Chinese Journal of Applied Chemistry ›› 2023, Vol. 40 ›› Issue (8): 1063-1076.DOI: 10.19894/j.issn.1000-0518.230048
• Review • Previous Articles Next Articles
Progress on Tuning the Geometric and Electronic Structure of Precious Metal Catalysts for Hydrogen Peroxide Production via Two-Electron Oxygen Reduction
Er-Gui LUO( ), Tao TANG, Yi WANG, Jun-Ming ZHANG, Yu-Hong CHANG, Tian-Jun HU, Jian-Feng JIA(
), Tao TANG, Yi WANG, Jun-Ming ZHANG, Yu-Hong CHANG, Tian-Jun HU, Jian-Feng JIA( )
)
- Key Laboratory of Magnetic Molecules and Magnetic Information Materials of Ministry of Education & School of Chemistry and Materials Science of Shanxi Normal University,Taiyuan 030032,China
-
Received:2023-03-06Accepted:2023-06-01Published:2023-08-01Online:2023-08-24 -
Contact:Er-Gui LUO,Jian-Feng JIA -
About author:jiajf@dns.sxnu.edu.cn
luogui1991@sxnu.edu.cn;
-
Supported by:the National Natural Science Foundation of China(22209102);the Natural Science Foundation of Shanxi Province(20210302124473)
CLC Number:
Cite this article
Er-Gui LUO, Tao TANG, Yi WANG, Jun-Ming ZHANG, Yu-Hong CHANG, Tian-Jun HU, Jian-Feng JIA. Progress on Tuning the Geometric and Electronic Structure of Precious Metal Catalysts for Hydrogen Peroxide Production via Two-Electron Oxygen Reduction[J]. Chinese Journal of Applied Chemistry, 2023, 40(8): 1063-1076.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: http://yyhx.ciac.jl.cn/EN/10.19894/j.issn.1000-0518.230048
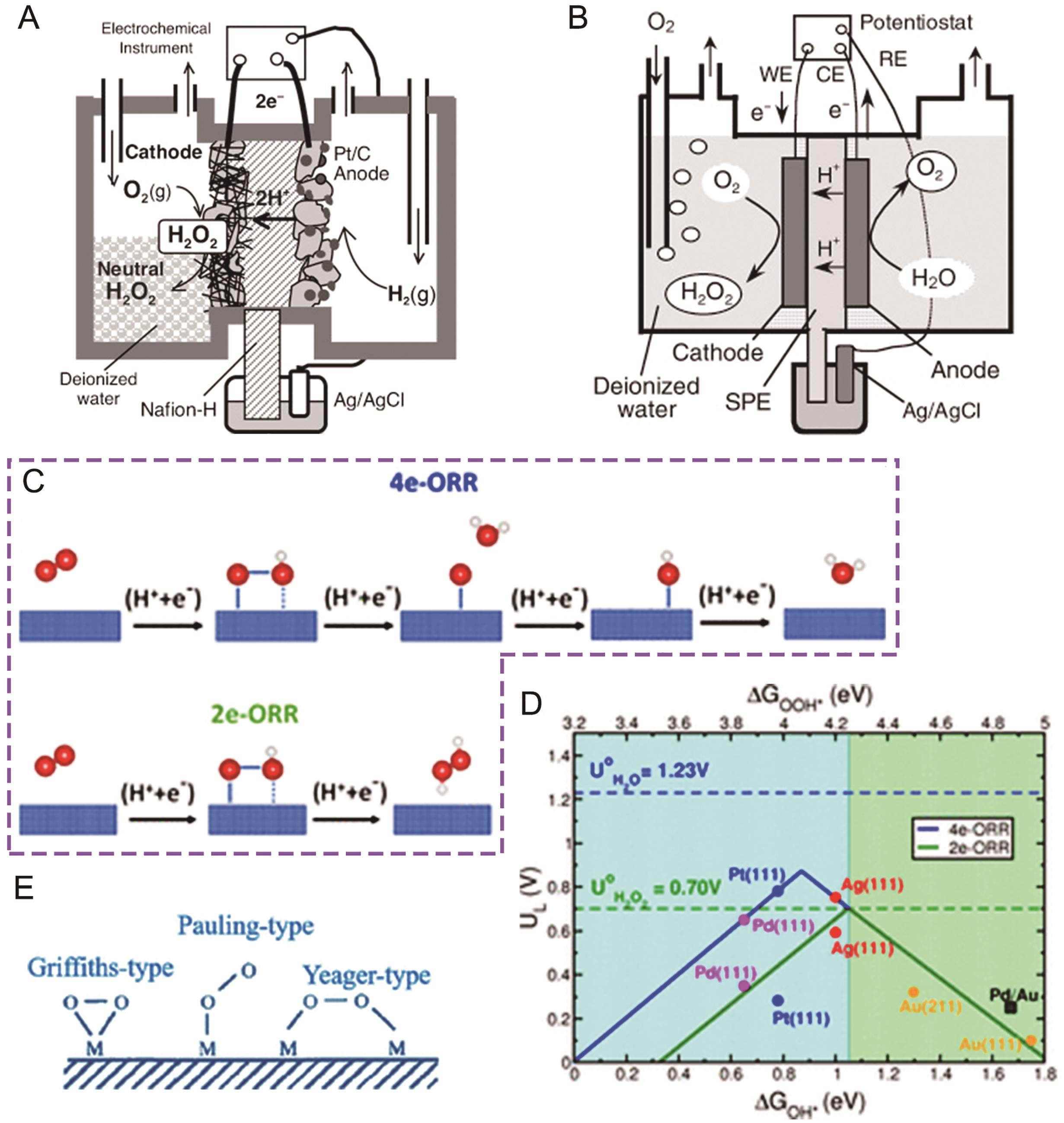
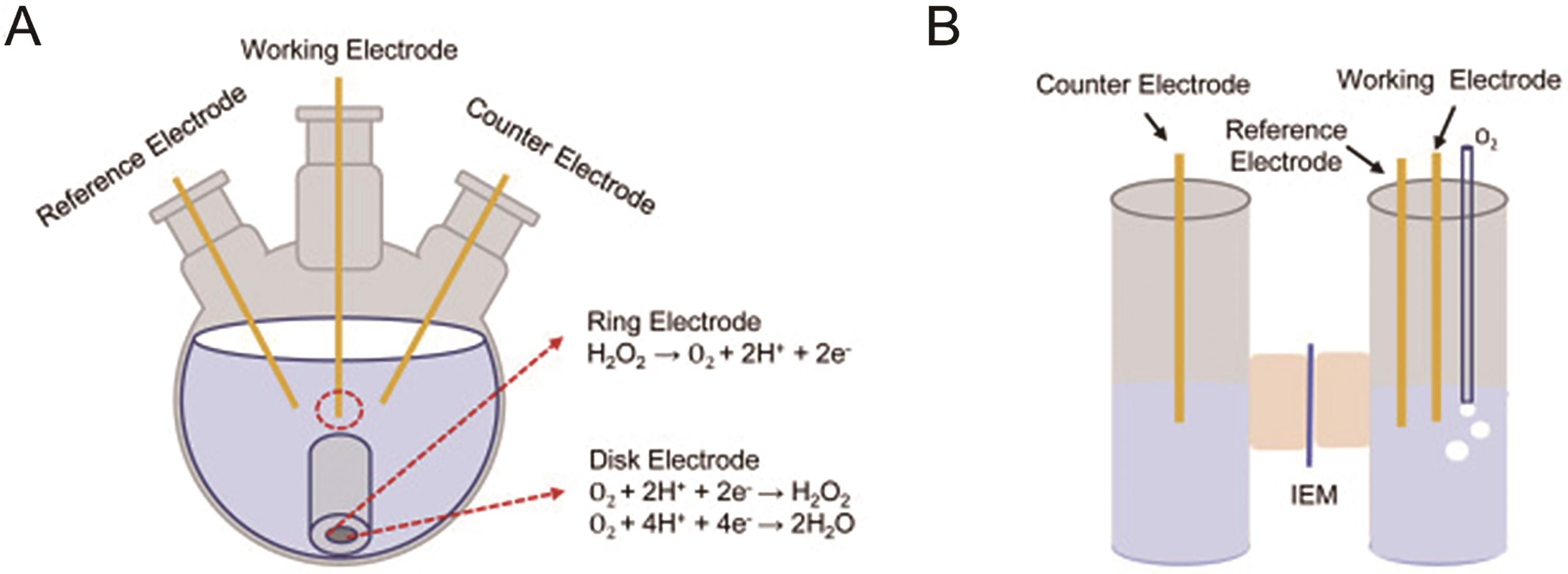
Fig.1 (A) Schematic illustration of a fuel cell for the synthesis of H2O2[22]; (B) Schematic illustration of a electrolysis cell for the synthesis of H2O2[23]; (C) Schematic diagram of the mechanisms for 4e-ORR and 2e-ORR[42]; (D) 4e-ORR (blue) and 2e-ORR (green) activity volcano curves[42]; (E) Schematic illustration of three modes of O2 adsorption[43]
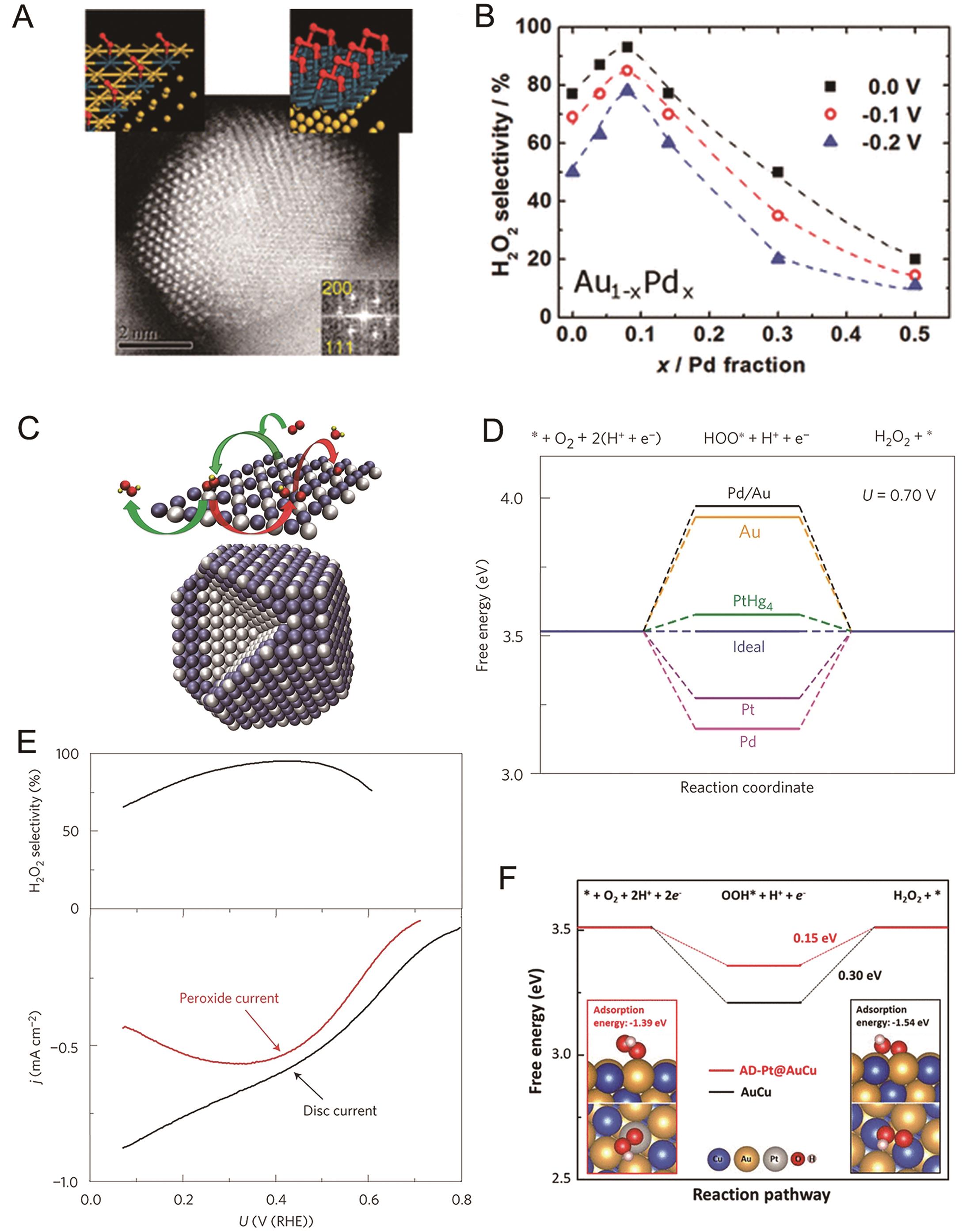
Fig.3 (A) HRTEM image of AuPd alloy and adsorption of O2 on Pd isolated atoms (left) and Pd monolayer (right) [62]; (B) H2O2 selectivity of AuPd alloy catalysts with varied Pd content[62]; (C) Schematic depiction of Pt-Hg alloy[27]; (D) Free-energy diagram for 2e-ORR to H2O2 on several metal surfaces[27]; (E) Polarization curve and corresponding H2O2 yield of Pt-Hg/C catalyst in O2-saturated 0.1 mol/L HClO4[27]; (F) Free-energy diagram for 2e-ORR to H2O2 on AuCu and AD-Pt@AuCu[64]
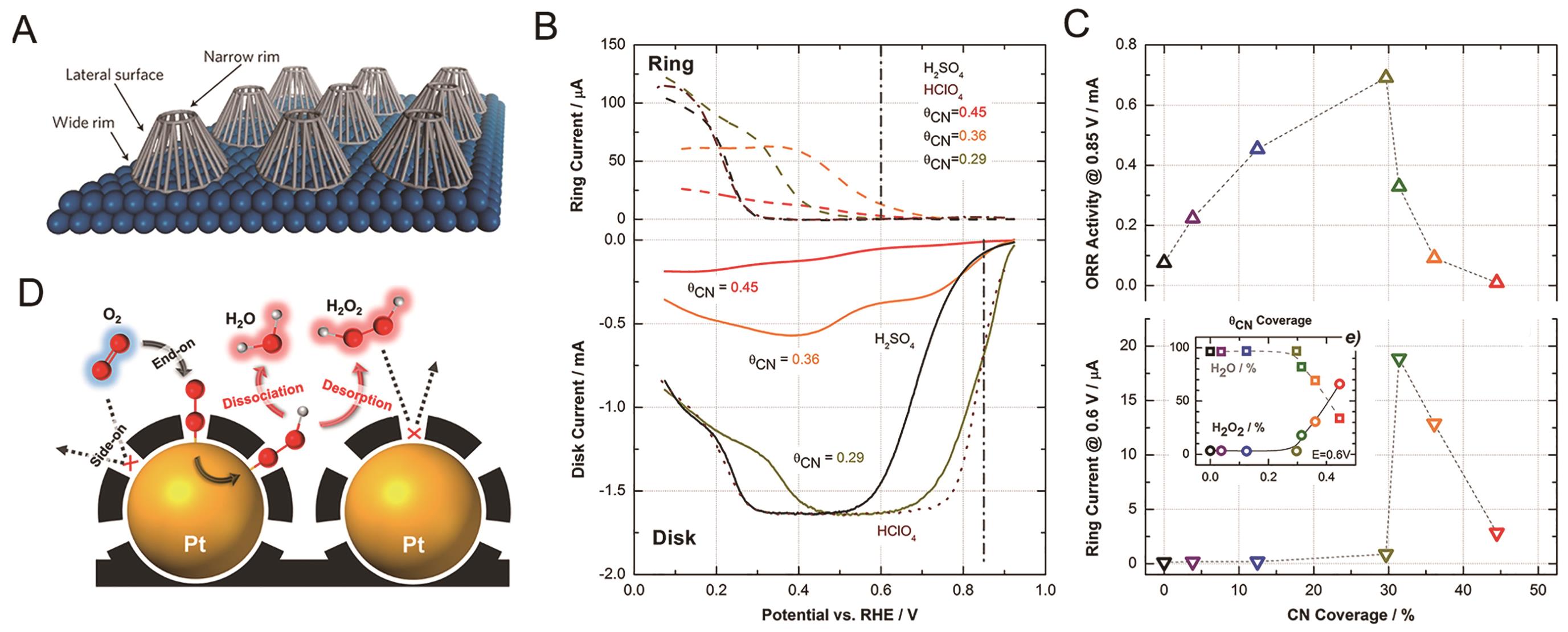
Fig.4 (A) Schematic diagram of Pt surface modified with calix[4]arene molecules[70]; (B) Polarization curve of CN--adsorbed Pt(111) electrode[67]; (C) ORR activity and ring current of CN--adsorbed Pt(111) electrode under different CN- coverage[67]; (D) The effect of carbon coating on the O2 adsorption mode and ORR pathway on Pt/C catalyst[72]

Fig.5 (A) ORR polarization curves and ring currents of Pt/TiN catalysts[73]; (B) Support effect in Pt SACs[74]; (C) Schematic illustration of the preparation of CuS x -supported Pt SACs (h-Pt1-CuS x )[75]; (D) ORR performance of h-Pt1-CuS x and control samples[75]; (E) AC-STEM image of Pt/HSC[76]; (F) ORR activity and (G) H2O2 selectivity of Pt/HSC and control samples[76]
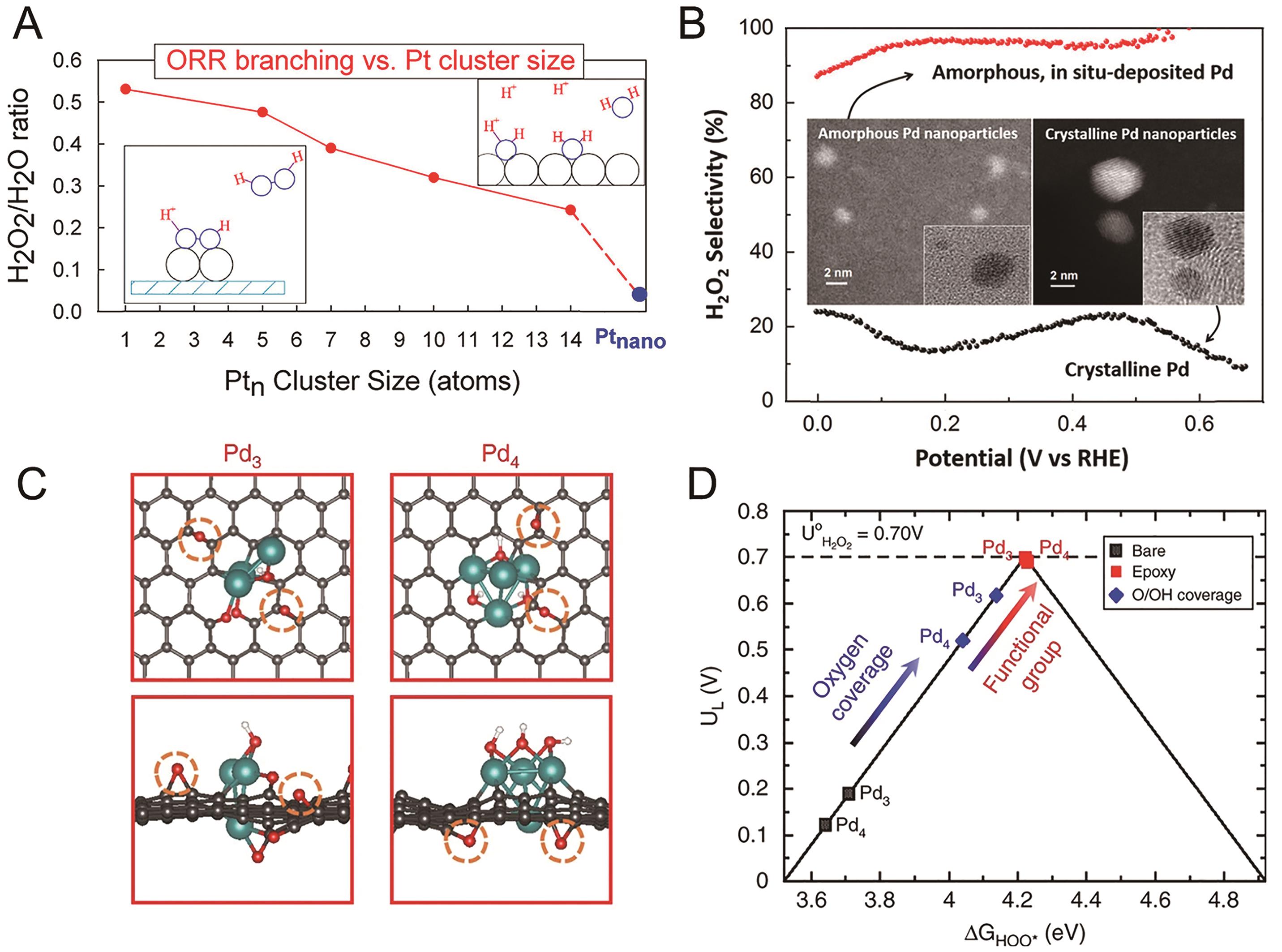
Fig.6 (A) Size dependence of H2O2 formation on Pt-based catalysts[83]; (B) H2O2 selectivity on crystalline Pd and amorphous Pd nanoparticles[84]; (C) Optimized structures of active sites in Pd δ+-OCNT catalyst and (D) activity volcano plot based on DFT calculations[53]
| 1 | CIRIMINNA R, ALBANESE L, MENEGUZZO F, et al. Hydrogen peroxide: a key chemical for today's sustainable development[J]. ChemSusChem, 2016, 9(24): 3374-3381. |
| 2 | LANE B S, BURGESS K. Metal-catalyzed epoxidations of alkenes with hydrogen peroxide[J]. Chem Rev, 2003, 103(7): 2457-2473. |
| 3 | HAGE R, LIENKE A. Applications of transition-metal catalysts to textile and wood-pulp bleaching[J]. Angew Chem Int Ed, 2005, 45(2): 206-222. |
| 4 | BRILLAS E, SIRES I, OTURAN M A. Electro-fenton process and related electrochemical technologies based on Fenton′s reaction chemistry[J]. Chem Rev, 2009, 109(12): 6570-6631. |
| 5 | CAMPOS-MARTIN J M, BLANCO-BRIEVA G, FIERRO J L. Hydrogen peroxide synthesis: an outlook beyond the anthraquinone process[J]. Angew Chem Int Ed, 2006, 45(42): 6962-6984. |
| 6 | GAO G, TIAN Y, GONG X, et al. Advances in the production technology of hydrogen peroxide[J]. Chin J Catal, 2020, 41(7): 1039-1047. |
| 7 | YI Y, WANG L, LI G, et al. A review on research progress in the direct synthesis of hydrogen peroxide from hydrogen and oxygen: noble-metal catalytic method, fuel-cell method and plasma method[J]. Catal Sci Technol, 2016, 6(6): 1593-1610. |
| 8 | EDWARDS J K, CARLEY A F, HERZING A A, et al. Direct synthesis of hydrogen peroxide from H2 and O2 using supported Au-Pd catalysts[J]. Faraday Discuss, 2008, 138: 225-239. |
| 9 | EDWARDS J K, HUTCHINGS G J. Palladium and gold-palladium catalysts for the direct synthesis of hydrogen peroxide[J]. Angew Chem Int Ed, 2008, 47(48): 9192-9198. |
| 10 | GHEEWALA C D, COLLINS B E, LAMBERT T H. An aromatic ion platform for enantioselective Bronsted acid catalysis[J]. Science, 2016, 351(6276): 961-965. |
| 11 | FLAHERTY D W. Direct synthesis of H2O2 from H2 and O2 on Pd catalysts: current understanding, outstanding questions, and research needs[J]. ACS Catal, 2018, 8(2): 1520-1527. |
| 12 | RANGANATHAN S, SIEBER V. Recent advances in the direct synthesis of hydrogen peroxide using chemical catalysis- a review[J]. Catalysts, 2018, 8(9): 379. |
| 13 | EDWARDS J K, FREAKLEY S J, LEWIS R J, et al. Advances in the direct synthesis of hydrogen peroxide from hydrogen and oxygen[J]. Catal Today, 2015, 248: 3-9. |
| 14 | YANG S, VERDAGUER-CASADEVALL A, ARNARSON L, et al. Toward the decentralized electrochemical production of H2O2: a focus on the catalysis[J]. ACS Catal, 2018, 8(5): 4064-4081. |
| 15 | LEWIS R J, HUTCHINGS G J. Recent advances in the direct synthesis of H2O2[J]. ChemCatChem, 2018, 11(1): 298-308. |
| 16 | JIANG Y, NI P, CHEN C, et al. Selective electrochemical H2O2 production through two-electron oxygen electrochemistry[J]. Adv Energy Mater, 2018, 8(31): 1801909. |
| 17 | WANG Y, WATERHOUSE G I N, SHANG L, et al. Electrocatalytic oxygen reduction to hydrogen peroxide: from homogeneous to heterogeneous electrocatalysis[J]. Adv Energy Mater, 2020, 11(15): 2003323. |
| 18 | OTSUKA K, YAMANAKA I. One step synthesis of hydrogen peroxide through fuel cell reaction[J]. Electrochim Acta, 1990, 35(2): 319-322. |
| 19 | YAMANAKA I, ONIZAWA T, TAKENAKA S, et al. Direct and continuous production of hydrogen peroxide with 93% selectivity using a fuel-cell system[J]. Angew Chem Int Ed, 2003, 42(31): 3653-3655. |
| 20 | YAMANAKA I, HASHIMOTO T, ICHIHASHI R, et al. Direct synthesis of H2O2 acid solutions on carbon cathode prepared from activated carbon and vapor-growing-carbon-fiber by a H2/O2 fuel cell[J]. Electrochim Acta, 2008, 53(14): 4824-4832. |
| 21 | JUNG E, SHIN H, HOOCH ANTINK W, et al. Recent advances in electrochemical oxygen reduction to H2O2: catalyst and cell design[J]. ACS Energy Lett, 2020, 5(6): 1881-1892. |
| 22 | YAMANAKA I, TAZAWA S, MURAYAMA T, et al. Catalytic synthesis of neutral H2O2 solutions from O2 and H2 by a fuel cell reaction[J]. ChemSusChem, 2008, 1(12): 988-992. |
| 23 | YAMANAKA I, MURAYAMA T. Neutral H2O2 synthesis by electrolysis of water and O2[J]. Angew Chem Int Ed, 2008, 47(10): 1900-1902. |
| 24 | ZHANG J, ZHANG H, CHENG M J, et al. Tailoring the electrochemical production of H2O2: strategies for the rational design of high-performance electrocatalysts[J]. Small, 2020, 16(15): e1902845. |
| 25 | WANG N, MA S, ZUO P, et al. Recent progress of electrochemical production of hydrogen peroxide by two-electron oxygen reduction reaction[J]. Adv Sci, 2021, 8(15): e2100076. |
| 26 | 喻奥, 马国铭, 朱龙涛, 等. 电化学还原二氧化碳合成碳材料电催化还原氧气合成过氧化氢[J]. 应用化学, 2022, 39(4): 657-665. |
| YU A, MA G M, ZHU L T, et al. Electrochemical reduction of carbon dioxide to carbon materials for two-electron oxygen reduction reaction[J]. Chin J Appl Chem, 2022, 39(4): 657-665. | |
| 27 | SIAHROSTAMI S, VERDAGUER-CASADEVALL A, KARAMAD M, et al. Enabling direct H2O2 production through rational electrocatalyst design[J]. Nat Mater, 2013, 12(12): 1137-1143. |
| 28 | VERDAGUER-CASADEVALL A, DEIANA D, KARAMAD M, et al. Trends in the electrochemical synthesis of H2O2: enhancing activity and selectivity by electrocatalytic site engineering[J]. Nano Lett, 2014, 14(3): 1603-1608. |
| 29 | CHEN S, CHEN Z, SIAHROSTAMI S, et al. Designing boron nitride islands in carbon materials for efficient electrochemical synthesis of hydrogen peroxide[J]. J Am Chem Soc, 2018, 140(25): 7851-7859. |
| 30 | MELCHIONNA M, FORNASIERO P, PRATO M. The rise of hydrogen peroxide as the main product by metal-free catalysis in oxygen reductions[J]. Adv Mater, 2019, 31(13): e1802920. |
| 31 | ZHOU Y, CHEN G, ZHANG J. A review of advanced metal-free carbon catalysts for oxygen reduction reactions towards the selective generation of hydrogen peroxide[J]. J Mater Chem A, 2020, 8(40): 20849-20869. |
| 32 | KIM H W, ROSS M B, KORNIENKO N, et al. Efficient hydrogen peroxide generation using reduced graphene oxide-based oxygen reduction electrocatalysts[J]. Nat Catal, 2018, 1(4): 282-290. |
| 33 | LU Z, CHEN G, SIAHROSTAMI S, et al. High-efficiency oxygen reduction to hydrogen peroxide catalysed by oxidized carbon materials[J]. Nat Catal, 2018, 1(2): 156-162. |
| 34 | SUN Y, SILVIOLI L, SAHRAIE N R, et al. Activity-selectivity trends in the electrochemical production of hydrogen peroxide over single-site metal-nitrogen-carbon catalysts[J]. J Am Chem Soc, 2019, 141(31): 12372-12381. |
| 35 | QIANG Z, CHANG J H, HUANG C P. Electrochemical generation of hydrogen peroxide from dissolved oxygen in acidic solutions[J]. Water Res, 2002, 36(1): 85-94. |
| 36 | KUO W G. Decolorizing dye wastewater with Fenton's reagent[J]. Water Res, 1992, 26(7): 881-886. |
| 37 | MIKLOS D B, REMY C, JEKEL M, et al. Evaluation of advanced oxidation processes for water and wastewater treatment-a critical review[J]. Water Res, 2018, 139: 118-131. |
| 38 | KREMER M L. The Fenton reaction. dependence of the rate on pH[J]. J Phys Chem A, 2003, 107(11): 1734-1741. |
| 39 | MUSTAIN W E, CHATENET M, PAGE M, et al. Durability challenges of anion exchange membrane fuel cells[J]. Energy Environ Sci, 2020, 13(9): 2805-2838. |
| 40 | MERLE G, WESSLING M, NIJMEIJER K. Anion exchange membranes for alkaline fuel cells: a review[J]. J Membrane Sci, 2011, 377(1): 1-35. |
| 41 | LI W, BONAKDARPOUR A, GYENGE E, et al. Drinking water purification by electrosynthesis of hydrogen peroxide in a power-producing PEM fuel cell[J]. ChemSusChem, 2013, 6(11): 2137-2143. |
| 42 | SIAHROSTAMI S, VILLEGAS S J, BAGHERZADEH MOSTAGHIMI A H, et al. A review on challenges and successes in atomic-scale design of catalysts for electrochemical synthesis of hydrogen peroxide[J]. ACS Catal, 2020, 10(14): 7495-7511. |
| 43 | GAO J, LIU B. Progress of electrochemical hydrogen peroxide synthesis over single atom catalysts[J]. ACS Mater Lett, 2020, 2(8): 1008-1024. |
| 44 | KOPER M T M. Theory of multiple proton-electron transfer reactions and its implications for electrocatalysis[J]. Chem Sci, 2013, 4(7): 2710-2723. |
| 45 | SIAHROSTAMI S, VERDAGUER-CASDEVALL A, KARAMAD M, et al. Activity and selectivity for O2 reduction to H2O2 on transition metal surfaces[J]. ECS Trans, 2013, 58(2): 53-62. |
| 46 | KULKARNI A, SIAHROSTAMI S, PATEL A, et al. Understanding catalytic activity trends in the oxygen reduction reaction[J]. Chem Rev, 2018, 118(5): 2302-2312. |
| 47 | ZAGAL J H, KOPER M T. Reactivity descriptors for the activity of molecular MN4 catalysts for the oxygen reduction reaction[J]. Angew Chem Int Ed, 2016, 55(47): 14510-14521. |
| 48 | SEH Z W, KIBSGAARD J, DICKENS C F, et al. Combining theory and experiment in electrocatalysis: insights into materials design[J]. Science, 2017, 355(6321): eaad4998. |
| 49 | TRIPKOVIC V, SKULASON E, SIAHROSTAMI S, et al. The oxygen reduction reaction mechanism on Pt(111) from density functional theory calculations[J]. Electrochim Acta, 2010, 55(27): 7975-7981. |
| 50 | MONTEMORE M M, VAN SPRONSEN M A, MADIX R J, et al. O2 activation by metal surfaces: implications for bonding and reactivity on heterogeneous catalysts[J]. Chem Rev, 2018, 118(5): 2816-2862. |
| 51 | CHENG J, LIBISCH F, CARTER E A. Dissociative adsorption of O2 on Al(111): the role of orientational degrees of freedom[J]. J Phys Chem Lett, 2015, 6(9): 1661-1665. |
| 52 | TANG J, ZHAO T, SOLANKI D, et al. Selective hydrogen peroxide conversion tailored by surface, interface, and device engineering[J]. Joule, 2021, 5(6): 1432-1461. |
| 53 | CHANG Q, ZHANG P, MOSTAGHIMI A H B, et al. Promoting H2O2 production via 2-electron oxygen reduction by coordinating partially oxidized Pd with defect carbon[J]. Nat Commun, 2020, 11(1): 2178. |
| 54 | JUNG E, SHIN H, LEE B H, et al. Atomic-level tuning of Co-N-C catalyst for high-performance electrochemical H2O2 production[J]. Nat Mater, 2020, 19(4): 436-442. |
| 55 | SHENG H, HERMES E D, YANG X, et al. Electrocatalytic production of H2O2 by selective oxygen reduction using earth-abundant cobalt pyrite (CoS2)[J]. ACS Catal, 2019, 9(9): 8433-8442. |
| 56 | SUN Y, SINEV I, JU W, et al. Efficient electrochemical hydrogen peroxide production from molecular oxygen on nitrogen-doped mesoporous carbon catalysts[J]. ACS Catal, 2018, 8(4): 2844-2856. |
| 57 | JIANG K, BACK S, AKEY A J, et al. Highly selective oxygen reduction to hydrogen peroxide on transition metal single atom coordination[J]. Nat Commun, 2019, 10(1): 3997. |
| 58 | ČOLIĆ V, YANG S, RÉVAY Z, et al. Carbon catalysts for electrochemical hydrogen peroxide production in acidic media[J]. Electrochim Acta, 2018, 272: 192-202. |
| 59 | MURRAY A T, VOSKIAN S, SCHREIER M, et al. Electrosynthesis of hydrogen peroxide by phase-transfer catalysis[J]. Joule, 2019, 3(12): 2942-2954. |
| 60 | ZHANG J, YANG H, GAO J, et al. Design of hierarchical, three-dimensional free-standing single-atom electrode for H2O2 production in acidic media[J]. Carbon Energy, 2020, 2(2): 276-282. |
| 61 | GAO J, YANG H B, HUANG X, et al. Enabling direct H2O2 production in acidic media through rational design of transition metal single atom catalyst[J]. Chem, 2020, 6(3): 658-674. |
| 62 | JIRKOVSKY J S, PANAS I, AHLBERG E, et al. Single atom hot-spots at Au-Pd nanoalloys for electrocatalytic H2O2 production[J]. J Am Chem Soc, 2011, 133(48): 19432-19441. |
| 63 | DEIANA D, VERDAGUER-CASADEVALL A, MALACRIDA P, et al. Determination of core-shell structures in Pd-Hg nanoparticles by STEM-EDX[J]. ChemCatChem, 2015, 7(22): 3748-3752. |
| 64 | SHI Q, ZHU W, ZHONG H, et al. Highly dispersed platinum atoms on the surface of AuCu metallic aerogels for enabling H2O2 production[J]. ACS Appl Energy Mater, 2019, 2(11): 7722-7727. |
| 65 | BRIMAUD S, ENGSTFELD A K, ALVES O B, et al. Structure-reactivity correlation in the oxygen reduction reaction: activity of structurally well defined AuxPt1- x/Pt(111) monolayer surface alloys[J]. J Electroanal Chem, 2014, 716: 71-79. |
| 66 | ZHENG Z, NG Y H, WANG D W, et al. Epitaxial growth of Au-Pt-Ni nanorods for direct high selectivity H2O2 production[J]. Adv Mater, 2016, 28(45): 9949-9955. |
| 67 | CIAPINA E G, LOPES P P, SUBBARAMAN R, et al. Surface spectators and their role in relationships between activity and selectivity of the oxygen reduction reaction in acid environments[J]. Electrochem Commun, 2015, 60: 30-33. |
| 68 | MARKOVIĆ N M, GASTEIGER H A, GRGUR B N, et al. Oxygen reduction reaction on Pt(111): effects of bromide[J]. J Electroanal Chem, 1999, 467(1/2): 157-163. |
| 69 | STAMENKOVIC V, M. MARKOVIC N, ROSS P N. Structure-relationships in electrocatalysis: oxygen reduction and hydrogen oxidation reactions on Pt(111) and Pt(100) in solutions containing chloride ions[J]. J Electroanal Chem, 2001, 500(1/2): 44-51. |
| 70 | GENORIO B, STRMCNIK D, SUBBARAMAN R, et al. Selective catalysts for the hydrogen oxidation and oxygen reduction reactions by patterning of platinum with calix[4]arene molecules[J]. Nat Mater, 2010, 9(12): 998-1003. |
| 71 | HE D, ZHONG L, GAN S, et al. Hydrogen peroxide electrosynthesis via regulating the oxygen reduction reaction pathway on Pt noble metal with ion poisoning[J]. Electrochim Acta, 2021, 371: 137721. |
| 72 | CHOI C H, KWON H C, YOOK S, et al. Hydrogen peroxide synthesis via enhanced two-electron oxygen reduction pathway on carbon-coated Pt surface[J]. J Phys Chem C, 2014, 118(51): 30063-30070. |
| 73 | YANG S, KIM J, TAK Y J, et al. Single-atom catalyst of platinum supported on titanium nitride for selective electrochemical reactions[J]. Angew Chem Int Ed, 2016, 55(6): 2058-2062. |
| 74 | YANG S, TAK Y J, KIM J, et al. Support effects in single-atom platinum catalysts for electrochemical oxygen reduction[J]. ACS Catal, 2017, 7(2): 1301-1307. |
| 75 | SHEN R, CHEN W, PENG Q, et al. High-concentration single atomic Pt sites on hollow CuSx for selective O2 reduction to H2O2 in acid solution[J]. Chem, 2019, 5(8): 2099-2110. |
| 76 | CHOI C H, KIM M, KWON H C, et al. Tuning selectivity of electrochemical reactions by atomically dispersed platinum catalyst[J]. Nat Commun, 2016, 7: 10922. |
| 77 | KIM J H, SHIN D, LEE J, et al. A general strategy to atomically dispersed precious metal catalysts for unravelling their catalytic trends for oxygen reduction reaction[J]. ACS Nano, 2020, 14(2): 1990-2001. |
| 78 | ZHAO J, FU C, YE K, et al. Manipulating the oxygen reduction reaction pathway on Pt-coordinated motifs[J]. Nat Commun, 2022, 13(1): 685. |
| 79 | BONAKDARPOUR A, DAHN T R, ATANASOSKI R T, et al. H2O2 release during oxygen reduction reaction on Pt nanoparticles[J]. Electrochem Solid-State Lett, 2008, 11(11): B208. |
| 80 | INABA M, YAMADA H, TOKUNAGA J, et al. Effect of agglomeration of Pt/C catalyst on hydrogen peroxide formation[J]. Electrochem Solid-State Lett, 2004, 7(12): A474. |
| 81 | GARA M, LABORDA E, HOLDWAY P, et al. Oxygen reduction at sparse arrays of platinum nanoparticles in aqueous acid: hydrogen peroxide as a liberated two electron intermediate[J]. Phys Chem Chem Phys, 2013, 15(44): 19487-19495. |
| 82 | YANG H, KUMAR S, ZOU S. Electroreduction of O2 on uniform arrays of Pt nanoparticles[J]. J Electroanal Chem, 2013, 688: 180-188. |
| 83 | VON WEBER A, BAXTER E T, WHITE H S, et al. Cluster size controls branching between water and hydrogen peroxide production in electrochemical oxygen reduction at Ptn/ITO[J]. J Phys Chem C, 2015, 119(20): 11160-11170. |
| 84 | WANG Y L, GURSES S, FELVEY N, et al. In situ deposition of Pd during oxygen reduction yields highly selective and active electrocatalysts for direct H2O2 production[J]. ACS Catal, 2019, 9(9): 8453-8463. |
| 85 | PRABHAKARAN V, ARGES C G, RAMANI V. Investigation of polymer electrolyte membrane chemical degradation and degradation mitigation using in situ fluorescence spectroscopy[J]. Proc Nat Acad Sci USA, 2012, 109(4): 1029-1034. |
| 86 | 李赫, 李宫, 宫雪, 等. Pt/C催化剂长时间ORR过程性能衰减的机理[J]. 应用化学, 2022, 39(10): 1564-1571. |
| LI H, LI G, GONG X, et al. Research on performance decay mechanism of Pt/C catalyst in long-term ORR test[J]. Chin J Appl Chem, 2022, 39(10): 1564-1571. | |
| 87 | LUO E, CHU Y, LIU J, et al. Pyrolyzed M-Nx catalysts for oxygen reduction reaction: progress and prospects[J]. Energy Environ Sci, 2021, 14(4): 2158-2185. |
| [1] | Yi-Ning DONG, He LI, Xue GONG, Ce HAN, Ping SONG, Wei-Lin XU. Research Progress of Non-Pt-Based Catalysts in Cathode Oxygen Reduction Reaction of Proton Exchange Membrane Fuel Cells [J]. Chinese Journal of Applied Chemistry, 2023, 40(8): 1077-1093. |
| [2] | Rui-Xue ZHENG, Qing-Lei MENG, Li ZHANG, Chang-Peng LIU, Wei XING, Mei-Ling XIAO. Hierarchically Porous Fe-N-C Catalysts for Efficient Electrocatalytic Oxygen Reduction Reaction [J]. Chinese Journal of Applied Chemistry, 2023, 40(8): 1187-1194. |
| [3] | Lian-Cheng HUI, Jian-Xing ZHUANG, Shun XIAO, Mei-Ping LI, Meng-Yuan JIN, Qing LYU. Nickel-Nitrogen-Doped Graphdiyne as an Efficient Catalyst for Oxygen Reduction [J]. Chinese Journal of Applied Chemistry, 2023, 40(8): 1205-1213. |
| [4] | Jing-Xia GAO, Zi-An WANG, Lian-Ming ZHANG, Jian-Ping LI. Research Progress of Macrocyclic Compounds in Highly Selective Molecular Imprinting Recognition System [J]. Chinese Journal of Applied Chemistry, 2023, 40(1): 24-39. |
| [5] | Dan WANG, Xian-Biao HOU, Xing-Kun WANG, Zhi-Cheng LIU, Huan-Lei WANG, Ming-Hua HUANG. Research Progress of Carbon‑Encapsulated Iron‑Based Nanoparticles Electrocatalysts for Zinc‑Air Batteries [J]. Chinese Journal of Applied Chemistry, 2022, 39(10): 1488-1500. |
| [6] | He LI, Gong LI, Xue GONG, Ming-Bo RUAN, Ce HAN, Ping SONG, Wei-Lin XU. Research on Performance Decay Mechanism of Pt/C Catalyst in Long‑Term ORR Test [J]. Chinese Journal of Applied Chemistry, 2022, 39(10): 1564-1571. |
| [7] | Jing TANG, Na ZHANG, Dong-Xu SHI, Fang-Hui ZHANG, Jian-Jie TANG. Synthesis of UiO-66-NH2Grafted Pyridineimine Cobalt Catalyst and Its Catalytic Performance in Ethylene Oligomerization [J]. Chinese Journal of Applied Chemistry, 2022, 39(02): 258-265. |
| [8] | LI Gong, JIN Long-Yi, YAO Peng-Fei, LIU Cong, XU Wei-Lin. Controllability Design of High Performance Oxygen Reduction Catalysts Supported by Platinum Nanoparticles Loaded on Mesoporous Carbon [J]. Chinese Journal of Applied Chemistry, 2021, 38(12): 1639-1646. |
| [9] | LIU Ning, WANG Danfeng, WU Suyun, LIU Shuilin, FU Lin, LIU Yuejin. Catalytic Synthesis of Bisphenol F over Short-Channeled Mesoporous Molecular Sieve Zr-Ce-SBA-15 Supported Acidic Ionic Liquids [J]. Chinese Journal of Applied Chemistry, 2020, 37(9): 1038-1047. |
| [10] | YAO Meiren, WANG Kangjun, ZHANG Yajing, WANG Dongping. Application of Sterically Hindered Chiral (Pyrrolidine Salen) Mn(III) Complex in Asymmetric Epoxidation of Alkenes [J]. Chinese Journal of Applied Chemistry, 2020, 37(8): 889-895. |
| [11] | WEI Zhenye, MENG Junling, WANG Haocong, ZHANG Wenwen, LIU Xiaojuan, MENG Jian. Improving the Electrocatalytic Activity of La2NiO4+δ Cathode by Surface Modification with Conformal Heterojunction [J]. Chinese Journal of Applied Chemistry, 2020, 37(8): 939-951. |
| [12] | LI Maosheng, CHEN Jinlong, TAO Youhua. Regio- and Stereoselective Ring-Opening Metathesis Polymerization of Amino Acid Functionalized Cyclooctenes [J]. Chinese Journal of Applied Chemistry, 2020, 37(3): 280-292. |
| [13] | LIU Qiang, ZHAO Zhenbo, ZHANG Chao, ZHAO Fengyu. Catalytic Conversion of γ-Valerolactone to 1,4-Pentanediol on CuZn/Al2O3 Catalyst [J]. Chinese Journal of Applied Chemistry, 2020, 37(11): 1285-1292. |
| [14] | WANG Lijia,TANG Yong. Research Progress on Asymmetric Synthesis of Donor-Acceptor Cyclopropanes and Their Enantioselective Ring-Opening/Annulation Reactions [J]. Chinese Journal of Applied Chemistry, 2018, 35(9): 1037-1056. |
| [15] | CHEN Si,SUN Lizhen,SHU Xinxin,ZHANG Jintao. Graphene-based Catalysts for Efficient Electrocatalytic Applications [J]. Chinese Journal of Applied Chemistry, 2018, 35(3): 272-285. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||