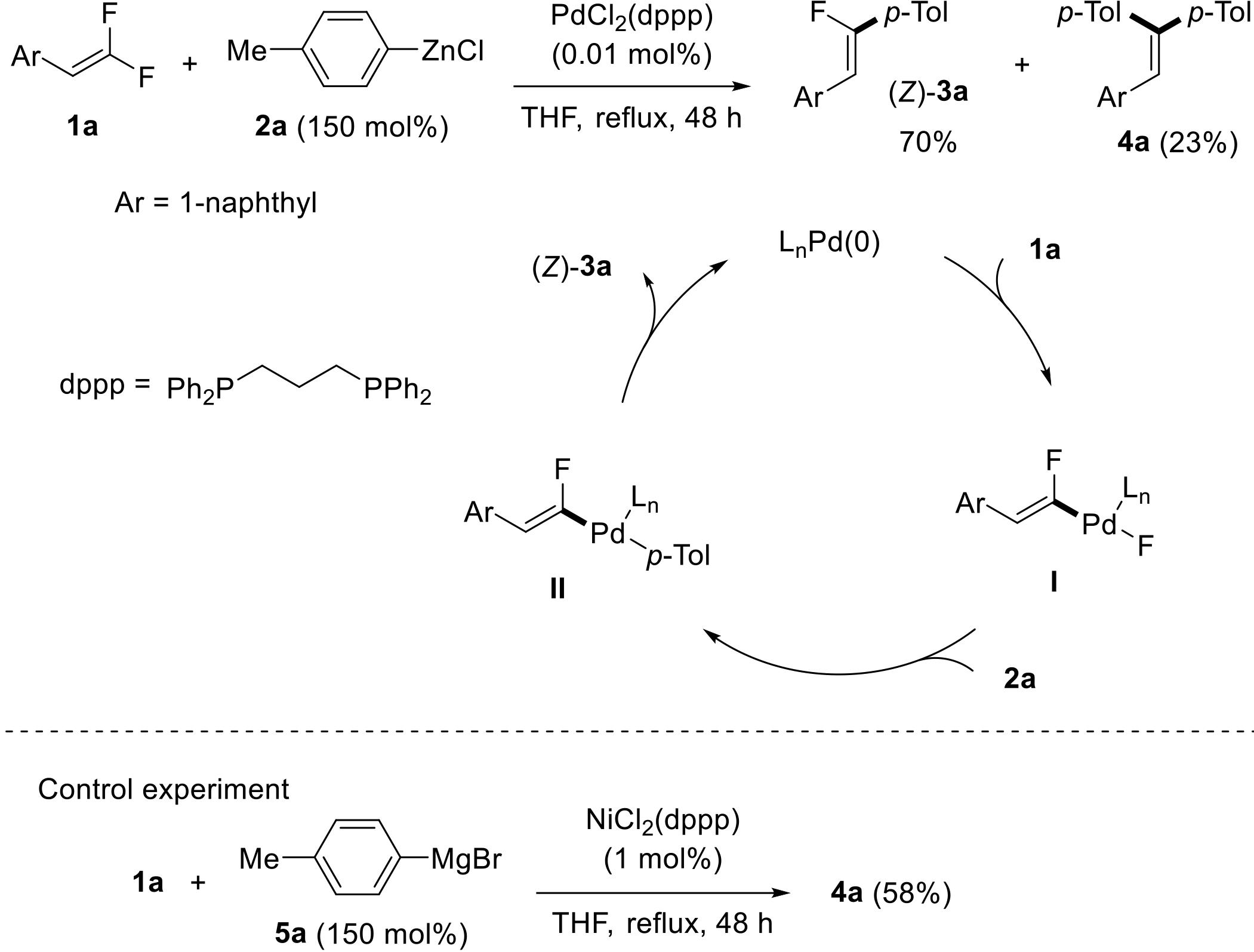
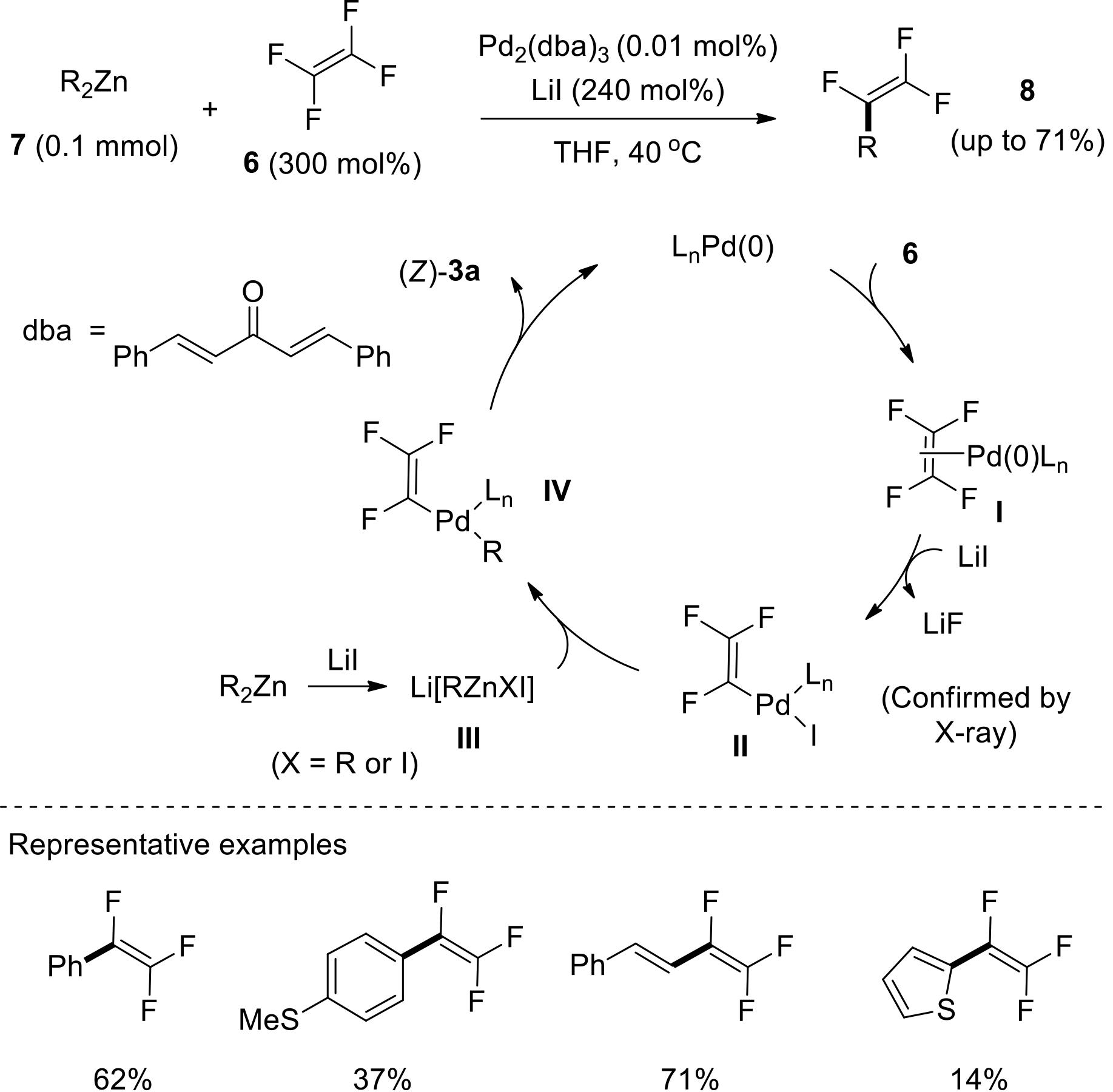
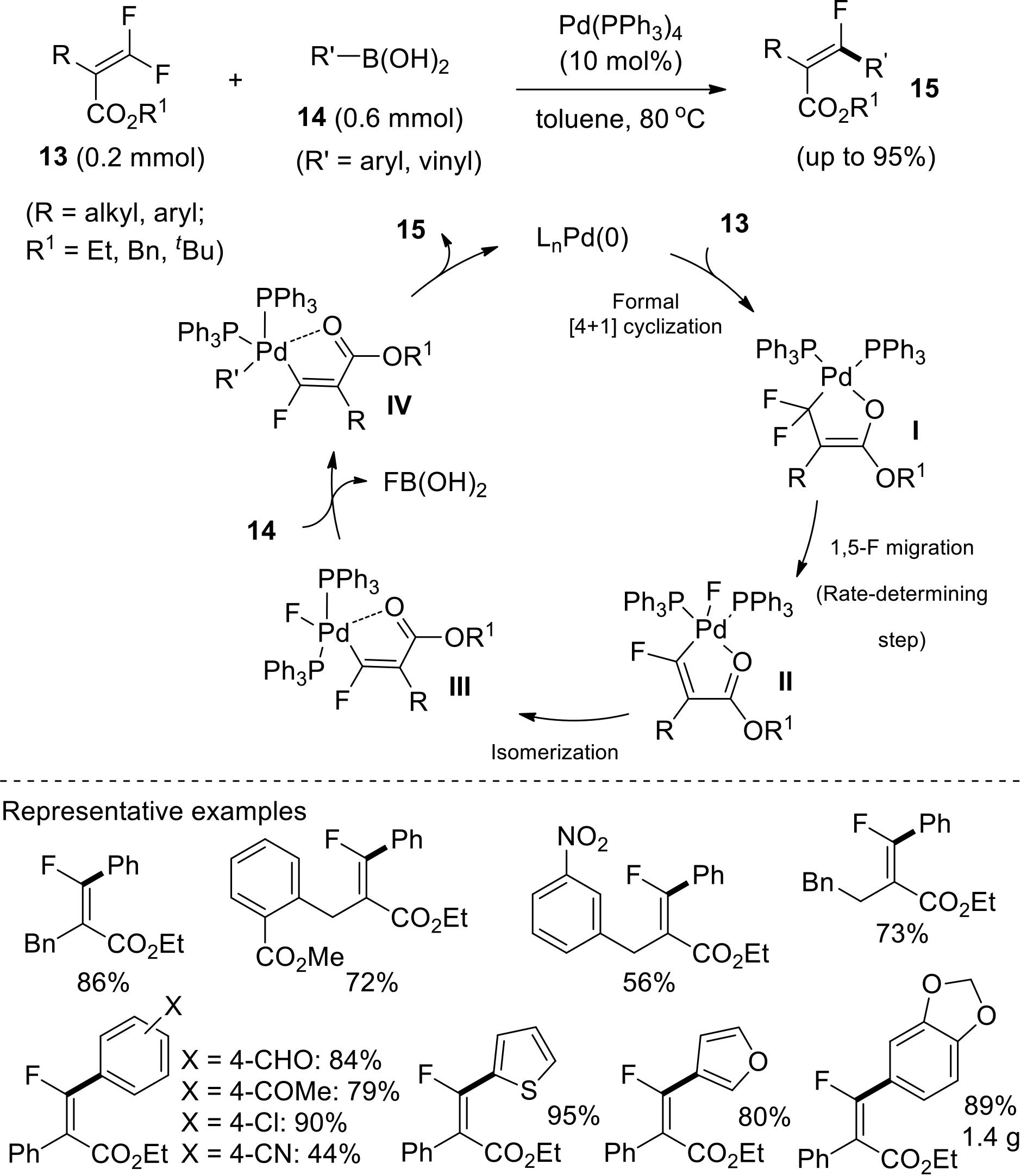
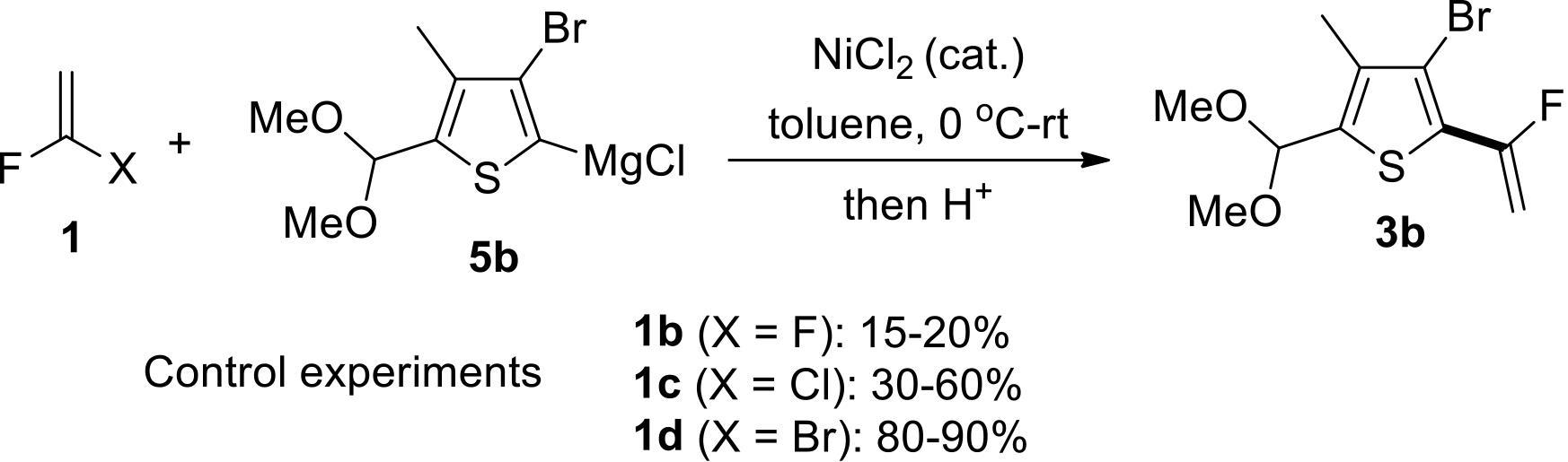应用化学 ›› 2022, Vol. 39 ›› Issue (5): 749-759.DOI: 10.19894/j.issn.1000-0518.210235
过渡金属催化氟代烯烃参与的交叉偶联反应进展
- 1.郑州工业应用技术学院药学与化学工程学院,郑州 451100
2.浙江师范大学化学与生命科学学院,金华 321004
3.河南大学化学化工学院,开封 475004
-
收稿日期:2021-05-13接受日期:2021-08-31出版日期:2022-05-01发布日期:2022-05-24 -
通讯作者:周列锦,曹中艳 -
基金资助:国家自然科学基金(21901233);河南省高等学校重点科研项目(21B350003)
Transition Metal⁃Catalyzed Cross⁃Coupling Reactions of Alkenyl Fluorides: Advances and Perspectives
Ya-Li FENG1, Lie-Jin ZHOU2( ), Zhong-Yan CAO3(
), Zhong-Yan CAO3( )
)
- 1.School of Pharmacy and Chemical Engineering,Zhengzhou University of Industrial Technology,Zhengzhou 451100,China
2.Key Laboratory of the Ministry of Education for Advanced Catalysis Materials,Department of Chemistry,Zhejiang Normal University,Jinhua 321004,China
3.College of Chemistry and Chemical Engineering,Henan University,Kaifeng 475004,China
-
Received:2021-05-13Accepted:2021-08-31Published:2022-05-01Online:2022-05-24 -
Contact:Lie-Jin ZHOU,Zhong-Yan CAO -
About author:zycao@henu.edu.cn
ljzhou@zjnu.cn
-
Supported by:the National Natural Science Foundation of China(21901233);the Key Scientific Research Projects of Colleges and Universities in Henan Province(21B350003)
摘要:
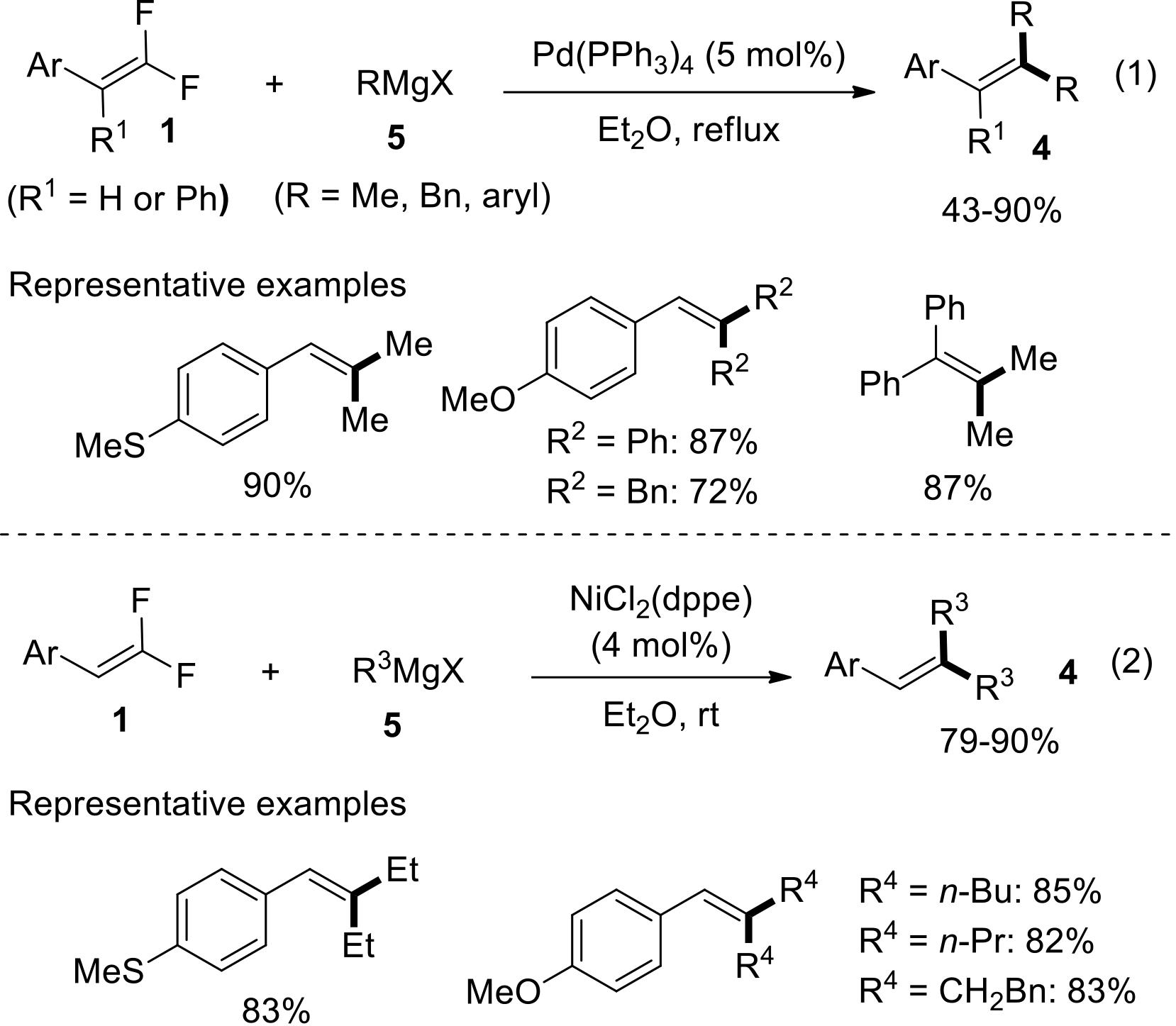
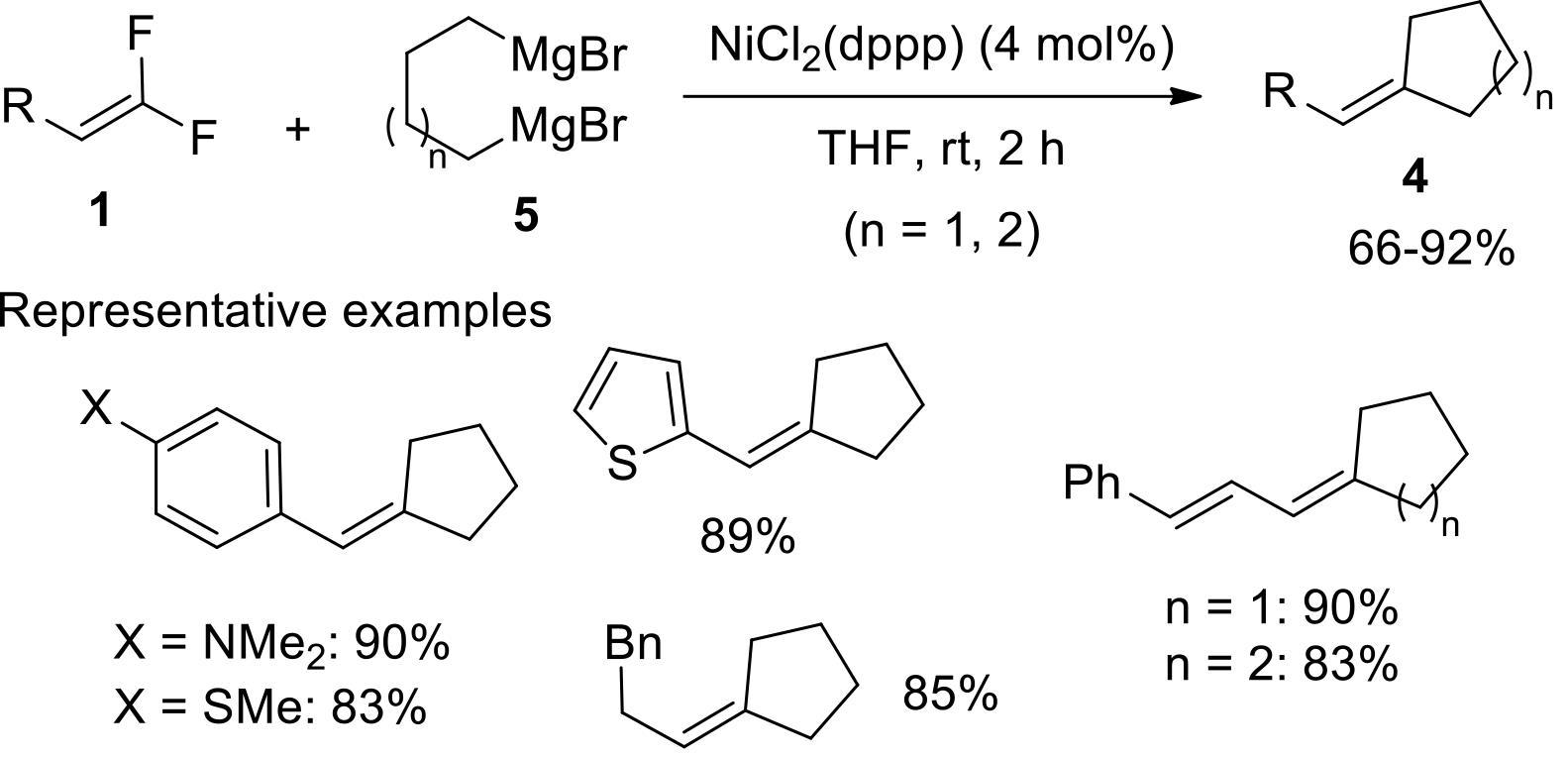
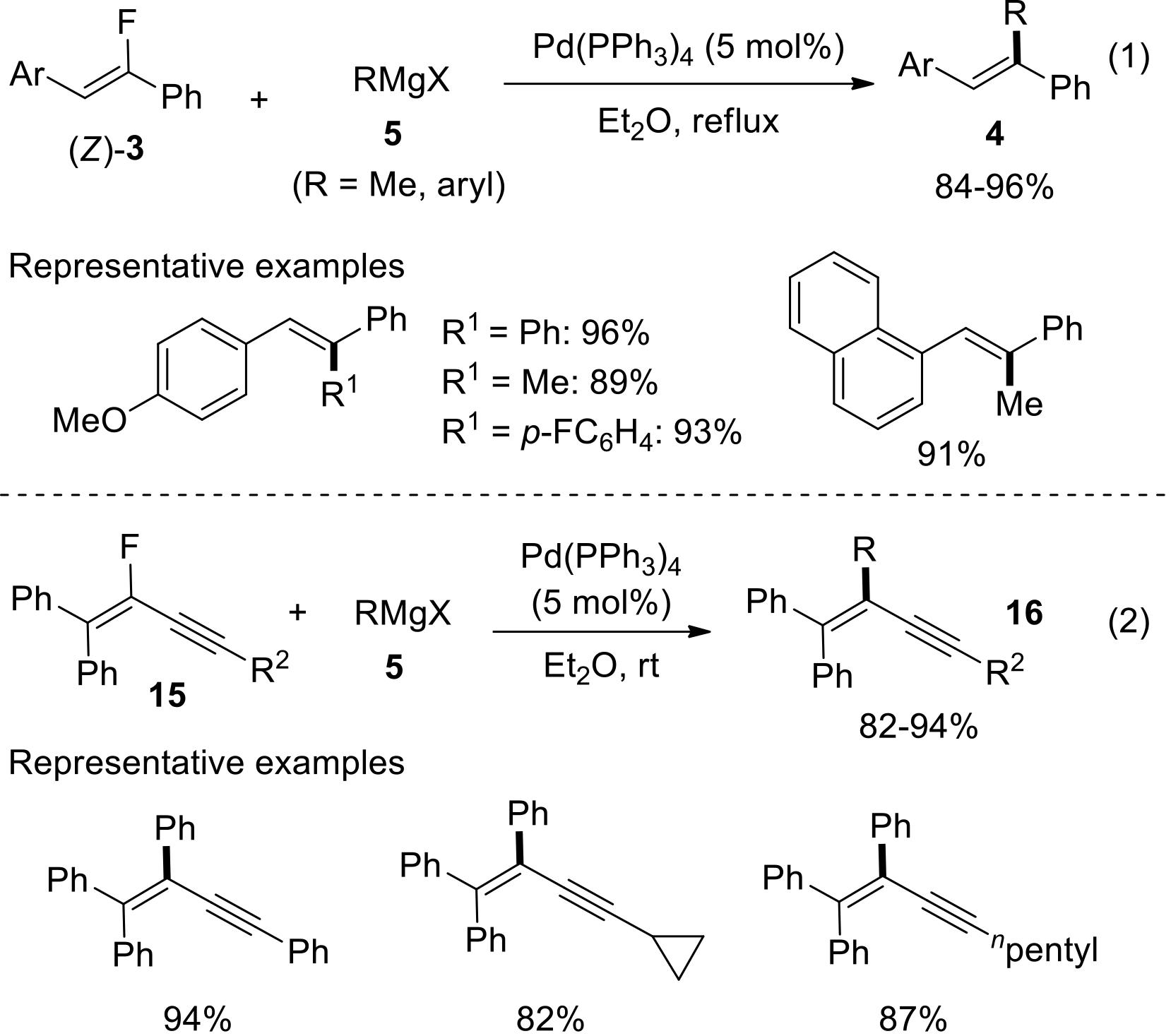
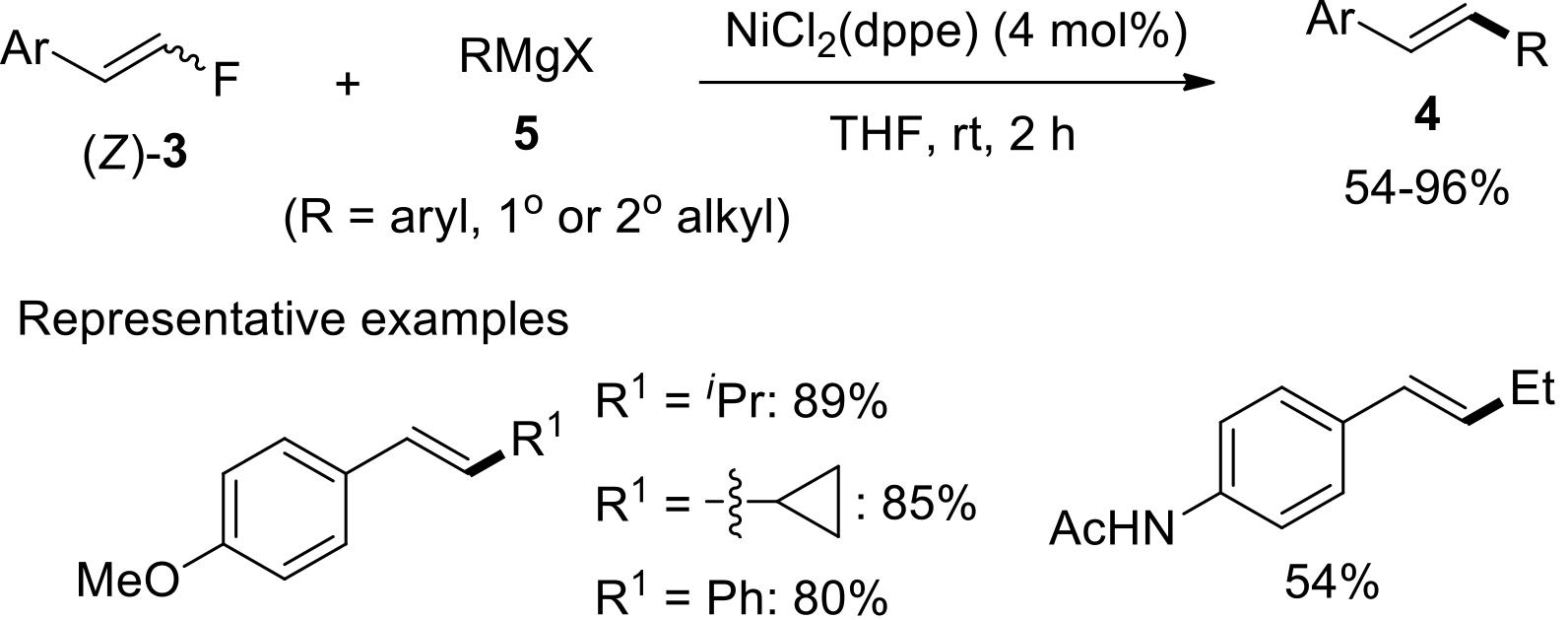
过渡金属催化(类)卤化物和不同金属试剂的交叉偶联反应是构建不同类型碳碳键和碳杂原子键的重要方法之一。该类反应一般使用活性较高的氯、溴、碘或类卤化物作为亲电试剂,尽管C—F键的键能较强,利用过渡金属直接活化较为惰性的芳基C—F键并参与实现的交叉偶联反应已有较多报道。此外,近期的研究表明,也可以通过直接活化烯基C—F键并催化实现该类底物参与不同类型的交叉偶联反应,从而进一步拓展了交叉偶联反应的底物适用范围,并应用于具有高附加值精细化学品的选择性合成。本文围绕钯或镍催化活化单氟或者多氟烯烃等底物参与的Negishi、Suzuki-Miyaura、Kumada、Hiyama和Sonogashira等5类交叉偶联反应,通过探讨已有方法的反应机理及其适用范围,综述了该领域的研究进展并进行了展望。
中图分类号:
引用本文
冯亚莉, 周列锦, 曹中艳. 过渡金属催化氟代烯烃参与的交叉偶联反应进展[J]. 应用化学, 2022, 39(5): 749-759.
Ya-Li FENG, Lie-Jin ZHOU, Zhong-Yan CAO. Transition Metal⁃Catalyzed Cross⁃Coupling Reactions of Alkenyl Fluorides: Advances and Perspectives[J]. Chinese Journal of Applied Chemistry, 2022, 39(5): 749-759.
使用本文
| 1 | DE MEIJERE A, DIEDERICH F. Metal-catalyzed cross-coupling reactions[M]. Weinheim: WILEY-VCH Verlag GmbH & Co. KGaA, 2004: 41-815. |
| 2 | MIYAURA N, SUZUKI A. Palladium-catalyzed cross-coupling reactions of organoboron compounds[J]. Chem Rev, 1995, 95(7): 2457-2483. |
| 3 | KANG S K, YAMAGUCHI T, KIM T H. Copper-catalyzed cross-coupling and carbonylative cross-coupling of crganostannanes and crganoboranes with hypervalent iodine compounds[J]. J Org Chem, 1996, 61: 9082-9083. |
| 4 | CHERNEY A H, KADUNCE N T, REISMAN S E. Enantioselective and enantiospecific transition-metal-catalyzed cross-coupling reactions of organometallic reagents to construct C—C bonds[J]. Chem Rev, 2015, 115(17): 9587-9652. |
| 5 | HERATH A, MOLTENI V, PAN S, et al. Generation and cross-coupling of crganozinc reagents in flow[J]. Org Lett, 2018, 20(23): 7429-7432. |
| 6 | ZHU F, WANG Z X. Nickel-catalyzed cross-coupling of aryl fluorides and organozinc reagents[J]. J Org Chem, 2014, 79(10): 4285-4292. |
| 7 | JANA R, PATHAK T P, SIGMAN M S. ChemInform abstract: advances in transition metal (Pd,Ni,Fe)-catalyzed cross-coupling reactions using alkyl-organometallics as reaction partners[J]. Chem Rev, 2011, 111(3): 1417-1492. |
| 8 | WOODWARD S, DAGORNE S. Modern organoaluminum reagents: preparation, structure, reactivity and use: topics in Organometallic Chemistry[M]. Berlin: Springer, 2003: 245-276. |
| 9 | MARUOKA K, YAMAMOTO H. Tetrahedron report number 239: organoaluminums in organic synthesis[J]. Tetrahedron, 1988, 44(16): 5001-5032. |
| 10 | CAREY F A, SANDBERG R J. Advanced organic chemistry part A: structure and mechanisms[M]. 5th Ed. New York:Springer,2007:258. |
| 11 | ZHANG X G, GUO P, HAN J F, et al. Cobalt fluorides: preparation, reactivity and applications in catalytic fluorination and C-F functionalization[J]. Chem Commun, 2020, 56(61): 8512-8523. |
| 12 | COATES G, REKHROUKH F, CRIMMIN M R. Breaking carbon-fluorine bonds with main group nucleophiles[J]. Synlett, 2019, 30(20): 2233-2246. |
| 13 | YIN H, ZABULA A V, SCHELTER E J. C-F→Ln/an interactions in synthetic F-element chemistry[J]. Dalton Trans, 2016, 45(15): 6313-6323. |
| 14 | AHRENS T, KOHLMANN J, AHRENS M, et al. Functionalization of fluorinated molecules by transition-metal-mediated C—F bond activation to access fluorinated building blocks[J]. Chem Rev, 2015, 115(2): 931-972. |
| 15 | OHASHI M, OGOSHI S. Palladium-catalyzed cross-coupling reactions of perfluoro organic compounds[J]. Catalysts, 2014, 4(3): 321-345. |
| 16 | STAHL T, KLARE H, OESTREICH M. ChemInform abstract: main-group lewis acids for C—F bond activation[J]. ChemInform, 2013, 3(7): 1578-1587. |
| 17 | KLAHN M, ROSENTHAL U. An update on recent stoichiometric and catalytic C—F bond cleavage reactions by lanthanide and group 4 transition-metal complexes[J]. ChemInform, 2012, 31(4): 1235-1244. |
| 18 | BRAUN T, WEHMEIEr F. C—F bond activation of highly fluorinated molecules at rhodium: from model reactions to catalysis[J]. Eur J Inorg Chem, 2011(5): 613-625. |
| 19 | CLOT E, EISENSTEIN O, JASIM N, et al. C—F and C—H bond activation of fluorobenzenes and fluoropyridines at transition metal centers: how fluorine tips the scales[J]. Acc Chem Res, 2011,44(5): 333-348. |
| 20 | AMII H, UNEYAMA K. C—F bond activation in organic synthesis[J]. Chem Rev, 2009, 109(5): 2119-2183. |
| 21 | BRAUN T, PERUTZ R N. Routes to fluorinated organic derivatives by nickel mediated C—F activation of heteroaromatics[J]. Chem Commun, 2002(23): 2749-2757. |
| 22 | KIPLINGER J L, RICHMOND T G, OSTERBERG C E. Activation of carbon-fluorine bonds by metal complexes[J]. Chem Rev, 1994, 94(2): 373-431. |
| 23 | KISO Y, TAMAO K, KUMADA M. Effects of the nature of halides on the alkyl group isomerization in the nickel-catalyzed cross-coupling of secondary alkyl Grignard reagents with organic halides [J]. Organomet Chem, 1973, 50(1): C12-C14. |
| 24 | AMII H, UNEYAMA K. C—F bond activation in organic synthesis[J]. Chem Rev, 2019, 109(5): 2119-2183. |
| 25 | SUN A D, LOVE J A. Cross coupling reactions of polyfluoroarenes via C—F activation[J]. Dalton Trans, 2010, 39(43): 10362-10374. |
| 26 | OHASHI M, OGOSHI S. Palladium-catalyzed cross-coupling reactions of perfluoro organic compounds[J]. Catalysts, 2014, 4: 321-345. |
| 27 | OHASHI M, OGOSHI, S. Transition-metal mediated transformations of tetrauoroethylene into various polyuorinated organic compounds[J]. J Synth Org Chem Jpn, 2016, 74(11): 1047-1057. |
| 28 | ZHANG X, CAO S. Recent advances in the synthesis and C—F functionalization of gem-difluoroalkenes[J]. Tetrahedron Lett, 2017, 58(5): 375-392. |
| 29 | ZHANG X J, CHENG Y M, ZHAO X W, et al. Catalytic asymmetric synthesis of monofluoroalkenes and gem-difluoroalkenes: advances and perspectives[J]. Org Chem Front, 2021, 8: 2315-2327. |
| 30 | WANG Q, TIAN P, CAO Z Y, et al. Copper-catalyzed remote direct thiocyanation of alkyl C(sp 3)-H bonds[J]. Adv Synth Catal, 2020, 362(18): 3851-3856. |
| 31 | ZHOU L J, WEI S, LEI Z, et al. Transition-metal-free α Csp 3—H cyanation of sulfonamides[J]. Chem Eur J, 2021, 27 (24): 7103-7107. |
| 32 | CAO Z Y, WANG W, LIAO K, et al.Catalytic enantioselective synthesis of cyclopropanes featuring vicinal all-carbon quaternary stereocenters with a CH2F group; study of the influence of C—F⋯H—N interactions on reactivity[J]. Org Chem Front, 2018, 5: 2960-2968. |
| 33 | SAEKI T, TAKASHIMA Y, TAMAO K. Nickel-and palladium-catalyzed cross-coupling reaction of polyfluorinated arenes and alkenes with Grignard reagents [J]. Synlett, 2005, 11: 1771-1774. |
| 34 | OHASHI M, KAMBARA T, HATANAKA T, et al. Palladium-catalyzed coupling reactions of tetrafluoroethylene with arylzinc compounds[J]. J Am Chem Soc, 2011, 133(10): 3256-3259. |
| 35 | OHASHI M, SAIJO H, SHIBATA M, et al. Palladium-catalyzed base-free Suzuki-Miyaura coupling reactions of fluorinated alkenes and arenes via a palladium fluoride key intermediate[J]. Eur J Org Chem, 2013(3): 443-447. |
| 36 | WANG Y, QI X, MA Q, et al. Stereoselective palladium-catalyzed base-free Suzuki-Miyaura cross-coupling of tetrasubstituted gem-difluoroalkenes: an experimental and computational study[J]. ACS Catal, 2021, 11(8): 4799-4809. |
| 37 | XIONG Y, HUANG T, JI X, et al. Nickel-catalyzed Suzuki-Miyaura type cross-coupling reactions of (2,2-difluorovinyl)benzene derivatives with arylboronic acids[J]. Org Biomol Chem, 2015, 13(27): 7389-7392. |
| 38 | QIU J, GYOROKOS A, TARASOW T M, et al. Grignard cross-coupling amenable to large scale production of alpha-fluorostyryl and alpha-fluorovinylthiophenes[J]. J Org Chem, 2008, 73(24): 9775-9777. |
| 39 | DAI W, XIAO J, JIN G, et al. Palladium-and nickel-catalyzed Kumada cross-coupling reactions of gem-difluoroalkenes and monofluoroalkenes with Grignard reagents[J]. J Org Chem, 2014, 79(21): 10537-10546. |
| 40 | DAI W, ZHANG X, ZHANG J, et al. Synthesis of exocyclic trisubstituted alkenes via nickel catalyzed Kumada-type cross-coupling reaction of gem-difluoroalkenes with di-Grignard reagents[J]. Adv Synth Catal, 2016, 358: 183-187. |
| 41 | SHI H, DAI W, WANG B, et al. Copper-and nickel-catalyzed cross-coupling reaction of monofluoroalkenes with tertiary, secondary, and primary alkyl and aryl Grignard reagents[J]. Organometallics, 2018, 37: 459-463. |
| 42 | SAIJO H, SAKAGUCHI H, OHASHI M, et al. Base-free Hiyama coupling reaction via a group 10 metal fluoride intermediate generated by C—F bond activation[J]. Organometallics, 2014, 33: 3669-3672. |
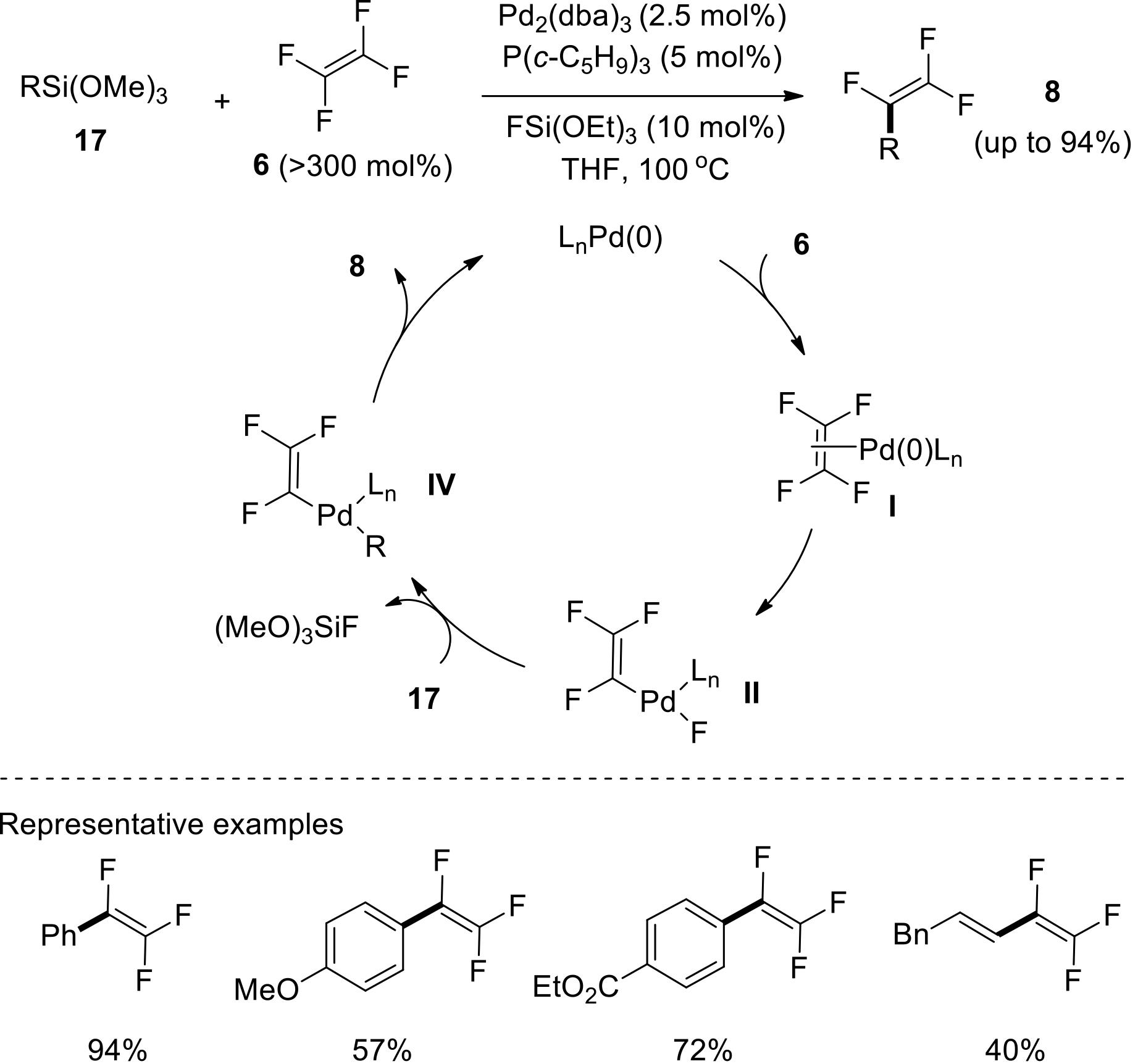
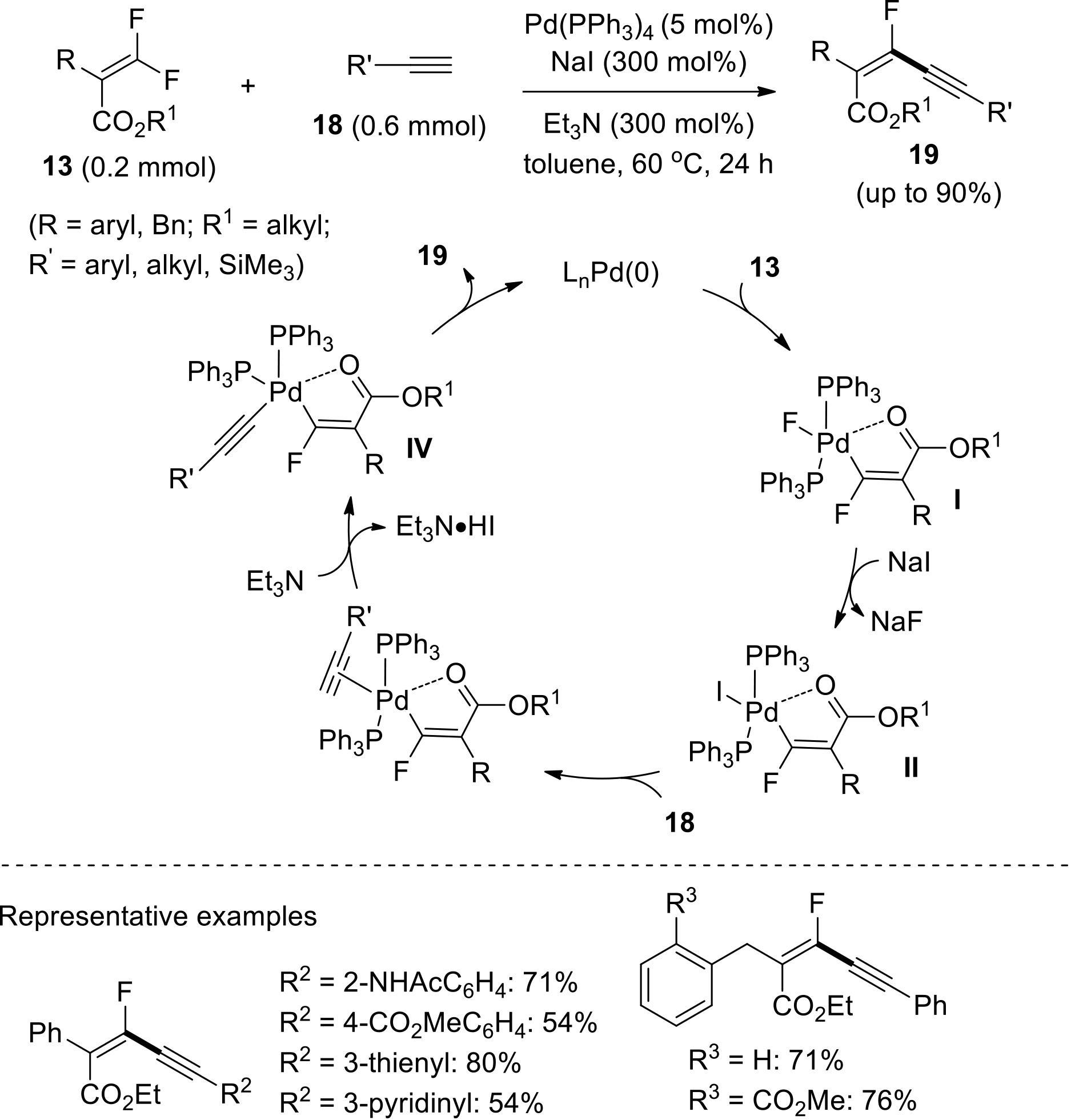
| 43 | MA Q, WANG Y, TSUI G C. Stereoselective palladium-catalyzed C—F bond alkynylation of tetrasubstituted gem-difluoroalkenes[J]. Angew Chem Int Ed, 2020, 59(28): 11293-11297. |
| [1] | 李威威,穆红亮,刘靖宇,李悦生. RCOO-取代的镍(Ⅱ)配合物单组分高效催化乙烯均聚和共聚反应[J]. 应用化学, 2018, 35(1): 89-101. |
| [2] | 周文俊,邓家英. 铁催化的交叉偶联反应研究进展[J]. 应用化学, 2016, 33(3): 245-266. |
| [3] | 冯翠兰, 刘建平, 桂建舟, 刘澜涛. 磁性纳米粒子负载钯催化剂在C—C键偶联反应中的应用[J]. 应用化学, 2015, 32(1): 19-26. |
| [4] | 冯翠兰, 徐海云, 刘澜涛, 王静, 刘瑛. 易磁分离磁性纳米Fe3O4在无溶剂和无配体条件下催化合成二芳醚[J]. 应用化学, 2014, 31(05): 536-540. |
| [5] | 王秀艳, 赵雪萍, 马树华. 浸渍法制备硅钨酸修饰的炭载钯催化剂电催化甲酸氧化[J]. 应用化学, 2013, 30(12): 1470-1475. |
| [6] | 穆红亮, 李彦国, 李悦生. 单组分酚膦中性镍催化乙烯均聚与共聚反应[J]. 应用化学, 2012, 29(12): 1381-1388. |
| [7] | 贺晓慧, 何福平, 聂华荣, 陈义旺, 王凯悌. 5-丁氧基亚甲基-2-降冰片烯的合成及其加成聚合反应[J]. 应用化学, 2011, 28(01): 10-15. |
| [8] | 沈娟章, 杨改秀, 唐亚文, 陆天虹. 直接甲酸燃料电池的研究进展[J]. 应用化学, 2010, 27(08): 869-874. |
| [9] | 陶李明, 刘文奇, 谭倪, 周芸. 5-甲基-2,3-二苯基-1,5-苯并硫氮杂卓-4(5H)-酮的合成[J]. 应用化学, 2010, 27(04): 494-496. |
| [10] | 苏新艳, 吴蕾, 徐洪耀. 钯催化Heck反应制备长共轭生色二苯乙烯衍生物[J]. 应用化学, 2008, 25(12): 1487-1489. |
| [11] | 郭孟萍, 周丽, 何仁. P⌒O环钯配合物的合成及对Suzuki交叉偶联反应的催化性能[J]. 应用化学, 2006, 23(5): 480-483. |
| [12] | 李三华, 刘蒲, 王岚. 壳聚糖席夫碱钯催化碘代苯与丙烯酸生成肉桂酸[J]. 应用化学, 2005, 22(5): 494-497. |
| [13] | 张磊, 崔元臣. 淀粉负载钯催化剂的制备及对Heck反应的催化性能[J]. 应用化学, 2005, 22(4): 440-444. |
| [14] | 薛行华, 王海华, 胡扬剑. 负载型二亚胺镍催化剂制备支化聚乙烯的结构与性能[J]. 应用化学, 2005, 22(1): 45-49. |
| [15] | 赫崇衡, 朱明, 金国林, 徐佩若, 汪仁. 氧化铈对Pd催化剂氧化活性和热稳定性的影响[J]. 应用化学, 2004, 21(2): 154-158. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||