Chinese Journal of Applied Chemistry ›› 2025, Vol. 42 ›› Issue (8): 1057-1069.DOI: 10.19894/j.issn.1000-0518.250259
• Review • Previous Articles Next Articles
Research Progress on the Application of Rare Earth Luminescent Materials in Immunoassay
Bin DAI1, Lin PENG2, Xue-Ning ZHU1, Te WANG3, Sai-Nan YANG1( ), Ling-Ling ZHANG3(
), Ling-Ling ZHANG3( )
)
- 1.Hunan Environment Biological Polytechnic,Hengyang 421000,China
2.Hunan Drug Inspection Center,Changsha 410000,China
3.State Key Laboratory of Rare Earth Resource Utilization,Changchun Institute of Applied Chemistry,Chinese Academy of Sciences,Changchun 130022,China
-
Received:2025-06-28Accepted:2025-07-08Published:2025-08-01Online:2025-08-11 -
Contact:Sai-Nan YANG,Ling-Ling ZHANG -
About author:zhangll@ciac.ac.cn
keepmoving9@163.com;
-
Supported by:Hunan Provincial Natural Science Foundation Project(2023JJ60207)
CLC Number:
Cite this article
Bin DAI, Lin PENG, Xue-Ning ZHU, Te WANG, Sai-Nan YANG, Ling-Ling ZHANG. Research Progress on the Application of Rare Earth Luminescent Materials in Immunoassay[J]. Chinese Journal of Applied Chemistry, 2025, 42(8): 1057-1069.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: http://yyhx.ciac.jl.cn/EN/10.19894/j.issn.1000-0518.250259
| Rare earth compound | Analysis technique | Target | Linear range/(ng·mL-1) | LOD/(ng·mL-1) | Ref. |
|---|---|---|---|---|---|
| Eu chelates | Time-resolved fluorescence immunoassay | African swine fever virus | 0.24~5 | 0.015 | [ |
| Sm chelates | Time-resolved fluorescence immunoassay | Lipoprotein(a) | 0~5 | 0.000 64 | [ |
| Eu chelates | Time-resolved fluorescence immunoassay | Aflatoxin B1 | 0.1~3.94 | 0.1 | [ |
| Eu cryptate | Homogenous time-resolved fluorescence | Bacterial protective antigen | 2~2 500 | 2 | [ |
| LiLuF4∶Ce/Tb NPs | Time-resolved fluorescence resonance energy transfer | Biotin | 0~152 600 | 210 | [ |
| Eu chelate | Time-resolved fluorescence resonance energy transfer | Cardiac troponin I | 0~1.16 | 0.097 | [ |
| Eu chelate | AlphaLISA | Staphylococcal enterotoxin B | 0.025~25 | 0.025 | [ |
Table 1 Application of rare earth luminescent compounds in time resolved fluorescence detection
| Rare earth compound | Analysis technique | Target | Linear range/(ng·mL-1) | LOD/(ng·mL-1) | Ref. |
|---|---|---|---|---|---|
| Eu chelates | Time-resolved fluorescence immunoassay | African swine fever virus | 0.24~5 | 0.015 | [ |
| Sm chelates | Time-resolved fluorescence immunoassay | Lipoprotein(a) | 0~5 | 0.000 64 | [ |
| Eu chelates | Time-resolved fluorescence immunoassay | Aflatoxin B1 | 0.1~3.94 | 0.1 | [ |
| Eu cryptate | Homogenous time-resolved fluorescence | Bacterial protective antigen | 2~2 500 | 2 | [ |
| LiLuF4∶Ce/Tb NPs | Time-resolved fluorescence resonance energy transfer | Biotin | 0~152 600 | 210 | [ |
| Eu chelate | Time-resolved fluorescence resonance energy transfer | Cardiac troponin I | 0~1.16 | 0.097 | [ |
| Eu chelate | AlphaLISA | Staphylococcal enterotoxin B | 0.025~25 | 0.025 | [ |
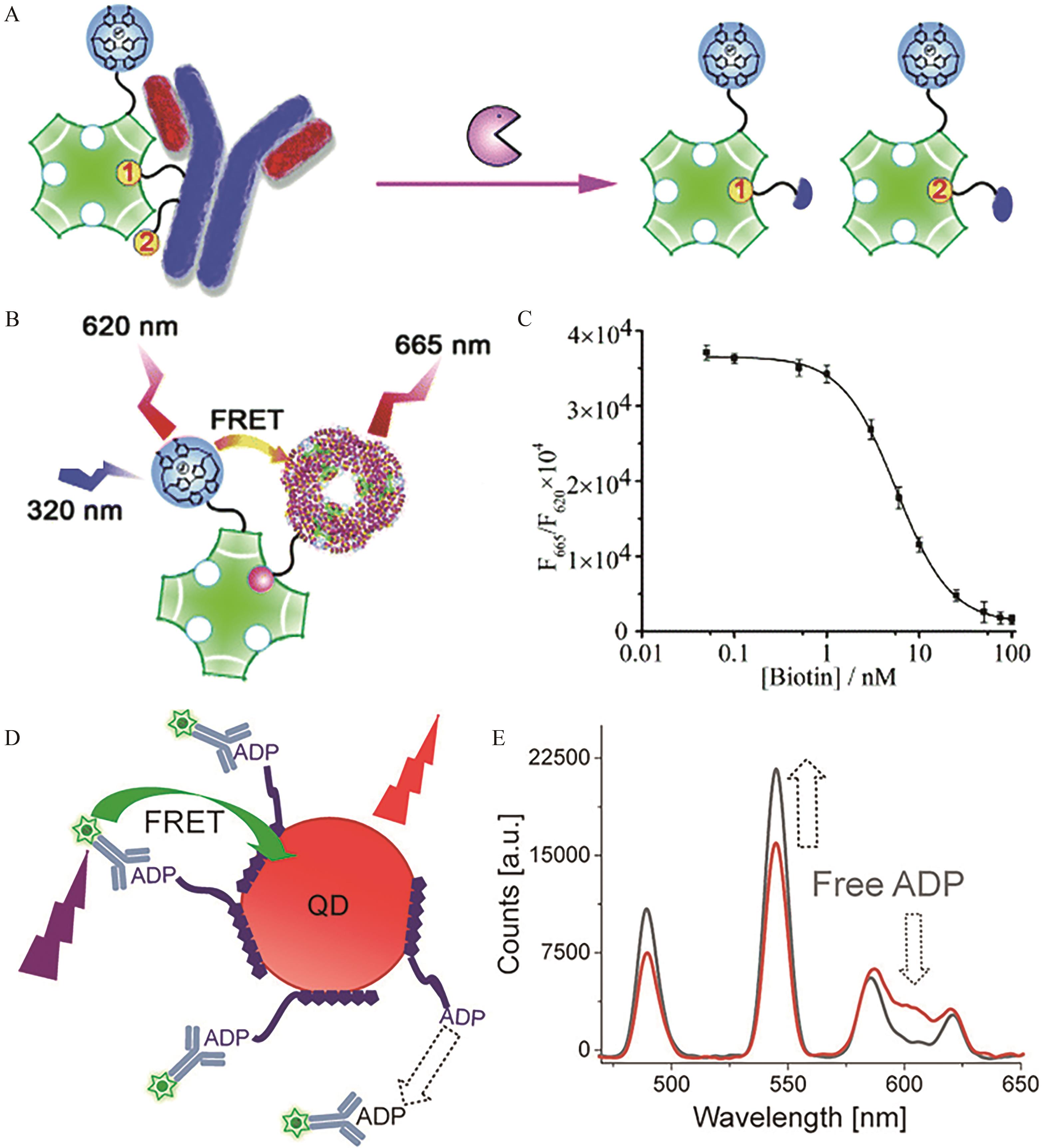
Fig.3 (A) Schematic diagram of the binding of free biotin and streptavidin; (B) Schematic diagrams of the principle for quantitative determination of biotin by TR-FRET. In the presence of free biotin, the binding probability of APC-labeled biotin decreases, resulting in weaker fluorescence intensity at 665 nm and stronger fluorescence intensity at 620 nm; (C) Curve diagram of the changes in fluorescence signal with biotin concentration[59]; (D) Schematic diagram of the ADP sensor: in the presence of free ADP, the FRET process between Tb-labeled antibody-bound ADP and QDs is weakened; (E) Spectra of the fluorophores of the ADP sensor[60]
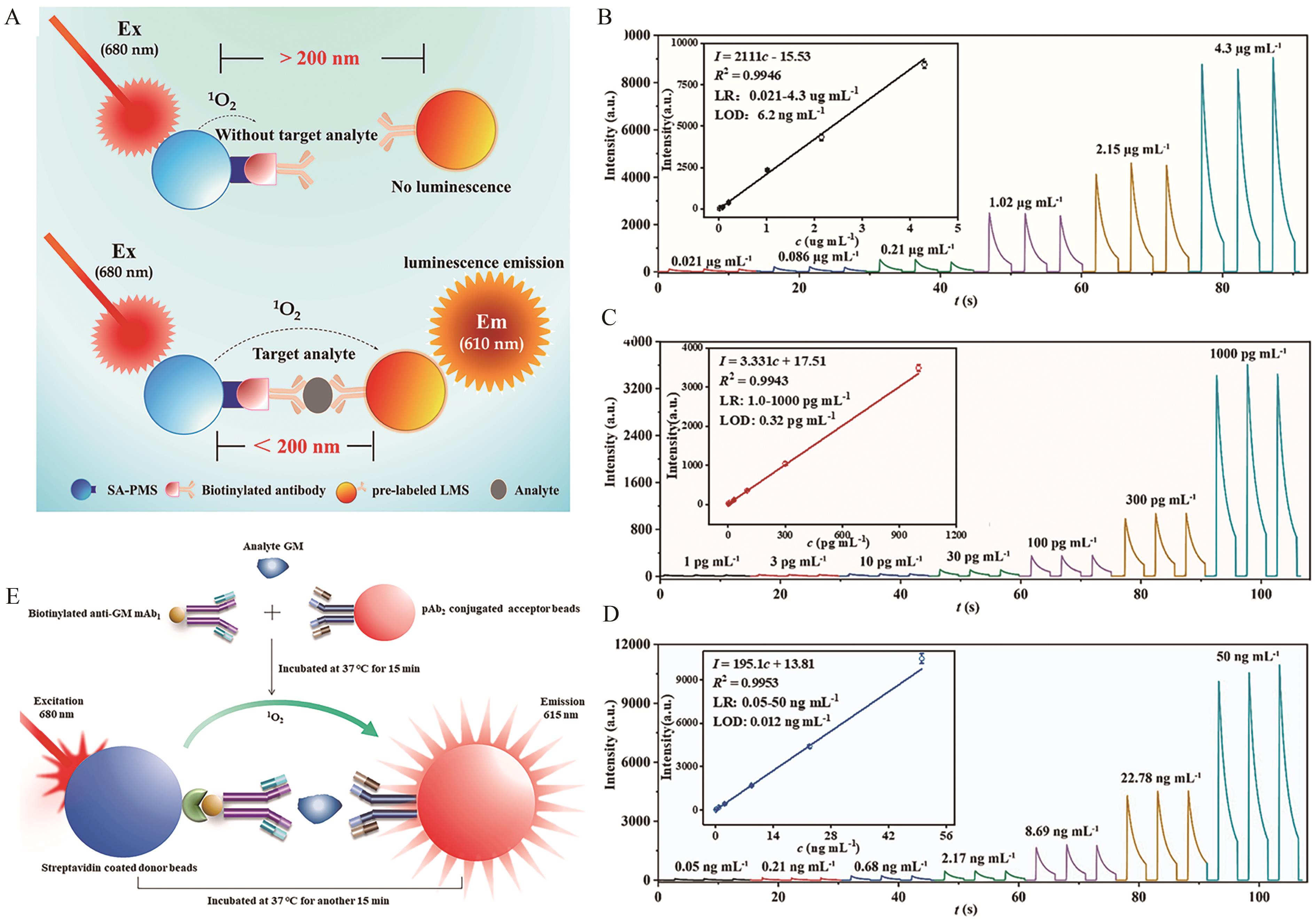
Fig.4 (A) Schematic diagram of the principle of light-initiated chemiluminescent assay technology; (B) Graph showing the relationship between CRP concentration and chemiluminescence signal based on light-initiated chemiluminescent assay technology; (C) Graph showing the relationship between IFN-γ concentration and chemiluminescence signal based on light-initiated chemiluminescent assay technology; (D) Graph showing the relationship between PCT concentration and chemiluminescence signal based on light-initiated chemiluminescent assay technology[66]; (E) Schematic diagram of light-initiated chemiluminescent assay of GM[67]
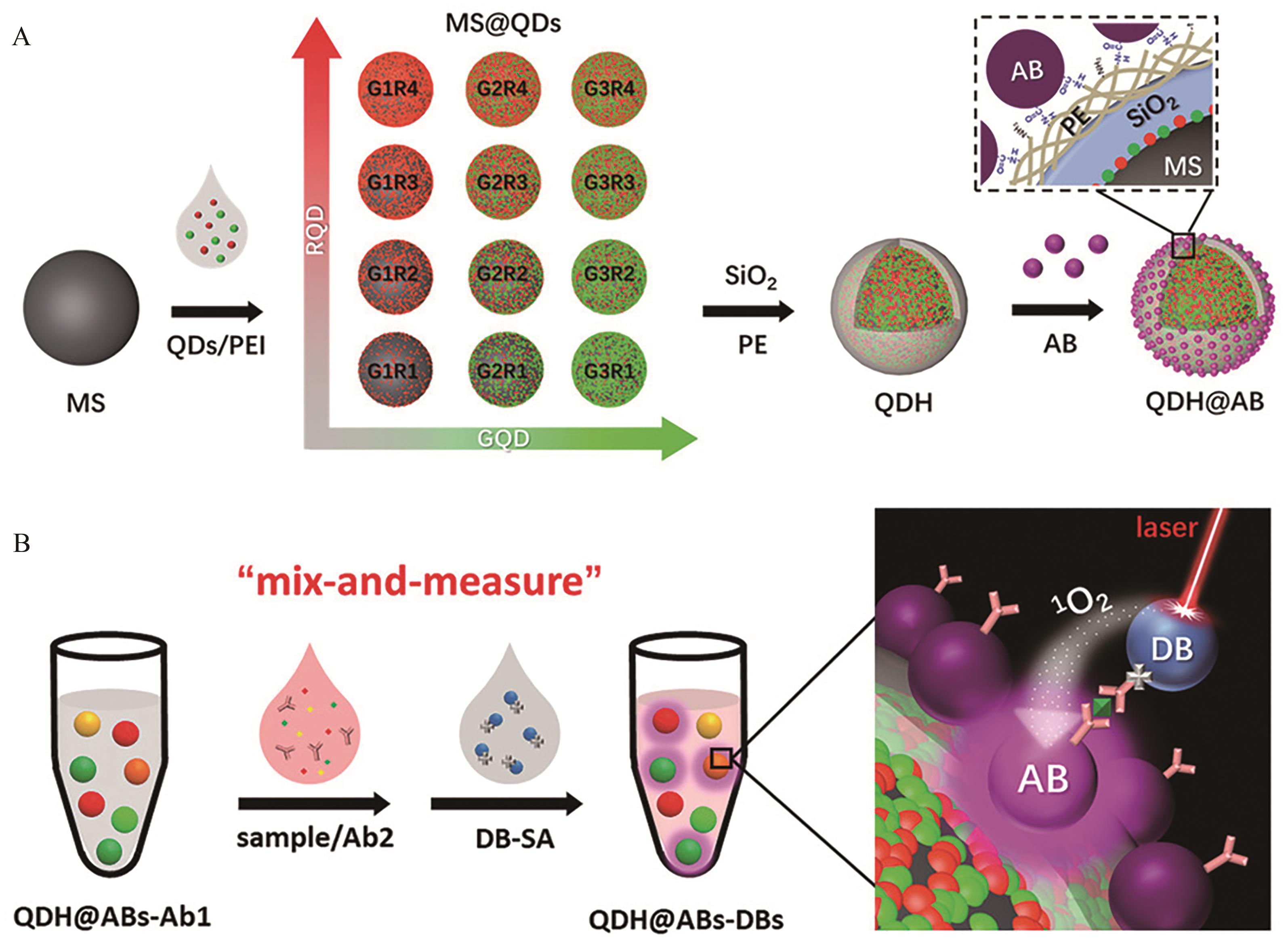
Fig.5 (A) Flow chart of microsphere encoding by quantum dots; (B) Schematic diagram of the principle of multi-LOCI technology for multiplex detection[73]
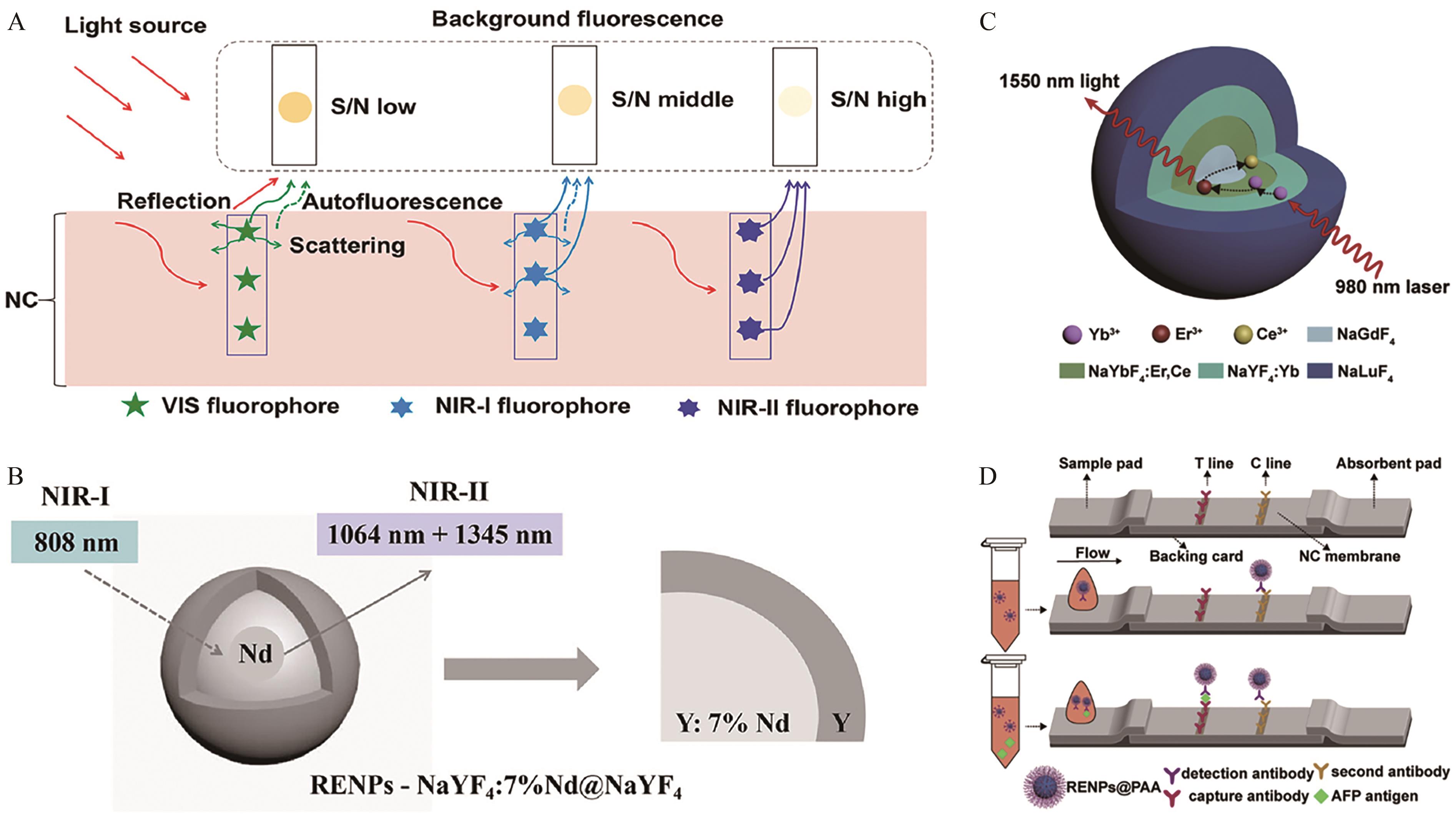
Fig.6 (A) Vertical cross-sectional view of the signal-to-noise ratio of different types of fluorescent probes in the LFA platform; (B) Structural diagram of NaYF4∶7%Nd@NaYF4 nanoparticles[78]; (C) Structural diagram of Yb,Er/Ce co-doped nanoparticles with 1550 nm emission under 980 nm excitation; (D) Schematic diagram of the lateral flow immunoassay platform based on NIR-Ⅱ emitting Yb,Er/Ce co-doped nanoparticles[79]
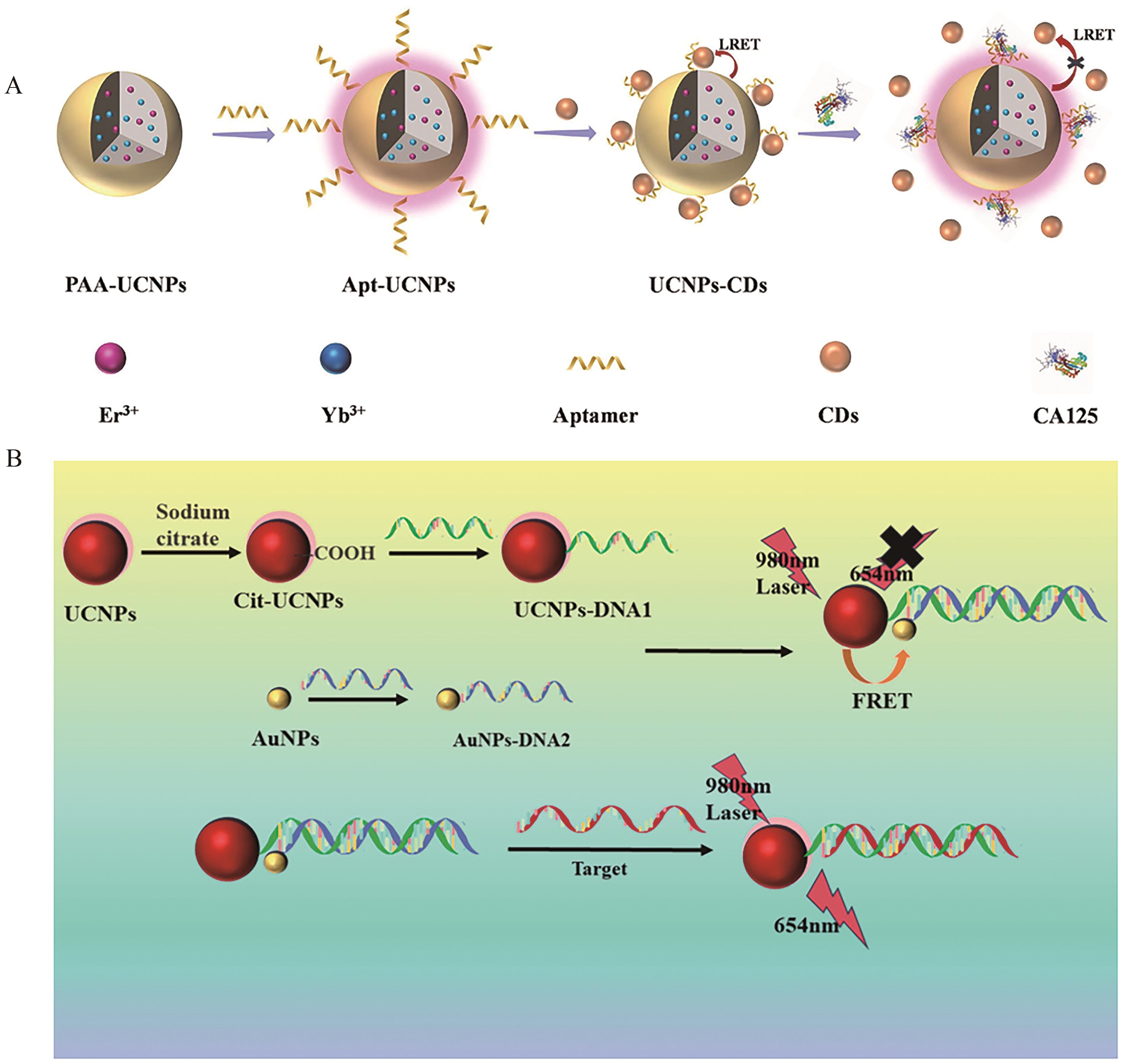
Fig.7 (A) Schematic illustration of the construction of rare earth upconversion nanoprobes and the CA125 sensing based on luminescence resonance energy transfer technology[82]; (B) Schematic diagram of miRNA-21 detection[83]
| [1] | FU H, JIANG Y, ZHANG M, et al. High-entropy rare earth materials: synthesis, application and outlook[J]. Chem Soc Rev, 2024, 53(4): 2211-2247. |
| [2] | LI Y, LIU Q, LI T, et al. Recent achievements in rare earth modified metal oxides for environmental and energy applications: a review[J]. Chin Chem Lett, 2024, 36(9): 110698. |
| [3] | LIU Y, LI Y, KOO S, et al. Versatile types of inorganic/organic NIR-Ⅱa/Ⅱb fluorophores: from strategic design toward molecular imaging and theranostics[J]. Chem Rev, 2022, 122(1): 209-268. |
| [4] | CHEISSON T, SCHELTER E J. Rare earth elements: Mendeleev′s bane, modern marvels[J]. Science, 2019, 363(6426): 489-493. |
| [5] | LI C, WANG P, HE M, et al. Rare earth-based nanomaterials in electrocatalysis[J]. Coord Chem Rev, 2023, 83(8): 574-594. |
| [6] | WANG S, ZHONG C, SHEN C, et al. Selective-crystallization strategy for the separation of rare earth elements: a minireview[J]. Coordi Chem Rev, 2025, 537: 216686. |
| [7] | PENG X X, WANG M X, ZHANG J L. Emerging frontiers in rare-earth element chemical biology[J]. Coord Chem Rev, 2024, 519: 216096. |
| [8] | ZHANG S, SAJI S E, YIN Z, et al. Rare-earth incorporated alloy catalysts: synthesis, properties, and applications[J]. Adv Mater, 2021, 33(16): 2005988. |
| [9] | COEY J M D. Perspective and prospects for rare earth permanent magnets[J]. Engineering, 2020, 6(2): 119-131. |
| [10] | DIAO H, YANG H, TAN T, et al. Navigating the rare earth elements landscape: challenges, innovations, and sustainability[J]. Minerals Eng, 2024, 216: 108889. |
| [11] | ZHANG H, ZHANG H. Special issue: rare earth luminescent materials[J]. Light: Sci Appl, 2022, 11(1): 260. |
| [12] | SU L, LIU X, NIU Q, et al. Photoresponsive lanthanide luminescent materials[J]. J Mater Chem C, 2024, 12(29): 10759-10774. |
| [13] | LIU J, KACZMAREK A M, VAN DEUN R. Advances in tailoring luminescent rare-earth mixed inorganic materials[J]. Chem Soc Rev, 2018, 47(19): 7225-7238. |
| [14] | ZHANG C, YIN Q, GE S, et al. Optical anti-counterfeiting and information storage based on rare-earth-doped luminescent materials[J]. Mater Res Bull, 2024, 176: 112801. |
| [15] | WANG H, YAO J, ZENG R. The luminescence modulation of rare earth-doped/containing lead-free double perovskites toward multifunctional applications: a review[J]. Nanoscale, 2024, 16(14): 6837-6852. |
| [16] | RAO R, ROWE E, SIEBENALLER R, et al. Multi-band luminescence from a rare earth-based two-dimensional material[J]. Matter, 2025, 8(2): 101929. |
| [17] | ZENG Z, SUN M, ZHANG S, et al. Rare-earth-based perovskite Cs2AgScCl6∶Bi for strong full visible spectrum emission[J]. Adv Funct Mate, 2022, 32(32): 2204780. |
| [18] | ZHANG X, ZHU Q, CHEN B, et al. Sensitizing full-spectrum lanthanide luminescence within a semiconductor CaZnOS host[J]. Adv Photon Res, 2021, 2(3): 2000089. |
| [19] | ZHANG Y, WEI P, LI Z, et al. Advancements in rare earth metal-organic frameworks: harnessing the power of photonics and beyond[J]. Coordin Chem Rev, 2024, 514: 215905. |
| [20] | SUN J, FU H, JING H, et al. Synergistic integration of halide perovskite and rare-earth ions toward photonics[J]. Adv Mater, 2025, 37(12): 2417397. |
| [21] | CHEN P, LI Z, LI D, et al. 2D rare earth material (EuOCl) with ultra-narrow photoluminescence at room temperature[J]. Small, 2021, 17(20): 2100137. |
| [22] | HARRISWANGLER C, FRíAS J C, ALBELDA M T, et al. Donor radii in rare-earth complexes[J]. Inorg Chem, 2023, 62(41): 17030-17040. |
| [23] | DARWISH I A, SUZUKI K, OGAWA H, et al. A prototype of ultrasensitive time-resolved fluoroimmunoassay for the quantitation of lead in plasma using a fluorescence-enhanced europium chelate label for the detection system[J]. RSC Adv, 2024, 14(13): 8671-8683. |
| [24] | KACZMAREK M T, ZABISZAK M, NOWAK M, et al. Lanthanides: schiff base complexes, applications in cancer diagnosis, therapy, and antibacterial activity[J]. Coord Chem Rev, 2018, 370: 42-54. |
| [25] | LI L, LI H, LIU Y, et al. Persistent- and mechanoluminescence of Er3+-doped NaYF4 for multipurpose use[J]. Small Methods, 2025, 9(5): 2401615. |
| [26] | LIU X, TU L, LI F, et al. Unravelling size-dependent upconversion luminescence in ytterbium and erbium codoped NaYF4 nanocrystals[J]. J Am Chem Soc, 2025, 147(7): 5955-5961. |
| [27] | PILCH A, WÜRTH C, KAISER M, et al. Shaping luminescent properties of Yb3+ and Ho3+ co-doped upconverting Core-Shell β-NaYF4 nanoparticles by dopant distribution and spacing[J]. Small, 2017, 13(47): 1701635. |
| [28] | SMARA Z, CHEROURA Y, BOYER D, et al. Energy transfer and luminescent properties of Eu3+, Tb3+, Eu3+-Yb3+ and Tb3+-Yb3+ doped α-NaYF4 nanophosphors prepared by coprecipitation route[J]. Opt Mater, 2020, 104: 109932. |
| [29] | JIA F, LI G, YANG B, et al. Investigation of rare earth upconversion fluorescent nanoparticles in biomedical field[J]. Nanotechnol Rev, 2019, 8(1): 1-17. |
| [30] | YANG M, GONG H, YANG D, et al. Research progress on rare earth up-conversion and near-infrared Ⅱ luminescence in biological applications[J]. Chin Chem Lett, 2024, 35(2): 108468. |
| [31] | ZHENG B, WANG H, PAN H, et al. Near-infrared light triggered upconversion optogenetic nanosystem for cancer therapy[J]. ACS Nano, 2017, 11(12): 11898-11907. |
| [32] | CHEN Y J, DOU C X, YIN P P, et al. U-type π-conjugated phosphorescent ligand sensitized lanthanide metal-organic frameworks for efficient white-light-emitting diodes[J]. Dalton T, 2023, 52(39): 13872-13877. |
| [33] | WEI X, CHUN F, LIU F, et al. Interfacing lanthanide metal-organic frameworks with ZnO nanowires for alternating current electroluminescence[J]. Small, 2024, 20(4): 2305251. |
| [34] | LI S, ZHOU L, ZHANG H. Investigation progresses of rare earth complexes as emitters or sensitizers in organic light-emitting diodes[J]. Light: Sci Appl, 2022, 11(1): 177. |
| [35] | HE J Y, WANG Y, CHEN X, et al. Air and thermally stable fluoride bridged rare-earth clusters showing intense photoluminescence and potential LED application[J]. Adv Mater, 2024, 36(47): 2406882. |
| [36] | PENG L, GUO H, REN B, et al. A novel dual-function fluorescence sensor Eu/CDs@MOF-808 for the sensitive detection of adenosine triphosphate and uric acid[J]. Chem Eng J, 2024, 500: 156811. |
| [37] | LIU J, ZHAO C, YANG J, et al. A novel hybrid lanthanide metal-organic frameworks based on porphyrin for rapid detection of iron ions[J]. Anal Chim Acta, 2024, 131: 9342961. |
| [38] | NING Y, ZHU M, ZHANG J L. Near-infrared (NIR) lanthanide molecular probes for bioimaging and biosensing[J]. Coordin Chem Rev, 2019, 399: 213028. |
| [39] | WANG M, MI C C, WANG W X, et al. Immunolabeling and NIR-excited fluorescent imaging of HeLa cells by using NaYF4∶Yb,Er upconversion nanoparticles[J]. ACS Nano, 2009, 3(6): 1580-1586. |
| [40] | YU Y, WANG X, JIA X, et al. Aptamer probes labeled with lanthanide-doped carbon nanodots permit dual-modal fluorescence and mass cytometric imaging[J]. Adv Sci, 2021, 8(24): 2102812. |
| [41] | RUGGIERI S, MIZZONI S, NARDON C, et al. Circularly polarized luminescence from new heteroleptic Eu(Ⅲ) and Tb(Ⅲ) complexes[J]. Inorg Chem, 2023, 62(23): 8812-8822. |
| [42] | WEN S, ZHOU J, ZHENG K, et al. Advances in highly doped upconversion nanoparticles[J]. Nat Commun, 2018, 9(1): 2415. |
| [43] | LIANG J, LI X, HUANG B, et al. Rapid, on-site quantitative determination of mycotoxins in grains using a multiple time-resolved fluorescent microsphere immunochromatographic test strip[J]. Biosens Bioelectron, 2024, 258: 116357. |
| [44] | CHEN C, LAI H, LIANG H, et al. A new method for detection african swine fever virus: time-resolved fluorescence immunoassay[J]. J Fluoresc, 2021, 31: 1291-1296. |
| [45] | MA H, MAO Q, ZHU Y, et al. Time-resolved fluorescence immunoassay (TRFIA) for thesimultaneous detection of hs-CRP and lipoprotein(a) in serum[J]. Biotechnol Appl Biochem, 2022, 69: 2617-2623. |
| [46] | HU X, YAO J, WANG F, et al. Eu3+-labeled IgG-based time-resolved fluoroimmunoassay for highly sensitivedetection of aflatoxin B1 in feed[J]. J Sci Food Agric, 2018, 98: 674-680. |
| [47] | COHEN N, MECHALY A, MAZOR O, et al. Rapid homogenous time-resolved fluorescence (HTRF) Immunoassay for anthrax detection[J]. J Fluoresc, 2014, 24: 795-801. |
| [48] | ZHANG S, XIE Y, HU X, et al. Time-resolved luminescent biodetection of biotin in infant formula based on lanthanide-doped LiLuF4 nanoparticles[J]. J Lumin, 2025, 285: 121312. |
| [49] | LEE K, KIM A, CHUN H, et al. Time-resolved ffuorescence resonance energy transfer-based lateral ffow immunoassay using a raspberry-type europium particle and a single membrane for the detection of cardiac troponin I[J]. Biosen Bioelectron, 2020, 163: 112284. |
| [50] | ZHAO J, LV Q, LIU P, et al. AlphaLISA for detection of staphylococcal enterotoxin B free from interference by protein A[J]. Toxicon, 2019, 165: 62-68. |
| [51] | SIITARI H, HEMMILÄ I, SOINI E, et al. Detection of hepatitis B surface antigen using time-resolved fluoroimmunoassay[J]. Nature, 1983, 301(5897): 258-260. |
| [52] | HU L M, LUO K, XIA J, et al. Advantages of time-resolved fluorescent nanobeads compared with fluorescent submicrospheres, quantum dots, and colloidal gold as label in lateral flow assays for detection of ractopamine[J]. Biosens Bioelectron, 2017, 91: 95-103. |
| [53] | CHEN J, GAO F, XU Z, et al. A terbium(Ⅲ) complex-based time-resolved luminescent probe for selenocysteine as an inhibitor of selenoproteins[J]. Chem Commun, 2024, 60(11): 1440-1443. |
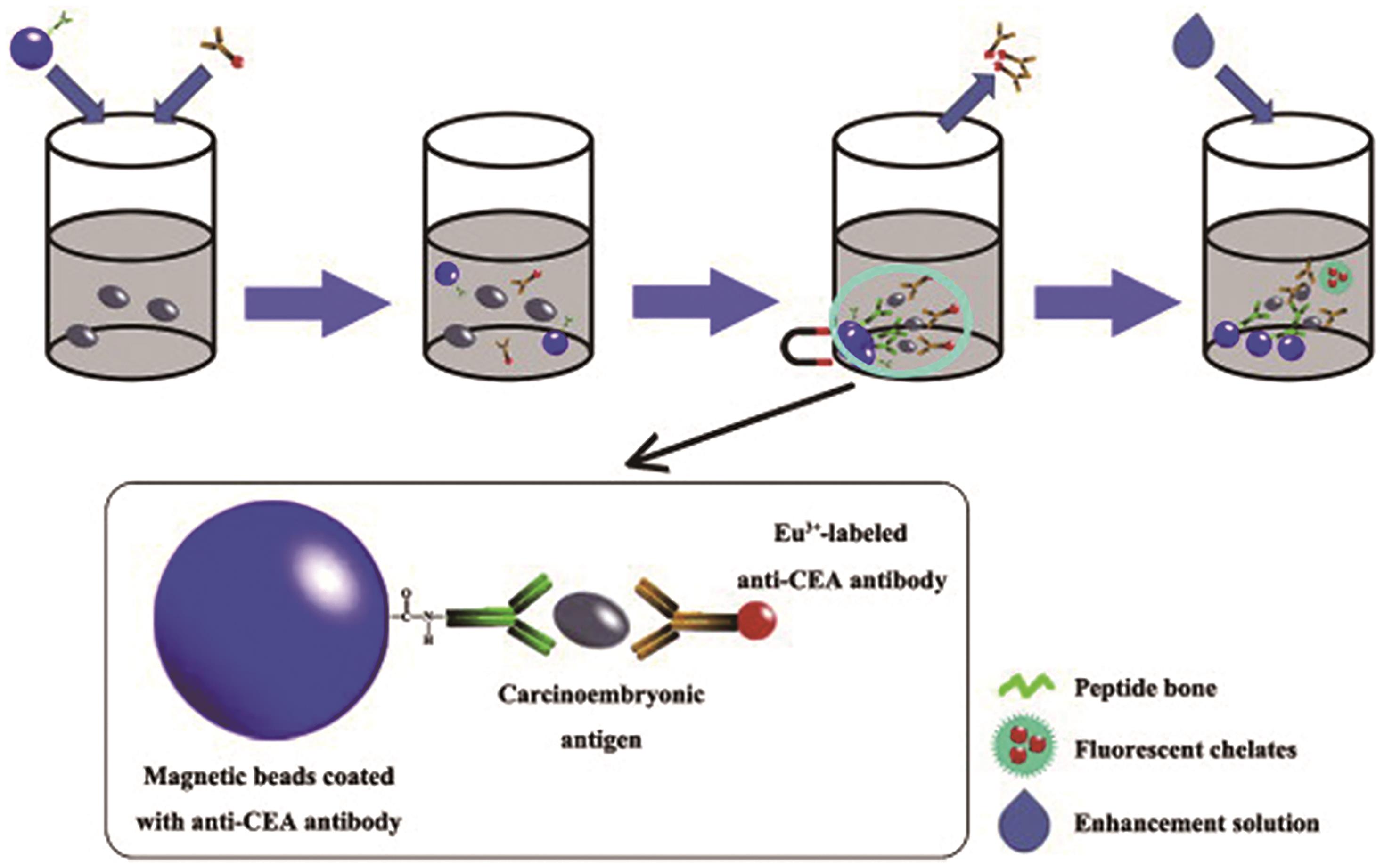
| [54] | HOU J Y, LIU T C, LIN G F, et al. Development of an immunomagnetic bead-based time-resolved fluorescence immunoassay for rapid determination of levels of carcinoembryonic antigen in human serum[J]. Anal Chim Acta, 2012, 734: 93-98. |
| [55] | LI M, WU T, WANG Y, et al. Highly selective and sensitive biological probe for cysteine detection based on fluorescence resonance energy transfer between lanthanide oxysulfide nanoparticles and Au nanoparticles[J]. Mater Res Bull, 2024, 169: 112520. |
| [56] | GORSHKOV K, MORALES VASQUEZ D, CHIEM K, et al. SARS-CoV-2 nucleocapsid protein TR-FRET assay amenable to high throughput screening[J]. ACS Pharmacol Transl Sci, 2022, 5(1): 8-19. |
| [57] | AKTER S, LAMMINMÄKI U. A 15-min non-competitive homogeneous assay for microcystin and nodularin based on time-resolved Förster resonance energy transfer (TR-FRET)[J]. Anal Bioanal Chem, 2021, 413(24): 6159-6170. |
| [58] | WESLEY N A, SKRAJNA A, SIMMONS H C, et al. Time resolved-fluorescence resonance energy transfer platform for quantitative nucleosome binding and footprinting[J]. Protein Sci, 2022, 31(6): e4339. |
| [59] | CHEN H, FENG Y, CAO Y, et al. A study on the detection of free and bound biotin based on TR-FRET technology[J]. Analyst, 2022, 147(2): 318-324. |
| [60] | DÍAZ S A, LASARTE-ARAGONES G, LOWERY R G, et al. Quantum dots as Förster resonance energy transfer acceptors of lanthanides in time-resolved bioassays[J]. ACS Appl Nano Mater, 2018, 1(6): 3006-3014. |
| [61] | CHENG F, LV B, HUANG Y, et al. Facile synthesis and characterization of fluorescent polystyrene nanospheres for homogeneous light-initiated chemiluminescence immunoassay[J]. J Fluoresc, 2024. https://doi.org/10.1007/s10895-024-03965-6. |
| [62] | LIU J, YIN J, YUAN H, et al. 1O2-activatable Eu3+-afterglow nanoprobe for highly sensitive detection of porphyria in whole blood[J]. J Rare Earth, 2022, 40(9): 1382-1388. |
| [63] | STAERZ S D, LISABETH E M, NJOMEN E, et al. Development of a cell-based AlphaLISA assay for high-throughput screening for small molecule proteasome modulators[J]. ACS Omega, 2023, 8(17): 15650-15659. |
| [64] | CHEN M, FANG H, WU J, et al. Establishment of trypsinogen-2 amplification luminescent proximity homogeneous assay and its application in acute pancreatitis[J]. J Fluoresc, 2024. https://doi.org/10.1007/s10895-024-03917-0. |
| [65] | LV W, LI Q, TANG Y, et al. AlphaLISA-based immunoassay for detection of troponin T in serum of patients with acute myocardial infarction[J]. J Fluoresc, 2025, 35(5): 3393-3403. |
| [66] | HUANG J, XIONG M, OU X, et al. Development of a light-initiated chemiluminescent assay platform for rapid detection of multiple inflammatory biomarkers[J]. Talanta, 2025, 295: 128297. |
| [67] | GUO Y, LI J. Sandwich-type homogeneous chemiluminescence immunoassay based on nanoparticle toward detection of Aspergillus galactomannan antigen[J]. Talanta, 2022, 243: 123392. |
| [68] | YAO S, XIAO W, CHEN H, et al. The combined detection of ovarian cancer biomarkers HE4 and CA125 by a fluorescence and quantum dot dual-signal immunoassay[J]. Anal Methods, 2019, 11(37): 4814-4821. |
| [69] | ZHANG H, LIU S, XIANG Q, et al. High-sensitivity triple-emission fluorescent probe for simultaneous detection of HCC and BC biomarkers and in situ imaging[J]. Sens Actuators B Chem, 2025, 440: 137932. |
| [70] | GAO M, LIAN H, YU L, et al. Rolling circle amplification integrated with suspension bead array for ultrasensitive multiplex immunodetection of tumor markers[J]. Anal Chim Acta, 2019, 1048: 75-84. |
| [71] | FENG Z, GUO Q, WANG Y, et al. Evolution of “On-Barcode” luminescence oxygen channeling immunoassay by exploring the barcode structure and the assay system[J]. ACS Omega, 2022, 7(2): 2344-2355. |
| [72] | SHENG S L, WANG Q, HUANG G, et al. Simultaneous determination of α-fetoprotein immune complexes and α-fetoprotein concentration in hepatocellular carcinoma using dual-label time-resolved immunofluorometric assays[J]. J Clin Lab Anal, 2009, 23(3): 179-185. |
| [73] | GUO Q, WANG Y, CHEN C, et al. Multiplexed luminescence oxygen channeling immunoassay based on dual-functional barcodes with a host-guest structure: a facile and robust suspension array platform[J]. Small, 2020, 16(17): 1907521. |
| [74] | WU Y, HU D, GAO D, et al. Miniature NIR-Ⅱ nanoprobes for active-targeted phototheranostics of brain tumors[J]. Adv Healthc Mater, 2022, 11(23): 2202379. |
| [75] | SONG Z, HAO Q, LI B, et al. NIR-Ⅱ fluorescence lateral flow immunosensor based on efficient energy transfer probe for point-of-care testing of tumor biomarkers[J]. Chin Chem Lett, 2025, 36(1): 109834. |
| [76] | XU M, LIN Y, LI Y, et al. Nanoprobe based on novel NIR-Ⅱ quinolinium cyanine for multimodal imaging[J]. Small, 2024, 20(49): 2406879. |
| [77] | SONG Z, GUO H, SUO Y, et al. Enhanced NIR-Ⅱ fluorescent lateral flow biosensing platform based on supramolecular host-guest self-assembly for point-of-care testing of tumor biomarkers[J]. ACS Appl Mater Interfaces, 2023, 15(44): 52038-52050. |
| [78] | SONG Z, SUO Y, DUAN S, et al. NIR-Ⅱ fluorescent nanoprobe-labeled lateral flow biosensing platform: a high-performance point-of-care testing for carcinoembryonic antigen[J]. Biosens Bioelectron, 2023, 224: 115063. |
| [79] | LI Y, KE J, LIU Q, et al. NIR-Ⅱ emitting rare-earth nanoparticles for a lateral flow immunoassay in hemolysis[J]. Sens Actuators B Chem, 2021, 345: 130380. |
| [80] | WILHELM S. Perspectives for upconverting nanoparticles[J]. ACS Nano, 2017, 11(11): 10644-10653. |
| [81] | LI H, LIU H, WONG K L, et al. Lanthanide-doped upconversion nanoparticles as nanoprobes for bioimaging[J]. Biomater Sci, 2024, 12(18): 4650-4663. |
| [82] | ZHANG X, WANG Y, DENG H, et al. An aptamer biosensor for CA125 quantification in human serum based on upconversion luminescence resonance energy transfer[J]. Microchem J, 2021, 161: 105761. |
| [83] | YANG L, CHE C, GUO M, et al. An Upconversion fluorescent resonant energy transfer biosensor for the detection of microRNA through DNA hybridization[J]. ACS Omega, 2024, 9(47): 47156-47166. |
| [1] | Rui-Qi ZHANG, Yu CHANG, Zuo-Jia LIU, Xue-Song LI, Li-Hua PAN. Rare Earth Fluorescent Probes for Rapid Detection of Hepatitis B Surface Antigen [J]. Chinese Journal of Applied Chemistry, 2024, 41(12): 1760-1769. |
| [2] | CHANG Yu, XIE Wenbing, ZHOU Shihong, LI Junling, LIU Baofeng, PAN Lihua. Synthesis and Spectroscopic Properties of a Cryptate Rare Earth Fluorescence Complex [J]. Chinese Journal of Applied Chemistry, 2017, 34(3): 361-366. |
| [3] | CHAI Yongzhen, CHANG Yu, SONG Ruiqin, LIU Wei, LI Yunhui, PAN Lihua. Synthesis and Characterization of Dimethyl 6,6'-Dimethyl-[2,2'-bipyridine]-4,4'-dicarboxylate [J]. Chinese Journal of Applied Chemistry, 2017, 34(2): 146-150. |
| [4] | LI Yun-Hui1, LI Hai-Yan1, PAN Li-Hua2*, XU Jing-Wei2, CHANG Yu2, DI Yan-Qing1, LIU Wen-Yu1. Preparation and Characterization of 4,4′-Dibromo -6,6′- bis(N,N-bis(ethoxycarbonylmethyl)amino methyl)- 2,2′-bipyridine [J]. Chinese Journal of Applied Chemistry, 2010, 27(09): 1008-1011. |
| [5] | Hou Shifa, Wang Wenyun. KINETIC STUDY ON THE Zn(Ⅱ)-HEMATOPORPHYRIN INCORPORATION REACTION IN MICELLAR MEDIUM [J]. Chinese Journal of Applied Chemistry, 1989, 0(6): 44-47. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||