Chinese Journal of Applied Chemistry ›› 2021, Vol. 38 ›› Issue (10): 1326-1339.DOI: 10.19894/j.issn.1000-0518.210359
• Review • Previous Articles Next Articles
Research Progress of Liquid Crystal Molecules for Application in Organic Solar Cells
Yong-Jie CUI1‡, Jia-Xin ZHONG1‡, Xun-Fan LIAO1,2( ), Yi-Wang CHEN1,2(
), Yi-Wang CHEN1,2( )
)
- 1State Key Laboratory for Modification of Chemical Fibers and Polymer Materials & College of Materials Science and Engineering,Donghua University,Shanghai 201620,China
2Institute of Advanced Scientific Research (iASR),Key Laboratory of Functional Organic Small Molecules for Ministry of Education,Jiangxi Normal University,Nanchang 330022,China
-
Received:2021-07-22Accepted:2021-09-05Published:2021-10-01Online:2021-10-15 -
Contact:Xun-Fan LIAO,Yi-Wang CHEN -
About author:ywchen@ncu.edu.cn; xfliao@jxnu.edu.cn -
Supported by:National Natural Science Foundation of China (NSFC)(51973032);the Fundamental Research Funds for the Central Universities and the Graduate Student Innovation Fund of Donghua University(CUSF-DH-D-2021008)
CLC Number:
Cite this article
Yong-Jie CUI, Jia-Xin ZHONG, Xun-Fan LIAO, Yi-Wang CHEN. Research Progress of Liquid Crystal Molecules for Application in Organic Solar Cells[J]. Chinese Journal of Applied Chemistry, 2021, 38(10): 1326-1339.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: http://yyhx.ciac.jl.cn/EN/10.19894/j.issn.1000-0518.210359
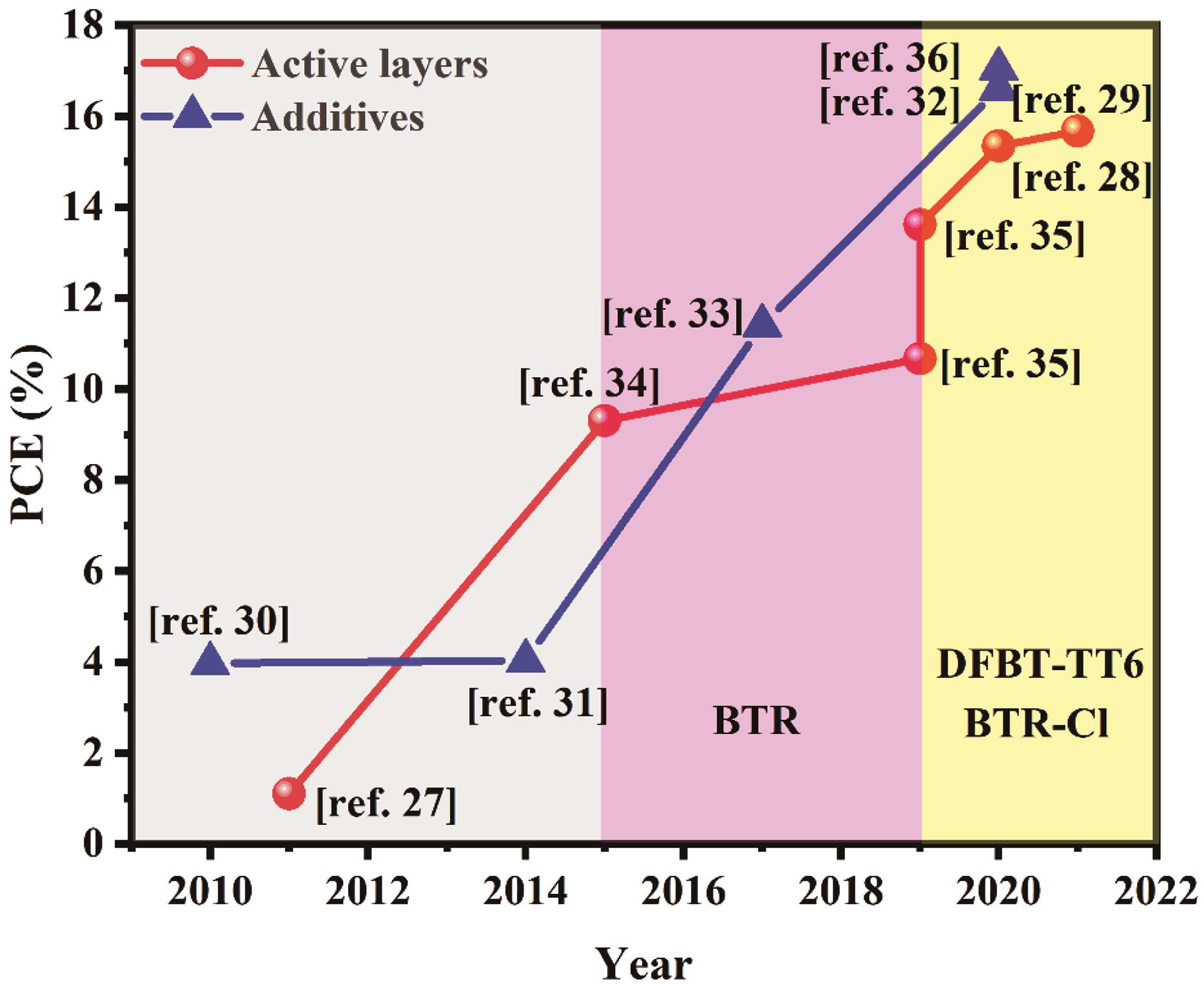
活性层 Active layer | 给体/受体质量比D/A mass ratio | 空穴传输层 Hole transport layer | 电子传输层 Electron transport layer | 开路电压 Voc/V | 短路电流Jsc/(mA·cm-2) | 填充因子FF/% | 能量转换效率PCE/% | 参考文献Ref. |
|---|---|---|---|---|---|---|---|---|
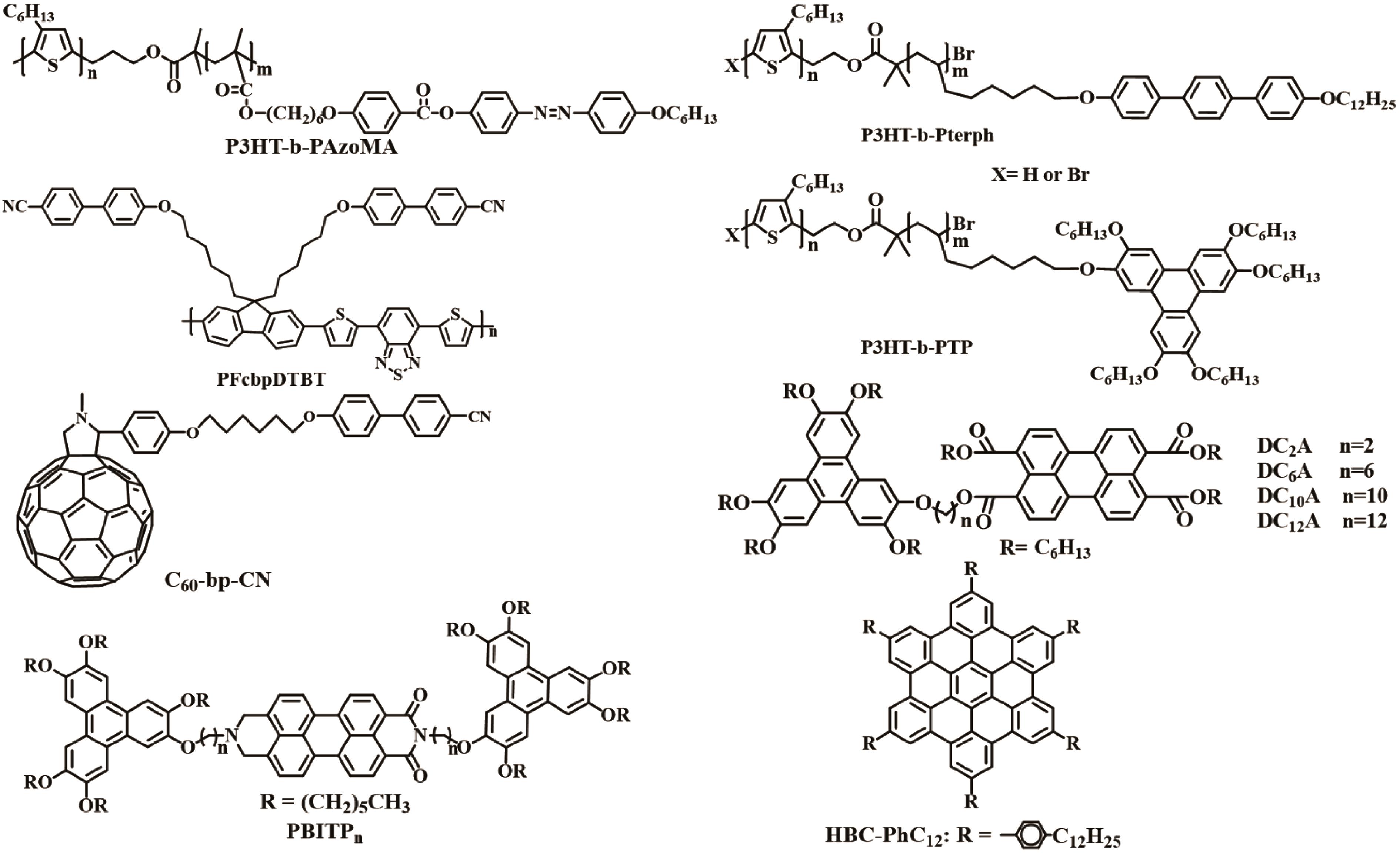
| PFcbpDTBT:PC61BM | 1∶3 | PEDOT:PSS | LiF | 0.68 | 3.38 | 48 | 1.10 | [ |
| P3HT:C60?bp?CN | 1∶1 | PEDOT:PSS | LiF | 0.52 | 5.5 | 23 | 0.65 | [ |
| P3HT?b?Pterph:PC61BM | 1∶1 | PEDOT:PSS | LiF | 0.54 | 4.42 | 23.6 | 0.56 | [ |
| P3HT?b?PTP:PC61BM | 1∶1 | PEDOT:PSS | LiF | 0.56 | 4.73 | 23.9 | 0.63 | [ |
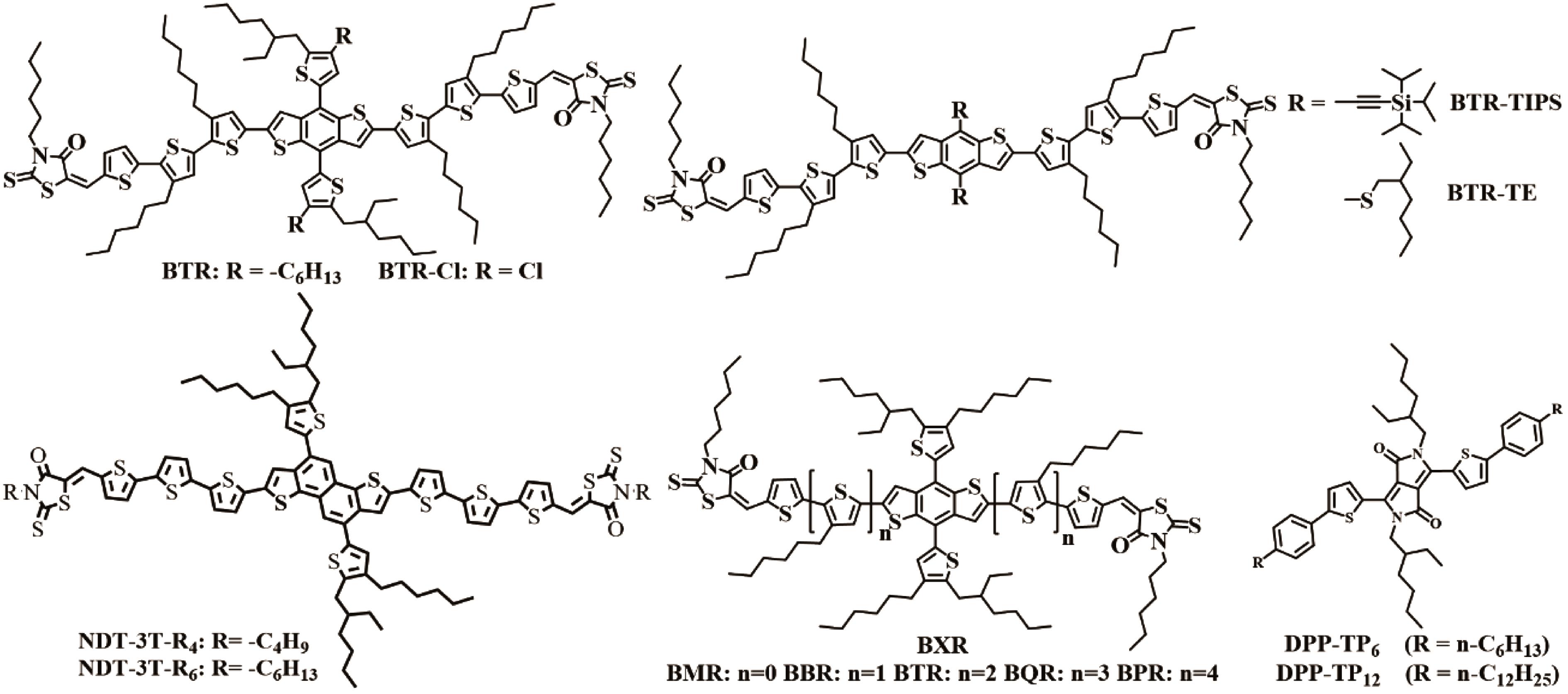
| BTR:PC71BM | 1∶1 | PEDOT:PSS | Ca | 0.90 | 13.90 | 74.1 | 9.3 | [ |
| BTR:Y6:PC71BM | 1.6∶0.7∶0.3 | PEDOT:PSS | Phen?NaDPO | 0.859 | 22.21 | 62 | 11.82 | [ |
| BTR:Y6 | 1.6∶1 | PEDOT:PSS | Phen?NaDPO | 0.85 | 22.25 | 56.4 | 10.67 | [ |
| BTR?Cl:Y6 | 1.6∶1 | PEDOT:PSS | Phen?NaDPO | 0.86 | 24.17 | 65.5 | 13.61 | [ |
| BTR?Cl:Y6:PC71BM | 1.8∶1∶0.1 | PEDOT:PSS | Phen?NaDPO | 0.837 | 23.75 | 77.11 | 15.34 | [ |
| BTR?Cl:Y6 | 1.6∶1 | PEDOT:PSS | ZrAcac | 0.829 | 23.42 | 68.9 | 13.48 | [ |
| BTR?Cl:Y6:anti?PDFC | 1.6∶1∶0.1 | PEDOT:PSS | ZrAcac | 0.837 | 23.97 | 72.6 | 14.56 | [ |
| BTR?Cl:Y6:syn?PDFC | 1.6∶1∶0.15 | PEDOT:PSS | ZrAcac | 0.839 | 23.41 | 71.8 | 14.09 | [ |
| BTR?Cl:Y6:PDFC?Ph | 1.6∶1∶0.1 | PEDOT:PSS | ZrAcac | 0.837 | 23.62 | 65 | 12.76 | [ |
| BTR?Cl:Y6:anti?PDFC:PC71BM | 1.7∶1∶0.1∶0.1 | PEDOT:PSS | ZrAcac | 0.836 | 25.01 | 74.9 | 15.67 | [ |
| BTR:Y6 | 1∶0.6 | PEDOT:PSS | Phen?NaDPO | 0.83 | 22.1 | 61 | 11.1 | [ |
| BTR?TE:Y6 | 1∶0.6 | PEDOT:PSS | Phen?NaDPO | 0.84 | 23.4 | 67 | 13.2 | [ |
| BTR?TIPS:Y6 | 1∶0.6 | PEDOT:PSS | Phen?NaDPO | 0.83 | 18.5 | 54 | 8.3 | [ |
| NDT?3T?R4:Y6 | 1∶0.6 | PEDOT:PSS | Ca | 0.76 | 17.02 | 71.4 | 9.2 | [ |
| NDT?3T?R6:Y6 | 1∶0.8 | PEDOT:PSS | Ca | 0.78 | 18.89 | 72.8 | 10.7 | [ |
| BQR:PC71BM | 1∶1 | PEDOT:PSS | Ca | 0.92 | 14.90 | 65 | 8.9 | [ |
| DPP?TP6:PC71BM | 1∶1 | PEDOT:PSS | LiF | 0.93 | 8.4 | 55 | 4.3 | [ |
Table 1 Device performance parameters of liquid crystal molecules as active layer of organic solar cells
活性层 Active layer | 给体/受体质量比D/A mass ratio | 空穴传输层 Hole transport layer | 电子传输层 Electron transport layer | 开路电压 Voc/V | 短路电流Jsc/(mA·cm-2) | 填充因子FF/% | 能量转换效率PCE/% | 参考文献Ref. |
|---|---|---|---|---|---|---|---|---|
| PFcbpDTBT:PC61BM | 1∶3 | PEDOT:PSS | LiF | 0.68 | 3.38 | 48 | 1.10 | [ |
| P3HT:C60?bp?CN | 1∶1 | PEDOT:PSS | LiF | 0.52 | 5.5 | 23 | 0.65 | [ |
| P3HT?b?Pterph:PC61BM | 1∶1 | PEDOT:PSS | LiF | 0.54 | 4.42 | 23.6 | 0.56 | [ |
| P3HT?b?PTP:PC61BM | 1∶1 | PEDOT:PSS | LiF | 0.56 | 4.73 | 23.9 | 0.63 | [ |
| BTR:PC71BM | 1∶1 | PEDOT:PSS | Ca | 0.90 | 13.90 | 74.1 | 9.3 | [ |
| BTR:Y6:PC71BM | 1.6∶0.7∶0.3 | PEDOT:PSS | Phen?NaDPO | 0.859 | 22.21 | 62 | 11.82 | [ |
| BTR:Y6 | 1.6∶1 | PEDOT:PSS | Phen?NaDPO | 0.85 | 22.25 | 56.4 | 10.67 | [ |
| BTR?Cl:Y6 | 1.6∶1 | PEDOT:PSS | Phen?NaDPO | 0.86 | 24.17 | 65.5 | 13.61 | [ |
| BTR?Cl:Y6:PC71BM | 1.8∶1∶0.1 | PEDOT:PSS | Phen?NaDPO | 0.837 | 23.75 | 77.11 | 15.34 | [ |
| BTR?Cl:Y6 | 1.6∶1 | PEDOT:PSS | ZrAcac | 0.829 | 23.42 | 68.9 | 13.48 | [ |
| BTR?Cl:Y6:anti?PDFC | 1.6∶1∶0.1 | PEDOT:PSS | ZrAcac | 0.837 | 23.97 | 72.6 | 14.56 | [ |
| BTR?Cl:Y6:syn?PDFC | 1.6∶1∶0.15 | PEDOT:PSS | ZrAcac | 0.839 | 23.41 | 71.8 | 14.09 | [ |
| BTR?Cl:Y6:PDFC?Ph | 1.6∶1∶0.1 | PEDOT:PSS | ZrAcac | 0.837 | 23.62 | 65 | 12.76 | [ |
| BTR?Cl:Y6:anti?PDFC:PC71BM | 1.7∶1∶0.1∶0.1 | PEDOT:PSS | ZrAcac | 0.836 | 25.01 | 74.9 | 15.67 | [ |
| BTR:Y6 | 1∶0.6 | PEDOT:PSS | Phen?NaDPO | 0.83 | 22.1 | 61 | 11.1 | [ |
| BTR?TE:Y6 | 1∶0.6 | PEDOT:PSS | Phen?NaDPO | 0.84 | 23.4 | 67 | 13.2 | [ |
| BTR?TIPS:Y6 | 1∶0.6 | PEDOT:PSS | Phen?NaDPO | 0.83 | 18.5 | 54 | 8.3 | [ |
| NDT?3T?R4:Y6 | 1∶0.6 | PEDOT:PSS | Ca | 0.76 | 17.02 | 71.4 | 9.2 | [ |
| NDT?3T?R6:Y6 | 1∶0.8 | PEDOT:PSS | Ca | 0.78 | 18.89 | 72.8 | 10.7 | [ |
| BQR:PC71BM | 1∶1 | PEDOT:PSS | Ca | 0.92 | 14.90 | 65 | 8.9 | [ |
| DPP?TP6:PC71BM | 1∶1 | PEDOT:PSS | LiF | 0.93 | 8.4 | 55 | 4.3 | [ |
活性层 Active layer | 添加剂含量 Additive contents/% | 空穴传输层 Hole transport layer | 电子传输层 Electron transport layer | 开路电压Voc/V | 短路电流Jsc/(mA·cm-2) | 填充因子FF/% | 能量转换效率PCE/% | 参考文献Ref. |
|---|---|---|---|---|---|---|---|---|
| P3HT:PC61BM:AZO1 | 5 | PEDOT:PSS | - | 0.53 | 16.48 | 30 | 2.44 | [ |
| P3HT:PC61BM:AZO2 | 5 | PEDOT:PSS | - | 0.48 | 16.73 | 31 | 2.40 | [ |
| P3HT:PC61BM:5CB | 3 | PEDOT:PSS | LiF | 0.604 | 8.86 | 61.13 | 3.27 | [ |
| P3HT:PC61BM:8CB | 4 | PEDOT:PSS | LiF | 0.604 | 9.64 | 63.94 | 3.72 | [ |
| P3HT:PC61BM:DLC1 | 3 | PEDOT:PSS | LiF | 0.65 | 9.30 | 63.60 | 3.90 | [ |
| P3HT:PC61BM:DLC2 | 3 | PEDOT:PSS | LiF | 0.67 | 9.15 | 64.94 | 3.97 | [ |
| P3HT:PC61BM:5CT | 3 | PEDOT:PSS | LiF | 0.621 | 9.87 | 56.6 | 3.5 | [ |
| P3HT:PC61BM:P3HT?b?Pterph | 3 | PEDOT:PSS | LiF | 0.60 | 9.58 | 58.1 | 3.34 | [ |
| P3HT:PC61BM:P3HT?b?PTP | 5 | PEDOT:PSS | LiF | 0.60 | 10.36 | 64.9 | 4.03 | [ |
| PTB7?Th:BTR:PC71BM | 25 | MoO3 | ZnO | 0.75 | 21.4 | 70 | 11.40 | [ |
| PTB7?Th:BTR:PC71BM | 10 | PEDOT:PSS | PFN | 0.78 | 19.23 | 72.21 | 10.83 | [ |
| PCE10:BTR:PC71BM | 20 | MoOx | ZnO | 0.794 | 17.62 | 64.52 | 10.88 | [ |
| PM6:BTR:Y6 | 5 | PEDOT:PSS | PFNBr | 0.839 | 25.8 | 76.7 | 16.6 | [ |
| P3HT:PC61BM:DFBT?TT6 | 4 | MoO3 | ZnO | 0.626 | 9.27 | 67.3 | 3.91 | [ |
| PM6:Y6:DFBT?TT6 | 3 | PEDOT:PSS | PDINO | 0.845 | 26.56 | 76 | 17.05 | [ |
| PTB7:DPP?TP6:PC71BM | 8 | MoO3 | ZnO | 0.73 | 15.54 | 69.2 | 7.85 | [ |
Table 2 Device performance parameters of liquid crystal molecules as additive of organic solar cells
活性层 Active layer | 添加剂含量 Additive contents/% | 空穴传输层 Hole transport layer | 电子传输层 Electron transport layer | 开路电压Voc/V | 短路电流Jsc/(mA·cm-2) | 填充因子FF/% | 能量转换效率PCE/% | 参考文献Ref. |
|---|---|---|---|---|---|---|---|---|
| P3HT:PC61BM:AZO1 | 5 | PEDOT:PSS | - | 0.53 | 16.48 | 30 | 2.44 | [ |
| P3HT:PC61BM:AZO2 | 5 | PEDOT:PSS | - | 0.48 | 16.73 | 31 | 2.40 | [ |
| P3HT:PC61BM:5CB | 3 | PEDOT:PSS | LiF | 0.604 | 8.86 | 61.13 | 3.27 | [ |
| P3HT:PC61BM:8CB | 4 | PEDOT:PSS | LiF | 0.604 | 9.64 | 63.94 | 3.72 | [ |
| P3HT:PC61BM:DLC1 | 3 | PEDOT:PSS | LiF | 0.65 | 9.30 | 63.60 | 3.90 | [ |
| P3HT:PC61BM:DLC2 | 3 | PEDOT:PSS | LiF | 0.67 | 9.15 | 64.94 | 3.97 | [ |
| P3HT:PC61BM:5CT | 3 | PEDOT:PSS | LiF | 0.621 | 9.87 | 56.6 | 3.5 | [ |
| P3HT:PC61BM:P3HT?b?Pterph | 3 | PEDOT:PSS | LiF | 0.60 | 9.58 | 58.1 | 3.34 | [ |
| P3HT:PC61BM:P3HT?b?PTP | 5 | PEDOT:PSS | LiF | 0.60 | 10.36 | 64.9 | 4.03 | [ |
| PTB7?Th:BTR:PC71BM | 25 | MoO3 | ZnO | 0.75 | 21.4 | 70 | 11.40 | [ |
| PTB7?Th:BTR:PC71BM | 10 | PEDOT:PSS | PFN | 0.78 | 19.23 | 72.21 | 10.83 | [ |
| PCE10:BTR:PC71BM | 20 | MoOx | ZnO | 0.794 | 17.62 | 64.52 | 10.88 | [ |
| PM6:BTR:Y6 | 5 | PEDOT:PSS | PFNBr | 0.839 | 25.8 | 76.7 | 16.6 | [ |
| P3HT:PC61BM:DFBT?TT6 | 4 | MoO3 | ZnO | 0.626 | 9.27 | 67.3 | 3.91 | [ |
| PM6:Y6:DFBT?TT6 | 3 | PEDOT:PSS | PDINO | 0.845 | 26.56 | 76 | 17.05 | [ |
| PTB7:DPP?TP6:PC71BM | 8 | MoO3 | ZnO | 0.73 | 15.54 | 69.2 | 7.85 | [ |

Fig.7 (a) J-V curve and structure of devices with different film thickness[33]; (b) Photoluminescence (PL) spectra of neat BTR, PTB7-Th, and blend PTB7-Th:BTR films under 640 nm light excitation. (c) Time-resolved PL (TRPL) spectra of neat PTB7-Th, BTR, and blend PTB7-Th:BTR films obtained[69]; (d) Transient photovoltage and (e) transient photocurrent of devices based on PM6:Y6, PM6:BTR:Y6 (0.95:0.05:1.2), and BTR:Y6 blends; (f) The schematic of morphology evolution from PM6:Y6 blend to PM6:BTR:Y6 blend[32]

Fig.8 (a) The chemical structure of DFBT-TT6; DSC traces of DFBT-TT6:PM6 blends and DFBT-TT6:Y6 blends; (c) Schematic diagram of the morphology evolution from the PM6:Y6 blend to the PM6:Y6:DFBT-TT6 blend; (d) J-V curves of devices[36]
| 1 | ARMIN A, LI W, SANDBERG O J, et al. A history and perspective of non-fullerene electron acceptors for organic solar cells[J]. Adv Energy Mater, 2021, 11(15): 20003570. |
| 2 | 黄飞, 薄志山, 耿延候, 等. 光电高分子材料的研究进展[J]. 高分子学报, 2020, 50(10): 988-1046. |
| HUANG F, BO Z S, GENG Y H, et al. Study on optoelectronic polymers: an overview and outlook[J]. Acta Polym Sin, 2020, 50(10): 988-1046. | |
| 3 | ZHAO F W, ZHANG H T, ZHANG R, et al. Emerging approaches in enhancing the efficiency and stability in non-fullerene organic solar cells[J]. Adv Energy Mater, 2020, 10(47): 2002746. |
| 4 | LU L Y, ZHENG T Y, WU Q H, et al. Recent advances in bulk heterojunction polymer solar cells[J]. Chem Rev, 2015, 115(23): 12666-12731. |
| 5 | CUI Y J, ZHU P P, LIAO X F, et al. Recent advances of computational chemistry in organic solar cell research[J]. J Mater Chem C, 2020, 8(45): 15920-15939. |
| 6 | KEARNS D, CALVIN M. Photovoltaic efect and photoconductivity in laminated organic systems[J]. J Chem Phys, 1958, 29(4): 950-951. |
| 7 | YUAN J, ZHANG H T, ZHANG R, et al. Reducing voltage losses in the A-DA′D-A acceptor-based organic solar cells[J]. Chem, 2020, 6(9): 2147-2161. |
| 8 | YUE Q H, LIU W Y, ZHU X Z. n-Type molecular photovoltaic materials: design strategies and device applications[J]. J Am Chem Soc, 2020, 142(27): 11613-11628. |
| 9 | LIU Q S, JIANG Y F, JIN K, et al. 18% efficiency organic solar cells[J]. Sci Bull, 2020, 65: 272-275. |
| 10 | KIM M, RYU S U, PARK S A, et al. Donor-acceptor-conjugated polymer for high-performance organic field-effect transistors: a progress report[J]. Adv Funct Mater, 2019, 30(20): 1904545. |
| 11 | CUI C H, LI Y F. High-performance conjugated polymer donor materials for polymer solar cells with narrow-bandgap nonfullerene acceptors[J]. Energy Environ Sci, 2019, 12(11): 3225-3246. |
| 12 | XU X P, ZHANG G J, LI Y, et al. The recent progress of wide bandgap donor polymers towards non-fullerene organic solar cells[J]. Chinese Chem Lett, 2019, 30(4): 809-825. |
| 13 | ZHENG Z, YAO H F, YE L, et al. PBDB-T and its derivatives: a family of polymer donors enables over 17% efficiency in organic photovoltaics[J]. Mater Today, 2020, 35: 115-130. |
| 14 | 吕敏, 周瑞敏, 吕琨, 等. 高结晶性小分子给体材料应用于全小分子有机太阳能电池中的研究进展[J]. 化学学报, 2021, 79(3): 284-302. |
| LV M, ZHOU R M, LV K, et al. Research progress of small molecule donors with high crystallinity in all small molecule organic solar cells[J]. Acta Chim Sin, 2021, 79(3): 284-302. | |
| 15 | DUAN C H, DING L M. The new era for organic solar cells: small molecular donors[J]. Sci Bull, 2020, 65(19):1597-1599. |
| 16 | TANG H, YAN C Q, HUANG J M, et al. Benzodithiophene-based small-molecule donors for next-generation all-small-molecule organic photovoltaics[J]. Matter, 2020, 3(5): 1403-1432. |
| 17 | STRALEY J P. Frank elastic constants of the hard-rod liquid crystal[J]. Phys Rev A, 1973, 8(4): 2181-2183. |
| 18 | CORNIL J, LEMAUR V, CALBERT J P, et al. Charge transport in discotic liquid crystals: a molecular scale description[J]. Adv Mater, 2002, 14(10): 726-729. |
| 19 | 杨琼芬, 聂汉, 陈自然, 等. 三唑和环戊烯苯并菲衍生物盘状液晶分子的电荷传输性质[J]. 物理学报, 2012, 61(6): 141-147. |
| YANG Q F, NIE H, CHEN Z R, et al. Charge transport properties of triazole or cyclopentene triphenylene derivative discogen molecules[J]. Acta Phys Sin, 2012, 61(6): 141-147. | |
| 20 | LYU X L, XIAO A Q, SHI D, et al. Liquid crystalline polymers: discovery, development, and the future[J]. Polymer, 2020, 202: 122740. |
| 21 | KUMAR M, KUMAR S. Liquid crystals in photovoltaics: a new generation of organic photovoltaics[J]. Polym J, 2016, 49(1): 85-111. |
| 22 | 林飞. 盘状液晶在有机光电器件中的应用[J]. 黑龙江科学, 2017, 8(14): 42-43. |
| LIN F. The application of discotic liquid crystal in organic optoelectronic devices[J]. Heilongjiang Sci, 2017, 8(14): 42-43. | |
| 23 | SCHMIDT-MENDE L, FECHTENKÖTTER A, MÜLLEN K, et al. Self-organized discotic liquid crystals for high-efficiency organic photovoltaics[J]. Science, 2001, 293(5532): 1119-1122. |
| 24 | GRELL M, BRADLEY D D C, INHASEKARUN M, et al. A glass-forming conjugated main-chain liquid crystal polymer for polarized electroiuminescence applications[J]. Adv Mater, 2010, 9(10): 798-802. |
| 25 | YAMABE K, GOTO H. Electrosynthesis of conducting polymers in lecithin liquid crystal reaction field[J]. Fibers Polym, 2018, 19(1): 248-253. |
| 26 | THOMPSON D C, TANTOL O, JALLAGEAS H, et al. Characterization of liquid crystal polymer (LCP) material and transmission lines on LCP substrates from 30 to 110 GHz[J]. IEEE Trans Microwave Theory Tech, 2004, 52(4): 1343-1352. |
| 27 | YAO K, CHEN Y W, CHRN L, et al. Mesogens mediated self-assembly in applications of bulk heterojunction solar cells based on a conjugated polymer with narrow band gap[J]. Macromolecules, 2011, 44(8): 2698-2706. |
| 28 | HU D Q, YANG Q G, CHEN H Y, et al. Efficiency all-small-molecule organic solar cells with improved fill factor enabled by a fullerene additive[J]. Energy Environ Sci, 2020, 13(7): 2134-2141. |
| 29 | SHAN T, DING K, YU L Y, et al. Spatially orthogonal 2D sidechains optimize morphology in all-small-molecule organic solar cells[J]. Adv Funct Mater, 2021, 31(24): 2100750. |
| 30 | JEONG S, KWON Y, CHOI B D, et al. Improved efficiency of bulk heterojunction poly(3-hexylthiophene):[6,6]-phenyl-C61-butyric acid methyl ester photovoltaic devices using discotic liquid crystal additives[J]. Appl Phys Lett, 2010, 96: 183305. |
| 31 | YUAN K, CHEN L, CHEN Y W. Photovoltaic performance enhancement of P3HT/PCBM solar cells driven by incorporation of conjugated liquid crystalline rod-coil block copolymers[J]. J Mater Chem C, 2014, 2(19): 3835-3845. |
| 32 | YAN C Q, TANG H, MA R J, et al. Synergy of liquid-crystalline small-molecule and polymeric donors delivers uncommon morphology evolution and 16.6% efficiency organic photovoltaics[J]. Adv Sci, 2020, 7(15): 2000149. |
| 33 | ZHANG G C, ZHANG K, YIN Q W, et al. High-performance ternary organic solar cell enabled by a thick active layer containing a liquid crystalline small molecule donor[J]. J Am Chem Soc, 2017, 139(6): 2387-2395. |
| 34 | SUN K, XIAO Z Y, LU S R, et al. A molecular nematic liquid crystalline material for high-performance organic photovoltaics[J]. Nat Commun, 2015, 6(1): 6013. |
| 35 | CHEN H Y, HU D Q, YANG Q G, et al. All-small-molecule organic solar cells with an ordered liquid crystalline donor[J]. Joule, 2019, 3(12): 3034-3047. |
| 36 | LIAO X F, HE Q N, ZHOU G Q, et al. Regulating favorable morphology evolution by a simple liquid-crystalline small molecule enables organic solar cells with over 17% efficiency and a remarkable Jsc of 26.56 mA/cm2[J]. Chem Mater, 2020, 33(1): 430-440. |
| 37 | ELIAS F, CLARKE S M, PECK R, et al. Nematic order drives phase separation in polydisperse liquid crystalline polymers[J]. Macromolecules, 2000, 33(6): 2060-2068. |
| 38 | KIM D, KYU T. Theoretical simulation of thermally induced phase separation in a main-chain liquid-crystalline polymer solution[J]. J Polym Sci Part B: Polym Phys, 2010, 41(9): 913-926. |
| 39 | MIJOVIC J, SY J W. Dipole dynamics and macroscopic alignment in molecular and polymeric liquid crystals by broad-band dielectric relaxation spectroscopy[J]. Macromolecules, 2000, 33(26): 7795-7802. |
| 40 | GANICZ T, STAŃCZYK W. Side-chain liquid crystal polymers (SCLCP): methods and materials. an overview[J]. Materials, 2009, 2(1): 95-128. |
| 41 | HAN D H, TONG X, ZHAO Y, et al. Block copolymers comprising pi-conjugated and liquid crystalline subunits: induction of macroscopic nanodomain orientation[J]. Angew Chem Int Ed Engl, 2010, 49(48): 9162-9165. |
| 42 | WANG P S, YAO K, CHEN L, et al. Self-assembled mesogens modified fullerene for efficiently stable bulk heterojunction solar cells[J]. Sol Energy Mater Sol Cells, 2012, 97: 34-42. |
| 43 | CHEN L, XIE C, CHEN Y W. Self-assembled conjugated polyelectrolyte-ionic liquid crystal complex as an interlayer for polymer solar cells: achieving performance enhancement via rapid liquid crystal-induced dipole orientation[J]. Macromolecules, 2014, 47(5): 1623-1632. |
| 44 | ZENG D L, TAHAR-DJEBBAR I, XIAO Y M, et al. Intertwined lamello-columnar coassemblies in liquid-crystalline side-chain π-conjugated polymers: toward a new class of nanostructured supramolecular organic semiconductors[J]. Macromolecules, 2014, 47(5): 1715-1731. |
| 45 | KONG X F, XIA L T, ZHANG H F, et al. Synthesis and investigation on liquid crystal and optical properties of dyads based on triphenylene and perylene[J]. RSC Adv, 2017, 7(28): 17030-17037. |
| 46 | KONG X F, HE Z Q, ZHANG Y N, et al. A mesogenic triphenylene-perylenetriphenylene triad[J]. Org Lett, 2011, 13(4): 764-767. |
| 47 | CRAATS A M V D, WARMAN J M, FECHTENKŐTTER A, et al. Record charge carrier mobility in a roomtemperature discotic liquid-crystalline derivative of hexabenzocoronene[J]. Adv Mater, 1999, 11(17): 1469-1472. |
| 48 | LI J L, KASTLER M, PISULA W, et al. Organic bulk-heterojunction photovoltaics based on alkyl substituted discotics[J]. Adv Funct Mater, 2007, 17(14): 2528-2533. |
| 49 | TANG H, CHEN H Y, YAN C Q, et al. Delicate morphology control triggers 14.7% efficiency all-small-molecule organic solar cells[J]. Adv Energy Mater, 2020, 10(27): 2001076. |
| 50 | ZHOU J Y, WAN X J, LIU Y S, et al. Small molecules based on benzo[1,2-b:4,5-b']dithiophene unit for high-performance solution-processed organic solar cells[J]. J Am Chem Soc, 2012, 134(39): 16345-16351. |
| 51 | YAO H F, YE L, ZHANG H, et al. Molecular design of benzodithiophene-based organic photovoltaic materials[J]. Chem Rev, 2016, 116(12): 7397-7457. |
| 52 | CHEN S H, FENG L W, JIA T, et al. High-performance polymer solar cells with efficiency over 18% enabled by asymmetric side chain engineering of non-fullerene acceptors[J]. Sci China Chem, 2021, 64: 1192-1199. |
| 53 | LI C, ZHOU J D, SONG J L, et al. Non-fullerene acceptors with branched side chains and improved molecular packing to exceed 18% efficiency in organic solar cells[J]. Nat Energy, 2021, 6(6): 605-613. |
| 54 | LIU Y S, WAN X J, WANG F, et al. High-performance solar cells using a solution-processed small molecule containing benzodithiophene unit[J]. Adv Mater, 2011, 23(45): 5387-5391. |
| 55 | HUO Y, ZHANG H L, ZHAN X W. Nonfullerene all-small-molecule organic solar cells[J]. ACS Energy Lett, 2019, 4(6): 1241-1250. |
| 56 | COLLINS S D, RAN N A, HEIBER M C, et al. Small is powerful: recent progress in solution-processed small molecule solar cells[J]. Adv Energy Mater, 2017, 7(10): 1602242. |
| 57 | KAN B, KAN Y Y, ZUO L J, et al. Recent progress on all-small molecule organic solar cells using small‐molecule nonfullerene acceptors[J]. InfoMat, 2021, 3(2): 175-200. |
| 58 | LI Z D, YAN C, XIAO L G, et al. Small molecule ternary solar cell with two synergistic electron acceptors for enhanced photovoltaic performance[J]. Org Electron, 2021, 93: 106135. |
| 59 | SUBBIAH J, LEE C J, MITCHELL V D, et al. Effect of side-chain modification on the active layer morphology and photovoltaic performance of liquid crystalline molecular materials[J]. ACS Appl Mater Interfaces, 2020, 13(1): 1086-1093. |
| 60 | LI H, WU Q, ZHOU R M, et al. Liquid‐crystalline small molecules for nonfullerene solar cells with high fill factors and power conversion efficiencies[J]. Adv Energy Mater, 2018, 9(6): 1803175. |
| 61 | GERAGHTY P B, LEE C, SUBBIAH J, et al. High performance p-type molecular electron donors for OPV applications via alkylthiophene catenation chromophore extension[J]. Beilstein J Org Chem, 2016, 12: 2298-2314. |
| 62 | BOURQUE A J, ENGMANN S, FUSTER A, et al. Morphology of a thermally stable small molecule OPV blend comprising a liquid crystalline donor and fullerene acceptor[J]. J Mater Chem A, 2019, 7(27): 16458-16471. |
| 63 | SHIN W, YASUDA T, WATANABE G, et al. Self-organizing mesomorphic diketopyrrolopyrrole derivatives for efficient solution-processed organic solar cells[J]. Chem Mater, 2013, 25(12): 2549-2556. |
| 64 | LIU J G, HAN Y C. The influence of additive property on performance of organic bulk heterojunction solar cells[J]. Polym Bull, 2012, 68(8): 2145-2174. |
| 65 | HUH Y H, PARK B. Power generating reflective-type liquid crystal displays using a reflective polariser and a polymer solar cell[J]. Sci Rep, 2015, 5: 11558. |
| 66 | IWAN A, BOHAREWICZ B, TAZBIR I, et al. Effect of chiral photosensitive liquid crystalline dopants on the performance of organic solar cells[J]. Solid-State Electron, 2015, 104: 53-60. |
| 67 | JEONG S, KWON Y, CHOI B D, et al. Effects of nematic liquid crystal additives on the performance of polymer solar cells[J]. Macromol Chem Phys, 2010, 211(23): 2474-2479. |
| 68 | ZHOU W H, SHI J M, LV L J, et al. A mechanistic investigation of morphology evolution in P3HT-PCBM films induced by liquid crystalline molecules under external electric field[J]. Phys Chem Chem Phys, 2015, 17: 387-397. |
| 69 | MA X L, ZHANG F J, AN Q S, et al. A liquid crystal material as the third component for ternary polymer solar cells with an efficiency of 10.83% and enhanced stability[J]. J Mater Chem A, 2017, 5(25): 13145-13153. |
| 70 | YIN A, ZHANG D Y, WANG J Q, et al. Mediated non-geminate recombination in ternary organic solar cells through a liquid crystal guest donor[J]. Front Chem, 2020, 8: 21. |
| 71 | LIAO X F, WU F Y, CHEN L, et al. Crystallization and optical compensation by fluorinated rod liquid crystals for ternary organic solar cells[J]. J Phys Chem C, 2016, 120(33): 18462-18472. |
| 72 | ZHOU W H, AI Q Y, ZHANG L, et al. Crystalline and active additive for optimization morphology and absorption of narrow bandgap polymer solar cells[J]. J Polym Sci Part A: Polym Chem, 2017, 55(4): 726-733. |
| [1] | Yan-Long SHEN, Li-Ye CHENG, Xiang-Ru MENG, Qiong LI, Lian-Yun DU, En-Peng WANG, Chang-Bao CHEN. Effects of Ginseng Continuous Soil Crop on Growth Development and Antioxidant System of Ginseng at Different Fertility Stages [J]. Chinese Journal of Applied Chemistry, 2023, 40(1): 109-115. |
| [2] | Xin HE, Cai-Yun JIANG, Tao DING, Yu-Ping WANG. Reserch Progress of Preparation of Ordered Surface Enhanced Raman Scattering Substrate [J]. Chinese Journal of Applied Chemistry, 2022, 39(8): 1167-1176. |
| [3] | Ai-Hua CHEN, Cheng-Yun ZHANG, Zi-Chao DENG, Ya-Lan SUN. Structure Control of Liquid Crystalline Block Copolymers in Liquid⁃Phase Self⁃assembly [J]. Chinese Journal of Applied Chemistry, 2021, 38(10): 1255-1267. |
| [4] | Dan WANG, Hai-Yan PENG, Xing-Ping ZHOU, Xiao-Lin XIE. Research Progress of Holographic Polymer/Liquid Crystal Composites [J]. Chinese Journal of Applied Chemistry, 2021, 38(10): 1268-1298. |
| [5] | SHI Yufang, BAI Yang, SUN Jinyu, LI Jun, ZHAO Minggen. Theoretical Investigation and Ultrafast Third-Order Nonlinear Optical Response of Novel Isomeric Pyrenyl Chalcones [J]. Chinese Journal of Applied Chemistry, 2019, 36(9): 1035-1043. |
| [6] | SUN Jinyu, WANG Yingjin, SHI Yufang, REN Guangming, ZHAO Minggen. Synthesis of Two Ferrocene-Based Chalcone Derivatives and Their Ultrafast Third-Order Nonlinear Optical Response [J]. Chinese Journal of Applied Chemistry, 2019, 36(3): 282-290. |
| [7] | SUN Jinyu,WANG Guilin,SHI Yufang,LIU Chengqi,ZHAO Minggen. Synthesis, Theoretical Investigation and Ultrafast Third-Order Nonlinear Optical Response of 2-(Pyren-1-yl)-1,8-naphthyridine [J]. Chinese Journal of Applied Chemistry, 2019, 36(10): 1172-1178. |
| [8] | Feng HONG, Yueru YIN, Yupeng TIAN, Jieying WU. A Novel Third-Order Non-Linear Optical Ruthenium(Ⅱ) Complex Based on Imidazo-phenanthroline Derivative:Design, Synthesis and DNA Binding Properties [J]. Chinese Journal of Applied Chemistry, 2018, 35(7): 818-824. |
| [9] | YE Lifang,WU Quanzhou. Modification of Ordered Macroporous Silica by a Functional Polymer Layer and Immobilization of Glucoamylase on the Macropore Walls [J]. Chinese Journal of Applied Chemistry, 2018, 35(11): 1309-1316. |
| [10] | CHEN Jiebo,XIE Weijie,WANG Lu,WANG Yiming,LEI Yufeng,WEI Yali. Synthesis of Ordered Mesoporous Ru-MgZr Composite Oxide Catalysts for Isomerization of Linoleic Acid [J]. Chinese Journal of Applied Chemistry, 2018, 35(11): 1342-1350. |
| [11] | SHI Yufang, SUN Jinyu, WANG Guilin, LIU Chengqi, ZHAO Minggen. Synthesis, Theoretical Investigation and Third-order Nonlinear Optical Properties of Novel Aromatic Chalcone Derivatives [J]. Chinese Journal of Applied Chemistry, 2016, 33(10): 1182-1188. |
| [12] | ZHANG Chen, LIU Huanhuan, CHEN Mingqing, LIU Shirong*. Rapid Fabrication of Three-dimensional Ordered Macroporous Titanium Dioxide via Vacuum Filling of Colloidal Crystal Templates [J]. Chinese Journal of Applied Chemistry, 2014, 31(12): 1441-1446. |
| [13] | CHE Guangbo1, YUAN Jing2, SU Bin1, LIU Chunbo2*, ZHAO Jing2, LI Lili2. Application of Inorganic Materials in Organic Solar Cells [J]. Chinese Journal of Applied Chemistry, 2013, 30(09): 977-985. |
| [14] | SUN Qian, YANG Yingchun*, YE Zhixiang, ZHANG Lin. Resonance Rayleigh Scattering and Resonance nonlinear Scattering Spectra of Hg(Ⅱ)-phen-CR Systems and Their Analytical Application [J]. Chinese Journal of Applied Chemistry, 2013, 30(04): 474-480. |
| [15] | MA Youmei, YANG Xiaoping*, JIA Xiaolong, GUO Lijuan. A Simple and Controllable Preparation of PbS Nanobelts [J]. Chinese Journal of Applied Chemistry, 2012, 29(05): 557-564. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||