Chinese Journal of Applied Chemistry ›› 2022, Vol. 39 ›› Issue (12): 1803-1817.DOI: 10.19894/j.issn.1000-0518.220053
• Review • Previous Articles Next Articles
Progress in Molecular Dynamics and Hansen Solubility Parameters of Low Molecular Weight Gels
Wan-Nian ZHANG1, Fang YU2,3, Shan-Lin ZHAO1,3, Zhi-Qiang ZHANG1( ), Yu-Peng HE1,2,3(
), Yu-Peng HE1,2,3( )
)
- 1.Key Laboratory for Functional Material,Educational Department of Liaoning Province,School of Chemical Engineering,University of Science and Technology Liaoning,Anshan 114051,China
2.State Key Laboratory of Fine Chemicals,Ningbo Institute of Dalian University of Technology,Ningbo 315016,China
3.Key Laboratory of Petrochemical Catalytic Science and Technology,Liaoning Petrochemical University,Fushun 113001,China
-
Received:2022-03-01Accepted:2022-08-03Published:2022-12-01Online:2022-12-13 -
Contact:Zhi-Qiang ZHANG,Yu-Peng HE -
About author:heyp_nbi@dlut.edu.cn
azzq@ustl.edu.cn
-
Supported by:the National Natural Science Foundation of China(21978124);the Key R&D Projects in Liaoning Province(2019JH2/10100005)
CLC Number:
Cite this article
Wan-Nian ZHANG, Fang YU, Shan-Lin ZHAO, Zhi-Qiang ZHANG, Yu-Peng HE. Progress in Molecular Dynamics and Hansen Solubility Parameters of Low Molecular Weight Gels[J]. Chinese Journal of Applied Chemistry, 2022, 39(12): 1803-1817.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: http://yyhx.ciac.jl.cn/EN/10.19894/j.issn.1000-0518.220053
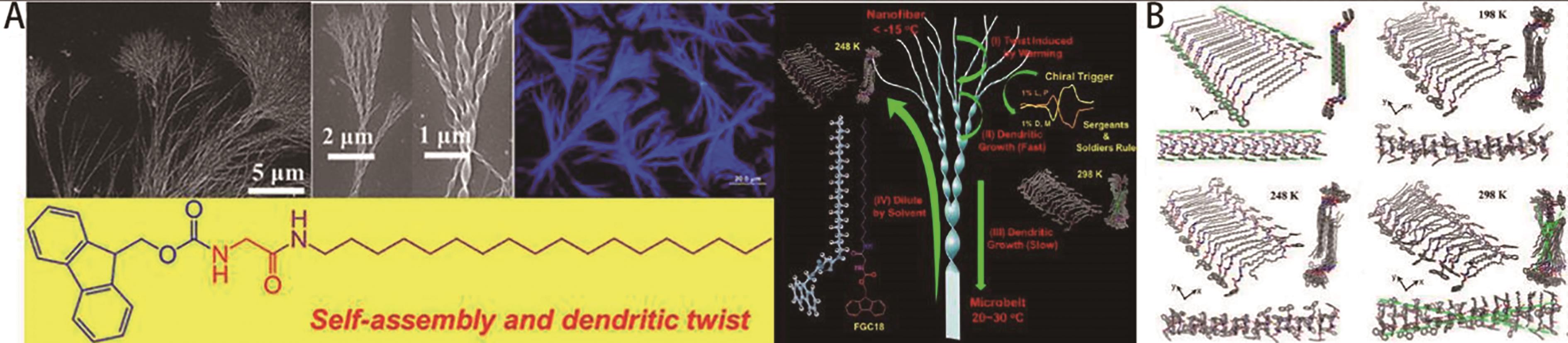
Fig.2 (A) Molecular structure of FGC18 and the schematic illustration of hierarchical dendrite twist structure formed by FGC18; (B) Molecular dynamics simulation snapshots of FGC18 at different temperatures[32]

Fig.3 (A) Calculation model and mechanism of the self-assembly of pure lipids; (B) Self-assembly of chiral lipids; (C) “Induced conformation rearrangement” mechanism of homochiral nanotube from heterochiral lipids[35]
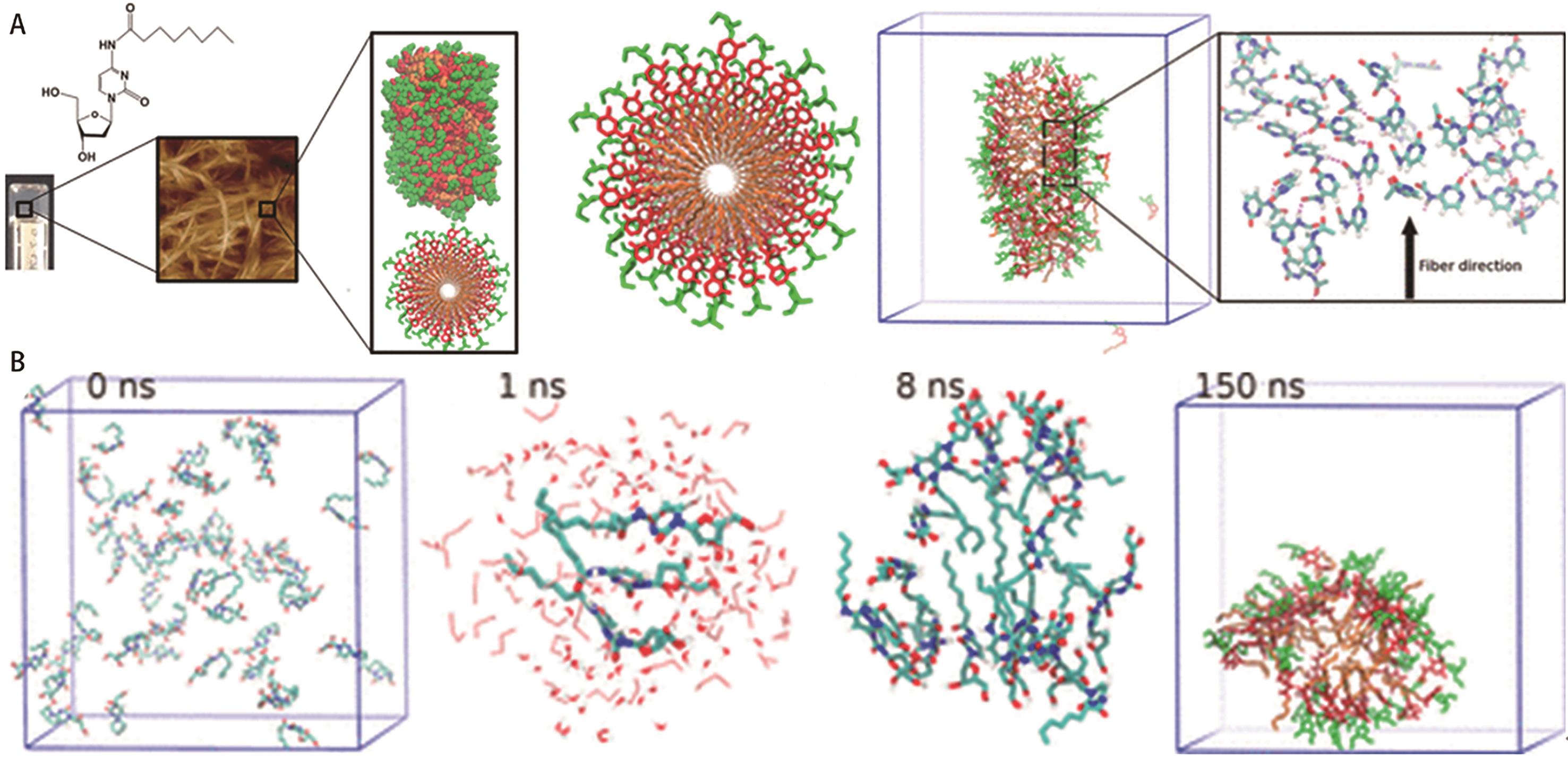
Fig.5 (A) Snapshots from an MD simulation of 160 pre-ordered gelator molecules in ethanol/water; (B) Snapshots from an MD simulation of the self-assembly of 50 gelator molecules in ethanol/water (V/V, 20/80) system[38]

Fig.6 (A) Molecular models illustrating the average configurations in the supramolecular columnar aggregates of BTECM[43]; (B) Snapshots of two tubules of sitosterol-oryzanol and sitosterol-CHEMS at the beginning or at the end of the course of the MD simulation[45]; (C) Hydrogen bonds, vdW interactions and π-π contacts between the ferulic acid groups of oryzanol on the interface of two tubules[45]
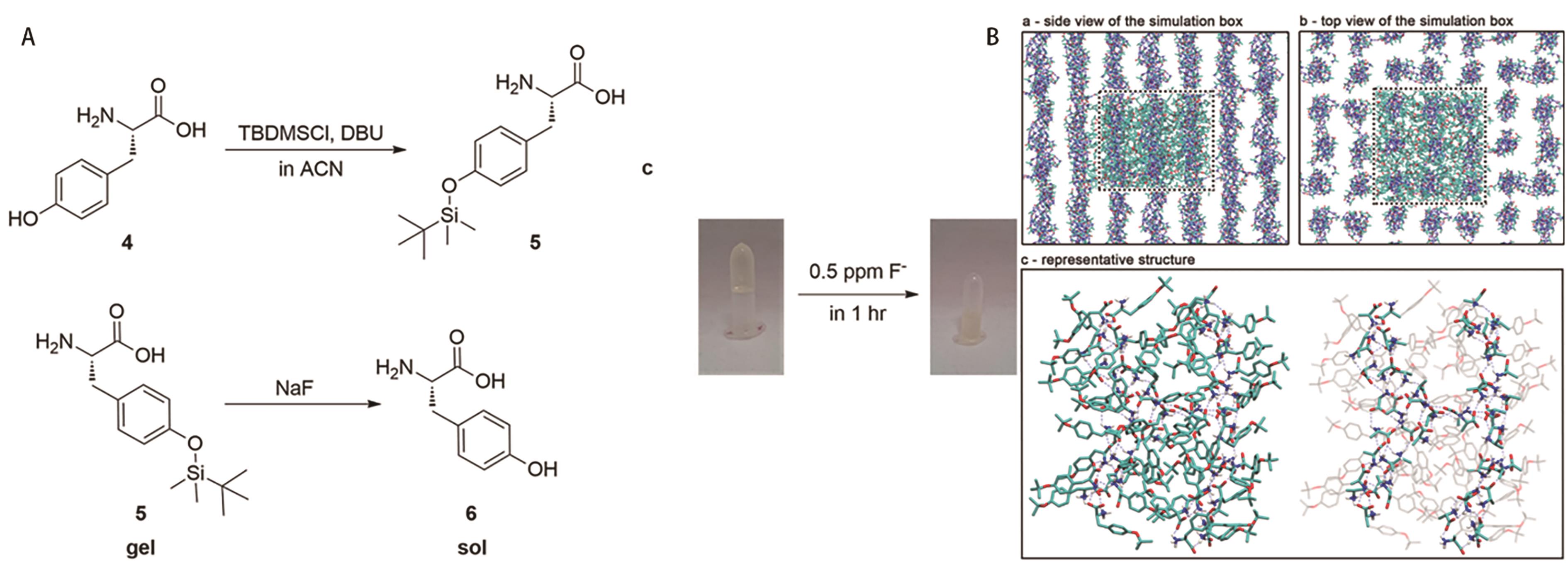
Fig.7 (A) Schematic diagram of the synthesis of LMWGs and fluoride ion response; (B) Snapshot of the self-assembly of the gel into fibrous structures after 1 μs MD simulation[46]
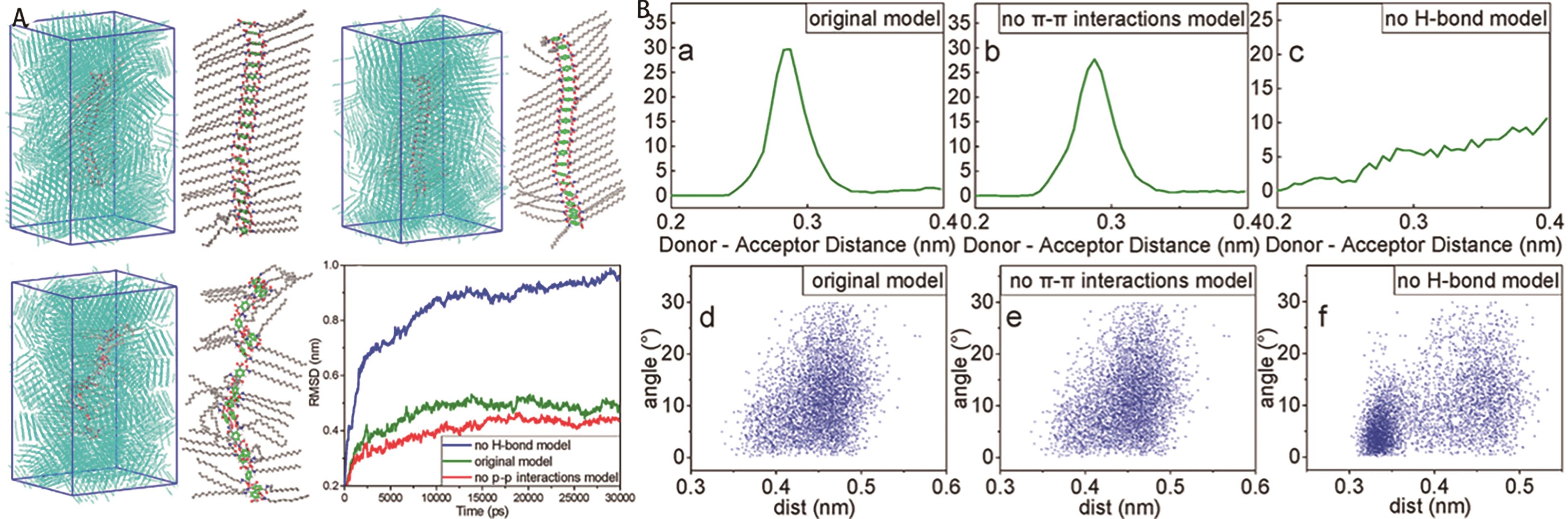
Fig.8 (A) The equilibrium states of gel self-assembly under different models by MD simulation; (B) The distribution of hydrogen bonds and π-π stacking under different models[47]
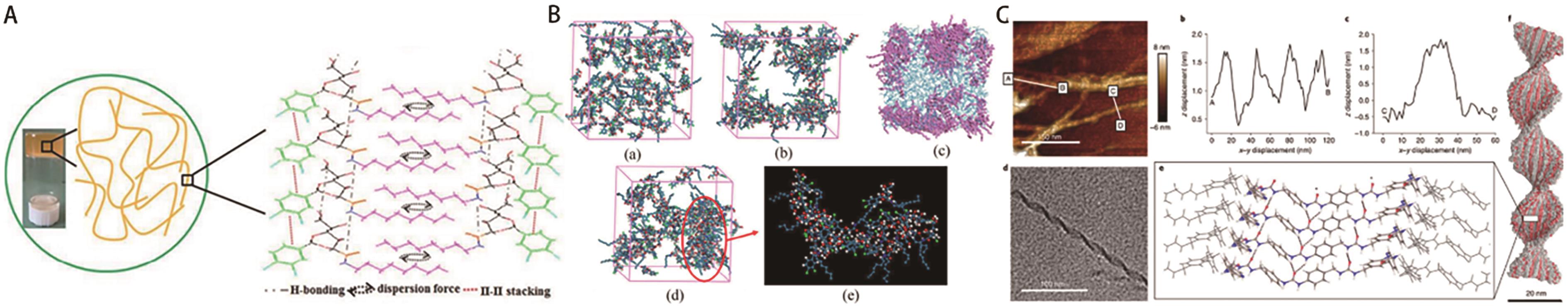
Fig.9 (A) Schematic of self-assembly of the 500SN gel; (B) Snapshots of simulation structures of the 500SN gel[52]; (C) The simulations indicate that four layers are required to produce a stable helicoid with the observed pitch and ribbon thickness[53]
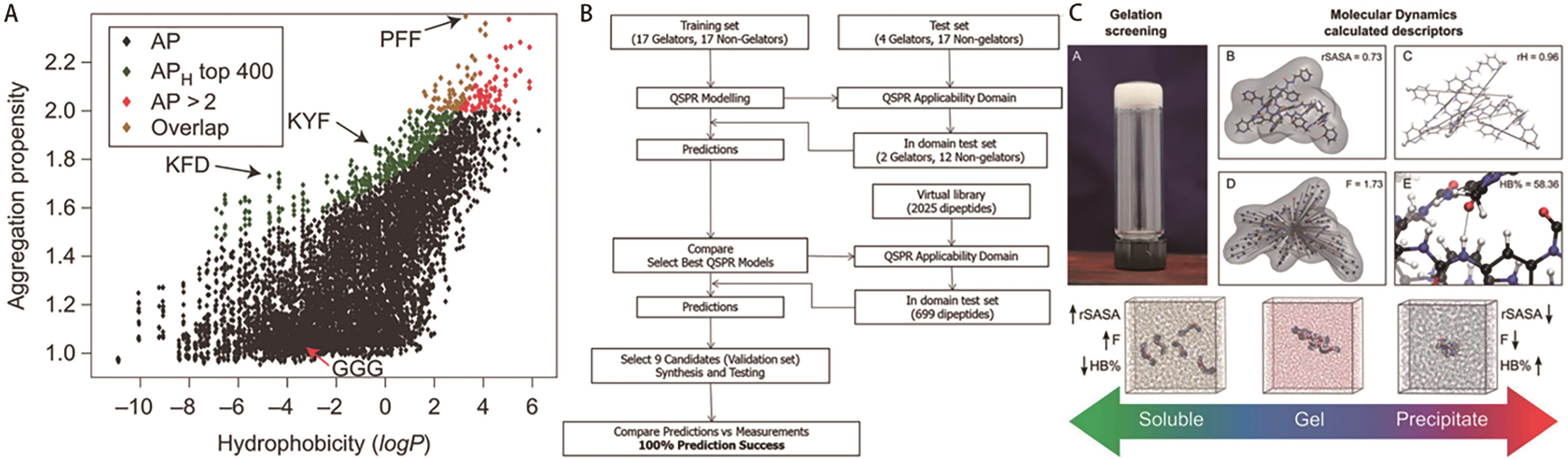
Fig.10 (A) Aggregation propensity (APH)[27]; (B) Overall QSPR modelling, synthesis and testing workflow[55]; (C) Prediction of general trends in gel state with descriptors rSASA, F and HB%[56]
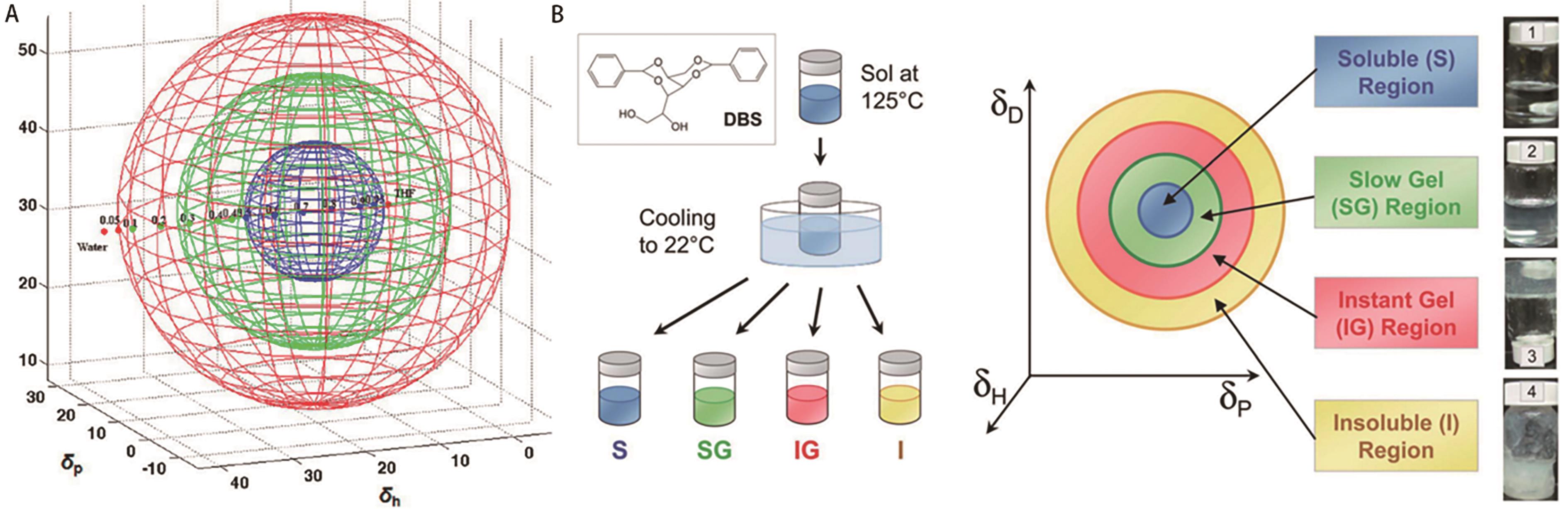
Fig.12 (A) Solubility data for 2.0% LMWGs in liquid mixtures represented in Hansen space with spheres/shells: blue (soluble), green (gel), and red (insoluble) [62]; (B) Schematic of the experimental setup (left) and the corresponding results in 3D Hansen space (right)[63]
| 1 | TERECH P, WEISS R G. Low molecular mass gelators of organic liquids and the properties of their gels[J]. Chem Rev, 1997, 97(8): 3133-3160. |
| 2 | LI Z, CAO J, LI H, et al. Self-assembled drug delivery system based on low-molecular-weight bis-amide organogelator: synthesis, properties and in vivo evaluation[J]. Drug Deliv, 2016, 23(8): 3168-3178. |
| 3 | LI J, GENG L, WANG G, et al. Self-healable gels for use in wearable devices[J]. Chem Mater, 2017, 29(21): 8932-8952. |
| 4 | 唐立宗, 张琳, 董云生, 等. 响应性水凝胶及其在生物医药领域应用研究进展[J]. 应用化学, 2021, 38(7): 743-753. |
| TANG L Z, ZHANG L, DONG Y S, et al. Research progress on responsive hydrogels and their applications in biomedicines[J]. Chinese J Appl Chem, 2021, 38(7): 743-753. | |
| 5 | LIM J Y C, GOH S S, LIOW S S, et al. Molecular gel sorbent materials for environmental remediation and wastewater treatment[J]. J Mater Chem A, 2019, 7(32): 18759-18791. |
| 6 | TRAUSEL F, VERSLUIS F, MAITY C, et al. Catalysis of supramolecular hydrogelation[J]. Acc Chem Res, 2016, 49(7): 1440-1447. |
| 7 | SHANG C, WANG G, HE M, et al. A high performance fluorescent arylamine sensor toward lung cancer sniffing[J]. Sens Actuators B: Chem, 2017, 241: 1316-1323. |
| 8 | TANAKA A, FUKUOKA Y, MORIMOTO Y, et al. Cancer cell death induced by the intracellular self-assembly of an enzyme-responsive supramolecular gelator[J]. J Am Chem Soc, 2015, 137(2): 770-775. |
| 9 | LIU X, FEI J, WANG A, et al. Transformation of dipeptide-based organogels into chiral crystals by cryogenic treatment[J]. Angew Chem Int Ed, 2017, 56(10): 2660-2663. |
| 10 | CHIVERS P R A, SMITH D K. Shaping and structuring supramolecular gels[J]. Nat Rev Mater, 2019, 4(7): 463-478. |
| 11 | DRAPER E R, ADAMS D J. How should multicomponent supramolecular gels be characterised?[J]. Chem Soc Rev, 2018, 47(10): 3395-3405. |
| 12 | BACANI R. Gel characterization: from molecules to nanostructure to macroproperties[M]. Nano Design for Smart Gels, 2019: 141-206. |
| 13 | WU S, ZHANG Q, DENG Y, et al. Assembly pattern of supramolecular hydrogel induced by lower critical solution temperature behavior of low-molecular-weight gelator[J]. J Am Chem Soc, 2020, 142(1): 448-455. |
| 14 | DAMA M, BERGER S. Study of an organogelator by diffusion-ordered NMR spectroscopy[J]. J Phys Chem B, 2013, 117(18): 5788-5791. |
| 15 | ADAMS D J, MORRIS K, CHEN L, et al. The delicate balance between gelation and crystallisation: structural and computational investigations[J]. Soft Matter, 2010, 6(17): 4144-4156. |
| 16 | CICCHI S, GHINI G, LASCIALFARI L, et al. Self-sorting chiral organogels from a long chain carbamate of 1-benzyl-pyrrolidine-3,4-diol[J]. Soft Matter, 2010, 6(8): 1655-1661. |
| 17 | WEISS R G. Controlling variables in molecular gel science: how can we improve the state of the art?[J]. Gels, 2018, 4(2): 25. |
| 18 | DRAPER E R, ADAMS D J. Low-molecular-weight gels: the state of the art[J]. Chem, 2017, 3(3): 390-410. |
| 19 | OTOISHI Y, SUGITA Y. Metabolite-protein interactions in bacterial cytoplasm: all-atomic cytoplasm model and theoretical study by large-scale molecular dynamics calculations[J]. Ensemble, 2017, 19: 75-80. |
| 20 | BABU S S, PRAVEEN V K, AJAYAGHOSH A. Functional pi-gelators and their applications[J]. Chem Rev, 2014, 114(4): 1973-2129. |
| 21 | ZHANG W, WANG K, WANG C, et al. Enhanced oil recovery: QM/MM based descriptors for anionic surfactant salt-resistance[J]. Colloid Surface A, 2022, 641: 128422. |
| 22 | 刘旭, 李杨可欣, 杜黎, 等 水凝胶的制备及仿生设计在能源领域应用的研究进展[J]. 应用化学, 2022, 39(1): 35-54. |
| LIU X, LI Y K X, DU L, et al. Bio⁃inspired hydrogels: synthesis, bionic design and applications in the field of energy storage and conversion[J]. Chinese J Appl Chem, 2022, 39(1): 35-54. | |
| 23 | 李胜男, 付俊. 水凝胶仿生柔性电子学[J]. 应用化学, 2022, 39(1): 55-73. |
| LI S N, FU J. Biomimetic flexible hydrogel electronics[J]. Chinese J Appl Chem, 2022, 39(1): 55-73. | |
| 24 | SULIMOV A V, KUTOV D C, KATKOVA E V, et al. New generation of docking programs: supercomputer validation of force fields and quantum-chemical methods for docking[J]. J Mol Graph Model, 2017, 78: 139-147. |
| 25 | YAN Y, TAO H, HE J, et al. The HDOCK server for integrated protein-protein docking[J]. Nat Protoc, 2020, 15(5): 1829-52. |
| 26 | JUMPER J, EVANS R, PRITZEL A, et al. Highly accurate protein structure prediction with AlphaFold[J]. Nature, 2021, 596(7873): 583-589. |
| 27 | FREDERIX P W, SCOTT G G, ABUL-HAIJA Y M, et al. Exploring the sequence space for (tri-)peptide self-assembly to design and discover new hydrogels[J]. Nat Chem, 2015, 7(1): 30-37. |
| 28 | KULKARNI C, KOREVAAR P A, BEJAGAM K K, et al. Solvent clathrate driven dynamic stereomutation of a supramolecular polymer with molecular pockets[J]. J Am Chem Soc, 2017, 139(39): 13867-13875. |
| 29 | MATHESON A, DALKAS G, MEARS R, et al. Stable emulsions of droplets in a solid edible organogel matrix[J]. Soft Matter, 2018, 14(11): 2044-2051. |
| 30 | XING P, CHEN H, XIANG H, et al. Selective coassembly of aromatic amino acids to fabricate hydrogels with light irradiation-induced emission for fluorescent imprint[J]. Adv Mater, 2018, 30(5): 1705633. |
| 31 | CHRISTOFF-TEMPESTA T, LEW A J, ORTONY J H. Beyond covalent crosslinks: applications of supramolecular gels[J]. Gels, 2018, 4(2): 40. |
| 32 | CAO H, YUAN Q, ZHU X, et al. Hierarchical self-assembly of achiral amino acid derivatives into dendritic chiral nanotwists[J]. Langmuir, 2012, 28(43): 15410-15417. |
| 33 | GREEN MARK M, PETERSON NORMAN C, SATO T, et al. A helical polymer with a cooperative response to chiral information[J]. Science, 1995, 268(5219): 1860-1866. |
| 34 | LANGEVELD-VOSS B M W, WATERVAL R J M, JANSSEN R A J, et al. Principles of “majority rules” and “sergeants and soldiers” applied to the aggregation of optically active polythiophenes: evidence for a multichain phenomenon[J]. Macromolecules, 1999, 32(1): 227-230. |
| 35 | ZHU X, JIANG Y, YANG D, et al. Homochiral nanotubes from heterochiral lipid mixtures: a shorter alkyl chain dominated chiral self-assembly[J]. Chem Sci, 2019, 10(13): 3873-3880. |
| 36 | HUDA M M, RAI N. Probing early-stage aggregation of low molecular weight gelator in an organic solvent[J]. J Phys Chem B, 2020, 124(11): 2277-2288. |
| 37 | SU T, HONG K H, ZHANG W, et al. Scaleable two-component gelator from phthalic acid derivatives and primary alkyl amines: acid-base interaction in the cooperative assembly[J]. Soft Matter, 2017, 13(22): 4066-4073. |
| 38 | ANGELEROU M G F, FREDERIX P, WALLACE M, et al. Supramolecular nucleoside-based gel: molecular dynamics simulation and characterization of its nanoarchitecture and self-assembly mechanism[J]. Langmuir, 2018, 34(23): 6912-6921. |
| 39 | DASTIDAR P. Supramolecular gelling agents: can they be designed?[J]. Chem Soc Rev, 2008, 37(12): 2699-2715. |
| 40 | CHUANG P H, TSENG Y H, GU Q, et al. Phase behavior and electron transfer properties of ferrocenyl cholesteryl N-formanidoformamide gelator: a computational study[J]. Colloid Polym Sci, 2015, 293(7): 2113-2119. |
| 41 | KULKARNI C, MEIJER E W, PALMANS A R A. Cooperativity scale: a structure-mechanism correlation in the self-assembly of benzene-1,3,5-tricarboxamides[J]. Acc Chem Res, 2017, 50(8): 1928-1936. |
| 42 | ALEGRE-REQUENA J V, SALDIAS C, INOSTROZA-RIVERA R, et al. Understanding hydrogelation processes through molecular dynamics[J]. J Mater Chem B, 2019, 7(10): 1652-1673. |
| 43 | SHEN Z, JIANG Y, WANG T, et al. Symmetry breaking in the supramolecular gels of an achiral gelator exclusively driven by pi-pi stacking[J]. J Am Chem Soc, 2015, 137(51): 16109-16115. |
| 44 | SHEN Z, WANG T, LIU M. Macroscopic chirality of supramolecular gels formed from achiral tris(ethyl cinnamate) benzene-1, 3, 5-tricarboxamides[J]. Angew Chem Int Ed, 2014, 53(49): 13424-13428. |
| 45 | DALKAS G, MATHESON A B, VASS H, et al. Molecular interactions behind the self-assembly and microstructure of mixed sterol organogels[J]. Langmuir, 2018, 34(29): 8629-8638. |
| 46 | AYKENT G, ZEYTUN C, MARION A, et al. Simple tyrosine derivatives act as low molecular weight organogelators[J]. Sci Rep, 2019, 9(1): 4893. |
| 47 | ZHANG W, ZHANG Z, ZHAO S, et al. Pyromellitic-based low molecular weight gelators and computational studies of intermolecular interactions: a potential additive for lubricant[J]. Langmuir, 2021, 37(9): 2954-2962. |
| 48 | SINGHA N, SRIVASTAVA A, PRAMANIK B, et al. Unusual confinement properties of a water insoluble small peptide hydrogel[J]. Chem Sci, 2019, 10(23): 5920-5928. |
| 49 | SHI L, HAN Q. Molecular dynamics study of the influence of solvents on the structure and mechanical properties of poly(vinyl alcohol) gels[J]. J Mol Model, 2018, 24(11): 325. |
| 50 | VYAS S, KHAMBETE M, GUDHKA R, et al. Network topology of LMWG cross-linked xyloglucan hydrogels for embedding hydrophobic nanodroplets: mechanistic insight and molecular dynamics[J]. Drug Deliv Transl Res, 2020, 10(4): 1076-1084. |
| 51 | GUILBAUD-CHEREAU C, DINESH B, SCHURHAMMER R, et al. Protected amino acid-based hydrogels incorporating carbon nanomaterials for near-infrared irradiation-triggered drug release[J]. ACS Appl Mater Interfaces, 2019, 11(14): 13147-13157. |
| 52 | WANG Y, YU Q, BAI Y, et al. Self-constraint gel lubricants with high phase transition temperature[J]. ACS Sustain Chem Eng, 2018, 6(11): 15801-15810. |
| 53 | JONES C D, SIMMONS H T D, HORNER K E, et al. Braiding, branching and chiral amplification of nanofibres in supramolecular gels[J]. Nat Chem, 2019, 11(4): 375-381. |
| 54 | BAI Y, YU Q, ZHANG J, et al. Soft-nanocomposite lubricants of supramolecular gel with carbon nanotubes[J]. J Mater Chem A, 2019, 7(13): 7654-7663. |
| 55 | GUPTA J K, ADAMS D J, BERRY N G. Will it gel? successful computational prediction of peptide gelators using physicochemical properties and molecular fingerprints[J]. Chem Sci, 2016, 7(7): 4713-4719. |
| 56 | VAN LOMMEL R, ZHAO J, DE BORGGRAEVE W M, et al. Molecular dynamics based descriptors for predicting supramolecular gelation[J]. Chem Sci, 2020, 11(16): 4226-4238. |
| 57 | JONES C D, KENNEDY S R, WALKER M, et al. Scrolling of supramolecular lamellae in the hierarchical self-assembly of fibrous gels[J]. Chem, 2017, 3(4): 603-628. |
| 58 | HANSEN C. Hansen solubility parameters: a user's handbook, Second Edition[M]. USA: CRC Press, 2012. |
| 59 | 刘建强, 马婧. 用汉森溶解度参数评估功能化石墨烯在溶剂中的分散性[J]. 过程工程学报, 2019, 19(1): 189-194. |
| LIU J Q, MA J. Evaluation of dispersion of functionalized graphene in solvents by Hansen solubility parameters[J]. Chinese J Process Eng, 2019, 19(1): 189-194. | |
| 60 | RAYNAL M, BOUTEILLER L. Organogel formation rationalized by Hansen solubility parameters[J]. Chem Commun, 2011, 47(29): 8271-8273. |
| 61 | ROSA NUNES D, RAYNAL M, ISARE B, et al. Organogel formation rationalized by Hansen solubility parameters: improved methodology[J]. Soft Matter, 2018, 14(23): 4805-4809. |
| 62 | YAN N, XU Z, DIEHN K K, et al. How do liquid mixtures solubilize insoluble gelators? self-assembly properties of pyrenyl-linker-glucono gelators in tetrahydrofuran-water mixtures[J]. J Am Chem Soc, 2013, 135(24): 8989-8999. |
| 63 | DIEHN K K, OH H, HASHEMIPOUR R, et al. Insights into organogelation and its kinetics from Hansen solubility parameters. Toward a priori predictions of molecular gelation[J]. Soft Matter, 2014, 10(15): 2632-2640. |
| 64 | LAN Y, CORRADINI M G, WEISS R G, et al. To gel or not to gel: correlating molecular gelation with solvent parameters[J]. Chem Soc Rev, 2015, 44(17): 6035-6058. |
| 65 | RIWAR L J, TRAPP N, KUHN B, et al. Substituent effects in parallel-displaced pi-pi stacking interactions: distance matters[J]. Angew Chem Int Ed, 2017, 56(37): 11252-11257. |
| 66 | EMAMIAN S, LU T, KRUSE H, et al. Exploring nature and predicting strength of hydrogen bonds: a correlation analysis between atoms-in-molecules descriptors, binding energies, and energy components of symmetry-adapted perturbation theory[J]. J Comput Chem, 2019, 40(32): 2868-2881. |
| 67 | LU T, CHEN Q. Van der Waals potential: an important complement to molecular electrostatic potential in studying intermolecular interactions[J]. J Mol Model, 2020, 26(11): 315. |
| 68 | WEISS R G. The past, present, and future of molecular gels. what is the status of the field, and where is it going?[J]. J Am Chem Soc, 2014, 136(21): 7519-7530. |
| [1] | YANG Kecheng, CUI Fengchao, LI Yunqi. Distribution and Dynamics of Water and Urea in Hydration Shell of Ribonuclease Sa:A Molecular Dynamics Simulation Study [J]. Chinese Journal of Applied Chemistry, 2018, 35(10): 1243-1248. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||