超疏水-超疏油材料在防污、防水、防油等领域有广泛的应用前景而引起人们极度关注。 本文用全氟辛酸溶液浸泡锌粉制得超疏水-超疏油锌粉,用聚乙烯醇胶将超疏水-超疏油锌粉粘合、固定到玻璃、木头、塑料、不锈钢、纸片、石头表面后可制得超疏水-超疏油表面,水滴、油滴在其表面的接触角均超过150°。 锌粉与全氟辛酸反应后生成Zn[CF3(CF2)6COO]2,氟代长链烷基的低表面能化学组成与微纳米粗糙结构的协调作用使其表现出超疏水、超疏油性能。 相关研究有望为超双疏材料的设计、制备及其在自清洁、防水防油及抗污等领域的应用提供借鉴。
Superhydrophobic-superoleophobic materials have received considerable attention due to their wide potential applications in the fields of anti-fouling, water-proof and oil-proof. In this paper, superhydrophobic-superoleophobic zinc spheres are first prepared by immersing the zinc spheres into the solution of perfluorooctanoic acid, and then the superhydrophobic-superoleophobic surfaces are obtained by pasting the obtained superhydrophobic-superoleophobic zinc spheres on substrates of glass, wood, plastic, stainless steel, paper and stone. The contact angles of water and oil droplets on these surfaces all exceed 150°. Zinc spheres react with perfluorooctanoic acid to form zinc perfluorooctanoate, and the coordinate effects of micro/nanometer rough structures and chemical composition with low surface energy of the long fluoroalkyl lead to the formation of superhydrophobicity and superoleophobicity. The research may offer a new idea for the design and fabrication of superamphiphobic materials, as well as their application in self-cleaning, waterproof, oil-proof and anti-contamination.
In the past decades, inspired by the self-cleaning and water repellency ability of lotus leaf[1,2], biomimetic construction of surfaces with special wettability have aroused worldwide interests[3,4,5,6]. Wetting behavior is a very important aspect of surface chemistry, which is often characterized by the measurements of contact angle(CA) and sliding angle(SA) of a liquid on the solid surface. For a solid surface, when the CA of water or oil on it is higher than 150°, it is defined as superhydrophobic or superoleophobic, respectively[7]. On the other hand, when the CA of water or oil on a surface is lower than 5°, it is normally called superhydrophilic or superoleophilic[8]. Likewise, a surface with superhydrophobic and superoleophobic properties is called superamphiphobic[9,10]. Recently, a great deal of interests have been focused on the development of superamphiphobic surfaces on account of their enormous and diverse potential applications in the fields of super-antiwetting[11], self-cleaning[12], anti-bacterial activity[13], corrosion resistance[14], and oil droplets manipulation[15]. To date, three strategies, “pre-roughening plus post-fluorinating”, “pre-fluorinating plus post-roughening” and combined approaches, have been employed to fabricate superamphiphobic surfaces[4,10].
Compared with conventional techniques of obtaining a superhydrophobic surface by roughening and modifying the surface with low surface energy[16], the construction of superoleophobic surface is much more difficult. As a matter of fact much lower surface energy and peculiarly shaped structures with “re-entrant”, “overhanging”, or “mushroom-like” are absolutely necessary in preparing superamphiphobic surfaces[17] because the surface tensions of oil is much smaller than that of water. Currently, techniques used for roughness in constructing superamphiphobic surfaces include etching[18], lithography[19], sputter deposition[20], sol-gel synthesis and so on[21,22]. Furthermore, low surface energy materials, such as 1 H,1 H,2 H,2 H-perfluorooctylphosphate(PFOP)[23], 1 H,1 H,2 H,2 H-perfluorooctanoic acid(PFOA)[18], 1 H,1 H,2 H,2 H-perfluorooctyl trichlorosilane(PFOTS)[24], 1 H,1 H,2 H,2 H-perfluorodecyl trichlorosilane(PFDTS)[25], 1 H,1 H,2 H,2 H-perfluorodecane-1-thiol(PFDSH)[22], and 1 H,1 H,2 H,2 H-perfluorodecyl acrylate(PFDAE) are also needed[26]. In the past decades, great achievements have been made in the fabrication of superamphiphobic materials. However, it still remains a challenge to create superamphiphobic surfaces because of the constructing process with severe conditions, tedious techniques, and expensive materials as well as poor durability. Therefore, how to design and construct superamphiphobic surface via a facile, low-cost, time-saving and effective way is still in high demand.
Jiang's group prepared superamphiphobic surfaces upon common engineering metals and their alloys by taking advantage of an electrochemical reaction in perfluorocarboxylic acid solution[27]. Subsequently, the group constructed a superamphiphobic surface on copper substrate by a one-pot method of electrodeposition by employing the electrolyte of ethanol solution of nonadecafluorodecanoic acid[28]. Compared with the traditional approaches of constructing superamphiphobic surfaces, the one-pot method presented above is simple and convenient, but it is time-consuming and a special apparatus as well as metal or alloys substrate is needed. Here, we present an approach for preparing superamphiphobic zinc particles by a facile, low cost of a one-step solution-immersion process. The zinc particles with superamphiphobicity can be applied to construct superhydrophobic and superoleophobic coating on many substrates, such as metal, glass, cotton fabric, stone, paper, wood strip and plastic. The simple approach may open up a new avenue for the industrial construction of superamphiphobic materials with self-cleaning and anticorrosion properties, especially for the oil pipelines of metal or metal alloys laid in deep sea water.
Zinc spheres with analytical purity were obtained from Beijing Pinggu Shuangyan Chemical Factory. Perfluorooctanoic acid(PFOA, CF3(CF2)8COOH) with a 90% purity was purchased from Aladdin Company(Shanghai, China). Polyvinyl alcohol(PVA 17-99) was supplied from Organic Chemical Plant of Beijing Eastern Petrochemical Company Limited. Golden dragon fish oil(ARAWANA, Xingping Food Industry Co., Ltd. Xianyang City, Shanxi Province), garlic acid(Yue Qian, Yueqian Food Industry Co., Ltd. Tengzhou City, Shandong Province ) and mustard oil(Xiangmanyu Condiment co., Ltd. Zhangye City, Gansu Province)
5.0 g zinc particles were immersed in a 50 mL ethanol solution of 0.02 mol/L PFOA under stirring condition at room temperature for 2 days. Then, zinc particles were taken out and thoroughly rinsed with ethanol for three times, and finally dried at 80 ℃ for 24 h.
Glue of polyvinyl alcohol(PVA) was prepared according to the following methods:5.0 g polyvinyl alcohol was first added into 100 mL beaker filled with 90.0 mL distilled water, then the beaker was placed into a water bath at 90 ℃ under stirring condition until the PVA was thoroughly dissolved.
Surface morphologies were obtained by using a Quanta 450 scanning electron microscopy(SEM, FEI, USA). The chemical composition of samples was investigated with a 200 Fourier-transform infrared spectrophotometer(FTIR, Thermo Nicolet ) and PHI-5702 X-ray photoelectron spectrometer(XPS, Physical Electronics, USA) using Al Kα radiation with a reference of C1 s at 284.6 eV. The CA and SA were measured with approximately 8 μL water/oil droplets by using a SL200KS contact angle meter(Kino, USA) equipped with a video camera and a tilting stage, and the reported value are the average of five measurements obtained from different position of one sample surface.
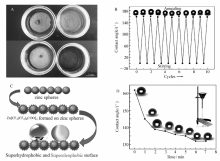
Fig.1A~1C shows SEM images of the pristine zinc particles without any further treatment. It was observed that the diameters of zinc spheres vary from to 12 μm.Fig.1C is an image of single zinc microsphere with higher resolution, it reveals that the surface of the pristine zinc sphere is relatively smooth. However, the surface of zinc microspheres becomes rough after being immersed in an ethanol solution of PFOA for 2 days, as shown inFig.1D~1F. After immersion, the diameters of zinc spheres do not change obviously, but the zinc carboxylate nanosheets start to grow upwards.Fig.1F is a higher resolution image, it shows that zinc microsphere surfaces are composed of numerous nanosheets with petal-like structures, which is 1~1.6 μm wide, and 100~500 nm apart. The morphologies of immersion treated zinc spheres with hierarchical structures of micro/nanometer scale are thought to give rise to the superhydrophobic/superoleophobic behaviors.
 | Fig.1 SEM images of pristine zinc spheres(A~C) and zinc spheres after being treated by solution immersion(D~F) at different magnifications |
The chemical reaction between zinc spheres and PFOA in ethanol solution is the same as the reaction between zinc foil and PFOA[27]. According to the literature[27], zinc particles can be naturally and slowly oxidized by dissolved oxygen in ethanol solution, but the n-perfluorooctanoic acid dissolved in ethanol can form an acidic environment which may catalyze the oxidization reaction of zinc spheres. Therefore, in the presence of PFOA, the spontaneous oxidation process between zinc and oxygen can be drastically accelerated. In PFOA solution, Zn2+ ions are continuously released from zinc spheres while O2 is reduced because of the following reaction (1) proceeds:
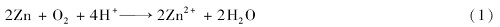
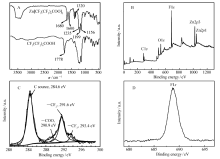
The released zinc ions can be immediately coordinated by PFOA molecules, forming zinc perfluorooctanoate due to the reaction (2) below:
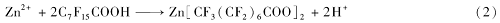
In the reaction process, zinc perfluorooctanoate is formed and slowly adsorbed onto the newly exposed surface of zinc particles until the coating of zinc perfluorocarboxylate with flower-sheets structures is fully formed on the surface of zinc spheres leading to a superamphiphobic surface, which prevents the continuous release of Zn2+ ions from zinc particles.
Pristine zinc spheres are originally superhydrophilic and superoleophilic, both water and oil droplets can spread over the surface completely, as shown in the insets ofFig.2A and Fig.2F, respectively. However, the wettability of zinc spheres is switched from superhydrophilicity to superhydrophobicity after being treated by solution immersion, and the water contact angle on the zinc spheres increased from approximately 0° to about 162°, as shown inFig.2A. Water droplets are hardly able to stick to the zinc spheres surface, the water droplets begin to roll off easily with a tight tilt, and the water sliding angle, which is defined as the critical angle where a water begins to slide down an inclined plate, is about 4°, as shown inFig.2B. Moreover, the superhydrophobic zinc spheres are also superoleophobic, and the contact angles of Golden Dragon Fish oil, garlic oil and mustard oil on the zinc spheres are about 155°, 158° and 151°, respectively,as shown inFig.2C~2E. In addition, the surface also exhibits super-wetting to some organic liquid with low surface tension and diesel oil, as shown inFig.2F~2H, the contact angle of dodecane(surface tension γ=25.3 mN/m), hexadecane( γ=27.5 mN/m) and diesel oil on the zinc spheres is about 151°, 160° and 150°, respectively, and the sliding angle of dodecane droplets on the zinc spheres is about 9°. Higher contact angle and lower sliding angle indicate that the surface of treated zinc particles exhibits self-cleaning property. It is well known that the superhydrophobicity/superoleophobicity is governed by the hierarchical micro-nanostructures and the low surface energy chemical composition. Hierarchical structures of zinc perfluorooctanoate not only decrease the contacting area between the solid and liquid but also decrease the continuity of the three-phase contact line at the solid liquid interface, and which eventually causes the performance of higher contact angle and lowers sliding angle[27]. Herein, FTIR was employed to verify the chemical composition of Zn[CF3(CF2)6COO]2 on zinc spheres. Compared with the FTIR spectra of PFOA inFig.3A, the free COO band of PFOA at 1778 cm-1 is completely disappeared and two new bands appear at 1680 and 1600 cm-1, which corresponds to the C=Ostretching vibration of coordinated COO moieties to Zn2+ ions formed during the immersion treatment of zinc spheres, and the bands at 1320, 1230, 1190, and 1145 cm-1 , standing for the C—F stretching vibration of the —CF3 and —CF2 groups do not obviously change. In addition to FTIR, XPS was also employed to further confirm the formation of Zn[CF3(CF2)6COO]2 on zinc spheres. As shown inFig.3B, elements contained on the superamphiphobic zinc surface are C, F, O and Zn, and the relative content of F and C is relatively high(about 34.2% and 42.3%, respectively).Fig.3C displays the high-resolution XPS of C1 s, there are three types of carbon in the treated zinc spheres besides the peak of carbon source locating at 284.6 eV, and the peaks at 290.9, 291.6 and 293.4 eV are assigned to the —COO-, —CF2 and —CF3 groups, respectively[27]. In addition, the strong F1 s peak locating at 688.5 eV is attributed to the fluorine atom of CF3 and CF2 groups. Both FTIR and XPS confirm that the product of Zn[CF3(CF2)6COO]2 is formed on zinc spheres by facile method of immersion process. The groups of —CF3 and —CF2 have the lowest surface tension of 6.7 and 18 mJ/m2, respectively[29,30] , and the high content of —CF3 and —CF2 groups in the long alkyl chains is very favorable for the formation of superamphiphobic zinc surface.
 | Fig.2 Optical micrographs of droplets of water(A.contact angle, B.sliding angle),cooking oil(C), garlic oil(D), mustard oil(E), dodecane(F), hexadecane(G) and diesel oil(H) on superamphiphobic surface of zinc spheres, respectively. Inset inFig.2A and 2F separately corresponds to the optical micrographs of droplets of water and dodecane on pristine zinc spheres, respectively |
 | Fig.3 (A)FTIR spectra of CF3(CF2)6COOH and Zn[CF3(CF2)6COO]2. XPS spectrum(B), high-resolutuon C1 s(C) and F1 s(D) peaks of the as-prepared superamphiphobic zinc spheres |
The results obtained from FTIR and XPS clearly show that the chemical composition of nanosheets on zinc spheres is Zn[CF3(CF2)6COO]2, and the superamphiphobic property of treated zinc sphere is derived from the combination effects of the hierarchical micro/nanostructures and the chemical composition of the low surface energy of Zn[CF3(CF2)6COO]2. In order to investigate the effect of superamphiphobicity on the buoyancy of zinc spheres in water and oil, pristine and superamphiphobic zinc spheres were put in water and diesel oil, respectively, consequently, zinc spheres with superamphiphobicity always keep floating on water and diesel oil while pristine zinc spheres without immersion treatment sank quickly into the bottom of water and diesel oil, as shown inFig.4A. The results indicate that the superamphiphobicity induced by immersion process can sharply enhance the buoyancy of zinc spheres and make them float on water and diesel. It is well known, water striders are capable of slipping quickly on water surface using their superior water repellent legs[31]. Superhydrophobic zinc spheres can float long time on water surface, however, when the zinc shperes floating on water surface were vigorously stirred for 30 min at room temperature, zinc spheres began to sink into bottom of water, and the wettability was changed from superhydrophobicity to superhydrophilicity. Interestingly, the superhydrophilic zinc spheres recovered to be superhydrophobic after being washed by ethanol and dried at 80 ℃ for 6 h. The reversible changes between superhydrophobicity to superhydrophilicity triggered by vigorous stirring and annealing could be cycled at least 10 times, and the corresponding water contact angles on superhydrophobic zinc spheres always maintained to be about (160±2)°, as shown inFig.4B.
As described above, higher contact angle and lower sliding angle indicate perfect superamphiphobicity of zinc spheres induced by solution immersion treatments. Herein, the formation mechanism of superamphiphobicity can be discussed on the basis of Cassie-Baxter equation[32]. When water or oil droplets were dripped on the surface of zinc spheres with micro/nanometer hierarchical structures and chemical composition of low surface energy, water or oil droplets could not penetrate into the grooves , but rather suspended on the micro/nano-sheets film due to the air trapped within the interstices of the microtextured surface. That is, the apparent contacts between water/oil droplets and solid of zinc spheres are actually a composite contacts between solid-liquid-air, and the liquid of water or oil is mainly supported by the trapped air underneath liquid. According to the Cassie equation (3)[32]:
Where f1 and f2 stands for the area fraction of solid and air on the composite interface, respectively. θ is the intrinsic contact angle of the flat and smooth surface which has identical chemical composition with rough surface, θ=110°(here, the flat and smooth surface was roughly imitated by a Si wafer coated by zinc)[28]. And θ* corresponds to the apparent water contact angle on Zn[CF3(CF2)6COO]2 composite surface, θ*=162°. With the known values, f1 and f2 were calculated to be about 0.074 and 0.926, respectively, which indicates that at the composite interface of solid-liquid-air, the fraction of air trapped in the interstices of microstructure is about 92.6%, and the fraction of solid is only 7.4%. As schematically illustrated inFig.4C, the large fraction of air trapped in the composite interface can effectively prevent the penetration of water or oil into grooves, and the properties of superamphiphobicity with higher contact angle and lower sliding angle were finally obtained.
Jiang's group successfully fabricated superamphiphobic coating by electrochemical reaction and electrodeposition, but the methods is only limited to metal or alloy substrates[28], and how to construct superamphiphobic surfaces without the limitation of substrates is still a big challenge. In order to evaluate the applications of superamphiphobic zinc spheres in practice, zinc spheres were pasted to the substrates of wood, plastic, glass slide, stainless steel, paper and stone by binder of PVA(PVA was first uniformally coated on substrates, and then the superamphiphobic zinc spheres were cast on substrates), with the subsequent treatment of annealing at 60 ℃ for 30 min and the substrates began to be superhydrophobic and superoleophobic, as shown inFig.5. The results indicate that it is feasible to construct superamphiphobic surfaces with antifouling and water/oil repellency properties on different kinds of substrates via simple solution immersion combining with subsequent treatment of pasting.
The mechanical stability is an essential requirement for the use of superamphiphobic materials in practical application. In order to test the mechanical stability of the superamphiphobic surfaces on glass substrate, sand grains(sieved by a 30 aperture mesh) flowed down from 3.0 cm height at a speed of 0.64 g/s and rubbed with the inclined glass, and the changes of water contact angles were recorded as the increases of friction time. As shown inFig.4D, with the increases of friction times, the water contact angle decreases sharply from 160° to about 132°, which reveals that the superamphiphobic coating formed by zinc spheres can be easily destroyed by the mechanical friction, and how to improve and strengthen the mechanical stability of superamphiphobic surfaces need to be further investigated.
In conclusion, we have developed a facile method for constructing superamphiphobic zinc spheres with high buoyancy via a facile solution-immersion technique. The combination effects of the flower-like hierarchical structures and the chemical composition with low surface energy of Zn[CF3(CF2)6COO]2 finally induce the superamphiphobic property. Pasting the superamphiphobic zinc spheres to the solid surfaces induces superhydrophobicity and superoleophobicity properties of surfaces. It is believed that the method described here may open a new avenue for the design and creation of superamphiphobic materials via simple solution immersion, and we believe that the zinc spheres with superamphiphobicity may be employed to construct anticorrosion, water-repellency, oil-proof and anti-fouling coatings on solid surfaces.
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|