应用化学 ›› 2023, Vol. 40 ›› Issue (5): 615-624.DOI: 10.19894/j.issn.1000-0518.220223
• 综合评述 • 下一篇
天然产物Polygalolides的全合成研究进展
- 1.贵州大学药学院,贵阳 550000
2.贵州医科大学,省部共建药用植物功效与利用国家重点实验室,贵阳 550014
-
收稿日期:2022-06-21接受日期:2023-02-13出版日期:2023-05-01发布日期:2023-05-26 -
通讯作者:何述钟 -
基金资助:贵州省科学技术基金(No. 黔科合基础-ZK[2021]一般039);贵州医科大学省部共建药用植物功效与利用国家重点实验室基金(No. FAMP202102K)
Research Progress of Total Synthesis of Polygalolides
Jia-Zheng LI1, Shu-Zhong HE2( )
)
- 1.School of Pharmacy Guizhou University,Guiyang 550000,China
2.State Key Laboratory of Functions and Applications of Medicinal Plants,Guizhou Medical University,Guiyang 550014,China
-
Received:2022-06-21Accepted:2023-02-13Published:2023-05-01Online:2023-05-26 -
Contact:Shu-Zhong HE -
About author:szhe@gzu.edu.cn
-
Supported by:the Science and Technology Foundation of Guizhou Province(No.the Science and Technology Foundation of Guizhou Province-ZK[2021]General 039) and the State Key Laboratory of Functions and Applications of Medicinal Plants, Guizhou Medical University(FAMP202102K)
摘要:
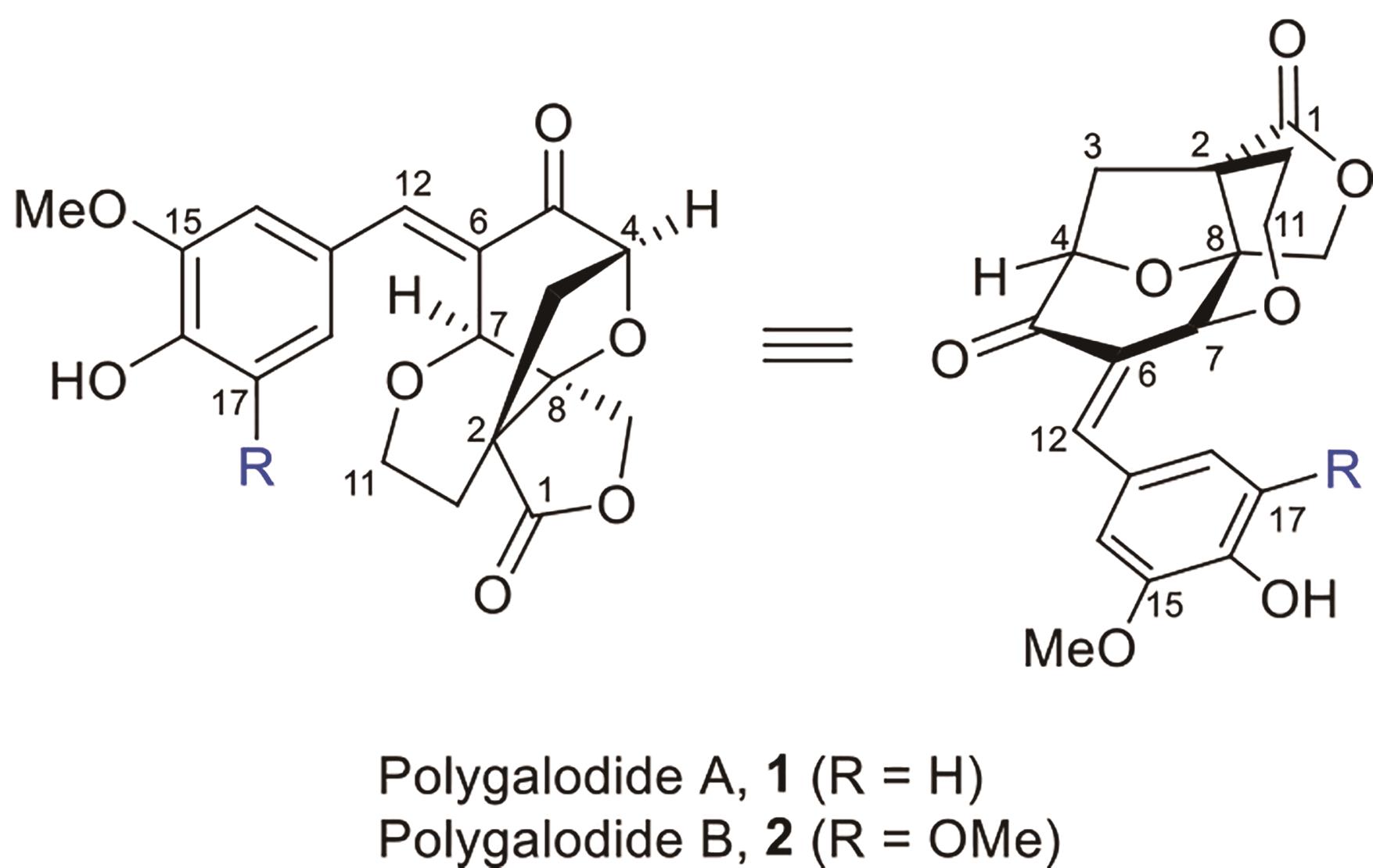
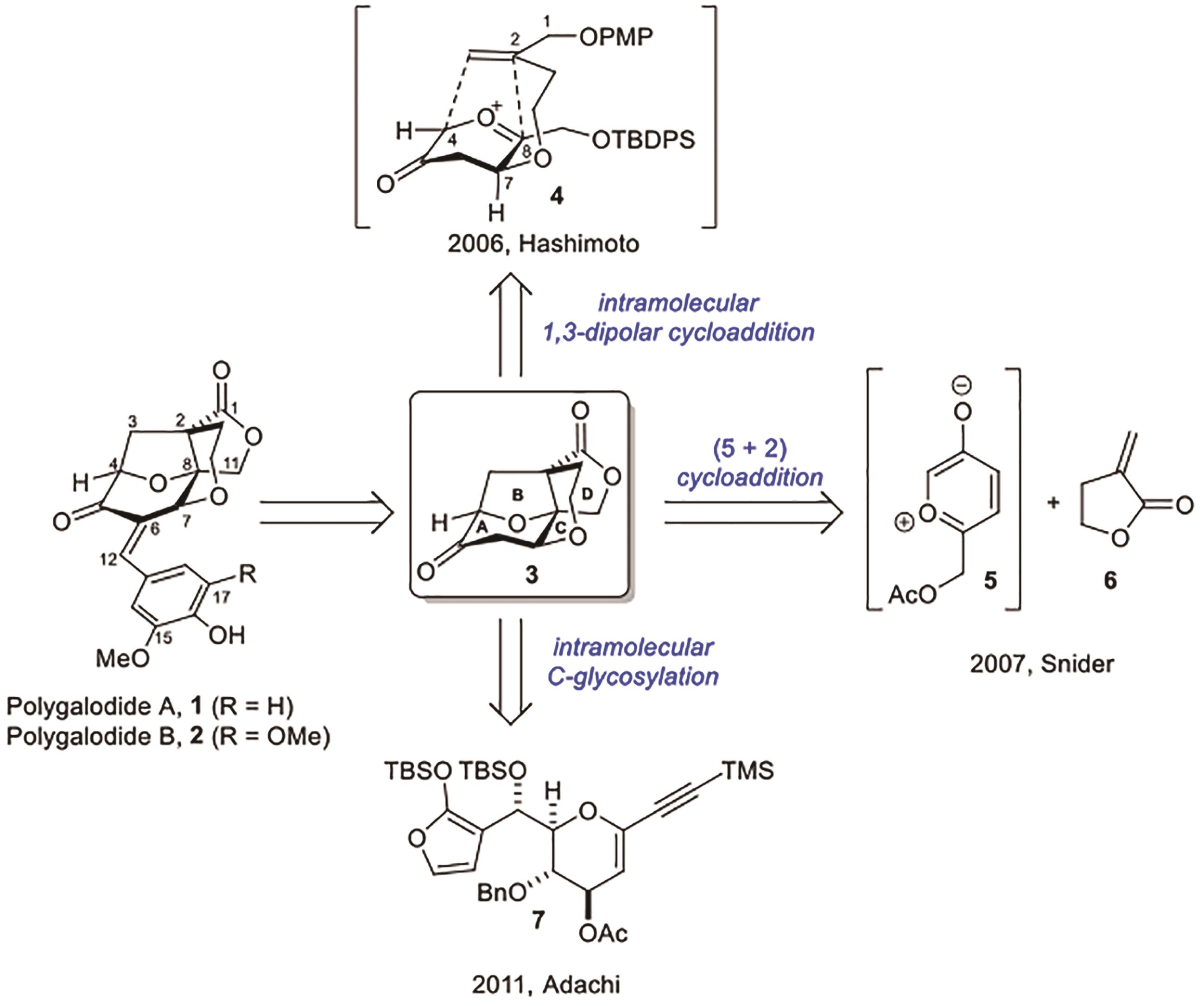
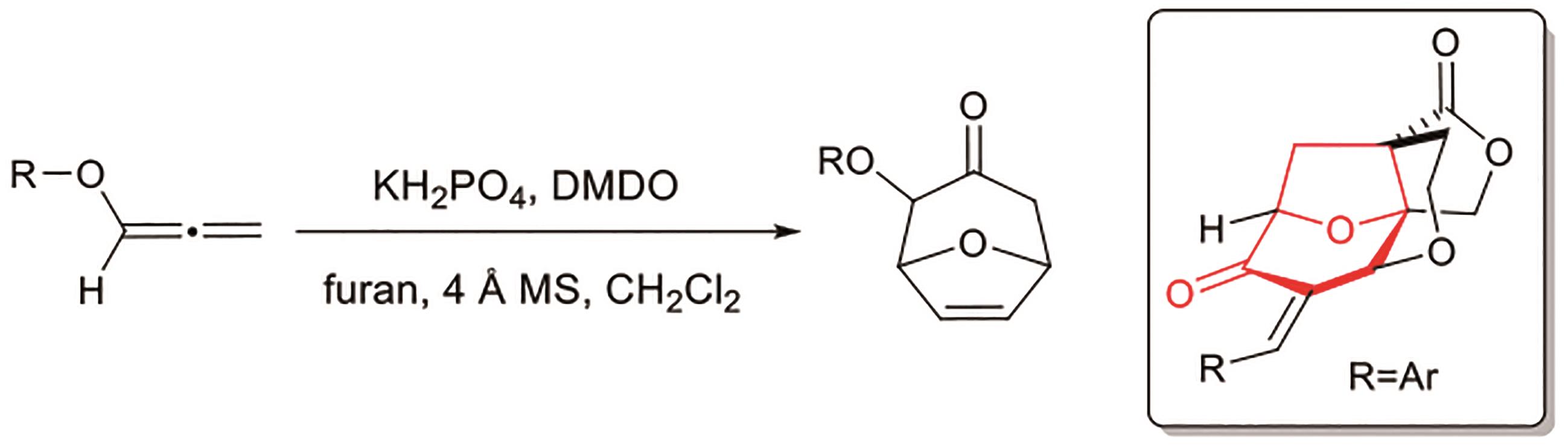
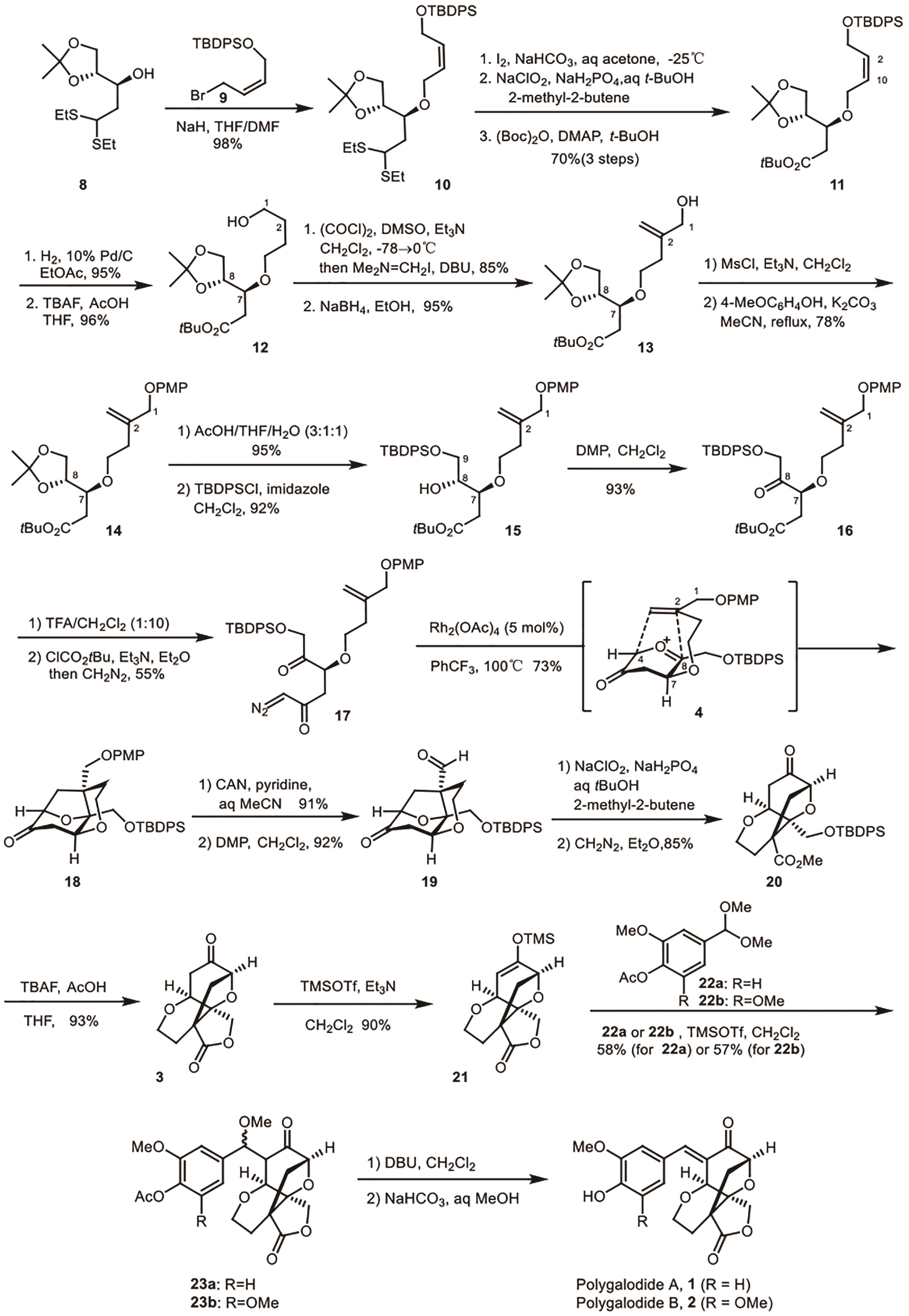
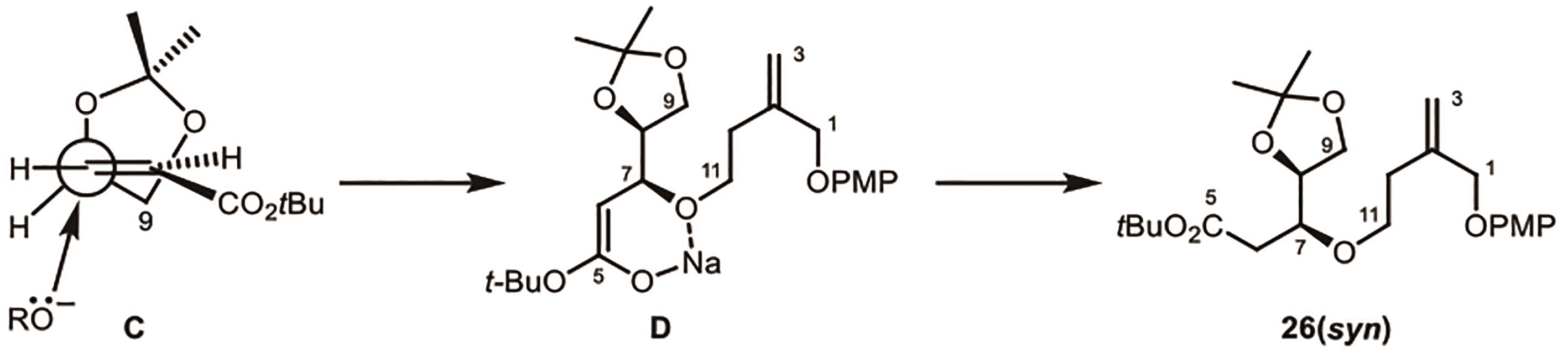
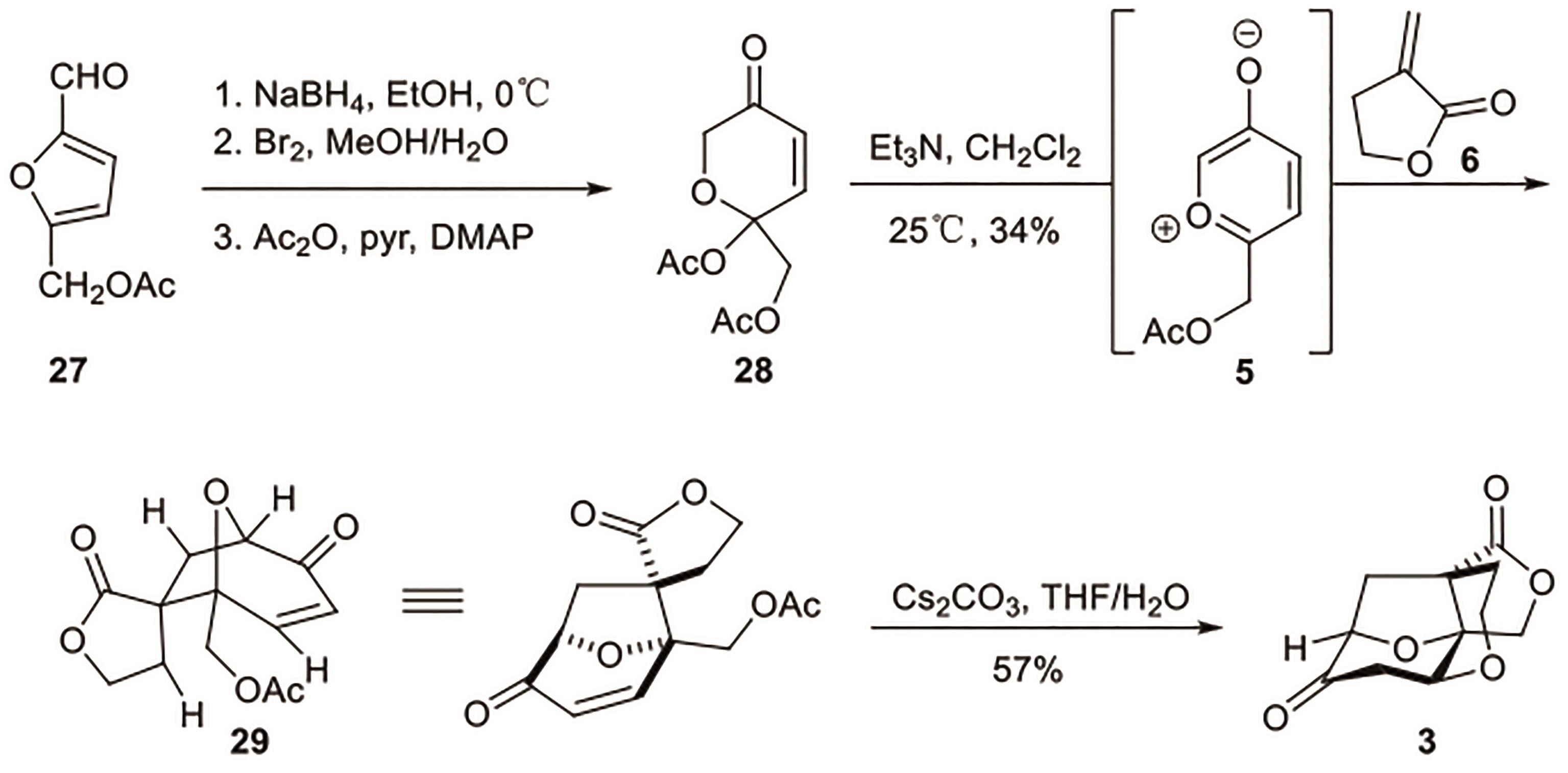
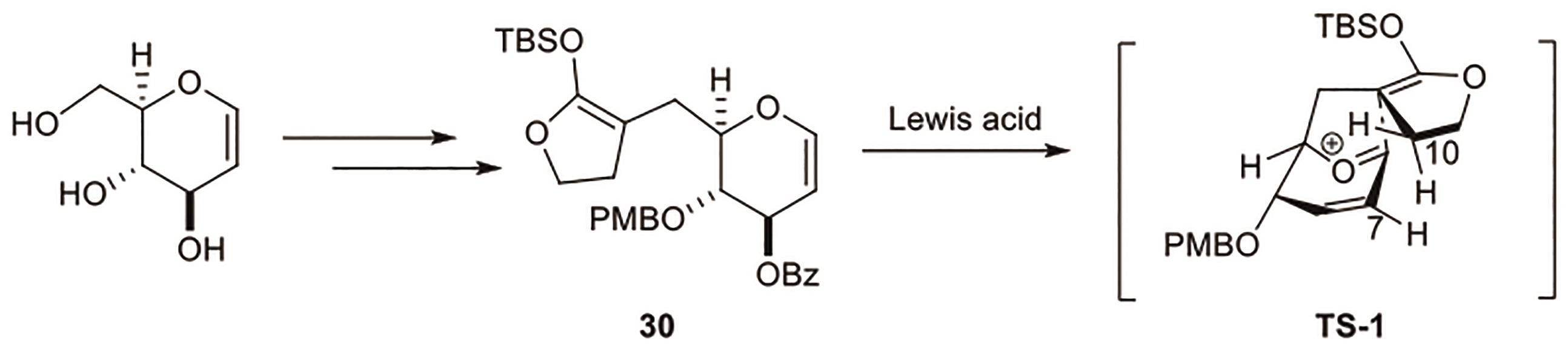
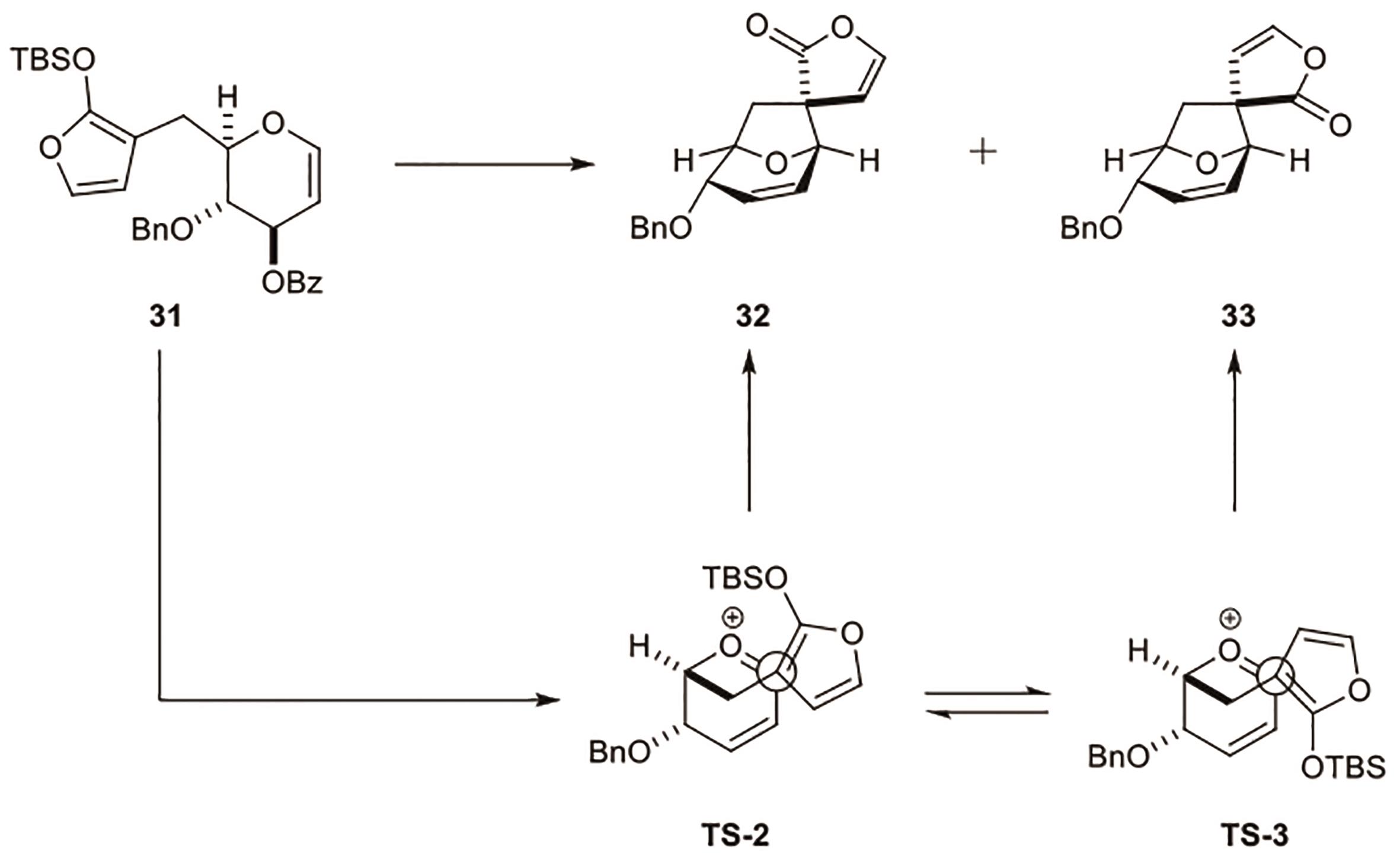
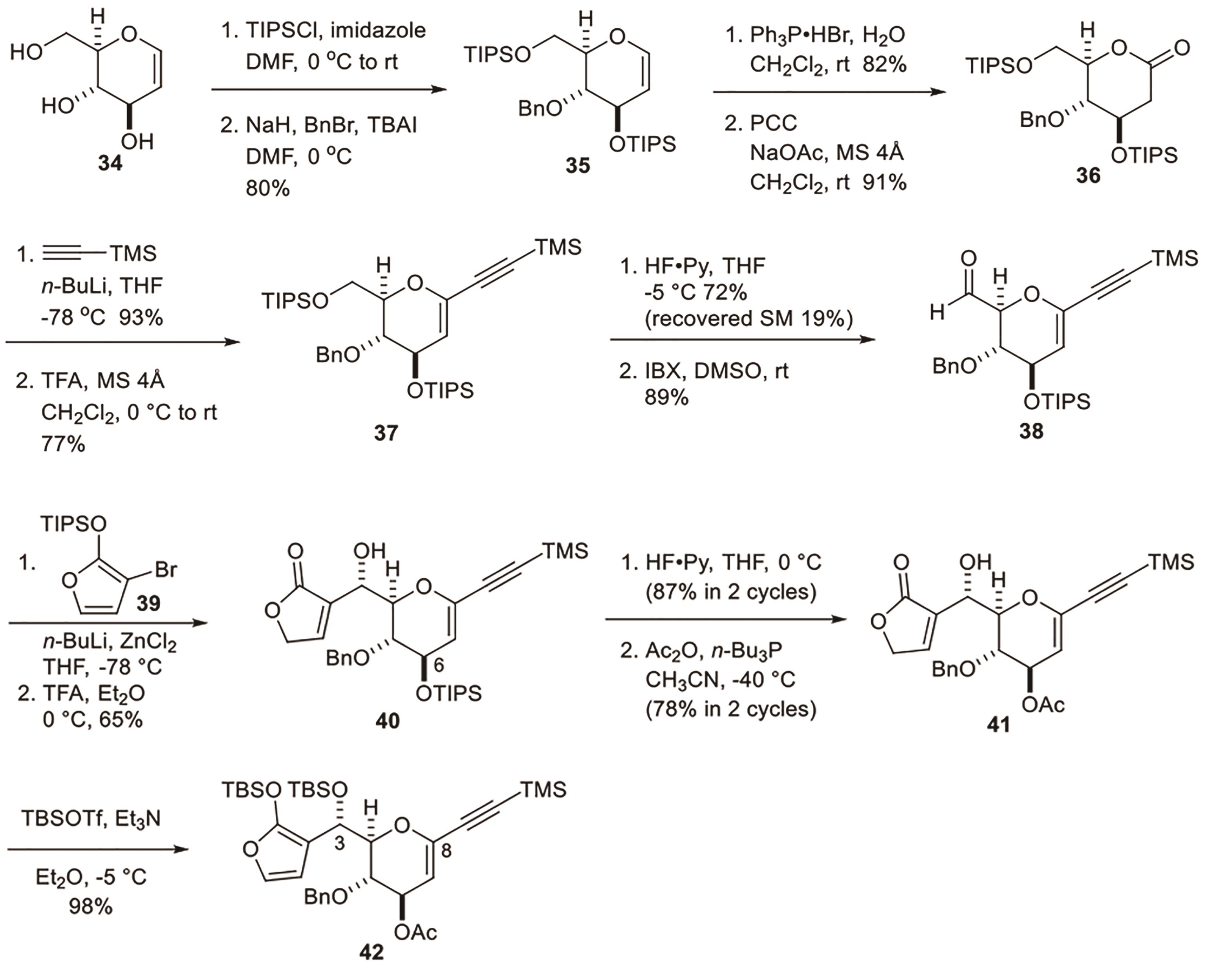
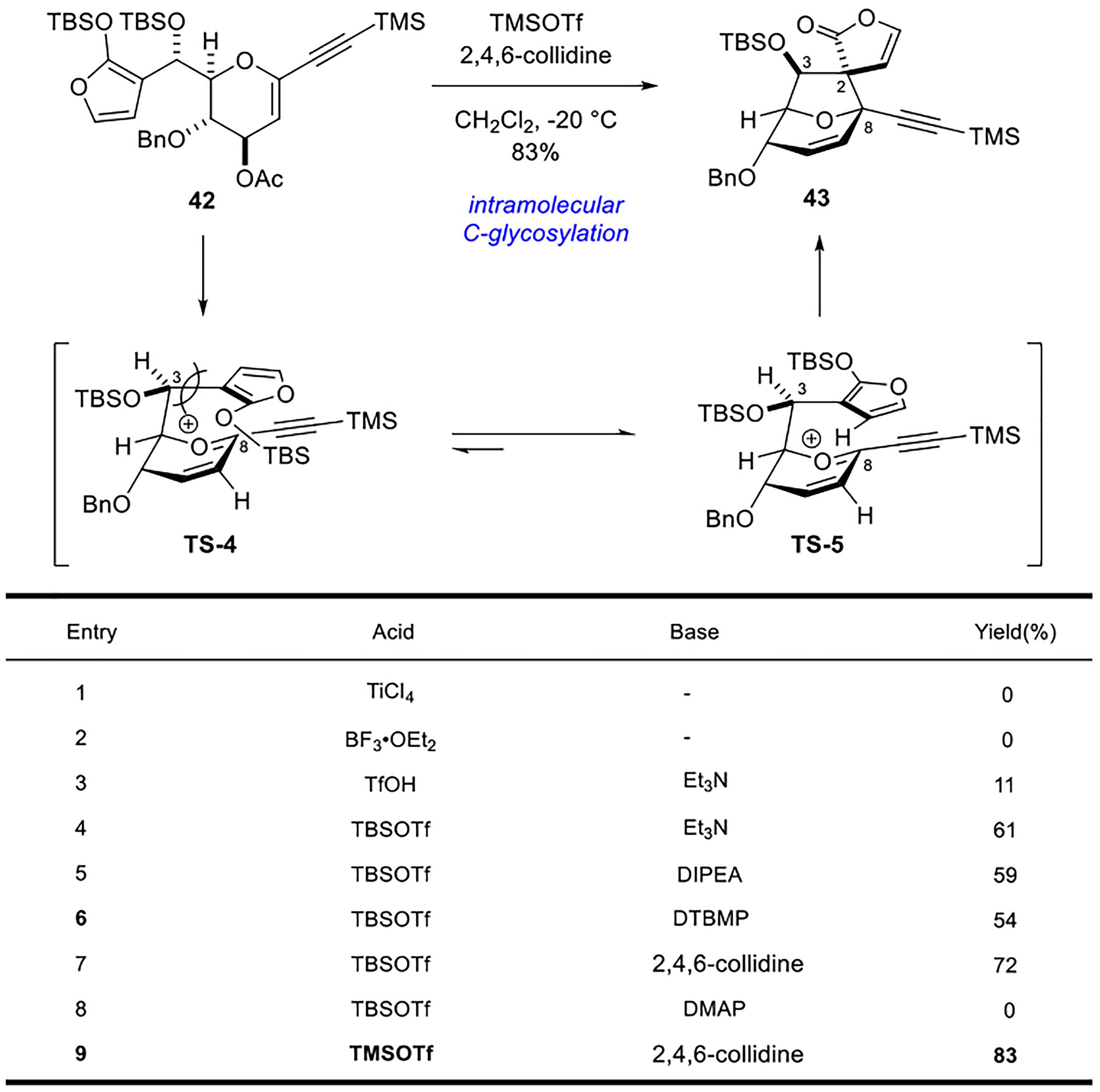
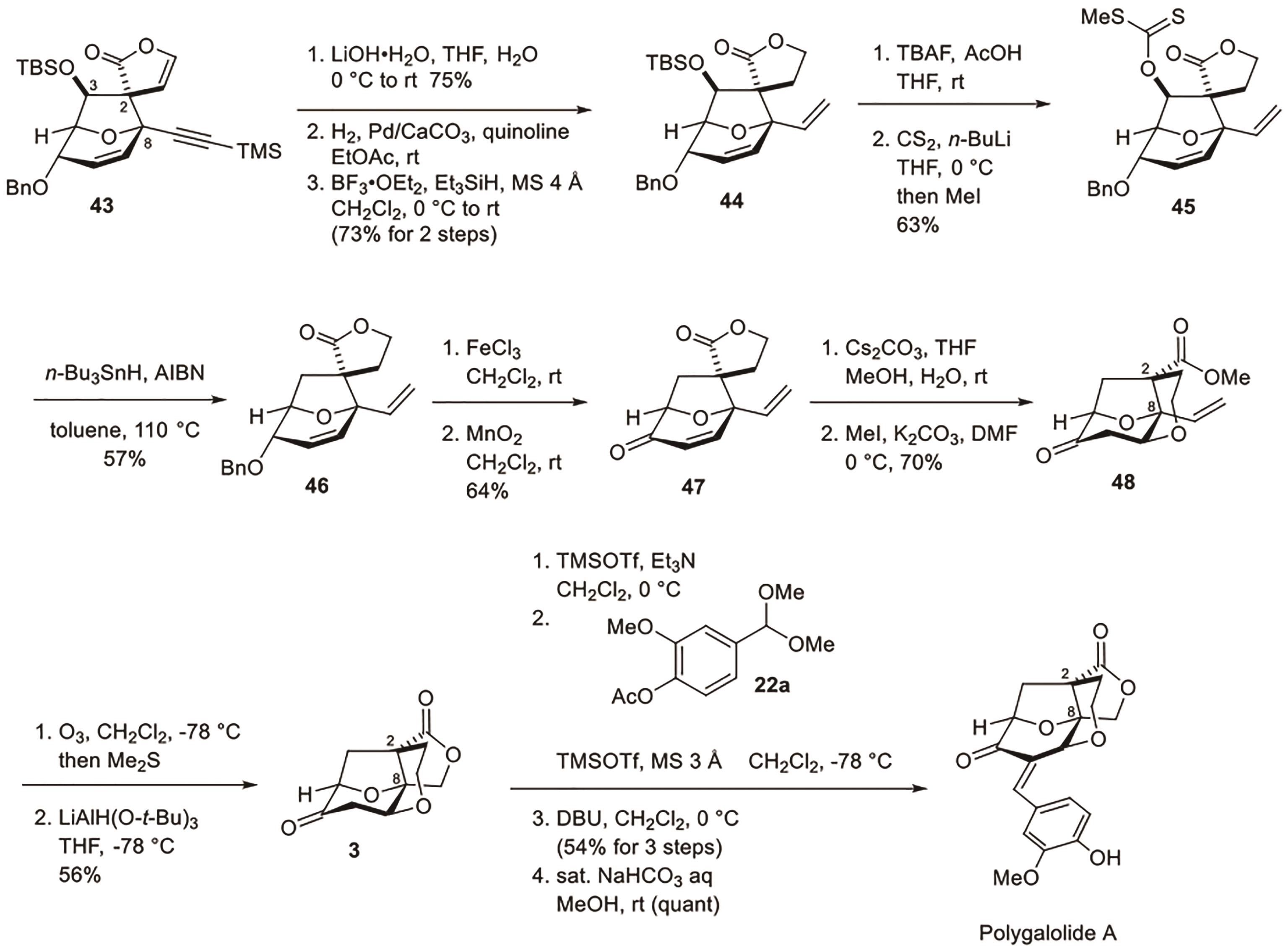
Polygalolide A和B是从药用植物黄花倒水莲(Polygala Falax Hemsl)的根茎中分离得到的2个新型酚类化合物。该类天然产物具有复杂紧凑的四环笼状骨架,包含1个高度氧化的环庚酮、3个含氧杂环及2个连续的桥头季碳中心等结构特征。本文综述了3个课题组分别利用(5+2)环加成、分子内(3+2)环加成和分子内亲核取代构建中心四环,后经向山羟醛缩合得到目标天然产物。这些工作为该分子生物活性的研究奠定了基础。
中图分类号:
引用本文
李佳铮, 何述钟. 天然产物Polygalolides的全合成研究进展[J]. 应用化学, 2023, 40(5): 615-624.
Jia-Zheng LI, Shu-Zhong HE. Research Progress of Total Synthesis of Polygalolides[J]. Chinese Journal of Applied Chemistry, 2023, 40(5): 615-624.
| 1 | 陈家宝, 潘为高, 罗彭, 等. 黄花倒水莲的研究进展[J]. 亚太传统医药, 2018, 14(5): 86-89. |
| CHEN J B, PAN W G, LUO P, et al. Research progress of Polygala Falax Hemsl[J]. Asia-pacific Trad Med, 2018, 14(5): 86-89. | |
| 2 | 王林海, 卢健棋, 庞延, 等. 黄花倒水莲药学研究及临床应用概述[J]. 辽宁中医杂志, 2018, 45(3): 648-651. |
| WANG L H, LU J Q, PANG Y, et al. Overview of the pharmaceutical research and clinical application of Polygala Falax Hemsl[J]. Liaoning J Trad Chin Med, 2018, 45(3): 648-651. | |
| 3 | 许立拔, 龙莉, 谢凤凤, 等. 黄花倒水莲药理研究进展[J]. 壮瑶药研究, 2019(1): 77-82, 106. |
| XU L B, LONG L, XIE F F, et al. Research progress on the pharmacology of Polygala Falax Hemsl[J]. Res Zhuang Yao Ethnic Med, 2019(1): 77-82, 106. | |
| 4 | MA W Z, WEI X Y, LING T J, et al. New phenolics from Polygala fallax[J]. Nat Prod, 2003, 66: 441-443. |
| 5 | NAKAMURA S, SUGANO Y, KIKUCHI F, et al. Total synthesis and absolute stereochemistry of polygalolide A and B[J]. Angew Chem Int Ed, 2006, 118(39): 6682-6685. |
| 6 | SUGANO Y, KIKUCHI F, TOITA A, et al. Total syntheses of (+)-polygalolide A and (+)-polygalolide B: elucidation of the absolute stereochemistry and biogenetic implications[J]. Chem Eur J, 2012, 18: 9682-9690. |
| 7 | SNIDER B B, WU X X, NAKAMURA S, et al. A short formal biomimetic synthesis of (±)-polygalolide A and B[J]. Org Lett, 2007, 9(5): 873-874. |
| 8 | ADACHI M, YAMADA H, ISOBE M, et al. Total synthesis of polygalolide A[J]. Org Lett, 2011, 13: 6532-6535. |
| 9 | YAMADA H, ADACHI M, ISOBE M, et al. Stereocontrolled total synthesis of polygalolide A[J]. Chem Asian J, 2013, 8: 1428-1435. |
| 10 | POHMAKOT M, YOTAPAN N, TUCHINDA P, et al. Stereoselective synthesis of b-carboethoxy-g-lactams via i-mino Mukaiyama aldol-type reaction of 1,4-bis(trimethylsilyloxy)-1,4-diethoxy-1,3-butadiene[J]. Tetrahedron, 2007(63): 4328-4337. |
| 11 | ROISNEL T, RUIZ J, KARRE N, et al. From protected beta-hydroxy acylsilanes to functionalized silyl enol ethers and applications in Mukaiyama aldol reactions[J]. Eur J Org Chem, 2016(4): 773-779. |
| 12 | BOUHLEL E, BEN B B. One pot synthesis of α,β-unsaturated ketones from the Mukaiyama aldol condensation[J]. Synth Commun, 1992(15): 2183-2186. |
| 13 | HUANG X, THOR W, FENG X Y, et al. (4+3) Cycloadditions of allenyl ether-deried oxygen-stabilized oxyallyls with furans[J]. Org Chem Front, 2020, 7: 255-260. |
| 14 | PADWA A. Catalyticdecomposition of diazo compounds as a method for generating carbonyl-ylide dipoles[J]. Helv Chim Acta, 2005, 88: 1357-1374. |
| 15 | PADWA A, BRODNEY M A, MARINO J P, et al. Utilization of the intramolecular cycloaddition-cationic π-cyclization of an isomünchnonederivative for the synthesis of (±)-lycopodine[J]. J Org Chem, 1997, 62: 78-87. |
| 16 | HODGSON D M, VILLALONGA B C. Studies towards a stereocontrolled synthesis of the tricarboxylate core of the zaragozic acids-squalestatins by a cycloaddition-rearrangement strategy[J]. Tetrahedron Lett, 2000, 41: 5597-5600. |
| 17 | CHEN B, REBECCA Y Y, CHENG K F, et al. Atandem metal carbene cyclization-cycloaddition approach to the pseudolaric acids[J]. J Org Chem, 2003, 68: 4195-4205. |
| 18 | MAGNUS P, SHEN L. Stereoselective synthesis of the “cyathin” diterpene skeleton via an intramolecular pyrylium ylide-alkene cyclization[J]. Tetrahedron Lett, 1999, 55: 3553-3560. |
| 19 | OGURI H, SCHREIBER S L. Skeletal diversity via a folding pathway: synthesis of indole alkaloid-like skeletons[J]. Org Lett, 2005, 7: 47-50. |
| 20 | WILLIAMS D R, BENBOW J W, ALLEN E E. Intramolecular dipolar addition of carbonyl ylides. studies of substituted bicycloundecanones[J].Tetrahedron Lett, 1990, 31: 6769-6772. |
| 21 | HODGSON D M, AVERY T D, DONOHUE A C. Stereoselective syntheses of cis-nemorensicacid and 4-hydroxy-cis-nemorensic acid via tandem carbonyl ylide formation-cycloaddition[J].Org Lett, 2002, 4:1809-1811. |
| 22 | TAKANO S, INOMATA K, SAMIZU K, et al. A convenient one-flask synthesis of α-methylenealdehydes from primary alcohols[J].Chem Lett, 1989: 1283-1284. |
| 23 | MURATA S, SUZUKI M, NOYORI R. A stereoselective aldol-type condensation of enol silyl ethers and acetals catalyzed by trimethylsilyl trifluoromethanesulfonate[J]. J Am Chem Soc, 1980, 102(9): 3248-3249. |
| 24 | GHOSH A K, BRINDISI M. Achmatowicz reaction and its application in the syntheses of bioactive molecules[J]. Rsc Adv, 2016, 6: 111564-111598. |
| 25 | POSTEMAM H D. Recent developments in the synthesis of C-glycosides[J]. Tetrahedron Lett, 1992, 48: 8545-8599. |
| 26 | DU Y, LINHARDT R J, VLAHOV I R. Recent advances in stereoselective C-glycoside synthesis[J]. Tetrahedron Lett, 1998, 54: 9913-9959. |
| 27 | SAEENG R, SIRION U, ISOBE M. Iodine catalyzes C-glycosidation of D-glucal with silylacetylene[J]. Tetrahedron Lett, 2003, 44: 6211-6215. |
| 28 | SAEENG R, ISOBE M. Synthesis of silylalleneglycosides and diene diglycosides by C-glycosidation of D-glucal with 1,4-bis(trimethylsilyl)-2-butyne[J]. Org Lett, 2005, 7: 1585-1588. |
| 29 | TANAKA S, TSUKIYAMA T, ISOBE M. Epimerization of C-1 alkynyl group on pyranose ring through dicobalt hexacarbonyl complexes[J]. Tetrahedron Lett, 1993, 34: 5757-5760. |
| 30 | HAMAJIM A A, ISOBE M. Total synthesis of ciguatoxin[J]. Angew Chem Int Ed, 2009, 48(16): 2941-2945. |
| 31 | 陈有双, 刘大胜, 黄文榜, 等. 烯醇硅醚的制备及其在羟醛(酮)缩合反应中的应用[J]. 广西化工, 1992(3): 20-27. |
| CHEN Y S, LIU D S, HUANG W B, et al. Preparation of silyl enol ethers and its application in aldol condensation reaction[J]. Guangxi Chem Ind, 1992(3): 20-27. | |
| 32 | 余孝其. 烯醇硅醚的制备及其在有机合成中的应用[J]. 化学试剂, 1992(1): 29-35. |
| YU X Q. Preparation of silyl enol ethers and its application in organic synthesis[J]. Chem Reag, 1992(1): 29-35. | |
| 33 | XIONG H, HUANG J, GHOSH S K, et al. Stereoselective intramolecular [4+3] cycloadditions of nitrogen-stabilized |
| chiral oxyallyl cations via epoxidation of N-tethered allenamides[J]. J Am Chem Soc, 2003, 125(42): 12694-12695. | |
| 34 | RAMESHKUMAR C, HSUNG R P. A tandem epoxidation/stereoselective intramolecular [4+3] cycloaddition reaction involving nitrogen-stabilized oxyallyl cations derived from chiral allenamides[J]. Angew Chem Int Ed, 2004, 43(5): 615-618. |
| [1] | 金宇婷, 支德福, 郭永泰, 隋悦, 孙蕾, 王倩, 赵龙铉, 赵春晖. 酚类衍生物的美白和抗氧化活性[J]. 应用化学, 2019, 36(11): 1257-1265. |
| [2] | 马涛, 杨金会, 牛明杰, 叶子平, 李方辉. 3种香叶基取代黄烷酮的合成及抗肿瘤活性[J]. 应用化学, 2017, 34(10): 1140-1149. |
| [3] | 杨成雄, 王士伟, 严秀平. 金属-有机骨架对苯二甲酸酯-铝吸附水中酚类化合物动力学和热力学[J]. 应用化学, 2016, 33(9): 1040-1046. |
| [4] | 吴锦明, 施磊, 沈爱宝, 汤艳峰, 陆天虹. H6PMo9V3O40/SiO2催化剂催化酚类化合物选择性硝化[J]. 应用化学, 2014, 31(04): 437-443. |
| [5] | 张应鹏, 陈宇涛, 杨云裳, 关晓. 双查尔酮Rhuschalcone Ⅰ的合成[J]. 应用化学, 2011, 28(06): 652-656. |
| [6] | 张成路, 王利东, 广东, 申洪江, 李元东, 王杨. 3-羰基-10-去甲基-12-甲氧基-13-甲基罗汉松烷的全合成[J]. 应用化学, 2009, 26(11): 1301-1304. |
| [7] | 钱华, 刘大斌, 王亮. 4-甲氧基-7H-呋喃[3,2-g][1]苯并吡喃-7-酮制备的新方法[J]. 应用化学, 2009, 26(09): 1084-1087. |
| [8] | 张成路, 孙春丽, 韩福忠, 刘林. (±)-13-羟基-8(14)-松香烯的全合成[J]. 应用化学, 2006, 23(8): 939-941. |
| [9] | 杨冰仪, 莫金垣, 邹小勇, 赖瑢, 黄宝美. 双通道毛细管电泳-电化学法同时检测硝酸根、亚硝酸根和氯酚类化合物[J]. 应用化学, 2005, 22(5): 484-488. |
| [10] | 朱燕云, 陈希慧, 黄初升, 刘红星, 凌新龙. 2种苯并吡喃类查尔酮天然产物的全合成[J]. 应用化学, 2005, 22(5): 560-562. |
| [11] | 范毅, 冯钰锜, 达世禄, 施治国, 徐丽. β-环糊精键合改性介孔硅胶的表征和吸附性能评价[J]. 应用化学, 2004, 21(9): 878-883. |
| [12] | 黄宇彬, 小松晃之, 土田英俊. 一种全合成型人工红血球的研究进展[J]. 应用化学, 2004, 21(11): 1081-1086. |
| [13] | 孟炜, 陈立功, 许艳杰, 古险峰, 宋芸. 3,7-二甲基-2-十三碳醇乙酸酯的全合成[J]. 应用化学, 2001, 18(12): 971-974. |
| [14] | 刘瑾, 李真. 甘薯象鼻虫信息素(E)-2-丁烯酸-(Z)-3-十二烯基酯的全合成[J]. 应用化学, 2001, 18(12): 998-1000. |
| [15] | 陈茹玉, 张成祥, 冯克胜, 程慕如. 4-芳氧基-4-氧代-1,3,4-二氮磷杂环戊-2-硫酮类化合物的合成及其除草活性[J]. 应用化学, 1996, 0(5): 100-102. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||