设计并合成了一系列新型含查尔酮-吲唑杂合衍生物,并对其体外肿瘤活性进行初步研究。 先以2,6-二氟苯腈和吗啉为起始原料,经过取代和环合两步反应合成4-吗啉-3-氨基-1 H-吲唑。 4-吗啉-3-氨基-1 H-吲唑与含羧基的查尔酮中间体与经过酰胺化反应制备了9个新型含查尔酮-吲唑杂合衍生物,其结构经傅里叶变换红外光谱仪(FTIR)、核磁共振波谱仪(NMR)、质谱(MS)和元素分析确证。 采用MTT法,以索拉菲尼为阳性对照药,以人胃癌细胞株(MKN45)和人肺癌细胞株(A549)为测试细胞株对目标化合物进行抗肿瘤活性评价。 结果表明,大部分目标化合物显示了较好的抗肿瘤活性,其中化合物4a和4d活性较好,其抑制人胃癌细胞株MKN45的IC50分别为2.65和3.55 μmol/L,均优于阳性对照药索拉菲尼(IC50=4.69 μmol/L)。
Co-corresponding author:DING Shi, lecturer; Tel/Fax:024-62202469; E-mail:dingshi_destiny@163.com; Research Interests:design and synthesis of antitumor agents
Nine novel indazole-chalcone hybrids(4a-4i) have been synthesized by condensation of substituted 4-(3-oxo-3-phenylprop-1-en-1-yl) benzoic acid with 4-morpholino-1 H-indazol-3-amine prepared from 2,6-difluorobenzonitrile by amination with morpholine and then cyclisation with hydrazine hydrate. Their chemical structures were confirmed by Fourier transform infrared spectrometer(FTIR), nuclear magnetic resonance spectroscopy(NMR), mass spectrometry(MS) and elemental analysis. Compounds(4a-4i) were screened for in vitro antiproliferative activities against human gastric cancer cell line(MKN45) and human lung adenocarcinoma cell line(A549). Most of the designed compounds were found to exhibit potential antiproliferative activities. Among them, compounds 4a and 4d exhibited remarkable inhibitory activity against MKN45 cell lines with IC50 value of 2.65 and 3.55 μmol/L, respectively, which were more potent than that of the positive control sorafenib(IC50=4.69 μmol/L).
In the past few decades, it has been found that compounds containing indazole structural units are an important class of heterocyclic compounds with broad pharmacological activities, such as anti-inflammatory, anti-microbial and anti-cancer activities[1,2,3]. Some indazole-based therapeutic agents have been approved for the treatment of cancers, for example, axtinib[4], linifanib[5], and pazopanib[6]. At the same time, chalcones are an important class of organic synthesis and drug synthesis intermediates due to their broad spectrum of biological activities including anticancer, antioxidant, anti-inflammatory and antiviral activities[7,8,9,10,11,12]. Previous research has not been fully considered to the compounds containing both chalcone and indazole units, which are often used as antitumor active compounds. We combined the chalcone with indazole by using the combination principles, and several fused heterocycle structures which showed excellent potencies were designed and synthesized in our laboratory[13,14]. In order to discover novel bio-activities compounds, we reported the synthesis of a series of substituted indazole-chalcone hybrids and evaluated for their in vitro antiproliferative activities against human gastric cancer cell line(MKN45) and human lung adenocarcinoma cell line(A549).
Unless specified otherwise, all starting materials and reagents were obtained from commercial suppliers without further purification. All melting points were taken on a Beijing Taike X-4 microscopy melting point apparatus and were uncorrected. Nuclear magnetic resonance spectroscopy(NMR) spectra were recorded on a Bruker Biospin 600 MHz or Bruker Biospin 300 MHz instrument(Bruker Bioscience, Billerica, MA) using tetramethylsilane(TMS) as the internal standard. IR spectra were recorded as KBr pellets on a PerkinElmer Spectrum one Fourier transform infrared spectrometer(FTIR)(Perkin-Elmer, MA). Mass spectrometry(MS) spectra were obtained on an 6460 QQQ mass spectrometer(Agilent, Palo Alto, CA) analysis system. Elemental analysis was carried out on a Carlo Erba 1108 analyser(Carlo Erba, Milan, Italy) and are found within the range of theoretical value.
1.2.1 Synthesis of 2 -fluoro-6 -morphinylbenzonitrile(1)
To the suspension of 2,6-difluorobenzonitrile(20.0 g, 144 mmol), K2CO3(40.0 g, 289 mmol) in dimethyl sulphoxide(80 mL), morpholine(13.2 g, 152 mmol) was added slowly. The mixture was heated at 90 ℃ for 3 h. After completion of the reaction as indicated by TLC, the reaction mixture was carefully poured into stirring water(500 mL), then filtered. The filter cake was washed with water, and dried to afford 18.0 g of 2-fluoro-6-morpholinobenzonitrile in 90.0% yield. mp 69~70 ℃; IR(KBr), σ/cm-1:3096, 2972, 2950, 2879, 2869, 2828, 2226(CN), 1605, 1567, 1474, 1447, 1373, 1257, 1230, 1159, 1114, 1082, 1058, 999, 927, 875, 798, 732, 690; MS(ESI) m/z(%):207.3[M+H]+; Anal. Calcd for C11H11FN2O(found)/%:C 64.07(63.99), H 5.38(5.42), N 13.58(13.63).
1.2.2 Synthesis of 3 -amine-4 -morpholino-1H -indazole(2)
A solution of 2-fluoro-6-morpholinobenzonitrile(15.0 g, 73 mmol) and hydrazine hydrate(80%, 50 mL) in N-Methyl pyrrolidone(50 mL) was heated at 70 ℃ for 25 h. After cooling to room temperature, the reaction mixture was carefully poured into cold water(400 mL), then filtered. The filter cake was washed with water, and dried to afford 14.50 g of 4-morpholino-1 H-indazol-3-amine in 97.0% yield. mp 206~208 ℃; IR(KBr), σ/cm-1:3475,3374,3272,2950,2854,2836,1603,1526,1509,1452,1389,1374,1356,1261,1236,1108,1060,1014,921,893,751,689;1H NMR(600 MHz,CDCl3), δ:7.30~7.17(m,1H,Ar—H),7.00(d, J=8.4 Hz,1H,Ar—H),6.58(d, J=7.2 Hz,1H,Ar—H),6.00~5.00(br,3H),3.97~3.86(m,4H,morpholine-H),3.18~3.03(m,4H,morpholine-H);MS(ESI) m/z(%):219.2[M+H]+; Anal. Calcd for C11H14N4O(found)/%:C 60.53(60.66),H 6.47(6.40),N 25.67(25.74).
1.2.3 General procedure for preparation of Chalcone-4 -formic acid intermediate(3)
Sodium hydroxide solution(1.0 mol/L, 40 mL) was added slowly to a suspension of 4-formylbenzoic acid(3.0 g) and acetophenone(2.5 g) in methanol(150 mL) with stirred at room temperature for 12 h, the reaction mixture was carefully poured into water(100 mL), adjusted to pH 2~4 with hydrochloric acid(1.0 mol/L) and then filtered. The filter cake was washed with water to neutrality and dried to afford compound 3 as a white solid powder.
4-(3-oxo-3-phenylpropenyl)-benzoic acid(3a):Yield 74.3%; mp 228~230 ℃; IR(KBr), σ/cm-1:2673,2557,1680.0,1606.7,1313.5,1215.2,1018.4,761.9,678.0; MS(ESI) m/z:251.1[M-H]-; Anal. Calcd for C16H12O3(found)/%:C 76.18(76.24),H 4.79(4.84).
4-[3-(4-methoxyphenyl)acryloyl]benzoic acid(3b):Yield 81.5%; mp 247~249 ℃; IR(KBr), σ/cm-1:3416,1674,1605,1423,1306,1173,1015,831,777;MS(ESI) m/z:281.1[M-H]-; Anal. Calcd for C17H14O4(found)/%:C 72.33(72.41),H 5.00(5.04).
4-[3-( p-tolyl)acryloyl)benzoic acid(3c):Yield 79.6%; mp 252~254 ℃; IR(KBr), σ/cm-1:3410,2365,1682,1605,1420,1302,1219,1022,766;MS(ESI) m/z:265.1[M-H]-; Anal. Calcd for C17H14O3(found)/%:C 76.68(76.73),H 5.30(5.34).
4-[3-(4-fluorophenyl)acryloyl]benzoic acid(3d):Yield 71.1%; mp 255~257 ℃; IR(KBr), σ/cm-1:3416,1684,1597,1502,1421,1225,1020,822,758;MS(ESI) m/z:269.1[M-H]-; Anal. Calcd for C16H11FO3(found)/%:C 71.11(71.18),H 4.10(4.12).
4-[3-(2-fluorophenyl)acryloyl]benzoic acid(3e):Yield 68.2%; mp 267~269 ℃; IR(KBr), σ/cm-1:3328,1693,1609,1414,1327,1281,1211,1111,1024,758,660; MS(ESI) m/z:269.1[M-H]-; Anal. Calcd for C16H11FO3(found)/%:C 71.11(71.15),H 4.10(4.14).
4-[3-(3-chlorophenyl)acryloyl]benzoic acid(3f):Yield 62.9%; mp 230~232 ℃; IR(KBr), σ/cm-1:3074,2679,2558,1676,1607,1423,1310,1209,775,719; MS(ESI) m/z:285.0[M-H]-; Anal. Calcd for C16H11ClO3(found)/%:C 67.03(67.09),H 3.87(3.91).
4-[3-(3,4-dimethoxyphenyl)acryloyl]benzoic acid(3g):Yield 73.4%; mp 218~220 ℃; IR(KBr), σ/cm-1:3078,2837,2556,1678,1591,1510,1423,1265,1157,1026,972,758; MS(ESI) m/z:311.1[M-H]-; Anal. Calcd for C18H16O5(found)/%:C 69.22(69.19),H 5.16(5.21).
4-[3-(2,4-dichlorophenyl)acryloyl]benzoic acid(3h):Yield 82.4%; mp 260~262 ℃; IR(KBr), σ/cm-1:3415,1690,1593,1416,1196,812,671; MS(ESI) m/z:319.0[M-H]-; Anal. Calcd for C16H10Cl2O3(found)/%:C 59.84(59.92),H 3.14(3.18).
4-(3-(2,6-dichloro-3-fluorophenyl)acryloyl)benzoic acid(3i):Yield 65.6%; mp 235~237 ℃; IR(KBr), σ/cm-1:2943,2833,2664,2546,1676,1591,1512,1423,1263,1159,1026,972,758; MS(ESI) m/z:337.0[M-H]-; Anal. Calcd for C16H9Cl2FO3(found)/%:C 56.67(60.66),H 2.67(2.68).
1.2.4 General procedure for preparation of target compounds(4a-4i)
A mixture of the 3-amino-4-morpholino-1 H-indazole(2.0 mmol), substituted chalcone-4-carboxylic acid(2.4 mmol), 2-(7-oxobenzotriazole)- N, N, N', N'-tetramethyluron hexafluorophosphate(HATU)(2.4 mmol) and triethylamine(TEA)(4.80 mmol) in 15 mL anhydrous N, N-dimethylformamide(DMF) was stirred at room temperature for 12 h. The reaction mixture was slowly added to potassium carbonate aqueous solution(20%, 100 mL), extracted by dichloromethane three times(50 mL×3), combined organic phase, which was washed three times with 20% aqueous potassium carbonate solution, the organic layer was washed two times with saturated saline, and dried over anhydrous sodium sulfate, then filtered and evaporated the dichloromethane under reduced-pressure to give crude product which was recrystallised from ethanol to give the target compounds.
3-[4-(3-amino-4-morpholino-1 H-indazole-1-carbonyl)phenyl]-1-phenylprop-2-en-1-one(4a):Yield 59.2%; mp 194~197 ℃; IR(KBr), σ/cm-1:3445,3406,2923,2851,1653,1624,1602,1434,1373,1336,1215,1118,1015,886,787,751;1H NMR(600 MHz,DMSO- d6), δ:8.20(d, J=7.5 Hz,2H),8.14~7.95(m,6H),7.83(d, J=15.6 Hz,1H),7.74~7.67(m,1H),7.65~7.51(m,3H),7.09(d, J=7.6 Hz,1H),6.07(s,2H),3.85(s,4H),3.01(s,4H);13C NMR(150 MHz,DMSO- d6), δ:189.57(s),166.23,152.98,148.88,143.27,142.19,137.82,137.55,136.31,133.71,131.55,130.86,129.24,129.01,128.51,124.12,114.42,114.10,111.56,66.34,53.57; MS(ESI) m/z(%):475.2[M+Na]+; Anal. Calcd for C27H24N4O3(found)/%:C 71.67(71.74),H 5.35(5.41),N 12.38(12.45).
3-[4-(3-amino-4-morpholino-1 H-indazole-1-carbonyl)phenyl]-1-(4-methoxyphenyl)prop-2-en-1-one(4b):Yield 52.3%; mp 177~179 ℃; IR(KBr), σ/cm-1:3413,2923,2846,1659,1608,1418,1388,1333,1259,1226,1168,1116,1020,886,825,760;1H NMR(600 MHz,DMSO- d6), δ:8.21(d, J=8.0 Hz,2H),8.14~7.97(m,6H),7.78(d, J=15.5 Hz,1H),7.61~7.48(m,1H),7.17~7.04(m,3H),6.07(s,2H),3.89(s,3H),3.85(s,4H),3.01(s,4H);13C NMR(150 MHz,DMSO- d6), δ:187.71,166.25,163.76,152.97,148.87,142.41,142.20,137.74,136.09,131.53,131.44,130.86,130.72,128.38,124.12,114.47,114.39,114.09,111.57,66.34,55.99,53.57; MS(ESI) m/z(%):505.2[M+Na]+; Anal. Calcd for C27H26N4O4(found)/%:C 69.70(69.77),H 5.43(5.41),N 11.61(11.68).
3-[4-(3-amino-4-morpholino-1 H-indazole-1-carbonyl)phenyl]-1-( p-tolyl)prop-2-en-1-one(4c):Yield 39.5%; mp 180~182 ℃; IR(KBr), σ/cm-1:3405,3291,2956,2840,1653,1624,1602,1544,1435,1373,1336,1242,1217,1118,1017,888,789,751,677;1H NMR(600 MHz,DMSO- d6), δ:8.15~7.96(m,8H),7.80(d, J=15.6 Hz,1H),7.56(t, J=7.9 Hz,1H),7.41(d, J=7.8 Hz,2H),7.09(d, J=7.7 Hz,1H),6.07(s,2H),3.85(s,4H),3.01(s,4H),2.42(s,3H);13C NMR(150 MHz,DMSO- d6), δ:188.96,166.24,152.97,148.88,144.19,142.90,142.19,137.63,136.21,135.32,131.54,130.86,129.80,129.16,128.45,124.14,114.41,114.09,111.56,66.34,53.57,21.60;MS(ESI) m/z(%):489.2[M+Na]+; Anal. Calcd for C28H26O3N4(found)/%:C 72.09(72.69),H 5.62(5.57),N 12.01(12.07).
3-[4-(3-amino-4-morpholino-1 H-indazole-1-carbonyl)phenyl]-1-(4-fluorophenyl)prop-2-en-1-one(4d):Yield 46.2%; mp 183~185 ℃; IR(KBr), σ/cm-1:3429,3401,2917,2851,1656,1605,1541,1500,1434,1412,1381,1328,1217,1155,1115,1023,889,828;1H NMR(600 MHz,DMSO- d6), δ:8.30(dd, J=8.5,5.6 Hz,2H),8.14~7.97(m,6H),7.83(d, J=15.6 Hz,1H),7.57(t, J=8.0 Hz,1H),7.43(t, J=8.7 Hz,2H),7.10(t, J=12.2 Hz,1H),6.07(s,2H),3.89~3.79(m,4H),3.02(s,4H);13C NMR(150 MHz,DMSO- d6), δ:188.08,166.39,165.55,152.98,148.88,143.43,142.18,137.50,136.35,134.51,132.05,131.55,130.84,128.54,123.88,116.26,114.42,114.10,111.56,66.33,53.57; MS(ESI) m/z(%):493.1[M+Na]+; Anal. Calcd for C27H23FN4O3(found)/%:C 68.93(68.99),H 4.93(4.98),N 11.91(11.98).
3-[4-(3-amino-4-morpholino-1 H-indazole-1-carbonyl)phenyl]-1-(2-fluorophenyl)prop-2-en-1-one(4e):Yield 41.1%; mp 172~174 ℃;IR(KBr), σ/cm-1:3450,2956,2923,2846,1660,1609,1547,1440,1383,1336,1264,1206,1017,888,760,661;1H NMR(600 MHz,DMSO- d6), δ:8.11(d, J=8.2 Hz,1H),7.99(d, J=8.3 Hz,2H),7.93(d, J=8.3 Hz,2H),7.84(td, J=7.5 Hz, J=1.4 Hz,1H),7.71(s,2H),7.61(dd, J=15.9 Hz, J=2.2 Hz,1H),7.56(t, J=8.0 Hz,1H),7.47~7.35(m,2H),7.09(d, J=7.8 Hz,1H),6.07(s,2H),3.96~3.73(m,4H),3.01(s,4H);13C NMR(150 MHz,DMSO- d6), δ:189.22,166.16,160.72,152.99,148.89,143.74,142.16,137.13,136.55,134.89,131.57,131.00,130.98,130.92,128.44,127.49,127.12,125.31,117.18,117.03,114.46,114.10,111.56,66.33,53.57; MS(ESI) m/z:493.1[M+Na]+; Anal. Calcd for C27H23O3FN4(found)/%:C 68.93(69.01),H 4.93(4.97),N 11.91(11.97).
3-[4-(3-amino-4-morpholino-1 H-indazole-1-carbonyl)phenyl]-1-(3-chlorophenyl)prop-2-en-1-one(4f):Yield 46.1%; mp 168~170 ℃; IR(KBr), σ/cm-1:3457,2923,2851,1660,1641,1602,1415,1382,1336,1240,1207,1115,886,839,795;1H NMR(600 MHz,DMSO- d6), δ:8.24(s,1H),8.03~8.18(m,5H),8.00(d, J= 8.2 Hz,2H),7.86(d, J=15.6 Hz,1H),7.77(dd, J=7.9,1.1 Hz,1H),7.64(t, J=7.9 Hz,1H),7.57(t, J=8.0 Hz,1H),7.09(d, J=7.8 Hz,1H),6.07(s,2H),3.93~3.78(m,4H),3.02(s,4H);13C NMR(150 MHz,DMSO- d6), δ:188.32,166.22,152.99,148.87,144.05,142.18,139.62,137.39,136.49,134.28,133.41,131.54,131.20,130.82,128.69,128.66,127.62,123.68,114.42,114.10,111.56,66.34,53.56; MS(ESI) m/z(%):509.1[M+Na]+; Anal. Calcd for C27H23ClN4O3(found)/%:C 66.60(66.69),H 4.76(4.83),N 11.51(11.62).
3-[4-(3-amino-4-morpholino-1 H-indazole-1-carbonyl)phenyl]-1-(3,4-dimethoxyphenyl)prop-2-en-1-one(4g):Yield 50.5%; mp 164~166 ℃; IR(KBr), σ/cm-1:3415,2917,2846,1648,1621,1604,1514,1418,1383,1259,1023,888,757;1H NMR(600 MHz,DMSO- d6), δ:8.14~8.06(m,2H),8.02(q, J=8.4 Hz,4H),7.96(dd, J=8.4,1.8 Hz,1H),7.79(d, J=15.6 Hz,1H),7.64(d, J=1.7 Hz,1H),7.56(t, J=8.0 Hz,1H),7.14(d, J=8.5 Hz,1H),7.08(d, J=7.8 Hz,1H),6.07(s,2H),3.89(s,3H),3.88(s,3H),3.86~3.82(m,4H),3.02(br,4H);13C NMR (150 MHz,DMSO- d6), δ:187.69,166.25,153.79,152.96,149.24,148.88,142.34,142.20,137.76,136.07,131.55,130.86,130.76,128.41,124.07,123.99,114.42,114.09,111.56,111.32,111.15,66.34,56.21,56.02,53.57; MS(ESI) m/z(%):535.2[M+Na]+; Anal. Calcd for C29H28N4O5(found)/%:C 67.96(68.03),H 5.51(5.58),N 10.93(11.01).
3-[4-(3-amino-4-morpholino-1 H-indazole-1-carbonyl)phenyl]-1-(2,4-dichlorophenyl)prop-2-en-1-one(4h):Yield 43.6%; mp 194~197 ℃; IR(KBr), σ/cm-1:3416,2917,2851,1673,1657,1590,1416,1376,1326,1241,1209,1111,1067,1019,888,826,763;1H NMR(600 MHz,DMSO- d6), δ:8.10(d, J=8.2 Hz,1H),7.97(d, J=8.3 Hz,2H),7.92(d, J=8.3 Hz,2H),7.82(d, J=1.7 Hz,1H),7.68(d, J=8.2 Hz,1H),7.61(dd, J=8.2 Hz, J=1.8 Hz,1H),7.55(dd, J=15.6 Hz, J=7.2 Hz,2H),7.42(d, J=16.2 Hz,1H),7.08(d, J=7.8 Hz,1H),6.06(s,2H),3.91~3.77(m,4H),3.01(s,4H);13C NMR(150 MHz,DMSO- d6), δ:192.59,166.08,153.00,148.88,145.74,142.16,137.58,136.91,136.74,136.25,131.75,131.57,131.31,130.89,130.17,128.58,128.10,127.87,114.45,114.09,111.56,66.33,53.56; MS(ESI) m/z:543.1[M+Na]+; Anal. Calcd for C27H22O3Cl2N4(found)/%:C 62.20(62.28),H 4.25(4.32),N 10.75(10.84).
3-[4-(3-amino-4-morpholino-1 H-indazole-1-carbonyl)phenyl]-1-(2,6-dichloro-3-fluorophenyl)prop-2-en-1-one(4i):Yield 52.7%; mp 196~198 ℃; IR(KBr), σ/cm-1:3441,2923,2851,1382,1240,1113,891,839;1H NMR(600 MHz,DMSO- d6), δ:8.10(d, J=8.2 Hz,1H),8.00~7.89(m,4H),7.75~7.63(m,2H),7.60~7.49(m,2H),7.33(d, J=16.4 Hz,1H),7.09(d, J=7.8 Hz,1H),6.05(s,2H),3.87~3.80(m,4H),3.01(s,4H);13C NMR(150 MHz,DMSO- d6), δ:191.61,166.01,162.67,156.92,153.00,148.88,148.07,142.14,139.20,137.15,136.55,131.58,130.87,130.62,128.82,127.62,125.98,119.18,119.02,118.48,114.47,114.09,111.55,66.32,53.56; MS(ESI) m/z:561.1[M+Na]+; Anal. Calcd for C27H21O3FCl2N4(found)/%:C 60.12(60.25),H 3.92(4.03),N 10.39(10.52).
The A549 or MKN45 cancer cell lines were cultured in minimum essential medium(MEM) supplement with 10% fetal bovine serum(FBS). Approximate 4×103 cells, suspended in MEM medium, were plated onto each well of a 96-well plate and incubated in 5% CO2 at 37 ℃ for 24 h. The tested compounds at the indicated final concentrations were added to the culture medium and the cell cultures were continued for 72 h. Fresh MTT was added to each well at a terminal concentration of 5 μg/mL, and incubated with cells at 37 ℃ for 4 h. The formazan crystals were dissolved in 100 mL DMSO each well, and the absorbency at 492 nm (for absorbance of MTT formazan) and 630 nm(for the reference wavelength) was measured with an ELISA reader. All compounds were tested three times in each of the cell lines. The results expressed as IC50(inhibitory concentration 50%) were the averages of three determinations and calculated by using the Bacus Laboratories Incorporated Slide Scanner(Bliss) software.
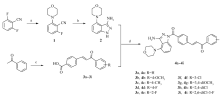
The synthetic methods for compounds 4a-4i are outlined in Scheme 1. The condensation of 2,6-difluorobenzonitrile with morpholine in dimethyl sulfoxide afforded intermediate 2-fluoro-6-morphinylbenzonitrile(1) as white crystalline solid. The cyclisation of compound 1 with hydrazine hydrate in N-methylpyrrolidone at reflux for 23 h gave 3-amine-4-morpholino-1 H-indazol(2). A series of substituted 4-(3-oxo-3-phenylprop-1-en-1-yl) benzoic acids(3) were synthesized by condensation of 4-formylbenzoic acid with different substituted acetophenone. The condensation of compound 2 with compound 3 in N, N-dimethylformamide afforded target compounds 4a-4i. Compounds 4a-4i were appropriately established by spectroscopic and analytical methods. All data were consistent with the structures of compounds 4a-4i, for example, IR shows the peak at about 1653 cm-1 results from the carbonyl stretching vibration of compound 4a. The1H NMR spectrum for compound 4a exhibits a broad singlet at δ 6.07, corresponding to the NH2 proton at position 3 of indazole. In the mass spectrum of compound 4a, the peak appeared at m/z 475.2([M+Na]+, 100%), which is in accordance with its molecular formula. IR,1H NMR, MS and elemental analyses of the target compounds confirmed their structural integrity. The existing chalcones in compounds 4a-4i could lead to E or Z isomeric forming. According to the reported motif of chalcones, the coupling constants of vinylic protons were observed for the E configuration( J>15.00 Hz) and Z configuration( J<12.00 Hz)[15,16]. The coupling constants( J≈15.6 Hz) of the vinylic system confirm the E-configuration for all synthesized compounds 4a-4i. For example, compound 4a showed a doublet peak at 7.83, which have coupling constant value 15.60 Hz corresponding to the E-configuration of chalcone. The E-configuration of compounds 4a-4i maybe due to the strong steric effects between the vinylic phenyl ring and the carbonyl group.
All the nine newly synthesized compounds(4a-4i) were screened for their in vitro antiproliferative activity by the MTT-based assay using sorafenib as a positive control. They were tested against two human cancer cell lines(MKN45 and A549). The results were expressed as IC50 values and summarized inTable 1. All the target compounds showed moderate-to-excellent antiproliferative activity against different cancer cells and some exhibited more or similar potent activities against certain cancer lines in comparison with sorafenib, indicating that the combination of indazole and chalcone moiety in one molecular maintained potent antiproliferative. More importantly, most of the compounds were more potent against MKN45 cell line than against A549 cell line(except for compounds 4f and 4i). The most promising compounds 4a and 4d exhibited more potent antiproliferative activity against A549 cell line than the positive control sorafenib with IC50 values of 2.65 and 3.55 μmol/L, respectively.
| Table 1 In vitro antiproliferative activity test of target compounds on MKN45 and A549 cell lines |
In summary, a series of novel indazole-chalcone hybrids were synthesized and investigated for their in vitro antiproliferative activity against two human cancer cell lines MKN45 and A549. Most compounds showed moderate-to-excellent activities against all cancer cell lines, indicating that the combination of indazole and chalcone moiety in one molecular maintained potent antiproliferative activities. The most two promising compounds 4a and 4d showed antiproliferative activities with IC50 values of 2.65 and 3.55 μmol/L against MKN45 cell lines, respectively. More importantly, it provided novel scaffolds for anticancer agents research. Further studies on structural optimization and biological activities about these derivatives are still underway in our laboratory and will be reported in the future.
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|