
Chinese Journal of Applied Chemistry ›› 2023, Vol. 40 ›› Issue (1): 9-23.DOI: 10.19894/j.issn.1000-0518.220138
• Review • Previous Articles Next Articles
Theoretical Research Progress of Single Atom Catalysts in Electrochemical Synthesis of Ammonia
Rong CAO1,2, Jie-Zhen XIA1,2, Man-Hua LIAO1,2, Lu-Chao ZHAO1,2, Chen ZHAO1,2, Qi WU1,2( )
)
- 1.Department of Physics,Tibet University,Lhasa 850000,China
2.Institute of Oxygen Supply,Tibet University,Lhasa 850000,China
-
Received:2022-04-16Accepted:2022-08-07Published:2023-01-01Online:2023-01-28 -
Contact:Qi WU -
About author:wuqi_zangda@163.com
-
Supported by:the National Natural Science Foundation of China(22168036);the Central for the Reform and Development of Local Colleges and Universities Funding Project(Zangcaijiaozhi[2021]1-21);Tibet University Postgraduate Students High-Level Talent Training Plan Project(2020-GSP-S039)
CLC Number:
Cite this article
Rong CAO, Jie-Zhen XIA, Man-Hua LIAO, Lu-Chao ZHAO, Chen ZHAO, Qi WU. Theoretical Research Progress of Single Atom Catalysts in Electrochemical Synthesis of Ammonia[J]. Chinese Journal of Applied Chemistry, 2023, 40(1): 9-23.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: http://yyhx.ciac.jl.cn/EN/10.19894/j.issn.1000-0518.220138
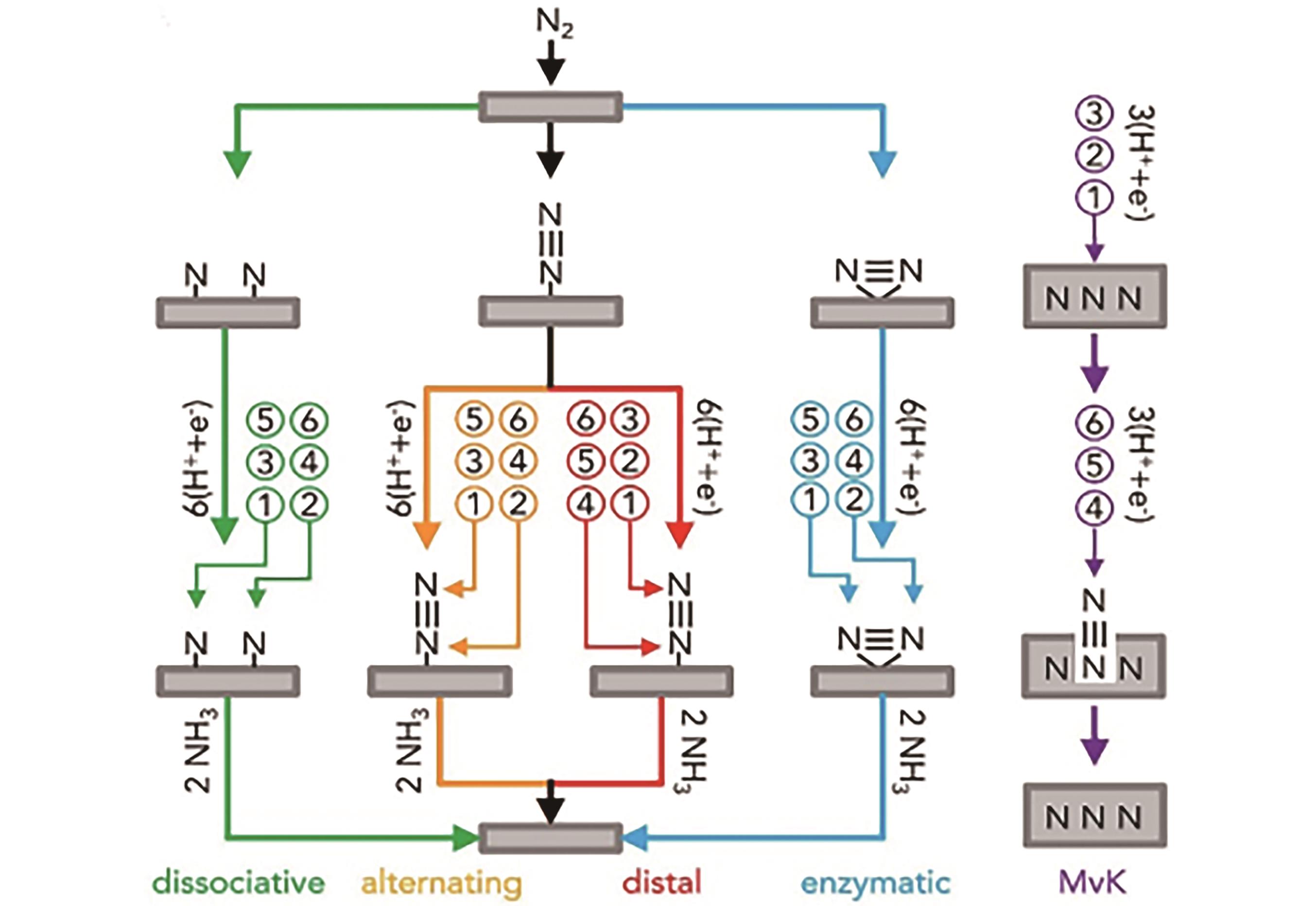
Fig.1 Schematic diagram of NRR reaction mechanism. Except for the purple arrows, the arrows of different colors in the figure show that N2 molecules are adsorbed on the catalyst surface in different ways from top to bottom. Then hydrogenation is performed on the N2 molecule in the order of ①-⑥ until the N2 molecule is reduced to form the NH3 molecule which is desorbed from the catalyst surface. In addition, the gray nitrogen-containing squares represent the metal nitride surfaces. When the lattice N atoms are reduced, a catalyst surface with vacancies is generated, and then the nitrogen gas reacts with the metal nitride to complete a catalytic cycle[40]
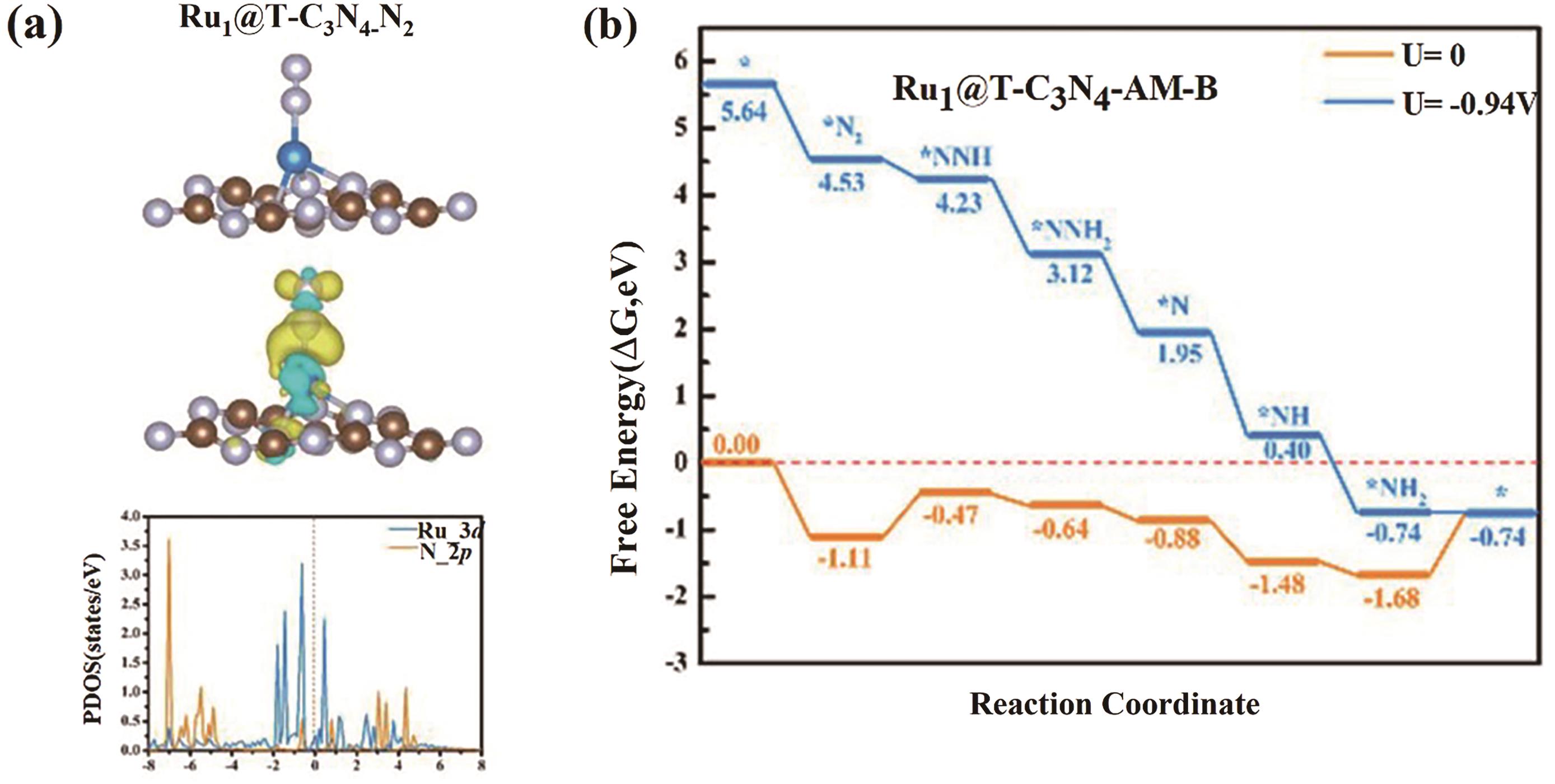
Fig.2 (a) Schematic diagram of the charge density difference and local density of states of N2 adsorption on the structurally stable Ru1@T-C3N4; (b) The free energy curve of the remote path on Ru1@T-C3N4[47]
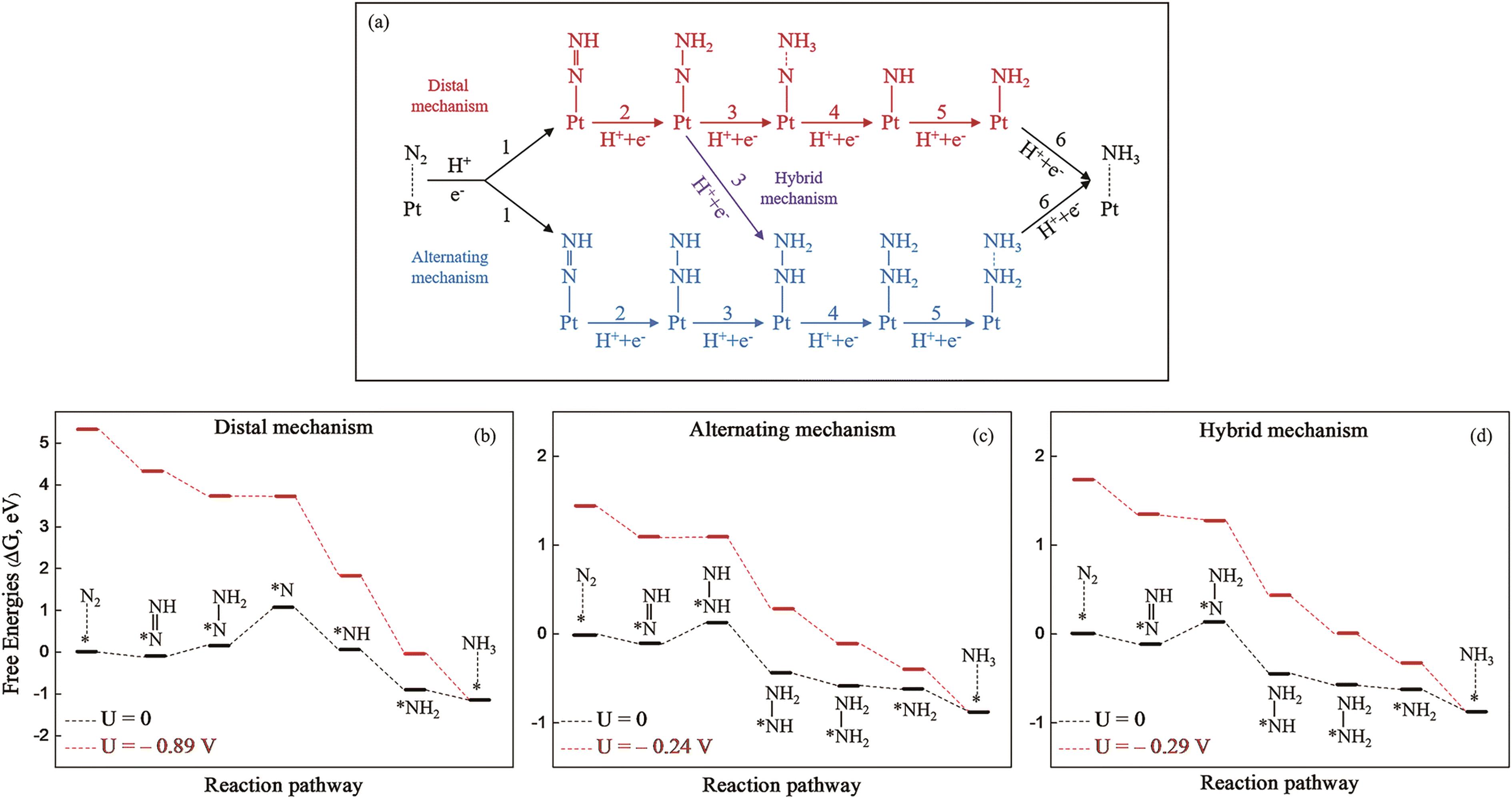
Fig.3 (a) Schematic illustration of three reaction mechanisms for electrochemical NRR on Pt/g-C3N4. Free energy distribution of NRR at 0 V (black) and UL (red) for Pt/g-C3N4via (b) distal, (c) alternation and (d) hybrid mechanisms[48]
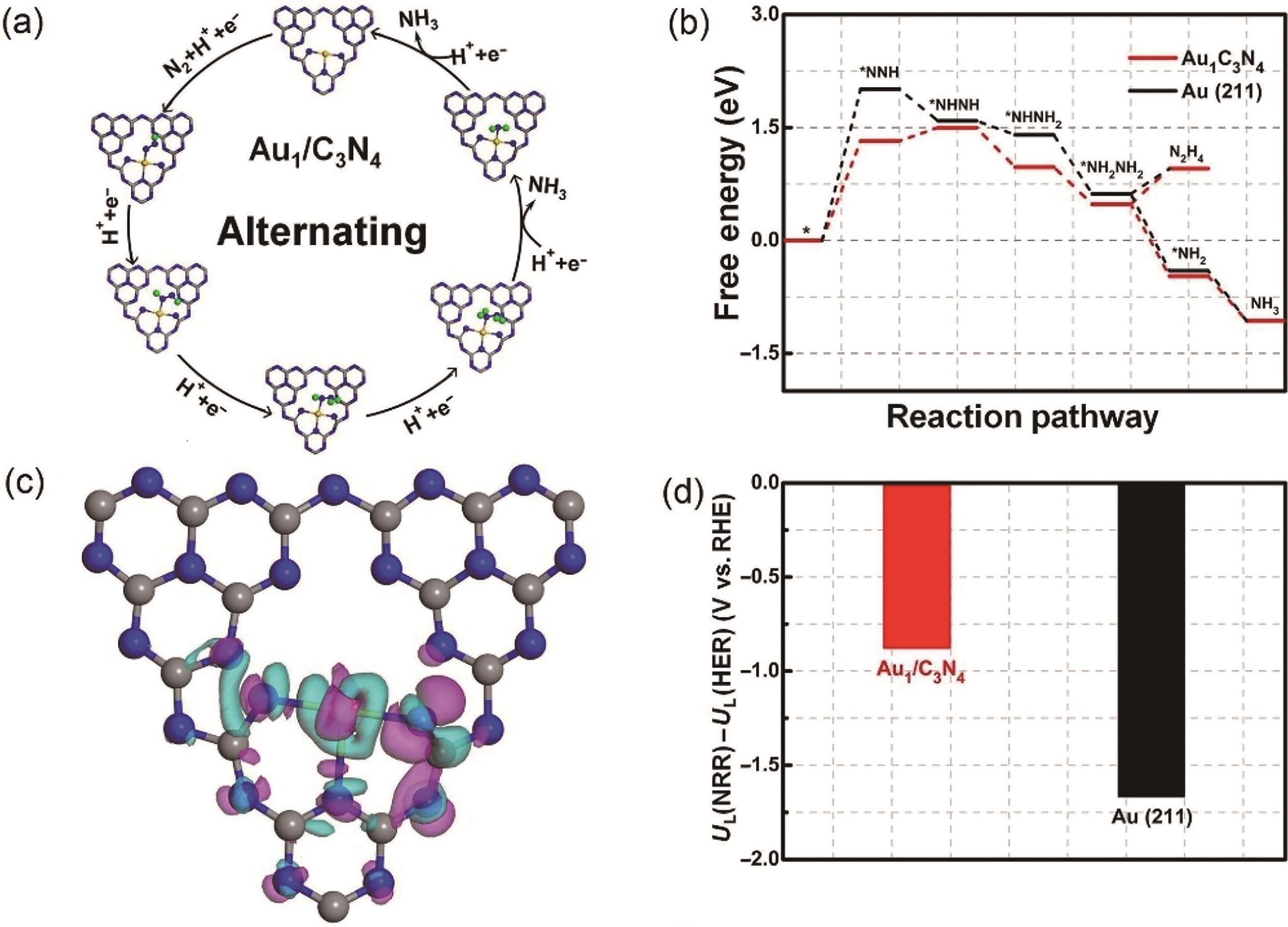
Fig.4 (a) Various reaction intermediates of NRR following alternate paths; (b) Free energy distribution of NRR on Au1/C3N4 and Au(211) under an alternate mechanism; (c) Charge density difference map of Au1/C3N4; (d) NRR and HER limiting potential[49]
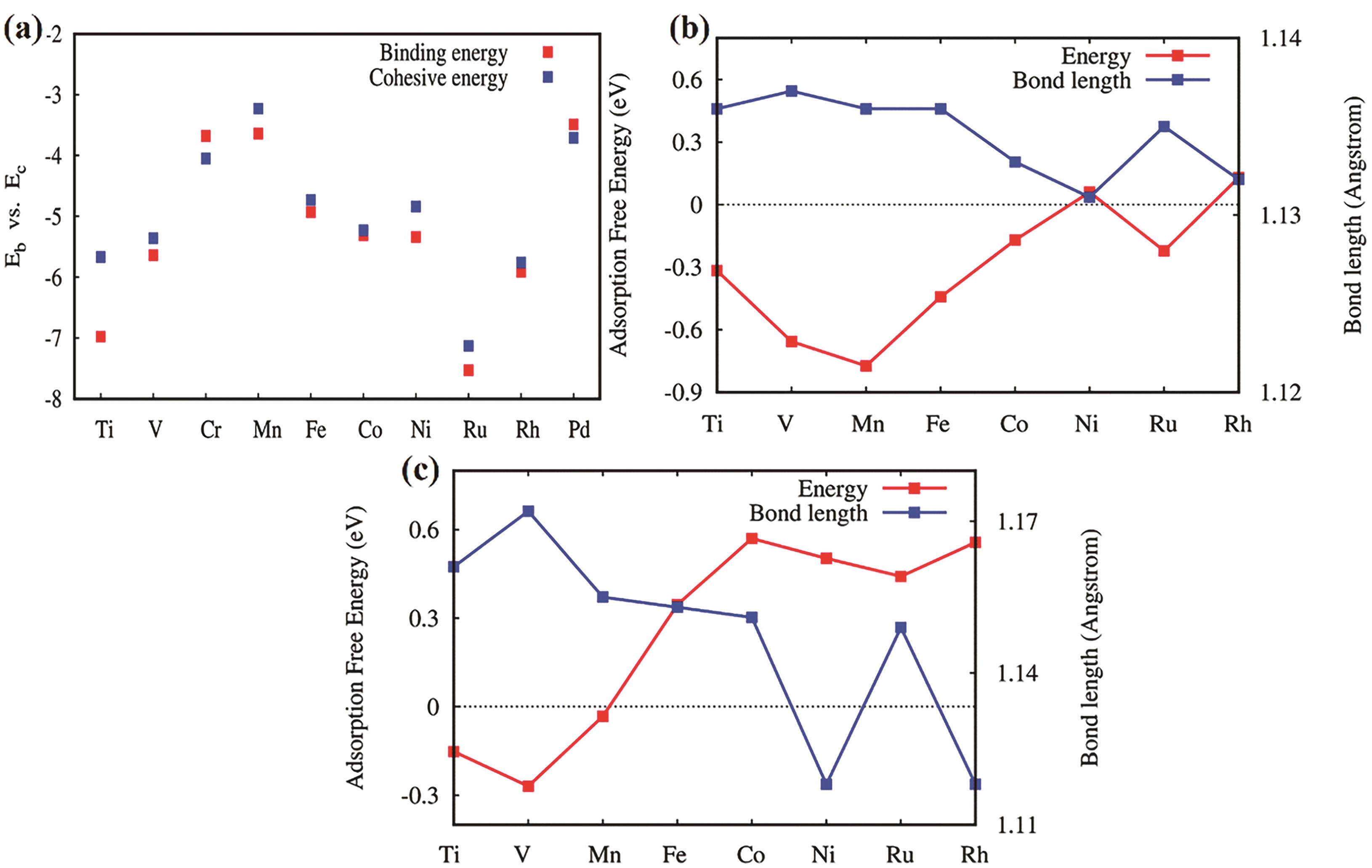
Fig.5 (a) The binding energies and corresponding cohesive energies of different metal atoms and MoP; the adsorption free energy (eV) and N—N bond length (0.1 nm) of N2 adsorbed on MoP modified by different metal atoms; (b) End-to-end adsorption; (c) Lateral adsorption[55]

Fig.6 (a) Limiting potential UL of Fe@N x for nitrogen reduction reaction (NRR); (b) Proportional relationship between limiting potential UL and N2H* adsorption energy[59]
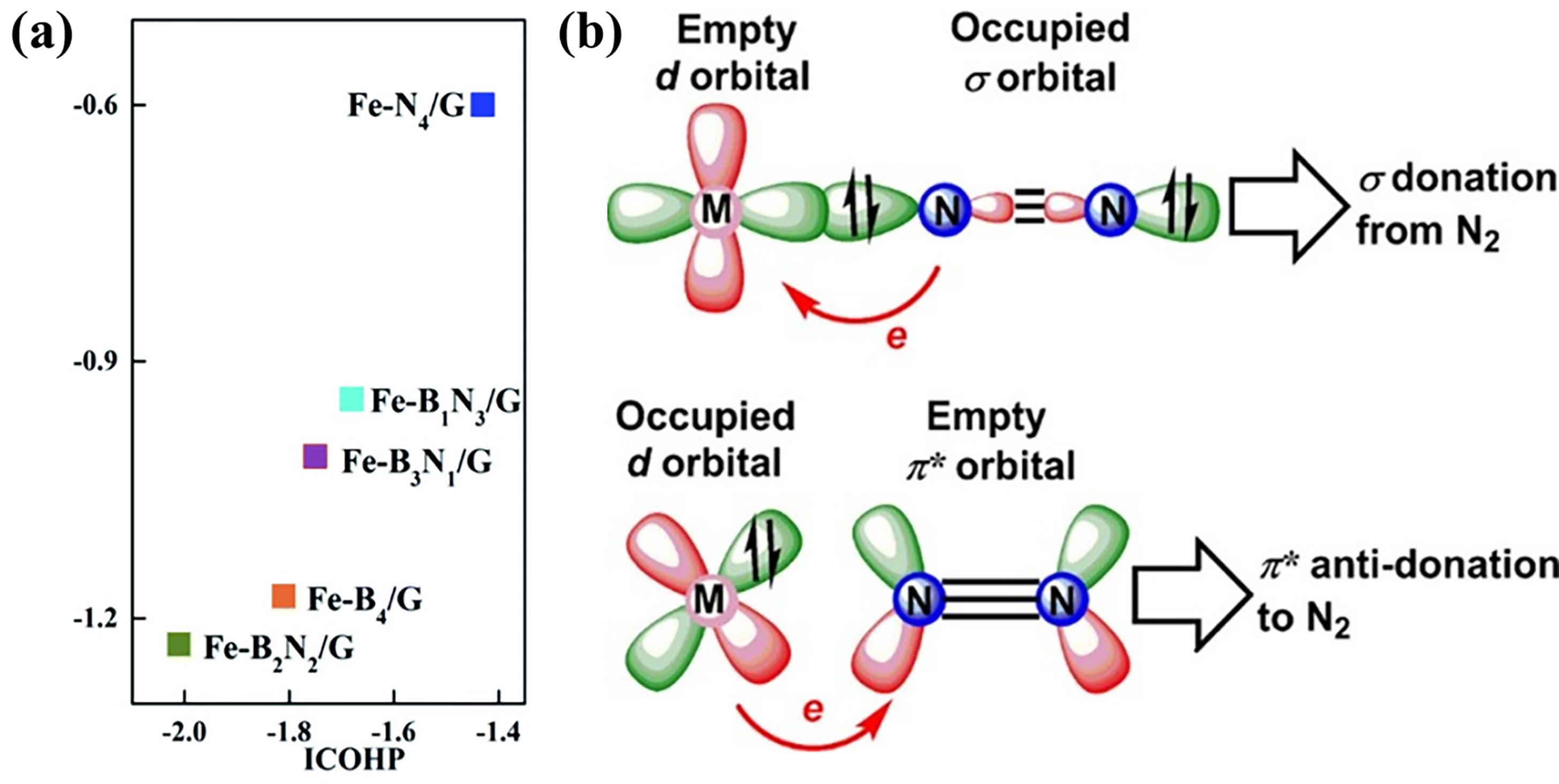
Fig.7 (a) Correlation between integrated crystal orbital Hamiltonian population (ICOHP) and N2 adsorption energy (ΔEads)[61]; (b) Schematic diagram of N2 immobilization and activation by transition metal catalysts[62]
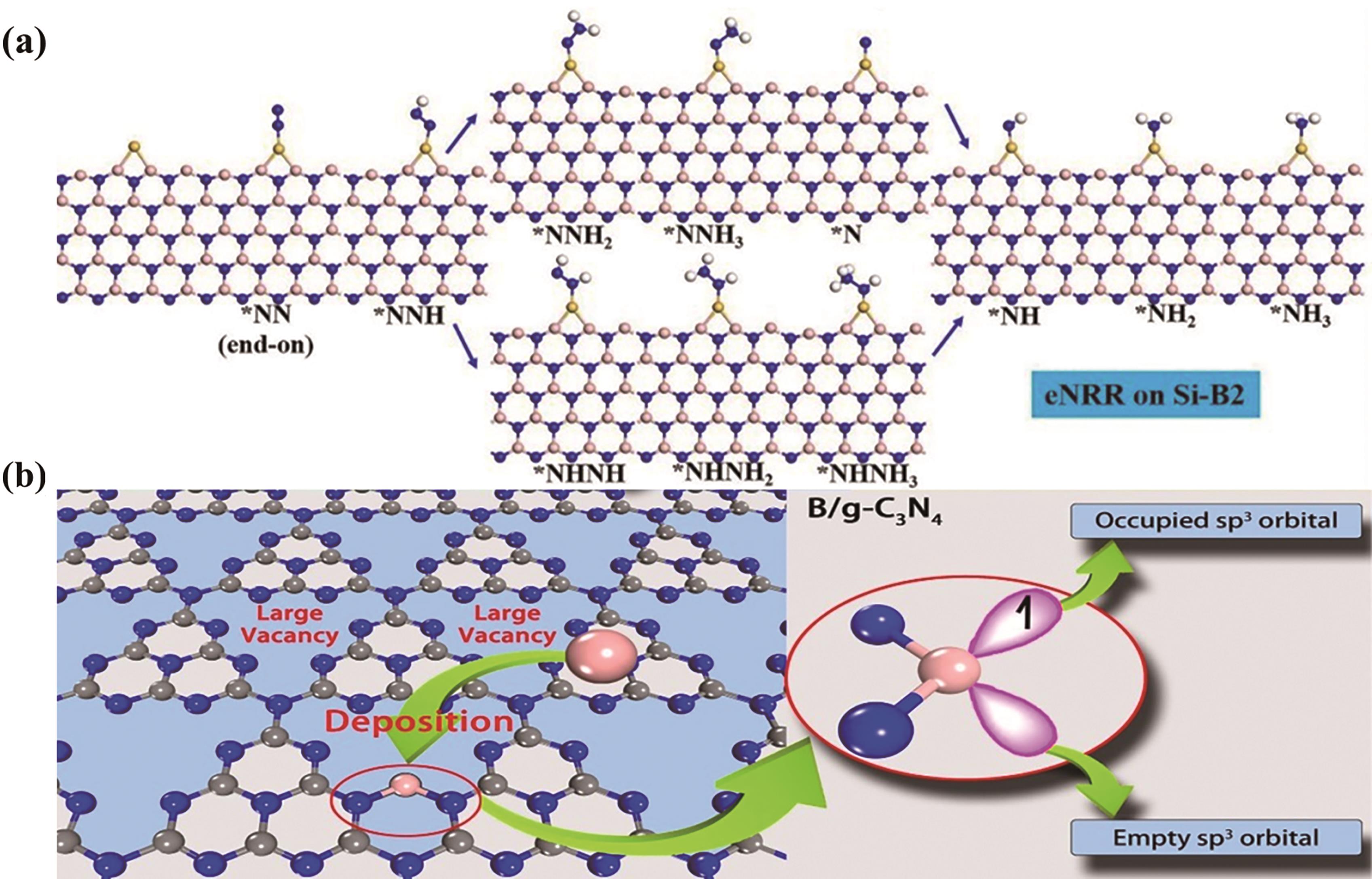
Fig.8 (a) Schematic illustration of the reaction mechanism of electrochemical NRR on Si-B2[65]. Colour scheme: pink (boron); blue (nitrogen); yellow (silicon); (b) Design concept of B/g-C3N4 as catalyst for N2 immobilization[66]. Colour scheme: pink (boron); blue (nitrogen); grey (silicon)
| Classification | Example | Overpotential/V | Mechanism | NH3 yield rate | FE/% | Electrolyte | Ref. |
|---|---|---|---|---|---|---|---|
| Precious metal catalyst | Ru/ZrO2@NC | -0.21 | Distal mechanism | 3.665 μg/(h·mg) | 21 | 0.1 mol/L HCl | [ |
| Rh SA/GDY | -0.20 | Distal mechanism | 74.15 μg/(h·cm2) | 20.36 | 0.1 mol/L K2SO4 | [ | |
| Rh1/MnO2 | — | — | 271.8 μg/(h·mg) | 73.3 | 9 mol/L K2SO4 | [ | |
| Pd-TiO2 | -0.5 | — | 17.4 μg/(h·mg) | 12.7 | 0.1 mol/L Na2SO4 | [ | |
| Pt/NiO | -0.2 | — | 20.59 μg/(h·mg) | 15.56 | 0.1 mol/L Na2SO4 | [ | |
| Ru1@T-C3N4 | -0.94 | Distal mechanism | — | — | — | [ | |
| Pt/g-C3N4 | -0.24 | Alternating mechanism | — | — | — | [ | |
| Au1/C3N4 | -0.88 | Alternating mechanism | — | — | — | [ | |
| Ru SACs/Cu x O y | -0.1~-0.2 | Distal mechanism | — | — | — | [ | |
| Ru@MoS2 | -0.33 | Enzymatic mechanism | — | — | — | [ | |
| Non-precious metal catalyst | Fe-TiO2 | -0.4 | Alternating mechanism | 25.47 μg/(h·mg) | 25.6 | 0.5 mol/L LiClO4 | [ |
| ZrPP | -0.38 | Enzymatic-consecutive hybrid path | — | — | — | [ | |
| Mn@C3N | -0.75 | Distal/alternating mechanism | — | — | — | [ | |
| W-VSe2 | -0.36 | Distal mechanism | — | — | — | [ | |
| W@g-C3N4 | -0.35 | Enzymatic mechanism | — | — | — | [ | |
| W/g-CN N | -0.29 | Distal mechanism | — | — | — | [ | |
| Mo@MoS2 | -0.28 | Distal mechanism | — | — | — | [ | |
| Mn@BCN NTs | -0.1 | Distal mechanism | — | — | — | [ | |
| Mo/MoS2 | -0.53 | Distal/alternating mechanism | — | — | — | [ | |
| Mn-MoP | -0.95 | Distal/alternating mechanism | — | — | — | [ | |
| Mo1N3 | -0.02 | Distal mechanism | — | — | — | [ | |
| Mo@BN | -0.19 | nzymatic mechanism | — | — | — | [ | |
| Mo@BCN | -0.42 | Enzymatic mechanism | — | — | — | [ | |
| Fe@N-C | -0.63 | Distal/alternating/enzymatic mechanism | — | — | — | [ | |
| Fe-N3/graphene | -0.25 | Distal/alternating/ enzymatic mechanism | — | — | — | [ | |
| Fe-B2N2 | -0.65 | Distal mechanism | — | — | — | [ | |
| Mn@g-N3C1O1 | -0.29 | Distal mechanism | — | — | — | [ | |
| W@BP | -0.40 | Distal mechanism | — | — | — | [ | |
| Ti@N4-garphene | -0.69 | Distal mechanism | — | — | — | [ | |
| V@BN | -0.25 | Enzymatic mechanism | — | — | — | [ | |
| CrB3C1-graphene | -0.13 | Distal mechanism | — | — | — | [ | |
| MnSA@VsN1 | -0.77 | Distal mechanism | — | — | — | [ | |
| Fe-B2N2 | -0.65 | Distal mechanism | — | — | — | [ | |
| FeB4-graphene | -0.55 | Distal mechanism | — | — | — | [ | |
| Mo/C9N4 | -0.40 | Distal mechanism | — | — | — | [ | |
| Mo-rTCNQ | -0.48 | Distal mechanism | — | — | — | [ | |
| MoPc/TcPc | -0.33/-0.54 | Distal/distal-alternating mechanism | — | — | — | [ | |
| Mo-PTA | -0.26 | Distal mechanism | — | — | — | [ | |
| Mo@MoS2 | -0.28 | Distal mechanism | — | — | — | [ | |
| W/Ti2-x C2O y | -0.11/-0.95 | Distal mechanism | — | — | — | [ | |
| W@g-C3N4 | -0.35 | Enzymatic mechanism | — | — | — | [ | |
| W@C9N4 | -0.25 | Distal mechanism | — | — | — | [ | |
| Non-metallic catalyst | B/g-C3N4 | -0.20 | Enzymatic mechanism | — | — | — | [ |
| B/C2N | -0.18 | Enzymatic mechanism | — | — | — | [ | |
| Si-BN | -1.06 | Distal/alternating mechanism | — | — | — | [ | |
| B@g-C3N4 | -0.2 | Enzymatic mechanism | — | — | — | [ | |
| N-doped@MOFs | -0.3 | Distal mechanism | 3.4×10-6 mol/(cm2·h) | 10.2 | 0.1 mol/L KOH | [ |
Table 1 Currently reported NRR single-atom catalysts
| Classification | Example | Overpotential/V | Mechanism | NH3 yield rate | FE/% | Electrolyte | Ref. |
|---|---|---|---|---|---|---|---|
| Precious metal catalyst | Ru/ZrO2@NC | -0.21 | Distal mechanism | 3.665 μg/(h·mg) | 21 | 0.1 mol/L HCl | [ |
| Rh SA/GDY | -0.20 | Distal mechanism | 74.15 μg/(h·cm2) | 20.36 | 0.1 mol/L K2SO4 | [ | |
| Rh1/MnO2 | — | — | 271.8 μg/(h·mg) | 73.3 | 9 mol/L K2SO4 | [ | |
| Pd-TiO2 | -0.5 | — | 17.4 μg/(h·mg) | 12.7 | 0.1 mol/L Na2SO4 | [ | |
| Pt/NiO | -0.2 | — | 20.59 μg/(h·mg) | 15.56 | 0.1 mol/L Na2SO4 | [ | |
| Ru1@T-C3N4 | -0.94 | Distal mechanism | — | — | — | [ | |
| Pt/g-C3N4 | -0.24 | Alternating mechanism | — | — | — | [ | |
| Au1/C3N4 | -0.88 | Alternating mechanism | — | — | — | [ | |
| Ru SACs/Cu x O y | -0.1~-0.2 | Distal mechanism | — | — | — | [ | |
| Ru@MoS2 | -0.33 | Enzymatic mechanism | — | — | — | [ | |
| Non-precious metal catalyst | Fe-TiO2 | -0.4 | Alternating mechanism | 25.47 μg/(h·mg) | 25.6 | 0.5 mol/L LiClO4 | [ |
| ZrPP | -0.38 | Enzymatic-consecutive hybrid path | — | — | — | [ | |
| Mn@C3N | -0.75 | Distal/alternating mechanism | — | — | — | [ | |
| W-VSe2 | -0.36 | Distal mechanism | — | — | — | [ | |
| W@g-C3N4 | -0.35 | Enzymatic mechanism | — | — | — | [ | |
| W/g-CN N | -0.29 | Distal mechanism | — | — | — | [ | |
| Mo@MoS2 | -0.28 | Distal mechanism | — | — | — | [ | |
| Mn@BCN NTs | -0.1 | Distal mechanism | — | — | — | [ | |
| Mo/MoS2 | -0.53 | Distal/alternating mechanism | — | — | — | [ | |
| Mn-MoP | -0.95 | Distal/alternating mechanism | — | — | — | [ | |
| Mo1N3 | -0.02 | Distal mechanism | — | — | — | [ | |
| Mo@BN | -0.19 | nzymatic mechanism | — | — | — | [ | |
| Mo@BCN | -0.42 | Enzymatic mechanism | — | — | — | [ | |
| Fe@N-C | -0.63 | Distal/alternating/enzymatic mechanism | — | — | — | [ | |
| Fe-N3/graphene | -0.25 | Distal/alternating/ enzymatic mechanism | — | — | — | [ | |
| Fe-B2N2 | -0.65 | Distal mechanism | — | — | — | [ | |
| Mn@g-N3C1O1 | -0.29 | Distal mechanism | — | — | — | [ | |
| W@BP | -0.40 | Distal mechanism | — | — | — | [ | |
| Ti@N4-garphene | -0.69 | Distal mechanism | — | — | — | [ | |
| V@BN | -0.25 | Enzymatic mechanism | — | — | — | [ | |
| CrB3C1-graphene | -0.13 | Distal mechanism | — | — | — | [ | |
| MnSA@VsN1 | -0.77 | Distal mechanism | — | — | — | [ | |
| Fe-B2N2 | -0.65 | Distal mechanism | — | — | — | [ | |
| FeB4-graphene | -0.55 | Distal mechanism | — | — | — | [ | |
| Mo/C9N4 | -0.40 | Distal mechanism | — | — | — | [ | |
| Mo-rTCNQ | -0.48 | Distal mechanism | — | — | — | [ | |
| MoPc/TcPc | -0.33/-0.54 | Distal/distal-alternating mechanism | — | — | — | [ | |
| Mo-PTA | -0.26 | Distal mechanism | — | — | — | [ | |
| Mo@MoS2 | -0.28 | Distal mechanism | — | — | — | [ | |
| W/Ti2-x C2O y | -0.11/-0.95 | Distal mechanism | — | — | — | [ | |
| W@g-C3N4 | -0.35 | Enzymatic mechanism | — | — | — | [ | |
| W@C9N4 | -0.25 | Distal mechanism | — | — | — | [ | |
| Non-metallic catalyst | B/g-C3N4 | -0.20 | Enzymatic mechanism | — | — | — | [ |
| B/C2N | -0.18 | Enzymatic mechanism | — | — | — | [ | |
| Si-BN | -1.06 | Distal/alternating mechanism | — | — | — | [ | |
| B@g-C3N4 | -0.2 | Enzymatic mechanism | — | — | — | [ | |
| N-doped@MOFs | -0.3 | Distal mechanism | 3.4×10-6 mol/(cm2·h) | 10.2 | 0.1 mol/L KOH | [ |
| 1 | CHEN X R, GUO Y T, DU X C, et al. Atomic structure modification for electrochemical nitrogen reduction to ammonia[J]. Adv Energy Mater, 2020, 10(3): 1903172. |
| 2 | GUO C X, RAN J R, VASILEFF A, et al. Rational design of electrocatalysts and photo (electro) catalysts for nitrogen reduction to ammonia (NH3) under ambient conditions[J]. Energy Environ Sci, 2018, 11(1): 45-56. |
| 3 | QIU Y, PENG X Y, LV F, et al. Single-atom catalysts for the electrocatalytic reduction of nitrogen to ammonia under ambient conditions[J].Chem Asian J, 2019, 14(16): 2770-2779. |
| 4 | WAN Y, XU J, LV R. Heterogeneous electrocatalysts design for nitrogen reduction reaction under ambient conditions[J]. Mater Today, 2019, 27: 69-90. |
| 5 | TAO H, CHOI C, DING L X, et al. Nitrogen fixation by Ru single-atom electrocatalytic reduction[J]. Chem, 2019, 5(1): 204-214. |
| 6 | WU T, ZHU X, XING Z, et al. Greatly improving electrochemical N2 reduction over TiO2 nanoparticles by iron doping[J]. Angew Chem Int Ed, 2019, 58(51): 18449-18453. |
| 7 | HUANG C X, LV S Y, LI C, et al. Single-atom catalysts based on two-dimensional metalloporphyrin monolayers for ammonia synthesis under ambient conditions[J]. Nano Res, 2022, 15(5): 4039-4047. |
| 8 | CHEN Y, ZHANG X, QIN J, et al. Theoretical screening of highly efficient single-atom catalysts for nitrogen reduction based on a defective C3N monolayer[J]. Int J Hydrog Energy, 2022, 47(8): 5292-5306. |
| 9 | WANG J, LUO Z, ZHANG X, et al. Single transition metal atom anchored on VSe2 as electrocatalyst for nitrogen reduction reaction[J]. Appl Surf Sci, 2022, 580: 152272. |
| 10 | HOU C C, ZOU L L, SUN L, et al. Single-atom iron catalysts on overhang-eave carbon cages for high-performance oxygen reduction reaction[J]. Angew Chem, 2020, 132(19): 7454-7459. |
| 11 | LIU J, JIAO M G, LU L L, et al. Ultrahigh-loading zinc single-atom catalyst for highly efficient oxygen reduction in both acidic and alkaline media[J]. Angew Chem Int Ed, 2019, 58(21): 7035-7039. |
| 12 | CHEN D C, CHEN Z W, ZHANG X X, et al. Recent developments of microenvironment engineering of single-atom catalysts for oxygen reduction toward desired activity and selectivity[J]. Adv Funct Mater, 2021, 31(45): 2103857. |
| 13 | WANG C, WANG D, LIU S, et al. Engineering the coordination environment enables molybdenum single-atom catalyst for efficient oxygen reduction reaction[J]. J Catal, 2020, 389: 150-156. |
| 14 | WAN C, DUAN X, HUANG Y. Molecular design of single-atom catalysts for oxygen reduction reaction[J]. Adv Energy Mater, 2020, 10(14): 1903815. |
| 15 | LEE W H, KO Y J, KIM J Y, et al. Single-atom catalysts for the oxygen evolution reaction: recent developments and future perspectives[J]. Chem Comm, 2020, 56(84): 12687-12697. |
| 16 | BAI L, HSU C S, ALEXANDER D T L, et al. A cobalt-iron double-atom catalyst for the oxygen evolution reaction[J]. J Am Chem Soc, 2019, 141(36): 14190-14199. |
| 17 | LI Y, WU Z S, LU P, et al. High-valence nickel single-atom catalysts coordinated to oxygen sites for extraordinarily activating oxygen evolution reaction[J]. Adv Sci, 2020, 7(5): 1903089. |
| 18 | LI Z, WANG Z, XI S, et al. Tuning the spin density of cobalt single-atom catalysts for efficient oxygen evolution[J]. ACS Nano, 2021, 15(4): 7105-7113. |
| 19 | XU Y, ZHANG W, LI Y, et al. A general bimetal-ion adsorption strategy to prepare nickel single atom catalysts anchored on graphene for efficient oxygen evolution reaction[J]. J Energy Chem, 2020, 43: 52-57. |
| 20 | CHEN D, CHEN Z, ZHANG X, et al. Exploring single atom catalysts of transition-metal doped phosphorus carbide monolayer for HER: a first-principles study[J]. J Energy Chem, 2021, 52: 155-162. |
| 21 | LIU X, ZHENG L, HAN C, et al. Identifying the activity origin of a cobalt single-atom catalyst for hydrogen evolution using supervised learning[J]. Adv Funct Mater, 2021, 31(18): 2100547. |
| 22 | GUTIC S J, DOBROTA A S, FAKO E, et al. Hydrogen evolution reaction-from single crystal to single atom catalysts[J]. Catalysts, 2020, 10(3): 290. |
| 23 | ZHOU K L, WANG Z, HAN C B, et al. Platinum single-atom catalyst coupled with transition metal/metal oxide heterostructure for accelerating alkaline hydrogen evolution reaction[J]. Nat Commun, 2021, 12(1): 1-10. |
| 24 | GAO G, BOTTLE S, DU A. Understanding the activity and selectivity of single atom catalysts for hydrogen and oxygen evolution via ab initial study[J]. Catal Sci Technol, 2018, 8(4): 996-1001. |
| 25 | LI M, WANG H, LUO W, et al. Heterogeneous single-atom catalysts for electrochemical CO2 reduction reaction[J]. Adv Mater, 2020, 32(34): 2001848. |
| 26 | CAO S, WEI S, WEI X, et al. Can N, S coordination promote single atom catalyst performance in CO2RR@Fe-N2S2 porphyrin versus Fe-N4 porphyrin[J]. Small, 2021, 17(29): 2100949. |
| 27 | HAN S G, MA D D, ZHU Q L. Atomically structural regulations of carbon-based single-atom catalysts for electrochemical CO2 reduction[J]. Small Methods, 2021, 5(8): 2100102. |
| 28 | ZHANG N, ZHANG X, TAO L, et al. Silver single-atom catalyst for efficient electrochemical CO2 reduction synthesized from thermal transformation and surface reconstruction[J]. Angew Chem Int Ed, 2021, 60(11): 6170-6176. |
| 29 | YANG H, WU Y, LI G, et al. Scalable production of efficient single-atom copper decorated carbon membranes for CO2 electroreduction to methanol[J]. J Am Chem Soc, 2019, 141(32): 12717-12723. |
| 30 | ZOU H, RONG W, WEI S, et al. Regulating kinetics and thermodynamics of electrochemical nitrogen reduction with metal single-atom catalysts in a pressurized electrolyser[J]. Proc Natl Acad Sci USA, 2020, 117(47): 29462-29468. |
| 31 | QIU Y, PENG X, LV F, et al. Single-atom catalysts for the electrocatalytic reduction of nitrogen to ammonia under ambient conditions[J]. Chem Asian J, 2019, 14(16): 2770-2779. |
| 32 | CHEN Z, ZHAO J, CABRERA C R, et al. Computational screening of efficient single-atom catalysts based on graphitic carbon nitride (g-C3N4) for nitrogen electroreduction[J]. Small Methods, 2019, 3(6): 1800368. |
| 33 | NIU H, WANG X, SHAO C, et al. Computational screening single-atom catalysts supported on g-CN for N2 reduction: high activity and selectivity[J]. ACS Sustain Chem Eng, 2020, 8(36): 13749-13758. |
| 34 | YANG T, SONG T T, ZHOU J, et al. High-throughput screening of transition metal single atom catalysts anchored on molybdenum disulfide for nitrogen fixation[J]. Nano Energy, 2020, 68: 104304. |
| 35 | ZHOU Y, WEI B, CAO H, et al. Electroreduction of nitrogen to ammonia by single-atom catalysis with synergistic boron-carbon nitrogen nanotubes[J]. J Environ Chem Eng, 2022, 10(3): 107752. |
| 36 | KIRLIN P S, GATES B C. Activation of the C—C bond provides a molecular basis for structure sensitivity in metal catalysis[J]. Nature, 1987, 325(6099): 38-40. |
| 37 | VIDAL V, THEOLIER A, THIVOLLE-CAZAT J, et al. Metathesis of alkanes catalyzed by silica-supported transition metal hydrides[J]. Science, 1997, 276(5309): 99-102. |
| 38 | LANZAFAME P, PERATHONERS, CENTI G, et al. Grand challenges for catalysis in the science and technology roadmap on catalysis for europe: moving ahead for a sustainable future[J]. Catal Sci Technol, 2017, 7(22): 5182-5194. |
| 39 | GUO D, WANG S, XU J, et al. Defect and interface engineering for electrochemical nitrogen reduction reaction under ambient conditions[J]. J Energy Chem, 2022, 65: 448-468. |
| 40 | MARTIN A J, SHINAGAWA T, PEREZ-RAMIREZ J. Electrocatalytic reduction of nitrogen: from Haber-Bosch to ammonia artificial leaf[J]. Chem, 2019, 5(2): 263-283. |
| 41 | LI L, CHANG X, LIN X, et al. Theoretical insights into single-atom catalysts[J]. Chem Soc Rev, 2020, 49(22): 8156-8178. |
| 42 | LIU D, CHEN M, DU X, et al. Development of electrocatalysts for efficient nitrogen reduction reaction under ambient condition[J]. Adv Funct Mater, 2021, 31(11): 2008983. |
| 43 | WU T, MELANDER M M, HONKALA K. Coadsorption of NRR and HER intermediates determines the performance of Ru-N4 toward electrocatalytic N2 reduction[J]. ACS Catal, 2022, 12(4): 2505-2512. |
| 44 | SHEN P, LI X, LUO Y, et al. Ultra-efficient N2 electroreduction achieved over a rhodium single-atom catalyst (Rh1/MnO2) in water-in-salt electrolyte[J]. Appl Catal B, 2022, 316: 121651. |
| 45 | CHEN H, DENG G, FENG Z, et al. Enhanced electrocatalytic performance of TiO2 nanoparticles by Pd doping toward ammonia synthesis under ambient conditions[J]. Chem Comm, 2022, 58(19): 3214-3217. |
| 46 | XIONG W, ZHOU M, LI H, et al. Electrocatalytic ammonia synthesis catalyzed by mesoporous nickel oxide nanosheets loaded with Pt nanoparticles[J]. Chinese J Catal, 2022, 43(5): 1371-1378. |
| 47 | CAO Y, GAO Y, ZHOU H, et al. Highly efficient ammonia synthesis electrocatalyst: single Ru atom on naturally nanoporous carbon materials[J]. Adv Theory Simul, 2018, 1(5): 1800018. |
| 48 | YIN H, LI S L, GAN L Y, et al. Pt-embedded in monolayer g-C3N4 as a promising single-atom electrocatalyst for ammonia synthesis[J]. J Mater Chem A, 2019, 7(19): 11908-11914. |
| 49 | WANG X, WANG W, QIAO M, et al. Atomically dispersed Au1 catalyst towards efficient electrochemical synthesis of ammonia[J]. Sci Bull, 2018, 63(19): 1246-1253. |
| 50 | CHEN S, LIU X, XIONG J, et al. Engineering strategies for boosting the nitrogen reduction reaction performance of MoS2 based electrocatalysts[J]. Mater Today, 2022: 100202. |
| 51 | MA F, SRINIVAS K, ZHANG X, et al. Mo2N quantum dots decorated N-doped graphene nanosheets as dual-functional interlayer for dendrite-free and shuttle-free lithium-sulfur batteries[J]. Adv Funct Mater, 2022: 2206113. |
| 52 | VASSEGHIAN Y, DOAN V D, NGUYEN T T T, et al. Flexible and high-sensitivity sensor based on Ti3C2-MoS2 MXene composite for the detection of toxic gases[J]. Chemosphere, 2022, 291: 133025. |
| 53 | ZHANG X, TIAN F, LAN X, et al. Building P-doped MoS2/g-C3N4 layered heterojunction with a dual-internal electric field for efficient photocatalytic sterilization[J]. Chem Eng J, 2022, 429: 132588. |
| 54 | ZHAO J, ZHAO J, CAI Q. Single transition metal atom embedded into a MoS2 nanosheet as a promising catalyst for electrochemical ammonia synthesis[J]. Phys Chem Chem Phys, 2018, 20(14): 9248-9255. |
| 55 | HAN M, WANG G, ZHANG H, et al. Theoretical study of single transition metal atom modified MoP as a nitrogen reduction electrocatalyst[J]. Phys Chem Chem Phys, 2019, 21(11): 5950-5955. |
| 56 | OU P, ZHOU X, MENG F, et al. Single molybdenum center supported on N-doped black phosphorus as an efficient electrocatalyst for nitrogen fixation[J]. Nanoscale, 2019, 11(28): 13600-13611. |
| 57 | ZHAO J, CHEN Z. Single Mo atom supported on defective boron nitride monolayer as an efficient electrocatalyst for nitrogen fixation: a computational study[J]. J Am Chem Soc, 2017, 139(36): 12480-12487. |
| 58 | HUANG Y, YANG T, YANG L, et al. Graphene-boron nitride hybrid-supported single Mo atom electrocatalysts for efficient nitrogen reduction reaction[J]. J Mater Chem A, 2019, 7(25): 15173-15180. |
| 59 | GUO X, GU J, HU X, et al. Coordination tailoring towards efficient single-atom catalysts for N2 fixation: a case study of iron-nitrogen-carbon (Fe@NC) systems[J]. Catal Today, 2020, 350: 91-99. |
| 60 | LI X F, LI Q K, CHENG J, et al. Conversion of dinitrogen to ammonia by FeN3-embedded graphene[J]. J Am Chem Soc, 2016, 138(28): 8706-8709. |
| 61 | JIAO D, LIU Y, CAI Q, et al. Coordination tunes the activity and selectivity of the nitrogen reduction reaction on single-atom iron catalysts: a computational study[J]. J Mater Chem A, 2021, 9(2): 1240-1251. |
| 62 | LI X, ZHOU Q, WANG S, et al. Tuning the coordination environment to effect the electrocatalytic behavior of a single-atom catalyst toward the nitrogen reduction reaction[J]. J Phys Chem C, 2021, 125(22): 11963-11974. |
| 63 | LIN X, LI L, CHANG X, et al. Black phosphorus-hosted single-atom catalyst for electrocatalytic nitrogen reduction[J]. Sci China Mater, 2021, 64(5): 1173-1181. |
| 64 | CHEN Z W, LU Z, CHEN L X, et al. Machine-learning-accelerated discovery of single-atom catalysts based on bidirectional activation mechanism[J]. Chem Catal, 2021, 1(1): 183-195. |
| 65 | GUO Z, QIU S, LI H, et al. Electrocatalytic nitrogen reduction performance of Si-doped 2D nanosheets of boron nitride evaluated via density functional theory[J]. Chem Cat Chem, 2021, 13(4): 1239-1245. |
| 66 | LING C, NIU X, LI Q, et al. Metal-free single atom catalyst for N2 fixation driven by visible light[J]. J Am Chem Soc, 2018, 140(43): 14161-14168. |
| 67 | MUKHERJEE S, CULLEN D A, KARAKALOS S, et al. Metal-organic framework-derived nitrogen-doped highly disordered carbon for electrochemical ammonia synthesis using N2 and H2O in alkaline electrolytes[J]. Nano Energy, 2018, 48: 217-226. |
| 68 | LI H, YU B, ZHUANG Z, et al. A small change in the local atomic environment for a big improvement in single-atom catalysis[J]. J Mater Chem A, 2021, 9(7): 4184-4192. |
| 69 | SURYANTO B H R, WANG D, AZOFRA L M, et al. MoS2 polymorphic engineering enhances selectivity in the electrochemical reduction of nitrogen to ammonia[J]. ACS Energy Lett, 2018, 4(2): 430-435. |
| 70 | CHOI C, BACK S, KIM N Y, et al. Suppression of hydrogen evolution reaction in electrochemical N2 reduction using single-atom catalysts: a computational guideline[J]. ACS Catal, 2018, 8(8): 7517-7525. |
| 71 | MA Z, CUI Z, XIAO C, et al. Theoretical screening of efficient single-atom catalysts for nitrogen fixation based on a defective BN monolayer[J]. Nanoscale, 2020, 12(3): 1541-1550. |
| 72 | ZAFARI M, KUMAR D, UMER M, et al. Machine learning-based high throughput screening for nitrogen fixation on boron-doped single atom catalysts[J]. J Mater Chem A, 2020, 8(10): 5209-5216. |
| 73 | GAO Z, HUANG H, XU S, et al. Regulating the coordination environment through doping N atoms for single-atom Mn electrocatalyst of N2 reduction with high catalytic activity and selectivity: a theoretical study[J]. Mol Catal, 2020, 493: 111091. |
| 74 | JIAO D, LIU Y, CAI Q, et al. Coordination tunes the activity and selectivity of the nitrogen reduction reaction on single-atom iron catalysts: a computational study[J]. J Mater Chem A, 2021, 9(2): 1240-1251. |
| 75 | JIANG Q, MENG Y, LI K, et al. Screening highly efficient hetero-diatomic doped PC6 electrocatalysts for selective nitrogen reduction to ammonia[J]. J Electrochem Soc, 2021, 168(11): 116519. |
| 76 | XUE Z, ZHANG X, QIN J, et al. Anchoring Mo on C9N4 monolayers as an efficient single atom catalyst for nitrogen fixation[J]. J Energy Chem, 2021, 57: 443-450. |
| 77 | LV S Y, HUANG C X, LI G, et al. Electrocatalytic mechanism of N2 reduction reaction by single-atom catalyst rectangular TM-TCNQ monolayers[J]. ACS Appl Mater Interfaces, 2021, 13(25): 29641-29653. |
| 78 | HUANG C X, LI G, YANG L M, et al. Ammonia synthesis using single-atom catalysts based on two-dimensional organometallic metal phthalocyanine monolayers under ambient conditions[J]. ACS Appl Mater Interfaces, 2020, 13(1): 608-621. |
| 79 | GAO L, WANG F, YU M, et al. A novel phosphotungstic acid-supported single metal atom catalyst with high activity and selectivity for the synthesis of NH3 from electrochemical N2 reduction: a DFT prediction[J]. J Mater Chem A, 2019, 7(34): 19838-19845. |
| 80 | DONG W, CHEN X, PENG J, et al. Recent progress on 2D transition metal compounds-based electrocatalysts for efficient nitrogen reduction[J]. Chem Res Chinese Univ, 2020, 36(4): 648-661. |
| 81 | WANG S, LI L, HUI K S, et al. Computational screening of single atoms anchored on defective Mo2CO2 MXene nanosheet as efficient electrocatalysts for the synthesis of ammonia[J]. Adv Eng Mater, 2021, 23(10): 2100405. |
| 82 | CHEN Z, ZHAO J, CABRERA C R, et al. Computational screening of efficient single-atom catalysts based on graphitic carbon nitride (g-C3N4) for nitrogen electroreduction[J]. Small Methods, 2019, 3(6): 1800368. |
| 83 | MENG Q, ZHANG L, WU J, et al. First-principles screening of single transition metal atoms anchored on two-dimensional C9N4 for the nitrogen reduction reaction[J]. Phys Chem Chem Phys, 2021, 23(14): 8784-8791. |
| 84 | LING C, NIU X, LI Q, et al. Metal-free single atom catalyst for N2 fixation driven by visible light[J]. J Am Chem Soc, 2018, 140(43): 14161-14168. |
| 85 | BHATTACHARYYA K, DATTA A. Visible light driven efficient metal free single atom catalyst supported on nanoporous carbon nitride for nitrogen fixation[J]. Phys Chem Chem Phys, 2019, 21(23): 12346-12352. |
| [1] | Xian WANG, Xiao-Long YANG, Rong-Peng MA, Chang-Peng LIU, Jun-Jie GE, Wei XING. Atomic Dispersion Ir‑N‑C Catalysts for Anode Anti‑poisoning Electrolysis in Fuel Cell [J]. Chinese Journal of Applied Chemistry, 2022, 39(8): 1202-1208. |
| [2] | HE Quan-Bao, HU Zheng, GE Ming. Research Progress on Photo-degradation of Antibiotics in Water by BiOX(X=Cl,Br,I) Composite Photocatalytic Materials [J]. Chinese Journal of Applied Chemistry, 2021, 38(7): 754-766. |
| [3] | LIU Lin-Chang, GUO Ya-Jun, ZHU Hong-Lin, MA Jing-Jing, LI Zhong-Yi, SHUI Miao, ZHENG Yue-Qing. Research Progress on Supported Ultrafine Nano-catalysts for Hydrolytic Dehydrogenation of Ammonia Borane [J]. Chinese Journal of Applied Chemistry, 2021, 38(11): 1405-1422. |
| [4] | YANG Zhengjun, LIU Bo, HUANG Pai, NIE Heran, ZHOU Guangyuan, ZHANG Jianfu. Effect of Pore Structures of Porous Polymer Microspheres on Catalytic Propylene Polymerization [J]. Chinese Journal of Applied Chemistry, 2020, 37(7): 746-755. |
| [5] | WU Shengli,LI Zongjun,GAO Xiang. Progress on Thiolation Reactions of Fullerenes [J]. Chinese Journal of Applied Chemistry, 2019, 36(4): 392-401. |
| [6] | HE Fagui,GAO Xiang. Application of Oxygen Nucleophiles Hydroxyl and Alkoxide Anions in Derivatization of Fullerenes [J]. Chinese Journal of Applied Chemistry, 2017, 34(5): 489-501. |
| [7] | LI Fei, JIANG Heng, ZHAO Shanlin, LI Ping. Product Analysis and Reaction Mechanism of High-Pressure Synthesis of Sulfurized Isobutylene by One-Step Method [J]. Chinese Journal of Applied Chemistry, 2015, 32(7): 771-776. |
| [8] | XU Liting, XING Na*, WU Qiong, MA Xitong, XING Yongheng*. Synthesis, Structure of an Iron(Ⅲ) Complex Containing N-Donor Ligand and Its Catalytic Activity for Cyclohexane Oxidation [J]. Chinese Journal of Applied Chemistry, 2014, 31(06): 707-714. |
| [9] | LIU Yu1, ZHANG Tianlong1, WANG Bozhou2, GE Zhongxue2, LI Hua1*. Mechanistic Synthesis of 3-Amino-4-amino-oxime Furazan Using Multivariate Curve Resolution Combined with On-line Infrared Spectroscopy [J]. Chinese Journal of Applied Chemistry, 2012, 29(09): 1075-1081. |
| [10] | ZHANG Shuli1, XIONG Xianfeng2, WANG Youbing2, WANG Bozhou2, GE Zhongxue2, LI Hua1*. Study on Synthetic Reaction Mechanism of Octogen Using Chemometrics and Infrared Spectroscopy [J]. Chinese Journal of Applied Chemistry, 2012, 29(06): 711-717. |
| [11] | LI Gao-Liang, HE Hui*. Studies on the reaction kinetics between nitrous acidand N,N-Dimethylhydroxylamine [J]. Chinese Journal of Applied Chemistry, 2010, 27(08): 916-923. |
| [12] | WANG Jian-Zhong1, ZHAO Hai-Yan1, XING Yong-Heng1*, WANG Qiang2, WANG Yong-Cheng2. Synthesis, Structure of 4-Iodo-3,5-dimethyl-pyrazole Potassium and Quantum Chemistry Calculation of Intermediate of Oximation [J]. Chinese Journal of Applied Chemistry, 2010, 27(07): 806-810. |
| [13] | DAI Yan, LI Bin-Dong, LUO Jun, LV Chun-Xu*, HU Yu-Feng. Synthesis of 2-Aromatic Amide Hexafluoroisoropanol [J]. Chinese Journal of Applied Chemistry, 2009, 26(09): 1090-1099. |
| [14] | Liu Yumin, Zhu Kaizheng, Liu Shetian, Yie Xingkai, Wu Yue. CoCuAl Hydrotalcite like Compounds:Synthesis and Use in the Catalytic Oxidation of p-Cresol Ⅱ.Catalytic Oxidation of p-Cresol [J]. Chinese Journal of Applied Chemistry, 1998, 0(2): 15-19. |
| [15] | Jiang Bo, Huang Guanglin. Reaction Mechanism of Radiation Induced Graft Polymerization on MgO Surface [J]. Chinese Journal of Applied Chemistry, 1997, 0(1): 95-97. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||