrap-MS的栀子甘草豉汤化学成分分析[J]. 广州化工, 2021, 49(8): 97-103. |
| CHEN M Q, WANG Y J, FENG F. Analysis of the chemical constituents of Gardenia Glycyrrhiza Glutinosa and Blac Bean Soup based on UPLC-Q-Exactive Orbitrap-MS[J]. Guangzhou Chem Ind, 2021, 49(8): 97-103. | |
| 29 | 王永丽, 黄广建, 刘从进, 等. UHPLC-Q-Exactive Orbitrap HRMS分析黄连解毒汤的化学成分及大鼠组织分布[J]. 中草药, 2022, 53(22): 6985-7000. |
| WANG Y L, HUANG G J, LIU C J, et al. Analysis of the chemical constituents and rat tissue distribution of Rhizoma Coptidis Toxin Relieving Tang by UHPLC-Q-Exactive Orbitrap HRMS[J]. Chin Tradit Herbal Drugs, 2022, 53(22): 6985-7000. | |
| 30 | 张烨, 邓琦, 魏敏, 等. 黄连花薹化学成分的UPLC-Q-Orbitrap HRMS鉴定[J]. 中国实验方剂学杂志, 2021, 27(15): 91-99. |
| ZHANG Y, DENG Q, WEI M, et al. UPLC-Q-Orbitrap HRMS for the identification of the chemical constituents of Rhizoma Coptidis Carex[J]. Chin J Exp Tradit Med Formulae, 2021, 27(15): 91-99. | |
| 31 | 胥爱丽, 肖观林, 毕晓黎, 等. 厚朴温中汤化学成分快速分析[J]. 中药新药与临床药理, 2021, 32(2): 252-258. |
| XU A L, XIAO G L, BI X L, et al. Rapid analysis of the chemical composition of Houpu Wenzhong Tang[J]. Tradit Chin Drugs Clin Remed Pharmacol, 2021, 32(2): 252-258. | |
| 32 | 张国升, 李前荣, 尹浩, 等. 气相色谱-飞行时间质谱法快速测定和鉴定芦根中阿魏酸的含量与结构[J]. 中草药, 2005(3): 333-335. |
| ZHANG G S, LI Q R, YIN H, et al. Rapid determination and structural characterization of ferulic acid in rehmanniaglutinosa by gas chromatography-time-of-flight mass spectrometry[J]. Chin Tradit Herbal Drugs, 2005(3): 333-335. | |
| 33 | 赵权, 张优, 陈影, 等. HPLC-VWD-Q-TOF-MS/MS法定性与定量分析枳实栀子豉汤成分[J]. 中成药, 2021, 43(11): 3067-3075. |
| ZHAO Q, ZHANG Y, CHEN Y, et al. Qualitative and quantitative analysis of the components of Citrus aurantium jasminoides and black beans soup by HPLC-VWD-Q-TOF-MS/MS[J]. Chin Tradit Herbal Drugs, 2021, 43(11): 3067-3075. | |
| 34 | 王月红, 王磊, 张洪兵, 等. 基于HPLC-Q/TOF-MS的六经头痛片血中移行成分研究[J]. 中草药, 2017, 48(20): 4151-4156. |
| WANG Y H, WANG L, ZHANG H B, et al. HPLC-Q/TOF-MS-based study of migratory componentsin the blood of Hexagram Headache Tablet[J]. Chin Tradit Herbal Drugs, 2017, 48(20): 4151-4156. | |
| 35 | 石坚宏, 姬丽婷, 骆启晗, 等. 石菖蒲化学成分、药理作用及质量标志物预测分析研究进展[J]. 中成药, 2021, 43(5): 1286-1290. |
| SHI J H, JI L T, LUO Q H, et al. Progress in the study of chemical composition, pharmacological effects and predictive analysis of quality markers of Acorus calamus[J]. Chin Tradit Pat Med, 2021, 43(5): 1286-1290. | |
| 36 | 马燕玲, 王一名, 初坤, 等. 超高效液相色谱串联质谱法测定葡萄酒中酚酸和酚醛类化合物[J]. 化学试剂, 2023, 45(2): 141-147. |
| MA Y L, WANG Y Y, CHU K, et al. Determination of phenolic acids and phenolic aldehydes in wine by ultra performance liquid chromatography tandem mass spectrometry[J]. Chem Reag, 2023, 45(2): 141-147. | |
| 37 | LEE E M, PARK S J, LEE J, et al. Highly geographical specificity of metabolomic traits among Korean domestic soybeans (Glycine max)[J]. Food Res Int, 2019, 120: 12-18. |
| 38 | 杨鹤年, 吴宿慧, 李寒冰, 等. 石菖蒲的研究进展及质量标志物预测分析[J]. 中国新药杂志, 2021, 30(13): 1213-1219. |
| YANG H N, WU S H, LI H B, et al. Research progress of Acorus calamus and analysis of quality markers prediction[J]. Chin New Drugs J, 2021, 30(13): 1213-1219. | |
| 39 | 郝艺铭, 霍金海, 王涛, 等. UPLC-Q-TOF/MS技术分析黄连中非生物碱类成分[J]. 中药材, 2020, 43(2): 354-358. |
| HAO Y M, HUO J H, WANG T, et al. Analysis of non-alkaloids in rhizoma coptidis by UPLC-Q-TOF/MS technique[J]. Chin Med Mat, 2020, 43(2): 354-358. | |
| 40 | 梁颖, 许文佳, 符策奕, 等. HPLC-MS/MS法同时测定枫蓼肠胃康合剂、胶囊中10种成分[J]. 中成药, 2019, 41(5): 983-987. |
| LIANG Y, XU W J, FU C Y, et al. Simultaneous determination of 10 components in Feng Polygonum Enterogastricum Combination and Capsules by HPLC-MS/MS method[J]. Chin Tradit Pat Med, 2019, 41(5): 983-987. | |
| 41 | 王彬, 裴科, 汪小莉, 等. 气相色谱-质谱联用测定石菖蒲中26种挥发性成分的研究[J]. 时珍国医国药, 2015, 26(11): 2627-2630. |
| WANG B, PEI K, WANG X L, et al. Determination of 26 volatile components in Acorus calamus by gas chromatography-mass spectrometry[J]. Lishizhen Med Mater Med, 2015, 26(11): 2627-2630. | |
| 42 | 邰佳, 邹俊波, 史亚军, 等. GC-MS分析水蒸气蒸馏法提取石菖蒲挥发油过程中油水分配规律[J]. 中草药, 2020, 51(1): 59-66. |
| TAI J, ZOU J B, SHI Y J, et al. GC-MS analysis of oil-water partitioning in the extraction of volatile oil from Acorus calamus by water vapor distillation[J]. Chin Tradit Herbal Drugs, 2020, 51(1): 59-66. |
| [1] | 胡轩, 吴同川, 艾鑫丹, 何兴悦, 徐弘康, 郑飞, 越皓, 戴雨霖. 液质联用结合网络药理学探究少腹逐瘀汤抗宫颈癌活性机理[J]. 应用化学, 2024, 41(5): 687-702. |
| [2] | 高英鑫, 徐伟, 张衍旭, 王野谌, 董雪莲. 灰色关联分析结合色度法评价不同产地黄连质量[J]. 应用化学, 2022, 39(6): 1000-1010. |
| [3] | 杜康, 周恒为, 丁明明, 叶峰, 石彤非. 聚类分析橡胶炭黑填充量与Yeoh模型参数的关联[J]. 应用化学, 2021, 38(6): 675-684. |
| [4] | 黄惠明, 李珊珊, 李海明, 吴水金, 李跃森, 林洪涛, 郑开斌. K-Means聚类分析法筛选柠檬香茅茎叶差异蛋白及鉴定[J]. 应用化学, 2020, 37(4): 455-463. |
| [5] | 方小伟,钟涛,姚国灿,高翔,杨美玲,李慧,乐长高,张兴磊. 脐橙果皮的室温和热辅助表面解吸常压化学电离质谱的比较[J]. 应用化学, 2015, 32(10): 1201-1207. |
| [6] | 徐景明, 宋崇立. 酰胺类萃取剂的模糊聚类分析[J]. 应用化学, 1992, 0(6): 71-76. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||