Chinese Journal of Applied Chemistry ›› 2023, Vol. 40 ›› Issue (2): 210-228.DOI: 10.19894/j.issn.1000-0518.220151
• Review • Previous Articles Next Articles
Research Progress of Sb-based Anode Materials for Potassium Ion Batteries
Xue-Jian SHI1,2, Wan-Qiang LIU2, Chun-Li WANG1( ), Yong CHENG1, Li-Min WANG1
), Yong CHENG1, Li-Min WANG1
- 1. State Key Laboratory of Rare Earth Resource Utilization,Changchun Institute of Applied Chemistry,CAS,Changchun 130022,China
2.School of Materials Science and Engineering,Changchun University of Science and Technology,Changchun 130012,China
-
Received:2022-04-23Accepted:2022-08-08Published:2023-02-01Online:2023-02-27 -
Contact:Chun-Li WANG -
About author:clwang@ciac.ac.cn
-
Supported by:the National Key R&D Program of China(2017YFE0198100);the National Natural Science Foundation of China(21975250);the Scientific and Technological Developing Project of Jilin Province(20200401031GX);the Open Project Program of Key Laboratory of Preparation and Application of Environmental Friendly Materials (Jilin Normal University), the Ministry of Education, China(2020005);the Open Program of State Key Laboratory of Metastable Materials Science and Technology (Yanshan University), China(202110)
CLC Number:
Cite this article
Xue-Jian SHI, Wan-Qiang LIU, Chun-Li WANG, Yong CHENG, Li-Min WANG. Research Progress of Sb-based Anode Materials for Potassium Ion Batteries[J]. Chinese Journal of Applied Chemistry, 2023, 40(2): 210-228.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: http://yyhx.ciac.jl.cn/EN/10.19894/j.issn.1000-0518.220151
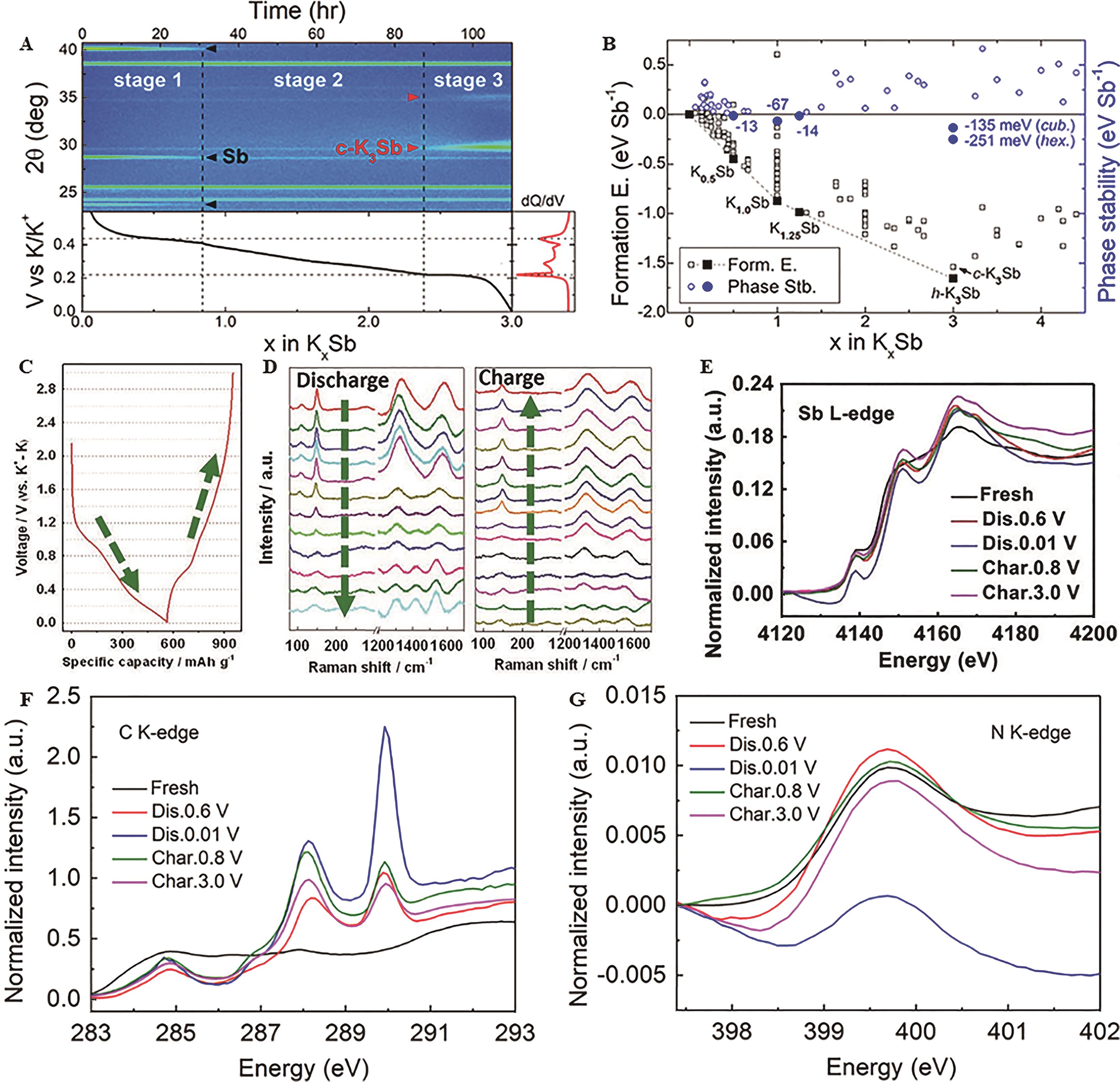
Fig.2 (A) In-situ XRD measurement of an Sb electrode for the first discharge; (B) Formation energies of K x Sb(0<x≤4.4) obtained by the ionic substitution method[10]; Phase stability is a relative energy of a given phase compared to that of all other materials with different compositions; K+ storage behavior of the as-prepared Sb@C PNFs integrated electrode: (C) Charge and discharge curves at 0.1 A/g, (D) The corresponding in-situ Raman spectra[11]; Ex-situ X-ray absorption near edge structure (XANES) spectra of (E) Sb L-edge, (F)C K-edge and (G) N K-edge of u-Sb@CNFs[12]
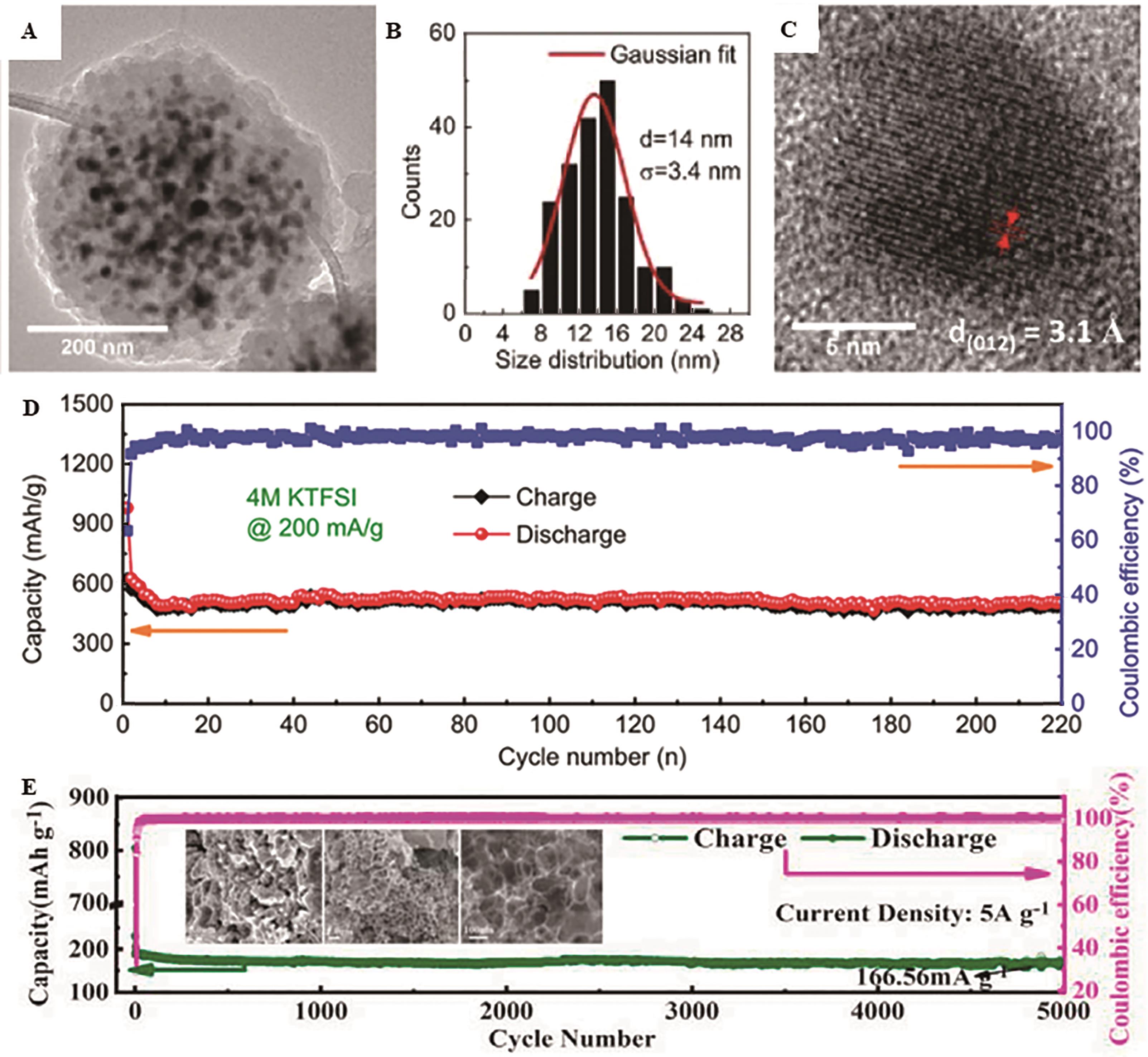
Fig.3 (A) TEM image of an individual Sb@CSN sphere and (B) corresponding size distribution curve of Sb nanoparticles from image (A); (C) HRTEM image of Sb@CSN; (D) The cycling performance at 0.2 A/g in the 4 mol/L KTFSI electrolyte for Sb@CSN anode[37]; (E) Cycling stability at 5 A/g of the 3D Se@Sb@C electrode, the insets are SEM images of the 3D Se@Sb@C after 5000 cycles[38]
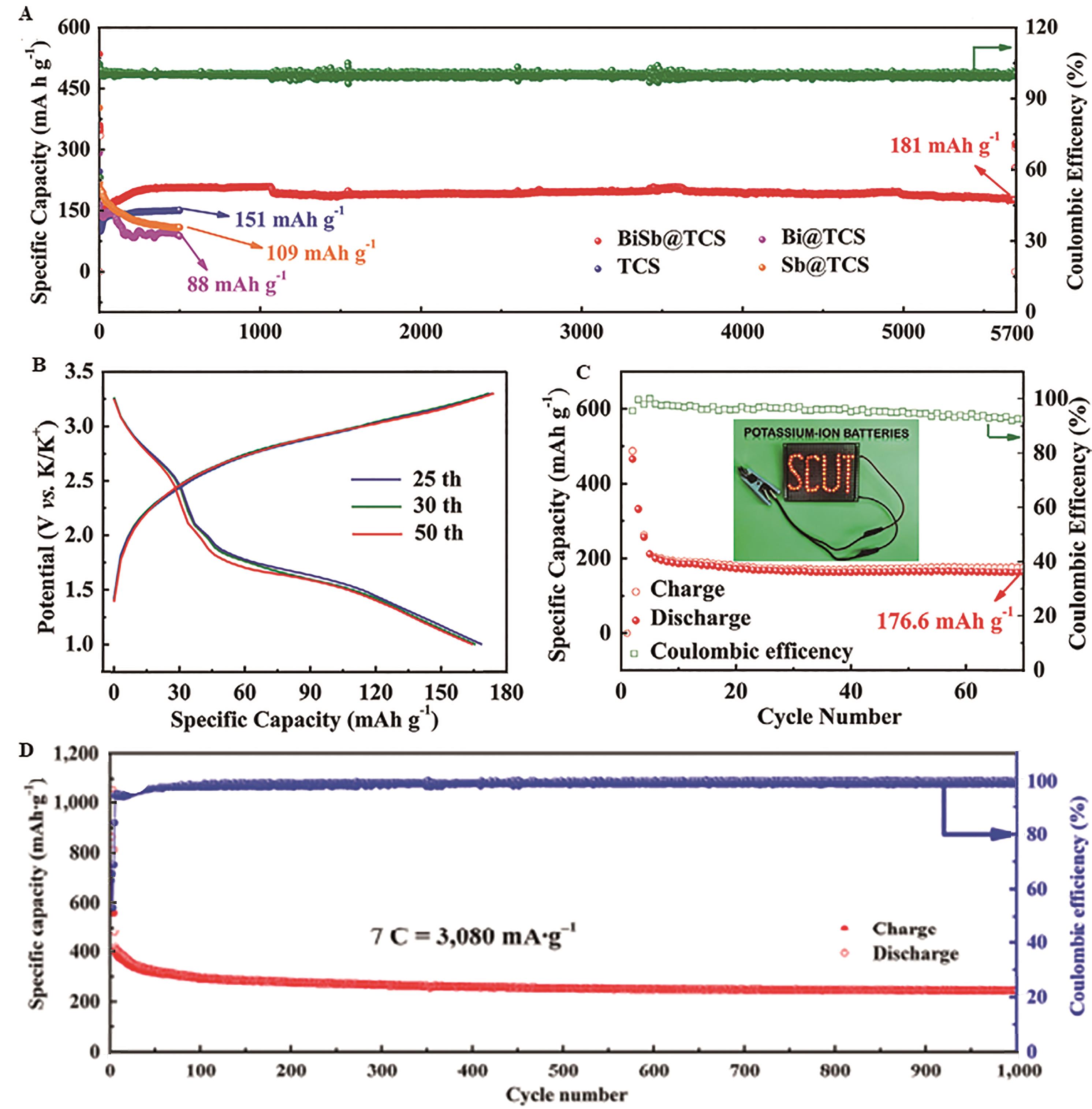
Fig.4 (A) Long-term cycling performances at 2 A/g for BiSb@TCS and control samples; Electrochemical performance of BiSb@TCS//Prussian white analogues full cells; (B) Galvanostatic charge/discharge profiles at 0.2 A/g; (C) Cycling performance at 0.2 A/g; The inset in (C) is photograph of lightening bulbs driven by the full cell[52]; (D) Long-term cycle performance of the FeSb@C/N?3DC/N electrode at a high rate of 7 C[53](0.308 A/g)
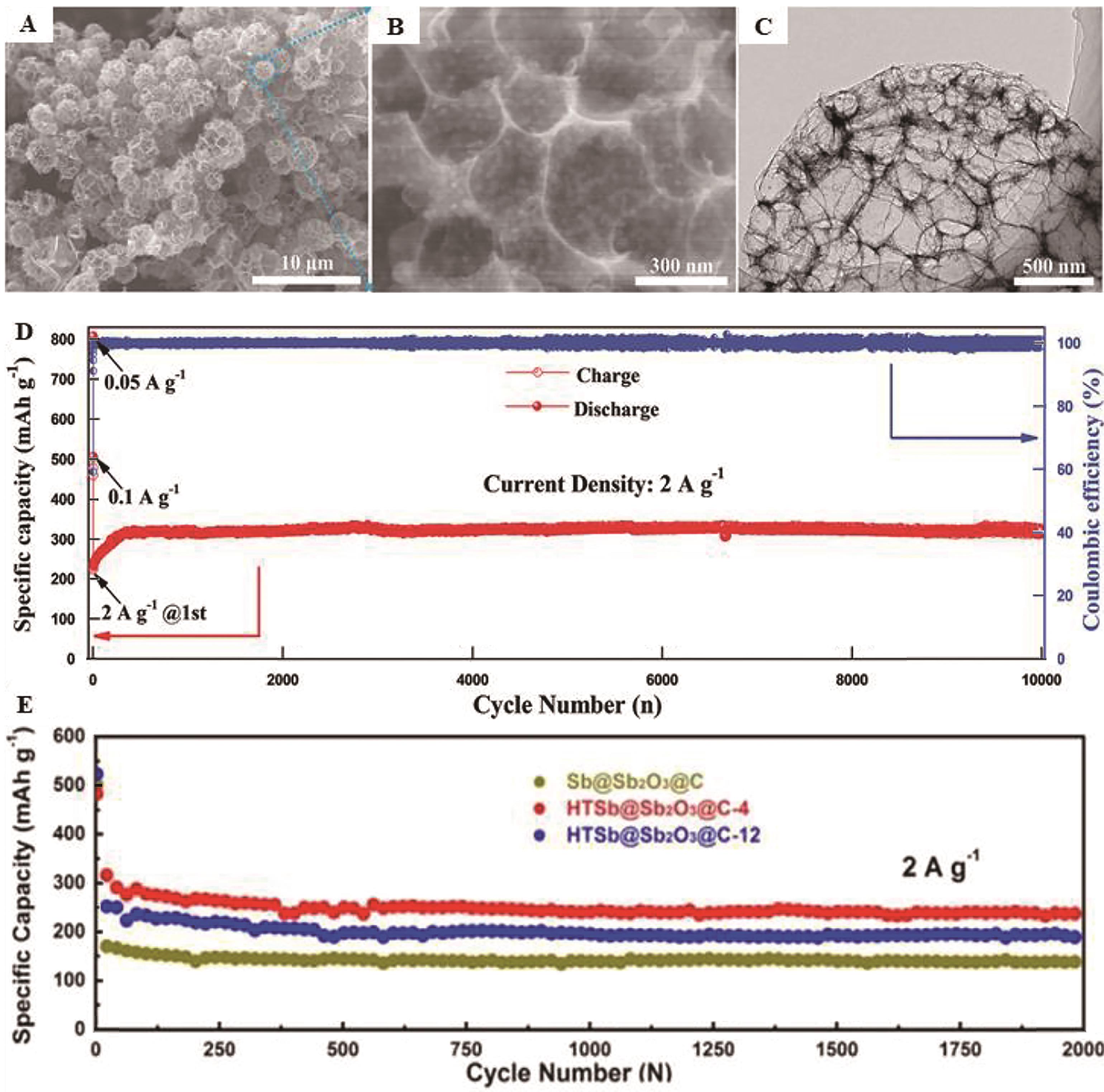
Fig.5 (A) and (B) HRTEM image, (C) TEM image Sb@Sb2O3@N-3DCHs; (D) Long-term cycling performance of Sb@Sb2O3@N-3DCHs at a current density of 2 A/g[58]; (E) Cycling performance at 0.1 A/g of HTSb@Sb2O3@C-4[57]
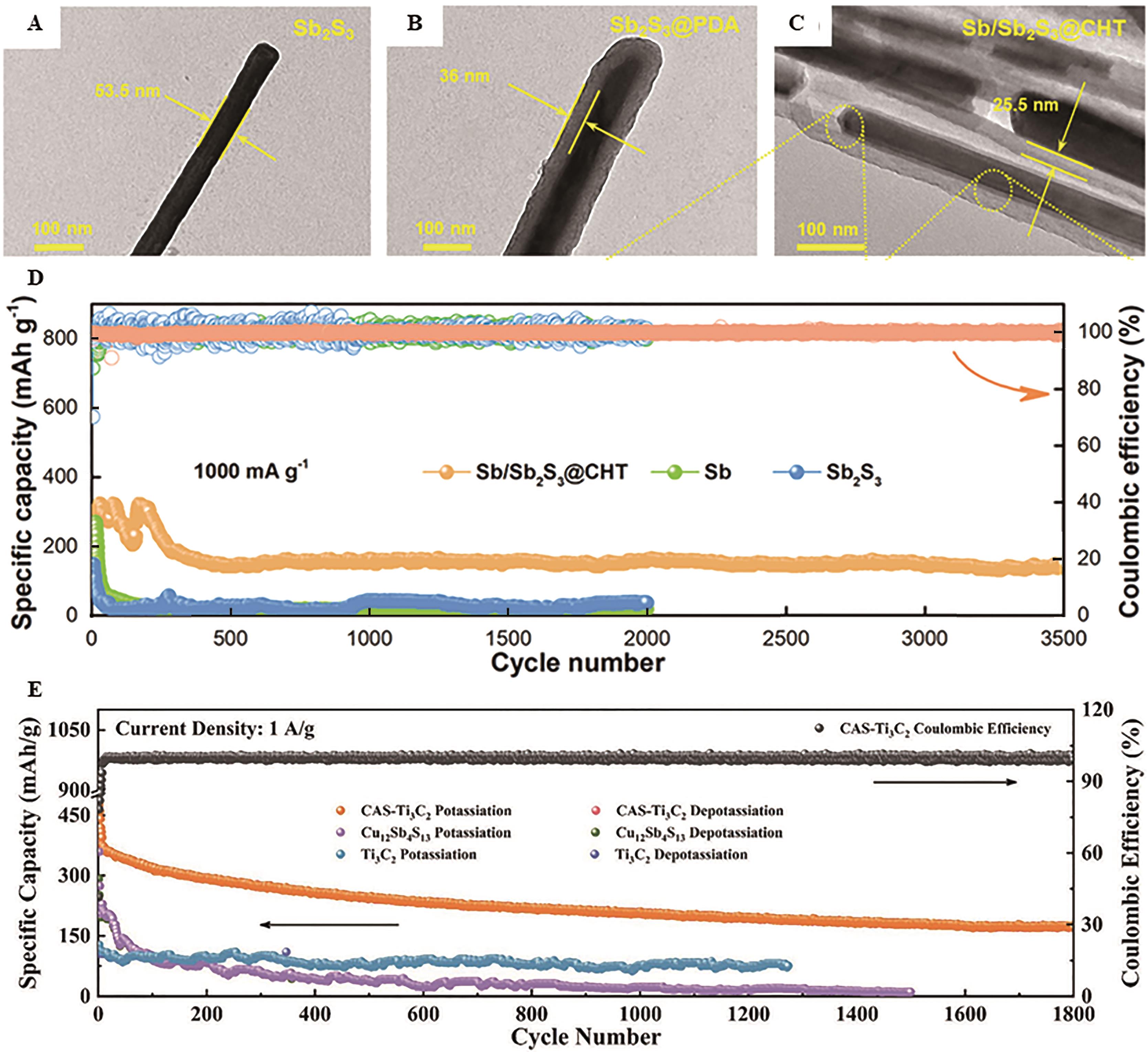
Fig.6 TEM images of (A) Sb2S3 nanorods, (B) Sb2S3@PDA and (C) Sb/Sb2S3@CHT, respectively; (D) Long cycle performance of commercial Sb powders, as-prepared Sb2S3 nanorods and Sb/Sb2S3@CHT at 1 A/g[64]; (E) Long cyclic performance of CAS-Ti3C2 composites, Cu12Sb4S13 quantum dots and Ti3C2 nanosheets[65]
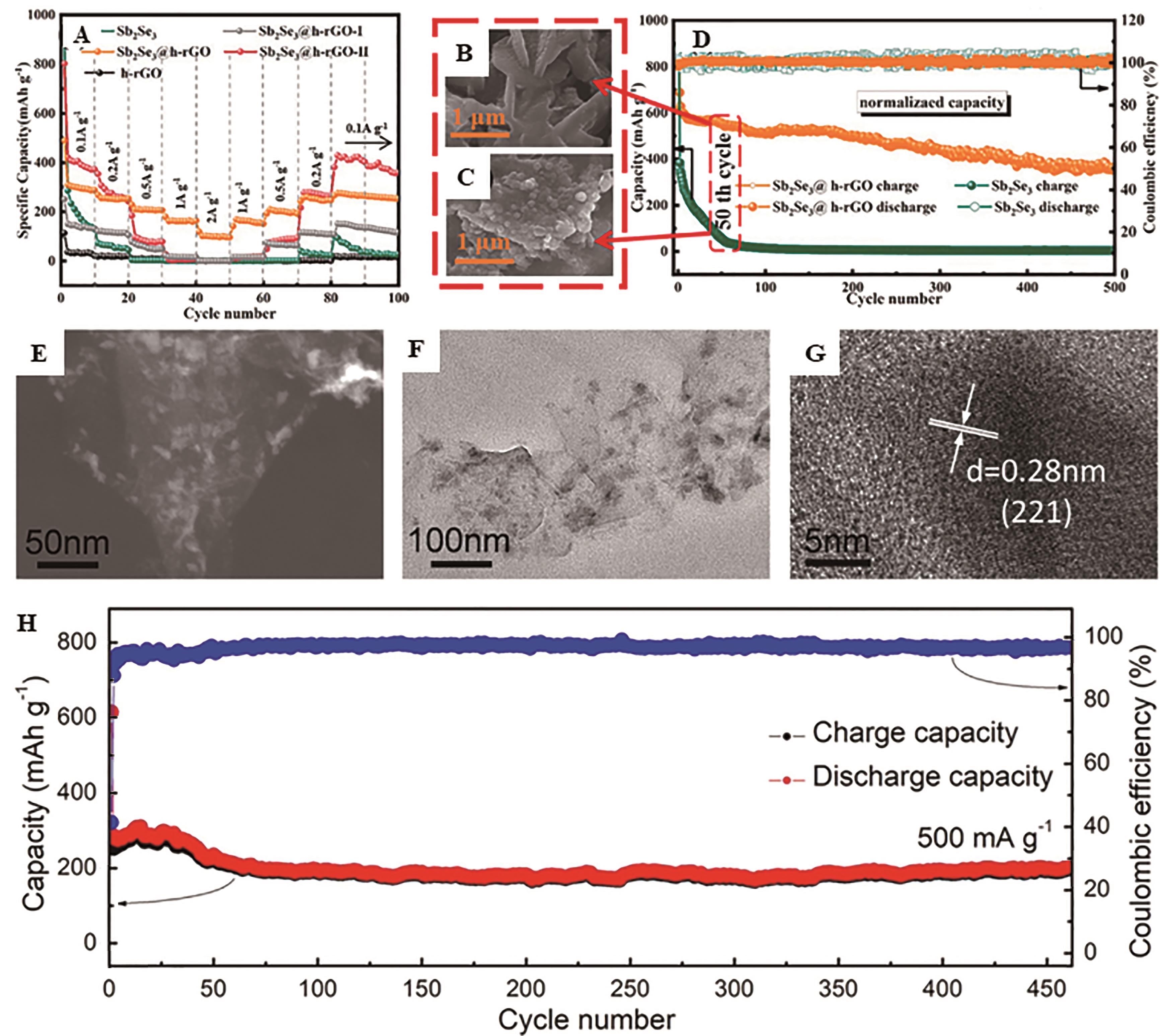
Fig.7 (A) Rate capability at the current densities from 0.05 to 2 A/g for h-rGO powder electrode, Sb2Se3 powder electrode, free-standing Sb2Se3@h-rGO, Sb2Se3@h-rGO-I and Sb2Se3@h-rGO-II electrodes; SEM images of (B) free-standing Sb2Se3@h-rGO electrode and (C) Sb2Se3 powder electrode after 50 repeated cycles[66]; (D) Cycling performances of free-standing Sb2Se3@h-rGO electrode and Sb2Se3 powder electrode at 0.1 A/g; (E) STEM images, (F) TEM image and (G) HRTEM image of the self-wrinkled Sb2Se3@RGO composites; The galvanostatic cycling performance of the self-wrinkled Sb2Se3@RGO composites at 0.5 A/g[67]
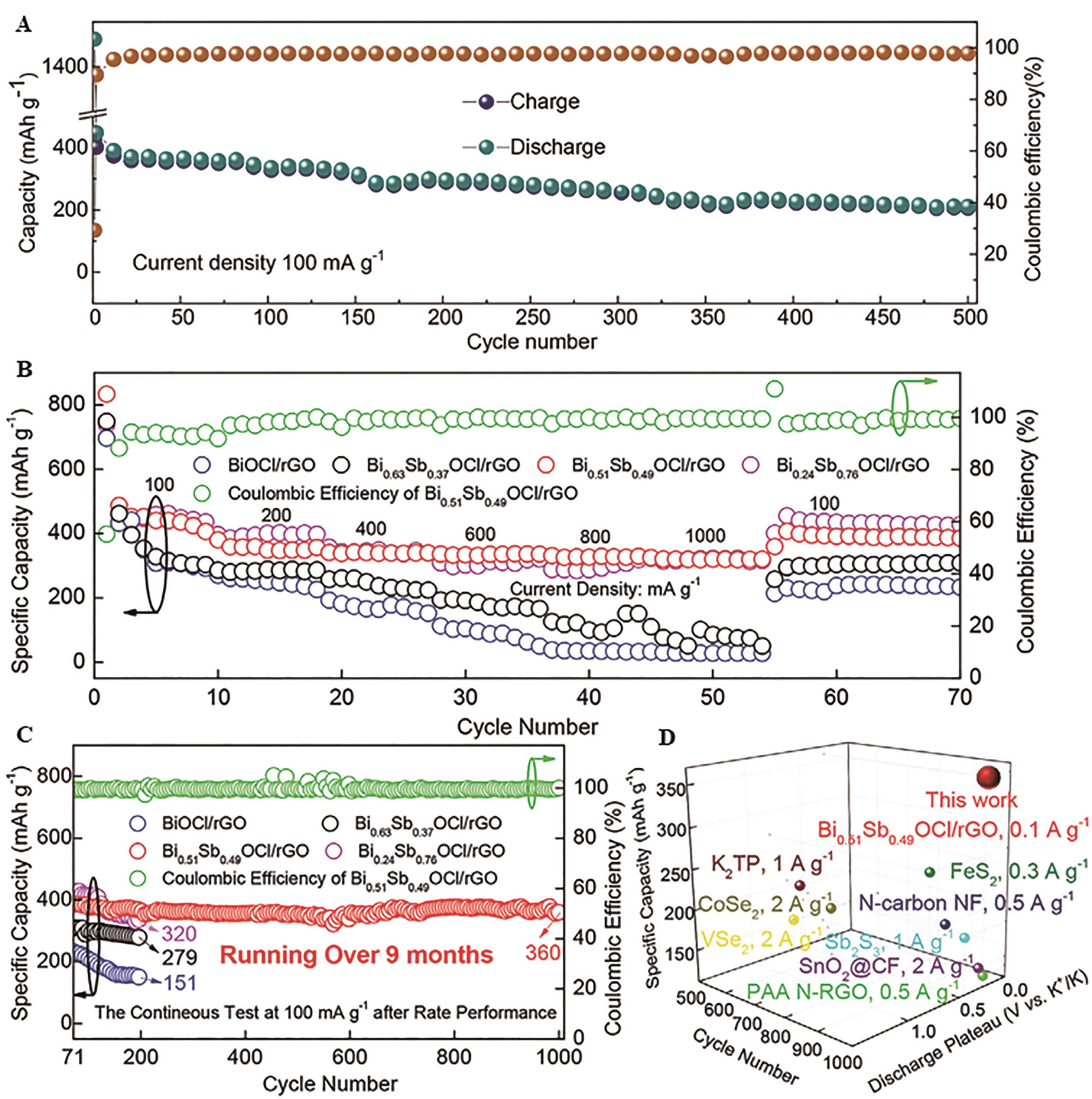
Fig.8 (A) Long-term cycling performance at a current density of 0.1 A/g of SbVO4@RGO[70]; (B) Rate performance of BiOCl/rGO, Bi0.63Sb0.37OCl/rGO, Bi0.51Sb0.49OCl/rGO and Bi0.24Sb0.76OCl/rGO; (C) The continuous test at 0.1 A/g after rate performance of BiOCl/rGO, Bi0.63Sb0.37OCl/rGO, Bi0.51Sb0.49OCl/rGO and Bi0.24Sb0.76OCl/rGO; (D) Comparison of the cycling performance, discharge plateau and specific capacity, between the Bi0.51Sb0.49OCl/rGO and other reported electrodes for PIBs with at least 500 cycles[68]
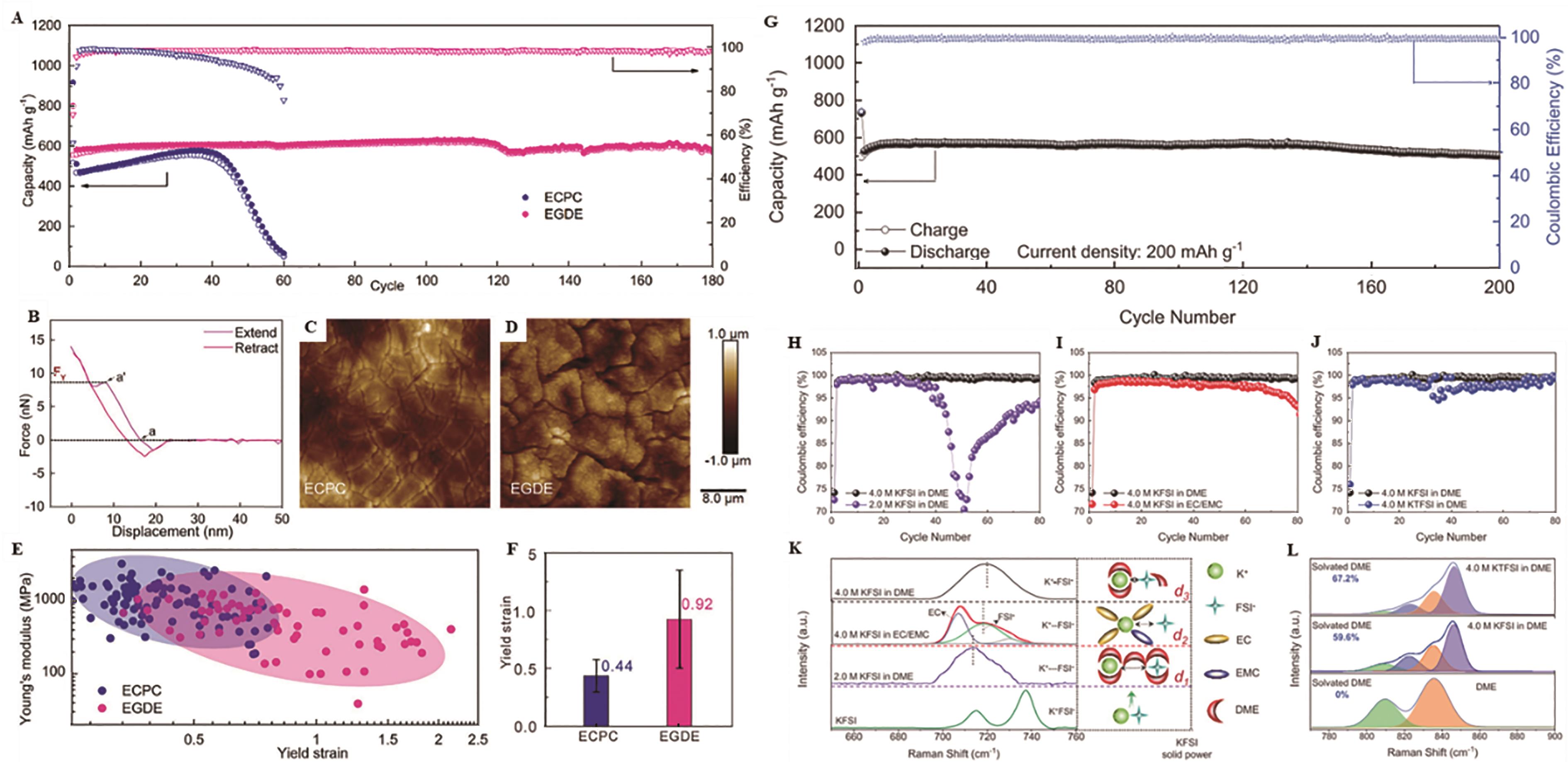
Fig.9 (A) Cyclic performance under 0.1 A/g in 1 mol/L KFSI/ECPC and 1 mol/L KFSI/EGDE electrolytes of Sb electrode; (B) Characteristic force curves obtained from the AFM tests on electrodeposited Sb electrodes; AFM topography images in (C) 1 mol/L KFSI/ECPC and (D) 1 mol/L KFSI/EGDE; (E) Distributions of Young′s modulus and yield strain of SEIs; (F) Histogram with an error bar of yield strain[73]; (G) Cycling performance of Sb anode at the current density of 0.2 A/g; (H-J) Comparison of Coulombic efficiency of Sb electrode in different electrolytes; (K) Raman spectra of FSI- anions using different KFSI concentrations or different solvents and the corresponding schematic interaction of the ions(i.e. K+, FSI-)and solvent; (L) Comparative Raman spectra showing the CH2 rocking/C—O stretching vibrations of DME solvent in different potassium salts (i.e. KFSI,KTFSI) was used in the DME-based electrolyte[74]
| Classify | Electrode material | Synthetic method | Electrolyte(concentration|solute salt|solvent) | Rate performance(current density|capacity) | Cycling performance (current density|cycle number|capacity) | Initial coulombic efficiency (ICE)/% | Ref. |
|---|---|---|---|---|---|---|---|
| Sb-based materials | Sb@RGO | Solvothermal+thermal reduction | 0.8 mol/L KPF6 in EC|PC | 0.1 A/g|381 mA·h/g | 0.5 A/g|200|210 mA·h/g | - | [ |
| Sb@CNFs | Ion-exchange+thermal reduction | 2 mol/L KFSI|DME | 2 A/g|121 mA·h/g | 1 A/g|1000|227 mA·h/g | 47 | [ | |
| Sb@C PNFs | Electrospinning+ thermal reduction | 1 mol/L KFSI in EC|DEC | 5 A/g|208.1 mA·h/g | 2 A/g|500|264 mA·h/g | 71.3 | [ | |
| u-Sb@CNFs | Electrospinning+ thermal reduction | 3 mol/L KFSI in DME | 5 A/g|145 mA·h/g | 1 A/g|2000|225 mA·h/g | 48.3 | [ | |
| Sb@NPMC | Electrospinning+ thermal reduction | 0.8 mol/L KPF6 in EC|DEC | 1 A/g|161 mA·h/g | 1 A/g|1500|130 mA·h/g | 50 | [ | |
| Sb/CNF-0.5 | Electrospinning | 1 mol/L KFSI in EC|DEC | - | 0.5 A/g|500|403 mA·h/g | 64.7 | [ | |
| Sb/hard carbon | Pyrolysis+ball-milling | 0.8 mol/L KPF6 in EC|DEC | - | 0.1 A/g|50|285 mA·h/g | 44 | [ | |
| Sb/CNS | Solvothermal | 1 mol/L KPF6 in EC|DMC | 2 A/g|101.4 mA·h/g | 0.2 A/g|600|247 mA·h/g | 48 | [ | |
| Sb@HCT | Calcination and reduction | 1 mol/L KTFSI in EC|DMC | 0.5 A/g|453.4 mA·h/g | 2 A/g|120|300.1 mA·h/g | - | [ | |
| Sb/DWCNT | - | 0.8 mol/L KFSI in EC|DEC | 0.88 A/g|453 mA·h/g | 0.11 A/g|30|491 mA·h/g | 88.4 | [ | |
| Sb-P-C | Ball-milling | 0.75 mol/L KPF6 in EC|DEC | - | 0.05 A/g|50|402 mAh/g | - | [ | |
| Sb@G@C | Freeze-dried | 3 mol/L KFSI in DME | 2 A/g|127 mA·h/g | 1 A/g|800|160 mA·h/g | 75.8 | [ | |
| Sb-G-C | Electrospinning | 0.8 mol/L KPF6 in EC|DEC | 1 A/g|120.83 mA·h/g | 0.1 A/g|100|204.95 mA·h/g | 46.84 | [ | |
| Sb/C/RGO | Freeze-dried | 1 mol/L KFSI|DME | 2 A/g|370 mA·h/g | 0.5 A/g|350|400 mA·h/g | 68 | [ | |
| Sb7@G3 | Ball-milling + ultrasonic+ | - | 1 A/g|340 mA·h/g | 0.2 A/g|100|292 mA·h/g | - | [ | |
| Sb/RGO | Freeze-dried | 0.8 mol/L KPF6 in EC|DMC | 1 A/g|180 mA·h/g | 1 A/g|100|140.4 mA·h/g | 60.3 | [ | |
| Sb/rGO | Freeze-dried+thermal reduction | 1 mol/L KPF6 in EC|PC | - | - | 61 | [ | |
| Sb@C-3DP | Ball-milling+thermal reduction | 5 mol/L KFSI in DME | 1 A/g|286 mA·h/g | 0.5 A/g|260|342 mA·h/g | 76.2 | [ | |
| 3D SbNPs@C | Template+freeze-dried +carbothermic reduction | 0.8 mol/L KPF6 in EC|DEC | 1 A/g|288 mA·h/g | 1 A/g|50|225 mA·h/g | 70 | [ | |
| Sb@PC | Liquid-solid absorption | 1 mol/L KPF6 in EC|DEC | 2 A/g|70 mA·h/g | 0.5 A/g|200|90 mA·h/g | 46.2 | [ | |
| Sb/C | - | 0.8 mol/L KFSI in EC|DEC | - | 0.044 A/g|30|365 mA·h/g | 68 | [ | |
| Sb@MCMB-3 | Ball-milling | 3 mol/L KFSI|DME | 5 A/g|293.9 mA·h/g | 1 A/g|500|300.1 mA·h/g | 54.8 | [ | |
| Sb-Co | Reduction precipitation | 0.8 mol/L KPF6 in EC|DEC | 6 A/g|377.8 mA·h/g | 0.06 A/g|100|402.7 mA·h/g | 67.9 | [ | |
| Sn-Sb LSM | Ball-milling | 4 mol/L KFSI in EMC | 5 A/g|118 mA·h/g | 0.5 A/g|150|296 mA·h/g | - | [ | |
| MS@C (MoS2/Sb@C) | Thermal reduction | 1 mol/L KFSI|DME | 2 A/g|235.4 mA·h/g | 2 A/g|1000|170.1 mA·h/g | 59.3 | [ | |
| Sb@CSN | Electrospray+thermal reduction | 4 mol/L KTFSI in EC|DEC | 0.1 A/g|566 mA·h/g | 0.2 A/g|220|504 mA·h/g | - | [ | |
| 3D Se@Sb@C | Template+CVD | 0.8 mol/L KFSI in EC|DEC | 10 A/g|107.7 mA·h/g | 5 A/g|5700|166.6 mA·h/g | 67.5 | [ | |
| NP-Sb | Vacuum-distillation | 0.8 mol/L KPF6 in EC|DEC | 0.5 A/g|265 mA·h/g | 0.1 A/g|50|318 mA·h/g | 71 | [ | |
| SbM-based materials | 3D FeSb@NC | Freeze-dried+thermal reduction | 0.8 mol/L KPF6 in EC|DEC | 2 A/g|119.7 mA·h/g | 0.5 A/g|1000|135 mA·h/g | - | [ |
| Cu2Sb@3DPC | Freeze-dried+thermal reduction | 0.8 mol/L KPF6 in EC|DEC | 5 A/g|148 mA·h/g | 0.1 A/g|100|260 mA·h/g | 20.4 | [ | |
| CoSb@3DPCs | Template+freeze-dried+thermal reduction | 0.8 mol/L KPF6 in EC|DEC | 5 A/g|134 mA·h/g | 1 A/g|500|287.5 mA·h/g | - | [ | |
| Bi0.5Sb0.5@P | Solution precipitation | 4 mol/L KFSI in DME | 6.5A/g|258.5 mA·h/g | 1 A/g|550|339.1 mA·h/g | - | [ | |
| BiSb@C | NaCl-template | 3 mol/L KFSI in DME | 2 A/g|246.8 mA·h/g | 0.5 A/g|1000|303.5 mA·h/g | 82 | [ | |
| BiSb@C | Freeze-dried+pyrolysis | 5 mol/L KFSI in DME | 2 A/g|152 mA·h/g | 0.5 A/g|600|320 mA·h/g | 70.2 | [ | |
| SnSb@C | NaCl template+thermal reduction | 0.8 mol/L KPF6 in EC|DEC | 10 A/g|58.3 mA·h/g | 0.1 A/g|100|293 mA·h/g | 56.2 | [ | |
| SnSb | High-energy ball-milling | 0.8 mol/L KFSI in EC|DEC | - | 0.1 A/g|40|282 mA·h/g | - | [ | |
| SnSb@MAC | Polyesterification | 4 mol/L KFSI in DME | 3.75 A/g|91.3 mA·h/g | 0.5 A/g|5000|200 mA·h/g | - | [ | |
| SnSb-G-C | Electrospinning+ carbonized | 0.8 mol/L KPF6 in EC|DEC | 1 A/g|207.27 mA·h/g | 0.1 A/g|100|275.14 mA·h/g | 52.53 | [ | |
| 3D SnSb@NC | NaCl template+ pyrolysis | 0.5 mol/L KPF6 in DME | 2 A/g|116.6 mA·h/g | 0.5 A/g|200|185.8 mA·h/g | - | [ | |
| BiSb@TCS | Spraying+thermal reduction | 3 mol/L KFSI|DME | 6 A/g|119.3 mA·h/g | 2 A/g|5700|181 mA·h/g | 64.8 | [ | |
| FeSb@C/N?3DC/N | Green salt template | 0.8 mol/L KPF6 in EC|DEC | 0.1 A/g|614 mA·h/g | 0.308 A/g|1000|245 mA·h/g | - | [ | |
| Sb x O y -based materials | BiSb@Bi2O3/SbO x @C | Template+pyrogenic decomposition | 0.8 mol/L KPF6 in EC|DEC | 2 A/g|111 mA·h/g | 1 A/g|500|214 mA·h/g | 34 | [ |
| Sb/Sb2O4/Fe3C | Freeze-dried+heat treatment | 1 mol/L KPF6 in DME | 2 A/g|135 mA·h/g | 2 A/g|1244|108 mA·h/g | - | [ | |
| PAA?N-RGO(PAA, H2Sb2O6·nH2O) | One-pot synthesis | 1 mol/L KPF6 in EC/DMC/EMC | 2 A/g|96 mA·h/g | 0.5 A/g|1000|124 mA·h/g | 51 | [ | |
HTSb @Sb2O3@C-4 | NaCl template+ pyrolysis | 0.8 mol/L KPF6 in EC|DMC | 0.1A/g|543.9 mA·h/g | 2 A/g|2000|273 mA·h/g | 21.6 | [ | |
| Sb@Sb2O3@N-3DCHs | Spray drying+heat treatment | 3 mol/L KFSI in DME | 5 A/g|239 mA·h/g | 2 A/g|10000|319 mA·h/g | - | [ | |
| Sb x S y -based materials | Sb2S3@C | Hydrothermal +carbonization | 1 mol/L KFSI in DME | 1 A/g|163 mA·h/g | 0.05 A/g|50|293 mA·h/g | - | [ |
| Sb2S3-SNG | Freeze-dried | 1 mol/L KPF6 in EC|DEC | 1 A/g|340 mA·h/g | 0.05 A/g|100|537 mA·h/g | 69.7 | [ | |
| (Bi,Sb)2S3 | In situ alloying | 3 mol/L KFSI in DME | 1 A/g|300 mA·h/g | 0.5 A/g|1000|353 mA·h/g | 51.9 | [ | |
| Sb2S3-rGO | Heat treatment | 1 mol/L KPF6 in EC|DEC | - | 0.05 A/g|50|110 mAh/g | - | [ | |
| Sb2S3@PPy | Sonication+freeze-dried | 0.8 mol/L KPF6 in EC|DEC | 2 A/g|220 mA·h/g | 1 A/g|50|157 mA·h/g | 63.7 | [ | |
| Sb/Sb2S3@CHT | Hydrothermal+ coating+carbonization | 4 mol/L KFSI in DME | 2 A/g|173.2 mA·h/g | 1 A/g|3500|147.5 mA·h/g | 62.3 | [ | |
| CAS-Ti3C2(Cu12Sb4S13, CAS) | Acoustic degradation +thermal injection | 0.8 mol/L KPF6 in EC|DEC | 5 A/g|163.3 mA·h/g | 1 A/g|1800|175.6 mA·h/g | 57.2 | [ | |
| Sb x Se y -based materials | Sb2Se3@h-rGO | Vacuum filtration+ thermal reduction | 0.8 mol/L KFSI in EC|DMC | 2 A/g|73 mA·h/g | 0.1 A/g|500|382.8 mA·h/g | 66 | [ |
| Sb2Se3@RGO | Hydrothermal+selenization | 0.8 mol/L KPF6 in EC|PC | 0.1 A/g|391.4 mA·h/g | 0.5 A/g|460|203.4 mA·h/g | 49 | [ | |
| Other Sb-based materials | Bi0.51Sb0.49OCl/rGO | Sol-gel+freeze-dried | 3 mol/L KFSI in DME | 1 A/g|319 mA·h/g | 0.1 A/g|1000|360 mA·h/g | 55.9 | [ |
| Sb2MoO6/rGO | Hydrothermal | 3 mol/L KFSI in DME | 0.2 A/g|381 mA·h/g | 0.5 A/g|100|247 mA·h/g | 55.7 | [ | |
| SbVO4@RGO | Solvothermal | 5 mol/L KFSI in EC| DMC | 0.1 A/g|447.9 mA·h/g | 0.1 A/g|500|210.1 mA·h/g | 29.2 | [ | |
| Opitimi cation of electrolyte | Micro-Sized Sb | Chemical reduction | 1 mol/L KFSI in EGDE | 5 A/g |225 mA·h/g | 0.1 A/g|180|573 mA·h/g | 69.4 | [ |
| Micro-Sized Sb | Commercial Sb powder | 4 mol/L KFSI in DME | 3 A/g|305 mA·h/g | 0.2 A/g|200|553 mA·h/g | - | [ |
Table 1 Comparison of the synthetic methods, electrolytes, and K+ storage performances of the different Sb-based materials
| Classify | Electrode material | Synthetic method | Electrolyte(concentration|solute salt|solvent) | Rate performance(current density|capacity) | Cycling performance (current density|cycle number|capacity) | Initial coulombic efficiency (ICE)/% | Ref. |
|---|---|---|---|---|---|---|---|
| Sb-based materials | Sb@RGO | Solvothermal+thermal reduction | 0.8 mol/L KPF6 in EC|PC | 0.1 A/g|381 mA·h/g | 0.5 A/g|200|210 mA·h/g | - | [ |
| Sb@CNFs | Ion-exchange+thermal reduction | 2 mol/L KFSI|DME | 2 A/g|121 mA·h/g | 1 A/g|1000|227 mA·h/g | 47 | [ | |
| Sb@C PNFs | Electrospinning+ thermal reduction | 1 mol/L KFSI in EC|DEC | 5 A/g|208.1 mA·h/g | 2 A/g|500|264 mA·h/g | 71.3 | [ | |
| u-Sb@CNFs | Electrospinning+ thermal reduction | 3 mol/L KFSI in DME | 5 A/g|145 mA·h/g | 1 A/g|2000|225 mA·h/g | 48.3 | [ | |
| Sb@NPMC | Electrospinning+ thermal reduction | 0.8 mol/L KPF6 in EC|DEC | 1 A/g|161 mA·h/g | 1 A/g|1500|130 mA·h/g | 50 | [ | |
| Sb/CNF-0.5 | Electrospinning | 1 mol/L KFSI in EC|DEC | - | 0.5 A/g|500|403 mA·h/g | 64.7 | [ | |
| Sb/hard carbon | Pyrolysis+ball-milling | 0.8 mol/L KPF6 in EC|DEC | - | 0.1 A/g|50|285 mA·h/g | 44 | [ | |
| Sb/CNS | Solvothermal | 1 mol/L KPF6 in EC|DMC | 2 A/g|101.4 mA·h/g | 0.2 A/g|600|247 mA·h/g | 48 | [ | |
| Sb@HCT | Calcination and reduction | 1 mol/L KTFSI in EC|DMC | 0.5 A/g|453.4 mA·h/g | 2 A/g|120|300.1 mA·h/g | - | [ | |
| Sb/DWCNT | - | 0.8 mol/L KFSI in EC|DEC | 0.88 A/g|453 mA·h/g | 0.11 A/g|30|491 mA·h/g | 88.4 | [ | |
| Sb-P-C | Ball-milling | 0.75 mol/L KPF6 in EC|DEC | - | 0.05 A/g|50|402 mAh/g | - | [ | |
| Sb@G@C | Freeze-dried | 3 mol/L KFSI in DME | 2 A/g|127 mA·h/g | 1 A/g|800|160 mA·h/g | 75.8 | [ | |
| Sb-G-C | Electrospinning | 0.8 mol/L KPF6 in EC|DEC | 1 A/g|120.83 mA·h/g | 0.1 A/g|100|204.95 mA·h/g | 46.84 | [ | |
| Sb/C/RGO | Freeze-dried | 1 mol/L KFSI|DME | 2 A/g|370 mA·h/g | 0.5 A/g|350|400 mA·h/g | 68 | [ | |
| Sb7@G3 | Ball-milling + ultrasonic+ | - | 1 A/g|340 mA·h/g | 0.2 A/g|100|292 mA·h/g | - | [ | |
| Sb/RGO | Freeze-dried | 0.8 mol/L KPF6 in EC|DMC | 1 A/g|180 mA·h/g | 1 A/g|100|140.4 mA·h/g | 60.3 | [ | |
| Sb/rGO | Freeze-dried+thermal reduction | 1 mol/L KPF6 in EC|PC | - | - | 61 | [ | |
| Sb@C-3DP | Ball-milling+thermal reduction | 5 mol/L KFSI in DME | 1 A/g|286 mA·h/g | 0.5 A/g|260|342 mA·h/g | 76.2 | [ | |
| 3D SbNPs@C | Template+freeze-dried +carbothermic reduction | 0.8 mol/L KPF6 in EC|DEC | 1 A/g|288 mA·h/g | 1 A/g|50|225 mA·h/g | 70 | [ | |
| Sb@PC | Liquid-solid absorption | 1 mol/L KPF6 in EC|DEC | 2 A/g|70 mA·h/g | 0.5 A/g|200|90 mA·h/g | 46.2 | [ | |
| Sb/C | - | 0.8 mol/L KFSI in EC|DEC | - | 0.044 A/g|30|365 mA·h/g | 68 | [ | |
| Sb@MCMB-3 | Ball-milling | 3 mol/L KFSI|DME | 5 A/g|293.9 mA·h/g | 1 A/g|500|300.1 mA·h/g | 54.8 | [ | |
| Sb-Co | Reduction precipitation | 0.8 mol/L KPF6 in EC|DEC | 6 A/g|377.8 mA·h/g | 0.06 A/g|100|402.7 mA·h/g | 67.9 | [ | |
| Sn-Sb LSM | Ball-milling | 4 mol/L KFSI in EMC | 5 A/g|118 mA·h/g | 0.5 A/g|150|296 mA·h/g | - | [ | |
| MS@C (MoS2/Sb@C) | Thermal reduction | 1 mol/L KFSI|DME | 2 A/g|235.4 mA·h/g | 2 A/g|1000|170.1 mA·h/g | 59.3 | [ | |
| Sb@CSN | Electrospray+thermal reduction | 4 mol/L KTFSI in EC|DEC | 0.1 A/g|566 mA·h/g | 0.2 A/g|220|504 mA·h/g | - | [ | |
| 3D Se@Sb@C | Template+CVD | 0.8 mol/L KFSI in EC|DEC | 10 A/g|107.7 mA·h/g | 5 A/g|5700|166.6 mA·h/g | 67.5 | [ | |
| NP-Sb | Vacuum-distillation | 0.8 mol/L KPF6 in EC|DEC | 0.5 A/g|265 mA·h/g | 0.1 A/g|50|318 mA·h/g | 71 | [ | |
| SbM-based materials | 3D FeSb@NC | Freeze-dried+thermal reduction | 0.8 mol/L KPF6 in EC|DEC | 2 A/g|119.7 mA·h/g | 0.5 A/g|1000|135 mA·h/g | - | [ |
| Cu2Sb@3DPC | Freeze-dried+thermal reduction | 0.8 mol/L KPF6 in EC|DEC | 5 A/g|148 mA·h/g | 0.1 A/g|100|260 mA·h/g | 20.4 | [ | |
| CoSb@3DPCs | Template+freeze-dried+thermal reduction | 0.8 mol/L KPF6 in EC|DEC | 5 A/g|134 mA·h/g | 1 A/g|500|287.5 mA·h/g | - | [ | |
| Bi0.5Sb0.5@P | Solution precipitation | 4 mol/L KFSI in DME | 6.5A/g|258.5 mA·h/g | 1 A/g|550|339.1 mA·h/g | - | [ | |
| BiSb@C | NaCl-template | 3 mol/L KFSI in DME | 2 A/g|246.8 mA·h/g | 0.5 A/g|1000|303.5 mA·h/g | 82 | [ | |
| BiSb@C | Freeze-dried+pyrolysis | 5 mol/L KFSI in DME | 2 A/g|152 mA·h/g | 0.5 A/g|600|320 mA·h/g | 70.2 | [ | |
| SnSb@C | NaCl template+thermal reduction | 0.8 mol/L KPF6 in EC|DEC | 10 A/g|58.3 mA·h/g | 0.1 A/g|100|293 mA·h/g | 56.2 | [ | |
| SnSb | High-energy ball-milling | 0.8 mol/L KFSI in EC|DEC | - | 0.1 A/g|40|282 mA·h/g | - | [ | |
| SnSb@MAC | Polyesterification | 4 mol/L KFSI in DME | 3.75 A/g|91.3 mA·h/g | 0.5 A/g|5000|200 mA·h/g | - | [ | |
| SnSb-G-C | Electrospinning+ carbonized | 0.8 mol/L KPF6 in EC|DEC | 1 A/g|207.27 mA·h/g | 0.1 A/g|100|275.14 mA·h/g | 52.53 | [ | |
| 3D SnSb@NC | NaCl template+ pyrolysis | 0.5 mol/L KPF6 in DME | 2 A/g|116.6 mA·h/g | 0.5 A/g|200|185.8 mA·h/g | - | [ | |
| BiSb@TCS | Spraying+thermal reduction | 3 mol/L KFSI|DME | 6 A/g|119.3 mA·h/g | 2 A/g|5700|181 mA·h/g | 64.8 | [ | |
| FeSb@C/N?3DC/N | Green salt template | 0.8 mol/L KPF6 in EC|DEC | 0.1 A/g|614 mA·h/g | 0.308 A/g|1000|245 mA·h/g | - | [ | |
| Sb x O y -based materials | BiSb@Bi2O3/SbO x @C | Template+pyrogenic decomposition | 0.8 mol/L KPF6 in EC|DEC | 2 A/g|111 mA·h/g | 1 A/g|500|214 mA·h/g | 34 | [ |
| Sb/Sb2O4/Fe3C | Freeze-dried+heat treatment | 1 mol/L KPF6 in DME | 2 A/g|135 mA·h/g | 2 A/g|1244|108 mA·h/g | - | [ | |
| PAA?N-RGO(PAA, H2Sb2O6·nH2O) | One-pot synthesis | 1 mol/L KPF6 in EC/DMC/EMC | 2 A/g|96 mA·h/g | 0.5 A/g|1000|124 mA·h/g | 51 | [ | |
HTSb @Sb2O3@C-4 | NaCl template+ pyrolysis | 0.8 mol/L KPF6 in EC|DMC | 0.1A/g|543.9 mA·h/g | 2 A/g|2000|273 mA·h/g | 21.6 | [ | |
| Sb@Sb2O3@N-3DCHs | Spray drying+heat treatment | 3 mol/L KFSI in DME | 5 A/g|239 mA·h/g | 2 A/g|10000|319 mA·h/g | - | [ | |
| Sb x S y -based materials | Sb2S3@C | Hydrothermal +carbonization | 1 mol/L KFSI in DME | 1 A/g|163 mA·h/g | 0.05 A/g|50|293 mA·h/g | - | [ |
| Sb2S3-SNG | Freeze-dried | 1 mol/L KPF6 in EC|DEC | 1 A/g|340 mA·h/g | 0.05 A/g|100|537 mA·h/g | 69.7 | [ | |
| (Bi,Sb)2S3 | In situ alloying | 3 mol/L KFSI in DME | 1 A/g|300 mA·h/g | 0.5 A/g|1000|353 mA·h/g | 51.9 | [ | |
| Sb2S3-rGO | Heat treatment | 1 mol/L KPF6 in EC|DEC | - | 0.05 A/g|50|110 mAh/g | - | [ | |
| Sb2S3@PPy | Sonication+freeze-dried | 0.8 mol/L KPF6 in EC|DEC | 2 A/g|220 mA·h/g | 1 A/g|50|157 mA·h/g | 63.7 | [ | |
| Sb/Sb2S3@CHT | Hydrothermal+ coating+carbonization | 4 mol/L KFSI in DME | 2 A/g|173.2 mA·h/g | 1 A/g|3500|147.5 mA·h/g | 62.3 | [ | |
| CAS-Ti3C2(Cu12Sb4S13, CAS) | Acoustic degradation +thermal injection | 0.8 mol/L KPF6 in EC|DEC | 5 A/g|163.3 mA·h/g | 1 A/g|1800|175.6 mA·h/g | 57.2 | [ | |
| Sb x Se y -based materials | Sb2Se3@h-rGO | Vacuum filtration+ thermal reduction | 0.8 mol/L KFSI in EC|DMC | 2 A/g|73 mA·h/g | 0.1 A/g|500|382.8 mA·h/g | 66 | [ |
| Sb2Se3@RGO | Hydrothermal+selenization | 0.8 mol/L KPF6 in EC|PC | 0.1 A/g|391.4 mA·h/g | 0.5 A/g|460|203.4 mA·h/g | 49 | [ | |
| Other Sb-based materials | Bi0.51Sb0.49OCl/rGO | Sol-gel+freeze-dried | 3 mol/L KFSI in DME | 1 A/g|319 mA·h/g | 0.1 A/g|1000|360 mA·h/g | 55.9 | [ |
| Sb2MoO6/rGO | Hydrothermal | 3 mol/L KFSI in DME | 0.2 A/g|381 mA·h/g | 0.5 A/g|100|247 mA·h/g | 55.7 | [ | |
| SbVO4@RGO | Solvothermal | 5 mol/L KFSI in EC| DMC | 0.1 A/g|447.9 mA·h/g | 0.1 A/g|500|210.1 mA·h/g | 29.2 | [ | |
| Opitimi cation of electrolyte | Micro-Sized Sb | Chemical reduction | 1 mol/L KFSI in EGDE | 5 A/g |225 mA·h/g | 0.1 A/g|180|573 mA·h/g | 69.4 | [ |
| Micro-Sized Sb | Commercial Sb powder | 4 mol/L KFSI in DME | 3 A/g|305 mA·h/g | 0.2 A/g|200|553 mA·h/g | - | [ |
| 1 | MASESE T, YOSHII K, YAMAGUCHI Y, et al. Rechargeable potassium-ion batteries with honeycomb-layered tellurates as high voltage cathodes and fast potassium-ion conductors[J]. Nat Commun, 2018, 9: 3823. |
| 2 | WU Y, B HUANG H, FENG Y Z, et al. The promise and challenge of phosphorus-based composites as anode materials for potassium-ion batteries[J]. Adv Mater, 2019, 31(50): 1901414. |
| 3 | LIAO J Y, CHEN C L, HU Q, et al. A low-strain phosphate cathode for high-rate and ultralong cycle-life potassium-ion batteries[J]. Angew Chem Int Ed, 2021, 60(48): 25575-25582. |
| 4 | MIN X, XIAO J, FANG M H, et al. Potassium-ion batteries: outlook on present and future technologies[J]. Energy Environ Sci, 2021, 14(4): 2186-2243. |
| 5 | TANG X, ZHOU D, LI P, et al. MXene-based dendrite-free potassium metal batteries[J]. Adv Mater, 2020, 32(4): 1906739. |
| 6 | 陈跃颖, 盘盈滢, 杜文卿, 等. 金属-有机框架在锂离子电池电极材料中的应用[J]. 材料研究与应用, 2022, 16(1): 68-80. |
| CHEN Y Y, PAN Y Y, DU W Q, et al. Application of metal-organic framework in electrode materials of lithium-ion batteries[J]. Mater Res Appl, 2022, 16(1): 68-80. | |
| 7 | YI Z, LIN N, ZHANG W Q, et al. Preparation of Sb nanoparticles in molten salt and their potassium storage performance and mechanism[J]. Nanoscale, 2018, 10(27): 13236-13241. |
| 8 | HUANG H W, WANG J W, YANG X F, et al. Unveiling the advances of nanostructure design for alloy-type potassium-ion battery anodes via in situ TEM[J]. Angew Chem Int Ed, 2020, 59(34): 14504-14510. |
| 9 | HE J, WEI Y, ZHAI T, et al. Antimony-based materials as promising anodes for rechargeable lithium-ion and sodium-ion batteries[J]. Mater Chem Front, 2018, 2(3): 437-455. |
| 10 | KO Y N, CHOI S H, KIM H, et al. One-pot formation of Sb-carbon microspheres with graphene sheets: potassium-ion storage properties and discharge mechanisms[J]. ACS Appl Mater Interfaces, 2019, 11(31): 27973-27981. |
| 11 | CAO K, LIU H, JIA Y, et al. Flexible antimony@carbon integrated anode for high‐performance potassium-ion battery[J]. Adv Mater Technol, 2020, 5(6): 2000199. |
| 12 | GE X, LIU S, QIAO M, et al. Enabling superior electrochemical properties for highly efficient potassium storage by impregnating ultrafine Sb nanocrystals within nanochannel-containing carbon nanofibers[J]. Angew Chem Int Ed, 2019, 58(41): 14578-14583. |
| 13 | LIU D Y, YANG L, CHEN Z Y, et al. Ultra-stable Sb confined into N-doped carbon fibers anodes for high-performance potassium-ion batteries[J]. Sci Bull, 2020, 65(12): 1003-1012. |
| 14 | ZHANG W M, MIAO W F, LIU X Y, et al. High-rate and ultralong-stable potassium-ion batteries based on antimony-nanoparticles encapsulated in nitrogen and phosphorus co-doped mesoporous carbon nanofibers as an anode material[J]. J Alloys Compd, 2018, 769: 141-148. |
| 15 | LIU H J, WANG Z J, WU Z H, et al. Direct tuning of meso-/micro-porous structure of carbon nanofibers confining Sb nanocrystals for advanced sodium and potassium storage[J]. J Alloys Compd, 2020, 833: 155127. |
| 16 | AHUJA V, SENTHILKUMAR B, SENGUTTUVAN P. Ultra-stable Sb/hard carbon composite anodes with synergistic alkali-ion storage performances[J]. Mater Res Bull, 2021, 144: 111491. |
| 17 | HAN Y, LI T Q, LI Y, et al. Stabilizing antimony nanocrystals within ultrathin carbon nanosheets for high-performance K-ion storage[J]. Energy Storage Mater, 2019, 20: 46-54. |
| 18 | LUO W, LI F, ZHANG W R, et al. Encapsulating segment-like antimony nanorod in hollow carbon tube as long-lifespan, high-rate anodes for rechargeable K-ion batteries[J]. Nano Res, 2019, 12(5): 1025-1031. |
| 19 | GABAUDAN V, TOUJA J, COT D, et al. Double-walled carbon nanotubes, a performing additive to enhance capacity retention of antimony anode in potassium-ion batteries[J]. Electrochem Commun, 2019, 105:106493. |
| 20 | SULTANA I, RAHMAN M M, LIU J N, et al. Antimony-carbon nanocomposites for potassium-ion batteries: insight into the failure mechanism in electrodes and possible avenues to improve cyclic stability[J]. J Power Sources, 2019, 413: 476-484. |
| 21 | LIU Q, FAN L, MA R F, et al. Super long-life potassium-ion batteries based on an antimony@carbon composite anode[J]. Chem Commun, 2018, 54(83): 11773-11776. |
| 22 | HUANG Z, DING S S, LI P C, et al. Flexible Sb-graphene-carbon nanofibers as binder-free anodes for potassium-ion batteries with enhanced properties[J]. Nanotechnol, 2021, 32(2):025401. |
| 23 | YANG X, ZHANG R Y, XU S F, et al. Graphene/amorphous carbon restriction structure for stable and long-lifespan antimony anode in potassium-ion batteries[J]. Chem Eur J, 2020, 26(26): 5818-5823. |
| 24 | LIU Q, FAN L, CHEN S H, et al. Antimony-graphite composites for a high-performance potassium-ion battery[J]. Energy Technol, 2019, 7(10): 1900634. |
| 25 | YANG X, ZHANG R Y. High-capacity graphene-confined antimony nanoparticles as a promising anode material for potassium-ion batteries[J]. J Alloys Compd, 2020, 834: 155191. |
| 26 | WANG L, JIA J J, WU Y, et al. Antimony/reduced graphene oxide composites as advanced anodes for potassium ion batteries[J]. J Appl Electrochem, 2018, 48(10): 1115-1120. |
| 27 | HE X D, LIU Z H, LIAO J Y, et al. A three-dimensional macroporous antimony@carbon composite as a high-performance anode material for potassium-ion batteries[J]. J Mater Chem A, 2019, 7(16): 9629-9637. |
| 28 | HAN C H, HAN K, WANG X P, et al. Three-dimensional carbon network confined antimony nanoparticle anodes for high-capacity K-ion batteries[J]. Nanoscale, 2018, 10(15): 6820-6826. |
| 29 | WANG H, WU X, QI X J, et al. Sb nanoparticles encapsulated in 3D porous carbon as anode material for lithium-ion and potassium-ion batteries[J]. Mater Res Bull, 2018, 103: 32-37. |
| 30 | GABAUDAN V, BERTHELOT R, STIEVANO L, et al. Inside the alloy mechanism of Sb and Bi electrodes for K-ion batteries[J]. J Phys Chem C, 2018, 122(32): 18266-18273. |
| 31 | JI F J, LIU T Q, LI Y Z, et al. Ball-milling strategy for fast and stable potassium-ion storage in antimony-carbon composite anodes[J]. ChemElectroChem, 2020, 7(22): 4587-4593. |
| 32 | ZHANG Y F, LI M, HUANG F B, et al. 3D porous Sb-Co nanocomposites as advanced anodes for sodium-ion batteries and potassium-ion batteries[J]. Appl Surf Sci, 2020, 499:143907. |
| 33 | DING H B, WANG J, FAN L, et al. Sn-Sb compounds with novel structure for stable potassium storage[J]. Chem Eng J, 2020, 395:125417. |
| 34 | ZHAO N, QIN J, CHU L J, et al. Heterogeneous interface of Se@Sb@C boosting potassium storage[J]. Nano Energy, 2020, 78. |
| 35 | CAO L, ZHANG B, XIA H F, et al. Hierarchical chrysanthemum-like MoS2/Sb heterostructure encapsulated into N-doped graphene framework for superior potassium-ion storage[J]. Chem Eng J, 2020, 387:124060. |
| 36 | ZHAO R Z, DI H X, WANG C X, et al. Encapsulating ultrafine Sb nanoparticles in Na+ pre-intercalated 3D porous Ti3C2TX MXene nanostructures for enhanced potassium storage performance[J]. ACS Nano, 2020, 14(10): 13938-13951. |
| 37 | ZHENG J, YANG Y, FAN X, et al. Extremely stable antimony-carbon composite anodes for potassium-ion batteries[J]. Energy Environ Sci, 2019, 12(2): 615-623. |
| 38 | ZHAO N, QIN J, CHU L, et al. Heterogeneous interface of Se@Sb@C boosting potassium storage[J]. Nano Energy, 2020, 78: 105345. |
| 39 | AN Y, TIAN Y, CI L, et al. Micron-sized nanoporous antimony with tunable porosity for high-performance potassium-ion batteries[J]. ACS Nano, 2018, 12(12): 12932-12940. |
| 40 | WANG Z, DONG K, WANG D, et al. Constructing N-doped porous carbon confined FeSb alloy nanocomposite with Fe-N-C coordination as a universal anode for advanced Na/K-ion batteries[J]. Chem Eng J, 2020, 384: 123327. |
| 41 | WANG D, MA Q, TIAN K H, et al. Ultrafine nano-scale Cu2Sb alloy confined in three-dimensional porous carbon as an anode for sodium-ion and potassium-ion batteries[J]. Int J Min Met Mater, 2021, 28(10): 1666-1674. |
| 42 | WANG D, MA Q, TIAN K, et al. Nanosized CoSb alloy confined in honeycomb carbon framework toward high‐property potassium-ion and sodium-ion batteries[J]. Energy Technol, 2021, 9(7): 2100095. |
| 43 | CHEN K T, TUAN H Y. Bi-Sb nanocrystals embedded in phosphorus as high-performance potassium ion battery electrodes[J]. ACS Nano, 2020, 14(9): 11648-11661. |
| 44 | WU Q, CHEN B, XIE H, et al. Bismuth-antimony alloy nanoparticles encapsulated in 3D carbon framework: synergistic effect for enhancing interfacial potassium storage[J]. Chem Eng J, 2022, 430(3): 132906. |
| 45 | XIONG P, WU J, ZHOU M, et al. Bismuth-antimony alloy nanoparticle@porous carbon nanosheet composite anode for high-performance potassium-ion batteries[J]. ACS Nano, 2020, 14(1): 1018-1026. |
| 46 | ZHAO H, ZHUANG C, XU J, et al. Synergistically enhanced sodium/potassium ion storage performance of SnSb alloy particles confined in three-dimensional carbon framework[J]. Ionics, 2020, 26(10): 5019-5028. |
| 47 | GABAUDAN V, BERTHELOT R, SOUGRATI M T, et al. SnSb vs.Sn: improving the performance of Sn-based anodes for K-ion batteries by synergetic alloying with Sb[J]. J Mater Chem A, 2019, 7(25): 15262-15270. |
| 48 | HSIEH Y Y, CHEN K T, TUAN H Y. A synergetic SnSb-amorphous carbon composites prepared from polyesterification process as an ultrastable potassium-ion battery anode[J]. Chem Eng J, 2021, 420(3): 130451. |
| 49 | HUANG Z, CHEN Z, DING S, et al. Multi-protection from nanochannels and graphene of SnSb-graphene carbon composites ensuring high properties for potassium-ion batteries[J]. Solid State Ionics, 2018, 324: 267-275. |
| 50 | SHI X, LIU W, ZHANG D, et al. Nanoscale localized growth of SnSb for general-purpose high performance alkali (Li, Na, K) ion storage[J]. Chem Eng J, 2022, 431(3): 134318. |
| 51 | WANG Z, DONG K, WANG D, et al. A nanosized SnSb alloy confined in N-doped 3D porous carbon coupled with ether-based electrolytes toward high-performance potassium-ion batteries[J]. J Mater Chem A, 2019, 7(23): 14309-14318. |
| 52 | HUANG C, XU A, LI G, et al. Alloyed BiSb nanoparticles confined in tremella-like carbon microspheres for ultralong-life potassium ion batteries[J]. Small, 2021, 17(23): e2100685. |
| 53 | LI Z, GAN Q, ZHANG Y, et al. FeSb@N-doped carbon quantum dots anchored in 3D porous N-doped carbon with pseudocapacitance effect enabling fast and ultrastable potassium storage[J]. Nano Res, 2021, 15(1): 217-224. |
| 54 | WANG Z, DUAN C, WANG D, et al. BiSb@Bi2O3/SbOx encapsulated in porous carbon as anode materials for sodium/potassium-ion batteries with a high pseudocapacitive contribution[J]. J Colloid Sci, 2020, 580: 429-438. |
| 55 | WANG J, CAO M, XU F, et al. A cage compound precursor-derived Sb/Sb2O4/Fe3C nanocomposite anchored on reduced graphene oxide as an anode for potassium ion batteries[J]. New J Chem, 2021, 45(2): 993-1000. |
| 56 | WANG B, DENG Z, XIA Y, et al. Realizing reversible conversion-alloying of Sb(V) in polyantimonic acid for fast and durable lithium- and potassium-ion storage[J]. Adv Energy Mater, 2019, 10(1): 1903119. |
| 57 | MA Q, SONG L, WAN Y, et al. Precise tuning of low-crystalline Sb@Sb2O3 confined in 3D porous carbon network for fast and stable potassium ion storage[J]. J Mater Sci Technol, 2021, 94: 123-129. |
| 58 | CHEN B, YANG L, BAI X, et al. Heterostructure engineering of core-shelled Sb@Sb2O3 encapsulated in 3D N-doped carbon hollow-spheres for superior sodium/potassium storage[J]. Small, 2021, 17(6): e2006824. |
| 59 | CHENG Y, YAO Z, ZHANG Q, et al. In situ atomic-scale observation of reversible potassium storage in Sb2S3@carbon nanowire anodes[J]. Adv Funct Mater, 2020, 30(52): 2005417. |
| 60 | LU Y, CHEN J. Robust self-supported anode by integrating Sb2S3 nanoparticles with S, N-codoped graphene to enhance K-storage performance[J]. Sci China Chem, 2017, 60(12): 1533-1539. |
| 61 | WANG J, FAN L, LIU Z, et al. In situ alloying strategy for exceptional potassium ion batteries[J]. ACS Nano, 2019, 13(3): 3703-3713. |
| 62 | LAKSHMI V, MIKHAYLOV A A, MEDVEDEV A G, et al. Probing electrochemical reactivity in an Sb2S3-containing potassium-ion battery anode: observation of an increased capacity[J]. J Mater Chem A, 2020, 8(22): 11424-11434. |
| 63 | SHI Y, LI F, ZHANG Y, et al. Sb(2)S(3)@PPy coaxial nanorods: a versatile and robust host material for reversible storage of alkali metal ions[J]. Nanomater, 2019, 9(4): 560. |
| 64 | WU Y, ZHENG J, TONG Y, et al. Carbon hollow tube-confined Sb/Sb2S3 nanorod fragments as highly stable anodes for potassium-ion batteries[J]. ACS Appl Mater Interfaces, 2021, 13(43): 51066-51077. |
| 65 | CAO Y, ZHANG Y, CHEN H, et al. Cu12Sb4S13 quantum dots/few-layered Ti3C2 nanosheets with enhanced K+ diffusion dynamics for efficient potassium ion storage[J]. Adv Funct Mater, 2021, 32(6): 2108574. |
| 66 | YANG Z, LI W, ZHANG G, et al. Constructing Sb—O—C bond to improve the alloying reaction reversibility of free-standing Sb2Se3 nanorods for potassium-ion batteries[J]. Nano Energy, 2022, 93: 106764. |
| 67 | YI Z, QIAN Y, JIANG S, et al. Self-wrinkled graphene as a mechanical buffer: a rational design to boost the K-ion storage performance of Sb2Se3 nanoparticles[J]. Chem Eng J, 2020, 379: 122352. |
| 68 | WANG J, WANG B, LU B. Nature of novel 2D van der Waals heterostructures for superior potassium ion batteries[J]. Adv Energy Mater, 2020, 10(24): 2000884. |
| 69 | WANG J, WANG B, LIU Z, et al. Nature of bimetallic oxide Sb2MoO6/rGO anode for high-performance potassium-ion batteries[J]. Adv Sci, 2019, 6(17): 1900904. |
| 70 | YI X, GE J, ZHOU J, et al. SbVO4 based high capacity potassium anode: a combination of conversion and alloying reactions[J]. Sci China Chem, 2020, 64(2): 238-244. |
| 71 | MADEC L, GABAUDAN V, GACHOT G, et al. Paving the way for K-ion batteries: role of electrolyte reactivity through the example of Sb-based electrodes[J]. ACS Appl Mater Interfaces, 2018, 10(40): 34116-34122. |
| 72 | YU S, PARK H, SIEGEL D J. Thermodynamic assessment of coating materials for solid-state Li, Na, and K batteries[J]. ACS Appl Mater Interfaces, 2019, 11(40): 36607-36615. |
| 73 | DU X, GAO Y, ZHANG B. Building elastic solid electrolyte interphases for stabilizing microsized antimony anodes in potassium ion batteries[J]. Adv Funct Mater, 2021, 31(26): 2102562. |
| 74 | ZHOU L, CAO Z, ZHANG J, et al. Electrolyte-mediated stabilization of high-capacity micro-sized antimony anodes for potassium-ion batteries[J]. Adv Energy Mater, 2021, 33(8): e2005993. |
| [1] | Yu MENG, Qing ZHANG, Wen-Hao PENG, Xiao-Fei ZHU, De-Feng ZHOU. Preparation and Electrochemical Performance of Pr0.8Sr0.2Fe0.7Ni0.3O3-δ ⁃Pr1.2Sr0.8Ni0.6Fe0.4O4+δ Composite Cathode [J]. Chinese Journal of Applied Chemistry, 2022, 39(5): 797-808. |
| [2] | WANG Chunli,SUN Lianshan,ZHONG Ming,WANG Limin,CHENG Yong. Research Progress of Transition Metal and Compounds for Lithium-Sulfur Batteries [J]. Chinese Journal of Applied Chemistry, 2020, 37(4): 387-404. |
| [3] | HUI Kanglong, FU Jipeng, GAO Tian, TANG Mingxue. Research Progress of Metal Sulfides in Rechargeable Batteries [J]. Chinese Journal of Applied Chemistry, 2020, 37(12): 1384-1402. |
| [4] | Zhaomin WANG, Zheng YI, Ming ZHONG, Yong CHENG, Limin WANG. Research Progress of Antimony-Based Anode Materials for Lithium Ion Batteries [J]. Chinese Journal of Applied Chemistry, 2018, 35(7): 745-755. |
| [5] | CHEN Lihui,WU Qiuhan,PAN Pei,SONG Zixuan,WANG Feng,DING Yu. Spinel Lithium Manganese Oxide Octahedral Nanoparticles with Excellent Electrochemical Performance as Cathode Materials for Lithium-Ion Batteries [J]. Chinese Journal of Applied Chemistry, 2018, 35(11): 1384-1390. |
| [6] | WAN Lu, FU Zhengbing. Preparation and Characterization of Nitrogen-doped Carbon-coated Lithium Titanate Anode for Lithium-ion Batteries [J]. Chinese Journal of Applied Chemistry, 2018, 35(1): 116-122. |
| [7] | JIAO Liansheng, MENG Lingju, WU Tongshun, LI Fenghua, NIU Li. Synthesis and Properties of Lithium Vanadium Phosphates/Reduced Graphene Oxide Composite as Cathode Materials [J]. Chinese Journal of Applied Chemistry, 2017, 34(6): 712-722. |
| [8] | FENG Xuejiao, CUI Hongmin, XIAO Zhengqiang, YAN Nanfu, LI Min. Synthesis of Porous Silicon Oxide/Silicon/Carbon Composite Material from Micro-SiO for Lithium Storage [J]. Chinese Journal of Applied Chemistry, 2017, 34(1): 76-82. |
| [9] | ZHU Xiaoming, FENG Yonglan, ZHANG Fuxing, YU Jiangxi, JIANG Wujiu, OU Yaping, KUANG Daizhi. Synthesis, Crystal Structure and Properties of Dinuclear Copper(Ⅱ) Complex {[Cu(Phen)(Nap)2]2·(EtOH)2·(H2O)2} (Phen=1,10-Phenanthroline; Nap=1-Naphthoic Acid; EtOH=Ethyl Alcohol) [J]. Chinese Journal of Applied Chemistry, 2016, 33(8): 932-938. |
| [10] | XU Shengnan, NA Zhaolin, YIN Dongming, WU Yaoming, WANG Limin. Performance of A Copper-Cerium Redox Flow Battery [J]. Chinese Journal of Applied Chemistry, 2016, 33(12): 1462-1464. |
| [11] | JIN Xiaoqing, CAO Jie, HU Zhongshan, FENG Xiaojuan, HAN Yuqi. Synthesis and Supercapacitor Properties of Graphene/Co-Ni Layered Double Hydroxides Composites [J]. Chinese Journal of Applied Chemistry, 2015, 32(5): 583-590. |
| [12] | WANG Feng,HU Xinliang,ZHANG Peng,ZHAO Shuangqi,DING Yu. Microwave Assisted Synthesis and Electrochemical Performance of Copper Ferrite as Anode Material for Lithium-ion Battery [J]. Chinese Journal of Applied Chemistry, 2015, 32(10): 1184-1189. |
| [13] | ZHANG Bin1,2, ZHANG Yibo1, YANG Xiangguang1*. Synthesis and Electrochemical Performance of Reduced Graphene Oxide Coated Cobalt Iron Oxide with Hierarchical Structure [J]. Chinese Journal of Applied Chemistry, 2014, 31(12): 1447-1452. |
| [14] | XU Pengpeng, YANG Jie, YU Dan, WANG Wei*. Application of Lanthanum in Polyanaline Fiber′s Electroless Plating and Its Electrochemical Properties [J]. Chinese Journal of Applied Chemistry, 2013, 30(05): 590-595. |
| [15] | LIAO Denghui1, CHEN Zhen1*, GUO Zhongcheng1, LU Lifang1. Preparation of New Stainless Steel Substrate Lead Dioxide-Tungsten Carbide Composite Inert Anode Material [J]. Chinese Journal of Applied Chemistry, 2013, 30(02): 196-202. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||