应用化学 ›› 2022, Vol. 39 ›› Issue (9): 1437-1446.DOI: 10.19894/j.issn.1000-0518.210544
硫酸铵母液除氯及其对结晶影响
自高勇1,2, 黄帮福1,2( ), 代蒙1,2, 杨征宇1,2, 文桢晶1,2, 李婉君1,2, 罗柳宾1,2
), 代蒙1,2, 杨征宇1,2, 文桢晶1,2, 李婉君1,2, 罗柳宾1,2
- 1.昆明理工大学冶金与能源工程学院,昆明 650093
2.云南省高校复杂铁资源清洁冶金重点实验室,昆明 650093
-
收稿日期:2021-11-23接受日期:2022-02-28出版日期:2022-09-01发布日期:2022-09-08 -
通讯作者:黄帮福 -
基金资助:云南省应用基础研究计划面上项目(202001AT070029);钢铁冶金及资源利用省部共建教育部重点实验室开放基金(FMRUlab-20-4)
Effect of Chlorine Removal on Crystallization in the Mother Liquor of Ammonium Sulfate
Gao-Yong ZI1,2, Bang-Fu HUANG1,2( ), Meng DAI1,2, Zheng-Yu YANG1,2, Zhen-Jing WEN1,2, Wan-Jun LI1,2, Liu-Bin LUO1,2
), Meng DAI1,2, Zheng-Yu YANG1,2, Zhen-Jing WEN1,2, Wan-Jun LI1,2, Liu-Bin LUO1,2
- 1.Faculty of Metallurgical and Energy Engineering,Kunming University of Science and Technology,Kunming 650093,China
2.Clean Metallurgy Key Laboratory of Complex Iron Resources,University of Yunnan Province,Kunming 650093,China
-
Received:2021-11-23Accepted:2022-02-28Published:2022-09-01Online:2022-09-08 -
Contact:Bang-Fu HUANG -
About author:kmusthbf@163.com
-
Supported by:the General Project of Applied Basic Research Program of Yunnan Province(202001AT070029);the Open Foundation of Key Laboratory of Iron and Steel Metallurgy and Resource Utilization of Ministry of Education(FMRUlab?20?4)
摘要:
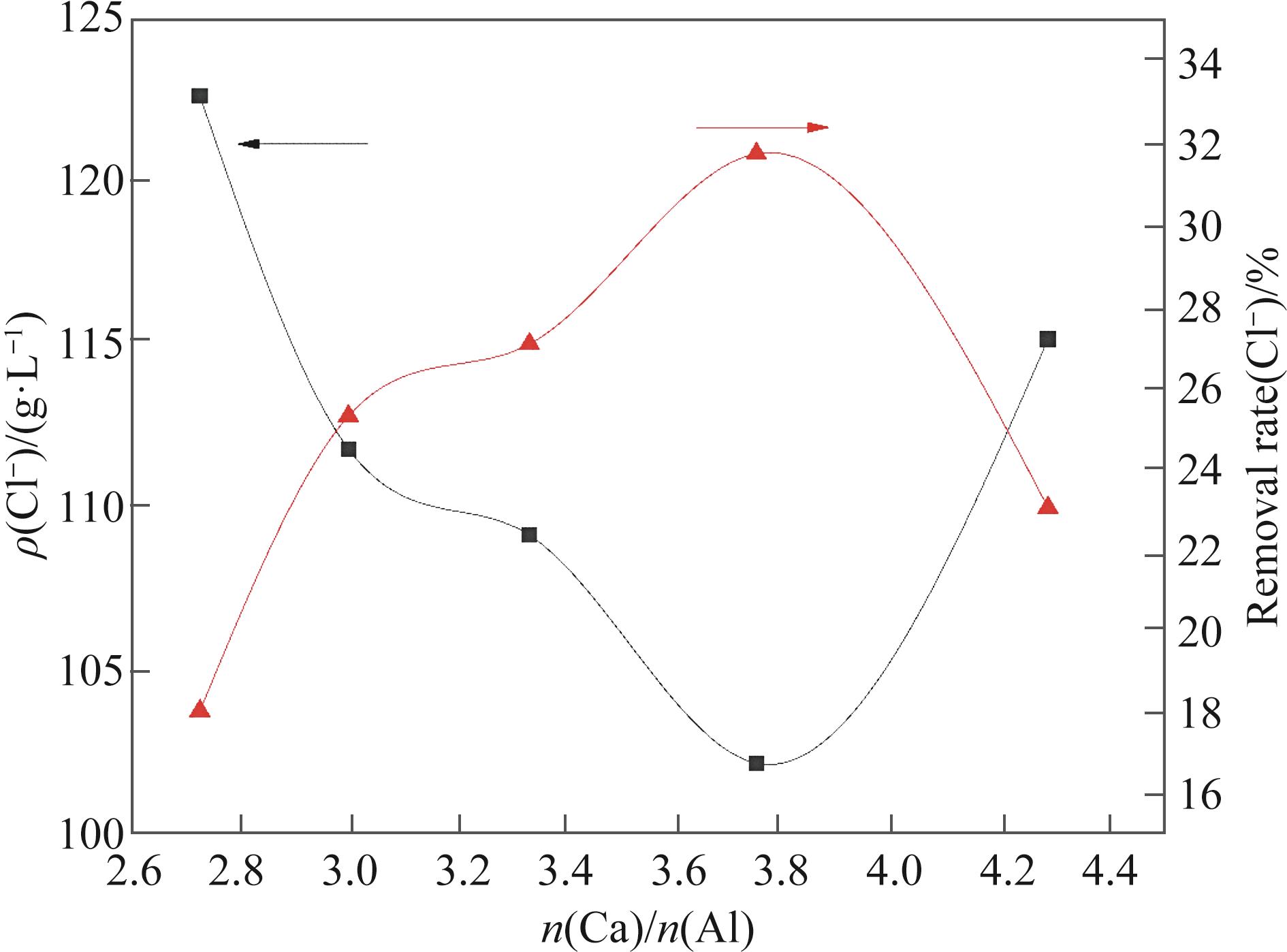
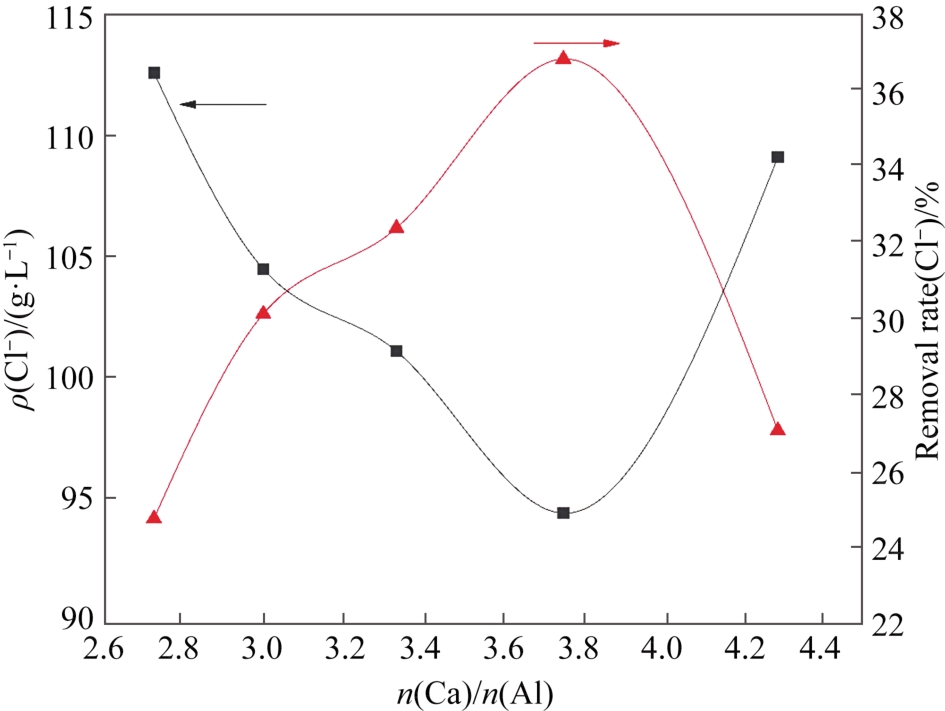
硫酸铵母液中Cl-不断循环富集导致设备腐蚀严重,同时影响硫酸铵结晶及品质。本文运用硫酸钙铝法和脱硫灰铝法分别对硫酸铵母液进行除氯研究,采用筛分法对晶体粒径进行分析、扫描电子显微镜(Scanning Electron Microscope, SEM)对晶体尺寸、形貌进行表征,X射线衍射仪(X-ray Diffractometer,XRD)分析晶体物相。研究表明:除氯剂最佳投加量为3.0 g硫酸钙和0.8 g偏铝酸钠,3.0 g脱硫灰和0.8 g偏铝酸钠,对应除氯率分别为31.70%和36.38%。在转速200 r/min,反应温度为75 ℃,两种除氯剂加入会使ρ(Cl-)快速下降,此为Cl-与Ca2+和AlO2-反应形成了不溶钙铝氯化合物;除氯剂加入过量会使NaAlO2发生双水解,解离出OH-,抑制Cl-与Ca2+、AlO2-反应,导致Cl-去除率下降。硫酸钙铝法所产生的钙铝氯化合物会附着在晶粒活性表面进而增大硫酸铵结晶介稳区宽度,抑制晶体正常生长,导致结晶量减少;脱硫灰铝法中杂质金属可将OH-消耗和减小硫酸铵结晶介稳区宽度,所含大量SO42-会使硫酸铵结晶量增加,但晶体纯度降低。相关研究结果可为减少氨法脱硫设备腐蚀及优化硫酸铵结晶提供参考。
中图分类号:
引用本文
自高勇, 黄帮福, 代蒙, 杨征宇, 文桢晶, 李婉君, 罗柳宾. 硫酸铵母液除氯及其对结晶影响[J]. 应用化学, 2022, 39(9): 1437-1446.
Gao-Yong ZI, Bang-Fu HUANG, Meng DAI, Zheng-Yu YANG, Zhen-Jing WEN, Wan-Jun LI, Liu-Bin LUO. Effect of Chlorine Removal on Crystallization in the Mother Liquor of Ammonium Sulfate[J]. Chinese Journal of Applied Chemistry, 2022, 39(9): 1437-1446.
序号 Num | 除氯剂 Dechlorination agent | 结晶量 Crystallization amount/g | 平均粒径 The average particle size/mm | C.V.值 C.V.value |
|---|---|---|---|---|
| 1 | —— | 138.600 | 1.151 | 509.247 |
| 2 | 3.0 g CaSO4+0.7 g NaAlO2 | 58.652 | 1.634 | 720.521 |
| 3 | 3.0 g CaSO4+0.8 g NaAlO2 | 54.311 | 1.602 | 768.410 |
| 4 | 3.0 g CaSO4+0.9 g NaAlO2 | 51.003 | 1.179 | 524.256 |
| 5 | 3.0 g CaSO4+1.0 g NaAlO2 | 48.011 | 1.568 | 685.040 |
| 6 | 3.0 g CaSO4+1.1 g NaAlO2 | 45.554 | 1.816 | 647.973 |
表1 硫酸钙铝法除氯对硫酸铵晶体结晶量、平均粒径和C.V.值影响
Table 1 Effect of dechlorination by calcium sulfate aluminum method on the crystalline amount, average particle size and C.V. value of ammonium sulfate crystals
序号 Num | 除氯剂 Dechlorination agent | 结晶量 Crystallization amount/g | 平均粒径 The average particle size/mm | C.V.值 C.V.value |
|---|---|---|---|---|
| 1 | —— | 138.600 | 1.151 | 509.247 |
| 2 | 3.0 g CaSO4+0.7 g NaAlO2 | 58.652 | 1.634 | 720.521 |
| 3 | 3.0 g CaSO4+0.8 g NaAlO2 | 54.311 | 1.602 | 768.410 |
| 4 | 3.0 g CaSO4+0.9 g NaAlO2 | 51.003 | 1.179 | 524.256 |
| 5 | 3.0 g CaSO4+1.0 g NaAlO2 | 48.011 | 1.568 | 685.040 |
| 6 | 3.0 g CaSO4+1.1 g NaAlO2 | 45.554 | 1.816 | 647.973 |
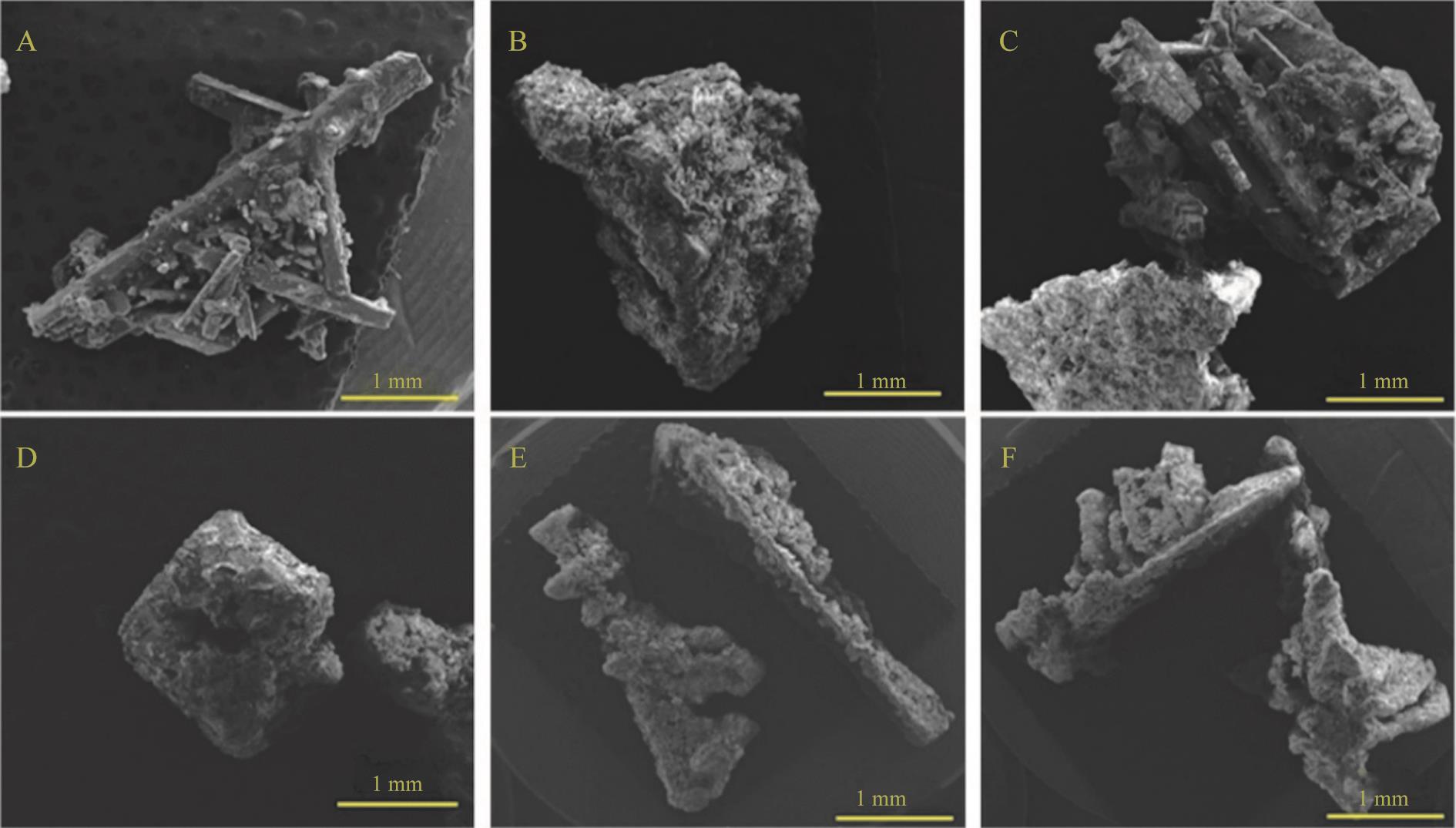
图2 硫酸钙铝法除氯对硫酸铵晶体形貌影响(A) 原液; (B) 3.0 g CaSO4+0.7 g NaAlO2;(C) 3.0 g CaSO4+0.8 g NaAlO2; (D) 3.0 g CaSO4+0.9 g NaAlO2; (E) 3.0 g CaSO4+1.0 g NaAlO2; (F) 3.0 g CaSO4+1.1 g NaAlO2
Fig.2 Effect of dechlorination by calcium sulfate aluminum on the morphology of ammonium sulfate crystals(A) Stock solution; (B) 3.0 g CaSO4+0.7 g NaAlO2; (C) 3.0 g CaSO4+0.8 g NaAlO2; (D) 3.0 g CaSO4+0.9 g NaAlO2; (E) 3.0 g CaSO4+1.0 g NaAlO2; (F) 3.0 g CaSO4+1.1 g NaAlO2
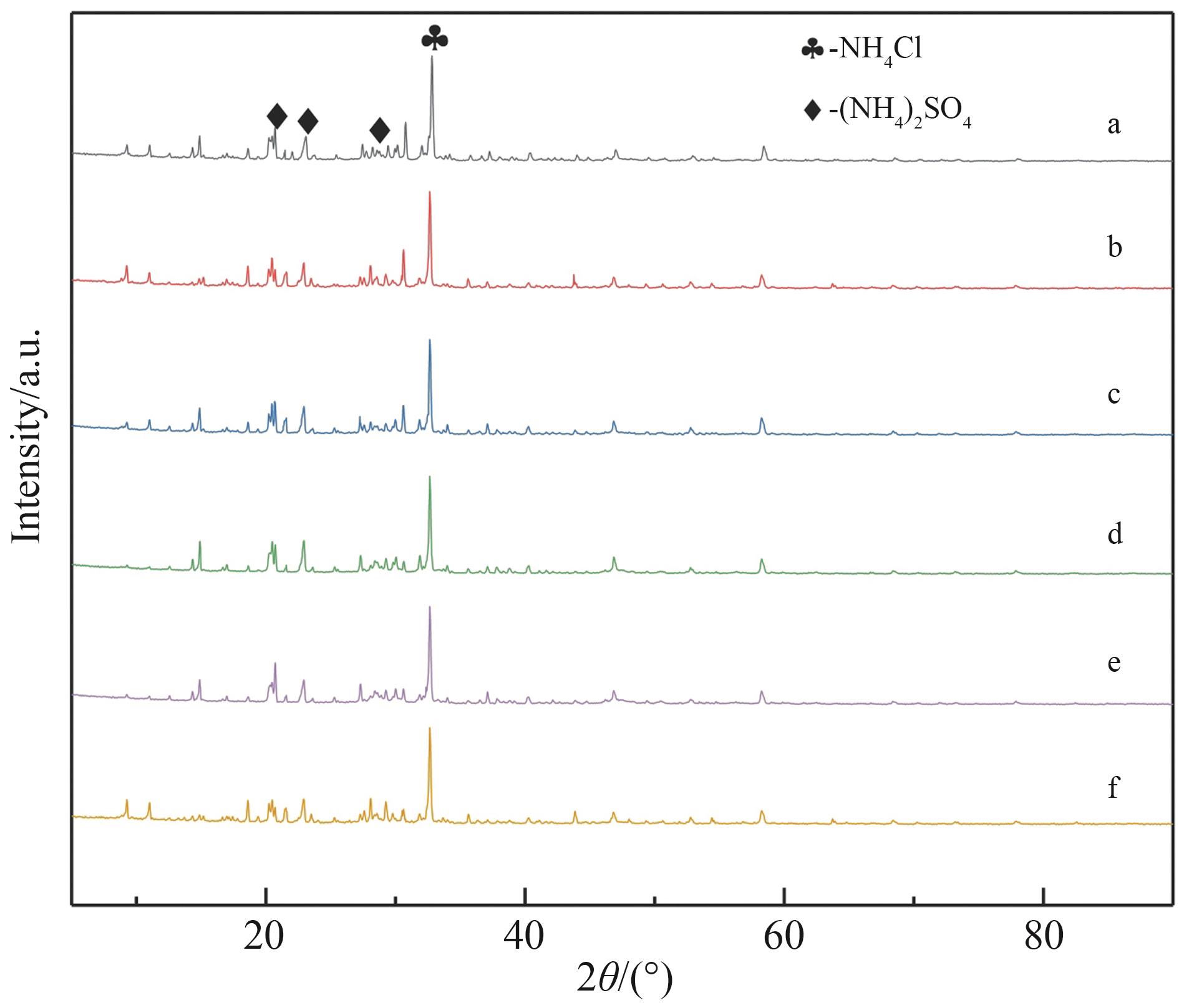
图3 硫酸钙铝法除氯后硫酸铵晶体的XRD衍射图谱a. 原液; b. 3.0 g CaSO4+0.7 g NaAlO2; c. 3.0 g CaSO4+0.8 g NaAlO2; d. 3.0 g CaSO4+0.9 g NaAlO2; e. 3.0 g CaSO4+1.0 g NaAlO2; f. 3.0 g CaSO4+1.1 g NaAlO2
Fig.3 XRD diffraction pattern of ammonium sulfate crystals after dechlorination by calcium sulfate aluminum methoda. Stock solution; b. 3.0 g CaSO4+0.7 g NaAlO2; c. 3.0 g CaSO4+0.8 g NaAlO2; d. 3.0 g CaSO4+0.9 g NaAlO2; e. 3.0 g CaSO4+1.0 g NaAlO2; f. 3.0 g CaSO4+1.1 g NaAlO2
序号 Num | 除氯剂 Dechlorination agent | 结晶量 Crystallization amount/g | 平均粒径 The average particle size/mm | C.V.值 C.V.value |
|---|---|---|---|---|
| 1 | —— | 138.600 | 1.151 | 509.247 |
| 2 | 3.0 g脱硫灰+0.7 g NaAlO2 3.0 g Desulfurization ash +0.7 g NaAlO2 | 149.840 | 1.150 | 407.790 |
| 3 | 3.0 g脱硫灰+0.8 g NaAlO2 3.0 g Desulfurization ash+0.8 g NaAlO2 | 145.614 | 1.112 | 427.378 |
| 4 | 3.0 g脱硫灰+0.9 g NaAlO2 3.0 g Desulfurization ash+0.9 g NaAlO2 | 163.662 | 1.091 | 397.555 |
| 5 | 3.0 g脱硫灰+1.0 g NaAlO2 3.0 g Desulfurization ash+1.0 g NaAlO2 | 153.138 | 1.063 | 382.120 |
| 6 | 3.0 g脱硫灰+1.1 g NaAlO2 3.0 g Desulfurization ash+1.1 g NaAlO2 | 141.383 | 1.092 | 403.825 |
表2 脱硫灰铝法除氯对硫酸铵晶体结晶量、平均粒径和C.V.值影响
Table 2 Effect of dechlorination by desulfurization ash aluminum method on the crystallization amount, average particle size and C.V. value of ammonium sulfate crystals
序号 Num | 除氯剂 Dechlorination agent | 结晶量 Crystallization amount/g | 平均粒径 The average particle size/mm | C.V.值 C.V.value |
|---|---|---|---|---|
| 1 | —— | 138.600 | 1.151 | 509.247 |
| 2 | 3.0 g脱硫灰+0.7 g NaAlO2 3.0 g Desulfurization ash +0.7 g NaAlO2 | 149.840 | 1.150 | 407.790 |
| 3 | 3.0 g脱硫灰+0.8 g NaAlO2 3.0 g Desulfurization ash+0.8 g NaAlO2 | 145.614 | 1.112 | 427.378 |
| 4 | 3.0 g脱硫灰+0.9 g NaAlO2 3.0 g Desulfurization ash+0.9 g NaAlO2 | 163.662 | 1.091 | 397.555 |
| 5 | 3.0 g脱硫灰+1.0 g NaAlO2 3.0 g Desulfurization ash+1.0 g NaAlO2 | 153.138 | 1.063 | 382.120 |
| 6 | 3.0 g脱硫灰+1.1 g NaAlO2 3.0 g Desulfurization ash+1.1 g NaAlO2 | 141.383 | 1.092 | 403.825 |
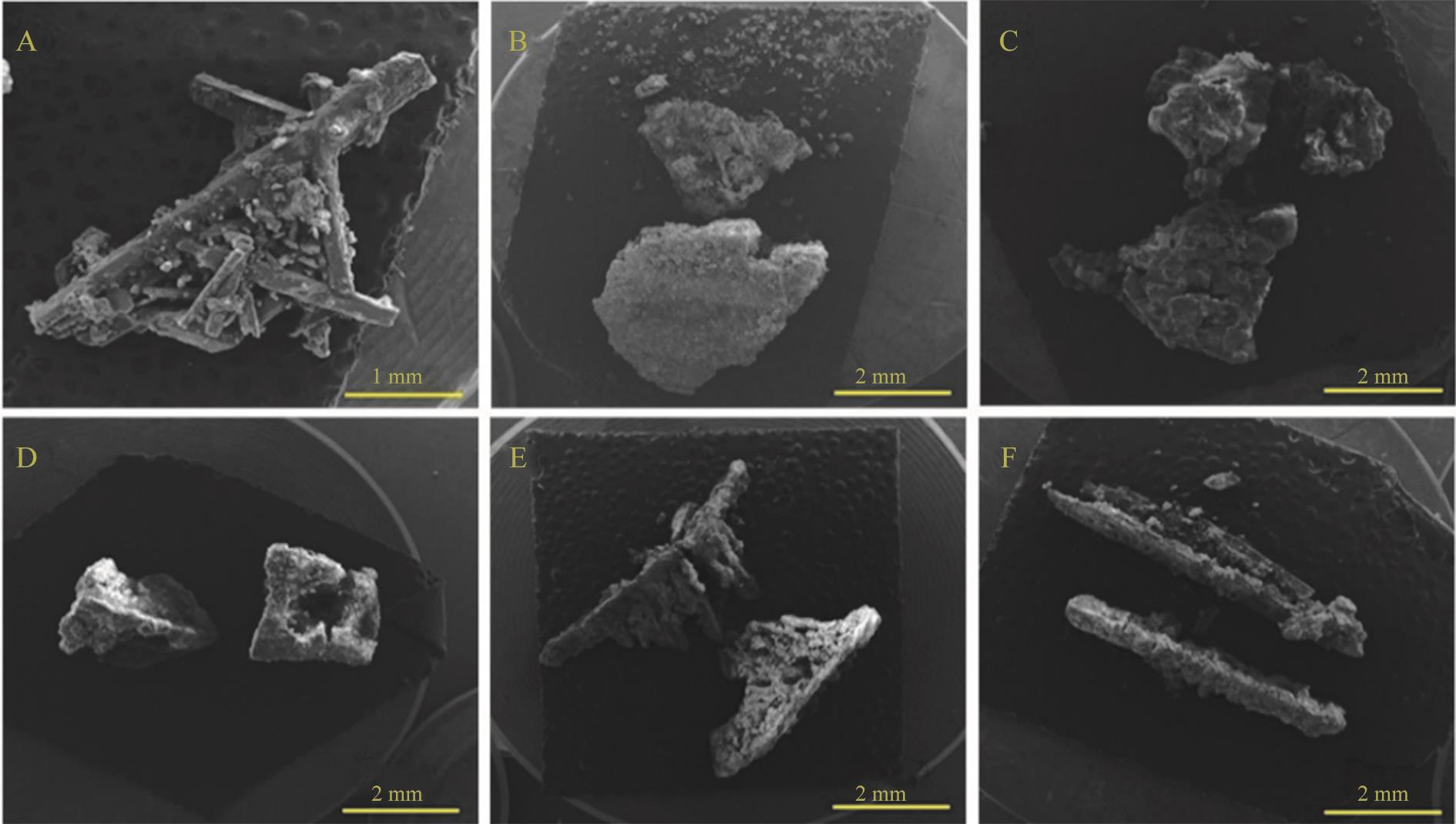
图5 脱硫灰铝法除氯对硫酸铵晶体形貌影响(A) 原液; (B) 3.0 g 脱硫灰+0.7 g NaAlO2; (C) 3.0 g 脱硫灰+0.8 g NaAlO2; (D) 3.0 g 脱硫灰+0.9 g NaAlO2; (E) 3.0 g 脱硫灰+1.0 g NaAlO2; (F) 3.0 g 脱硫灰+1.1 g NaAlO2
Fig.5 Effect of dechlorination by desulfurization ash aluminum method on the morphology of ammonium sulfate crystals(A) Stock solution; (B) 3.0 g Desulfurization ash +0.7 g NaAlO2; (C) 3.0 g Desulfurization ash+0.8 g NaAlO2; (D) 3.0 g Desulfurization ash +0.9 g NaAlO2; (E) 3.0 g Desulfurization ash+1.0 g NaAlO2; (F) 3.0 g Desulfurization ash+1.1 g NaAlO2

图6 脱硫灰铝法除氯后硫酸铵晶体XRD衍射图谱a. 原液; b. 3.0 g 脱硫灰+0.7 g NaAlO2; c. 3.0 g 脱硫灰+0.8 g NaAlO2; d. 3.0 g 脱硫灰+0.9 g NaAlO2; e. 3.0 g 脱硫灰+1.0 g NaAlO2; f. 3.0 g 脱硫灰+1.1 g NaAlO2
Fig.6 XRD diffraction pattern of ammonium sulfate crystals after dechlorination by desulfurization ash aluminum methoda. Stock solution; b. 3.0 g Desulfurization ash +0.7 g NaAlO2; c. 3.0 g Desulfurization ash +0.8 g NaAlO2; d. 3.0 g Desulfurization ash +0.9 g NaAlO2; e. 3.0 g Desulfurization ash +1.0 g NaAlO2; f. 3.0 g Desulfurization ash +1.1 g NaAlO2
| 1 | 赵春丽, 吴铁, 伯鑫, 等. 钢铁行业烧结烟气脱硫现状及协同治理对策建议[J]. 环境工程, 2014, 32(10): 76-78, 103. |
| ZHAO C L, WU T, BO X, et al. The status quo of Sinteing flue gas desulfurization of iron & steel industry and pollutants co-treatment suggestion[J]. Environ Eng, 2014, 32(10): 76-78, 103. | |
| 2 | 田凡, 车帅, 任栋. 我国钢铁行业烧结烟气脱硫的概况[J]. 冶金能源, 2017, 36(S1): 115-117. |
| TIAN F, CHE S, REN D. General situation of sintering flue gas desulfurization in China′s iron and steel industry[J]. Energy Metall Ind, 2017, 36(S1): 115-117. | |
| 3 | 石峥, 任晓芬, 张子平, 等. 烧结工序烟气治理方法[J]. 山东农业大学学报(自然科学版), 2020, 51(1): 168-173. |
| SHI Z, REN X F, ZHANG Z P, et al. Summary of flue gas treatment methods in sintering process[J]. J Shandong Agric Univ (Nat Sci Ed), 2020, 51(1): 168-173. | |
| 4 | 竹涛, 伊能静, 王礼锋, 等. 烧结烟气脱硫脱硝技术进展[J]. 河北冶金, 2019(S1): 7-10. |
| ZHU T, YI N J, WANG L F, et al. Progress in sintering flue gas desulfurization and denitrification technology[J]. Hebei Metall, 2019(S1): 7-10. | |
| 5 | 闫伯骏, 邢奕, 路培, 等. 钢铁行业烧结烟气多污染物协同净化技术研究进展[J]. 工程科学学报, 2018, 40(7): 767-775. |
| YAN B J,XING Y,LU P, et al. A critical review on the research progress of multi-pollutant collaborative control technologies of sintering flue gas in the iron and steel industry[J]. Chinese J Eng, 2018, 40(7): 767-775. | |
| 6 | 屈荷叶, 吴伟, 鲁果, 等. 某钢厂烧结机脱硝除尘超低排放技术应用探讨[J]. 中国环保产业, 2020(3): 47-50. |
| QU H Y, WU W, LU G, et al. Discussion on the application of sintering machine denitrification and dedusting technology for ultra-low emissions in a steel mill[J]. China Environ Prot Ind, 2020(3): 47-50. | |
| 7 | 金平, 王昊辰, 李磊, 等. 烟气脱硫技术现状及展望[J]. 当代化工, 2019, 48(1): 119-121, 126. |
| JIN P, WANG H C, LI L, et al. Status and prospect of flue gas desulfurization[J]. Contemp Chem Ind, 2019, 48(1): 119-121, 126. | |
| 8 | 杜家芝, 曹顺安. 湿法烟气脱硫技术的现状与进展[J]. 应用化工, 2019, 48(6): 1495-1500. |
| DU J Z,CAO S A. Research status and progress of wet flue gas desulfurization technology[J]. Appl Chem Ind, 2019, 48(6): 1495-1500. | |
| 9 | 罗海兵, 李啸. 烧烟气氨法脱硫技术介绍与分析[J]. 化工装备技术, 2011, 32(6): 25-27, 41. |
| LUO H B, LI X. Introduction and analysis of sintering flue gas ammonia desulfurization[J]. Chem Equip Technol, 2011, 32(6): 25-27, 41. | |
| 10 | 王海风, 张春霞, 齐渊洪. 氨法脱硫研究进展[J]. 环境工程, 2010, 28(6): 55-58,62. |
| WANG H F, ZHANG C X, QI Y H. Status of research on desulfurization by ammonia method[J]. Environ Eng, 2010, 28(6): 55-58,62. | |
| 11 | LI L, HUANG B F, ZHANG G F, et al. Optimization of ammonium sulfate crystals based on orthogonal design[J]. J Cryst Growth, 2021, 570. DOI:10.1016/j.jcrysgro.2021.126217. |
| 12 | 付志浩, 徐佳欢, 汪丹, 等. 烟道气氨法脱硫液对Q235碳钢腐蚀研究[J]. 腐蚀科学与防护技术, 2019, 31(5): 521-525. |
| FU Z H, XU J H, WANG D, et al. Corrosion of Q235 carbon steel in ammonia-based flue gas desulfurization slurry[J]. Corros Sci Prot Technol, 2019, 31(5): 521-525. | |
| 13 | 张海鹏, 张晓蕾, 刘建新, 等. 氨法脱硫硫酸铵浆液腐蚀及防护对策[J]. 全面腐蚀控制, 2019, 33(5): 11-16. |
| ZHANG H P, ZHANG X L, LIU J X, et al. Corrosion and protection of ammonium sulfate slurry in ammonia desulfurization[J]. Total Corrosion Control, 2019, 33(5): 11-16. | |
| 14 | 段威, 姚宣, 陈鸥, 等. 燃煤电厂脱硫废水氯离子脱除技术研究进展[J]. 盐科学与化工, 2020, 49(4): 16-19. |
| DUAN W, YAO X, CHEN O, et al. Recent advances on dechlorination technology for flue gas desulfurization wastewater of coat-fired power plants[J]. J Salt Sci Chem Ind, 2020, 49(4): 16-19. | |
| 15 | CUI L, LI G P, LI Y H, et al. Electrolysis-electrodialysis process for removing chloride ion in wet flue gas desulfurization wastewater (DW): influencing factors and energy consumption analysis[J]. Chem Eng Res Des, 2017, 123(1): 240-247. |
| 16 | LUO Z W, WANG D, ZHU D M, et al. Separation of fluoride and chloride ions from ammonia-based flue gas desulfurization slurry using a two-stage electrodialysis[J]. Chem Eng Res Des, 2019, 147(1): 73-82. |
| 17 | 袁靖淞, 罗正维, 郭牧林, 等. 电渗析法分离烟气氨法脱硫浆液中的氯离子[J]. 水处理技术, 2016, 42(11): 93-97, 103. |
| YUAN J S, LUO Z W, GUO M L, et al. Removal of chloride from ammonia-based flue gas desulfurization slurry by electrodialysis[J]. Technol Water Treat, 2016, 42(11): 93-97, 103. | |
| 18 | 武杰, 柴涛, 房亚玲. 高氯含量废水中氯离子的去除研究[J]. 现代化工, 2016, 36(4): 101-103. |
| WU J, CHAI T, FANG Y L. Removal of chlorine ion from high chlorine waste water[J]. Mod Chem Ind, 2016, 36(4): 101-103. | |
| 19 | 马双忱, 刘亚争, 马岚, 等. 焙烧镁铝水滑石吸附脱硫废水中高浓度氯离子的基础研究[J]. 煤炭学报, 2019, 44(2): 611-617. |
| MA S C, LIU Y Z, MA L, et al.Adsorption and ion-exchange behavior of calcined Mg-Al layered double hy-droxide for high concentration Cl- from flue gas desulfurization wastewater[J]. J China Coal Soc, 2019, 44(2): 611-617. | |
| 20 | 杨涛, 刘发强. 超高石灰铝法脱除废水中氯离子的影响因素分析[J]. 石化技术与应用, 2017, 35(1): 37-39, 47. |
| YANG T, LIU F Q. Influential factors analysis of removal of chloride ion in wastewater by ultra high lime aluminum method[J]. Petrochem Technol Appl, 2017, 35(1): 37-39, 47. | |
| 21 | 党红娟, 祁福平, 雷朝红, 等. 烟气氨法脱硫浆液Cl-含量测定的试验研究[J]. 中氮肥, 2017(1): 72-74, 80. |
| DANG H J, QI F P, LEI C H, et al. Experimental study on determination of Cl- content in flue gas ammonia desulfurization grout[J]. M-sized Nitrogen Fertil Prog,2017(1): 72-74, 80. | |
| 22 | 刘宝树, 种悦晖, 孙华, 等. 均一化大颗粒硫酸铵结晶高效制备工艺研究[J]. 无机盐工业, 2017, 49(6): 77-80. |
| LIU B S, CHONG Y H, SUN H, et al.High efficiency preparation of homogeneous large granular ammonium sulfate crystals[J]. Inorg Chem Ind, 2017, 49(6): 77-80. | |
| 23 | 万雅曼, 齐鸣斋, 袁萍, 等. 硫铵蒸发结晶的影响因素研究与优化[J]. 上海化工, 2014, 39(1): 11-15. |
| WAN Y M, QI M Z, YUAN P. Study and optimization of effect factors for ammonium sulfate evaporating crystallization[J]. Shanghai Chem Ind, 2014, 39(1): 11-15. | |
| 24 | 卢勇, 赵彦龙, 孙耀华, 等. 钙铝沉淀法除氯及沉淀再应用的研究进展[J]. 安徽化工, 2021, 47(5): 5-7, 10. |
| LU Y, ZHAO Y L, SUN Y H, et al.Research progress of calcium-aluminum precipitation for chloride ion removal and precipitation reapplication[J]. Anhui Chem Ind, 2021, 47(5): 5-7, 10. |
| [1] | 曹友錋, 庞烜, 项盛, 王天昶, 冯立栋, 边新超, 李杲, 陈学思. 溶剂诱导的聚乳酸/聚乳酸衍生物共结晶行为[J]. 应用化学, 2021, 38(1): 60-68. |
| [2] | 杨正军, 刘博, 黄湃, 聂赫然, 周光远, 张健夫. 多孔聚合物微球孔结构对丙烯催化聚合性能的影响[J]. 应用化学, 2020, 37(7): 746-755. |
| [3] | 张艳平, 薛冬峰. 钠和钾离子对磷酸二氢根拉曼光谱的影响[J]. 应用化学, 2020, 37(7): 823-829. |
| [4] | 苗中硕,门永锋. 快速扫描量热法研究聚对苯二甲酸-1,4-环己烷二甲醇酯的结晶和熔融行为[J]. 应用化学, 2020, 37(6): 642-649. |
| [5] | 何玉芳, 颜品萍, 黄宝铨, 罗富彬, 李红周, 钱庆荣, 陈庆华. 聚乙二醇/氮化硼复合材料相变导热性能及其结晶行为[J]. 应用化学, 2020, 37(6): 650-657. |
| [6] | 张予东,高芸,张磊,李庆华. 聚-4-乙基苯酚抗氧剂对聚丙烯结晶行为的影响[J]. 应用化学, 2019, 36(5): 539-547. |
| [7] | 衣彦林, 梁秋菊, 李令东, 刘剑刚, 韩艳春. 小分子优先结晶构筑聚合物/非富勒烯共混体系互穿网络结构[J]. 应用化学, 2019, 36(4): 423-430. |
| [8] | 钟振兴, 刘桂兰, 李永海, 李连国, 黄燕, 张光宇, 苏朝晖. 1,2-丁二醇共聚对聚对苯二甲酸乙二醇酯结晶行为及力学性能的影响[J]. 应用化学, 2019, 36(2): 170-175. |
| [9] | 孟繁志, 赵忠夫, 刘伟, 张春庆, 陈平, 白振民, 师悦. 高性能氢化星型聚(苯乙烯-b-丁二烯-b-苯乙烯)嵌段共聚物阴离子交换膜的制备、结构与性能[J]. 应用化学, 2019, 36(10): 1135-1146. |
| [10] | 孟繁志, 赵忠夫, 刘伟, 张春庆, 陈平, 白振民, 师悦. 高性能氢化星型聚(苯乙烯-b-丁二烯-b-苯乙烯)嵌段共聚物阴离子交换膜的制备、结构与性能[J]. 应用化学, 2019, 36(10): 0-0. |
| [11] | 雷小梅, 罗发亮, 沈志远, 司朋飞. 4,4'-二羟基二苯硫醚对聚甲醛结晶和熔融行为的影响[J]. 应用化学, 2017, 34(2): 180-186. |
| [12] | 孙彬, 刘焱龙, 边新超, 张宝, 周林尧, 冯立栋, 李杲, 陈志明. 高耐热聚乳酸立体复合材料的制备[J]. 应用化学, 2016, 33(9): 1033-1039. |
| [13] | 项盛, 邵俊, 冯立栋, 李杲, 陈学思, 边新超, 刘凤岐. 光学纯度对聚左旋乳酸结晶与熔融行为的影响[J]. 应用化学, 2016, 33(8): 887-893. |
| [14] | 边新超, 冯立栋, 陈志明, 陈学思. 聚乳酸的非等温结晶行为[J]. 应用化学, 2016, 33(7): 766-773. |
| [15] | 李晓萱, 陈涛, 伍胜利. 水性聚氨酯/功能化石墨烯纳米复合材料的非等温结晶动力学[J]. 应用化学, 2015, 32(11): 1319-1326. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||