应用化学 ›› 2023, Vol. 40 ›› Issue (5): 640-652.DOI: 10.19894/j.issn.1000-0518.220304
AB2型Laves相储氢合金研究进展
修海祥1,2, 刘万强1( ), 尹东明2(
), 尹东明2( ), 程勇2, 王春丽2, 王立民2
), 程勇2, 王春丽2, 王立民2
- 1.长春理工大学材料科学与工程学院,长春 130022
2.中国科学院长春应用化学研究所,稀土资源利用国家重点实验室,长春 130022
-
收稿日期:2022-09-15接受日期:2023-03-08出版日期:2023-05-01发布日期:2023-05-26 -
通讯作者:刘万强,尹东明 -
基金资助:内蒙古自治区重大科技专项(2021ZD0029);国家重点研发计划(2020YFE0204500)
Research Progress of AB2 Laves Phase Hydrogen Storage Alloys
Hai-Xiang XIU1,2, Wan-Qiang LIU1( ), Dong-Ming YIN2(
), Dong-Ming YIN2( ), Yong CHENG2, Chun-Li WANG2, Li-Min WANG2
), Yong CHENG2, Chun-Li WANG2, Li-Min WANG2
- 1.School of Materials Science and Engineering,Changchun University of Science and Technology,Changchun 130022,China
2.State Key Laboratory of Rare Earth Resources Utilization,Changchun Institute of Applied Chemistry,Chinese Academy of Sciences,Changchun 130022,China
-
Received:2022-09-15Accepted:2023-03-08Published:2023-05-01Online:2023-05-26 -
Contact:Wan-Qiang LIU,Dong-Ming YIN -
About author:dmyin@ciac.ac.cn
wqliu1979@126.com
-
Supported by:the Major Science and Technology Project of Inner Mongolia(2021ZD0029);the National Key R&D Program of China(2020YFE0204500)
摘要:
AB2型储氢合金因其具有理论储氢容量高、循环寿命长以及性价比高等优点引起研究者的广泛研究兴趣。但是,AB2型储氢合金还存在活化困难、易毒化以及平台高等缺点阻碍了其实际应用。近年,针对AB2型合金的缺点,研究者们进行了大量的改性研究,并取得了很大进展。本文综述了AB2型储氢合金近30年以来的研究进展情况,重点介绍了改善其储氢性能的方法,提出了AB2型合金今后的重点研究方向。
中图分类号:
引用本文
修海祥, 刘万强, 尹东明, 程勇, 王春丽, 王立民. AB2型Laves相储氢合金研究进展[J]. 应用化学, 2023, 40(5): 640-652.
Hai-Xiang XIU, Wan-Qiang LIU, Dong-Ming YIN, Yong CHENG, Chun-Li WANG, Li-Min WANG. Research Progress of AB2 Laves Phase Hydrogen Storage Alloys[J]. Chinese Journal of Applied Chemistry, 2023, 40(5): 640-652.
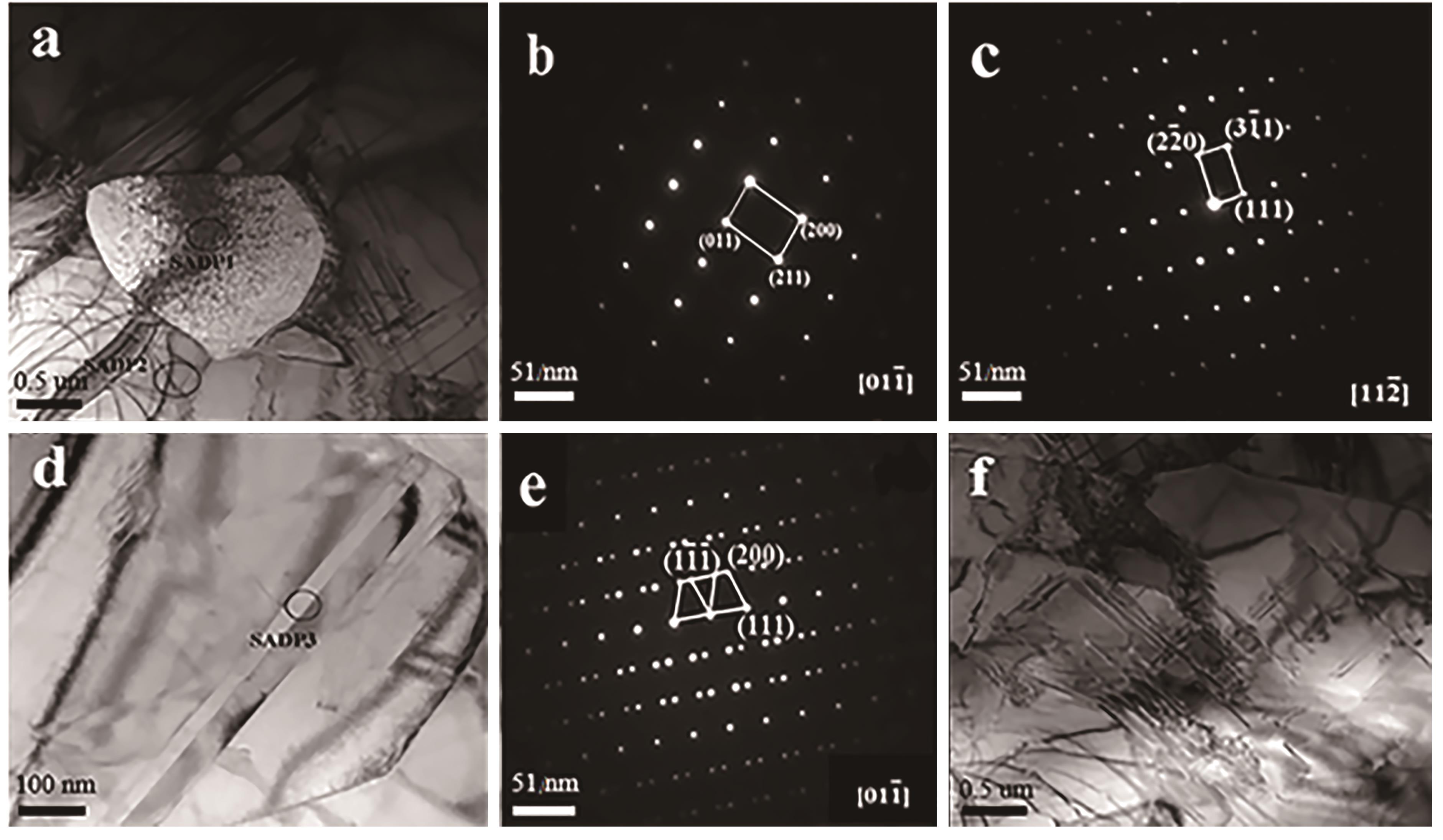
图3 合金显微组织的TEM相(a、d、f),选区电子衍射SADP1中V的衍射斑点(b),SADP2中ZrV2的衍射斑点(c),SADP3中ZrV2孪晶衍射斑点(e)[40]
Fig.3 TEM microstructures of Zr0.9Ti0.1V1.7 (a, d, f), diffraction spots of V in SADP1 (b), diffraction spots of ZrV2 in SADP2 (c), twin diffraction spots of ZrV2 in SADP3 (e)[40]
| Element | Add methods | Master alloy | Capacity | Activation performance | Platform slope | |
|---|---|---|---|---|---|---|
| Sc | Substituted Zr | ZrMn0.6V0.2Ni1.2Co0.1 | Increased | Easy | Unknown | [ |
| Y | Substituted Zr | ZrFe2 | Increased | Easy | Increased | [ |
| La | Doped | Ti1.02Cr1.1Mn0.3Fe0.6 | Increased | Easy | Increased | [ |
| Ho | Doped | Ti1.02Cr1.1Mn0.3Fe0.6 | Increased | Easy | Increased | [ |
| Ce | Doped | Ti0.8Zr0.2Cr0.75Mn1.25 | Increased | Easy | Increased | [ |
| Pr | Substituted La | LaMgNi4 | Increased | Easy | Unknown | [ |
| Nb | Substituted La | LaMgNi4 | Increased | Easy | Unknown | [ |
| Sm | Substituted La | LaMgNi4 | Increased | Easy | Unknown | [ |
表1 不同稀土元素对AB2型Laves相储氢合金性能的影响
Table 1 Effects of different rare earth elements on properties of AB2 Laves phase hydrogen storage alloys
| Element | Add methods | Master alloy | Capacity | Activation performance | Platform slope | |
|---|---|---|---|---|---|---|
| Sc | Substituted Zr | ZrMn0.6V0.2Ni1.2Co0.1 | Increased | Easy | Unknown | [ |
| Y | Substituted Zr | ZrFe2 | Increased | Easy | Increased | [ |
| La | Doped | Ti1.02Cr1.1Mn0.3Fe0.6 | Increased | Easy | Increased | [ |
| Ho | Doped | Ti1.02Cr1.1Mn0.3Fe0.6 | Increased | Easy | Increased | [ |
| Ce | Doped | Ti0.8Zr0.2Cr0.75Mn1.25 | Increased | Easy | Increased | [ |
| Pr | Substituted La | LaMgNi4 | Increased | Easy | Unknown | [ |
| Nb | Substituted La | LaMgNi4 | Increased | Easy | Unknown | [ |
| Sm | Substituted La | LaMgNi4 | Increased | Easy | Unknown | [ |
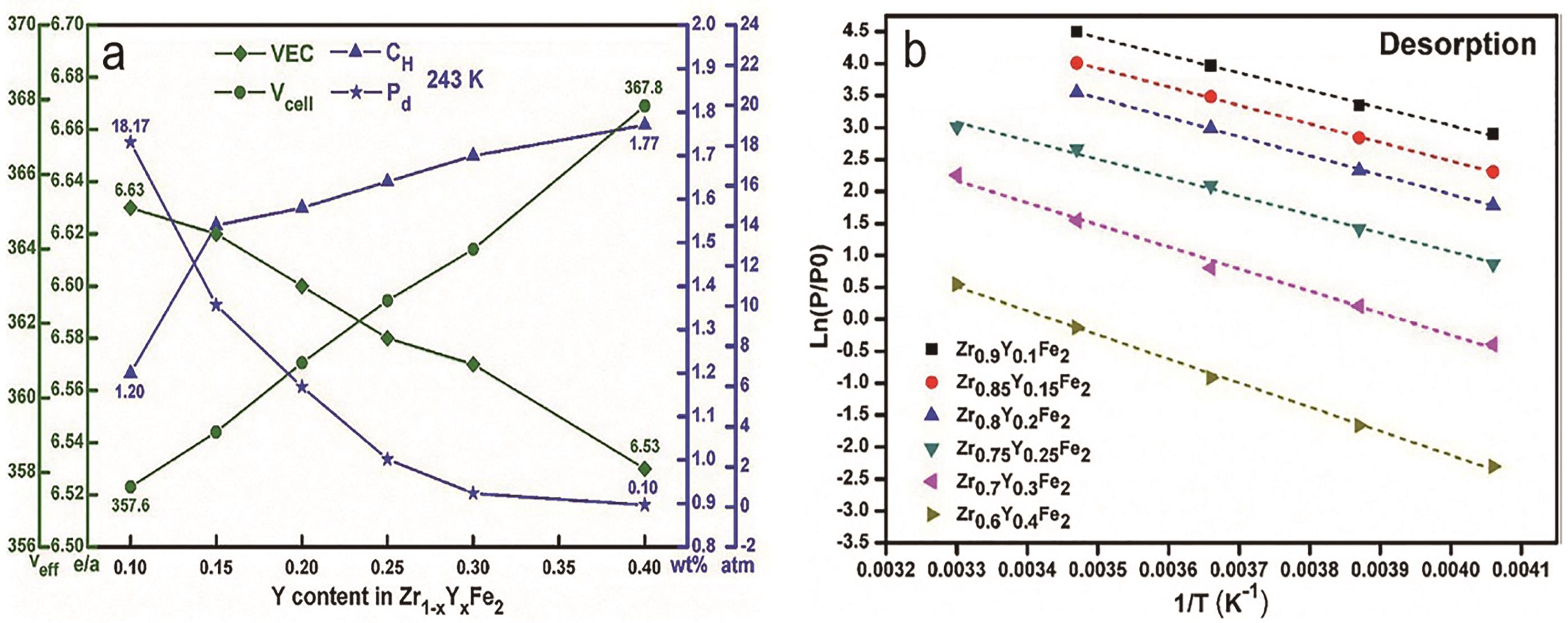
图4 晶胞体积、价电子浓度、储氢容量和解离压力与Y含量的关系(a), Zr-Y-Fe化合物lnP与1/T的拟合图(b)[45]Fig.?4 The cell volume, valence electron concentration, capacity and dehydriding pressure as a function of Y content (a), the fitting plots between lnP and 1/T of Zr-Y-Fe compounds (b)[45]
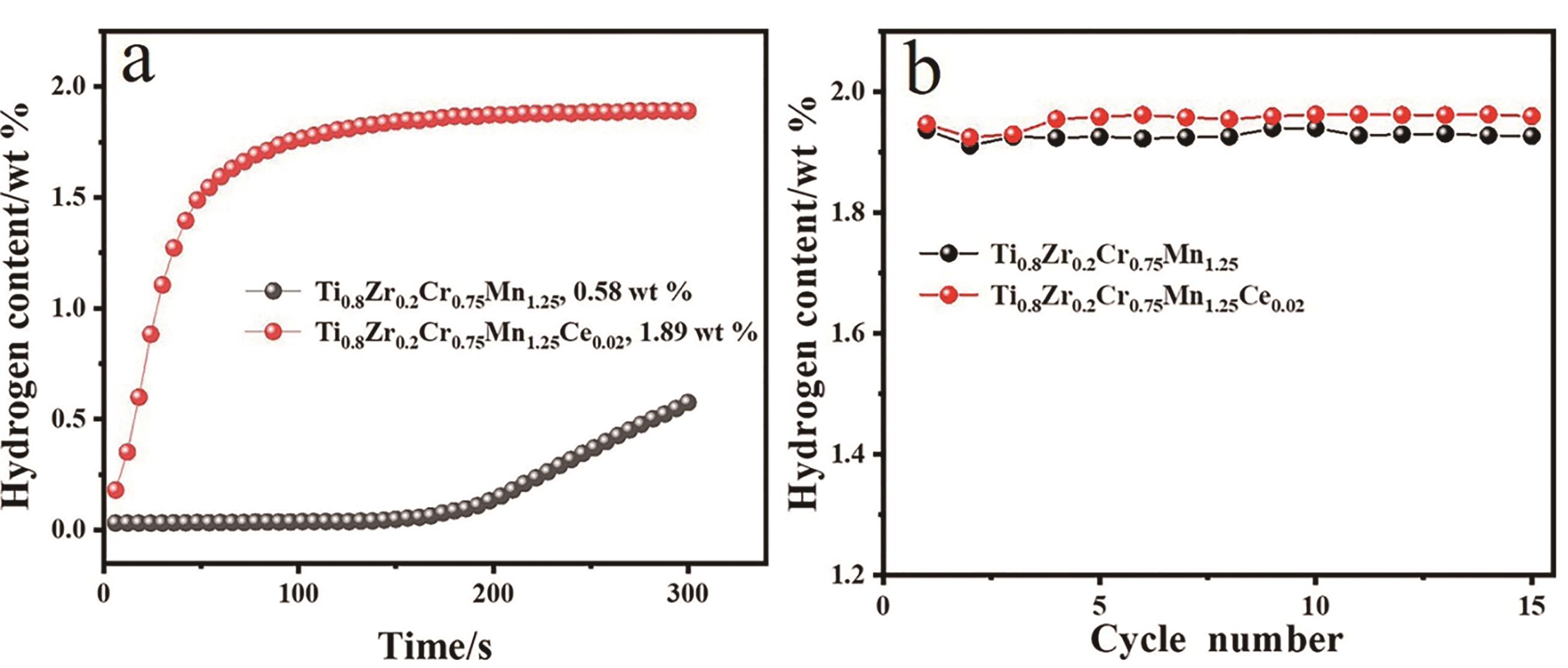
图5 Ti0.8Zr0.2Cr0.75Mn1.25和Ti0.8Zr0.2Cr0.75Mn1.25Ce0.02合金的首次吸氢动力学曲线(a)和循环稳定性(b)[47]
Fig.5 First hydrogen absorption kinetic curves (a), cycle stability (b) of Ti0.8Zr0.2Cr0.75Mn1.25 and Ti0.8Zr0.2Cr0.75Mn1.25Ce0.02 alloys[47]
| Element | Add methods | Master alloy | Capacity | Pressure of platform | |
|---|---|---|---|---|---|
| Mo | Substituted Fe | YFe2 | Decreased | Increased | [ |
| Al | Substituted Fe | YFe2 | Decreased | Unknown | [ |
| Ti | Substituted Zr | ZrFe2 | Decreased | Decreased | [ |
| Mn | Substituted Fe | ZrFe2 | Increased | Decreased | [ |
| Zr | Substituted Ti | TiCr2 | Increased | Decreased | [ |
表2 不同金属元素置换对于AB2储氢合金储氢性能的影响
Table 2 Effect of different metal element replacement on hydrogen storage properties of AB2 hydrogen storage alloy
| Element | Add methods | Master alloy | Capacity | Pressure of platform | |
|---|---|---|---|---|---|
| Mo | Substituted Fe | YFe2 | Decreased | Increased | [ |
| Al | Substituted Fe | YFe2 | Decreased | Unknown | [ |
| Ti | Substituted Zr | ZrFe2 | Decreased | Decreased | [ |
| Mn | Substituted Fe | ZrFe2 | Increased | Decreased | [ |
| Zr | Substituted Ti | TiCr2 | Increased | Decreased | [ |
| Alloy | Capacity before poisoning/(m·g-1) | Capacity before poisoning/(m·g-1) | Capacity retention rate/% |
|---|---|---|---|
| ZrCr0.8Fe1.2 | 154.1 | 0 | 0 |
| ZrCr0.6Fe1.4 | 141.5 | 80.3 | 56.7 |
| ZrCr0.4Fe1.6 | 122.9 | 98.3 | 79.7 |
| ZrCr0.2Fe1.8 | 96.0 | 38.0 | 39.6 |
表3 ZrCr2-x Fe x 合金在空气中的抗中毒性能[65]
Table 3 Impurity poisoning resistance of the ZrCr2-x Fe xalloy in air[65]
| Alloy | Capacity before poisoning/(m·g-1) | Capacity before poisoning/(m·g-1) | Capacity retention rate/% |
|---|---|---|---|
| ZrCr0.8Fe1.2 | 154.1 | 0 | 0 |
| ZrCr0.6Fe1.4 | 141.5 | 80.3 | 56.7 |
| ZrCr0.4Fe1.6 | 122.9 | 98.3 | 79.7 |
| ZrCr0.2Fe1.8 | 96.0 | 38.0 | 39.6 |
| 1 | 徐硕, 余碧莹.中国氢能技术发展现状与未来展望[J]. 北京理工大学学报(社会科学版), 2021, 23(6): 1-12. |
| XU S, YU B Y. Current development and prospect of hydrogen energy technology in China[J]. J B Inst Technol (Soc Sci Ed), 2021, 23(6): 1-12. | |
| 2 | 王丽娜, 时哲, 王玉辉. 氢能产业发展现状及展望[J]. 工业炉, 2022, 44(1): 24-28. |
| WANG L N, SHI Z, WANG Y H. Current situation and prospect of hydrogen energy industry[J]. Ind Furnace, 2022, 44(1): 24-28. | |
| 3 | 刘翠伟, 裴业斌, 韩辉, 等. 氢能产业链及储运技术研究现状及发展趋势[J]. 油气储运, 2022, 41(5): 498-514. |
| LIU C W, PEI Y B, HAN H, et al. Research status and development trend of hydrogen energy industry chain and the storage and transportation technologies[J]. Oil Gas Storage Transportation, 2022, 41(5): 498-514. | |
| 4 | 雷超, 李韬. 碳中和背景下氢能利用关键技术及发展现状[J]. 发电技术, 2021, 42(2): 207-217. |
| LEI C, LI T. Key technologies and development status of hydrogen energy utilization under the background of carbon neutrality[J]. Power Generation Technol, 2021, 42(2): 207-217. | |
| 5 | 杨春. 我国氢能产业发展现状及战略政策动态研究[J]. 电工技术, 2022(2): 20-22. |
| YANG C. Research on the development status and strategic policy trends of chinas hydrogen energy[J]. Electr Eng, 2022(2): 20-22. | |
| 6 | 周健, 邓一荣, 李晓源. 中国氢能发展的现状、挑战与建议[J]. 环境科学与管理, 2022, 47(3): 15-19. |
| ZHOU J, DENG Y R, LI X Y. Current status, challenges and suggestions of hydrogen power promotion in China[J]. Environ Sci Manage, 2022, 47(3): 15-19. | |
| 7 | ZHAO S, LIANG L, LIU B, et al. Superior dehydrogenation performance of α-AlH3 catalyzed by Li3N: realizing 8.0 wt.% capacity at 100 ℃[J]. Small, 2022, 18(17): 2107983. |
| 8 | DING N, LI Y, LIANG F, et al. Highly efficient hydrogen storage capacity of 2.5 wt.% above 0.1 MPa using Y and Cr codoped V-based alloys[J]. ACS Appl Energy Mater, 2022, 5(3): 3282-3289. |
| 9 | LING L, WANG C, REN M, et al. Unraveling the synergistic catalytic effects of TiO2 and Pr6O11 on superior dehydrogenation performances of α-AlH3[J]. ACS Appl Mater Interfaces, 2021, 13(23): 26998-27005. |
| 10 | ZHENG W, SONG W, WU T, et al. Experimental investigation and thermodynamic modeling of the ternary Ti-Fe-Mn system for hydrogen storage applications[J]. J Alloys Compd, 2022, 891: 161957. |
| 11 | LU X, ZHANG L, ZHENG J, et al. Construction of carbon covered Mg2NiH4 nanocrystalline for hydrogen storage[J]. J Alloys Compd, 2022, 905: 164-169. |
| 12 | YU H, YANG X, JIANG X, et al. LaNi5.5 particles for reversible hydrogen storage in N-ethylcarbazole[J]. Nano Energy, 2021, 80: 105476. |
| 13 | TÉLIZ E, ABBOUD M, FACCIO R, et al. Hydrogen storage in AB2 hydride alloys: diffusion processes analysis[J]. J Electroanal Chem, 2020, 879: 114781. |
| 14 | LIU H, ZHANG J, SUN P, et al. Effect of oxygen on the hydrogen storage properties of TiFe alloys[J]. J Energy Storage, 2022, 55: 105543. |
| 15 | SUJAN G K, PAN Z, LI H, et al. An overview on TiFe intermetallic for solid-state hydrogen storage: microstructure, hydrogenation and fabrication processes[J]. Crit Rev Solid State Mater Sci, 2020, 45(5): 410-427. |
| 16 | GRIGOROVA E, TZVETKOV P, TODOROVA S, et al. Facilitated synthesis of Mg2Ni based composites with attractive hydrogen sorption properties[J]. Materials, 2021, 14(8): 1936. |
| 17 | YOUP S M, KWAK Y J, CHOI E. Rate-controlling steps for the hydriding reaction of the intermetallic compound Mg2Ni[J]. J Nanosci Nanotechnol, 2020, 20(11): 7010-7017. |
| 18 | BAUM Z J, DIAZ L L, KONOVALOVA T, et al. Materials research directions toward a green hydrogen economy: a review[J]. ACS Omega, 2022, 7(37): 32908-32935. |
| 19 | YE Y, YUE Y, LU J, et al. Enhanced hydrogen storage of a LaNi5 based reactor by using phase change materials[J]. Renew Energy, 2021, 180: 734-743. |
| 20 | 吴朝玲. 无钕稀土LPC系贮氢合金电极材料的开发研究[D]. 成都: 四川大学, 2003. |
| WU C L. Research on Nd-free LPC-type hydrogen storage alloys for hydride electrodes[D]. Chengdu: Sichuan University, 2003. | |
| 21 | 李学军, 崔舜, 周增林, 等. 稀土(镁)系AB2型贮氢合金的研究进展[J]. 材料导报, 2008(7): 77-81. |
| LI X J, CUI S, ZHOU Z L, et al. Research development of RE-(Mg)-system AB2-type hydrogen storage alloys[J]. Mater Rev, 2008(7): 77-81. | |
| 22 | YARTYS V A, LOTOTSKYY M V. Laves type intermetallic compounds as hydrogen storage materials: a review[J]. J Alloys Compd, 2022: 165219. |
| 23 | 秦高梧, 谢红波, 潘虎成, 等. 一类介于晶体与准晶体之间的有序结构[J]. 金属学报, 2018, 54(11): 1490-1502. |
| QIN G W, XIE H B, PAN H C, et al. A new class of ordered structure between crystals and quasicrystals[J]. Acta Metall Sin, 2018, 54(11): 1490-1502. | |
| 24 | 赵栋梁, 韩忠刚, 翟亭亭, 等. TiFe基合金储氢活化性能研究进展[J]. 稀有金属, 2020, 44(4): 337-351. |
| ZHAO D L, HAN Z G, ZHAI T T, et al. Advances in activation property of hydrogen storage for TiFe-based alloy[J]. Chin J Rare Met, 2020, 44(4): 337-351. | |
| 25 | YU X, XIA B, WU Z, et al. Phase structure and hydrogen sorption performance of Ti-Mn-based alloys[J]. Mater Sci Eng A, 2004, 373(1/2): 303-308 |
| 26 | 苏强. AB2型Laves相储氢合金吸氢机理及掺杂元素作用机制的研究[D]. 南宁: 广西大学, 2004. |
| SU Q. Study on Absorb-hydrogen mechanism and functions of substitute element in AB2 type Laves phase hydrogen storage alloys[D]. Nanning: Guangxi University, 2004. | |
| 27 | SCHÜLKE M, KISS G, PAULUS H, et al. Complex surface analytical investigations on hydrogen absorption and desorption processes of a TiMn2-based alloy[J]. Anal Bioanal Chem, 2009, 393: 1843-1856. |
| 28 | XIUMEI G, ERDONG W, SUCHENG W. Hydrogen storage properties of Laves phase Ti1- xZrx(Mn0.5Cr0.5)2 alloys[J]. Rare Met, 2006, 25(6): 218-223. |
| 29 | 张羊换, 王国清, 董小平, 等. AB2型Laves相贮氢合金的研究进展[J]. 金属功能材料, 2005(4): 25-30. |
| ZHANG Y H, WANG G Q, DONG X P, et al. Development research of AB2-type Laves phase hydrogen storage alloy[J]. Met Funct Mater, 2005(4): 25-30. | |
| 30 | 邓庆洲, 陈德敏, 杨柯, 等. 一种快速凝固AB2型储氢合金的球磨改性[J]. 物理化学学报, 1999(5): 420-425. |
| DENG Q Z, CHEN D M, YANG K, et al. Modification of a rapidly solidified AB2 type hydrogen storage alloy by ball-milling[J]. Acta Phys-Chim Sin, 1999(5): 420-425. | |
| 31 | 杨晓光. Zr-Cr-Ni系AB2型Laves相贮氢合金及其电化学性能的研究[J]. 材料导报, 1996(1): 78. |
| YANG X G. Study on the electrochemical properties of Zr-Cr-Ni AB2 type Laves phase hydrogen storage alloys[J]. Mater Rev, 1996(1): 78. | |
| 32 | WANG X L, SUDA S. Surface characteristics of fluorinated hydriding alloys[J]. J Alloys Compd, 1995, 231(1/2): 380-386. |
| 33 | 刘新, 全钰泰. 碱液对AB2型储氢合金活化性能的影响[J]. 渝州大学学报(自然科学版), 2001(3): 50-52. |
| LIU X, QUAN Y T. The effect of alkaline solution on activation of AB2 hydrogen storage alloy[J]. J Chongqing Technol Business Univ (Nat Sci Ed), 2001(3): 50-52. | |
| 34 | 邢磊, 李一鸣, 张羊换, 等. 快淬-退火La4MgNi19合金的电化学储氢性能及其失效行为[J]. 稀有金属, 2017, 41(12): 1318-1326. |
| XING L, LI Y M, ZHANG Y H, et al. Electrochemical hydrogen storage performances and degradation behavior of rapidquenching-annealed La4MgNi19 alloy[J]. Chin J Rare Met, 2017, 41(12): 1318-1326. | |
| 35 | 刘泽民, 张素银, 邵诚, 等. 无稀土MnBi永磁合金的研究进展[J]. 科技资讯, 2016, 14(34): 80-84. |
| LIU Z M, ZHANG S Y, SHAO C, et al. The research progress of rare-earth free permanent magnetic alloy in MnBi[J]. Sci Technol Inf, 2016, 14(34): 80-84. | |
| 36 | 张羊换, 李平, 王新林, 等. 快淬对AB2型贮氢合金电化学性能与微观结构的影响[J]. 功能材料, 2004(3): 298-301. |
| ZHANG Y H, LI P, WANG X L, et al. The effects of rapid quenching on the electrochemical characteristics and microstructures of AB2 Laves phase electrode alloys[J]. J Funct Mater, 2004(3): 298-301. | |
| 37 | 张羊换, 李平, 王新林, 等. 熔体快淬对AB2型Laves相储氢电极合金循环寿命的影响[J]. 稀有金属材料与工程, 2004(12): 1321-1324. |
| ZHANG Y H, LI P, WANG X L, et al. The effect of rapid quenching on the cycle lives of H2-storage electrode alloys with AB2 Laves phase[J]. Rare Met Mater Eng, 2004(12): 1321-1324. | |
| 38 | ZHANG Y, ZHANG W, SONG X, et al. Effects of spinning rate on structures and electrochemical hydrogen storage performances of RE-Mg-Ni-Mn-based AB2-type alloys[J]. T Nonferr Met Soc, 2016, 26(12): 3219-3231. |
| 39 | 翟亭亭. La-Mg-Ni系AB2型贮氢合金的结构、贮氢性能及容量衰退机理研究[D]. 沈阳: 东北大学, 2015. |
| ZHAI T T. Structure, hydrogen storage properties anddegradation mechanism of capacity of La-Mg-Ni system AB2 type alloys[D]. Shenyang: Northeastern University, 2015. | |
| 40 | 张云龙. 非计量比Zr基Laves相合金的微结构及储氢性能[D]. 西安: 西北工业大学, 2017. |
| ZHANG Y L. Microstructures and hydrogen storage properties of non-stoichiometric Zr-based Laves phase alloys[D]. Xi′an: Northwestern Polytechnical University, 2017. | |
| 41 | 刘海镇, 徐丽, 郭秀梅, 等. Ti-Mn系AB2型Laves相储氢合金研究[J]. 稀有金属, 2019, 43(9): 928-934. |
| LIU H Z, XU L, GUO X M, et al. Hydrogen storage properties of Ti-Mn based AB2-type Laves phase alloys[J]. Chin J Rare Met, 2019, 43(9): 928-934. | |
| 42 | YOUNG K, OUCHI T, KOCH J, et al. Compositional optimization of vanadium-free hypo-stoichiometric AB2 metal hydride alloy for Ni/MH battery application[J]. J Alloys Compd, 2012, 510(1): 97-106. |
| 43 | 姜婉婷, 罗永春, 赵磊, 等. 稀土元素对R-Y-Ni系A2B7型无镁储氢合金微观结构和电化学性能的影响[J]. 无机化学学报, 2018, 34(10): 1817-1825. |
| JIANG W T, LUO Y C, ZHAO L, et al. Effect of rare earth elements on the microstructure and electrochemical properties of Mg-free R-Y-Ni based A2B7-type hydrogen storage alloys[J]. Chin J Inorg Chem, 2018, 34(10): 1817-1825. | |
| 44 | LI K, LUO Y, WANG W, et al. Effects of scandium on hydrogen storage and electrochemical properties of AB2-type Zr1- xScxMn0.6V0.2Ni1.2Co0.1(x=0~1) alloys[J]. J Chin Rare Earth Soc, 2013, 31(4): 442-449. |
| 45 | QIN C, WANG H, LIU J, et al. Tuning hydrogen storage thermodynamic properties of ZrFe2 by partial substitution with rare earth element Y[J]. Int J Hydrog Energy, 2021, 46(35): 18445-18452. |
| 46 | YAO Z, LIU L, XIAO X, et al. Effect of rare earth doping on the hydrogen storage performance of Ti1.02Cr1.1Mn0.3Fe0.6 alloy for hybrid hydrogen storage application[J]. J Alloys Compd, 2018, 731: 524-530. |
| 47 | ZHOU L, LI W, HU H, et al. Ce-doped TiZrCrMn alloys for enhanced hydrogen storage[J]. Energy Fuels, 2022, 36(7): 3997-4005. |
| 48 | 尚宏伟. 稀土镁基AB2型LaMgNi4系贮氢合金电化学性能及气态储氢性能的研究[D]. 包头: 内蒙古科技大学, 2013. |
| SHAGN H W. An investigation on the electrochemical performance andgaseous hydrogen storage performance of RE-Mg based LaMgNi4-type hydrogen storage alloy[D]. Baotou: Inner Mongolia University of Science and Technology, 2013. | |
| 49 | SIVOV R B, ZOTOV T A, VERBETSKY V N. Interaction of ZrFe2 doped with Ti and Al with hydrogen[J]. Inorg. Mater, 2010, 46(4): 372-376. |
| 50 | 杨康, 蒋利军, 苑慧萍, 等. Ce替代Y对Y1- xCexFe2(x=0,0.15,0.25和0.50)合金吸氢性能的影响[J]. 稀有金属, 2017, 41(11): 1202-1207. |
| YANG K, JIANG L J, YUAN H P, et al. Hydrogen absorption property of Y1- xCexFe2(x=0, 0.15, 0.25 and 0.50) alloys with Ce substitution for Y[J]. Chin J Rare Met, 2017, 41(11): 1202-1207. | |
| 51 | ZOTOV T, MOVLAEV E, MITROKHIN S, et al. Interaction in (Ti,Sc) Fe2-H2 and (Zr,Sc) Fe2-H2 systems[J]. J Alloys Compd, 2008, 459(1/2): 220-224. |
| 52 | ZHAO S, WANG H, LIU J. Exploring the hydrogen-induced amorphization and hydrogen storage reversibility of Y(Sc)0.95Ni2 Laves phase compounds[J]. Materials, 2021, 14(2): 276. |
| 53 | SIVOV R, ZOTOV T, VERBETSKY V. The influence Y, Gd and Dy doping on hydrogen sorption properties of ZrFe2[J]. Interact Hydrogen Isot Struct Mater (IHISM-08 Junior), Sarov, 2009: 201-208. |
| 54 | WONG D F, YOUNG K, NEI J, et al. Effectsof Nd-addition on the structural, hydrogen storage, andelectrochemical properties of C14 metal hydride alloys[J]. J Alloys Compd, 2015, 647: 507-518. |
| 55 | 庞浩良. 元素替代对Y系AB2型储氢合金结构和性能的影响研究[D]. 广州: 华南理工大学, 2018. |
| PANG H L. Alloying substitution on structure and properties of Y based AB2 hydrogen storage alloys[D]. Guangzhou: South China University of Technology, 2018. | |
| 56 | 黎子鸣. YFe2基新型稀土合金结构与性能的关系[D]. 广州: 华南理工大学, 2019. |
| LI Z M. The relation between structure and properties in novel YFe2-based rare earth alloys[D]. Guangzhou: South China University of Technology, 2019. | |
| 57 | QIN C, ZHOU C, OUYANG L, et al. High-pressure hydrogen storage performances of ZrFe2 based alloys with Mn, Ti, and V addition[J]. Int J Hydrogen Energy, 2020, 45(16): 9836-9844. |
| 58 | 李吉刚, 盛鹏, 徐丽, 等. (Ti1- xZrx)yCr2.0- zVz合金制备和吸放氢性能的研究[J]. 中国科技论文, 2017, 12(24): 2761-2766. |
| LI J G, SHENG P, XU L, et al. Preparation and hydrogen absorption/desorption properties of (Ti1- xZrx)yCr2.0- zVz alloys[J]. China Sci Paper, 2017, 12(24): 2761-2766. | |
| 59 | AOKI K, LI H W, DILIXIATI M, et al. Formation of crystalline and amorphous hydrides by hydrogenation of C15 Laves phase YFe2[J]. Mater Sci Eng, 2007, 449: 2-6. |
| 60 | AOKI K, LI X, MASUMOTO T. Factors controlling hydrogen-induced amorphization of C15Laves compounds[J]. Acta Metallurgica Et Materialia, 1992, 40(7): 1717-1726. |
| 61 | CHUNG U I, KIM Y G, LEE J Y. General features of hydrogen-induced amorphization in RM2 (R=rare earth, M=transition element) Laves phases[J]. Philos Mag B, 1991, 63(5): 1119-1130. |
| 62 | LI J, GUO Y, JIANG X, et al. Hydrogen storage performances, kinetics and microstructure of Ti1.02Cr1.0Fe0.7- xMn0.3Alx alloy by Al substituting for Fe[J]. Renew Energy, 2020, 153: 1140-1154. |
| 63 | 吴天栋. Zr基Laves相储氢合金的构效关系及抗毒化行为[D]. 西安: 西北工业大学, 2017. |
| WU T D. Structure-activity relationship of Zr-based Laves phase hydrogen storage alloys and poisoning resistance against gaseous impurities[D]. Xi′an: Northwestern Polytechnical University, 2017. | |
| 64 | 张思方. 元素替代对Zr基AB2型贮氢合金相结构与电化学性能的影响[D]. 长沙: 中南大学, 2008. |
| ZHANG S F. Effect of elemental substitution on phase structure and electrochemical properties of Zr-based AB2 hydrogen storage alloys[D]. Changsha: Central South University, 2008. | |
| 65 | 李晋平, 李冬梅, 郎卫平, 等. ZrCr2- xFex合金表面性质及中毒研究[J]. 矿冶工程, 1994(4): 61-63. |
| LI J P, LI D M, LANG W P, et al. Study on surface properties and toxicity of ZrCr2- xFex alloy[J]. Min Metall Eng, 1994(4): 61-63. | |
| 66 | LUAN B, CUI N, ZHAO H, et al. Effects of potassium-boron addition on the performance of titanium based hydrogen storage alloy electrodes[J]. Int J Hydrogen Energy, 1996, 21(5): 373-379. |
| 67 | YU X, WU Z, HUANG T, et al. Effect of carbon addition on activation and hydrogen sorption characteristics of TiMn1.25Cr0.25 alloy[J]. Mater Chem Phys, 2004, 83(2/3): 273-277. |
| 68 | YOUNG K, OUCHI T, HUANG B, et al. Effects of B, Fe, Gd, Mg, and C on the structure, hydrogen storage, and electrochemical properties of vanadium-free AB2 metal hydride alloy[J]. J Alloys Compd, 2012, 511(1): 242-250. |
| 69 | DRAŠNER A, BLAẐINA Ẑ. The influence of Si and Ge on the hydrogen sorption properties of the intermetallic compound ZrCr2[J]. J Alloys Compd, 1993, 199(1/2): 101-104. |
| 70 | ZOTOV T A, SIVOV R B, MOVLAEV E A, et al. IMC hydrides with high hydrogen dissociation pressure[J]. J Alloys Compd, 2011, 509: S839-S843. |
| 71 | LIU S, LU G. Interaction of cationic vesicle with ribonucleotides (AMP, ADP, and ATP) and physicochemical characterization of DODAB/ribonucleotides complexes[J]. Biophys Chem, 2007, 127(1/2): 19-27. |
| 72 | YOUNG K, REICHMAN B, FETCENKO M A. Electrochemical performance of AB2 metal hydride alloys measured at -40 ℃[J]. J Alloys Compd, 2013, 580: S349-S352. |
| [1] | 陈新, 平郑骅, 丁雅娣, 龙英才. 填充Silicalite-Ⅰ沸石的PDMS橡胶醇-水分离膜[J]. 应用化学, 1994, 0(3): 21-25. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||