
Chinese Journal of Applied Chemistry ›› 2023, Vol. 40 ›› Issue (2): 188-209.DOI: 10.19894/j.issn.1000-0518.220175
• Review • Previous Articles Next Articles
Research Progress of Controlling Lithium-Sulfur Batteries by Electrocatalysts under Lean Electrolyte Conditions
Lu-Fei WANG1, Meng-Meng ZHEN1( ), Bo-Xiong SHEN2(
), Bo-Xiong SHEN2( )
)
- 1.School of Energy and Environmental Engineering,Hebei University of Technology,Tianjin 300071,China
2.School of Chemical Engineering and Technology,Hebei University of Technology,Tianjin 300071,China
-
Received:2022-05-10Accepted:2022-08-04Published:2023-02-01Online:2023-02-27 -
Contact:Meng-Meng ZHEN,Bo-Xiong SHEN -
About author:shenbx@hebut.edu.cn
zhenmengmeng@hebut.edu.cn;
-
Supported by:the National Natural Science Foundation of China(51702236);the Natural Science Foundation of Hebei Province(B2021202052)
CLC Number:
Cite this article
Lu-Fei WANG, Meng-Meng ZHEN, Bo-Xiong SHEN. Research Progress of Controlling Lithium-Sulfur Batteries by Electrocatalysts under Lean Electrolyte Conditions[J]. Chinese Journal of Applied Chemistry, 2023, 40(2): 188-209.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: http://yyhx.ciac.jl.cn/EN/10.19894/j.issn.1000-0518.220175
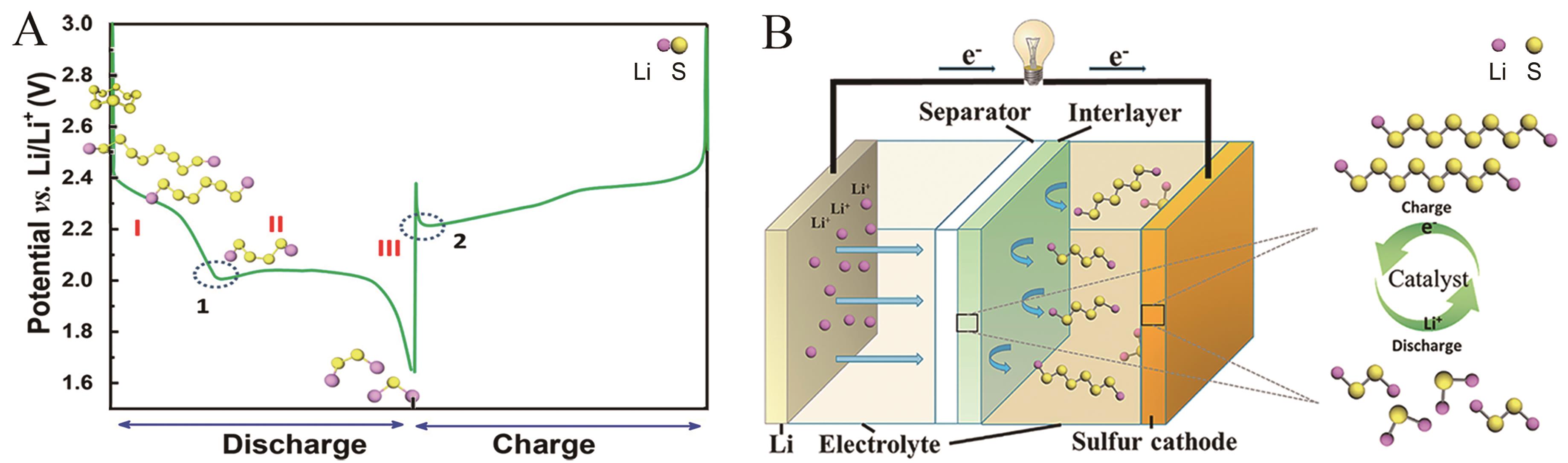
Fig.1 (A) Typical charge-discharge curves for non-aqueous LSBs[14]; (B) Illustration of the catalytic mechanism during charge-discharge process in LSBs[15]

Fig.2 (A) Transmission electron microscopy (TEM) image of black phosphorus (BP) that is centrifuged at 4000 r/min; (B) TEM image of BP centrifuged at 8000 r/min (BP-8K); (C) High-resolution TEM (HRTEM) image and corresponding selected area electron diffraction (SAED) pattern (inset) of BP-8K; (D-F) TEM and HRTEM images of BPQDs; (G) Raman spectra of bulk BP (red) and BPQD (blue) on a silicon (Si) substrate; (H) Initial discharge-charge profiles at 0.1 C between 1.7 and 2.8 V vs.Li+/Li; (I) Cyclic performance of the PCNF/S/BPQD at 0.1 C for 200 cycles; (porous carbon nanofibers,PCNFs)[47]
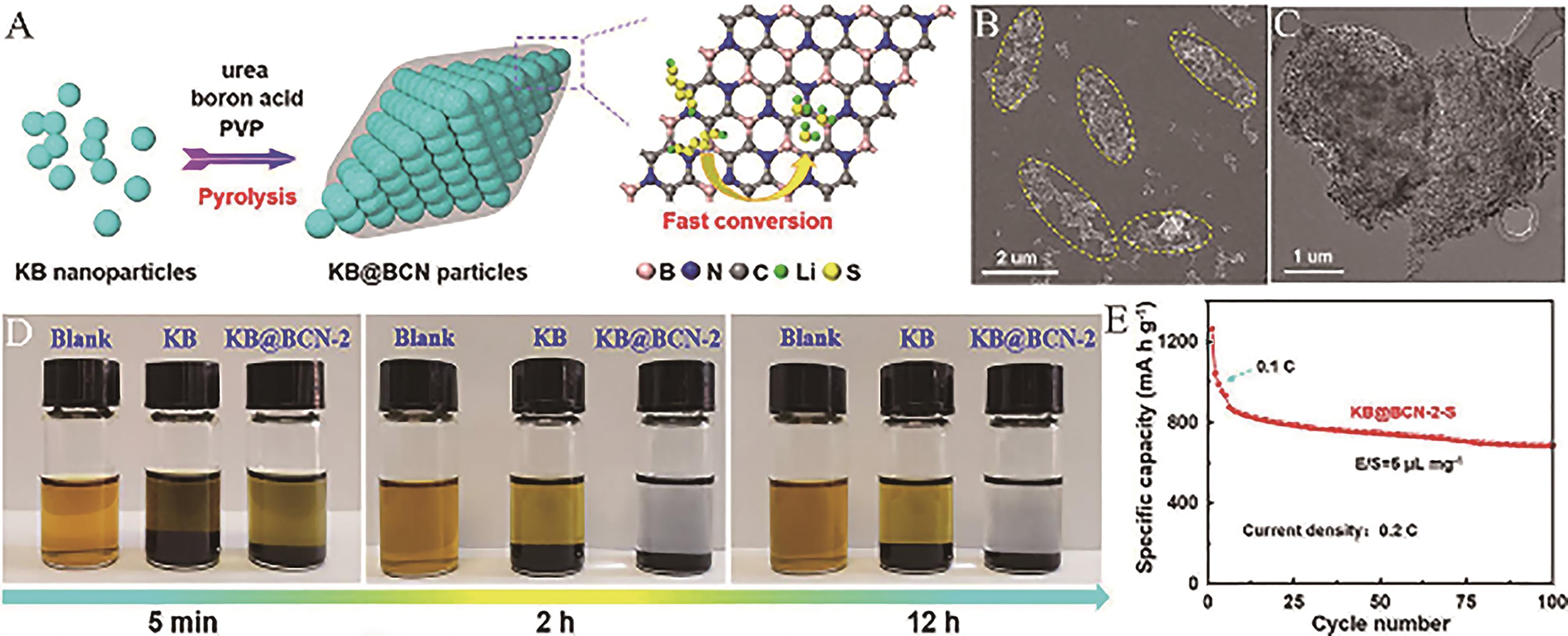
Fig.3 (A) Schematic illustration of the synthesis process of porous carbon@borocarbonitride (KB@BCN); (B) SEM and (C) TEM images of KB@BCN-2; (D) Optical photographs of an adsorption experiment between Li2S6 solution (0.003 mol/L) and KB@BCN-2 or KB; (E) Cycle performance of KB@BCN-2-S at 0.2 C with a low E/S=5 μL/mg[53]
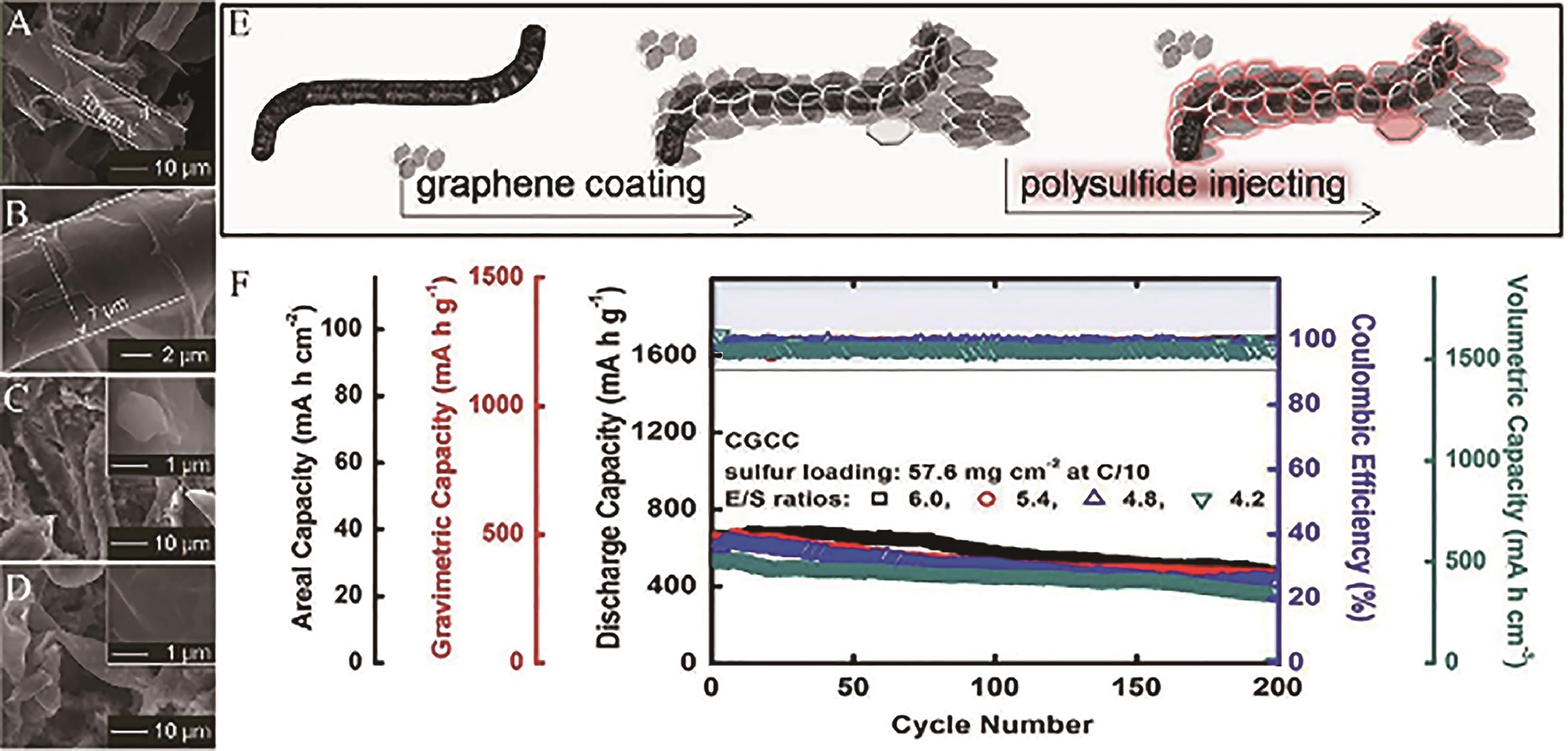
Fig.4 (A) Low-magnification S and (B) high-magnification SEM images of CGCC; (C) SEM images of CGCC cathode; (D) SEM images of cycled CGCC cathodes; (E) Illustration of the synthesis route of the CGCC cathode; (F) Cyclability performance of CGCC cathodes[49]
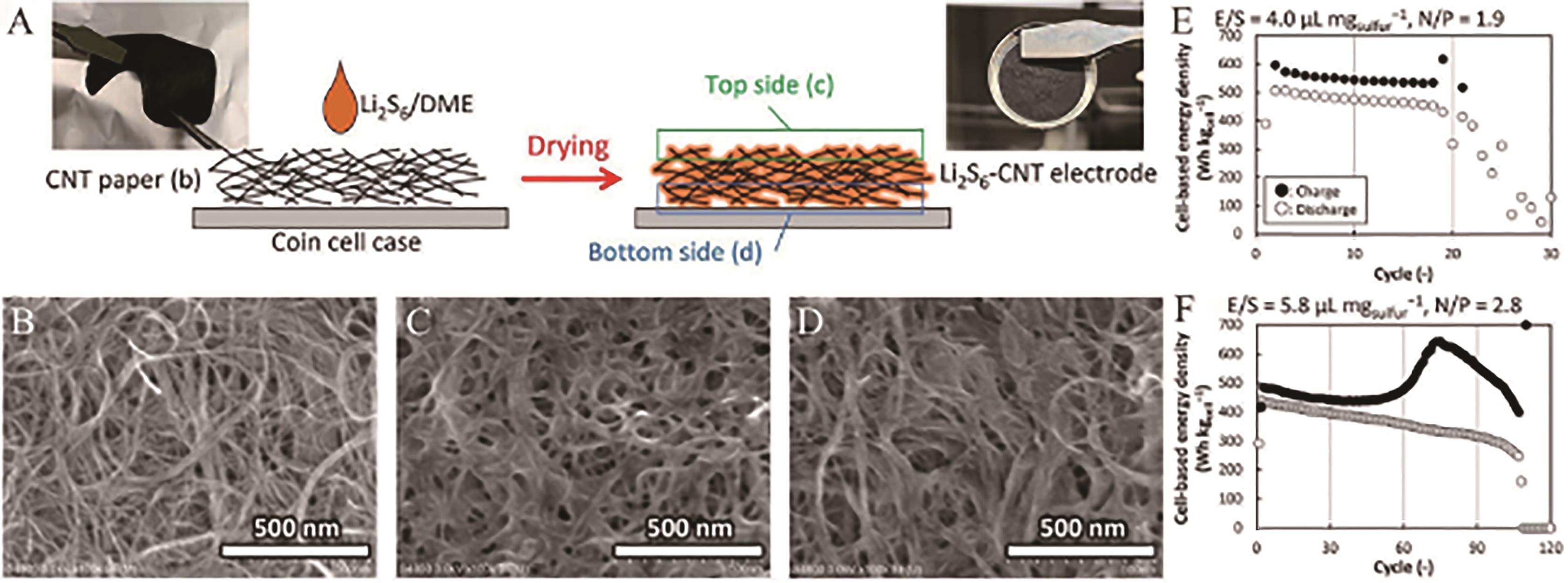
Fig.5 (A) Schematic and digital images of the CNT paper and the Li2S6-CNT electrode; SEM images of (B) the CNT paper and (C, D) the Li2S6-CNT electrode from the top (c) and the bottom (d); Energy density of the Li||Li2S6-CNT full cells shown in (E) and (F) [56]
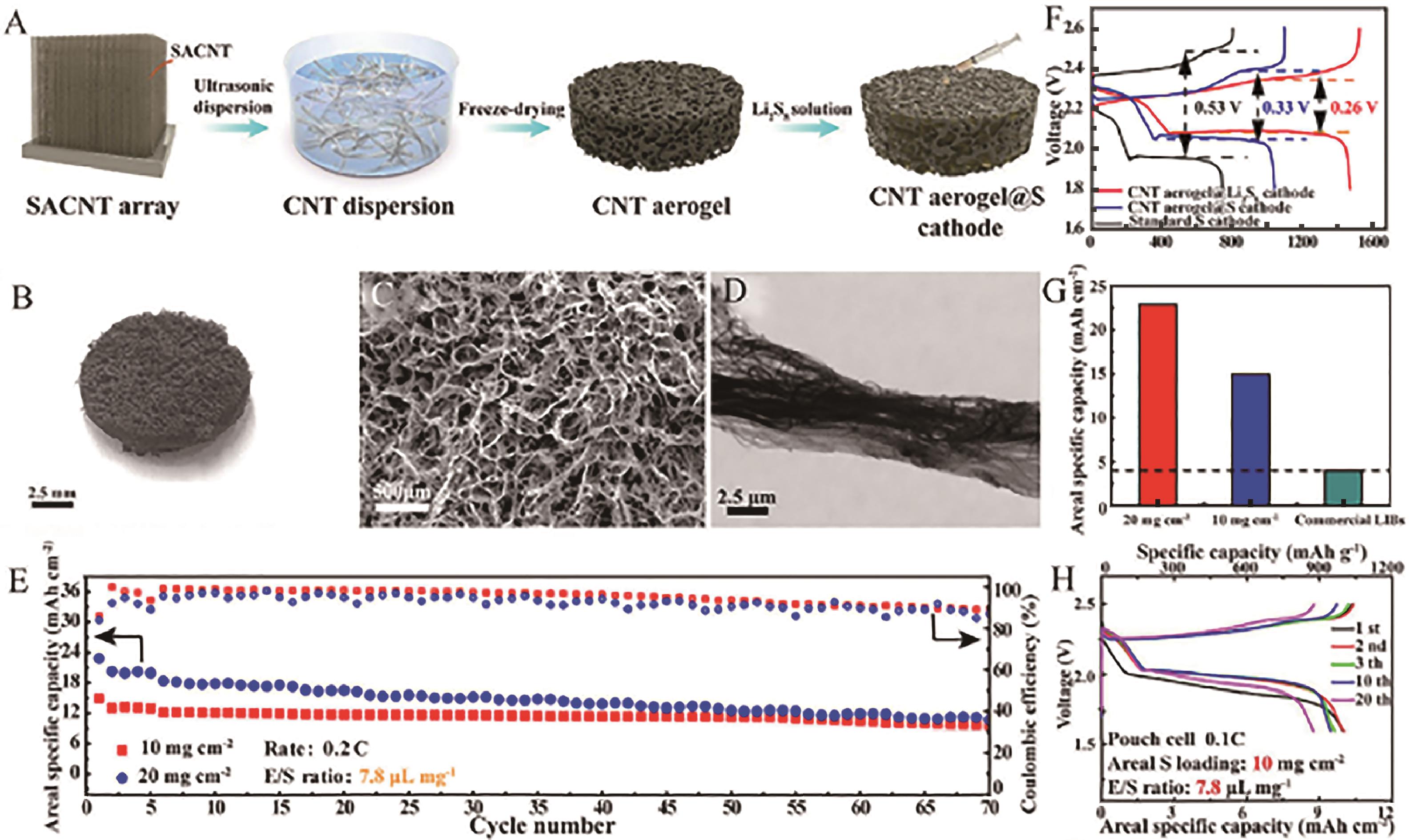
Fig.6 (A) Schematic of the fabrication process of a CNT aerogel@Li2S8 cathode; (B) Digital photo of a CNT aerogel; (C, D) SEM images of a CNT aerogel@Li2S8 cathode; (E) CNT aerogel@Li2S8 cathode with high S loading area specific capacity at 0.2 C; (F) Voltage profiles of different cathodes; (G) Areal specific capacities of the CNT aerogel@Li2S8 cathodes with high S loadings compared to commercial LIBs; (H) Charge and discharge curves of a CNT aerogel@Li2S8 pouch cell at 0.1 C[57]

Fig.7 SEM images of (A) NCNFs and (B) VNCNFs; (C) and (D) TEM images of VNCNFs; (E) Schematic illustration of the synthetic process of asymmetric bifunctional VNCNFs membrane and diagram of the S/VNCNFs||VNCNFs/Li full cell configuration; (F) The long-term cycle life of the S/VNCNFs||VNCNFs/Li full cell with a sulfur load of 8.3 mg/cm2; (G) The areal capacity of the S/VNCNFs||VNCNFs/Li full cell with a high sulfur load of 15.27?mg/cm2 at 1 mA/cm2. (Li-S, lithium-sulfur; PECVD, plasma-enhanced chemical vapor deposition; SEM, scanning electron microscopy)[60]

Fig.8 (A) Schematic illustration of the synthesis process of S@WLC-CNTs electrodes; (B-D) SEM images of the WLC-CNTs host; (E) SEM image of the S@WLC-CNTs cathode with sulfur loading of 17.3 mg/cm2; (F-G) Cyclability of S@WLC-CNTs electrodes with different thicknesses and sulfur content at the 0.1 C[62]
| Material | E/S ratio (μL·mg-1) | Sulfur loading/ (mg·cm-2) | Current/number of cycles | Discharge capacity/ (mA·h·g-1) | Area capacity/ (mA·h·cm-2) | Ref. |
|---|---|---|---|---|---|---|
| PCNF/S/BPQDs | 6.5 | 8.0 | 0.1 C/200 | 550 | 4.4 | [ |
| KB@BCN-2-S | 5.0 | 4.0 | 0.2 C/100 | 677 | 2.7 | [ |
| CGCC | 4.2 | 57.6 | 0.1 C/200 | 539 | 31.0 | [ |
| HCFF-S | 3.53 | 21.2 | 3.55 (mA/cm2)/150 | 698 | 14.8 | [ |
| CNTaerogel@Li2S8 | 7.8 | 20.0 | 0.2 C/70 | 535 | 10.7 | [ |
| S/NF@VG | 4.8 | 13.0 | 0.1 C/100 | 846 | ~11 | [ |
| S/VNCNFs||VNCNFs/Li | 4.0 | 15.27 | 1.0 (mA/cm2)/50 | 851 | 13.0 | [ |
| S@WLC-CNTs | 6.0 | 52.4 | 0.1 C/100 | 692 | 36.2 | [ |
Table 1 The electrochemical performance of nonmetallic catalysts in LSB under lean electrolyte
| Material | E/S ratio (μL·mg-1) | Sulfur loading/ (mg·cm-2) | Current/number of cycles | Discharge capacity/ (mA·h·g-1) | Area capacity/ (mA·h·cm-2) | Ref. |
|---|---|---|---|---|---|---|
| PCNF/S/BPQDs | 6.5 | 8.0 | 0.1 C/200 | 550 | 4.4 | [ |
| KB@BCN-2-S | 5.0 | 4.0 | 0.2 C/100 | 677 | 2.7 | [ |
| CGCC | 4.2 | 57.6 | 0.1 C/200 | 539 | 31.0 | [ |
| HCFF-S | 3.53 | 21.2 | 3.55 (mA/cm2)/150 | 698 | 14.8 | [ |
| CNTaerogel@Li2S8 | 7.8 | 20.0 | 0.2 C/70 | 535 | 10.7 | [ |
| S/NF@VG | 4.8 | 13.0 | 0.1 C/100 | 846 | ~11 | [ |
| S/VNCNFs||VNCNFs/Li | 4.0 | 15.27 | 1.0 (mA/cm2)/50 | 851 | 13.0 | [ |
| S@WLC-CNTs | 6.0 | 52.4 | 0.1 C/100 | 692 | 36.2 | [ |
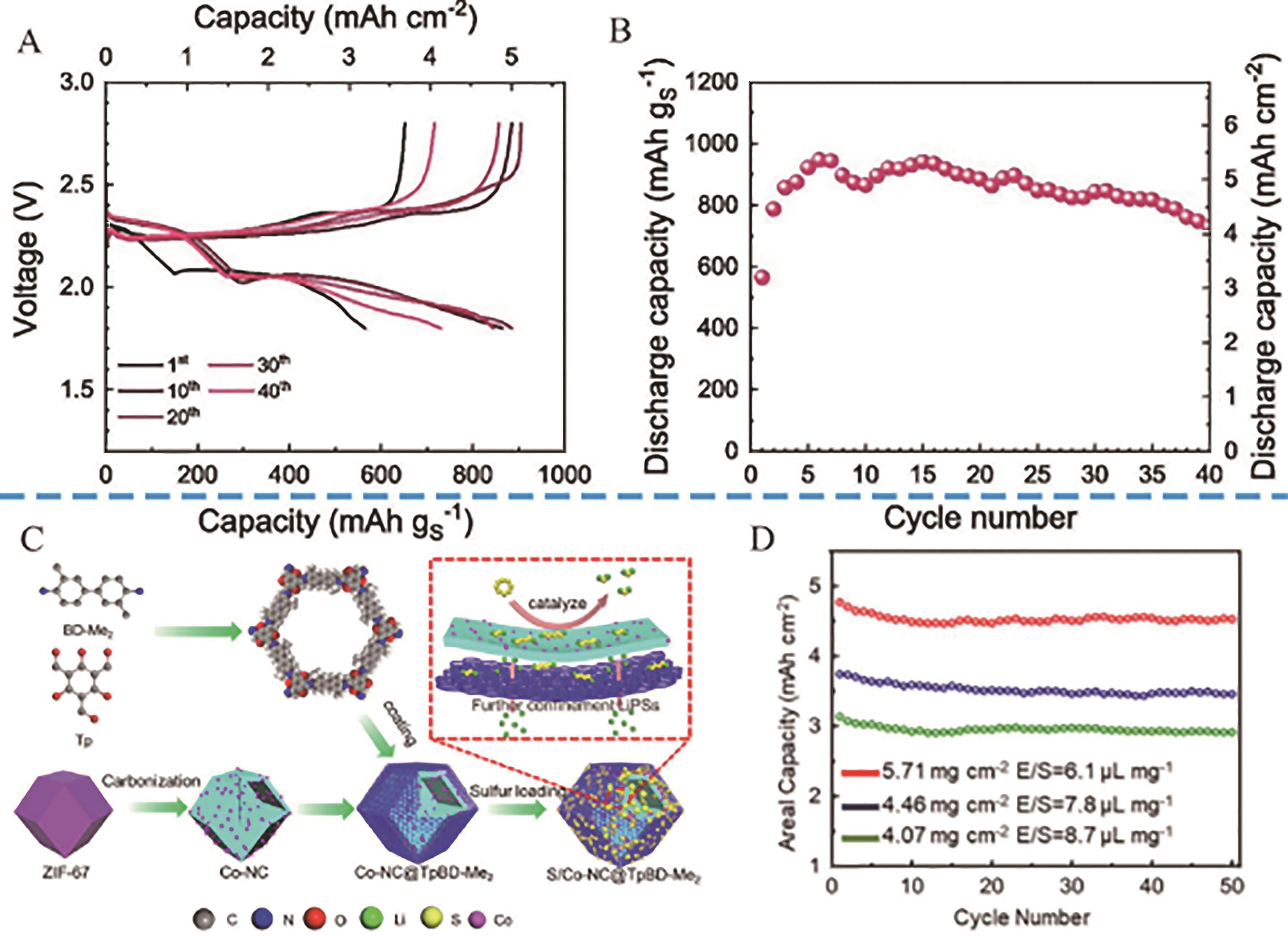
Fig.9 (A) Voltage profiles and (B) cycling trend at the constant current rate of C/20 of the Li|S∶Au 97∶3(m/m) cell with sulfur loading over the electrode of 5.7 mg/cm2 and E/S ratio of 5 μL/mg[67]; (C) Preparation procedure of Co-NC@TpBD-Me2 cathode and its working mechanism as sulfur cathode; (D) Cycling of S/Co-NC@TpBD-Me2 cathode at 0.2 C under high sulfur loadings[69]
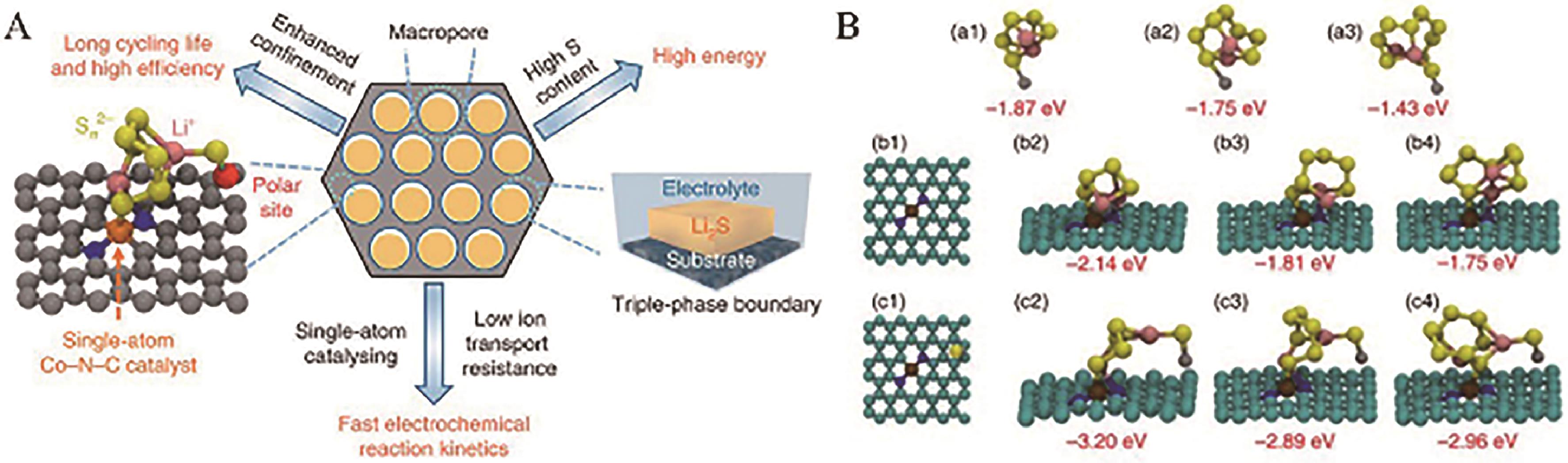
Fig.10 (A) Design strategy of macroporous host with DEB sites; (B) Optimized configurations of Li2S4, Li2S6 and Li2S8 absorption on ZnS (a1-a3), the Co-N-C surface (b1-b4) and the ZnS, Co-N-C surface (c1-c4); The yellow, pink, silver, brown, blue and cyan balls denote the S, Li, Zn, Co, N and C atoms, respectively; The 3D ordered macroporous sulfur host with ZnS and Co-N-C DEB sites (“3d-omsh/ZnS,Co-N-C”)[71]
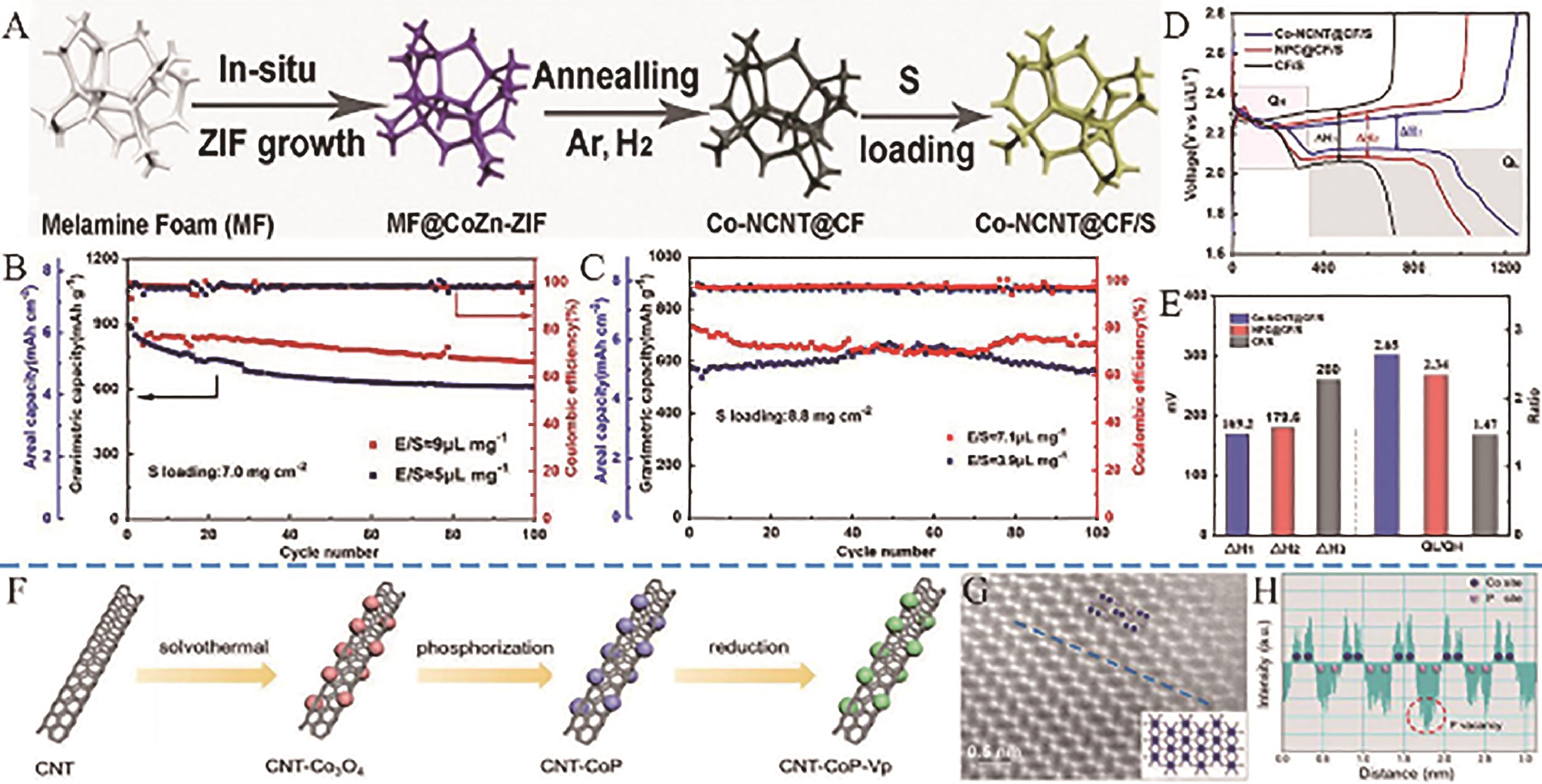
Fig.11 (A) Schematic diagrams of the synthetic procedure of Co-NCNT@CF/S; (B) Cycling performance of Co-NCNT@CF/S electrode under different E/S ratios at 0.1?C; (C) The cycling performance of the Co-NCNT@CF/S cathode at 0.1 C; (D) Initial charge-discharge profiles of different electrodes with a 0.1?C current rate; (E) Values of ΔH and QL/QH calculated from the charge/discharge curves[73]; (F) Schematic illustration of the synthesis of CNT-CoP-Vp; (G) STEM-HADDF image of CNT-CoP-Vp-1M, the insert image is the theoretical model of CoP; (H) The intensity of Co and P atomic columns along blue dotted line in (G) [75]
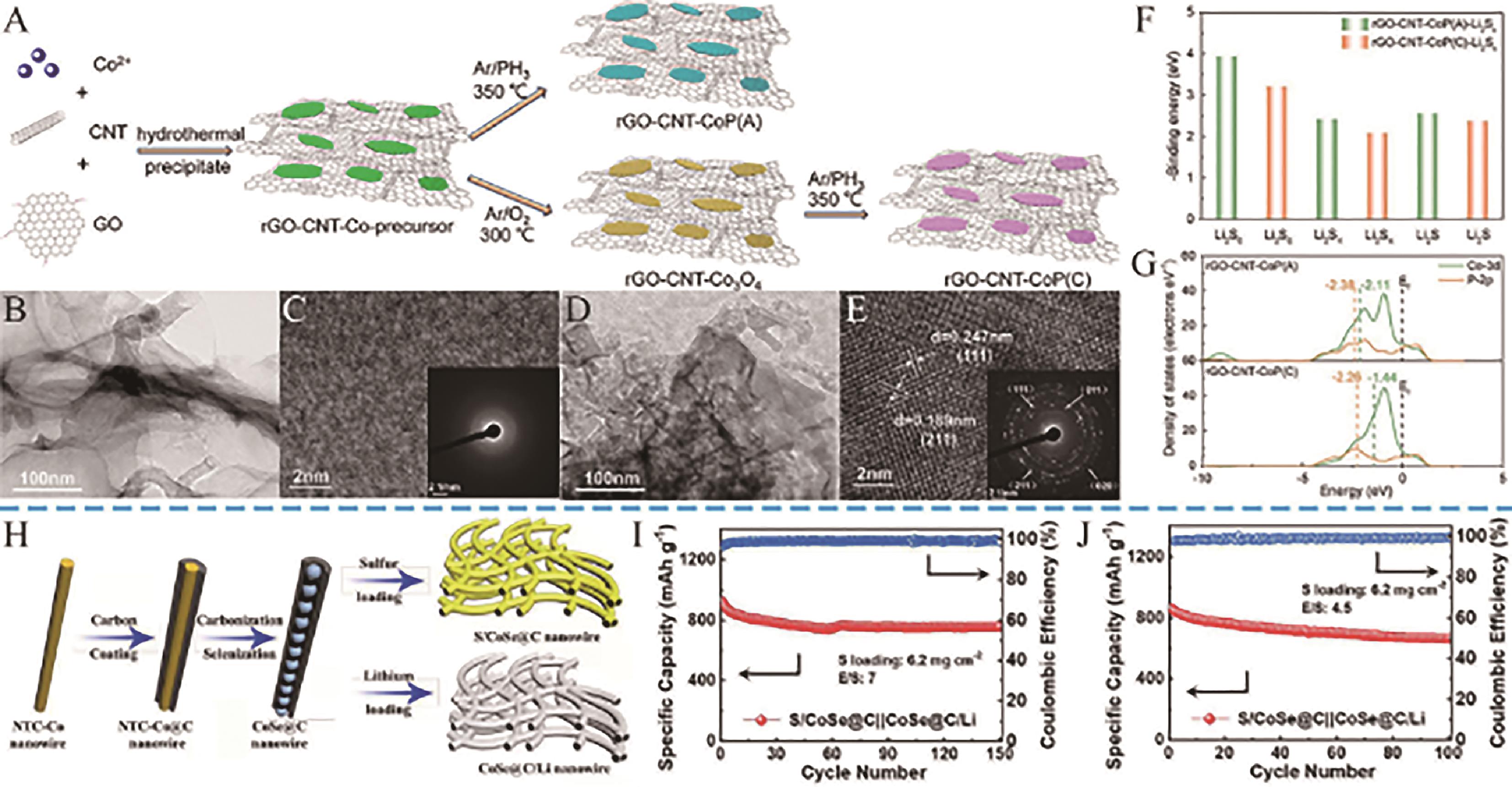
Fig.12 (A) Schematic Illustration of the Fabrication of rGO-CNT-CoP(A) and rGO-CNT-CoP(C) Composite; (B, C) TEM, HRTEM images of rGO-CNT-CoP(A); (D, E) TEM, HRTEM images of rGO-CNT-CoP(C); (F) Binding energies of Li2S6, Li2S4, and Li2S on rGO-CNT-CoP (A) and rGO-CNT-CoP (C); (G) Density of states of d bands of Co and p bands of P[76]; (H) Schematic of the synthesis route for S/CoSe@C and CoSe@C/Li; (I) Cycling stability of the S/CoSe@C||CoSe@ C/Li cell at 0.2 C rate; (J) Cycling stability of the S/CoSe@C||CoSe@C/Li cell at 0.1 C rate. Nitrilotriacetic acid (NTC)[78]
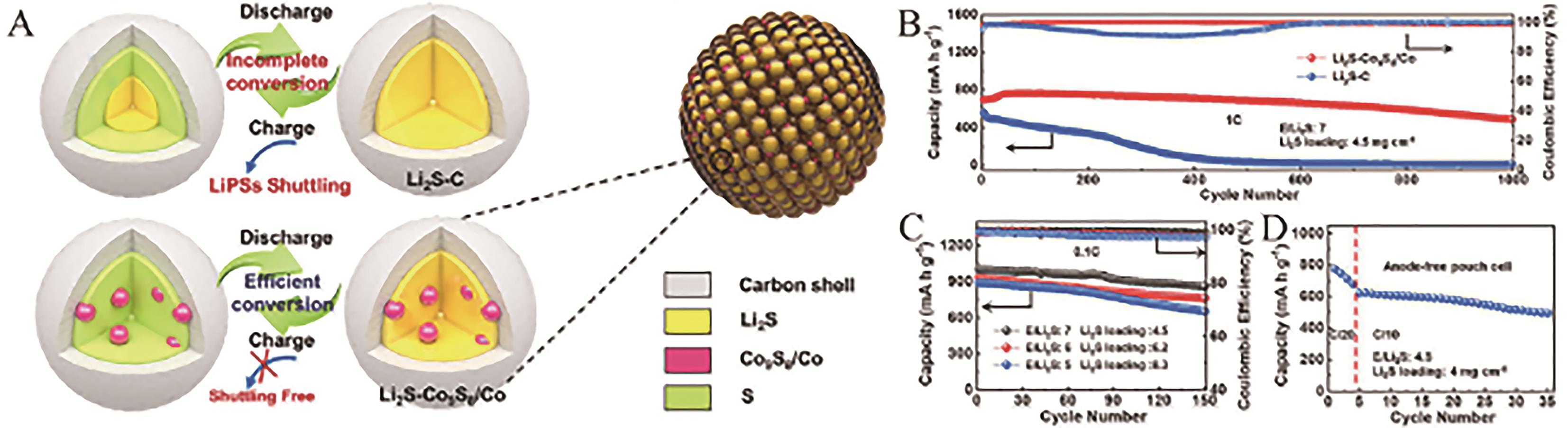
Fig.13 (A) Schematic illustration of the advantages of Li2S-Co9S8/Co in LSBs; (B) Cycling stability of the Li2S-Co9S8/Co cathode at 1 C rate; (C) Cycling stability of the Li2S-Co9S8/Co cathode with various Li2S loadings at 0.1 C rate; (D) Cycling stability of the Li2S-Co9S8/Co-Te cathode in the Ni||Li2S pouch cell[81]
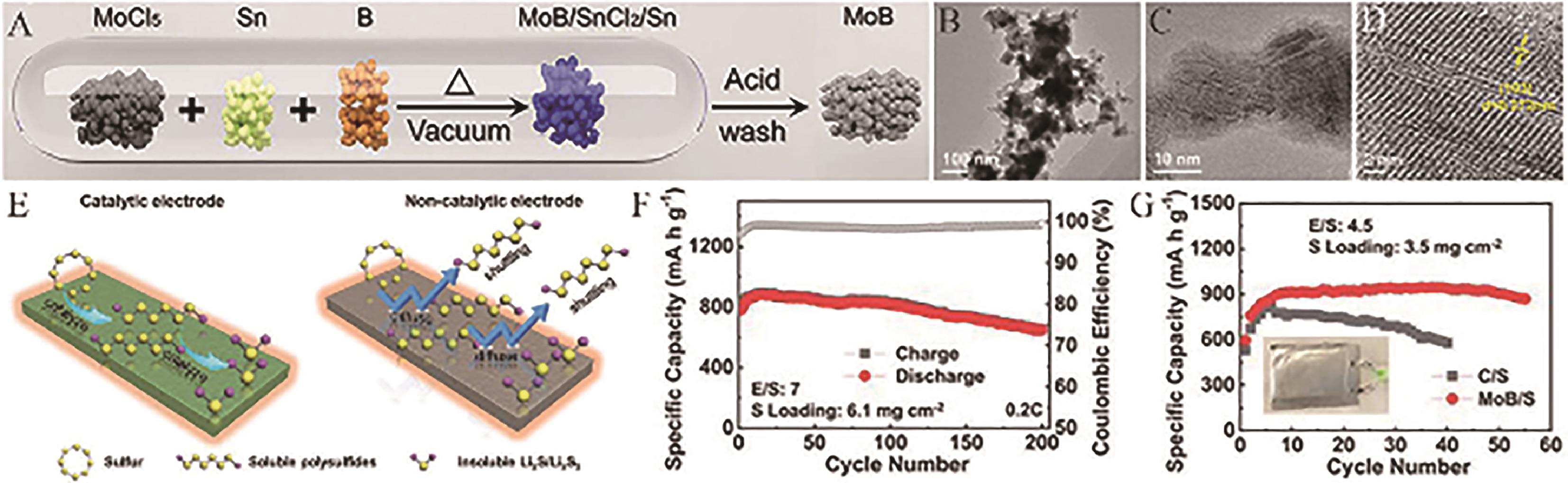
Fig.14 (A) Schematic diagram of the fabrication process of MoB; (B, C) Low-magnification TEM images of MoB; (D) HRTEM images of MoB; (E) Schematic illustration of the advantages of catalytic electrode in LSBs; (F) Cycling stability of the MoB/S cathode in coin cell at 0.2 C rate; (G) A comparative cycling stability of the MoB/S and C/S cathodes in pouch cells with an E/S ratio of 4.5 μL/mg and a sulfur loading of 3.5 mg/cm2 at C/20 rate[82]

Fig.15 (A) Synthesis processes of the schematic illustration of the Co-MoSe2/MXene; (B-D) Electrochemical performance of S/Co-MoSe 2 /MXene monolith cathodes in lean electrolyte at 0.1 C, (B) Long-term cycling stability at the E/S ratio of 5.0 μL/mg; (C) Gravimetric and volumetric capacities and (D) areal capacities at an E/S ratio of 3.5 μL/mg[90]
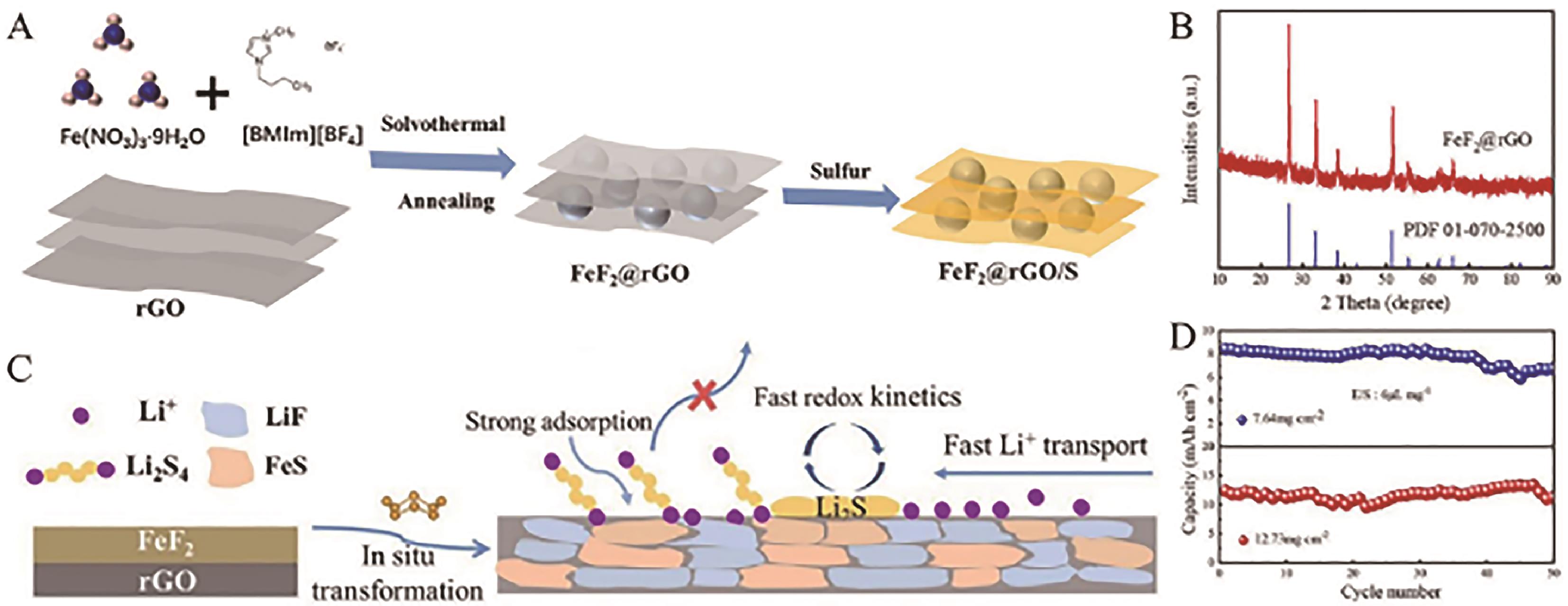
Fig.16 (A) FeF2@rGO synthetic route diagram; (B) XRD patterns of FeF2@rGO; (C) Schematic diagram of the cathode catalytic conversion process during cycle; (D) Cycling performances of FeF2@rGO cathode and rGO cathode under high sulfur loading at 0.1 C[96]
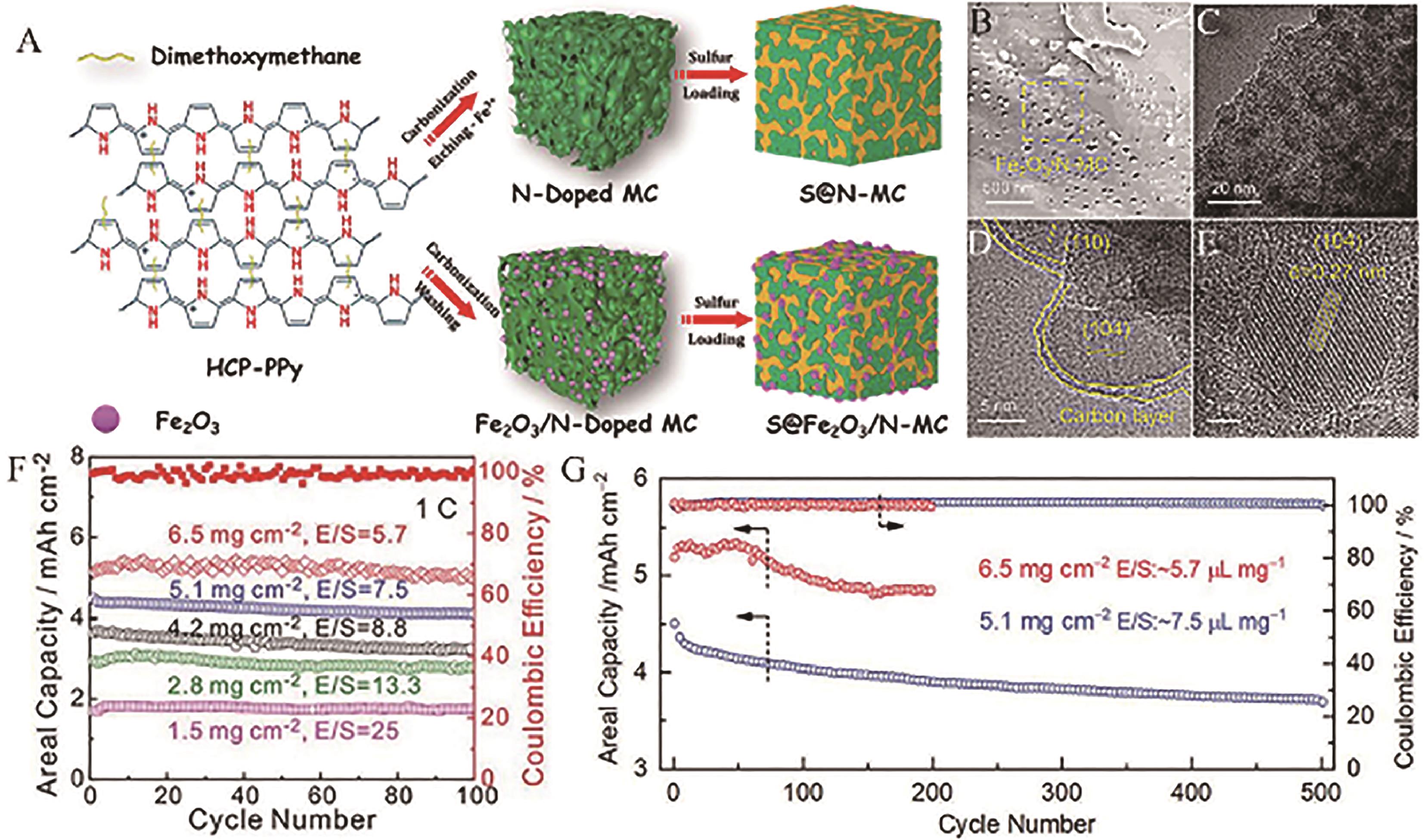
Fig.17 (A) Schematic illustration for the synthesis of Fe2O3/N-MC, N-MC and their application in LSBs; (B) SEM image and (C) TEM image and (D, E) HRTEM images of S@Fe2O3/N-MC; (F) The short cycling performance of S@Fe2O3/N-MC cathode under different E/S ratio and sulfur loading and (G) long-term cycling performance under high areal loading (5.1 and 6.5 mg/cm2) at 1.0 C[97]
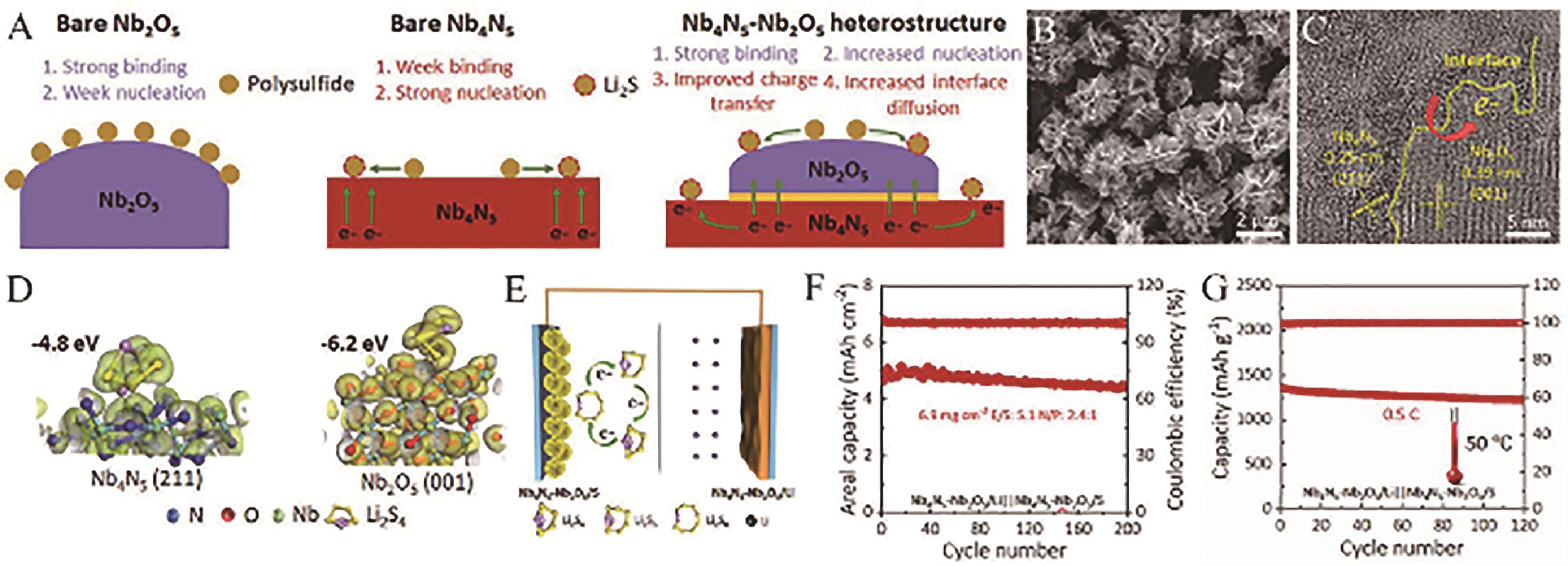
Fig.18 (A) Catalytic mechanism and (B) SEM image and (C) HRTEM image of Nb4N5-Nb2O5 heterostructures; (D) Optimized geometries and their corresponding binding energies of Li2S4 on Nb4N5 (211) and Nb2O5 (001) surfaces; (E) Schematic configuration of Nb4N5-Nb2O5 heterostructures based full batteries; (F) Areal capacity of Nb4N5-Nb2O5/Li||Nb4N5-Nb2O5/S battery obtained at 0.3 C with high sulfur loading of 6.9 mg/cm2 and (G) Cycling performance of the full battery operated at an elevated temperature of 50 ℃[100]
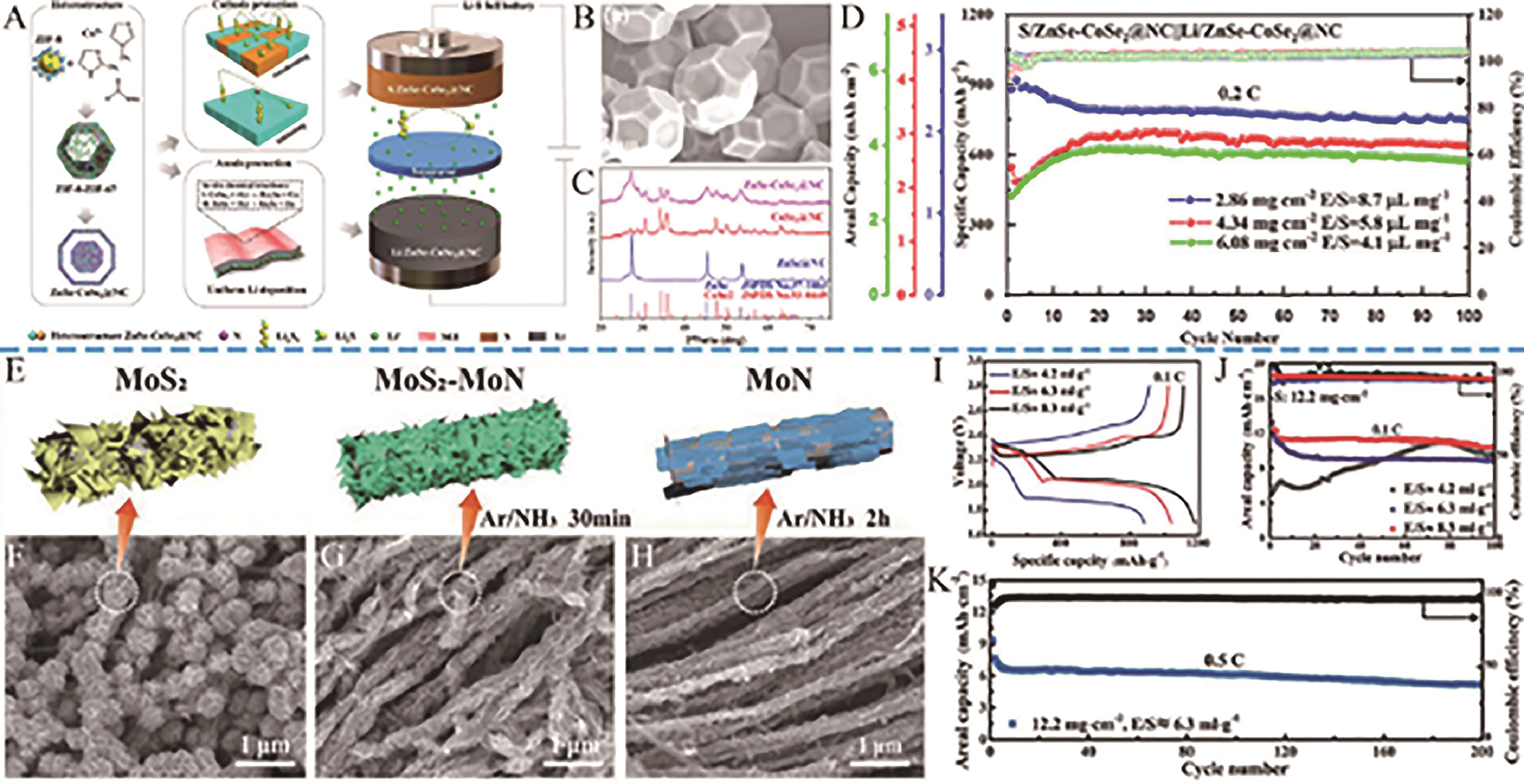
Fig.19 (A) The working principle of the Li-S full battery based on the “two-in-one” ZnSe-CoSe2@NC hosts; (B) SEM images of ZnSe-CoSe2@NC; (C) XRD pattern of ZnSe-CoSe2@NC, CoSe2@NC and ZnSe@NC; (D) Cycle performance of the full cell under high sulfur loading and lean electrolyte[101]; (E) Schematic illustration of MoS2, MoS2-MoN, and MoN nanosheets grown on nitrogen-doped carbon nanotube (NCNT) vertically; SEM images of (F) MoS2, (G) MoS2-MoN and (H) MoN hosts; (I) Galvanostatic charge/discharge curves for different E/S ratios with sulfur loadings of 12.2 mg/cm2; (J) Cycling performances of MoS2-MoN/S cathodes with sulfur loadings of 12.2 mg/cm2 with different E/S ratios at 0.1 C for 100 cycles; (K) Cycling performance of MoS2-MoN/S cathode at 0.5 C for 200 cycles[102]
| Material | E/S ratio/ (μL·mg-1) | Sulfur loading/ (mg·cm-2) | Current/number of cycles | Discharge capacity/ (mA·h·g-1) | Area capacity/ (mA·h·cm-2) | Ref. |
|---|---|---|---|---|---|---|
| m(S)∶m(Au)=97∶3 | 5.0 | 5.7 | 0.05 C/40 | 730 | 4.2 | [ |
| S/Co-NC@TpBD-Me2 | 6.1 | 5.71 | 0.2 C/50 | 793 | 4.53 | [ |
| ZnS and Co-N-C DEB sites | 4.0 | 6.25 | 83.33 (mA/g)/80 | 1 257 | 7.86 | [ |
| Co-NCNT@CF/S | 3.9 | 8.8 | 0.1 C/100 | 579 | 5.10 | [ |
| S/CNT-CoP-Vp | 5.0 | 7.7 | 0.4 (mA/cm2)/30 | 1 043 | 8.03 | [ |
| S/rGO-CNT-CoP(A) | 7.0 | 5.3 | 0.8 (mA/cm2)/50 | 833 | 4.43 | [ |
| S/CoSe@C||CoSe@C/Li | 4.5 | 6.2 | 0.1 C/100 | 680 | 4.2 | [ |
| N-CoSe2/S | 4.4 | 10.2 | 0.2 C/70 | ~790 | ~8.1 | [ |
| Li2S-Co9S8/Co | 5.0 | 8.3(Li2S) | 0.1 C/150 | 653 | - | [ |
| MoB/S | 7.0 | 6.1 | 0.2 C/200 | 647 | 3.95 | [ |
| MoS2-x /rGO | 5.0 | 5.6 | 0.5 (mA/cm2)/100 | 730 | 4.1 | [ |
| S/Co-MoSe2/MXene | 3.5 | 9.9 | 0.1 C/50 | 606 | ~6.0 | [ |
| FeNC/wG | 7.8 | 5.39 | 0.5 C/50 | 776 | 4.18 | [ |
| FeHCF-A | 5.6 | 5.2 | 0.2 C/180 | 865 | 4.5 | [ |
| FeF2@rGO/S | 6.0 | 12.73 | 0.1 C/50 | 882 | 11.2 | [ |
| S@Fe2O3/N-MC | 5.7 | 6.5 | 1.0 C/200 | 745 | 4.84 | [ |
| CoSe-ZnSe@G | 3.0 | 7.7 | 0.01 C/40 | 532 | 4.1 | [ |
| Nb4N5-Nb2O5 | 5.1 | 6.9 | 0.3 C/200 | 725 | 5.0 | [ |
| ZnSe-CoSe2@NC | 4.1 | 6.08 | 0.2 C/100 | 642 | 4.16 | [ |
| MoS2-MoN | 4.2 | 12.2 | 0.1 C/78 | 893 | 10.9 | [ |
Table 2 Electrochemical performance of metal catalysts for LSB under lean electrolyte
| Material | E/S ratio/ (μL·mg-1) | Sulfur loading/ (mg·cm-2) | Current/number of cycles | Discharge capacity/ (mA·h·g-1) | Area capacity/ (mA·h·cm-2) | Ref. |
|---|---|---|---|---|---|---|
| m(S)∶m(Au)=97∶3 | 5.0 | 5.7 | 0.05 C/40 | 730 | 4.2 | [ |
| S/Co-NC@TpBD-Me2 | 6.1 | 5.71 | 0.2 C/50 | 793 | 4.53 | [ |
| ZnS and Co-N-C DEB sites | 4.0 | 6.25 | 83.33 (mA/g)/80 | 1 257 | 7.86 | [ |
| Co-NCNT@CF/S | 3.9 | 8.8 | 0.1 C/100 | 579 | 5.10 | [ |
| S/CNT-CoP-Vp | 5.0 | 7.7 | 0.4 (mA/cm2)/30 | 1 043 | 8.03 | [ |
| S/rGO-CNT-CoP(A) | 7.0 | 5.3 | 0.8 (mA/cm2)/50 | 833 | 4.43 | [ |
| S/CoSe@C||CoSe@C/Li | 4.5 | 6.2 | 0.1 C/100 | 680 | 4.2 | [ |
| N-CoSe2/S | 4.4 | 10.2 | 0.2 C/70 | ~790 | ~8.1 | [ |
| Li2S-Co9S8/Co | 5.0 | 8.3(Li2S) | 0.1 C/150 | 653 | - | [ |
| MoB/S | 7.0 | 6.1 | 0.2 C/200 | 647 | 3.95 | [ |
| MoS2-x /rGO | 5.0 | 5.6 | 0.5 (mA/cm2)/100 | 730 | 4.1 | [ |
| S/Co-MoSe2/MXene | 3.5 | 9.9 | 0.1 C/50 | 606 | ~6.0 | [ |
| FeNC/wG | 7.8 | 5.39 | 0.5 C/50 | 776 | 4.18 | [ |
| FeHCF-A | 5.6 | 5.2 | 0.2 C/180 | 865 | 4.5 | [ |
| FeF2@rGO/S | 6.0 | 12.73 | 0.1 C/50 | 882 | 11.2 | [ |
| S@Fe2O3/N-MC | 5.7 | 6.5 | 1.0 C/200 | 745 | 4.84 | [ |
| CoSe-ZnSe@G | 3.0 | 7.7 | 0.01 C/40 | 532 | 4.1 | [ |
| Nb4N5-Nb2O5 | 5.1 | 6.9 | 0.3 C/200 | 725 | 5.0 | [ |
| ZnSe-CoSe2@NC | 4.1 | 6.08 | 0.2 C/100 | 642 | 4.16 | [ |
| MoS2-MoN | 4.2 | 12.2 | 0.1 C/78 | 893 | 10.9 | [ |
| 1 | KONG L, JIN Q, ZHANG X T, et al. Towards full demonstration of high areal loading sulfur cathode in lithium-sulfur batteries[J]. J Energy Chem, 2019, 39: 17-22. |
| 2 | LI J, YUAN Y, JIN H, et al. One-step nonlinear electrochemical synthesis of TexSy@PANI nanorod materials for Li-TexSy battery[J]. Energy Stor Mater, 2019, 16: 31-36. |
| 3 | BRUCE P G, FREUNBERGER S A, HARDWICK L J, et al. Li-O2 and Li-S batteries with high energy storage[J]. Nat Mater, 2012, 11(1): 19-29. |
| 4 | LU Y, RONG X, HU Y, et al. Research and development of advanced battery materials in China[J]. Energy Stor Mater, 2019, 23: 144-153. |
| 5 | PANG Q, LIANG X, KWOK C Y, et al. Advances in lithium-sulfur batteries based on multifunctional cathodes and electrolytes[J]. Nat Energy, 2016, 1(9): 16132. |
| 6 | LIU Y, ZHU Y, CUI Y, et al. Challenges and opportunities towards fast-charging battery materials[J]. Nat Energy, 2019, 4(7): 540-550. |
| 7 | ZHAO M, LI B Q, PENG H J, et al. Lithium-sulfur batteries under lean electrolyte conditions: challenges and opportunities[J]. Angew Chem Int Ed Engl, 2020, 59(31): 12636-12652. |
| 8 | ZHANG M, YU C, ZHAO C, et al. Cobalt-embedded nitrogen-doped hollow carbon nanorods for synergistically immobilizing the discharge products in lithium-sulfur battery[J]. Energy Stor Mater, 2016, 5: 223-229. |
| 9 | BHARGAV A, BELL M E, CUI Y, et al. Polyphenylene tetrasulfide as an inherently flexible cathode material for rechargeable lithium batteries[J]. ACS Appl Energy Mater, 2018, 1(11): 5859-5864. |
| 10 | HE J, LV W, CHEN Y, et al. Tellurium-impregnated porous cobalt-doped carbon polyhedra as superior cathodes for lithium-tellurium batteries[J]. ACS Nano, 2017, 11(8): 8144-8152. |
| 11 | LU X, ZHANG Q, WANG J, et al. High performance bimetal sulfides for lithium-sulfur batteries[J]. Chem Eng J, 2019, 358: 955-961. |
| 12 | HUANG J, ZHANG Q, WEI F. Multi-functional separator/interlayer system for high-stable lithium-sulfur batteries:progress and prospects[J]. Energy Stor Mater, 2015, 1: 127-145. |
| 13 | LI G, WANG S, ZHANG Y, et al. Revisiting the role of polysulfides in lithium-sulfur batteries[J]. Adv Mater, 2018, 30(22): 1705590. |
| 14 | SURIYAKUMAR S, STEPHAN A M. Mitigation of polysulfide shuttling by interlayer/permselective separators in lithium-sulfur batteries[J]. ACS Appl Energy Mater, 2020, 3(9): 8095-8129. |
| 15 | HE J, MANTHIRAM A. A review on the status and challenges of electrocatalysts in lithium-sulfur batteries[J]. Energy Stor Mater, 2019, 20: 55-70. |
| 16 | SHI H, LV W, ZHANG C, et al. Functional carbons remedy the shuttling of polysulfides in lithium-sulfur batteries: confining, trapping, blocking, and breaking up[J]. Adv Funct Mater, 2018, 28(38): 1800508. |
| 17 | VAN NOORDEN R. Sulphur back in vogue for batteries[J]. Nature, 2013, 498(7455): 416-417. |
| 18 | MANTHIRAM A, FU Y, SU Y. Challenges and prospects of lithium-sulfur batteries[J]. Acc Chem Res, 2013, 46(5): 1125-1134. |
| 19 | LIANG X, HART C, PANG Q, et al. A highly efficient polysulfide mediator for lithium-sulfur batteries[J]. Nat Commun, 2015, 6(1): 5682. |
| 20 | ZHOU J, LIU X, ZHU L, et al. Deciphering the modulation essence of p bands in Co-based compounds on Li-S chemistry[J]. Joule, 2018, 2(12): 2681-2693. |
| 21 | MOON S, JUNG Y H, JUNG W K, et al. Encapsulated monoclinic sulfur for stable cycling of Li-S rechargeable batteries[J]. Adv Mater, 2013, 25(45): 6547-6553. |
| 22 | HAGEN M, HANSELMANN D, AHLBRECHT K, et al. Lithium-sulfur cells: the gap between the state-of-the-art and the requirements for high energy battery cells[J]. Adv Energy Mater, 2015, 5(16): 1401986. |
| 23 | CHUNG S H, CHANG C H, MANTHIRAM A. Progress on the critical parameters for lithium-sulfur batteries to be practically viable[J]. Adv Funct Mater, 2018, 28(28): 1801188. |
| 24 | LV D, ZHENG J, LI Q, et al. High energy density lithium-sulfur batteries: challenges of thick sulfur cathodes[J]. Adv Energy Mater, 2015, 5(16): 1402290. |
| 25 | JI X, LEE K T, NAZAR L F. A highly ordered nanostructured carbon-sulphur cathode for lithium-sulphur batteries[J]. Nat Mater, 2009, 8(6): 500-506. |
| 26 | FU Y, ZU C, MANTHIRAM A. In situ-formed Li2S in lithiated graphite electrodes for lithium-sulfur batteries[J]. J Am Chem Soc, 2013, 135(48): 18044-18047. |
| 27 | SUN L, LI M, JIANG Y, et al. Sulfur nanocrystals confined in carbon nanotube network as a binder-free electrode for high-performance lithium sulfur batteries[J]. Nano Lett, 2014, 14(7): 4044-4049. |
| 28 | HE J, CHEN Y, LI P, et al. Three-dimensional CNT/graphene-sulfur hybrid sponges with high sulfur loading as superior-capacity cathodes for lithium-sulfur batteries[J]. J Mater Chem A, 2015, 3(36): 18605-18610. |
| 29 | FU Y, MANTHIRAM A. Enhanced cyclability of lithium-sulfur batteries by a polymer acid-doped polypyrrole mixed ionic-electronic conductor[J]. Chem Mater, 2012,24(15): 3081-3087. |
| 30 | WANG T, KRETSCHMER K, CHOI S, et al. Fabrication methods of porous carbon materials and separator membranes for lithium-sulfur batteries: development and future perspectives[J]. Small Methods, 2017, 1(8): 1700089. |
| 31 | XU Z, KIM J, KANG K. Carbon nanomaterials for advanced lithium sulfur batteries[J]. Nano Today, 2018, 19: 84-107. |
| 32 | LIU X, HUANG J, ZHANG Q, et al. Nanostructured metal oxides and sulfides for lithium-sulfur batteries[J]. Adv Mater, 2017, 29(20): 1601759. |
| 33 | CHEN J, YUAN R, FENG J, et al. Conductive Lewis base matrix to recover the missing link of Li2S8 during the sulfur redox cycle in Li-S battery[J]. Chem Mater, 2015, 27(6): 2048-2055. |
| 34 | PENG H, HOU T, ZHANG Q, et al. Strongly coupled interfaces between a heterogeneous carbon host and a sulfur-containing guest for highly stable lithium-sulfur batteries: mechanistic insight into capacity degradation[J]. Adv Mater Interfaces, 2014, 1(7): 1400227. |
| 35 | ZHEN M, JIANG K, GUO S, et al. Suitable lithium polysulfides diffusion and adsorption on CNTs@TiO2-bronze nanosheets surface for high-performance lithium-sulfur batteries[J]. Nano Res, 2022, 15(2): 933-941. |
| 36 | WANG J, WANG C, ZHEN M. Template-free synthesis of multifunctional Co3O4 nanotubes as excellent performance electrode materials for superior energy storage[J]. Chem Eng J, 2019, 356: 1-10. |
| 37 | CHEN T, MA L, CHENG B, et al. Metallic and polar Co9S8 inlaid carbon hollow nanopolyhedra as efficient polysulfide mediator for lithium-sulfur batteries[J]. Nano Energy, 2017, 38: 239-248. |
| 38 | PENG H, ZHANG G, CHEN X, et al. Enhanced electrochemical kinetics on conductive polar mediators for lithium-sulfur batteries[J]. Angew Chem Int Ed, 2016, 55(42): 12990-12995. |
| 39 | ZHOU F, LI Z, LUO X, et al. Low cost metal carbide nanocrystals as binding and electrocatalytic sites for high performance Li-S batteries[J]. Nano Lett, 2018, 18(2): 1035-1043. |
| 40 | SUN Z, ZHANG J, YIN L, et al. Conductive porous vanadium nitride/graphene composite as chemical anchor of polysulfides for lithium-sulfur batteries[J]. Nat Commun, 2017, 8(1): 14627. |
| 41 | ZHONG Y, CHAO D, DENG S, et al. Confining sulfur in integrated composite scaffold with highly porous carbon fibers/vanadium nitride arrays for high-performance lithium-sulfur batteries[J]. Adv Funct Mater, 2018, 28(38): 1706391. |
| 42 | PANG Q, KWOK C Y, KUNDU D, et al. Lightweight metallic MgB2 mediates polysulfide redox and promises high-energy-density lithium-sulfur batteries[J]. Joule, 2019, 3(1): 136-148. |
| 43 | ZHENG J, TIAN J, WU D, et al. Lewis acid-base interactions between polysulfides and metal organic framework in lithium sulfur batteries[J]. Nano Lett, 2014, 14(5): 2345-2352. |
| 44 | PENG H, HUANG J, ZHAO M, et al. Nanoarchitectured graphene/CNT@porous carbon with extraordinary electrical conductivity and interconnected micro/mesopores for lithium-sulfur batteries[J]. Adv Funct Mater, 2014, 24(19): 2772-2781. |
| 45 | XU T, SONG J, GORDIN M L, et al. Mesoporous carbon-carbon nanotube-sulfur composite microspheres for high-areal-capacity lithium-sulfur battery cathodes[J]. ACS Appl Mater Inter, 2013, 5(21): 11355-11362. |
| 46 | HOU Y, REN Y, ZHANG S, et al. 3D S@MoS2@reduced graphene oxide aerogels cathode for high-rate lithium-sulfur batteries[J]. J Alloy Compd, 2021, 852: 157011. |
| 47 | XU Z, LIN S, ONOFRIO N, et al. Exceptional catalytic effects of black phosphorus quantum dots in shuttling-free lithium sulfur batteries[J]. Nat Commun, 2018, 9(1): 4164. |
| 48 | LI L, CHEN L, MUKHERJEE S, et al. Phosphorene as a polysulfide immobilizer and catalyst in high-performance lithium-sulfur batteries[J]. Adv Mater, 2017, 29(2): 1602734. |
| 49 | CHUNG S, MANTHIRAM A. Designing lithium-sulfur cells with practically necessary parameters[J]. Joule, 2018, 2(4): 710-724. |
| 50 | ZHANG J, LI J, WANG W, et al. Microemulsion assisted assembly of 3D porous S/Graphene@g-C3N4 hybrid sponge as free-standing cathodes for high energy density Li-S batteries[J]. Adv Energy Mater, 2018, 8(14): 1702839. |
| 51 | LEI W, ZHANG H, WU Y, et al. Oxygen-doped boron nitride nanosheets with excellent performance in hydrogen storage[J]. Nano Energy, 2014, 6: 219-224. |
| 52 | FAN Y, YANG Z, HUA W, et al. Functionalized boron nitride nanosheets/graphene interlayer for fast and long-life lithium-sulfur batteries[J]. Adv Energy Mater, 2017, 7(13): 1602380. |
| 53 | YU X, LI W, HE B, et al. Porous carbon/borocarbonitride hybrid with enhanced tap density as a polar host for ultralong life lithium-sulfur batteries[J]. Chem Eng J, 2022, 430: 132987. |
| 54 | FANG R, ZHAO S, HOU P, et al. 3D Interconnected electrode materials with ultrahigh areal sulfur loading for Li-S batteries[J]. Adv Mater, 2016, 28(17): 3374-3382. |
| 55 | YEN Y, CHUNG S. Lean-electrolyte lithium-sulfur electrochemical cells with high-loading carbon nanotube/nanofiber-polysulfide cathodes[J]. Chem Commun, 2021, 57(16): 2009-2012. |
| 56 | YOSHIE Y, HORI K, MAE T, et al. High-energy-density Li-S battery with positive electrode of lithium polysulfides held by carbon nanotube sponge[J]. Carbon, 2021, 182: 32-41. |
| 57 | FANG Z, LUO Y, WU H, et al. Mesoporous carbon nanotube aerogel-sulfur cathodes: a strategy to achieve ultrahigh areal capacity for lithium-sulfur batteries via capillary action[J]. Carbon, 2020, 166: 183-192. |
| 58 | SONG J, GORDIN M L, XU T, et al. Strong lithium polysulfide chemisorption on electroactive sites of nitrogen-doped carbon composites for high-performance lithium-sulfur battery cathodes[J]. Angew Chem Int Ed, 2015, 54(14): 4325-4329. |
| 59 | LIN J, MO Y, LI S, et al. Nitrogen-doped porous carbon fiber/vertical graphene as an efficient polysulfide conversion catalyst for high-performance lithium-sulfur batteries[J]. J Mater Chem A, 2022, 10(2): 690-698. |
| 60 | ZHANG Y, WU Z, WANG S, et al. Complex permittivity-dependent plasma confinement-assisted growth of asymmetric vertical graphene nanofiber membrane for high-performance Li-S full cells[J]. InfoMat, 2022: e12294. |
| 61 | WANG J, JIANG K, SHEN B, et al. Synergetic effect of nitrogen/sulfur dual-doped hierarchically porous carbon networks for Li-S batteries[J]. ACS Sustain Chem Eng, 2020, 8(2): 749-758. |
| 62 | WANG N, ZHANG X, JU Z, et al. Thickness-independent scalable high-performance Li-S batteries with high areal sulfur loading via electron-enriched carbon framework[J]. Nat Commun, 2021, 12(1): 4519. |
| 63 | ZHANG L, LIU D, MUHAMMAD Z, et al. Single nickel atoms on nitrogen-doped graphene enabling enhanced kinetics of lithium-sulfur batteries[J]. Adv Mater, 2019, 31(40): 1903955. |
| 64 | MA F, WAN Y, WANG X, et al. Bifunctional atomically dispersed Mo-N2/C nanosheets boost lithium sulfide deposition/decomposition for stable lithium-sulfur batteries[J]. ACS Nano, 2020, 14(8): 10115-10126. |
| 65 | PEI G X, LIU X Y, WANG A, et al. Ag alloyed Pd single-atom catalysts for efficient selective hydrogenation of acetylene to ethylene in excess ethylene[J]. ACS Catal, 2015, 5(6): 3717-3725. |
| 66 | AL SALEM H, BABU G, V RAO C, et al. Electrocatalytic polysulfide traps for controlling redox shuttle process of Li-S batteries[J]. J Am Chem Soc, 2015, 137(36): 11542-11545. |
| 67 | MARANGON V, Di LECCE D, BRETT D J L, et al. Characteristics of a gold-doped electrode for application in high-performance lithium-sulfur battery[J]. J Energy Chem, 2022, 64: 116-128. |
| 68 | LI Y, FAN J, ZHENG M, et al. A novel synergistic composite with multi-functional effects for high-performance Li-S batteries[J]. Energ Environ Sci, 2016, 9(6): 1998-2004. |
| 69 | DU B, LUO Y, YANG Y, et al. COFs-confined multifunctional sulfur-host design towards high-performance lithium-sulfur batteries[J]. Chem Eng J, 2022, 442: 135823. |
| 70 | WEI SEH Z, SUN Y, ZHANG Q, et al. Designing high-energy lithium-sulfur batteries[J]. Chem Soc Rev, 2016, 45(20): 5605-5634. |
| 71 | ZHAO C, XU G, YU Z, et al. A high-energy and long-cycling lithium-sulfur pouch cell via a macroporous catalytic cathode with double-end binding sites[J]. Nat Nanotechnol, 2021, 16(2): 166-173. |
| 72 | ZHEN M, ZUO X, WANG J, et al. An integrated cathode with bi-functional catalytic effect for excellent-performance lithium-sulfur batteries[J]. Nano Res, 2019, 12(5): 1017-1024. |
| 73 | WANG D, MA K, HAO J, et al. Multifunction Co-Nx species to manipulate polysulfides conversion kinetics toward highly efficient lithium-sulfur batteries[J]. Nano Energy, 2021, 89: 106426. |
| 74 | LIN H, ZHANG S, ZHANG T, et al. Elucidating the catalytic activity of oxygen deficiency in the polysulfide conversion reactions of lithium-sulfur batteries[J]. Adv Energy Mater, 2018, 8(30): 1801868. |
| 75 | SUN R, BAI Y, BAI Z, et al. Phosphorus vacancies as effective polysulfide promoter for high-energy-density lithium-sulfur batteries[J]. Adv Energy Mater, 2022, 12(12): 2102739. |
| 76 | SUN R, BAI Y, LUO M, et al. Enhancing polysulfide confinement and electrochemical kinetics by amorphous cobalt phosphide for highly efficient lithium-sulfur batteries[J]. ACS Nano, 2021, 15(1): 739-750. |
| 77 | YUAN H, PENG H, LI B, et al. Conductive and catalytic triple-phase interfaces enabling uniform nucleation in high-rate lithium-sulfur batteries[J]. Adv Energy Mater, 2019, 9(1): 1802768. |
| 78 | HE J, MANTHIRAM A. 3D CoSe@C aerogel as a host for dendrite-free lithium-metal anode and efficient sulfur cathode in Li-S full cells[J]. Adv Energy Mater, 2020, 10(41): 2002654. |
| 79 | YE Z, JIANG Y, LI L, et al. A high-efficiency CoSe electrocatalyst with hierarchical porous polyhedron nanoarchitecture for accelerating polysulfides conversion in Li-S batteries[J]. Adv Mater, 2020, 32(32): 2002168. |
| 80 | WANG M, FAN L, SUN X, et al. Nitrogen-doped CoSe2 as a bifunctional catalyst for high areal capacity and lean electrolyte of Li-S battery[J]. ACS Energy Lett, 2020, 5(9): 3041-3050. |
| 81 | HE J, BHARGAV A, MANTHIRAM A. High-performance anode-free Li-S batteries with an integrated Li2S-electrocatalyst cathode[J]. ACS Energy Lett, 2022, 7(2): 583-590. |
| 82 | HE J, BHARGAV A, MANTHIRAM A. Molybdenum boride as an efficient catalyst for polysulfide redox to enable high-energy-density lithium-sulfur batteries[J]. Adv Mater, 2020, 32(40): 2004741. |
| 83 | QIAN J, XING Y, YANG Y, et al. Enhanced electrochemical kinetics with highly dispersed conductive and electrocatalytic mediators for lithium-sulfur batteries[J]. Adv Mater, 2021, 33(25): 2100810. |
| 84 | ZHEN M, GUO S, SHEN B. Constructing defect-rich MoS2/N-doped carbon nanosheets for catalytic polysulfide conversion in lithium-sulfur batteries[J]. ACS Sustain Chem Eng, 2020, 8(35): 13318-13327. |
| 85 | LIN H, YANG L, JIANG X, et al. Electrocatalysis of polysulfide conversion by sulfur-deficient MoS2 nanoflakes for lithium-sulfur batteries[J]. Energ Environ Sci, 2017, 10(6): 1476-1486. |
| 86 | YE H, SUN J, LIM X F, et al. Mediator-assisted catalysis of polysulfide conversion for high-loading lithium-sulfur batteries operating under the lean electrolyte condition[J]. Energy Stor Mater, 2021, 38: 338-343. |
| 87 | FANG Y, PAN J, HE J, et al. Structure re-determination and superconductivity observation of bulk 1T MoS2[J]. Angew Chem Int Ed, 2018, 57(5): 1232-1235. |
| 88 | HUANG X, ZHAO Z, CAO L, et al. High-performance transition metal-doped Pt3Ni octahedra for oxygen reduction reaction[J]. Science, 2015, 348(6240): 1230-1234. |
| 89 | LIANG X, RANGOM Y, KWOK C Y, et al. Interwoven MXene nanosheet/carbon-nanotube composites as Li-S cathode hosts[J]. Adv Mater, 2017, 29(3): 1603040. |
| 90 | WANG W, HUAI L, WU S, et al. Ultrahigh-volumetric-energy-density lithium-sulfur batteries with lean electrolyte enabled by cobalt-doped MoSe2/Ti3C2Tx MXene bifunctional catalyst[J]. ACS Nano, 2021, 15(7): 11619-11633. |
| 91 | LIU Y, LIU S, LI G, et al. High volumetric energy density sulfur cathode with heavy and catalytic metal oxide host for lithium-sulfur battery[J]. Adv Sci, 2020, 7(12): 1903693. |
| 92 | KIM J, KIM S J, JUNG E, et al. Atomic structure modification of Fe-N-C catalysts via morphology engineering of graphene for enhanced conversion kinetics of lithium-sulfur batteries[J]. Adv Funct Mater, 2022, 32(19): 2110857. |
| 93 | ZHU Y, LI G, LUO D, et al. Unsaturated coordination polymer frameworks as multifunctional sulfur reservoir for fast and durable lithium-sulfur batteries[J]. Nano Energy, 2021, 79: 105393. |
| 94 | XIN S, LI J, CUI H, et al. Self-templating synthesis of prismatic-like N-doped carbon tubes embedded with Fe3O4 as a high-efficiency polysulfide-anchoring-conversion mediator for high performance lithium-sulfur batteries[J]. Chem Eng J, 2021, 410: 128153. |
| 95 | LU J, WANG Z, GUO Y, et al. Ultrathin nanosheets of FeOOH with oxygen vacancies as efficient polysulfide electrocatalyst for advanced lithium-sulfur batteries[J]. Energy Stor Mater, 2022, 47: 561-568. |
| 96 | SUN X, TIAN D, SONG X, et al. In situ conversion to construct fast ion transport and high catalytic cathode for high-sulfur loading with lean electrolyte lithium-sulfur battery[J]. Nano Energy, 2022, 95: 106979. |
| 97 | LU Y, QIN J L, SHEN T, et al. Hypercrosslinked polymerization enabled N-doped carbon confined Fe2O3 facilitating Li polysulfides interface conversion for Li-S batteries[J]. Adv Energy Mater, 2021, 11(42): 2101780. |
| 98 | YE Z, JIANG Y, YANG T, et al. Engineering catalytic CoSe-ZnSe heterojunctions anchored on graphene aerogels for bidirectional sulfur conversion reactions[J]. Adv Sci, 2022, 9(1): 2103456. |
| 99 | YE C, JIAO Y, JIN H, et al. 2D MoN-VN heterostructure to regulate polysulfides for highly efficient lithium‐sulfur batteries[J]. Angew Chem Int Ed, 2018, 57(51): 16703-16707. |
| 100 | SHI H, QIN J, LU P, et al. Interfacial engineering of bifunctional niobium(V)-based heterostructure nanosheet toward high efficiency lean-electrolyte lithium-sulfur full batteries[J]. Adv Funct Mater, 2021, 31(28): 2102314. |
| 101 | XU J, XU L, ZHANG Z, et al. Heterostructure ZnSe-CoSe2 embedded with yolk-shell conductive dodecahedral as two-in-one hosts for cathode and anode protection of lithium-sulfur full batteries[J]. Energy Stor Mater, 2022, 47: 223-234. |
| 102 | WANG S, FENG S, LIANG J, et al. Insight into MoS2-MoN heterostructure to accelerate polysulfide conversion toward high-energy-density lithium-sulfur batteries[J]. Adv Energy Mater, 2021, 11(11): 2003314. |
| [1] | LI Yan, GONG Shilei, CHE Ying, JIN Li'e, Yan Guolan. Hydroxyl Radical Scavenging Activity and Kinetics of Vitamin C [J]. Chinese Journal of Applied Chemistry, 2015, 32(8): 948-954. |
| [2] | REN Yinghui1,2*, LI Wen2, ZHANG Xianbo2, ZHAO Fengqi1, YI Jianhua1, MA Haixia2, XU Kangzhen2, SONG Jirong2,3. Nonisothermal Decomposition Kinetics and Thermal Safety of Ag2(BTATz)·2H2O(BTATz=3,6-Bis(1-H-1,2,3,4-tetrazole-5-amino)-1,2,4,5-tetrazine) [J]. Chinese Journal of Applied Chemistry, 2013, 30(09): 1036-1041. |
| [3] | LI Gao-Liang, HE Hui*. Studies on the reaction kinetics between nitrous acidand N,N-Dimethylhydroxylamine [J]. Chinese Journal of Applied Chemistry, 2010, 27(08): 916-923. |
| [4] | Zhong Hui, Guo Linghong. Synthetic Kinetics of Solid State Reaction of H2TiO2 Type Ion exchangers [J]. Chinese Journal of Applied Chemistry, 1998, 0(3): 103-105. |
| [5] | Wei Yunyang, Lu Chunxu, Lu Ming, Wang Yu, Li Zhiping. Products of Cyclisation and Mannich Condensation of Methylenedinitramine [J]. Chinese Journal of Applied Chemistry, 1994, 0(5): 104-106. |
| [6] | Zou Wenqiao, Feng Yangjie, Li Bing. STUDY ON THE KINETICS OF IMMOBILIZED ENZYME REACTION WITH INTERNAL DIFFUSION LIMITATION USING SINGLE SIZE PARTICLE [J]. Chinese Journal of Applied Chemistry, 1993, 0(3): 16-19. |
| [7] | Huang Yongchang, Chen Houlong. STUDY ON THE ANODIC REACTION OF CHLORINE EVOLUTION ON Ti/β-PbO2ELECTRODE [J]. Chinese Journal of Applied Chemistry, 1990, 0(2): 25-29. |
| [8] | Zhu Penwei, Chang Mian. ON THE CALCULATION OF TG DATA OF SOME ABS RESINS [J]. Chinese Journal of Applied Chemistry, 1989, 0(1): 91-94. |
| [9] | Wu Zhiyuan, Zhou Yunhong, Gao Rong . A STUDY ON OPEN CIRCUIT VOLTAGE OF OXYGEN ELECTRODE ON CARBON SUBSTRATE [J]. Chinese Journal of Applied Chemistry, 1988, 0(2): 38-43. |
| [10] | Tan Weihong, Peng Shaoyi, Tan Changyu. KINETIC STUDY OF CONSECUTIVE HYDROGENATION OF ACETYLENE [J]. Chinese Journal of Applied Chemistry, 1988, 0(1): 47-51. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||