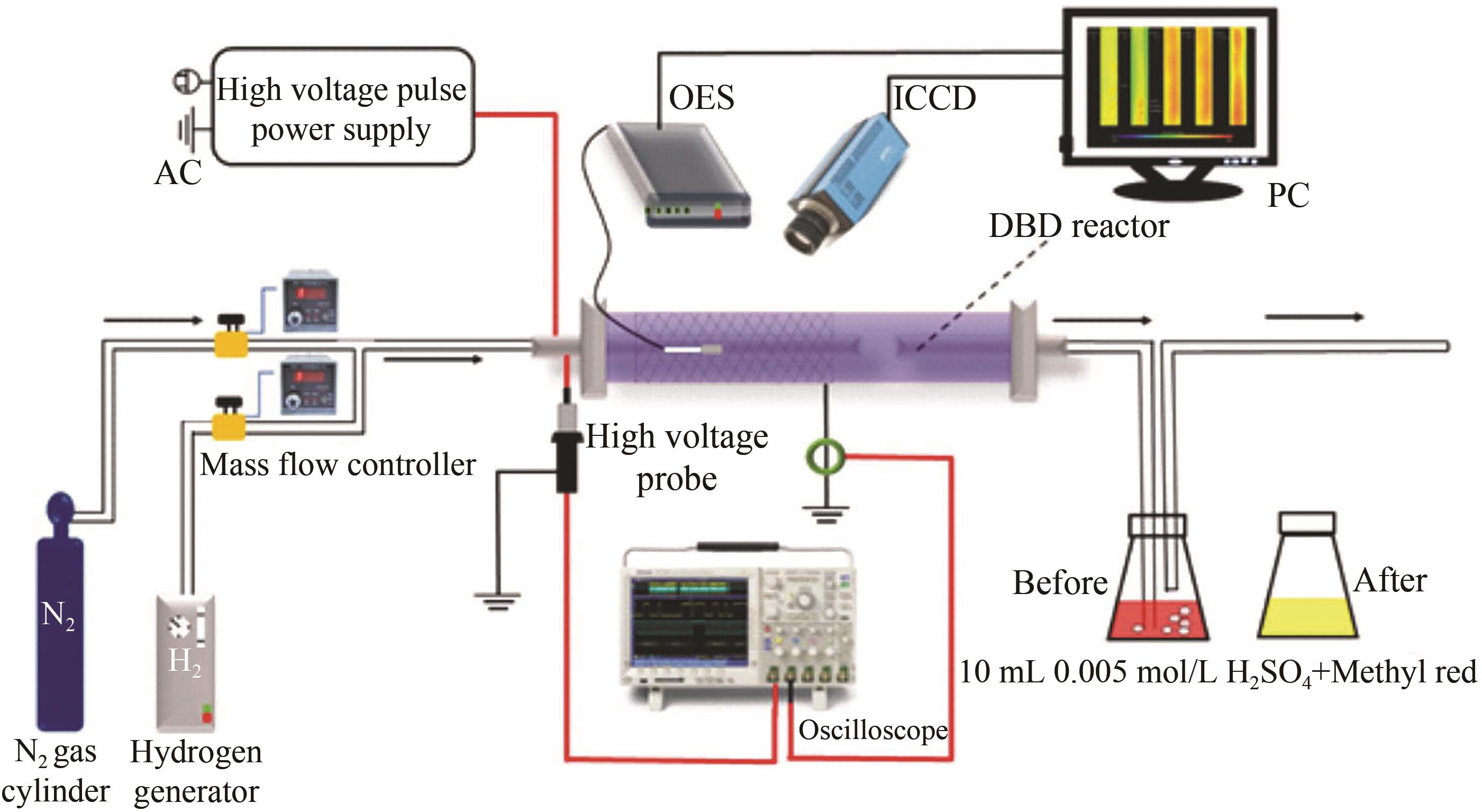
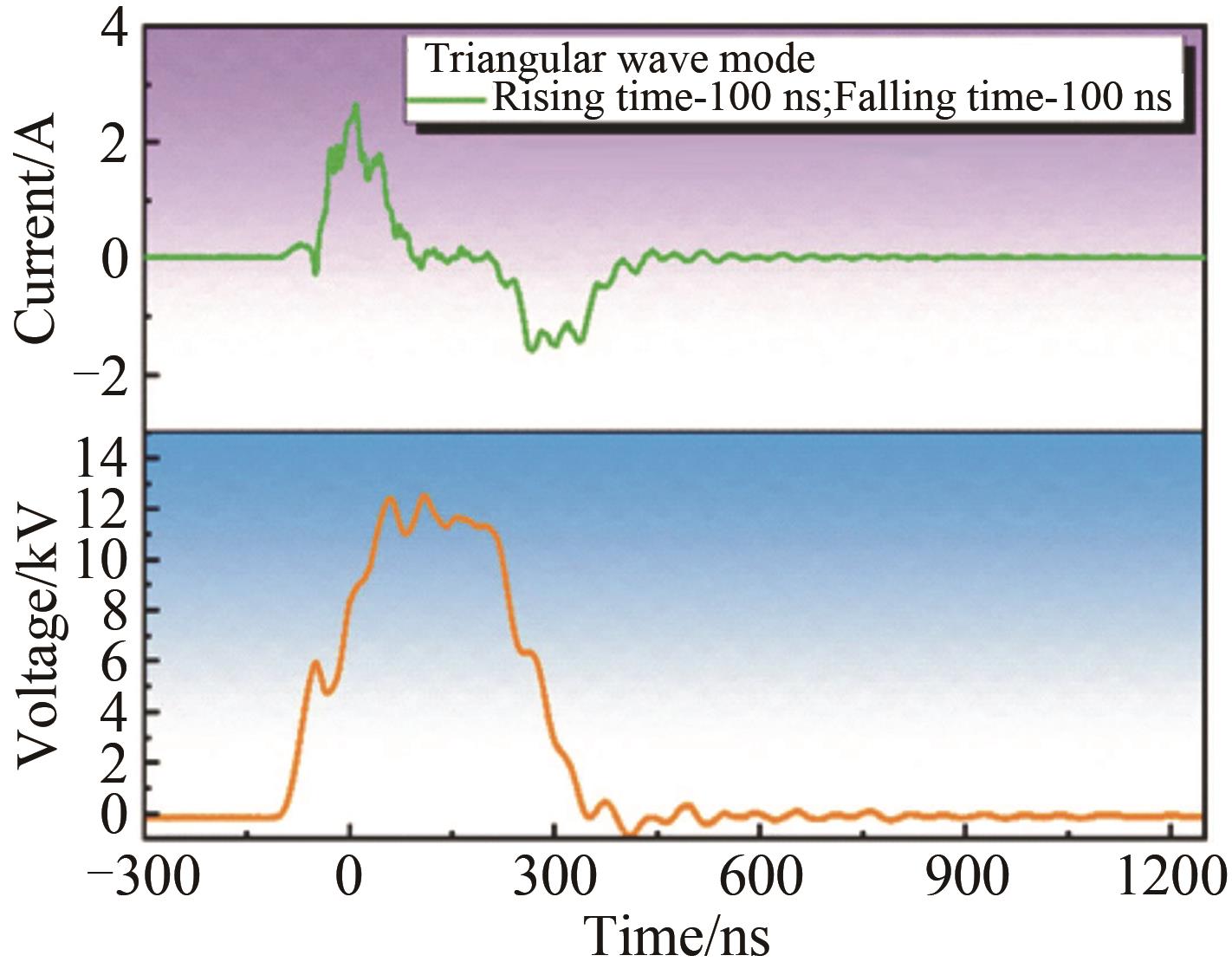
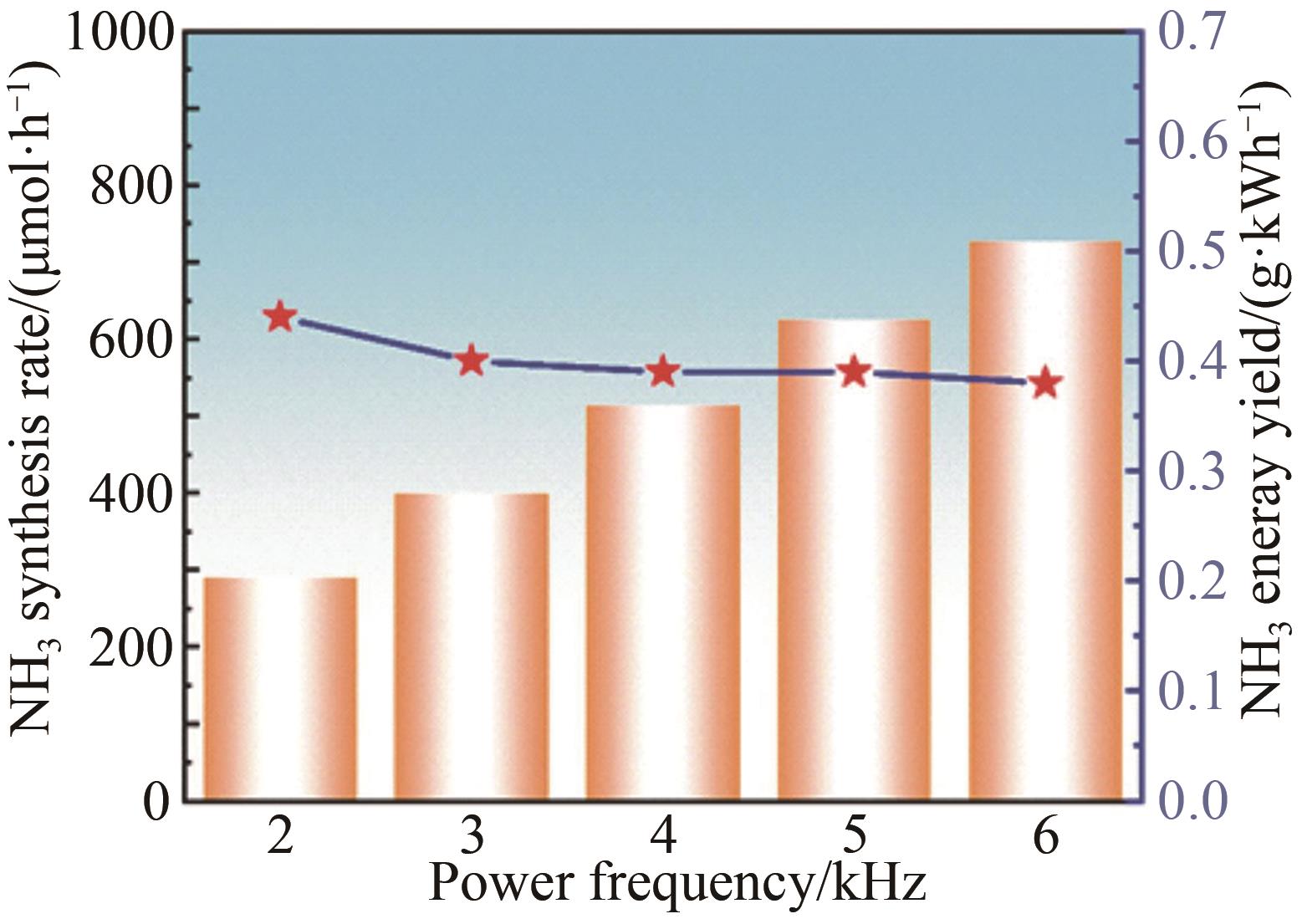
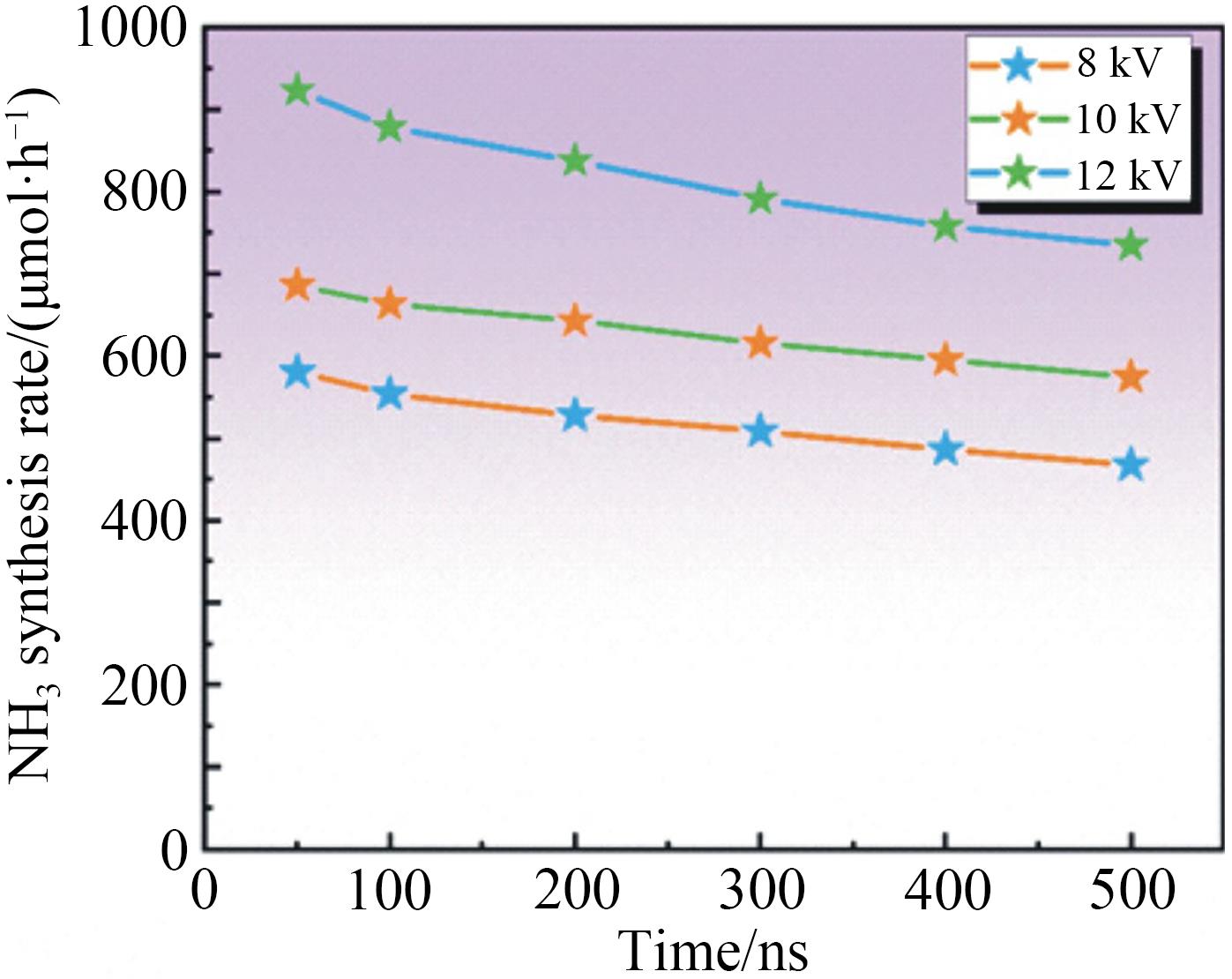
Chinese Journal of Applied Chemistry ›› 2023, Vol. 40 ›› Issue (2): 268-276.DOI: 10.19894/j.issn.1000-0518.220204
• Full Papers • Previous Articles Next Articles
Optimization of Process Parameters for Ammonia Synthesis by Nanosecond Pulsed Dielectric Barrier Discharge Plasma
Yang LIU, Hai-Bao ZHANG( ), Qiang CHEN
), Qiang CHEN
- Laboratory of Plasma Physics and Materials,Beijing Institute of Graphic Communication,Beijing 102600,China
-
Received:2022-06-08Accepted:2022-10-12Published:2023-02-01Online:2023-02-27 -
Contact:Hai-Bao ZHANG -
About author:hbzhang@bigc.edu.cn
-
Supported by:the National Natural Science Foundation of China(11875090);Beijing Municipal National Science Foundation(1192008);Beijing Municipal Education Commission Project(KM202010015003)
CLC Number:
Cite this article
Yang LIU, Hai-Bao ZHANG, Qiang CHEN. Optimization of Process Parameters for Ammonia Synthesis by Nanosecond Pulsed Dielectric Barrier Discharge Plasma[J]. Chinese Journal of Applied Chemistry, 2023, 40(2): 268-276.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: http://yyhx.ciac.jl.cn/EN/10.19894/j.issn.1000-0518.220204
| Horizontal factor | A | B | C | D |
|---|---|---|---|---|
| Peak voltage/ kV | Power frequency/ kHz | Total gas flow/ (mL·min-1) | V(N2)∶V(H2) | |
| 1 | 8 | 2 | 200 | 1∶3 |
| 2 | 10 | 3 | 180 | 1∶2 |
| 3 | 12 | 4 | 160 | 1∶1 |
| 4 | 14 | 5 | 140 | 2∶1 |
| 5 | 16 | 6 | 120 | 3∶1 |
Table 1 Levels of the factors used in the orthogonal experiment
| Horizontal factor | A | B | C | D |
|---|---|---|---|---|
| Peak voltage/ kV | Power frequency/ kHz | Total gas flow/ (mL·min-1) | V(N2)∶V(H2) | |
| 1 | 8 | 2 | 200 | 1∶3 |
| 2 | 10 | 3 | 180 | 1∶2 |
| 3 | 12 | 4 | 160 | 1∶1 |
| 4 | 14 | 5 | 140 | 2∶1 |
| 5 | 16 | 6 | 120 | 3∶1 |
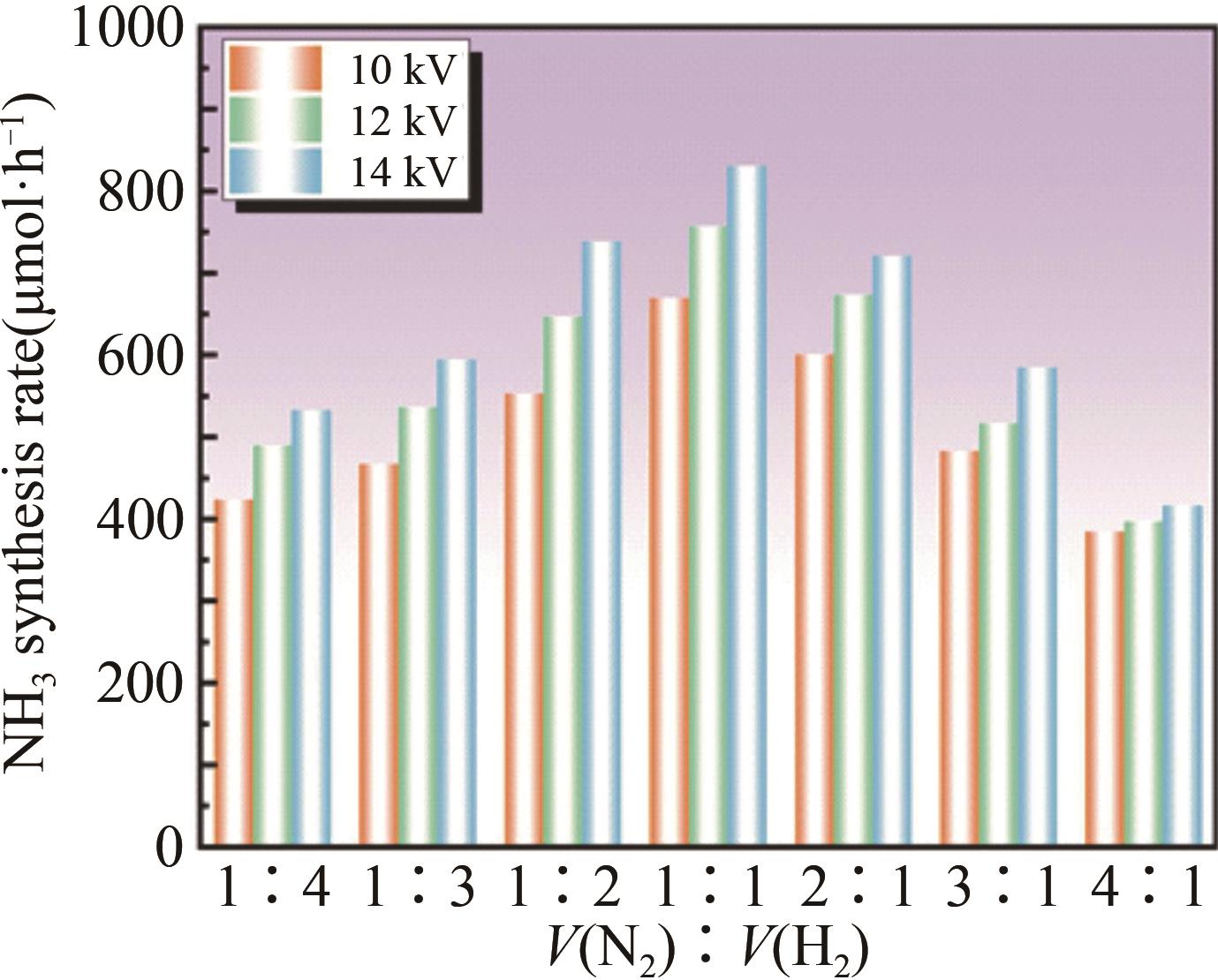
| Experiment number | A | B | C | D | Ammonia synthesis rate/ (μmol·h-1) | Energy yield/ (g?kWh-1) |
|---|---|---|---|---|---|---|
| Peak voltage/kV | Power frequency/kHz | Total gas flow/(mL·min-1) | V(N2)∶V(H2) | |||
| 1 | 8 | 2 | 200 | 1∶3 | 135.75 | 0.28 |
| 2 | 8 | 3 | 160 | 2∶1 | 107.14 | 0.30 |
| 3 | 8 | 4 | 120 | 1∶2 | 190.48 | 0.36 |
| 4 | 8 | 5 | 180 | 3∶1 | 239.20 | 0.30 |
| 5 | 8 | 6 | 140 | 1∶1 | 350.88 | 0.28 |
| 6 | 10 | 2 | 120 | 2∶1 | 180.45 | 0.32 |
| 7 | 10 | 3 | 180 | 1∶2 | 272.73 | 0.23 |
| 8 | 10 | 4 | 140 | 3∶1 | 194.81 | 0.20 |
| 9 | 10 | 5 | 200 | 1∶1 | 483.22 | 0.30 |
| 10 | 10 | 6 | 160 | 1∶3 | 441.72 | 0.26 |
| 11 | 12 | 2 | 140 | 1∶2 | 221.13 | 0.26 |
| 12 | 12 | 3 | 200 | 3∶1 | 218.45 | 0.20 |
| 13 | 12 | 4 | 160 | 1∶1 | 442.26 | 0.29 |
| 14 | 12 | 5 | 120 | 1∶3 | 413.79 | 0.25 |
| 15 | 12 | 6 | 180 | 2∶1 | 545.45 | 0.26 |
| 16 | 14 | 2 | 160 | 3∶1 | 244.90 | 0.29 |
| 17 | 14 | 3 | 120 | 1∶1 | 371.13 | 0.38 |
| 18 | 14 | 4 | 180 | 1∶3 | 413.79 | 0.18 |
| 19 | 14 | 5 | 140 | 2∶1 | 383.80 | 0.13 |
| 20 | 14 | 6 | 200 | 1∶2 | 666.67 | 0.27 |
| 21 | 16 | 2 | 180 | 1∶1 | 423.53 | 0.26 |
| 22 | 16 | 3 | 140 | 1∶3 | 365.48 | 0.20 |
| 23 | 16 | 4 | 200 | 2∶1 | 456.85 | 0.20 |
| 24 | 16 | 5 | 160 | 1∶2 | 692.31 | 0.24 |
| 25 | 16 | 6 | 120 | 3∶1 | 264.71 | 0.09 |
| NH3 synthesis rate/(μmol·h-1) | ||||||
| k1 | 204.69 | 241.15 | 392.19 | 354.11 | ||
| k2 | 314.59 | 266.99 | 378.94 | 408.70 | ||
| k3 | 368.22 | 339.64 | 364.24 | 414.20 | ||
| k4 | 416.06 | 442.46 | 303.22 | 334.74 | ||
| k5 | 440.58 | 453.89 | 246.02 | 232.41 | ||
| Range R1 | 235.89 | 212.74 | 146.17 | 181.79 | ||
| Order of factors | A>B>D>C | |||||
| Energy yield/(g?kWh-1) | ||||||
| k1 | 0.30 | 0.28 | 0.25 | 0.23 | ||
| k2 | 0.26 | 0.26 | 0.25 | 0.27 | ||
| k3 | 0.25 | 0.25 | 0.22 | 0.30 | ||
| k4 | 0.25 | 0.24 | 0.21 | 0.24 | ||
| k5 | 0.20 | 0.23 | 0.21 | 0.22 | ||
| Range R2 | 0.10 | 0.05 | 0.04 | 0.08 | ||
| Order of factors | A>D>B>C | |||||
Table 2 Orthogonal experimental results and rang analysis of NH3 synthesis by nanosecond pulsed dielectric barrier discharge plasma
| Experiment number | A | B | C | D | Ammonia synthesis rate/ (μmol·h-1) | Energy yield/ (g?kWh-1) |
|---|---|---|---|---|---|---|
| Peak voltage/kV | Power frequency/kHz | Total gas flow/(mL·min-1) | V(N2)∶V(H2) | |||
| 1 | 8 | 2 | 200 | 1∶3 | 135.75 | 0.28 |
| 2 | 8 | 3 | 160 | 2∶1 | 107.14 | 0.30 |
| 3 | 8 | 4 | 120 | 1∶2 | 190.48 | 0.36 |
| 4 | 8 | 5 | 180 | 3∶1 | 239.20 | 0.30 |
| 5 | 8 | 6 | 140 | 1∶1 | 350.88 | 0.28 |
| 6 | 10 | 2 | 120 | 2∶1 | 180.45 | 0.32 |
| 7 | 10 | 3 | 180 | 1∶2 | 272.73 | 0.23 |
| 8 | 10 | 4 | 140 | 3∶1 | 194.81 | 0.20 |
| 9 | 10 | 5 | 200 | 1∶1 | 483.22 | 0.30 |
| 10 | 10 | 6 | 160 | 1∶3 | 441.72 | 0.26 |
| 11 | 12 | 2 | 140 | 1∶2 | 221.13 | 0.26 |
| 12 | 12 | 3 | 200 | 3∶1 | 218.45 | 0.20 |
| 13 | 12 | 4 | 160 | 1∶1 | 442.26 | 0.29 |
| 14 | 12 | 5 | 120 | 1∶3 | 413.79 | 0.25 |
| 15 | 12 | 6 | 180 | 2∶1 | 545.45 | 0.26 |
| 16 | 14 | 2 | 160 | 3∶1 | 244.90 | 0.29 |
| 17 | 14 | 3 | 120 | 1∶1 | 371.13 | 0.38 |
| 18 | 14 | 4 | 180 | 1∶3 | 413.79 | 0.18 |
| 19 | 14 | 5 | 140 | 2∶1 | 383.80 | 0.13 |
| 20 | 14 | 6 | 200 | 1∶2 | 666.67 | 0.27 |
| 21 | 16 | 2 | 180 | 1∶1 | 423.53 | 0.26 |
| 22 | 16 | 3 | 140 | 1∶3 | 365.48 | 0.20 |
| 23 | 16 | 4 | 200 | 2∶1 | 456.85 | 0.20 |
| 24 | 16 | 5 | 160 | 1∶2 | 692.31 | 0.24 |
| 25 | 16 | 6 | 120 | 3∶1 | 264.71 | 0.09 |
| NH3 synthesis rate/(μmol·h-1) | ||||||
| k1 | 204.69 | 241.15 | 392.19 | 354.11 | ||
| k2 | 314.59 | 266.99 | 378.94 | 408.70 | ||
| k3 | 368.22 | 339.64 | 364.24 | 414.20 | ||
| k4 | 416.06 | 442.46 | 303.22 | 334.74 | ||
| k5 | 440.58 | 453.89 | 246.02 | 232.41 | ||
| Range R1 | 235.89 | 212.74 | 146.17 | 181.79 | ||
| Order of factors | A>B>D>C | |||||
| Energy yield/(g?kWh-1) | ||||||
| k1 | 0.30 | 0.28 | 0.25 | 0.23 | ||
| k2 | 0.26 | 0.26 | 0.25 | 0.27 | ||
| k3 | 0.25 | 0.25 | 0.22 | 0.30 | ||
| k4 | 0.25 | 0.24 | 0.21 | 0.24 | ||
| k5 | 0.20 | 0.23 | 0.21 | 0.22 | ||
| Range R2 | 0.10 | 0.05 | 0.04 | 0.08 | ||
| Order of factors | A>D>B>C | |||||
| 1 | GALLOWAY J N, TOWNSEND A R, ERISMAN J W, et al. Transformation of the nitrogen cycle: recent trends, questions, and potential solutions[J]. Science, 2008, 320(5878): 889-892. |
| 2 | LICHT S, CUI B, WANG B, et al. Ammonia synthesis by N2 and steam electrolysis in molten hydroxide suspensions of nanoscale Fe2O3[J]. Science, 2015, 45(43): 637-640. |
| 3 | 刘化章. 合成氨工业: 过去、现在和未来——合成氨工业创立100周年回顾、启迪和挑战[J]. 化工进展, 2013, 32(9):1995-2005. |
| LIU H Z. Synthetic ammonia industry: past, present and future—review, enlightenment and challenges of the 100th anniversary of synthetic ammonia industry[J]. Prog Chem Ind, 2013, 32(9): 1995-2005. | |
| 4 | CHEN C, ARYAFAR H, LOVEGROVE K M, et al. Modeling of ammonia synthesis to produce supercritical steam for solar thermochemical energy storage[J]. Sol Energy, 2017, 155: 363-371. |
| 5 | VALERA-MEDINA A, XIAO H, OWEN-JONES M, et al. Ammonia for power[J]. Prog Energ Combus, 2018, 69: 63-102. |
| 6 | YAPICIOGLU A, DINCER I. A review on clean ammonia as a potential fuel for power generators[J]. Renew Sust Energ Rev, 2019, 103: 96-108. |
| 7 | WANG W, PATIL B, HEIJKERS S, et al. Nitrogen fixation by gliding arc plasma: better insight by chemical kinetics modelling[J]. Chem Sus Chem, 2017, 10(10): 2145-2157. |
| 8 | ROBERT S. Catalytic synthesis of ammonia—a “never-ending story”[J]. Angew Chem Int Ed, 2003, 42: 2004-2008. |
| 9 | 韩慧. 高气压非平衡等离子体化学合成氨的研究[D]. 大连: 大连海事大学, 2002. |
| HAN H. Research on chemical synthesis of ammonia by high pressure non-equilibrium plasma[D]. Dalian: Dalian Maritime University, 2002. | |
| 10 | 诸葛绍渊. 常压介质阻挡放电等离子体协同催化合成氨应用基础研究[D]. 杭州: 浙江工业大学, 2015. |
| ZHUGE S Y. Basic research on the application of atmospheric pressure dielectric barrier discharge plasma synergistic catalytic synthesis of ammonia[D]. Hongzhou: Zhejiang University of Technology, 2015. | |
| 11 | TANABE Y, NISHIBAYASHI Y. Developing more sustainable processes for ammonia synthesis[J]. Coord Chem Rev, 2013, 257(17/18): 2551-2564. |
| 12 | WANG Y L, CRAVEN M, YU X T, et al. Plasma-enhanced catalytic synthesis of ammonia over a Ni/Al2O3 catalyst at near-room temperature: insights into the importance of the catalyst surface on the reaction mechanism[J]. ACS Catal, 2019, 9: 10780. |
| 13 | SAADATJOU N, JAFARI A, SAHEBDELFAR S. Ruthenium nano-catalysts for ammonia synthesis: a review[J]. Chem Eng Commun, 2015, 202(4): 420-448. |
| 14 | MARNELLOS G, STOUKIDES M. Ammonia synthesis at atmospheric pressure[J]. Science, 1998, 282(5386): 98-100. |
| 15 | KITANO M, INOUE Y, YAMAZAKI Y, et al. Ammonia synthesis using a stable electride as an electron donor and reversible hydrogen store[J]. Nat Chem, 2012, 4(11): 934-940. |
| 16 | MEHTA P, BARBOUN P, HERRERA F A, et al. Overcoming ammonia synthesis scaling relations with plasma-enabled catalysis[J]. Nat Chem, 2018, 1(4): 269-275. |
| 17 | HONG J, PRAWER S, MURPHY A B. Plasma catalysis as an alternative route for ammonia production: status, mechanisms, and prospects for progress[J]. ACS Sustain Chem Eng, 2018, 6, 15-31. |
| 18 | 郑硕. 介质阻挡放电等离子体协同催化合成氨的实验研究[D]. 北京: 北京交通大学, 2019. |
| ZHENG S. Experimental study on synergistic catalytic synthesis of ammonia by dielectric barrier discharge plasma[D]. Beijing: Beijing Jiaotong University, 2019. | |
| 19 | PATIL B S, CHERKASOV N, LANG J, et al. Low temperature plasma-catalytic NOx synthesis in a packed DBD reactor: effect of support materials and supported active metal oxides[J]. Appl Catal B: Environ, 2016, 194: 123-133. |
| 20 | BAI M, ZHANG Z, BAI X, et al. Plasma synthesis of ammonia with a microgap dielectric barrier discharge at ambient pressure[J]. IEEE T Plasma Sci, 2003, 31(6): 1285-1291. |
| 21 | SHAH J, WU T, LUCERO J, et al. Non-thermal plasma synthesis of ammonia over Ni-MOF-74[J]. ACS Sustain Chem Eng, 2019, 7: 377-383. |
| 22 | JAVISHK S, WANG W, ANNEMIE B, et al. Ammonia synthesis by radio frequency plasma catalysis: revealing the underlying mechanisms[J]. ACS Appl Energy Mater, 2018, 1: 4824-4839. |
| 23 | UYAMA H, NAKAMURA T, TANAKA S, et al. Catalytic effect of iron wires on the syntheses of ammonia and hydrazine in a radio-frequency discharge[J]. Plasma Chem Plasma P, 1993, 13(1): 117-131. |
| 24 | YIN K S, VENUGOPALAN M. Plasma chemical synthesis. I. effect of electrode material on the synthesis of ammonia[J]. Plasma Chem Plasma P, 1983, 3(3): 343-350. |
| 25 | SUGIYAMA K, AKAZAWA K, OSHIMA M, et al. Ammonia synthesis by means of plasma over MgO catalyst[J]. Plasma Chem Plasma P, 1986, 6(2): 179-193. |
| 26 | UYAMA H, MATSUMOT O. Synthesis of ammonia in high-frequency discharges. II. synthesis of ammonia in a microwave discharge under various conditions[J]. Plasma Chem Plasma P, 1989, 9(3): 13-24. |
| 27 | NAKAJIMA J, SEKIGUCHI H. Synthesis of ammonia using microwave discharge at atmospheric pressure[J]. Thin Solid Films, 2008, 516(13): 4446-4451. |
| 28 | 陈杰, 梁华, 魏彪, 等. 参数化纳秒脉冲电源激励下表面介质阻挡放电特性[J]. 高电压技术, 2019, 45(10): 3365-3374. |
| CHEN J, LIANG H, WEI B, et al. Discharge characteristics of surface dielectric barrier discharge driven by parameterized nanosecond pulsed power supply[J]. High Voltage Eng, 2019, 45(10): 3365-3374. | |
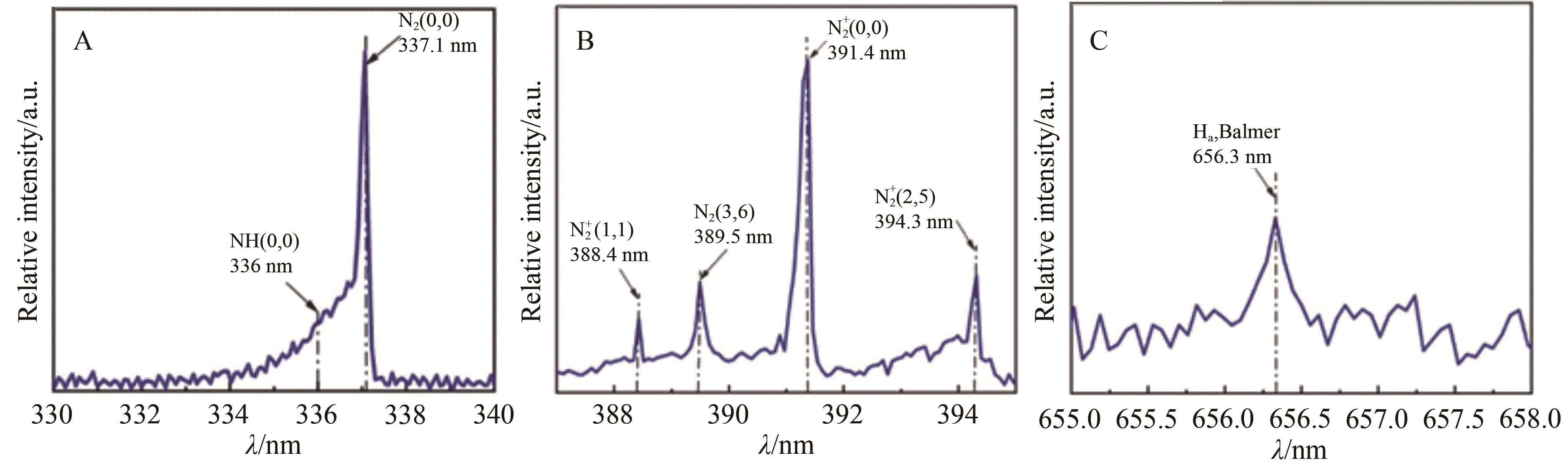
| 29 | 康少芬, 张帅, 陈晓晓, 等. 纳秒脉冲介质阻挡放电等离子体氮还原合成氨的研究[J]. 高电压技术, 2021, 47(1): 368-375. |
| KANG S F, ZHANG S, CHEN X X, et al. Research on nitrogen reduction of synthetic ammonia by nanosecond pulsed dielectric barrier discharge plasma[J]. High Voltage Eng, 2021, 47(1): 368-375. | |
| 30 | LITTLE J, TAKASHIMA K, NISHIHARA M, et al. Separation control with nanosecond-pulse-driven dielectric barrier discharge plasma actuators[J]. AIAA J, 2012, 50(2): 350-365. |
| 31 | MIZUSHIMA T, MATSUMOTO K, OHKITA H, et al. Catalytic effects of metal-loaded membrane-like alumina tubes on ammonia synthesis in atmospheric pressure plasma by dielectric barrier discharge[J]. Plasma Chem Plasma P, 2007, 27(1): 1-11. |
| 32 | PATIL B S, KAATHOVEN A S R V, PEETERS F J J, et al. Deciphering the synergy between plasma and catalyst support for ammonia synthesis in a packed dielectric barrier discharge reactor[J]. J Phys D Appl Phys, 2020, 53(14): 144003. |
| 33 | XIE Q, ZHUGE S, SONG X, et al. Non-thermal atmospheric plasma synthesis of ammonia in a DBD reactor packed with various catalysts[J]. J Phys D Appl Phys, 2020, 53(6): 064002. |
| 34 | KIM H H, TERAMOTO Y, OGATA A, et al. Atmospheric-pressure nonthermal plasma synthesis of ammonia over ruthenium catalysts[J]. Plasma Process Polym, 2017, 14(6): 1600157-1600179. |
| 35 | PENG P, CHENG Y, HATZENBLLER R, et al. Ru-based multifunctional mesoporous catalyst for low-pressure and non-thermal plasma synthesis of ammonia[J]. Int J Hydrogen Energ, 2017, 42(30): 19056-19066. |
| 36 | WAGNER H E, BRANDENBURG R, KOZLOV K V, et al. The barrier discharge[J]. Vacuum, 2003, 71(3): 417-436. |
| 37 | TU X, GALLON H J, WHITEHEAD J C. Transition behavior of packed-bed dielectric barrier discharge in argon[J]. IEEE T Plasma Sci, 2011, 39(11): 2172-2173. |
| 38 | HONG J, PANCHESHNYI S, TAM E, et al. Corrigendum: kinetic modelling of NH3 production in N2-H2 non-equilibrium atmospheric-pressure plasma catalysis[J]. J Phys D Appl Phys, 2018, 51(10): 1450-1459. |
| 39 | GAO M X, ZHANG Y, WANG H Y, et al. Mode transition of filaments in packed-bed dielectric barrier discharges[J]. Catalysts, 2018, 8(6): 248. |
| 40 | GÓMEZ-RAMÍREZ A, COTRINO J, LAMBERT R M, et al. Efficient synthesis of ammonia from N2 and H2 alone in a ferroelectric packed-bed DBD reactor[J]. Plasma Sources Sci Technol, 2015, 24(6): 065011. |
| 41 | ZHANG C, LIU Z P, HU P. Stepwise addition reactions in ammonia synthesis: a first principles study[J]. J Chem Phys, 2001, 115: 609-611. |
| 42 | LIN B, WEI K, NI J, et al. KOH activation of thermally modified carbon as a support of Ru catalysts for ammonia synthesis[J]. ChemCatChem, 2013, 5(7): 1941-1947. |
| [1] | Rong CAO, Jie-Zhen XIA, Man-Hua LIAO, Lu-Chao ZHAO, Chen ZHAO, Qi WU. Theoretical Research Progress of Single Atom Catalysts in Electrochemical Synthesis of Ammonia [J]. Chinese Journal of Applied Chemistry, 2023, 40(1): 9-23. |
| [2] | LUO Ce, YAN Yan, LI Jian, LIU Ting, BAI Huan-Huan, LU Fan, FENG Jing. Determination of Trace Iron in High Purity Titanium by Methane Dynamic Reaction-Inductively Coupled Plasma Mass Spectrometry Standard Addition Method [J]. Chinese Journal of Applied Chemistry, 2021, 38(7): 874-880. |
| [3] | LIU Yang, ZHANG Hai-Bao, CHEN Qiang. Research Progress on Ammonia Synthesis Using Low Temperature Plasma [J]. Chinese Journal of Applied Chemistry, 2021, 38(6): 622-636. |
| [4] | ZHANG Chun-Hua, ZHAO Xiao-Bo, LI Yue-Jun, SUN Da-Wei. Preparation of (BiO)2CO3-Bi-TiO2 Composite Nanofibers and Its Photocatalytic Degradation of Antibiotics [J]. Chinese Journal of Applied Chemistry, 2021, 38(1): 99-106. |
| [5] | BI Yipiao, GONG Xue, YANG Fa, RUAN Mingbo, SONG Ping, XU Weilin. Polyvalent MnOx/C Electrocatalyst for Highly Efficient Nitrogen Reduction Reaction [J]. Chinese Journal of Applied Chemistry, 2020, 37(9): 1048-1055. |
| [6] | LI Yuejun, CAO Tieping, SUN Dawei, ZHAO Yanhui, BAI Benang. Preparation of Ternary Ho3+-TiO2/Bi Plasmonic Composite Fibers for Photocatalytic H2 Production under Visible Light Irradiation [J]. Chinese Journal of Applied Chemistry, 2020, 37(5): 570-578. |
| [7] | SU Fengmei, ZHANG Da, LIANG Feng. Progress in Preparation and Modification of Nano-catalytic Materials by Low-Temperature Plasma [J]. Chinese Journal of Applied Chemistry, 2019, 36(8): 882-891. |
| [8] | SHEN Shuchang, PENG Cheng, WANG Di. Preparation Solid Phase Extraction Packing Material Based on Ligand Bonded Montmorillonite and Its Performance on Heavy Metal Ion Adsorption [J]. Chinese Journal of Applied Chemistry, 2019, 36(6): 717-725. |
| [9] | ZHU Deqin, ZHENG Shouyang, SHENG Yu. Flame-retardant Synergistic Effect of Synergists on Intumescent Flame-retardant Wood Flour-Polypropylene Composites [J]. Chinese Journal of Applied Chemistry, 2017, 34(2): 195-203. |
| [10] | GUO Yanyan, LIU Ying*, HU Ruisheng, GAO Guanjun, SUN Hongjuan. Preparation and Optimization of SnCl2-ZnCl2/C Mercury-free Catalyst for Acetylene Hydrochlorination [J]. Chinese Journal of Applied Chemistry, 2014, 31(05): 624-626. |
| [11] | DAI Yan1,2, LV Chunxu3, LI Bindong3*, TAN Weihong1,2*. Microwave-Assisted Synthesis of 2-(4-Hydroxy-phenyl)-1,1,1,3,3,3-hexafluoro-propan-2-ol via Potassium Hydroxide as the Catalyst [J]. Chinese Journal of Applied Chemistry, 2013, 30(12): 1438-1442. |
| [12] | REN Jie*, LIAO Ruirui, YANG Wu, LI Yan, GAO Jinzhang. Fabrication of Superhydrophobic Iron-surface by Using Glow Discharge Electrolysis Plasma [J]. Chinese Journal of Applied Chemistry, 2013, 30(02): 208-213. |
| [13] | HU Shuanghui, LV Yijun, YAN Wenjuan, WANG Baowei*. Process for Conversion of Methane to C2 Hydrocarbons in Gliding Arc Discharge Plasma Reactor [J]. Chinese Journal of Applied Chemistry, 2013, 30(01): 61-66. |
| [14] | CAO Huihui, PENG Jingdong*, ZHANG Lei. Quantitative Determination of Bezafibrate in Rat Plasma Using HPLC-MS [J]. Chinese Journal of Applied Chemistry, 2012, 29(05): 591-596. |
| [15] | REN Jie*, TAO Lihong, GAO Jinzhang, LI Yan, LIU Hongwei, LI Yaping. Synthesis of composite superabsorbent of poly (acrylic acid sodium)/ humic acid by using glow discharge electrolysis plasma [J]. Chinese Journal of Applied Chemistry, 2012, 29(04): 376-382. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||