应用化学 ›› 2023, Vol. 40 ›› Issue (4): 583-596.DOI: 10.19894/j.issn.1000-0518.220320
钠离子电池层状过渡金属氧化物正极材料研究进展
师文君, 孙中辉( ), 宋忠乾, 许佳楠, 韩冬雪, 牛利(
), 宋忠乾, 许佳楠, 韩冬雪, 牛利( )
)
- 广州大学化学化工学院,分析科学技术研究中心,广州 510006
-
收稿日期:2022-10-07接受日期:2023-01-10出版日期:2023-04-01发布日期:2023-04-17 -
通讯作者:孙中辉,牛利 -
基金资助:国家自然科学基金(22204028);广州市科技计划项目(202201010245)
Research Progress of Layered Transition Metal Oxides Cathode Materials for Sodium-ion Batteries
Wen-Jun SHI, Zhong-Hui SUN( ), Zhong-Qian SONG, XU-Jia NAN, Dong-Xue HAN, Li NIU(
), Zhong-Qian SONG, XU-Jia NAN, Dong-Xue HAN, Li NIU( )
)
- Center for Advanced Analytical Science,School of Chemistry and Chemical Engineering,Guangzhou University,Guangzhou 510006,China
-
Received:2022-10-07Accepted:2023-01-10Published:2023-04-01Online:2023-04-17 -
Contact:Zhong-Hui SUN,Li NIU -
About author:lniu@gzhu.edu.cn;
cczhsun@gzhu.edu.cn
-
Supported by:the National Natural Science Foundation of China(22204028);Guangzhou Science and Technology Plan Proiect(202201010245)
摘要:
钠离子电池层状过渡金属氧化物正极材料具有价格低廉、比容量相对高的特点,是未来大型储能电站等能源转型设施的重要候选者,与锂离子电池在市场中的应用场景互为补充,为能源转型提供了有力支持,钠离子电池以Na+特有的理化性质而具有极大的开发潜力。然而,层状过渡金属氧化物正极材料在充放电过程伴随着钠离子的嵌入、脱出会产生一系列不利于其电化学性能的变化,如过渡金属溶解、结构相转变、相对较低的能量密度和较差的空气稳定性与循环稳定性,因此对正极材料的结构与性能进行优化变得尤为重要。近10年来许多研究学者针对层状正极材料的失效机制进行了结构上的优化,得到了性能相对良好的正极材料,报道了当前层状过渡金属氧化物正极材料的电化学性能失效机制、改性手段的现状,对钠离子层状氧化物正极材料面临的挑战进行了总结,并对未来发展需要解决的关键问题做出了展望。
中图分类号:
引用本文
师文君, 孙中辉, 宋忠乾, 许佳楠, 韩冬雪, 牛利. 钠离子电池层状过渡金属氧化物正极材料研究进展[J]. 应用化学, 2023, 40(4): 583-596.
Wen-Jun SHI, Zhong-Hui SUN, Zhong-Qian SONG, XU-Jia NAN, Dong-Xue HAN, Li NIU. Research Progress of Layered Transition Metal Oxides Cathode Materials for Sodium-ion Batteries[J]. Chinese Journal of Applied Chemistry, 2023, 40(4): 583-596.
| Cationradius/nm | Ar/(g·mol-1) | E/V(νs.SHE) | mp/℃ | Capacity/(mA·h·g-1) | Relative cost | |
|---|---|---|---|---|---|---|
| Lithium ion | 0.076 | 6.9 | -2.71 | 180.5 | 3 829 | 1 |
| Sodium ion | 0.098 | 23 | -3.04 | 97.7 | 1 165 | 0.7 |
表1 Na+与Li+的理化性质对比[25]
Table 1 Comparison of physicochemical properties of sodium ions and lithium ions[25]
| Cationradius/nm | Ar/(g·mol-1) | E/V(νs.SHE) | mp/℃ | Capacity/(mA·h·g-1) | Relative cost | |
|---|---|---|---|---|---|---|
| Lithium ion | 0.076 | 6.9 | -2.71 | 180.5 | 3 829 | 1 |
| Sodium ion | 0.098 | 23 | -3.04 | 97.7 | 1 165 | 0.7 |
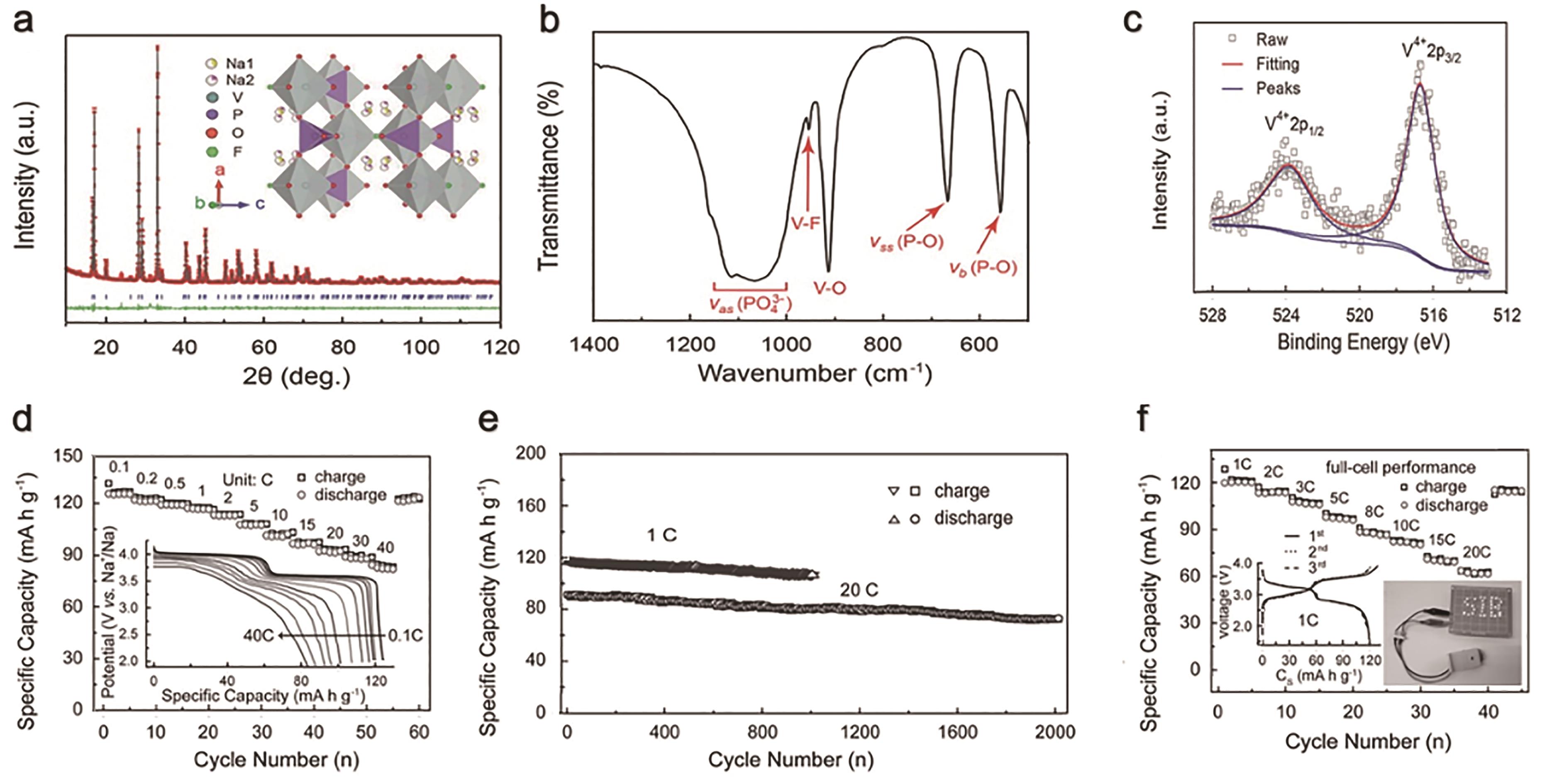
图3 (a) NVPF-NTP的X射线衍射图;(b) 红外光谱图;(c) 高分辨率V2p 的X射线光电子图谱;(d) 0.1~40 C的倍率性能和相应的充放电曲线;(e)1和2 C电流密度下的长循环稳定性;(f)Sb-CNT//NVPF-NTP全电池性能[44]
Fig.3 (a) PXRD pattern for NVPF-NTP;(b)FT-IR; (c) High-resolution V2p XPS spectrum; (d) Rate capabilities from 0.1 to 40 C and the corresponding GCD curves; (e) The cycling stabilities at different rates of 1 C for 1000 cycles and 20 C for 2000 cycles; (f) The electrochemical performance of Sb-CNT//NVPF-NTP full cell[44]
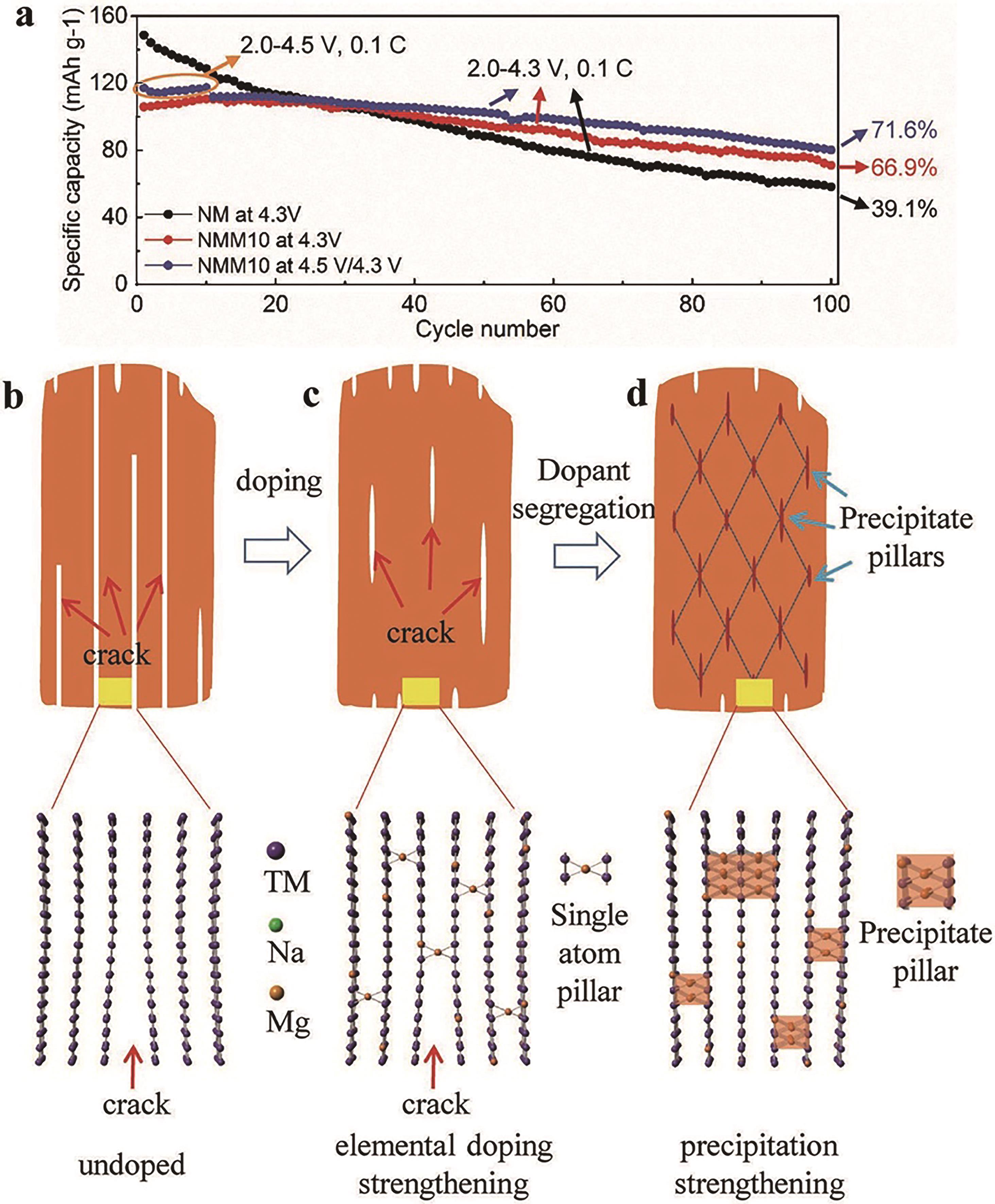
图4 (a) 不同充放电电压区间的循环性能;(b) 未引入掺杂元素的层状材料;(c) 常规掺杂元素;(d) 引入高密度纳米沉淀相构建三维网络结构有效抑制开裂[63]
Fig.4 (a) Capacity retentions as a function of cycle numbers; (b) The undoped P2-NM sample; (c) The conventional doped sample; (d) By forming high density of nanoprecipitates, the 3D network structure can effectively suppress bulk cracking[63]
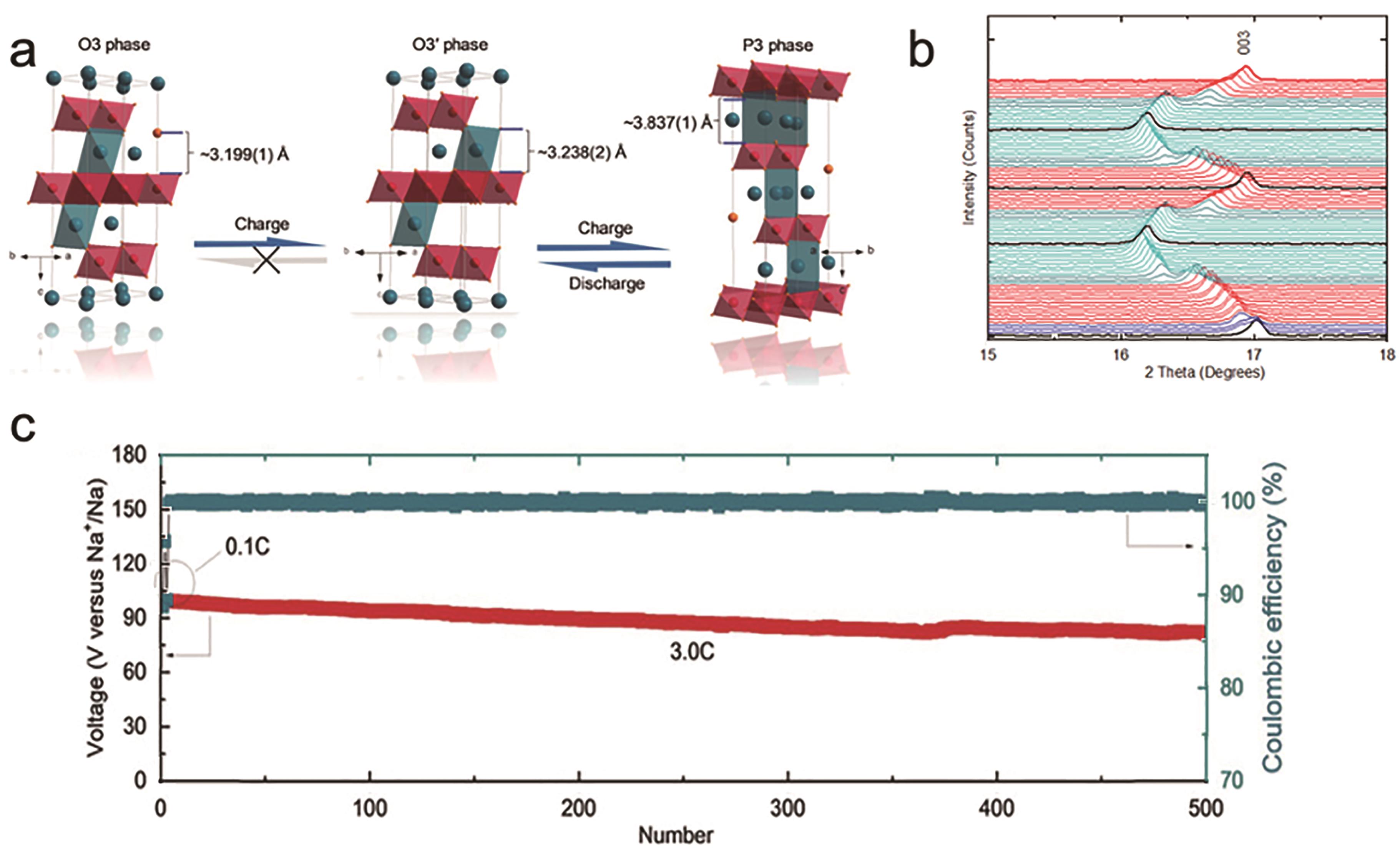
图5 (a) 高熵正极材料晶体结构演化;(b) 充放电过程中(003)峰值的演化;(c) 在3 C下的放电容量和库伦效率[32]
Fig.5 (a) HEO cathode crystal structure evolution; (b) The evolution (003) peaks during the charge-discharge process; (c) Retention of the discharge capacity and Coulombic efficiency at rates of 3 C[29]
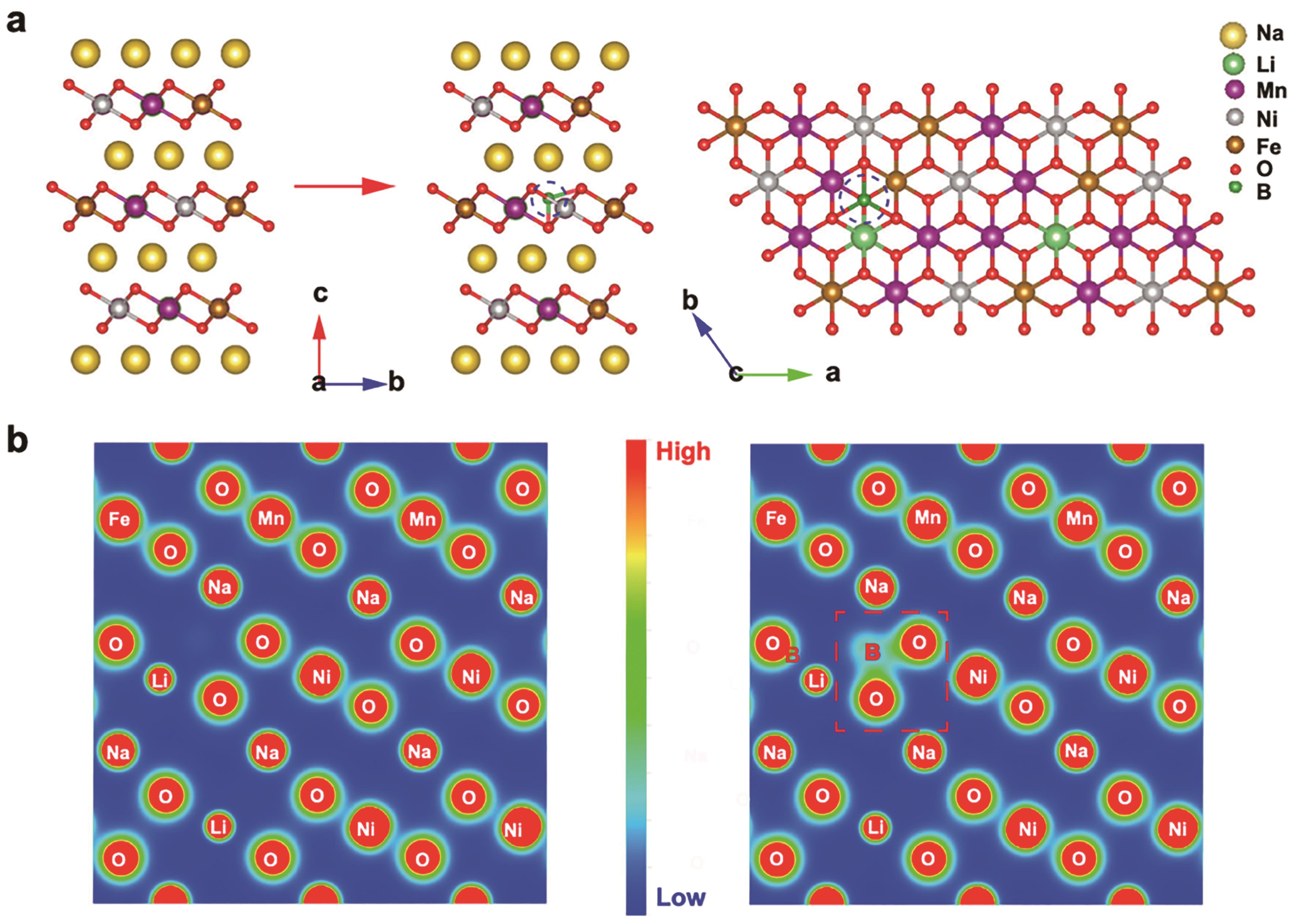
图6 (a) NLNFM和NLNFMB结构示意图;(b) NLNFM和NLNFMB电荷密度轮廓图[90]
Fig.6 (a) Schematic structures of NLNFM and NLNFMB; (b) Contour maps of charge density on corresponding planes in NLNFM and NLNFMB[90]
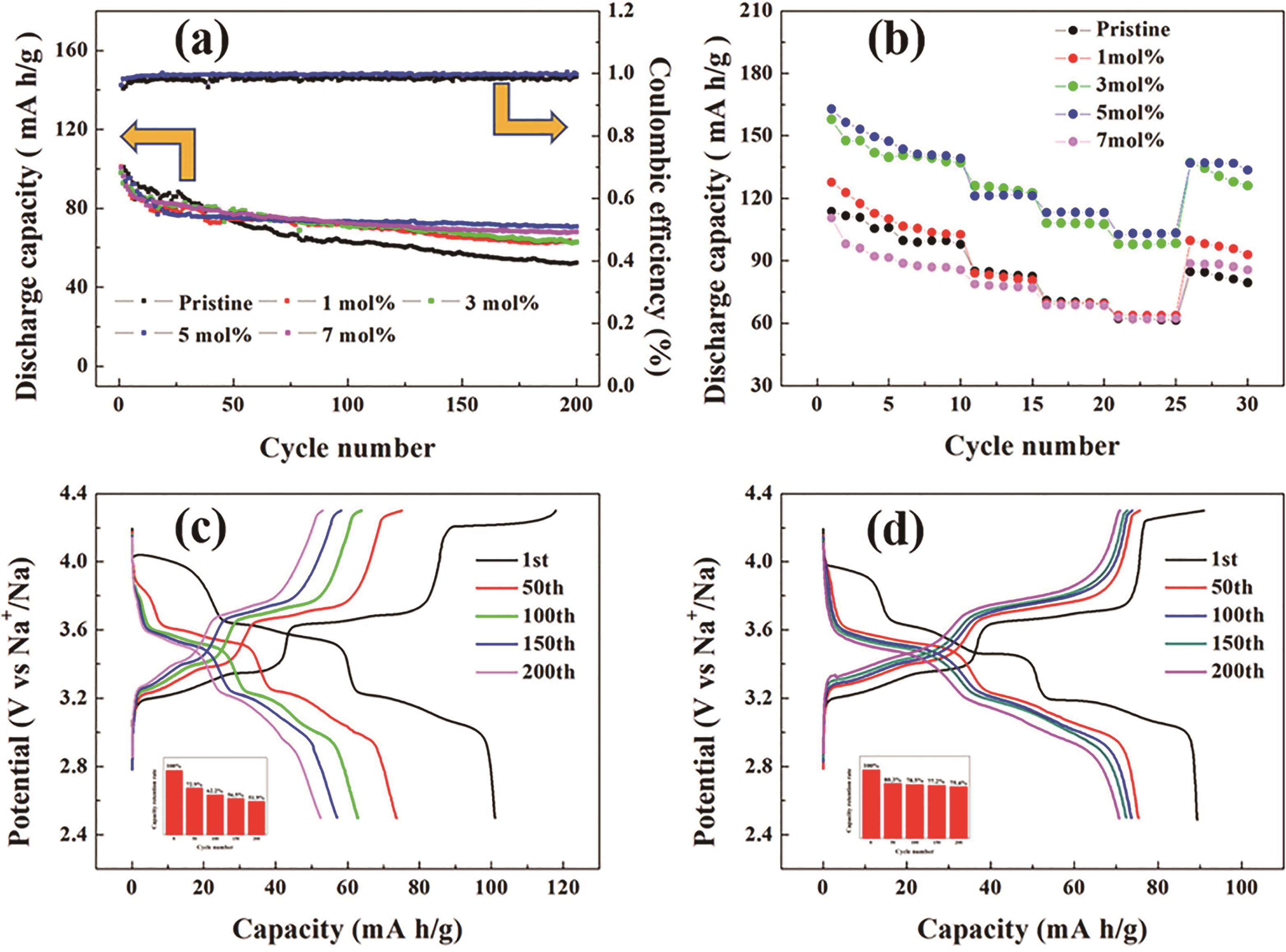
图7 (a) 原始样品和氧化锌包覆样品在0.5 C电流密度下的循环性能;(b) 原始样品和氧化锌包覆(Zn2+与NNMO摩尔比分别为0.01、0.03、0.05和0.07)样品在不同电流密度(0.1、0.2、0.5、1、2和0.1 C)下的倍率性能;(c) 原始样品和(d) 5%氧化锌包覆电极在0.5 C电流密度下的不同循环的比容量与电压曲线[95]
Fig.7 (a) Cycle performances of pristine and ZnO-coated samples at a current density of 0.5 C; (b) Rate performances of pristine and ZnO-coated (molar ratio of Zn2+ and NNMO=0.01, 0.03, 0.05 and 0.07) samples at different current densities (0.1, 0.2, 0.5, 1, 2 and 0.1 C); Specific capacity vs. voltage curves for the (c) pristine and (d) 5% ZnO-coated electrodes at different cycles at a current density of 0.5 C with corresponding capacity retention rate vs. cycle number[95]
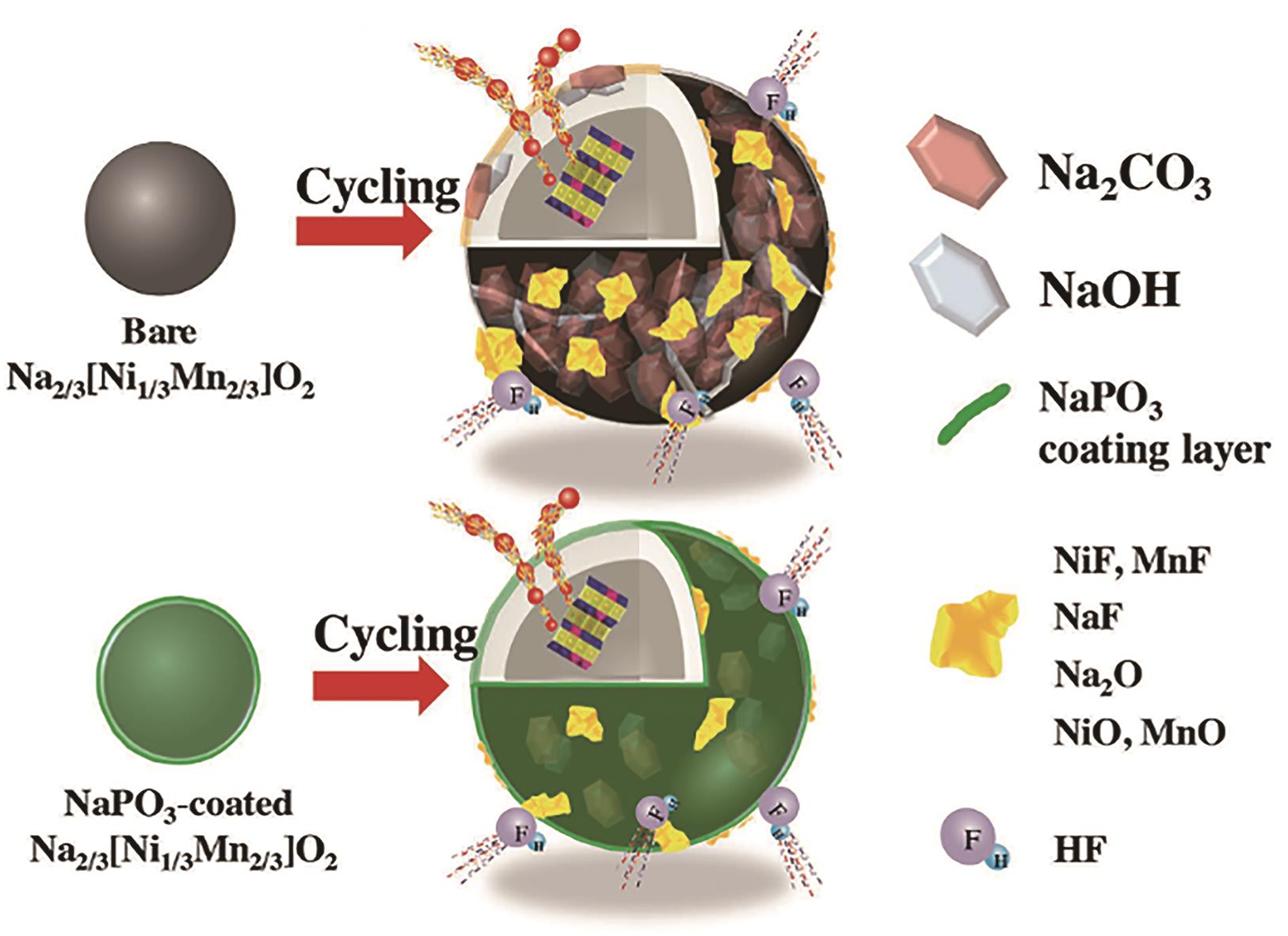
图8 原始和包覆磷酸钠的Na2/3[Ni1/3Mn2/3]O2表面副产物示意图[96]
Fig.8 Schematic illustration of byproducts on the surface of bare and NaPO3-coated Na2/3[Ni1/3Mn2/3]O2[96]
| 1 | WHITTINGHAM M S. Electrical energy storage and intercalation chemistry[J]. Science, 1976, 192(4244): 1126-1127. |
| 2 | TARASCON J M, ARMAND M. Issues and challenges facing rechargeable lithium batteries[J]. Nature, 2001, 414(6861): 359-367. |
| 3 | TIAN Y S, ZENG G B, RUTT A, et al. Promises and challenges of next-generation “beyond Li-ion” batteries for electric vehicles and gird decarbonization[J]. Chem Rev, 2021, 121(3): 1623-1669. |
| 4 | CHEN Y Q, KANG Y Q, ZHAO Y, et al. A review of lithium-ion battery safety concerns: the issues, strategies, and testing standards[J]. J Energy Chem, 2021(59): 83-99. |
| 5 | SUN F, WANG H, QU Z B, et al. Carboxyl-dominant oxygen rich carbon for improved sodium ion storage: synergistic enhancement of adsorption and intercalation mechanisms[J]. Adv Energy Mater, 2021, 11(4): 2002981. |
| 6 | JIN Q Z, LI W, WANG K L, et al. Tailoring 2D heteroatom-doped carbon nanosheets with dominated pseudocapacitive behaviors enabling fast and high-performance sodium storage[J]. Adv Funct Mater, 2020, 30(14): 1909907. |
| 7 | WANG H J, LAN J L, YUAN H C, et al. Chemical grafting-derived N,P Co-doped hollow microporous carbon spheres for high-performance sodium-ion battery anodes[J]. Appl Surface Sci, 2020, 518: 746221. |
| 8 | REN D S, FENG X N, LIU L S, et al. Investigating the relationship between internal short circuit and thermal runaway of lithium-ion batteries under thermal abuse condition[J]. Energy Storage Mater, 2021, 34: 563-573. |
| 9 | KIM D Y, LI O L, KANG J. Novel synthesis of highly phosphorus-doped carbon as an ultrahigh-rate anode for sodium ion batteries[J]. Carbon, 2020(168): 448-457. |
| 10 | YANG L, HU M X, QIAN L, et al. Salt and sugar derived high power carbon microspheres anode with excellent low-potential capacity[J]. Carbon, 2020(163): 288-296 |
| 11 | HU Y S, LU Y X. 2019 Nobel prize for the Li-ion batteries and new opportunities and challenges in Na-ion batteries[J]. ACS Energy Lett, 2019, 4(11): 2689-2690. |
| 12 | YABUUCHI N, KUBOAT K, DAHBI M, et al. Research development on sodium-ion batteries[J]. Chem Rev, 2014, 114(23): 11636-11682. |
| 13 | ALVIN S, CHANDRA C, KIM J. Extended plateau capacity of phosphorus-doped hard carbon used as an anode in Na- and K-ion batteries[J]. Chem Eng J, 2020(391): 123576. |
| 14 | ZHAO C L, LU Y X, CHEN L Q, et al. Ni-based cathode materials for Na-ion batteries[J]. Nano Res, 2019, 12(9): 2018-2030. |
| 15 | MIZUSHIMA K, JONES P C, WISEMAN P J, et al. LixCoO2 (0<x<1): a new cathode material for batteries of high energy density[J]. Mater Res Bull, 1980, 15(6): 783-789. |
| 16 | ZHOU G M, LI F, CHEN H M. Progress in flexible lithium batteries and future prospects[J]. Energy Environ Sci, 2014, 7(4): 1307-1338. |
| 17 | YANG Q F, YAO Z G, LAI C Z, et al. Pre-pulverizing Ni-rich layered oxide cathodes via “liquid explosive” infiltration toward endurable 4.5 V lithium batteries[J]. Energy Storage Mater, 2022(50): 819-828. |
| 18 | LI S, SUN Y P, LI N, et al. Porosity development at Li-rich layered cathodes in all-solid-state battery during in situ delithiation[J]. Nano Lett, 2022, 22(12): 4905-4911. |
| 19 | LEWIS J A, CAVALLARO K A, LIU Y, et al. The promise of alloy anodes for solid-state batteries[J]. Joule, 2022, 6(7): 1418-1430. |
| 20 | STEVENS D A, DAHN J R. High capacityanode materials for rechargeable sodium-ion batteries[J]. J Electrochem Soc, 2000, 147(4): 1271-1273. |
| 21 | CHEN M Z, ZHANG Y Y, XING G C, et al. Building high power density of sodium-ion batteries: importance of multidimensional diffusion pathways in cathode materials[J]. Front Chem, 2020(8): 152. |
| 22 | NAYAK P K, YANG L T, BREHM W, et al. From lithium-ion to sodium-ion batteries: advantages, challenges, and surprises[J]. Angew Chem Int Ed, 2018, 57(1): 102-120. |
| 23 | MUNOZ-MARQUEZ M A, SAUREL D, JUAN L G, et al. Na-ion batteries for large scale applications: areview on anode materials and solid electrolyte interphase formation[J]. Adv Energy Mater, 2017, 7(20): 1700463. |
| 24 | KLAVETTER K C, GARCIA S, DAHAL N, et al. Li- and Na-reduction products of meso-Co3O4 form high-rate, stably cycling battery anode materials[J]. J Mater Chem A, 2014, 2(34): 14209-14221. |
| 25 | SLATER M D, KIM D, LEE E, et al. Sodium-ion batteries[J]. Adv Funct Mater, 2013, 23(8): 947-958. |
| 26 | 杨馨蓉, 车海英, 杨柯, 等. 硬碳负极材料的热稳定性及其钠离子电池安全性能评测[J]. 过程工程学报, 2022, 22(4): 552-560. |
| YANG X R, CHE H Y, YANG K, et al. Evaluation of safety performance and thermal stability of hard carbon anode for sodium-ion battery[J]. Chin J Process Eng, 2022, 22(4): 552-560. | |
| 27 | 杨梦华, 岳丽宏. 基于COMSOL的锂离子电池热失控仿真与防控[J]. 新能源进展, 2022, 10(4): 375- 382. |
| YANG M H, YUE L H. Thermal runaway simulation and prevention and control of lithium-ion batteries based on COMSOL[J]. Adv New Renewable En, 2022, 10(4): 375- 382. | |
| 28 | FANG C, HUANG Y H, ZHANG W X, et al. Routes to high energy cathodes of sodium-ion batteries[J]. Adv Energy Mater, 2016, 6(5): 1501727. |
| 29 | ZHAO C L, YAO Z P, WANG Q D, et al. Revealing high Na-content P2-typed layered oxides as advanced sodium-ion cathodes[J]. J Am Chem Soc, 2020, 142(12): 5742-5750. |
| 30 | KOMABA S, YABUUCHI N, NAKAYAMA T, et al. Study on the reversible electrode reaction of Na1- xNi0.5Mn0.5O2 for a rechargeable sodium-ion battery[J]. Inorg Chem, 2012, 51(11): 6211-6220. |
| 31 | MU L Q, XU S Y, LI Y M, et al. Prototype sodium-ion batteries using an air-stable and Co/Ni-Free O3-layered metal oxide cathode[J]. Adv Mater, 2015, 27(43): 6928-6933. |
| 32 | ZHAO C L, DING F X, LU Y X, et al. High-entropy layered oxide cathodes for sodium-ion batteries[J]. Angew Chem Int Ed, 2020, 59(1): 264-269. |
| 33 | KUBOTA K, YABUUCHI N, YOSHIDA H, et al. Layered oxides as positive electrode materials for Na-ion batteries[J]. Mrs Bull, 2014, 39(5): 416-422. |
| 34 | FENG J, LUO S H, CAI K X, et al. Research progress of tunnel-type sodium manganese oxide cathodes for SIBs[J]. Chin Chem Lett, 2022, 33(5): 2316-2326. |
| 35 | DELMAS C, FOUASSIER C, HAGENMULLER P. Structural classification and properties of the layered oxides[J]. Physica B+C, 1980, 99(1/2/3/4): 81-85. |
| 36 | BERTHELOT R, CARLIER D, DELMAS C. Electrochemical investigation of the P2-NaxCoO2 phase diagram[J]. Nat Mater, 2011, 10(1): 74-80 |
| 37 | ZHAO C L, WANG Q D, YAO Z P, et al. Rational design of layered oxide materials for sodium-ion batteries[J]. Science, 2020, 370(6517): 708. |
| 38 | YABUUCHI N, KOMABA S. Recent research progress on iron- and manganese-based positive electrode materials for rechargeable sodium batteries[J]. Sci Technol Adv Mater, 2014, 15(4): 043501. |
| 39 | XU S Y, WANG Y S, BEN L B, et al. Fe-based tunnel-type Na0.61Mn0.27Fe0.34Ti0.39O2 designed by a new strategy as a cathode material for sodium-ion batteries[J]. Adv Energy Mater, 2015, 5(22): 1501156. |
| 40 | LING J, KARUPPIAH C, KRISHNAN S G, et al. Phosphate polyanion materials as high-voltage lithium-ion battery cathode: a review[J]. Energy Fuels, 2021, 35(13): 10428-10450. |
| 41 | LAN Y Q, YAO W J, HE X L, et al. Mixed polyanionic compounds as positive electrodes for low-cost electrochemical energy storage[J]. Angew Chem Int Ed, 2020, 59(24): 9255-9262. |
| 42 | YANG W, LIU Q, ZHAO Y S, et al. Progress on Fe-based polyanionic oxide cathodes materials toward grid-scale energy storage for sodium-ion batteries[J]. Small Methods, 2022, 6(9): 2200555. |
| 43 | LI H X, XU M, ZHANG Z, et al. Engineering of polyanion type cathode materials for sodium-ion batteries: toward higher energy/power density[J]. Adv Funct Mater, 2020, 30(28): 2000473. |
| 44 | GUO J Z, WANG P F, WU X L, et al. High-energy/power and low-temperature cathode for sodium-ion batteries: in situ XRD study and superior full-cell performance[J]. Adv Mater, 2017, 29(33): 1701968. |
| 45 | WANG L, SONG J, QIAO R M, et al. Rhombohedral prussianwhite as cathode for rechargeable sodium-ion batteries[J]. J Am Chem Soc, 2015, 137(7): 2548-2554. |
| 46 | ZHAO C L, YAO Z P, ZHOU D, et al. Constructing Na-ion cathodes via alkali-site substitution[J]. Adv Funct Mater, 2020, 30(17): 1910840. |
| 47 | QIAN J F, WU C, CAO Y L, et al. Prussian blue cathode materials for sodium-ion batteries and other ion batteries[J]. Adv Energy Mater, 2018, 8(17): 1702619. |
| 48 | YOU Y, YAO H R, XIN S, et al. Subzero-temperature cathode for a sodium-ion battery[J]. Adv Mater, 2016, 28(33): 7243. |
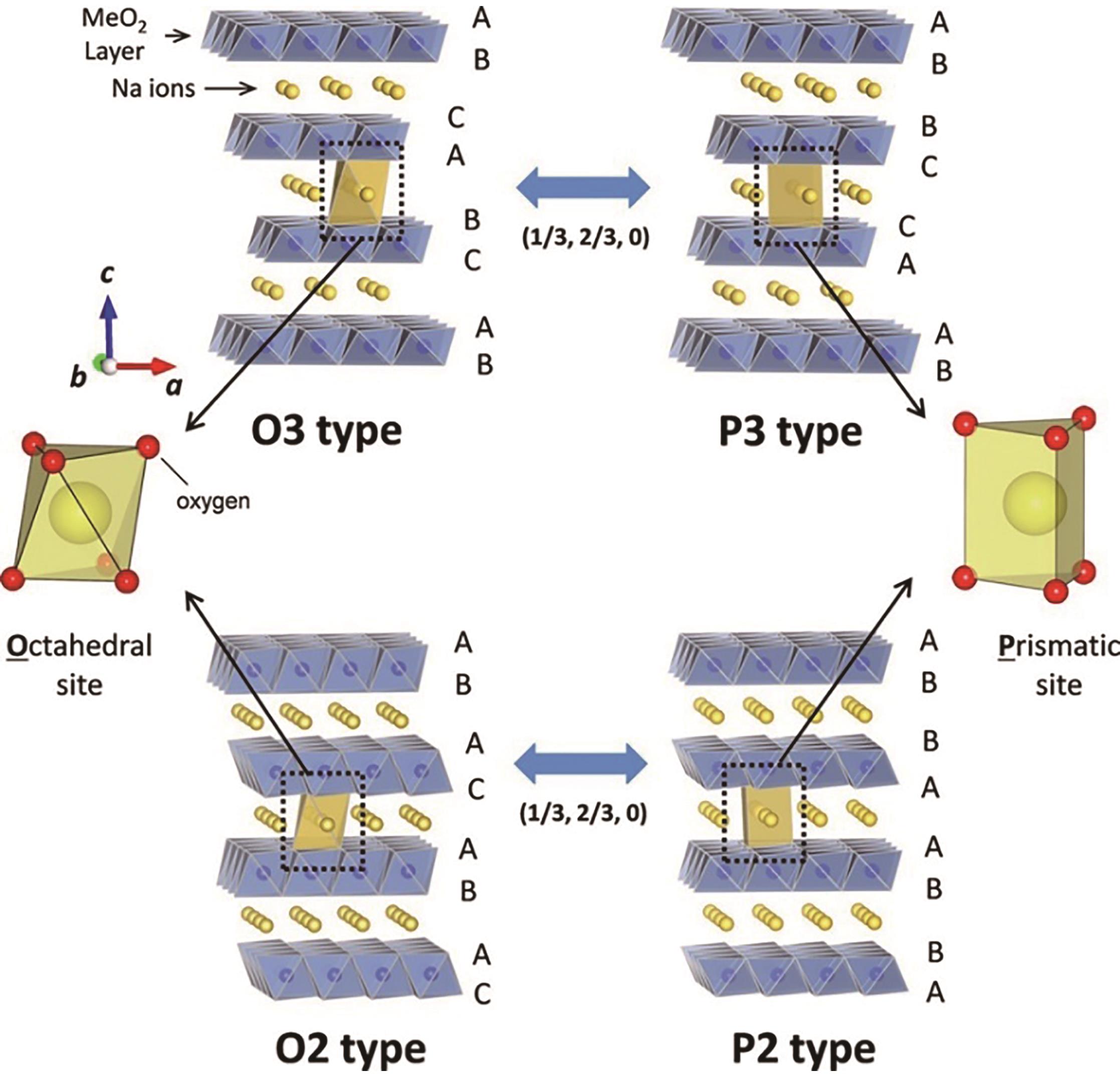
| 49 | SUN Y, GUO S H, ZHOU H S. Adverse effects of interlayer-gliding in layered transition-metal oxides on electrochemical sodium-ion storage[J]. Energ Environ Sci, 2019, 12(3): 825-840. |
| 50 | YABUUCHI N, YANO M, YOSHIDA H, et al. Synthesis and electrode performance of O3-type NaFeO2-NaNi1/2Mn1/2O2 solid solution for rechargeable sodium batteries[J]. J Electrochem Soc, 2013, 160(5): A3131-A3137. |
| 51 | SATHIYA M, HEMALATHA K, RAMESHA K, et al. Synthesis, structure, and electrochemical properties of the layered sodium insertion cathode material: NaNi1/3Mn1/3Co1/3O2[J]. Chem Mater, 2012, 24(10): 1846-1853. |
| 52 | WANG X F, TAMARU M, OKUBO M, et al. Electrode properties of P2-Na2/3MnyCo1- yO2 as cathode materials for sodium-ion batteries[J]. J Phys Chem C, 2013, 117(30): 15545-15551. |
| 53 | SHI Y S, LI S, GAO A, et al. Probing the structural transition kinetics and charge compensation of the P2-Na0.78Al0.05Ni0.33Mn0.60O2 cathode for sodium ion batteries[J]. ACS Appl Mater Inter, 2019, 11(27): 24122-24131. |
| 54 | YABUUCHI N, KAJIYAMA M, IWATATE J, et al. P2-type NaxFe1/2Mn1/2O2 made from earth-abundant elements for rechargeable Na batteries[J]. Nat Mater, 2012, 11(6): 512-517. |
| 55 | LIU Z B, SHEN J D, FENG S H, et al. Ultralow volume change of P2-type layered oxide cathode for Na-ion batteries with controlled phase transition by regulating distribution of Na+[J]. Angew Chem Int Ed, 2021, 60(38): 20960-20969. |
| 56 | CHEN Z, BALAVHANDRAN R, LEK-HENG C, et al. Effects of transition-metal mixing on Na ordering and kinetics in layered P2 oxides[J]. Phys Rev Appl, 2017, 7(6): 064003. |
| 57 | LI X, MA H, SU D, et al. Direct visualization of the jahn-teller effect coupled to Na ordering in Na5/8MnO2[J]. Nat Mater, 2014, 13(6): 586-592. |
| 58 | DE BOISSE B M, LIU G D, MA J T, et al. Intermediate honeycomb ordering to trigger oxygen redox chemistry in layered battery electrode[J]. Nat Commun, 2016(7): 11397. |
| 59 | CHEN J. Na+/vacancy disordering promises high-rate Na-ion batteries[J]. Acta Phys-Chim Sin, 2019, 35(4): 347-348. |
| 60 | MU L Q, FENG X, KOU R H, et al. Deciphering the cathode-electrolyte interfacial chemistry in sodium layered cathode materials[J]. Adv Energy Mater, 2018, 8(34): 1801975. |
| 61 | ZUO W H, LIU X S, QIU J M, et al. Engineering Na+-layer spacings to stabilize Mn-based layered cathodes for sodium-ion batteries[J]. Nat Commun, 2021, 12(1): 4903. |
| 62 | WANG P F, YOU Y, YIN Y X, et al. Suppressing the P2-O2 phase transition of Na0.67Mn0.67Ni0.33O2 by magnesium substitution for improved sodium-ion batteries[J]. Angew Chem Int Ed, 2016, 55(26): 7445-7449. |
| 63 | WANG K, WAN H, YAN P F, et al. Dopant segregation boosting high-voltage cyclability of layered cathode for sodium ion batteries[J]. Adv Mater, 2019, 31(46): 1904816. |
| 64 | CHEN C, DING M L, YAN T R, et al. Anionic redox activities boosted by aluminum doping in layered sodium-ton battery electrode[J]. Small Methods, 2022, 6(3): 2101524. |
| 65 | SATO T, YOSHIKAWA K, ZHAO W, et al. Efficient stabilization of Na storage reversibility by Ti integration into O3-type NaMnO2[J]. Energy Mater Adv, 2021(1): 143-154. |
| 66 | SHEN Q Y, LIU Y C, ZHAO D, et al. Transition-metal vacancy manufacturing and sodium-site doping enable a high-performance layered oxide cathode through cationic and anionic redox chemistry[J]. Adv Funct Mater, 2021, 31(51): 2106923. |
| 67 | PENG B, CHEN Y X, WANG F, et al. Unusualsite-selective doping in layered cathode strengthens electrostatic cohesion of alkali-metal layer for practicable sodium-ion full cell[J]. Adv Mater, 2022, 34(6): 2103210. |
| 68 | FY H W, WANG Y P, FAN G Z, et al. Synergetic stability enhancement with magnesium and calcium ion substitution for Ni/Mn-based P2-type sodium-ion battery cathodes[J]. Chem Sci, 2022, 13(3): 726-736. |
| 69 | WALCZAK K, PLEWA A, GHICA C, et al. NaMn0.2Fe0.2Co0.2Ni0.2Ti0.2O2 high-entropy layered oxideexperimental and theoretical evidence of high electrochemical performance in sodium batteries[J]. Energy Storage Mater, 2022(47): 500-514. |
| 70 | SARKAR A, VELASCO L, WANG D, et al. High entropy oxides for reversible energy storage[J]. Nat Commun, 2018, 9(1): 3400 |
| 71 | YAN S X, LUO S H, YANG L, et al. Novel P2-type layered medium-entropy ceramics oxide as cathode material for sodium-ion batteries[J]. J Adv Ceram, 2022, 11(1): 158-171. |
| 72 | WALCZAK K, PLEWA A, GHICA C, et al. NaMn0.2Fe0.2Co0.2Ni0.2Ti0.2O2 high-entropy layered oxide-experimental and theoretical evidence of high electrochemical performance in sodium batteries[J]. Energy Storage Mater, 2022(47): 500-514. |
| 73 | RONG X H, HU E Y, LU Y X, et al. Anionic redox reaction-induced high-capacity and low-strain cathode with suppressed phase transition[J]. Joule, 2019, 3(2): 503-517. |
| 74 | LI Z Y, GAO R, ZHANG J C, et al. New insights into designing high-rate performance cathode materials for sodium ion batteries by enlarging the slab-spacing of the Na-ion diffusion layer[J]. J Mater Chem A, 2016, 4(9): 3453-3461. |
| 75 | SU J C, PEI Y, YANG Z H, et al. First-principles investigation on the structural, electronic properties and diffusion barriers of Mg/Al doped NaCoO2 as the cathode material of rechargeable sodium batteries[J]. RSC Adv, 2015, 5(25): 27229-27234. |
| 76 | HAN S C, LIM H, JEONG J, et al. Ca-doped NaxCoO2 for improved cyclability in sodium ion batteries[J]. J Power Sources, 2015(277): 9-16. |
| 77 | YU H J, REN Y, XIAO D D, et al. An ultrastable anode for long-life room-temperature sodium-ion batteries[J]. Angew Chem Int Ed, 2014, 53(34): 8963-8969. |
| 78 | HAMANI D, ATI M, TARASCOM J M, et al. NaxVO2 as possible electrode for Na-ion batteries[J], Electrochem Commun, 2011, 13(9): 938-941. |
| 79 | BRACONNIER J J, DELMAS C, HAGENMULLER P. Etude par desintercalation electrochimique des systemes NaxCrO2 et NaxNiO2[J]. Mater Res Bull, 1982, 17(8): 993-1000. |
| 80 | BILLAUD J, CHEMENT R J, ARMSTRONG A R, et al. β-NaMnO2: a high-performance cathode for sodium-ion batteries[J]. J Am Chem Soc, 2014, 136(49): 17243-17248. |
| 81 | KIKKAWA S, MIYAZAKI S, KOIZUMI M. Deintercalated NaCoO2 and LiCoO2[J], J Soild State Chem, 1986, 62(1): 35-39. |
| 82 | HAN M H, GONZALO E, CASAS-CABANAS M, et al. Structural evolution and electrochemistry of monoclinic NaNiO2 upon the first cycling process[J]. J Power Sources, 2014(258): 266-271. |
| 83 | AGUESSE F, DEL AMO J M L, OTAEGUI L, et al. Structural and electrochemical analysis of Zn doped Na3Ni2SbO6 cathode for Na-ion battery[J]. J Power Sources, 2016(336): 186-195. |
| 84 | MU L Q, XU S Y, LI Y M, et al. Prototype sodium-ion batteries using an air-stable and Co/Ni-free O3-layered metal oxide cathode[J]. Adv Mater, 2015, 27(43): 6928. |
| 85 | BAI X, SATHIYA M, MENDOZA-SENCHEZ B, et al. Anionic redox activity in a newly Zn-doped sodium layered oxide P2-Na2/3Mn1- yZnyO2 (0<y<0.23)[J]. Adv Energy Mater, 2018, 8(32): 1802379. |
| 86 | SATHIYA M, JACQUET Q, DOUBLET M L, et al. A chemical approach to raise cell voltage and suppress phase transition in O3 sodium layered oxide electrodes[J]. Adv Energy Mater, 2018, 8(11): 1702599. |
| 87 | YUAN D D, LIANG X M, WU L, et al. A Honeycomb-layered Na3Ni2SbO6: a high-rate and cycle-stable cathode for sodium-ion batteries[J]. Adv Mater, 2014, 26(36): 6301-6306. |
| 88 | LU J L, CAO B, HU B W, et al. Heavy fluorination via ion exchange achieves high-performance Li-Mn-O-F layered cathode for Li-ion batteries[J]. Small, 2022, 18(6): 2103499. |
| 89 | ZHAO C, YANG Q, GENG F, et al. Restraining oxygen loss and boosting reversible oxygen redox in a P2-type oxide cathode by trace anion substitution[J]. ACS Appl Mater Interfaces, 2021, 13(1): 360. |
| 90 | GUO Y J, WANG P F, NIU Y B, et al. Boron-doped sodium layered oxide for reversible oxygen redox reaction in Na-ion battery cathodes[J]. Nat Commun, 2021(12): 5276. |
| 91 | BIANCHINI M, GONZALO E, DREWETT N E, et al. Layered P2-O3 sodium-ion cathodes derived from earth abundant elements[J]. J Mater Chem A, 2018, 6(8): 3552-3559. |
| 92 | ZHOU Y N, WANG P F, NIU Y B, et al. A P2/P3 composite layered cathode for high-performance Na-ion full batteries[J]. Nano Energy, 2019(55): 143-150. |
| 93 | JO C H, JO J H, YASHIRO H, et al. Bioinspired surface layer for the cathode material of high-energy-density sodium-ion batteries[J]. Adv Energy Mater, 2018, 8(13): 1702942. |
| 94 | JIANG Y, YANG Z Z, LI W H, et al. Nanoconfined carbon-coated Na3V2(PO4)3 particles in mesoporous carbon enabling ultralong cycle life for sodium-ion batteries[J]. Adv Energy Mater, 2015, 5(10): 1402104. |
| 95 | YANG Y Q, DANG R B, WU K, et al. Semiconductor material ZnO-coated P2-type Na2/3Ni1/3Mn2/3O2 cathode materials for sodium-ion batteries with superior electrochemical performance[J]. J Phys Chem C, 2020, 124(3): 1780-1787. |
| 96 | JO J H, CHOI J U, KONAROV A, et al. Sodium-ion batteries: building effective layered cathode materials with long-term cycling by modifying the surface via sodium phosphate[J]. Adv Funct Mater, 2018, 28(14): 1705968. |
| 97 | ZHAO L N, ZHAO H L, WANG J, et al. Micro/nano Na3V2(PO4)3/N-doped carbon composites with a hierarchical porous structure for high-rate pouch-type sodium-ion full-cell performance[J]. ACS Appl Mater Interfaces, 2021, 13(7): 8445-8454. |
| 98 | PENG M H, ZHANG D T, ZHENG L M, et al. Hierarchical Ru-doped sodium vanadium fluorophosphates hollow microspheres as a cathode of enhanced superior rate capability and ultralong stability for sodium-ion batteries[J]. Nano Energy, 2017(31): 64-73. |
| 99 | MAO Q J, GAO R, LI Q Y, et al. O3-type NaNi0.5Mn0.5O2 hollow microbars with exposed {010} facets as highperformance cathode materials for sodium-ion batteries[J]. Chem Eng J, 2020(382): 122978. |
| 100 | LIU Y C, SHEN Q Y, ZHAO X D, et al. Hierarchical engineering of porous P2-Na2/3Ni1/3Mn2/3O2 nanofibers assembled by nanoparticles enables superior sodium-ion storage cathodes[J]. Adv Funct Mater, 2019, 30(6): 1907837. |
| 101 | LI Y M, YANG Z Z, XU S Y, et al. Air-stable copper-based P2-Na7/9Cu2/9Fe1/9Mn2/3O2 as a new positive electrode material for sodium-ion batteries[J]. Adv Sci, 2015, 2(6): 1500031. |
| 102 | ZHANG Z L, LIU M, XIE Y Y, et al. Superstructurednanocrystals/dual-doped mesoporous carbon anodes for high-performance sodium-ion batteries[J]. Inorg Chem, 2022, 61(3): 8887-8897. |
| [1] | 王恩通, 杨林芳. 高比容量锂离子电池正极材料LiNi0.6Co0.2Mn0.2O2的制备及性能[J]. 应用化学, 2022, 39(8): 1209-1215. |
| [2] | 赵莹, 邵奕嘉, 李罗钱, 任建伟, 廖世军. 富锂正极材料的衰减机理及循环稳定性提升的研究进展[J]. 应用化学, 2022, 39(02): 205-222. |
| [3] | 张和,张梦诗,廖世军. 富锂三元层状正极材料的研究进展[J]. 应用化学, 2018, 35(11): 1277-1288. |
| [4] | 陈丽辉,吴秋晗,潘佩,宋子轩,王锋,丁瑜. 尖晶石型八面体结构锰酸锂的制备及其电化学性能[J]. 应用化学, 2018, 35(11): 1384-1390. |
| [5] | 杜鑫川, 黄岗, 王立民. 五氧化二钒空心球制备及其作为镁二次电池正极材料[J]. 应用化学, 2015, 32(12): 1462-1464. |
| [6] | 杜鑫川, 黄岗, 王立民. 五氧化二钒空心球制备及其作为镁二次电池正极材料[J]. 应用化学, 2015, 32(12): 0-0. |
| [7] | 贺冬华, 唐安平, 申洁, 徐国荣, 刘立华, 令玉林. 锂离子电池电极材料磷酸氧钒锂的研究进展[J]. 应用化学, 2014, 31(10): 1115-1122. |
| [8] | 刘建本, 莫如宝, 吴显明. 锂离子正极材料Li(Ni1/3Co1/3Mn1/3)0.95Al0.05O2的制备及电化学性能[J]. 应用化学, 2014, 31(04): 462-468. |
| [9] | 王洪, 张伟德. 富锂正极材料Li[Li0.2Mn0.4Fe0.4]O2的表面包覆改性[J]. 应用化学, 2013, 30(06): 705-709. |
| [10] | 唐安平, 刘立华, 徐国荣, 申洁, 令玉林. 锂离子电池硼酸盐电极材料的研究进展[J]. 应用化学, 2012, 29(11): 1221-1230. |
| [11] | 王洪, 杨驰, 郭春泰, 王大兴, 孙礼安. 表面包覆保护的新型正极材料LiFeBO3电化学性能[J]. 应用化学, 2011, 28(07): 821-825. |
| [12] | 张静温, 张蕾蕾, 吴超勇, 张新波, 王立民. 新方法合成LiFePO4/C[J]. 应用化学, 2010, 27(11): 1356-1358. |
| [13] | 粟智,刘丛,徐茂文. 单斜层状结构锂离子电池正极材料LiYxMn1-xO2的合成及电化学性能[J]. 应用化学, 2010, 27(02): 220-226. |
| [14] | 杨辉, 李娟. 锂离子电池纳米结构正极材料LiV3O8的制备及性能[J]. 应用化学, 2009, 26(08): 989-992. |
| [15] | 丁春桃, 莫祥银, 王克宇, 沈健. 电池级Co3O4的制备与表征[J]. 应用化学, 2008, 25(5): 543-547. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||