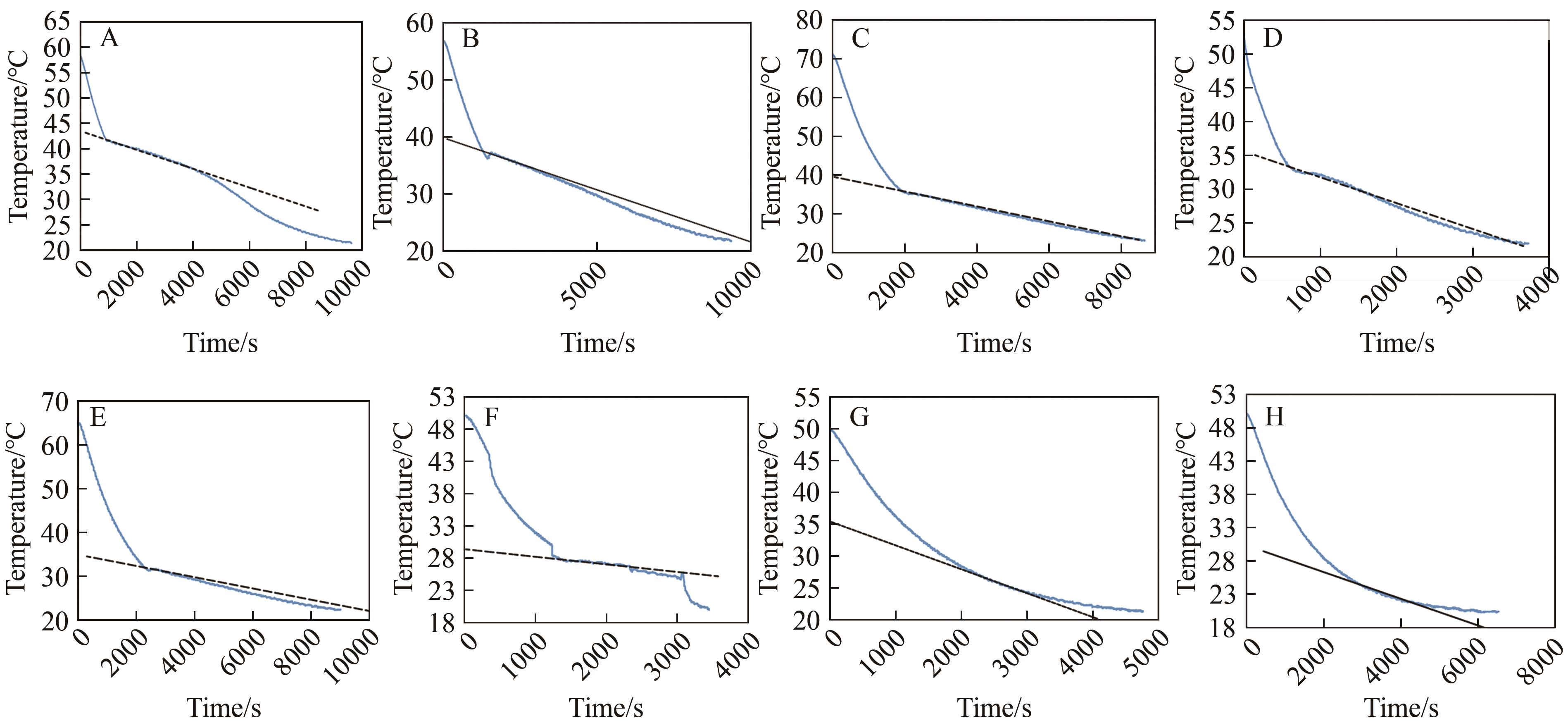
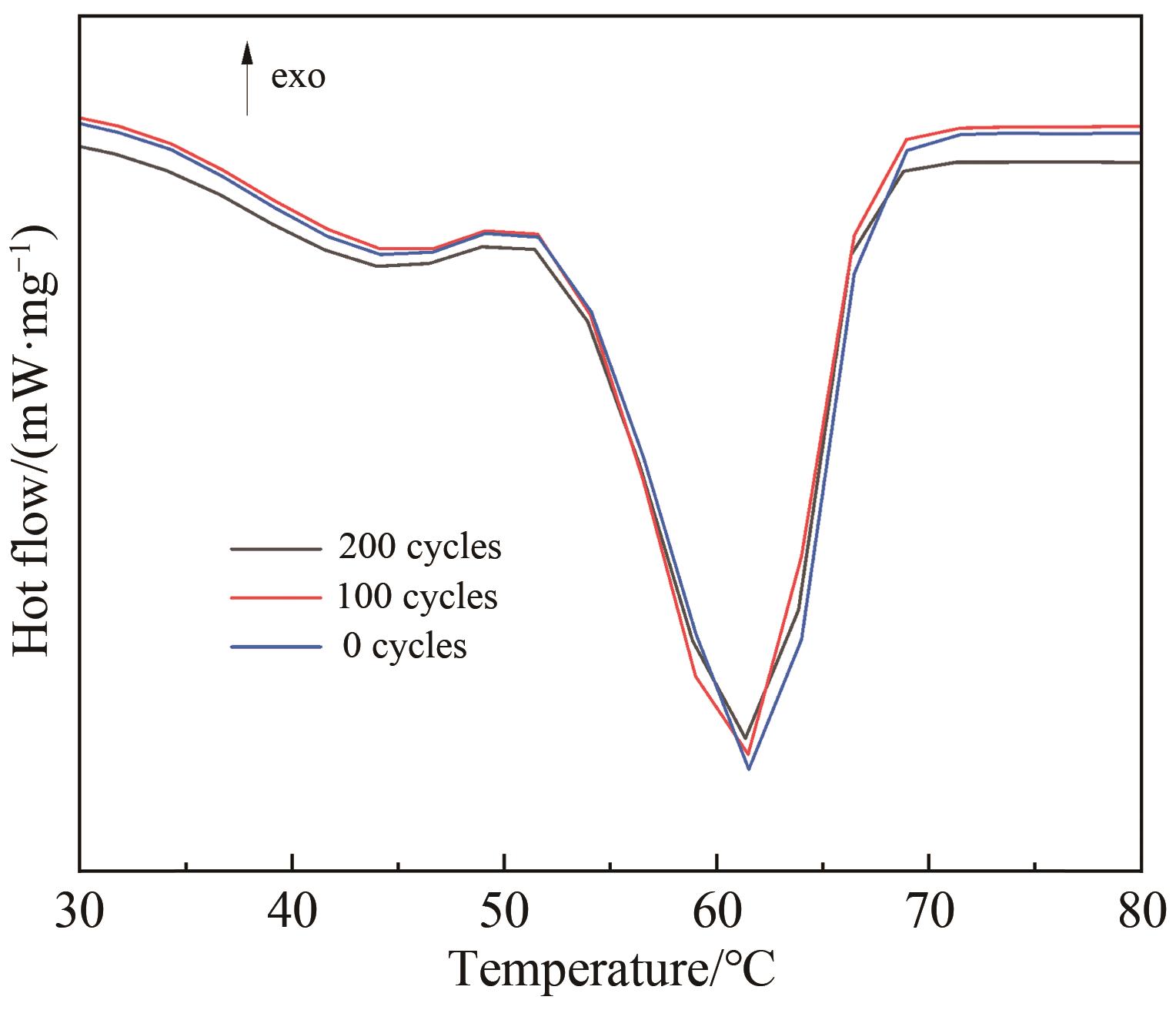
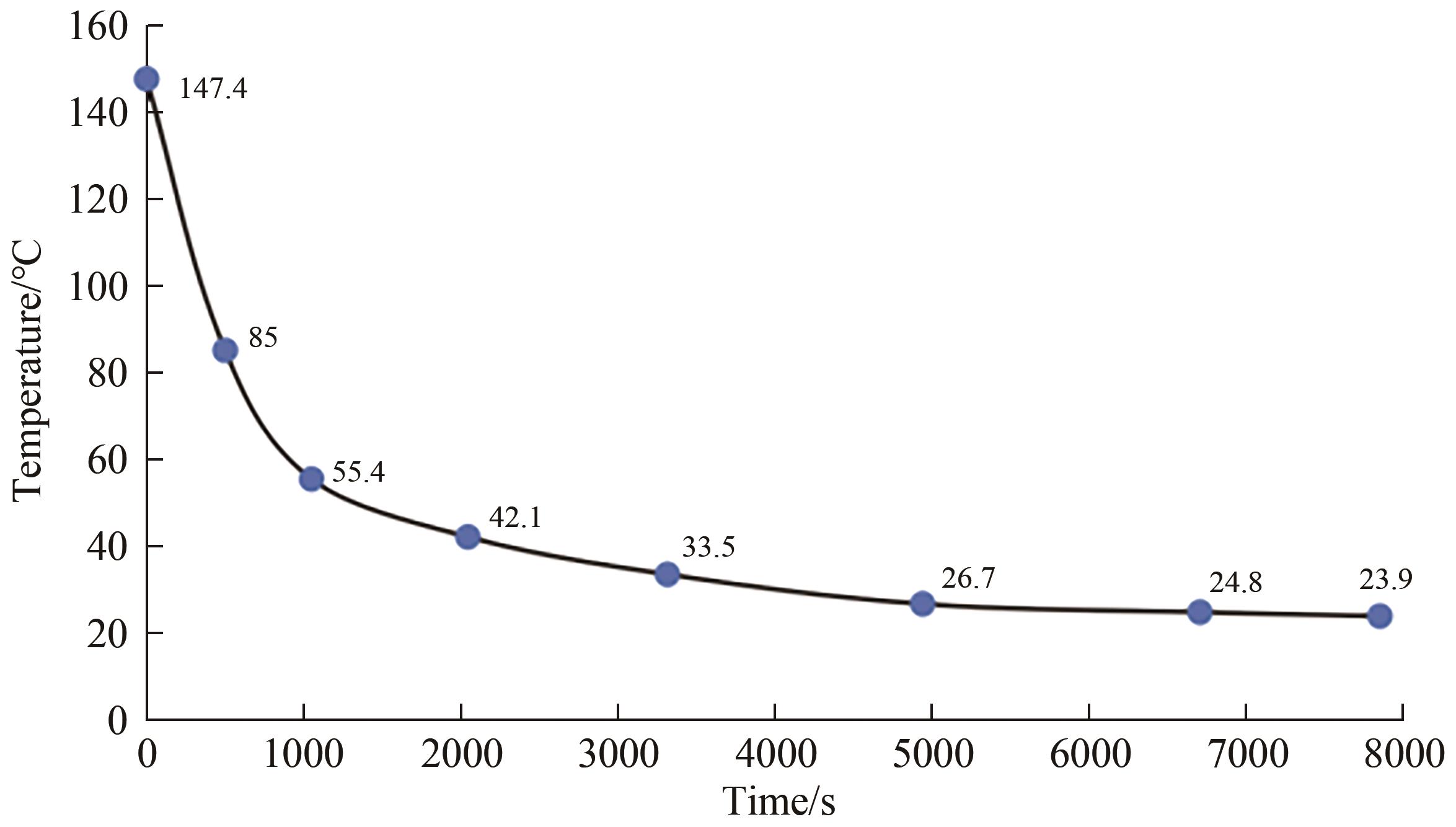
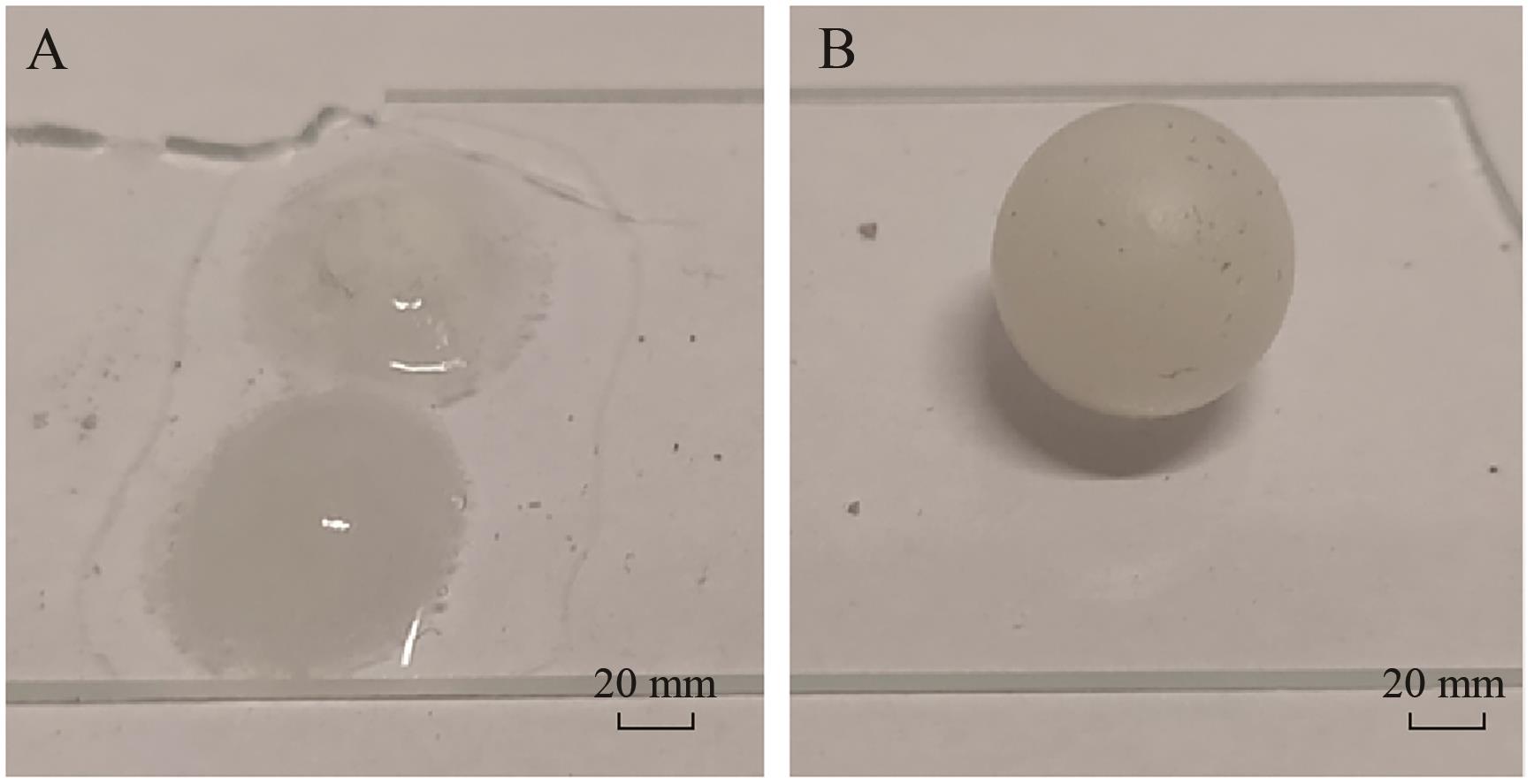
Chinese Journal of Applied Chemistry ›› 2024, Vol. 41 ›› Issue (12): 1780-1789.DOI: 10.19894/j.issn.1000-0518.240173
• Full Papers • Previous Articles Next Articles
Design and Application Research of Shape-Stabilized Phase Change Materials for Building Energy Conservation
Jie-Wei HE1( ), Shao-Wei DUAN2, Xiao-Chuan LI1
), Shao-Wei DUAN2, Xiao-Chuan LI1
- 1.School of Architecture and Engineering,Shaoyang Polytechnic,Shaoyang 422000,China
2.School of Civil Engineering,Central South University of Forestry and Technology,Changsha 410004,China
-
Received:2024-05-30Accepted:2024-08-15Published:2024-12-01Online:2025-01-02 -
Contact:Jie-Wei HE -
About author:373652240@qq.com
-
Supported by:Shaoyang Science and Technology Bureau Project(2023ZD0145);Shaoyang Vocational and Technical College Teaching Research and Reform Project(23JG013);the Scientific Research Project of Hunan Provincial Department of Education(24B1123)
CLC Number:
Cite this article
Jie-Wei HE, Shao-Wei DUAN, Xiao-Chuan LI. Design and Application Research of Shape-Stabilized Phase Change Materials for Building Energy Conservation[J]. Chinese Journal of Applied Chemistry, 2024, 41(12): 1780-1789.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: http://yyhx.ciac.jl.cn/EN/10.19894/j.issn.1000-0518.240173
| No | m(raw material)/g∣w(raw material)/% | |||||
|---|---|---|---|---|---|---|
| Na2SO4·10H2O | Na2HPO4·12H2O | KCl | CMC | Deionized water | Graphite | |
| 1 | 2.100∣19.4 | 6.303∣58.2 | 0.427∣3.9 | 0.500∣4.6 | 1.000∣9.2 | 0.500∣4.6 |
| 2 | 2.000∣20.1 | 6.000∣61.3 | 0.500∣5.1 | 0.090∣0.9 | 0.700∣7.2 | 0.500∣5.1 |
| 3 | 2.500∣23.2 | 6.500∣60.4 | 0.500∣4.6 | 0.070∣0.7 | 0.700∣6.5 | 0.500∣4.6 |
Table 1 Inorganic phase change material raw material ratio
| No | m(raw material)/g∣w(raw material)/% | |||||
|---|---|---|---|---|---|---|
| Na2SO4·10H2O | Na2HPO4·12H2O | KCl | CMC | Deionized water | Graphite | |
| 1 | 2.100∣19.4 | 6.303∣58.2 | 0.427∣3.9 | 0.500∣4.6 | 1.000∣9.2 | 0.500∣4.6 |
| 2 | 2.000∣20.1 | 6.000∣61.3 | 0.500∣5.1 | 0.090∣0.9 | 0.700∣7.2 | 0.500∣5.1 |
| 3 | 2.500∣23.2 | 6.500∣60.4 | 0.500∣4.6 | 0.070∣0.7 | 0.700∣6.5 | 0.500∣4.6 |
| No | m(raw material)/g∣w(raw material)/% | ||||
|---|---|---|---|---|---|
| C17H34O2 | C12H24O2 | C12H26O | SiO2 | SEBS | |
| 1 | 1∣9.6 | 9∣86.5 | 0.1∣1.0 | 0.1∣1.0 | 0.2∣1.9 |
| 2 | 2∣19.2 | 8∣77.0 | 0.1∣1.0 | 0.1∣1.0 | 0.2∣1.9 |
| 3 | 3∣28.9 | 7∣67.3 | 0.1∣1.0 | 0.1∣1.0 | 0.2∣1.9 |
| 4 | 4∣38.5 | 6∣57.7 | 0.1∣1.0 | 0.1∣1.0 | 0.2∣1.9 |
| 5 | 5∣48.1 | 5∣48.1 | 0.1∣1.0 | 0.1∣1.0 | 0.2∣1.9 |
| 6 | 6∣57.7 | 4∣38.5 | 0.1∣1.0 | 0.1∣1.0 | 0.2∣1.9 |
| 7 | 7∣67.3 | 3∣28.9 | 0.1∣1.0 | 0.1∣1.0 | 0.2∣1.9 |
| 8 | 8∣77.0 | 2∣19.2 | 0.1∣1.0 | 0.1∣1.0 | 0.2∣1.9 |
| 9 | 9∣86.5 | 1∣9.6 | 0.1∣1.0 | 0.1∣1.0 | 0.2∣1.9 |
Table 2 Organic phase change material raw material ratio
| No | m(raw material)/g∣w(raw material)/% | ||||
|---|---|---|---|---|---|
| C17H34O2 | C12H24O2 | C12H26O | SiO2 | SEBS | |
| 1 | 1∣9.6 | 9∣86.5 | 0.1∣1.0 | 0.1∣1.0 | 0.2∣1.9 |
| 2 | 2∣19.2 | 8∣77.0 | 0.1∣1.0 | 0.1∣1.0 | 0.2∣1.9 |
| 3 | 3∣28.9 | 7∣67.3 | 0.1∣1.0 | 0.1∣1.0 | 0.2∣1.9 |
| 4 | 4∣38.5 | 6∣57.7 | 0.1∣1.0 | 0.1∣1.0 | 0.2∣1.9 |
| 5 | 5∣48.1 | 5∣48.1 | 0.1∣1.0 | 0.1∣1.0 | 0.2∣1.9 |
| 6 | 6∣57.7 | 4∣38.5 | 0.1∣1.0 | 0.1∣1.0 | 0.2∣1.9 |
| 7 | 7∣67.3 | 3∣28.9 | 0.1∣1.0 | 0.1∣1.0 | 0.2∣1.9 |
| 8 | 8∣77.0 | 2∣19.2 | 0.1∣1.0 | 0.1∣1.0 | 0.2∣1.9 |
| 9 | 9∣86.5 | 1∣9.6 | 0.1∣1.0 | 0.1∣1.0 | 0.2∣1.9 |
| No | Wax melting point/℃ | m(raw material)/g∣w(raw material)/% | |
|---|---|---|---|
| Wax | SEBS | ||
| 1 | 20 | 16∣80.0 | 4∣20.0 |
| 2 | 32 | 16∣80.0 | 4∣20.0 |
| 3 | 48 | 16∣80.0 | 4∣20.0 |
Table 3 Material ratio of phase change encapsulation materials
| No | Wax melting point/℃ | m(raw material)/g∣w(raw material)/% | |
|---|---|---|---|
| Wax | SEBS | ||
| 1 | 20 | 16∣80.0 | 4∣20.0 |
| 2 | 32 | 16∣80.0 | 4∣20.0 |
| 3 | 48 | 16∣80.0 | 4∣20.0 |
| No | m(raw material)/g∣w(raw material)/% | Phase change material (piece) | m(raw material)/g∣w(raw material)/% | Phase change material (piece) | ||
|---|---|---|---|---|---|---|
| CaHPO4·0.5H2O | Deionized water | CaHPO4·0.5H2O | Deionized water | |||
| 1 | 18.0∣57.1 | 13.5∣42.9 | 0 | 149.4∣57.1 | 112.1∣42.9 | 0 |
| 2 | 15.5∣57.2 | 11.6∣42.8 | 4 | 128.7∣57.2 | 96.3∣42.8 | 33 |
| 3 | 14.2∣57.0 | 10.7∣43.0 | 6 | 117.9∣57.0 | 88.8∣43.0 | 50 |
| 4 | 12.9∣56.8 | 9.8∣43.0 | 8 | 107.1∣56.8 | 81.4∣43.2 | 67 |
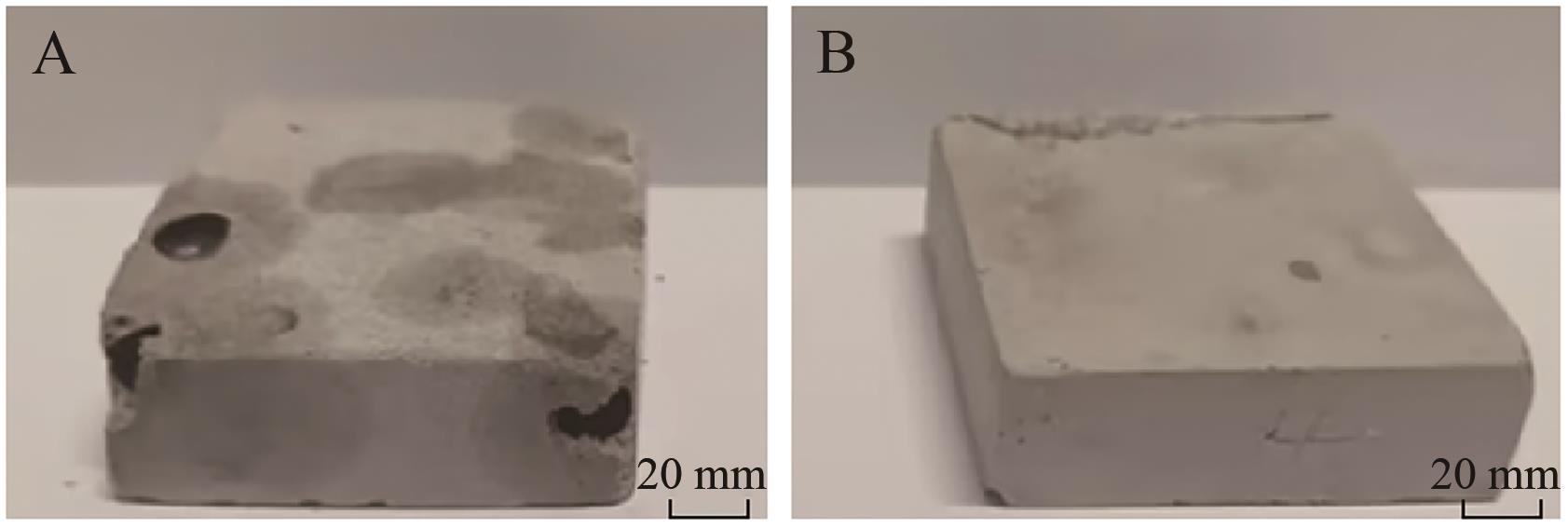
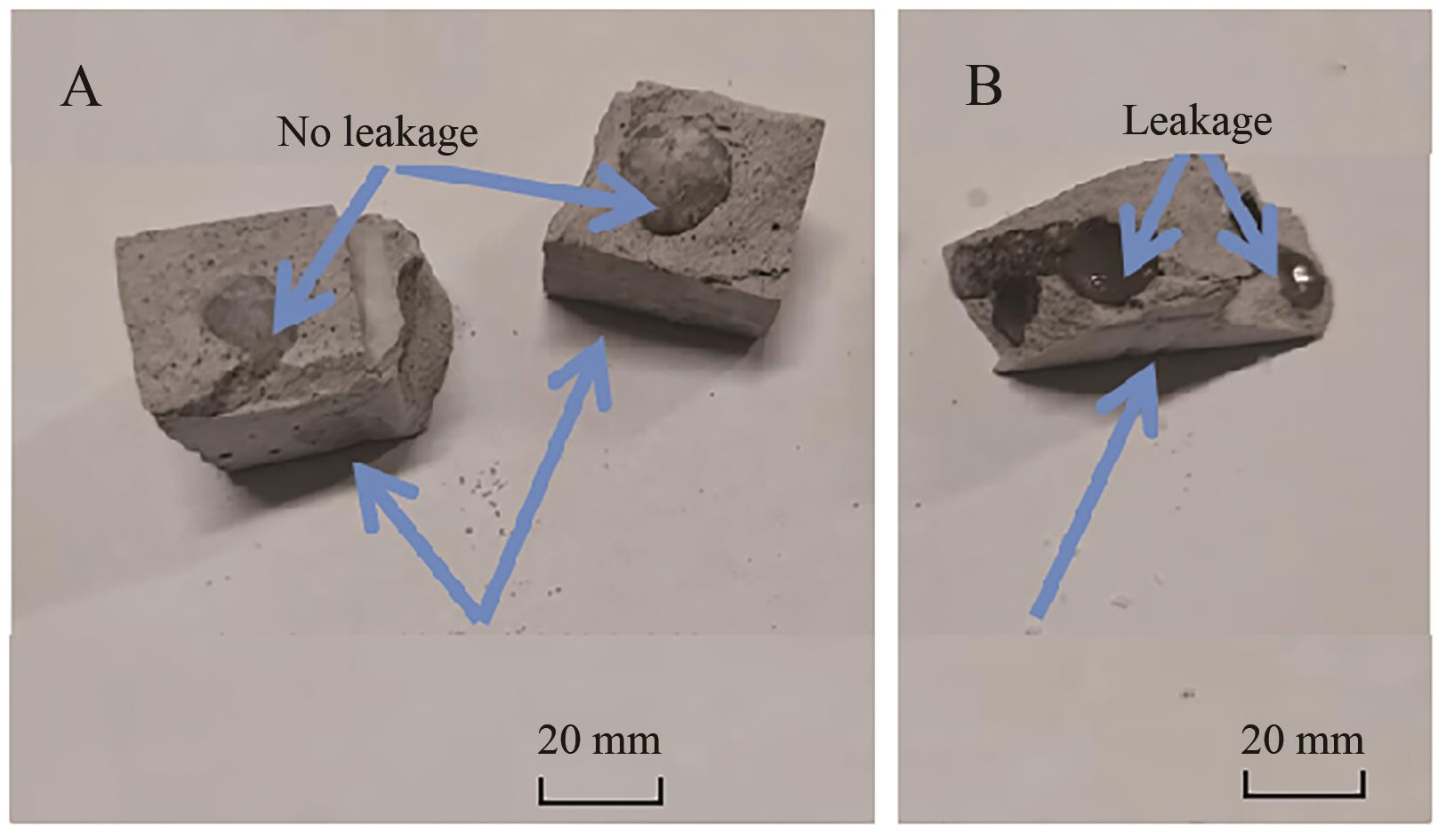
Table 4 Raw material ratio of model bricks
| No | m(raw material)/g∣w(raw material)/% | Phase change material (piece) | m(raw material)/g∣w(raw material)/% | Phase change material (piece) | ||
|---|---|---|---|---|---|---|
| CaHPO4·0.5H2O | Deionized water | CaHPO4·0.5H2O | Deionized water | |||
| 1 | 18.0∣57.1 | 13.5∣42.9 | 0 | 149.4∣57.1 | 112.1∣42.9 | 0 |
| 2 | 15.5∣57.2 | 11.6∣42.8 | 4 | 128.7∣57.2 | 96.3∣42.8 | 33 |
| 3 | 14.2∣57.0 | 10.7∣43.0 | 6 | 117.9∣57.0 | 88.8∣43.0 | 50 |
| 4 | 12.9∣56.8 | 9.8∣43.0 | 8 | 107.1∣56.8 | 81.4∣43.2 | 67 |
| No | m(raw material)/g∣w(raw material)/% | Phase transition temperature/℃ | Latent heat of phase transformation/(kJ·kg-1) | |
|---|---|---|---|---|
| C17H34O2 | C12H24O2 | |||
| 1 | 1∣9.6 | 9∣86.5 | 34.5~41.5 | 187.0 |
| 2 | 2∣19.2 | 8∣77.0 | 31.0~37.5 | 184.0 |
| 3 | 3∣28.9 | 7∣67.3 | 28.0~36.0 | 181.0 |
| 4 | 4∣38.5 | 6∣57.7 | 26.5~33.5 | 178.0 |
| 5 | 5∣48.1 | 5∣48.1 | 25.5~32.0 | 175.0 |
| 6 | 6∣57.7 | 4∣38.5 | 24.5~28.5 | 172.0 |
| 7 | 7∣67.3 | 3∣28.9 | 23.5~27.5 | 169.0 |
| 8 | 8∣77.0 | 2∣19.2 | 21.5~24.5 | 166.0 |
| 9 | 9∣86.5 | 1∣9.6 | / | 163.0 |
Table 5 Phase transition temperature and latent heat of phase change materials with different raw material ratios
| No | m(raw material)/g∣w(raw material)/% | Phase transition temperature/℃ | Latent heat of phase transformation/(kJ·kg-1) | |
|---|---|---|---|---|
| C17H34O2 | C12H24O2 | |||
| 1 | 1∣9.6 | 9∣86.5 | 34.5~41.5 | 187.0 |
| 2 | 2∣19.2 | 8∣77.0 | 31.0~37.5 | 184.0 |
| 3 | 3∣28.9 | 7∣67.3 | 28.0~36.0 | 181.0 |
| 4 | 4∣38.5 | 6∣57.7 | 26.5~33.5 | 178.0 |
| 5 | 5∣48.1 | 5∣48.1 | 25.5~32.0 | 175.0 |
| 6 | 6∣57.7 | 4∣38.5 | 24.5~28.5 | 172.0 |
| 7 | 7∣67.3 | 3∣28.9 | 23.5~27.5 | 169.0 |
| 8 | 8∣77.0 | 2∣19.2 | 21.5~24.5 | 166.0 |
| 9 | 9∣86.5 | 1∣9.6 | / | 163.0 |
| Phase change materials | Number of cycles/frequency | Latent heat/(kJ·kg-1) |
|---|---|---|
| C17H34O2-C12H24O2 | 0 | 172.0 |
| 100 | 169.6 | |
| 200 | 167.3 |
Table 6 Thermal performance of CCTPF-PW after different cycles
| Phase change materials | Number of cycles/frequency | Latent heat/(kJ·kg-1) |
|---|---|---|
| C17H34O2-C12H24O2 | 0 | 172.0 |
| 100 | 169.6 | |
| 200 | 167.3 |
| Samples | λ/(W·m-1·K-1) |
|---|---|
| SiO2·nH2O | 0.075 |
| C17H34O2-C12H24O2 | 0.256 |
| C17H34O2-C12H24O2/SiO2 | 0.145 |
Table 7 Thermal conductivity of phase change materials
| Samples | λ/(W·m-1·K-1) |
|---|---|
| SiO2·nH2O | 0.075 |
| C17H34O2-C12H24O2 | 0.256 |
| C17H34O2-C12H24O2/SiO2 | 0.145 |
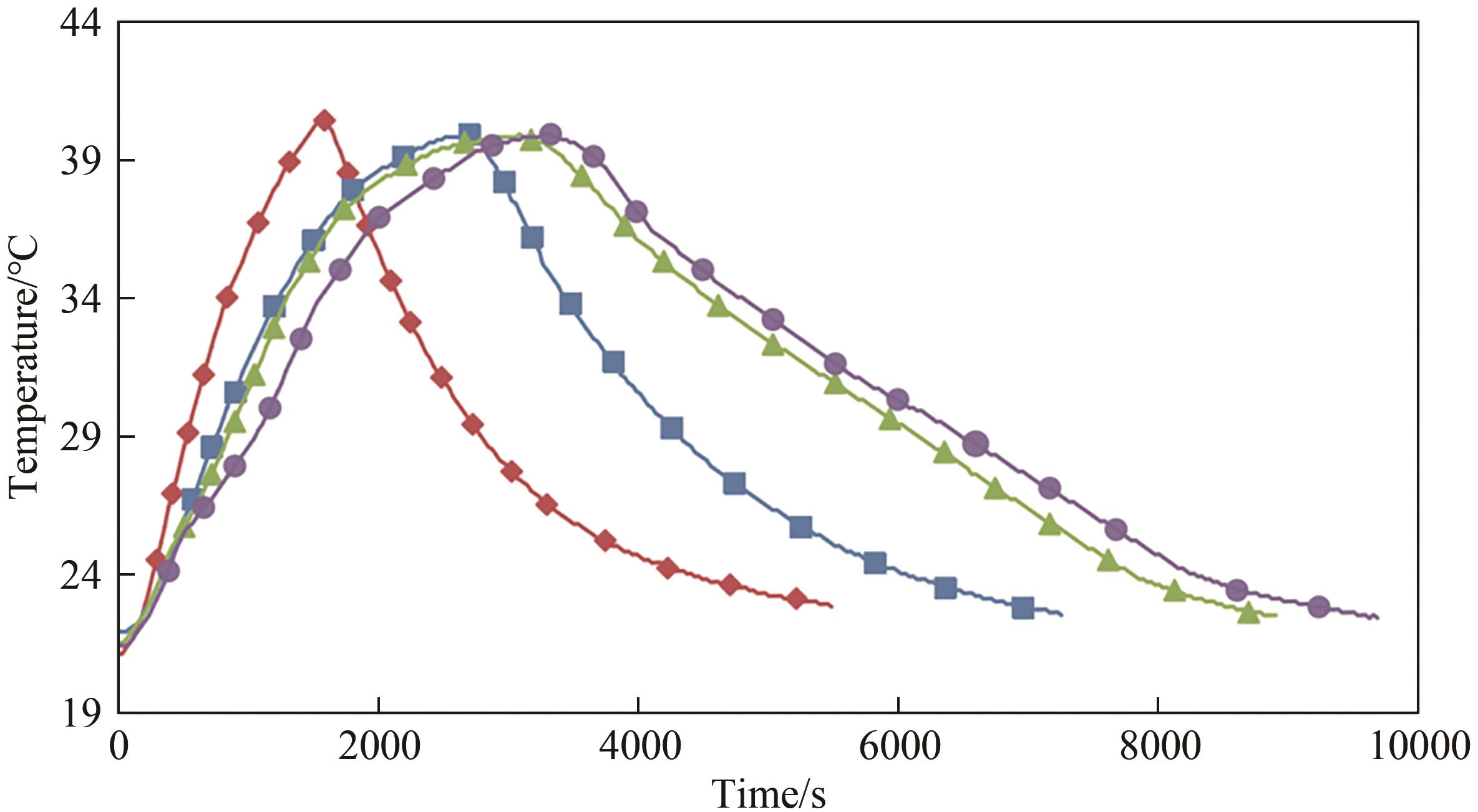
Fig.7 Simulation of the variation of temperature in a room over timeNote: Red, blue, green, and purple respectively represent the temperature changes over time in a simulated room constructed with no added phase change material bricks, 4 added small ball phase change material bricks, 6 added small ball phase change material bricks, and 8 added small ball phase change material bricks
| 1 | 张华民. 高效大规模化学储能技术研究开发现状及展望[J]. 电源技术, 2007(8): 587-591. |
| ZHANG H M. Research and development status and prospects of efficient large scale chemical energy storage technology[J]. Power Suppl Technol, 2007(8): 587-591. | |
| 2 | 李国俭. 相变储能材料开发与封装技术研究进展[J]. 热力发电, 2023, 52(2): 23-31. |
| LI G J. Research progress in the development and packaging technology of phase change energy storage materials[J]. Therm Power Generation, 2023, 52(2): 23-31. | |
| 3 | 陈中华, 马丽丽, 余飞. 有机相变储能材料及其复合化研究进展[J]. 化工新型材料, 2010(9): 42-44, 64. |
| CHEN Z H, MA L L, YU F. Research progress on organic phase change energy storage materials and their composites[J]. New Chem Mater, 2010(9): 42-44, 64. | |
| 4 | 陈爱英, 汪学英, 曹学增. 相变储能材料的研究进展与应用[J]. 材料导报, 2003, 17(5): 42-44, 72. |
| CHEN A Y, WANG X Y, CAO X Z. Research progress and application of phase change energy storage materials[J]. Mater Rev, 2003, 17(5): 42-44, 72. | |
| 5 | 曾关跃, 高专, 熊玉竹. 多壁碳纳米管负载银/微晶纤维素定型复合相变材料的制备及性能研究[J]. 材料导报, 2023(23): 231-236. |
| ZENG G Y, GAO Z, XIONG Y Z. Preparation and performance study of multi walled carbon nanotube loaded silver/microcrystalline cellulose shaped composite phase change materials[J]. Mater Introduct, 2023(23): 231-236. | |
| 6 | REDDY V J, AKHILA K, DIXIT P, et al. Thermal buffering performance evaluation of fatty acids blend/fatty alcohol based eutectic phase change material and simulation[J]. J Energy Storage, 2021, 38: 102499. |
| 7 | 叶志林, 魏婷, 易红玲, 等. 癸酸-棕榈酸/膨胀珍珠岩定型相变材料的制备与热性能[J]. 华东理工大学学报(自然科学版), 2017, 43(4): 495-500. |
| YE Z L, WEI T, YI H L, et al. Preparation and thermal properties of decanoic palmitic acid/expanded perlite phase change materials[J]. J East China Univ Sci Technol (Nat Sci Ed), 2017, 43(4): 495-500. | |
| 8 | 舒钊, 钟珂, 肖鑫, 等. 硅藻土基脂肪酸定型相变材料的制备与表征[J]. 硅酸盐学报, 2022, 50(6): 1652-1660. |
| SHU Z, ZHONG K, XIAO X, et al. Preparation and characterization of diatomaceous earth based fatty acid phase change materials[J]. J Chin Ceramic Soc, 2022, 50(6): 1652-1660. | |
| 9 | 陈云博, 李昕怡, 毛志平, 等. 纳米甲壳素基Pickering乳液辅助构筑相变微胶囊[J]. 日用化学工业(中英文), 2022, 52(12):1286-1292. |
| CHEN Y B, LI X Y, MAO Z P, et al. Construction of phase change microcapsules assisted by nano chitin based Pickering lotion[J]. Daily Chem Ind (Eng Chin), 2022, 52(12): 1286-1292. | |
| 10 | 徐众, 李军, 吴恩辉, 等. 添加提钒尾渣对膨胀石墨/石蜡复合相变材料稳定性和导电性的影响[J]. 应用化学, 2022, 39(3): 461-469. |
| XU Z, LI J, WU E H, et al. The effect of adding vanadium extraction tailings on the stability and conductivity of expanded graphite/paraffin composite phase change materials[J]. Chin J Appl Chem, 2022, 39(3): 461-469. | |
| 11 | XIANG B, YANG Z B, ZHANG J. ASA/SEBS/paraffin composites as phase change material for potential cooling and heating applications in building[J]. Polym Adv Technol, 2021, 32(1): 420-427. |
| 12 | 吴秋萍, 蔡昆廷, 王元康, 等. Co/C微波吸收性能及Co/C-聚氨酯相变复合材料的微波-热转换性能[J]. 应用化学, 2021, 38(12): 1588-1598. |
| WU Q P, CAI K T, WANG Y K, et al. Microwave absorption performance of Co/C and microwave thermal conversion performance of Co/C-polyurethane phase change composite materials[J]. Chin J Appl Chem, 2021, 38 (12): 1588-1598. | |
| 13 | 陈曦, 郑楠, 刘凌志, 等. 长链烷酸接枝羟丙基纤维素相变材料的制备及其性能[J]. 应用化学, 2015, 32(5): 535-541. |
| CHEN X, ZHENG N, LIU L Z, et al. Preparation and properties of long-chain alkanoic acid grafted hydroxypropyl cellulose phase change materials[J]. Chin J Appl Chem, 2015, 32(5): 535-541. | |
| 14 | 吴秋萍, 蔡昆廷, 王元康, 等. Co/C微波吸收性能及Co/C-聚氨酯相变复合材料的微波-热转换性能[J]. 应用化学, 2021, 38(12): 969-975. |
| WU Q P, CAI K T, WANG Y K, et al. Microwave absorption performance of Co/C and microwave thermal conversion performance of Co/C-polyurethane phase change composite materials[J]. Chin J Appl Chem, 2021, 38(12): 969-975. |
| [1] | Zhong XU, Jun LI, En-Hui WU, Yan JIANG. Influence of Vanadium Tailings on the Thermal Stability and Electrical Conductivity of Expanded Graphite/ Paraffin Composite Phase Change Materials [J]. Chinese Journal of Applied Chemistry, 2022, 39(3): 461-469. |
| [2] | WU Qiu-Ping, CAI Kun-Ting, WANG Yuan-Kang, SUN Kai, YANG Jin-Bo, HAN Song-Bai, LIU Yun-Tao. Microwave Absorption Properties of Co/C and Microwave-Heat Conversion Properties of Co/C-Polyurethane Phase Change Composites [J]. Chinese Journal of Applied Chemistry, 2021, 38(12): 0-0. |
| [3] | WU Qiu-Ping, CAI Kun-Ting, WANG Yuan-Kang, SUN Kai, YANG Jin-Bo, HAN Song-Bai, LIU Yun-Tao. Microwave Absorption Properties of Co/C and Microwave-Heat Conversion Properties of Co/C-Polyurethane Phase Change Composites [J]. Chinese Journal of Applied Chemistry, 2021, 38(12): 1588-1598. |
| [4] | AN Pinping, LUO Fubin, HUANG Baoquan, XIAO Liren, QIAN Qingrong, LI Hongzhou, CHEN Qinghua. Properties of Thermal Conductivity Enhanced Polyethylene Glycol-Based Phase Change Composites [J]. Chinese Journal of Applied Chemistry, 2020, 37(1): 46-53. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||