应用化学 ›› 2022, Vol. 39 ›› Issue (5): 736-748.DOI: 10.19894/j.issn.1000-0518.210144
抗污界面构建及其电化学生物传感应用进展
黎振华1,3, 诸颖1,2, 陈静1,2, 宋世平1,2( )
)
- 1.中国科学院上海应用物理研究所物理生物研究室,中国科学院微观界面与探测重点实验室,上海 201800
2.中国科学院上海高等研究员基础交叉研究中心,上海同步辐射光源,张江实验室,上海 201210
3.中国科学院大学,北京 100049
-
收稿日期:2021-03-25接受日期:2021-06-23出版日期:2022-05-01发布日期:2022-05-24 -
通讯作者:宋世平 -
基金资助:国家重点基础研究发展计划质量专项项目(2018YFF0212803);国家自然科学基金项目(21974147)
Advances in Construction and Electrochemical Biosensing of Antifouling Interfaces
Zhen-Hua LI1,3, Ying ZHU1,2, Jing CHEN1,2, Shi-Ping SONG1,2( )
)
- 1.Division of Physical Biology,CAS Key Laboratory of Interfacial Physics and Technology,Shanghai Institute of Applied Physics,Chinese Academy of Sciences,Shanghai 201800,China
2.The Interdisciplinary Research Center,Shanghai Synchrotron Radiation Facility,Zhangjiang Laboratory,Shanghai Advanced Research Institute,Chinese Academy of Sciences,Shanghai 201210,China
3.University of Chinese Academy of Sciences,Beijing 100049,China
-
Received:2021-03-25Accepted:2021-06-23Published:2022-05-01Online:2022-05-24 -
Contact:Shi-Ping SONG -
About author:songsp@sari.ac.cn
-
Supported by:the National Key R&D Program of China(2018YFF0212803);the National Natural Science Foundation of China(21974147)
摘要:
电化学生物传感器具有灵敏度高、便携性好、响应快速和易于集成等优点,在临床检测方面有很大应用潜力,并在可穿戴健康监测领域得到了快速发展。但在实际临床生物样本检测中,非靶标生物物质会在电极表面产生非特异性吸附(即生物污染),影响了电化学生物传感器的性能。因此,构建具有防污染能力的传感界面(抗污界面),防止非靶标物质吸附到电极表面,对于扩大电化学生物传感器的实际应用范围,实现在复杂生物样本中的检测至关重要。本文概述了物理、化学和生物抗污电极界面的构建及其在临床相关生物标志物检测中的应用,为电化学生物传感器实际应用性能的提升提供技术参考,并通过对界面抗污原理和存在问题的探讨,对抗污界面发展前景和未来趋势予以展望。
中图分类号:
引用本文
黎振华, 诸颖, 陈静, 宋世平. 抗污界面构建及其电化学生物传感应用进展[J]. 应用化学, 2022, 39(5): 736-748.
Zhen-Hua LI, Ying ZHU, Jing CHEN, Shi-Ping SONG. Advances in Construction and Electrochemical Biosensing of Antifouling Interfaces[J]. Chinese Journal of Applied Chemistry, 2022, 39(5): 736-748.
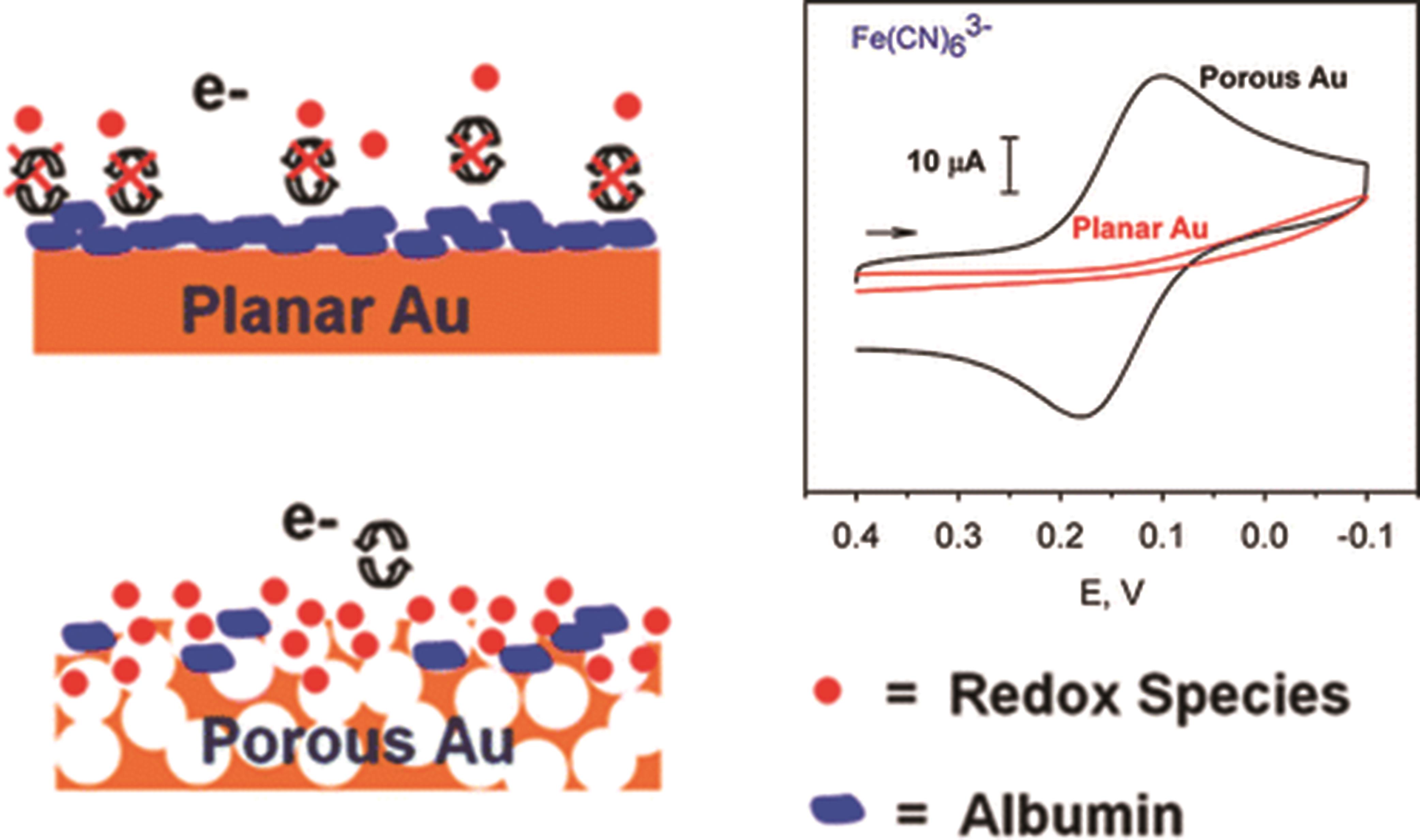
图1 纳米金孔和平面金界面在溶液中存在白蛋白和小氧化还原分子时的示意图[13]
Fig.1 Simplified graphical representation of the surface of nanoporous gold and planar gold in the presence of albumin and a small redox molecule in solution[13]
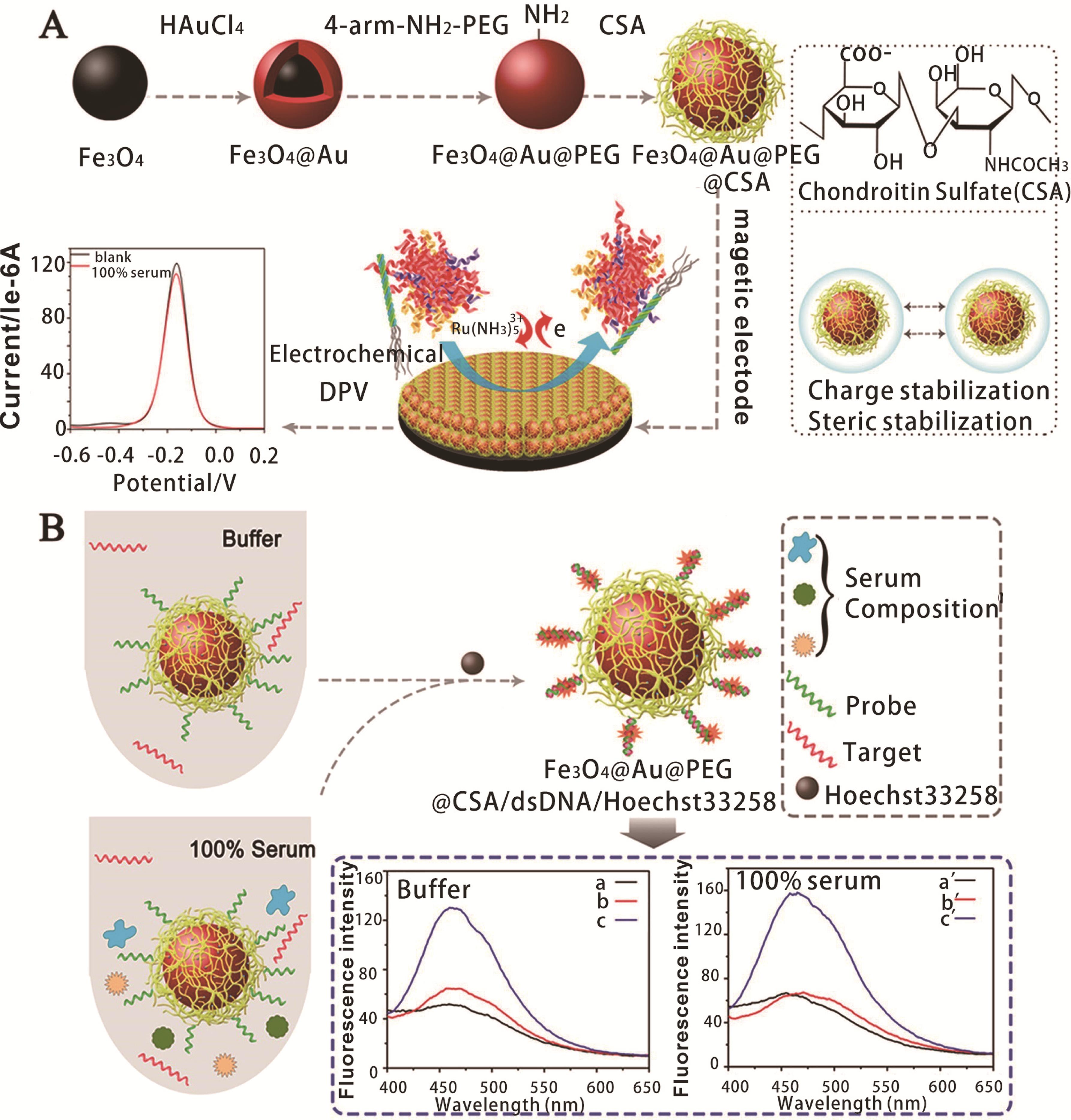
图2 Fe3O4@Au@PEG@CSA NPs的制备和稳定性示意图及其抗污性能的检测步骤(A)和磁性NPs在疾病标记检测中的应用(B)[35]
Fig.2 Illustration of the fabrication and stabilization of Fe3O4@Au@PEG@CSA NPs and procedure for detection of its antifouling performance (A) and the application of magnetic NPs in disease marker detection (B)[35]
| 抗污策略 | 抗污原理 | 抗污组成 | 靶标分子 | 检测基质 | 线性范围 | 检测限 | 参考文献 |
|---|---|---|---|---|---|---|---|
| Antifouling strategies | Principle | Antifouling component | Target | Matrix | Linear range | Detection limit | Ref. |
| 物理抗污 Physical | 孔径限制 Pore size restriction and filtration | 纳米多孔金 | 脱氧核糖核酸 | 牛血清白蛋白 | 10~200 nmol/L | - a | [ |
| Nanoporous gold film | DNA | BSA | |||||
| 纳米多孔氧化铝膜 | 癌抗原15?3 | 全血 | 60~240 U/mL | 52 U/mL | [ | ||
| AAO nanoporous membrane | CA15?3 | Blood | |||||
| 化学抗污Chemical | 水化层 Hydration layer | PEG和糖胺聚糖抗污层 | 人乳头瘤病毒DNA | 100%血清 | 0.001~10 nmol/L | - | [ |
| PEG and CSA layer | HPV DNA | 100% serum | |||||
| 苯基磷酰胆碱抗污层 | 肿瘤坏死因子α | 磷酸缓冲盐溶液 | 0.1~150 pg/mL | 0.1 pg/mL | [ | ||
| PPC layer | TNF?α | PBS | |||||
| 生物抗污Biological | 空间位阻 | DNA纳米结构 | 凝血酶 | 磷酸缓冲盐溶液 | 100~ 100000 pmol/L | 100 pmol/L | [ |
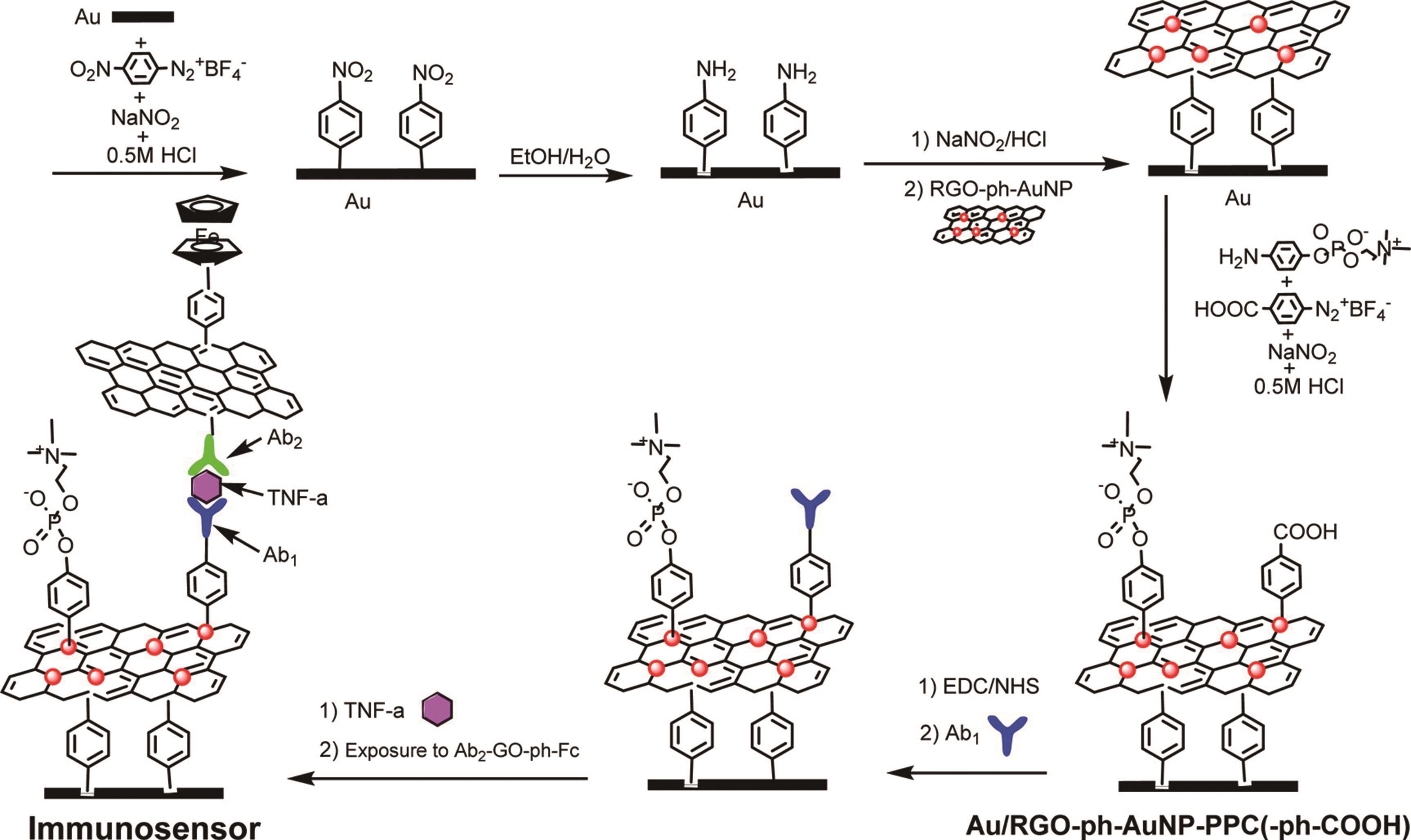
| Steric hindrance | DNA nanostructure | Thrombin | PBS | ||||
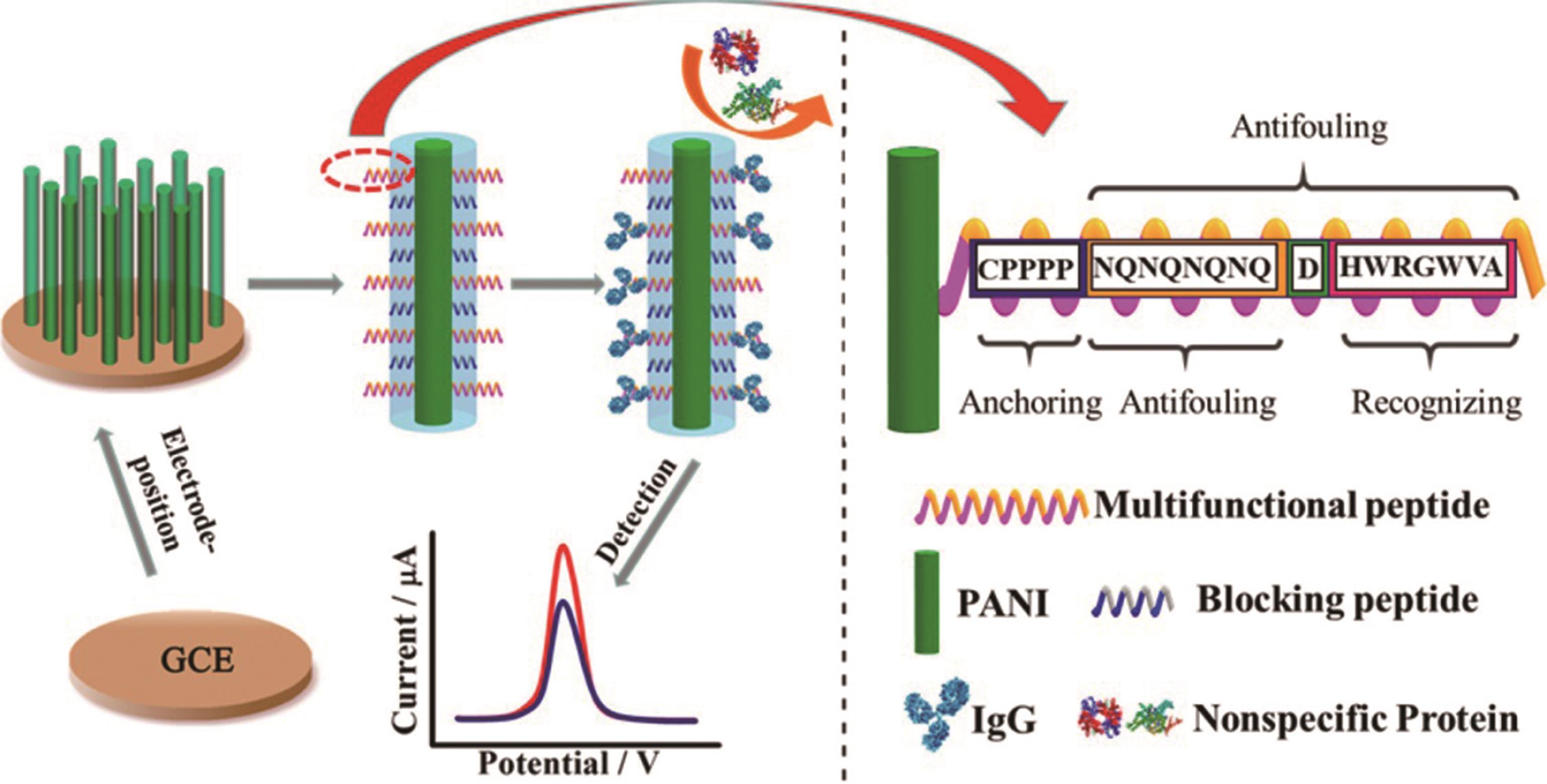
| 水化层 | 多功能肽 | 免疫球蛋白G | 血清 | - | 0.26 ng/mL | [ | |
| Hydration layer | Multifunctional peptide | Immunoglobulin G | Serum | ||||
| 孔径限制 | 多孔纳米复合物涂层 | 白细胞介素6 | 血浆 | - | 23 pg/mL | [ | |
| Pore size restriction | Porous nanocomposite coating | Interleukin 6 | Plasma |
表1 不同抗污界面及其电化学生物传感器的抗污和检测性能
Table 1 Antifouling and detection performance of different antifouling interfaces and their electrochemical biosensors
| 抗污策略 | 抗污原理 | 抗污组成 | 靶标分子 | 检测基质 | 线性范围 | 检测限 | 参考文献 |
|---|---|---|---|---|---|---|---|
| Antifouling strategies | Principle | Antifouling component | Target | Matrix | Linear range | Detection limit | Ref. |
| 物理抗污 Physical | 孔径限制 Pore size restriction and filtration | 纳米多孔金 | 脱氧核糖核酸 | 牛血清白蛋白 | 10~200 nmol/L | - a | [ |
| Nanoporous gold film | DNA | BSA | |||||
| 纳米多孔氧化铝膜 | 癌抗原15?3 | 全血 | 60~240 U/mL | 52 U/mL | [ | ||
| AAO nanoporous membrane | CA15?3 | Blood | |||||
| 化学抗污Chemical | 水化层 Hydration layer | PEG和糖胺聚糖抗污层 | 人乳头瘤病毒DNA | 100%血清 | 0.001~10 nmol/L | - | [ |
| PEG and CSA layer | HPV DNA | 100% serum | |||||
| 苯基磷酰胆碱抗污层 | 肿瘤坏死因子α | 磷酸缓冲盐溶液 | 0.1~150 pg/mL | 0.1 pg/mL | [ | ||
| PPC layer | TNF?α | PBS | |||||
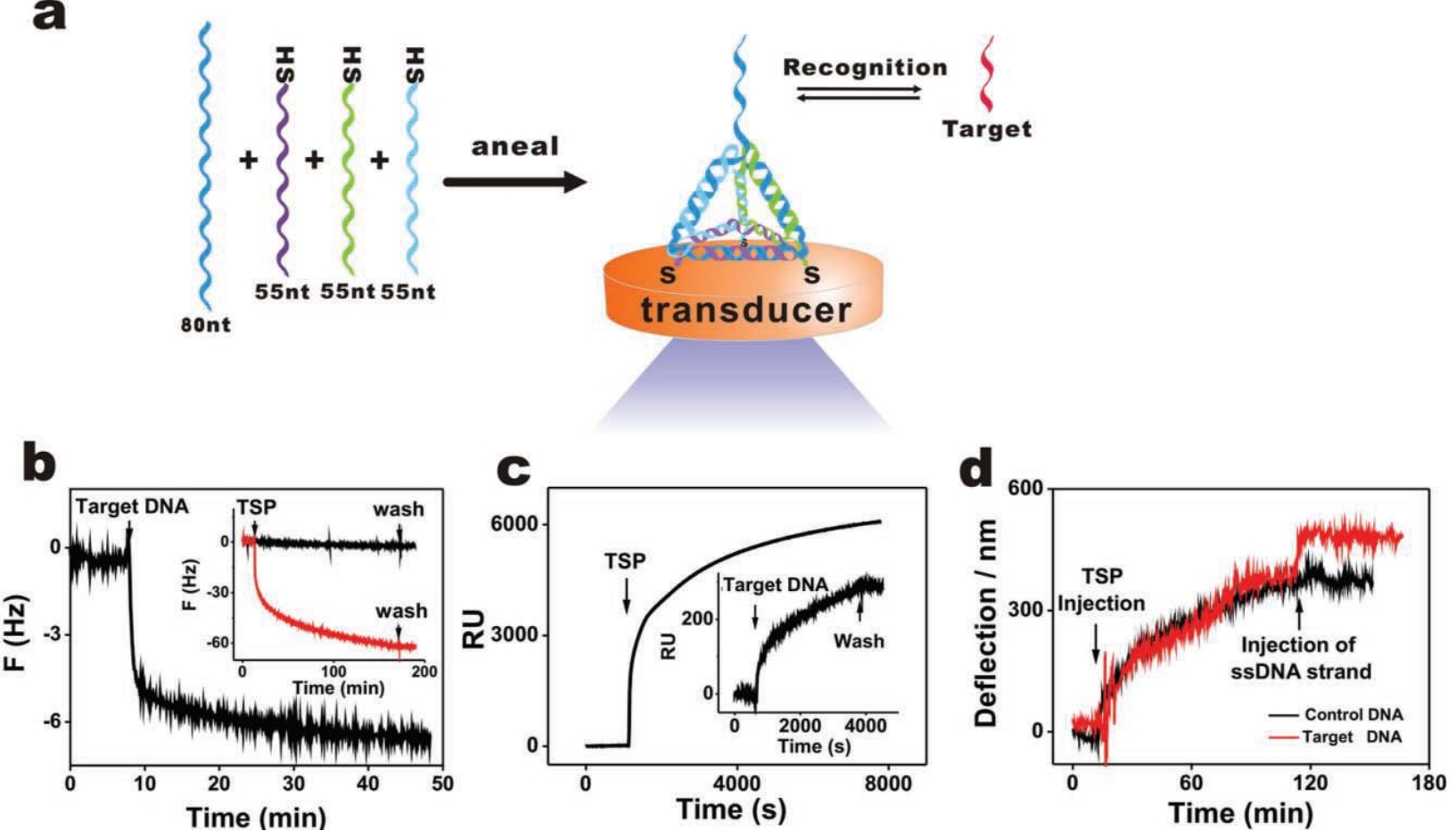
| 生物抗污Biological | 空间位阻 | DNA纳米结构 | 凝血酶 | 磷酸缓冲盐溶液 | 100~ 100000 pmol/L | 100 pmol/L | [ |
| Steric hindrance | DNA nanostructure | Thrombin | PBS | ||||
| 水化层 | 多功能肽 | 免疫球蛋白G | 血清 | - | 0.26 ng/mL | [ | |
| Hydration layer | Multifunctional peptide | Immunoglobulin G | Serum | ||||
| 孔径限制 | 多孔纳米复合物涂层 | 白细胞介素6 | 血浆 | - | 23 pg/mL | [ | |
| Pore size restriction | Porous nanocomposite coating | Interleukin 6 | Plasma |
| 1 | CLARK C L J, LYONS C. Electrode systems for continuous monitoring in cardiovascular surgery[J]. Ann NY Acad Sci, 1962, 102: 29-45. |
| 2 | KIM J, CAMPBELL A S, DE AVILA B E, et al. Wearable biosensors for healthcare monitoring[J]. Nat Biotechnol, 2019, 37(4): 389-406. |
| 3 | HERNANDEZ-RODRIGUEZ J F, ROJAS D, ESCARPA A. Electrochemical sensing directions for next-generation healthcare: trends, challenges, and frontiers[J]. Anal Chem, 2021, 93(1): 167-183. |
| 4 | MONDAL S, ZEHRA N, CHOUDHRUY A, et al. Wearable sensing devices for point of care diagnostics[J]. ACS Appl Bio Mater, 2020, 4(1): 47-70. |
| 5 | YANG A, YAN F. Flexible electrochemical biosensors for health monitoring[J]. ACS Appl Electron Mater, 2020, 3(1): 53-67. |
| 6 | WISNIEWSKI N M F, REICHERT W M. Characterization of implantable biosensor membrane biofouling[J]. Fresenius J Anal Chem, 2000, 366: 611-621. |
| 7 | LIU X, XIAO T, WU F, et al. Ultrathin cell-membrane-mimic phosphorylcholine polymer film coating enables large improvements for in vivo electrochemical detection[J]. Angew Chem Int Ed Engl, 2017, 56(39): 11802-11806. |
| 8 | WANG X, SUN M, ZHAO Y, et al. In situ electrochemical generation of reactive chlorine species for efficient ultrafiltration membrane self-cleaning[J]. Environ Sci Technol, 2020, 54(11): 6997-7007. |
| 9 | CALLOW J A, CALLOW M E. Trends in the development of environmentally friendly fouling-resistant marine coatings[J]. Nat Commun, 2011, 2: 244-253. |
| 10 | KRISTENSEN J B, MEYER R L, LAURSEN B S, et al. Antifouling enzymes and the biochemistry of marine settlement[J]. Biotechnol Adv, 2008, 26(5): 471-481. |
| 11 | OSTUNI O, CHAPMAN R G, HOLMLIN R E, et al. A survey of structure-property relationships of surfaces that resist the adsorption of protein[J]. Langmuir, 2001, 17: 5605-5620. |
| 12 | CHEN S, LI L, ZHAO C, et al. Surface hydration: principles and applications toward low-fouling/nonfouling biomaterials[J]. Polymer, 2010, 51(23): 5283-5293. |
| 13 | PATEL J, RADHAKRISHNAN L, ZHAO B, et al. Electrochemical properties of nanostructured porous gold electrodes in biofouling solutions[J]. Anal Chem, 2013, 85(23): 11610-11618. |
| 14 | DAGGUMAT P, MATHRU Z, SEKER E. Effect of nanoporous gold thin film morphology on electrochemical DNA sensing[J]. Anal Chem, 2015, 87(16): 8149-8156. |
| 15 | DAGGUMATI P, MATHARU Z, WANG L, et al. Biofouling-resilient nanoporous gold electrodes for DNA sensing[J]. Anal Chem, 2015, 87(17): 8618-8622. |
| 16 | SILVA T A, KHAN M R K, FATIBELLO-FIHO O, et al. Simultaneous electrochemical sensing of ascorbic acid and uric acid under biofouling conditions using nanoporous gold electrodes[J]. J Electroanal Chem, 2019, 846: 113160-113168. |
| 17 | RUIZ-CLAVIJO A, CABALLERO-CALERO O, MARTIN-GONZALEZ M. Revisiting anodic alumina templates: from fabrication to applications[J]. Nanoscale, 2021, 13(4): 2227-2265. |
| 18 | 孙小彤, 陈南, 梁含雪, 等. 阳极氧化铝模板限域制备一维杂化纳米材料及其多样化应用的研究进展[J]. 应用化学, 2020, 37(2): 123-133. |
| SUN X T, CHEN N, LIANG H X, et al. Progress of fabrication of one-dimensional hybrid nanomaterials by template-confined growth and their diverse applications[J]. Chinese J Appl Chem, 37(2): 123-133. | |
| 19 | RAJEEV G, PRIETO S B, MARSAL L F, et al. Advances in nanoporous anodic alumina-based biosensors to detect biomarkers of clinical significance: a review[J]. Adv Health Mater, 2018, 7(5): 1700904-1700921. |
| 20 | ESCOSURA-MUNIZ A, MERKOCI A. A nanochannel/nanoparticle-based filtering and sensing platform for direct detection of a cancer biomarker in blood[J]. Small, 2011, 7(5): 675-682. |
| 21 | ESCOSURA-MUNIZ A, CHUNGLOK W, SURAREUNGCHAI W, et al. Nanochannels for diagnostic of thrombin-related diseases in human blood[J]. Biosens Bioelectron, 2013, 40(1): 24-31. |
| 22 | OSTUNI E, CHAPMAN G R, HOLMLIN E R, et al. A survey of structure⁃property relationships of surfaces that resist the adsorption of protein[J]. Langmuir, 2001, 17: 5605-5620. |
| 23 | PRIME K L, WHITESIDES M G. Self-assembled organic monolayers: model systems for studying adsorption of proteins at surfaces[J]. Science, 1991, 252: 1164-1167. |
| 24 | XU M, LUO X, DAVISA J J. The label free picomolar detection of insulin in blood serum[J]. Biosens Bioelectron, 2013, 39(1): 21-25. |
| 25 | BEDATTY F F C, PATIL A V, BUENO P R, et al. Optimized diagnostic assays based on redox tagged bioreceptive interfaces[J]. Anal Chem, 2015, 87(24): 12137-12144. |
| 26 | BRYAN T, LUO X, BUENO P R, et al. An optimised electrochemical biosensor for the label-free detection of C-reactive protein in blood[J]. Biosens Bioelectron, 2013, 39(1): 94-98. |
| 27 | PATIL A V, BEDATTY F F C, BUENO P R, et al. Immittance electroanalysis in diagnostics[J]. Anal Chem, 2015, 87(2): 944-950. |
| 28 | LI Q, TOFARIS G K, DAVIS J J. Concentration-normalized electroanalytical assaying of exosomal markers[J]. Anal Chem, 2017, 89(5): 3184-3190. |
| 29 | SANTOS A, BUENO P R, DAVIS J J. A dual marker label free electrochemical assay for Flavivirus dengue diagnosis[J]. Biosens Bioelectron, 2018, 100: 519-525. |
| 30 | KHOR S M, LIU G, FAIRMAN C, et al. The importance of interfacial design for the sensitivity of a label-free electrochemical immuno-biosensor for small organic molecules[J]. Biosens Bioelectron, 2011, 26(5): 2038-2044. |
| 31 | LIU G, KHOR S M, IYENGAR S G, et al. Development of an electrochemical immunosensor for the detection of HbA1c in serum[J]. Analyst, 2012, 137(4): 829-832. |
| 32 | LUO X, XU Q, JAMES T, et al. Redox and label-free array detection of protein markers in human serum[J]. Anal Chem, 2014, 86(11): 5553-5558. |
| 33 | WANG W, FAN X, XU S, et al. Low fouling label-free DNA sensor based on polyethylene glycols decorated with gold nanoparticles for the detection of breast cancer biomarkers[J]. Biosens Bioelectron, 2015, 71: 51-56. |
| 34 | HUI N, SUN X, NIU S, et al. PEGylated polyaniline nanofibers: antifouling and conducting biomaterial for electrochemical DNA sensing[J]. ACS Appl Mater Interfaces, 2017, 9(3): 2914-2923. |
| 35 | CHEN L, LV S, LIU M, et al. Low-fouling magnetic nanoparticles and evaluation of their potential application as disease markers assay in whole serum[J]. ACS Appl Nano Mater, 2018, 1(6): 2489-2495. |
| 36 | PIDHATIKA B, RODENSTEIN M, CHEN Y, et al. Comparative stability studies of poly(2-methyl-2-oxazoline) and poly(ethylene glycol) brush coatings[J]. Biointerphases, 2012, 7: 1-16. |
| 37 | DU Y, GAO J, CHEN T, et al. Understanding the oxidative stability of antifouling polymer brushes[J]. Langmuir, 2017, 33(29): 7298-7304. |
| 38 | LOWE S, SIMPSON N O, CONNAL L A. Antibiofouling polymer interfaces: poly(ethylene glycol) and other promising candidates[J]. Polym Chem, 2015, 6: 198-212. |
| 39 | LI L, CHEN S, JIANG S S. Protein interactions with oligo(ethylene glycol) (OEG) self-assembled monolayers: OEG stability, surface packing density and protein adsorption[J]. J Biomater Sci Polym Ed, 2007, 18(11): 1415-1427. |
| 40 | YANG W, XUE H, CARR L R, et al. Zwitterionic poly(carboxybetaine) hydrogels for glucose biosensors in complex media[J]. Biosens Bioelectron, 2011, 26(5): 2454-2459. |
| 41 | LUO X, XU M, FREEMAN C, et al. Ultrasensitive label free electrical detection of insulin in neat blood serum[J]. Anal Chem, 2013, 85(8): 4129-4134. |
| 42 | GUI A L, LUAIS E, PETERSON J R, et al. Zwitterionic phenyl layers: finally, stable, anti-biofouling coatings that do not passivate electrodes[J]. ACS Appl Mater Interfaces, 2013, 5(11): 4827-4835. |
| 43 | JIANG C, ALAM M T, PARKER S G, et al. Strategies to achieve control over the surface ratio of two different components on modified electrodes using aryldiazonium salts[J]. Langmuir, 2016, 32(10): 2509-2517. |
| 44 | JIANG C, MORAES SILVA S, FAN S, et al. Aryldiazonium salt derived mixed organic layers: from surface chemistry to their applications[J]. J Electroanal Chem, 2017, 785: 265-278. |
| 45 | JIANG C, ALAM M T, SILVA S M, et al. Unique sensing interface that allows the development of an electrochemical immunosensor for the detection of tumor necrosis factor α in whole blood[J]. ACS Sens, 2016, 1(12): 1432-1438. |
| 46 | QI M, ZHANG Y, CAO C, et al. Decoration of reduced graphene oxide nanosheets with aryldiazonium salts and gold nanoparticles toward a label-free amperometric immunosensor for detecting cytokine tumor necrosis factor-alpha in live cells[J]. Anal Chem, 2016, 88(19): 9614-9621. |
| 47 | WEI H, NI S, CAO C, et al. Graphene oxide signal reporter based multifunctional immunosensing platform for amperometric profiling of multiple cytokines in serum[J]. ACS Sens, 2018, 3(8): 1553-1561. |
| 48 | LI H, DAUPHIN-DUCHARME P, ARROYO-CURRAS N, et al. A biomimetic phosphatidylcholine-terminated monolayer greatly improves the in vivo performance of electrochemical aptamer-based sensors[J]. Angew Chem Int Ed Engl, 2017, 56(26): 492-7495. |
| 49 | ANDEEL E, LANGE S C, PUJARI S P, et al. Systematic comparison of zwitterionic and non-zwitterionic antifouling polymer brushes on a bead-based platform[J]. Langmuir, 2019, 35(5): 1181-1191. |
| 50 | ZHENG L, SUNDARAM H S, WEI Z, et al. Applications of zwitterionic polymers[J]. React Funct Polym, 2017, 118: 51-61. |
| 51 | SUBBIAHDOSS G, ZENG G, ASLAN H, et al. Antifouling properties of layer by layer DNA coatings[J]. Biofouling, 2019, 35(1): 75-88. |
| 52 | MCQUISTAN A, ZAITOUNA A J, ECHEVERRIA E, et al. Use of thiolated oligonucleotides as anti-fouling diluents in electrochemical peptide-based sensors[J]. Chem Commun, 2014, 50(36): 4690-4692. |
| 53 | SEEEMAN N. Nucleic acid junctions and lattices[J]. J Theor Biol, 1982, 99: 237-247. |
| 54 | GE Z, LIN M, WANG P, et al. Hybridization chain reaction amplification of microRNA detection with a tetrahedral DNA nanostructure-based electrochemical biosensor[J]. Anal Chem, 2014, 86(4): 2124-2130. |
| 55 | SU J, LIU W, CHEN S, et al. A carbon-based DNA framework nano-bio interface for biosensing with high sensitivity and a high signal-to-noise ratio[J]. ACS Sens, 2020, 5(12): 3979-3987. |
| 56 | WEN Y, PEI H, WAN Y, et al. DNA nanostructure-decorated surfaces for enhanced aptamer-target binding and electrochemical cocaine sensors[J]. Anal Chem, 2011, 83(19): 7418-7423. |
| 57 | ZHOU G, LIN M, SONG P, et al. Multivalent capture and detection of cancer cells with DNA nanostructured biosensors and multibranched hybridization chain reaction amplification[J]. Anal Chem, 2014, 86(15): 7843-7848. |
| 58 | LI Z, ZHAO B, WANG D, et al. DNA nanostructure-based universal microarray platform for high-efficiency multiplex bioanalysis in biofluids[J]. ACS Appl Mater Interfaces, 2014, 6(20): 17944-17953. |
| 59 | PEI H, LU N, WEN Y, et al. A DNA nanostructure-based biomolecular probe carrier platform for electrochemical biosensing[J]. Adv Mater, 2010, 22(42): 4754-4758. |
| 60 | WEN Y, PEI H, SHEN Y, et al. DNA nanostructure-based Interfacial engineering for PCR-free ultrasensitive electrochemical analysis of microRNA[J]. Sci Rep, 2012, 2: 867. |
| 61 | HUI L, XU A, LIU H. DNA-based nanofabrication for antifouling applications[J]. Langmuir, 2019, 35(38): 12543- 12549. |
| 62 | DAS J, CEDERQUIST K B, ZARAGOZA A A, et al. An ultrasensitive universal detector based on neutralizer displacement[J]. Nat Chem, 2012, 4(8): 642-648. |
| 63 | CHEN S, CAO Z, JIANG S. Ultra-low fouling peptide surfaces derived from natural amino acids[J]. Biomaterials, 2009, 30(29): 5892-5896. |
| 64 | EDERTH T, LERM M, ORIHUELA B, et al. Resistance of zwitterionic peptide monolayers to biofouling[J]. Langmuir, 2019, 35(5): 1818-1827. |
| 65 | SAKALA G P, RECHES M. Peptide-based approaches to fight biofouling[J]. Adv Mater Interfaces, 2018, 5(18): 1800073-1800098. |
| 66 | CUI M, WANG Y, JIAO M, et al. Mixed self-assembled aptamer and newly designed zwitterionic peptide as antifouling biosensing interface for electrochemical detection of alpha-fetoprotein[J]. ACS Sens, 2017, 2(4): 490-494. |
| 67 | CUI M, WANG Y, WANG H, et al. A label-free electrochemical DNA biosensor for breast cancer marker BRCA1 based on self-assembled antifouling peptide monolayer[J]. Sens Actuators B:Chem, 2017, 244: 742-749. |
| 68 | WANG G, HAN R, SU X, et al. Zwitterionic peptide anchored to conducting polymer PEDOT for the development of antifouling and ultrasensitive electrochemical DNA sensor[J]. Biosens Bioelectron, 2017, 92: 396-401. |
| 69 | LIU N, HUI N, DAVIS J J, et al. Low fouling protein detection in complex biological media supported by a designed multifunctional peptide[J]. ACS Sens, 2018, 3(6): 1210-1216. |
| 70 | SABATE DEL RIO J, HENRY O Y F, JOLLY P, et al. An antifouling coating that enables affinity-based electrochemical biosensing in complex biological fluids[J]. Nat Nanotechnol, 2019, 14(12): 1143-1149. |
| 71 | 司晓晶, 朱文菁, 李香. 聚对氨基苯磺酸/石墨烯电化学修饰电极检测药物中氧氟沙星[J]. 应用化学, 2020, 37(6): 726-732. |
| SI X J, ZHU W J, LI X, et al. Determination of ofloxacin in medicine via poly(p-aminobenzene sulfonic acid)/graphene electrochemical modified electrode[J]. Chinese J Appl Chem, 2020, 37(6): 726-732 | |
| 72 | TAVALLAIE R, MCCARROLL J, GRAND M, et al. Nucleic acid hybridization on an electrically reconfigurable network of gold-coated magnetic nanoparticles enables microRNA detection in blood[J]. Nat Nanotechnol, 2018, 13(11): 1066-1071. |
| 73 | TENZER S, DOCTER D, KUHAREV J, et al. Rapid formation of plasma protein corona critically affects nanoparticle pathophysiology[J]. Nat Nanotechnol, 2013, 8(10): 772-781. |
| 74 | KONG H, ZHANG J, LI J, et al. Genetically encoded X-ray cellular imaging for nanoscale protein localization[J]. Nat Sci Rev, 2020, 7(7): 1218-1227. |
| [1] | 李铸衡,刘霞,刘殿骏,王振新. 蛋白质微阵列芯片在临床分析中的应用[J]. 应用化学, 2016, 33(11): 1253-1264. |
| [2] | 杨惠宇, 张昊, 徐美玲, 干高龙, 陈克乐, 于源华. 干式生化膜片的制备及其应用[J]. 应用化学, 2015, 32(8): 963-968. |
| [3] | 刘平, 朱亚琦, 马洁, 薛颖, 吴国华. 氧化苏木精复合电极测定牛奶中残留青霉素[J]. 应用化学, 2006, 23(5): 519-523. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||